Abstract
Background
Chronic obstructive pulmonary disease (COPD) is one of the leading causes of morbidity and mortality worldwide and is characterized by a partially reversible airflow limitation. Currently, many studies put forward that COPD is associated with both genetic and environmental factors. It has been reported that germline mutations in telomerase are risk factors for COPD susceptibility. In this study, we validated the association between TERT polymorphisms and COPD risk with a case–control study in the Chinese Li population.
Methods
A total of 279 COPD patients and 290 control individuals were recruited. We identified five single nucleotide polymorphisms (SNPs) in TERT that were associated with COPD. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated in logistic regression models after adjusting for age and gender to assess the association.
Results
In the genetic model analysis, we found the “C/T‐T/T” genotype of rs10069690 in TERT was associated with an increased COPD risk in the dominant model (p = 0.046); the rs2853677 in TERT was significantly associated with increased COPD risk based on the codominant model (“A/G” genotype, p = 0.033), dominant model (A/G‐G/G genotype, p = 0.0091), and log‐additive model (p = 0.023). The rs2853676 in TERT could increase the risk of COPD in the dominant model (“C/T‐T/T” genotype, p = 0.026) and in the Log‐additive model (p = 0.022).
Conclusion
Our data shed new light on the association between TERT SNPs and COPD susceptibility in the Chinese Li population.
Keywords: Chinese Li population, chronic obstructive pulmonary disease, single nucleotide polymorphism (SNP), susceptibility, TERT
1. INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is a complex respiratory disease that seriously affects human respiration, characterized by incomplete reversible airflow limitation, and the physiological signs of COPD are cough, sputum, shortness of breath, or dyspnea (Vestbo et al., 2013; Vogelmeier et al., 2017). COPD plays an important role in the major causes of global morbidity and mortality (Murray et al., 2013; Toy, Gallagher, Stanley, Swensen, & Duh, 2010). According to the study of COPD in China, in rural areas and urban areas in China to COPD as the main cause of death were ranked third, fourth (Fang, Wang, & Bai, 2011). COPD is formed by complex environmental and genetic factors. Although smoking is identified as a major risk factor, due to individual differences, only a small number of smokers develop COPD, which suggesting that genetic susceptibility may play an important role (Hallberg et al., 2008; Mathers & Loncar, 2006). DeMeo et al. (2004) have estimated the heritability of COPD and Silverman et al. (1998) have proved that an increased risk of COPD for first‐degree relatives of probands with COPD. However, the current pathogenesis of COPD based on molecular genetics remains unclear. At this stage we can try to find some genes that cause the risk of COPD in genetic polymorphisms (Silverman et al., 2011).
Telomere is a DNA‐protein structure located at the end of the chromosome that is protecting the chromosome integrity and controlling the cell cycle. Telomere will gradually shorten with cell division, age growth, when shortened to a certain extent cause cell senescence and apoptosis (Armanios, 2013). Telomerase is a basic nucleoprotein reverse transcriptase (TERT; OMIM: 187270). Telomere DNA can be added to the eukaryotic chromosome terminal. Prolonged telomeres not only maintain the chromosome hereditary stability but also enhance the cell's ability (Kim et al., 2009; Young, 2010). Stanley et al. have shown that the carriers of germline TERT mutations are associated with an increased risk of emphysema. Therefore, the TERT gene may contribute to the development of COPD.
In this case–control study, we performed an extensive association analysis to assess the roles of TERT polymorphisms and haplotypes on COPD susceptibility in a Chinese Li Nationality of Hainan Province. We conducted the relationship between five TERT single nucleotide polymorphisms (SNPs) and COPD risk, which could identify several markers to improve patient survival and guide intervention decisions.
2. MATERIALS AND METHODS
2.1. Ethical compliance
All human samples in this study have been approved by the local ethics committee of People's Hospital of Hainan Province. This investigation was conducted in accordance with the Declaration of Helsinki and following national and international guidelines. All participants agreed to participate, and signed the informed consent.
2.2. Subject enrollment
A total of 279 patients with COPD from Li Nationality in Hainan Province of China were recruited in our study (134 women, 145 males), who were diagnosed by the People's Hospital of Hainan Province, and diagnostic criteria are based on WHO Global initiative for chronic Obstructive Lung Disease (GOLD) (Rabe et al., 2007). We excluded subjects who are clearly suffering from other significant respiratory or pulmonary diseases, such as pneumonia, tuberculosis, lung cancer, etc. All cases were validated, and the patient had no age, sex, smoking status, or disease stage limitation.
We recruited 290 unrelated people as a control group (142 females, 148 males), they are the Li nationality, living in Hainan Province of China. The physical condition of these subjects was diagnosed by the same hospital, and we chose the subjects with normal lung function, no lung‐related diseases, other chronic diseases and disorders, and severe endocrine, metabolic, and nutritional disorders.
2.3. Demographic and clinical data
For the subjects, we provide a standardized epidemiological questionnaire (Yan, Wang, Bo, & Li, 2017) to collect demographic and personal data. After sorting the collected information, we select some variables for statistical analysis, including: age, gender, smoking status. In addition, smoking status was recorded as current, former, or never smoking by standardized epidemiological questionnaire. Current smokers were individuals who smoked at least one cigarette per day in the past 12 months, even he/she had quitted within a year. Former smokers were individuals who had ceased smoking more than 1 year earlier. Never smokers were individuals who had never smoked regularly.
We collected patients and normal people 5 ml of peripheral venous blood as experimental specimens, and to ensure that patients do not receive any treatment before the collection.
2.4. SNP assay design and genotyping
Using the public HapMap databases, we randomly selected five SNPs on the basis of their allele frequencies, location, and disease relevance etc. All SNPs in TERT (GenBank: NG_009265.1) which have been validated with minor allele frequency (MAF)> 5% in the 1,000 genome (http://www.internationalgenome.org/). A total of five SNPs in the TERT gene were selected for further genotyping.
We collected the subjects about 5 ml of venous blood in EDTA tubes and stored in a −80℃ refrigerator. Extraction of genomic DNA from peripheral blood using the E.Z.N.A. ® Blood Mini Kit II (Omega Bio‐tek, Inc, USA), and then concentration was detected by the NanoDrop 2000 (Thermo Scientific, Waltham, MA, USA). Genotyping of all SNPs was performed using MassARRAY Nanodispenser (Agena Bioscience, San Diego, CA, USA and MassARRAY iPLEX platform (Agena Bioscience, San Diego, CA, USA), the experimental procedure was performed according to the manufacturer's protocol (Gabriel, Ziaugra, & Tabbaa, 2009). Primers and multiplex reactions were designed using the Agena Bioscience Assay Design Suite V2.0 software (https://agenacx.com/online-tools/), the corresponding primer for each SNPs is shown in Table 1. Data management and analysis were performed using Sequenom Typer 4.0 software (Gabriel et al., 2009; Thomas et al., 2007).
Table 1.
PCR primers
| SNP_ID | 1st‐PCRP | 2nd‐PCRP | UEP_SEQ |
|---|---|---|---|
| rs2075786 | ACGTTGGATGCAGGTTACACACGTGGTGAG | ACGTTGGATGCGCCACTCTTGACTTTCCAA | GGCAAAGAGCAGCAGGAGCC |
| rs10069690 | ACGTTGGATGCCTGTGGCTGCGGTGGCTG | ACGTTGGATGATGTGTGTTGCACACGGGAT | GGGATCCTCATGCCA |
| rs2242652 | ACGTTGGATGACAGCAGGACACGGATCCAG | ACGTTGGATGAGGCTCTGAGGACCACAAGA | GTCGGAGGACCACAAGAAGCAGC |
| rs2853677 | ACGTTGGATGATCCAGTCTGACAGTCGTTG | ACGTTGGATGGCAAGTGGAGAATCAGAGTG | GGGTAATCAGAGTGCACCAG |
| rs2853676 | ACGTTGGATGTGTCTCCTGCTCTGAGACC | ACGTTGGATGCAAAACTAAGACCCAAGAGG | AGATGGAAGTCTGACGAAGGC |
Abbreviations: PCRP, PCR primers; SNP, Single‐nucleotide polymorphism; UEP SEQ, Unextended mini‐sequencing primer.
2.5. Statistical analysis
Data analysis was performed according to the standard statistical analysis procedure (Lykouras et al., 2008). Microsoft Excel and the SPSS software version 19.0 (SPSS Inc., Chicago, IL, USA) were used for statistical analyses. The Hardy‐Weinberg equilibrium test was performed on the control group using the exact test to evaluate the reliability of the control group. Differences in demographic characteristics between the cases and controls were assessed using the chi‐square test (categorical variables) and Student's t test (continuous variables). In addition, χ 2 test was used to calculate the allele frequencies and genotype frequencies of the case and control group. ORs (odds ratios) and 95% CI (95% confidence interval) were calculated using unconditional logistic regression analysis with adjustment for age, gender, and smoking, and used to assess the association between SNPs and COPD risk in the four models (genotype, dominance, recessive, and additive) (Bland & Altman, 2000). All p‐values are two‐sided in this study, and a value of p ≤ 0.05 was defined as statistically significant. The Haploview4.2 software package and the SHEsis software platform (http://www.nhgg.org/analysis/) software were employed to analyze the linkage disequilibrium, haplotype construction, and genetic association of polymorphic loci (Barrett, Fry, Maller, & Daly, 2005; Shi & He, 2005).
3. RESULTS
The genotypes of 569 participants were analyzed in this study, including 279 patients with COPD and 290 healthy individuals. Basic characteristics (gender, age, and smoking status) of the cases and controls are shown in Table 2. There were no significant differences in gender (p = 0.823) or smoking status (p = 0.286) between the cases and the controls. Nevertheless, statistically significant differences in age were observed, patients with COPD were significantly older than controls (69.05 ± 9.732 years vs 56.13 ± 14.054 years, p < 0.050).
Table 2.
General characteristics the of this study population
| Variables | Case | Control | p‐value |
|---|---|---|---|
| (N = 279) | (N = 290) | ||
| Sex | 0.823 | ||
| Female | 134 (48%) | 142 (49%) | |
| Male | 145 (52%) | 148 (51%) | |
| Age, year(mean ± SD) | 69.05 ± 9.732 | 56.13 ± 14.054 | <0.05 |
| Smoking | 0.286 | ||
| Yes | 92 (33.3%) | 84 (29.2%) | |
| No | 184 (66.7%) | 204 (70.8%) |
p‐values were calculated by Wald test and p < 0.05 indicates statistical significance.
Smoking (yes): including current smokers and former smoker; Smoking (no): including individuals who have never smoked regularly.
The basic characteristics of the five SNPs of TERT in our study are listed in Table 3, and include the association between five SNPs genotype and COPD risk. By the exact test, the five SNPs in the control group were in Hardy‐Weinberg (p > 0.05). The call rate of SNPs was above 98.5% in cases and controls. However, there were no statistically significant differences in patients and controls in allele frequency distributions of SNPs. In other words, there is no statistically significant association between allele and COPD risk.
Table 3.
Frequency distributions of TERT alleles and their associations with COPD
| SNPs | Position | Locus | Alleles (A/B) | MAF | HWE p value | OR 95% CI | p value | |
|---|---|---|---|---|---|---|---|---|
| Case | Control | |||||||
| rs2075786 | 1266310 | 5p15.33 | G/A | 0.189 | 0.181 | 0.162 | 1.06 (0.78–1.43) | 0.721 |
| rs10069690 | 1279790 | 5p15.33 | T/C | 0.156 | 0.120 | 0.153 | 1.36 (0.96–1.91) | 0.081 |
| rs2242652 | 1280028 | 5p15.33 | A/G | 0.154 | 0.128 | 0.107 | 1.24 (0.89–1.74) | 0.206 |
| rs2853677 | 1287194 | 5p15.33 | G/A | 0.369 | 0.321 | 0.281 | 1.24 (0.97–1.58) | 0.085 |
| rs2853676 | 1288547 | 5p15.33 | T/C | 0.159 | 0.121 | 0.279 | 1.38 (0.99–1.94) | 0.059 |
Abbreviations: Alleles A/B, Minor/major alleles; CI, confidence interval; HWE, Hardy‐Weinberg Equilibrium; MAF, minor allele frequency; ORs: odds ratios; SNPs, single nucleotide polymorphisms.
p < 0.05 indicates statistical significance.
Furthermore, we used the genetic model to analyze the association between the five SNPs of TERT and the risk of COPD, which were performed by logistic tests and listed in Table 4. The “C/T‐T/T” genotype of rs10069690 was analyzed in the dominant model for COPD with an increased risk (OR, 1.56; 95% CI, 1.01–2.43; p = 0.046). The “A/G” genotype at the rs2853677 increased the risk of COPD in the codominant model (OR, 1.6 9; 95% CI, 1.12–2.53; p = 0.033), and the SNP in the dominant model, log‐additive model both associated with COPD risk (OR, 1.67; 95% CI, 1.13–2.46; p = 0.009; OR, 1.39; 95% CI, 1.04–1.86; p = 0.023). For the rs2853676 SNP, “C/T‐T/T” genotype increased the risk of COPD in the dominant model (OR, 1.66; 95% CI, 1.06–2.59; p = 0.026), and both increased the risk of COPD in the log‐additive model (OR, 1.56; 95% CI, 1.06–2.29; p = 0.022). The other two SNPs had no statistically significant association with COPD risk. All SNPs susceptibility analysis of COPD was performed after adjustment by age, gender, and smoking. After application of the Bonferroni correction (p < 0.05/5 TERT variants × 4 genetic models = 0.05/20 = 0.0025), we found no SNPs significantly associated with the risk of COPD.
Table 4.
Genotypic model analysis of relationship between SNPs and COPD
| SNP | Model | Genotype | Cases | Controls | OR (95% CI) | p‐value | AIC | BIC |
|---|---|---|---|---|---|---|---|---|
| rs2075786 | Codominant | A/A | 181 (66.5%) | 197 (68.9%) | 1 | 0.970 | 636.2 | 662.1 |
| A/G | 81 (29.8%) | 76 (26.6%) | 1.05 (0.69–1.61) | |||||
| G/G | 10 (3.7%) | 13 (4.5%) | 1.01 (0.38–2.69) | |||||
| Dominant | A/A | 181 (66.5%) | 197 (68.9%) | 1 | 0.820 | 634.2 | 655.8 | |
| A/G‐G/G | 91 (33.5%) | 89 (31.1%) | 1.05 (0.70–1.57) | |||||
| Recessive | A/A‐A/G | 262 (96.3%) | 273 (95.5%) | 1 | 0.990 | 634.2 | 655.8 | |
| G/G | 10 (3.7%) | 13 (4.5%) | 1.00 (0.38–2.63) | |||||
| Log‐additive | – | – | – | 1.03 (0.73–1.45) | 0.850 | 634.2 | 655.8 | |
| rs10069690 | Codominant | C/C | 192 (71.1%) | 224 (78.3%) | 1 | 0.062 | 624.9 | 650.9 |
| C/T | 73 (27%) | 55 (19.2%) | 1.69 (1.07–2.67) | |||||
| T/T | 5 (1.8%) | 7 (2.5%) | 0.70 (0.18–2.65) | |||||
| Dominant | C/C | 192 (71.1%) | 224 (78.3%) | 1 | 0.046 | 624.5 | 646.1 | |
| C/T‐T/T | 78 (28.9%) | 62 (21.7%) | 1.56 (1.01–2.43) | |||||
| Recessive | C/C‐C/T | 265 (98.2%) | 279 (97.5%) | 1 | 0.480 | 628.0 | 649.6 | |
| T/T | 5 (1.8%) | 7 (2.5%) | 0.62 (0.16–2.34) | |||||
| Log‐additive | – | – | – | 1.36 (0.92–2.02) | 0.120 | 626.1 | 647.7 | |
| rs2242652 | Codominant | G/G | 197 (71.4%) | 221 (77%) | 1 | 0.140 | 636.9 | 662.9 |
| G/A | 74 (26.8%) | 58 (20.2%) | 1.50 (0.95–2.35) | |||||
| A/A | 5 (1.8%) | 8 (2.8%) | 0.62 (0.17–2.24) | |||||
| Dominant | G/G | 197 (71.4%) | 221 (77%) | 1 | 0.140 | 636.6 | 658.3 | |
| G/A‐A/A | 79 (28.6%) | 66 (23%) | 1.38 (0.90–2.13) | |||||
| Recessive | G/G‐G/A | 271 (98.2%) | 279 (97.2%) | 1 | 0.370 | 638.0 | 659.6 | |
| A/A | 5 (1.8%) | 8 (2.8%) | 0.56 (0.15–2.03) | |||||
| Log‐additive | – | – | – | 1.22 (0.83–1.79) | 0.310 | 637.7 | 659.4 | |
| rs2853677 | Codominant | A/A | 104 (37.7%) | 137 (47.6%) | 1 | 0.033 | 635.2 | 661.2 |
| A/G | 142 (51.5%) | 117 (40.6%) | 1.69 (1.12–2.53) | |||||
| G/G | 30 (10.9%) | 34 (11.8%) | 1.61 (0.85–3.07) | |||||
| Dominant | A/A | 104 (37.7%) | 137 (47.6%) | 1 | 0.009 | 633.3 | 654.9 | |
| A/G‐G/G | 172 (62.3%) | 151 (52.4%) | 1.67 (1.13–2.46) | |||||
| Recessive | A/A‐A/G | 246 (89.1%) | 254 (88.2%) | 1 | 0.500 | 639.6 | 661.3 | |
| G/G | 30 (10.9%) | 34 (11.8%) | 1.23 (0.67–2.25) | |||||
| Log‐additive | – | – | – | 1.39 (1.04–1.86) | 0.023 | 634.9 | 656.6 | |
| rs2853676 | Codominant | C/C | 197 (71.4%) | 224 (77.8%) | 1 | 0.073 | 636.8 | 662.8 |
| C/T | 70 (25.4%) | 58 (20.1%) | 1.60 (1.00–2.55) | |||||
| T/T | 9 (3.3%) | 6 (2.1%) | 2.23 (0.67–7.46) | |||||
| Dominant | C/C | 197 (71.4%) | 224 (77.8%) | 1 | 0.026 | 635.1 | 656.8 | |
| C/T‐T/T | 79 (28.6%) | 64 (22.2%) | 1.66 (1.06–2.59) | |||||
| Recessive | C/C‐C/T | 267 (96.7%) | 282 (97.9%) | 1 | 0.250 | 638.7 | 660.4 | |
| T/T | 9 (3.3%) | 6 (2.1%) | 2.00 (0.60–6.65) | |||||
| Log‐additive | – | – | – | 1.56 (1.06–2.29) | 0.022 | 634.9 | 656.5 |
Abbreviations: AIC, Akaike's information criterion; BIC, Bayesian information criterion; CI, confidence interval; ORs, odds ratios; SNPs, single nucleotide polymorphisms.
p‐values were calculated by Wald test and p < 0.05 indicates statistical significance.
Finally, we used linkage disequilibrium analysis (LD) to select SNPs that fell into the same LD block to construct haplotypes, we analyzed the haplotype structure of the TERT gene,LD was firmed pairwise between five SNPs, and two SNPs (rs10069690 and 2242652) were detected in single LD blocks (Figure 1). The Pearson χ 2 test was used to determine the correlation between haplotype and COPD. Based on the analysis, no positive result was obtained as shown in Table 5.
Figure 1.
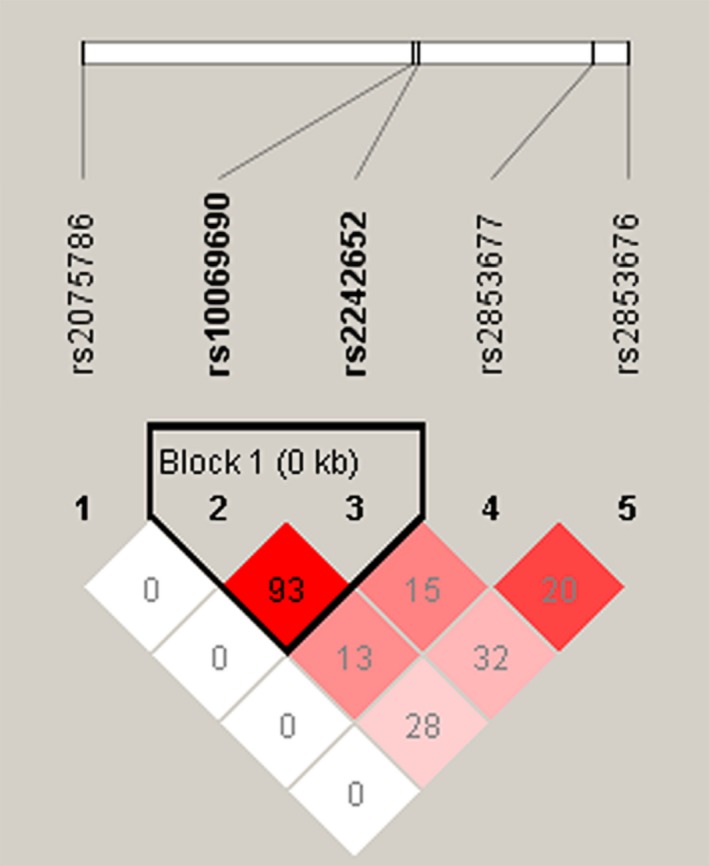
Linkage disequilibrium plots containing five SNPs from TERT gene
Table 5.
Haplotype analysis results in this study
| SNPs | Haplotype | Frequency | Before adjusted | After adjusted | |||
|---|---|---|---|---|---|---|---|
| Case | Control | OR(95% CI) | p‐value | OR(95% CI) | p a‐value | ||
| rs10069690/rs2242652 | CG | 0.846 | 0.872 | 1 | – | 1 | – |
| TA | 0.152 | 0.121 | 1.30 (0.92–1.82) | 0.130 | 1.29 (0.88–1.91) | 0.206 | |
Abbreviations: CI, confidence interval; ORs, odds ratios; SNPs, single nucleotide polymorphisms.
p‐values < 0.05 indicates statistical significance; p a: adjusted by gender and age.
4. DISCUSSION
In this study, we analyzed the relationship between genetic variants of TERT and COPD in the Chinese Li population. We found that TERT SNPs (rs10069690, rs2853677, rs2853676) were associated with COPD risk.
Chronic inflammation is a prominent feature of COPD (Celli & MacNee, 2004; Cosio & Agusti, 2010; Sin & Man, 2005). The main reason for this is the excessive inflammation of the airway in response to chronic irritants such as smoking, which in turn can lead to airway remodeling and destruction of lung parenchyma (Amsellem et al., 2011). This persistent inflammation may not only be a major driver factor of COPD progression, but may also lead to systemic complications with poor outcomes, such as cardiovascular disease, weight loss, and muscle dysfunction (Bolton et al., 2004; Sabit et al., 2007; Sin & Man, 2005). It is well known that the genetic factors contribute prominently to the susceptibility to COPD and many studies have proved on correlation between the genes and COPD. It has been confirmed that the hedgehog interacting protein (HHIP), cholinergic receptor nicotinic alpha 5 subunit (CHRNA5), and trafficking protein particle complex 9 (TRAPPC9) are associated with susceptibility to COPD (Boueiz et al., 2017; Manichaikul et al., 2014). Recently, several studies have suggested that telomeres may play a pivotal role in the pathogenesis of COPD (Chilosi, Poletti, & Rossi, 2012; Lee, Sandford, Man, & Sin, 2011; Tsuji, Aoshiba, & Nagai, 2010). Compared to control subjects, patients with COPD have shorter telomeres (Mui et al., 2009). Telomeres are the structures of DNA proteins that protect the ends of chromosomes. The shortening and dysfunction of telomeres occurs with cell division and aging, which means that DNA damage causes cell senescence and apoptosis (Armanios, 2013; Harley, Futcher, & Greider, 1990). Telomerase is the enzyme complex which can generate and maintain telomeres. It is comprised of telomerase reverse transcriptase (TERT) and an RNA template, telomerase RNA component (TERC), which provide the template for telomere repeat addition (Gansner & Rosas, 2013). Lung disease is the primary life‐threatening presentation in patients with telomerase mutations (Parry, Alder, Qi, Chen, & Armanios, 2011). The mutations in the telomerase cause telomere shortening, and have their most common clinical manifestation in lung disease. Alder JK et al. studied telomerase‐deficient mice. And the results showed that although mice with short telomeres have no baseline histologic defects, they are more susceptible to developing emphysema after cigarette smoke exposure. (Alder et al., 2011). Stanley et al. demonstrated that deleterious telomerase mutations are a risk factor by examining their frequency in smokers with COPD. They found that 3 of 292 patients with severe COPD carried deleterious TERT mutation (1%), and all of them fell in conserved motifs within the telomerase reverse transcriptase domain (Stanley et al., 2015). However, we have not found any evidence for the correlation on heredity between TERT and COPD susceptibility in previous study. To the best of our knowledge, this is the first study to evaluate the association of these SNPs in TERT gene with the risk of COPD in Chinese Han population. Our results suggested that TERT may be the genetic risk factor of COPD.
There still existed several limitations in the present study that should be considered when interpreting the results. First, genetic associations with COPD were not able to elucidate causal mechanisms. After the Bonferroni correction (p < 0.05/20), there was no SNPs significantly associated with the risk of COPD. So we need a greater number of loci and a larger sample size would be needed for additional evidence regarding the role of these genes in COPD. Second, the effects of secondhand smoke or the non‐smoking individuals were not considered. Hence, further research would analyze the relationship between COPD and the detailed smoking status, including secondhand smoking, smoking intensity, and air smog. Moreover, in the present study, the detection was not performed in heterozygous patients due to the limited sample size. Further study should enroll a large sample size to verify the strength of the study.
In general, we proofed that the three SNPs (rs10069690, rs2853677, and rs2853676) polymorphisms in TERT are associated with an increased risk of COPD in patients from the Li ethnicity, living in Hainan Province for the first time. Our results suggest that the complex genetic control of telomere biology and telomerase plays a key role in the development of COPD. These results may also help to raise awareness of the differences among populations in COPD etiology, and it shows that the genetic variation in TERT gene plays a complex role in the occurrence of COPD. The interaction of TERT gene locus may be more important than single locus. To sum up, in conclusion, our results may provide new data for COPD risk screening, revealing new candidate genes and new ideas for studying the mechanisms of follow‐up to COPD.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (No. 81660013 and No. 81160008). We thank all of the participants for their involvement in this study. We are very grateful to the clinicians and other hospital staff for providing blood samples and data collection for this study.
Ding Y, Li Q, Wu C, et al. TERT gene polymorphisms are associated with chronic obstructive pulmonary disease risk in the Chinese Li population. Mol Genet Genomic Med. 2019;7:e773 10.1002/mgg3.773
Yipeng Ding and Quanni Li are joint first authors.
Contributor Information
Yipeng Ding, Email: dryipengding@163.com.
Pingdong Xie, Email: cwcxpd.ok@163.com.
REFERENCES
- Alder, J. K. , Guo, N. , Kembou, F. , Parry, E. M. , Anderson, C. J. , Gorgy, A. I. , … Armanios, M. (2011). Telomere length is a determinant of emphysema susceptibility. American Journal of Respiratory and Critical Care Medicine, 184(8), 904–912. 10.1164/rccm.201103-0520OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsellem, V. , Gary‐Bobo, G. , Marcos, E. , Maitre, B. , Chaar, V. , Validire, P. , … Adnot, S. (2011). Telomere dysfunction causes sustained inflammation in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine, 184(12), 1358–1366. 10.1164/rccm.201105-0802OC [DOI] [PubMed] [Google Scholar]
- Armanios, M. (2013). Telomeres and age‐related disease: How telomere biology informs clinical paradigms. Journal of Clinical Investigation, 123(3), 996–1002. 10.1172/jci66370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett, J. C. , Fry, B. , Maller, J. , & Daly, M. J. (2005). Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics, 21(2), 263–265. 10.1093/bioinformatics/bth457 [DOI] [PubMed] [Google Scholar]
- Bland, J. M. , & Altman, D. G. (2000). Statistics notes. The odds ratio. British Medical Journal, 320(7247), 1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton, C. E. , Ionescu, A. A. , Shiels, K. M. , Pettit, R. J. , Edwards, P. H. , Stone, M. D. , … Shale, D. J. (2004). Associated loss of fat‐free mass and bone mineral density in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine, 170(12), 1286–1293. 10.1164/rccm.200406-754OC [DOI] [PubMed] [Google Scholar]
- Boueiz, A. , Lutz, S. M. , Cho, M. H. , Hersh, C. P. , Bowler, R. P. , Washko, G. R. , … DeMeo, D. L. (2017). Genome‐wide association study of the genetic determinants of emphysema distribution. American Journal of Respiratory and Critical Care Medicine, 195(6), 757–771. 10.1164/rccm.201605-0997OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celli, B. R. , MacNee, W. , Agusti, A. , Anzueto, A. , Berg, B. , Buist, A. S. , … ZuWallack, R. (2004). Standards for the diagnosis and treatment of patients with COPD: A summary of the ATS/ERS position paper. European Respiratory Journal, 23(6), 932–946. 10.1183/09031936.04.00014304 [DOI] [PubMed] [Google Scholar]
- Chilosi, M. , Poletti, V. , & Rossi, A. (2012). The pathogenesis of COPD and IPF: Distinct horns of the same devil? Respiratory Research, 13, 3 10.1186/1465-9921-13-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosio, B. G. , & Agusti, A. (2010). Update in chronic obstructive pulmonary disease 2009. American Journal of Respiratory and Critical Care Medicine, 181(7), 655–660. 10.1164/rccm.201001-0111UP [DOI] [PubMed] [Google Scholar]
- DeMeo, D. L. , Carey, V. J. , Chapman, H. A. , Reilly, J. J. , Ginns, L. C. , Speizer, F. E. , … Silverman, E. K. (2004). Familial aggregation of FEF(25–75) and FEF(25–75)/FVC in families with severe, early onset COPD. Thorax, 59(5), 396–400. 10.1136/thx.2003.012856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang, X. , Wang, X. , & Bai, C. (2011). COPD in China: The burden and importance of proper management. Chest, 139(4), 920–929. 10.1378/chest.10-1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel, S. , Ziaugra, L. , & Tabbaa, D. (2009). SNP genotyping using the Sequenom MassARRAY iPLEX platform. Current Protocols in Human Genetics, Chapter 2, Unit 2.12. 10.1002/0471142905.hg0212s60 [DOI] [PubMed] [Google Scholar]
- Gansner, J. M. , & Rosas, I. O. (2013). Telomeres in lung disease. Translational Research, 162(6), 343–352. 10.1016/j.trsl.2013.04.001 [DOI] [PubMed] [Google Scholar]
- Hallberg, J. , Dominicus, A. , Eriksson, U. K. , Gerhardsson de Verdier, M. , Pedersen, N. L. , Dahlbäck, M. , … Svartengren, M. (2008). Interaction between smoking and genetic factors in the development of chronic bronchitis. American Journal of Respiratory and Critical Care Medicine, 177(5), 486–490. 10.1164/rccm.200704-565OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley, C. B. , Futcher, A. B. , & Greider, C. W. (1990). Telomeres shorten during ageing of human fibroblasts. Nature, 345(6274), 458–460. 10.1038/345458a0 [DOI] [PubMed] [Google Scholar]
- Kim, J. , Kang, J. W. , Park, J. H. , Choi, Y. , Choi, K. S. , Park, K. D. , … Kim, H. S. (2009). Biological characterization of long‐term cultured human mesenchymal stem cells. Archives of Pharmacal Research, 32(1), 117–126. 10.1007/s12272-009-1125-1 [DOI] [PubMed] [Google Scholar]
- Lee, J. , Sandford, A. , Man, P. , & Sin, D. D. (2011). Is the aging process accelerated in chronic obstructive pulmonary disease? Current Opinion in Pulmonary Medicine, 17(2), 90–97. 10.1097/MCP.0b013e328341cead [DOI] [PubMed] [Google Scholar]
- Lykouras, D. , Sampsonas, F. , Kaparianos, A. , Karkoulias, K. , Tsoukalas, G. , & Spiropoulos, K. (2008). Human genes in TB infection: Their role in immune response. Monaldi Archives for Chest Disease, 69(1), 24–31. 10.4081/monaldi.2008.408 [DOI] [PubMed] [Google Scholar]
- Manichaikul, A. , Hoffman, E. A. , Smolonska, J. , Gao, W. , Cho, M. H. , Baumhauer, H. , … Barr, R. G. (2014). Genome‐wide study of percent emphysema on computed tomography in the general population. The multi‐ethnic study of atherosclerosis Lung/SNP health association resource study. American Journal of Respiratory and Critical Care Medicine, 189(4), 408–418. 10.1164/rccm.201306-1061OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers, C. D. , & Loncar, D. (2006). Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med, 3(11), e442 10.1371/journal.pmed.0030442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mui, T. S. Y. , Man, J. M. , McElhaney, J. E. , Sandford, A. J. , Coxson, H. O. , Birmingham, C. L. , … Sin, D. D. (2009). Telomere length and chronic obstructive pulmonary disease: Evidence of accelerated aging. Journal of the American Geriatrics Society, 57(12), 2372–2374. 10.1111/j.1532-5415.2009.02589.x [DOI] [PubMed] [Google Scholar]
- Murray, C. J. , Atkinson, C. , Bhalla, K. , Birbeck, G. , Burstein, R. , Chou, D. , … Murray, W. S. (2013). The state of US health, 1990–2010: Burden of diseases, injuries, and risk factors. JAMA, 310(6), 591–608. 10.1001/jama.2013.13805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry, E. M. , Alder, J. K. , Qi, X. , Chen, J. J. , & Armanios, M. (2011). Syndrome complex of bone marrow failure and pulmonary fibrosis predicts germline defects in telomerase. Blood, 117(21), 5607–5611. 10.1182/blood-2010-11-322149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabe, K. F. , Hurd, S. , Anzueto, A. , Barnes, P. J. , Buist, S. A. , Calverley, P. , … Zielinski, J. (2007). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. American Journal of Respiratory and Critical Care Medicine, 176(6), 532–555. 10.1164/rccm.200703-456SO [DOI] [PubMed] [Google Scholar]
- Sabit, R. , Bolton, C. E. , Edwards, P. H. , Pettit, R. J. , Evans, W. D. , McEniery, C. M. , … Shale, D. J. (2007). Arterial stiffness and osteoporosis in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine, 175(12), 1259–1265. 10.1164/rccm.200701-067OC [DOI] [PubMed] [Google Scholar]
- Shi, Y. Y. , & He, L. (2005). SHEsis, a powerful software platform for analyses of linkage disequilibrium, haplotype construction, and genetic association at polymorphism loci. Cell Research, 15(2), 97–98. 10.1038/sj.cr.7290272 [DOI] [PubMed] [Google Scholar]
- Silverman, E. , Chapman, H. , Drazen, J. , Weiss, S. , Rosner, B. , Campbell, E. , … Speizer, F. (1998). Genetic epidemiology of severe, early‐onset chronic obstructive pulmonary disease. Risk to relatives for airflow obstruction and chronic bronchitis. American Journal of Respiratory and Critical Care Medicine, 157(6 Pt 1), 1770–1778. 10.1164/ajrccm.157.6.9706014 [DOI] [PubMed] [Google Scholar]
- Silverman, E. K. , Vestbo, J. , Agusti, A. , Anderson, W. , Bakke, P. S. , Barnes, K. C. , … Crapo, J. D. (2011). Opportunities and challenges in the genetics of COPD 2010: An International COPD Genetics Conference report. COPD: Journal of Chronic Obstructive Pulmonary Disease, 8(2), 121–135. 10.3109/15412555.2011.558864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin, D. D. , & Man, S. F. (2005). Chronic obstructive pulmonary disease as a risk factor for cardiovascular morbidity and mortality. Proceedings of the American Thoracic Society, 2(1), 8–11. 10.1513/pats.200404-032MS [DOI] [PubMed] [Google Scholar]
- Stanley, S. E. , Chen, J. J. L. , Podlevsky, J. D. , Alder, J. K. , Hansel, N. N. , Mathias, R. A. , … Armanios, M. (2015). Telomerase mutations in smokers with severe emphysema. Journal of Clinical Investigation, 125(2), 563–570. 10.1172/jci78554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, R. K. , Baker, A. C. , DeBiasi, R. M. , Winckler, W. , LaFramboise, T. , Lin, W. M. , … Garraway, L. A. (2007). High‐throughput oncogene mutation profiling in human cancer. Nature Genetics, 39(3), 347–351. 10.1038/ng1975 [DOI] [PubMed] [Google Scholar]
- Toy, E. L. , Gallagher, K. F. , Stanley, E. L. , Swensen, A. R. , & Duh, M. S. (2010). The economic impact of exacerbations of chronic obstructive pulmonary disease and exacerbation definition: A review. COPD: Journal of Chronic Obstructive Pulmonary Disease, 7(3), 214–228. 10.3109/15412555.2010.481697 [DOI] [PubMed] [Google Scholar]
- Tsuji, T. , Aoshiba, K. , & Nagai, A. (2010). Alveolar cell senescence exacerbates pulmonary inflammation in patients with chronic obstructive pulmonary disease. Respiration, 80(1), 59–70. 10.1159/000268287 [DOI] [PubMed] [Google Scholar]
- Vestbo, J. , Hurd, S. S. , Agustí, A. G. , Jones, P. W. , Vogelmeier, C. , Anzueto, A. , … Rodriguez‐Roisin, R. (2013). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. American Journal of Respiratory and Critical Care Medicine, 187(4), 347–365. 10.1164/rccm.201204-0596PP [DOI] [PubMed] [Google Scholar]
- Vogelmeier, C. F. , Criner, G. J. , Martinez, F. J. , Anzueto, A. , Barnes, P. J. , Bourbeau, J. , … Agusti, A. (2017). Global strategy for the diagnosis, management and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Respirology, 22(3), 575–601. 10.1111/resp.13012 [DOI] [PubMed] [Google Scholar]
- Yan, R. , Wang, Y. , Bo, J. , & Li, W. (2017). Healthy lifestyle behaviors among individuals with chronic obstructive pulmonary disease in urban and rural communities in China: A large community‐based epidemiological study. International Journal of Chronic Obstructive Pulmonary Disease, 12, 3311–3321. 10.2147/copd.s144978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, N. S. (2010). Telomere biology and telomere diseases: Implications for practice and research. Hematology American Society Hematology Education Program, 2010, 30–35. 10.1182/asheducation-2010.1.30 [DOI] [PMC free article] [PubMed] [Google Scholar]


