Abstract
Background
MicroRNA‐33a (miR‐33a) plays the role of the tumor suppressor gene by regulating the expression level of downstream genes. However, the effects of miR‐33a in renal cell cancer (RCC) remain unknown. Our study was designed to investigate the expression level and potential function of miR‐33a in RCC.
Methods
RT‐qPCR was applied to measure the levels of miR‐33a in RCC tissues and cell lines. Western blotting and luciferase reporter assay were used to detect the relationship between miR‐33a and Mouse double minute 4 (MDM4) in RCC cells. CCK‐8 and flow cytometry were applied to detected cell viability and cell cycle. Animal models and TUNEL assay were applied to detect the effect of miR‐33a on the growth of RCC and cell apoptosis.
Results
We found that the levels of miR‐33a were significantly decreased in RCC tissues and cell lines. Moreover, the low expression of miR‐33a in RCC patients indicated a shorter overall survival (OS). Notably, MDM4 as a direct target of miR‐33a in RCC, the expression level of MDM4 was significantly increased in RCC cells group than the control group. Furthermore, miR‐33a overexpression significantly inhibited RCC cells growth than the control group, while the inhibitory effects of miR‐33a were reversed upon the overexpression of MDM4. Luciferase reporter assays showed that there was a direct interaction between miR‐33a and 3′ UTR of MDM4 mRNA. In vivo, tumor volumes and weight were significantly decreased in the transfected miR‐33a mimics group than the control group.
Conclusion
Taken together, our study indicates that miR‐33a inhibits RCC cell growth by targeting MDM4.
Keywords: cell proliferation, MDM4, MiR‐33a, renal cell cancer
1. INTRODUCTION
Renal cell cancer (RCC) remains one of the most common urogenital tumors in men and women (Chen et al., 2016; Siegel, Miller, Miller, & Jemal, 2017), and it accounts for more than 3% of adult malignancies (Chen et al., 2016). Although the curative methods are surgical tumor or radical resection for renal cell carcinoma, more than 30% patients with localized RCC develop metastasis and recurrence after surgical resection (Akhavan et al., 2015; Li et., 2016; Pantuck, Zisman, & Belldegrun, 2001). Therefore, better tumor biomarkers and therapeutic targets for diagnosis and treatment of RCC are necessary.
MicroRNAs (miRNAs) are comprised of 22 nucleotides and abundant family of evolutionarily conserved small, noncoding, and endogenous RNAs, which are closely associated with processes of cell biology (Bartel, 2009; Du et al., 2015; Li, Wu, et al., 2016; Wei, Wang, Wang, Ye, & Chen, 2016). Recently, a large number of studies found that abnormal miRNAs expression is closely related to tumor pathogenesis and they are promising therapeutic targets or biomarkers for cancers (Bartel, 2009; Dallavalle et al., 2016; Du et al., 2015; Henrique & Jeronimo, 2017; Li, Wu, et al., 2016; Wei et al., 2016).
miR‐33a has been defined as a tumor suppressor miRNA in a variety of cancers including prostate cancer, gallbladder cancer, melanoma, osteosarcoma, and breast cancer through directly targeting downstream genes (Huang et al., 2018; Kang, Li, Li, Zhao, He, & Shi, 2018; Karatas et al., 2017; Li et al., 2018; Zhang et al., 2015, 2016; Zhou et al., 2015). However, the clinical significance and the effect of miR‐33a in RCC remain poorly understood.
The P53 (OMIM 191,170), a tumor suppressor, plays a pivotal role in a variety of physiological processes (AJ, 1997; Vogelstein, 2000). Inactivation of P53 function is critical to tumorigenesis, progression, and metastasis. Mouse double minute 4(MDM4) protein (OMIM 602,704) can inhibit P53 transcriptional activity directly as MDM4 contains a P53 binding domain in a variety of malignancies (Gansmo et al., 2015; Li & Lozano, 2013; Marine & Jochemsen, 2016). The activity of P53 is reduced by MDM4 overexpression, which contributes to tumorigenesis (Gansmo et al., 2015; Jeffreena Miranda et al., 2017). Furthermore, MDM4 is overexpressed in prostate cancer, gastric cancer, hematologic malignancies, lung cancer, etc (Bao, Song, Xu, Qu, & Xue, 2016; Cao, Xu, & Li, 2015; Gansmo et al., 2015; Marine & Jochemsen, 2016; Miranda et al., 2017; Xiong et al., 2016; Xu et al., 2016). Those studies demonstrated that MDM4 might be closely involved in tumorigenesis. However, the role of MDM4 in RCC remains unclear. Moreover, miR‐33a as a suppressor gene downregulating the expression of MDM4 in RCC has not yet been reported. Therefore, we explored the clinical significance of miR‐33a and its roles in the pathogenesis of RCC, and implored it is as a promising biomarker and therapeutic target for RCC.
2. MATERIALS AND METHODS
2.1. Human tissue samples
The study protocol was approved by the Local Ethics Committees of Guizhou Provincial People's Hospital, and all the patients were approved and signed the written informed consent. The samples of patient‐matched RCC and control (adjacent normal renal tissues) were obtained from patients who underwent laparoscopic or open nephrectomy at Guizhou Provincial People's Hospital between 2007 and 2011. Thirty cases of RCC tissues were identified as renal clear cell cancer by histopathological analysis. Those patients with a history of any other types of tumors or received chemo‐ or radiotherapy before surgery were excluded. All tissues were stored at −80℃ after obtaining.
2.2. Cell culture
Normal primary renal tubular HK‐2 cell lines and RCC cell lines (Caki‐1, ACHN and 786‐O) were purchased from China Center For Type Culture Collection (Wuhan, China). HK‐2 cells were cultured in RPMI 1640 medium (Gibco, USA) and RCC cells were cultured in DMEM medium (Gibco, USA) with 10% fetal calf serum (FCS, Gibco) in an atmosphere of 5% CO2 at 37ºC in cell humidified incubator.
2.3. Cell transfection
miR‐33a inhibitor, mimics, and negative control of miR‐33a (inhibitor‐ and mimics‐NC) were purchased from RiboBio (Guangzhou, China). MDM4 siRNAs and nontargeting siRNA were purchased from GenePharma (Shanghai, China). In brief, 50nM of miR‐33a mimics and 100nM of miR‐33a inhibitor were transfected into the 786‐O, ACHN, and Caki‐1 RCC cells by Lipofectamine 3000 (Invitrogen, China) following the manufacturer's protocol in 6‐well cell plate. Hundred nanomolar of MDM4 siRNA was used to knockdown of endogenous MDM4 following the manufacturer's protocol.
2.4. Real‐time quantitative RT‐PCR (qPCR)
TRIzol reagents (Invitrogen, USA) were used to extract the total RNA following the manufacturer's protocol. cDNAs were compounded by the PrimeScript RT reagent kit (Takara). Next, qPCRs were conducted using 7,500 Real‐Time PCR System (Applied Biosystems) with SYBR Green PCR Master mix (Applied Biosystems, USA) for mRNA analysis, GAPDH was employed to normalize the expression of MDM4. qPCRs were conducted using the TaqMan MicroRNA Reverse Transcription kit (Applied Biosystems, USA) for miRNA analysis, U6 was employed to normalize the expression of miR‐33a. The primer sequences are shown below:
miR‐33a (F: 5′‐GGTGCATTGTAGTTGCATTGC‐3′, R: 5′‐GTGCAGGGTCCG AGGTATTC‐3′); U6 (5′‐GCGCGTCGTGAAGCGTTC‐3′, R: 5′‐GTGCAGGGTCCG AGGT‐3′); MDM4 (F: 5′‐CTCAGTGTCAACATCTGACAG‐3′, R: 5′‐CATATGCTG CTCCTGCTGATC‐3′); GAPDH (F:5′‐ATGGGGAAGGTGAAGGTCG‐3′, R: 5′‐GGGGTCATT GATGGCAACAATA‐3′).
2.5. Western blotting
RIPA buffer was used to lysing RCC cells to extract total protein. The Bradford assay (Bio‐Rad, USA) was applied to measure the protein concentration of each samples. Next, 10% SDS‐PAGE gel was used to separate the protein, and electrotransferred to ECL nitrocellulose membranes, and BSA with 0.1% Tris‐buffered saline‐Tween 20 (TBST) was used to block the membranes for 2 hr. TBST was used to wash the membranes of each samples and incubated overnight with MDM4 primary antibody (1:2000 dilution) and GAPDH primary antibody (1:3,000 dilution). Subsequently incubated with matched secondary antibodies (1:3,000 dilution) for 2 hr at room temperate after the membranes were washed with TBST. Finally, ECL western blot analysis substrate was used to quantify the results.
2.6. Luciferase reporter assay
The bioinformatics algorithms from Targetscan and miRwalk were used. Wild type 3′‐UTR of MDM4 and mutant controls were constructed and inserted into the psiCheck2 Luciferase vector (Promega, USA). Next, the MDM4‐mutant or MDM4‐wild type and miR‐33a mimics were co‐transduced into cells by lipofectamine 3,000. After 48 hr, luciferase activity was detected using a Dual‐Luciferase Reporter Assay System (Promega Corporation Madison, USA) based on the manufacturer's protocol.
2.7. CCK‐8 assay
Caki‐1 and 786‐O cells were cultured in 96‐well cell plates with 1 × 105 cells/well density for 24 hr after transfection. CCK‐8 assays were used to measure the effects of miR‐33a and MDM4 on RCC cells growth based on the manufacturer's protocol. The results were performed from three independent experiments with triplicate.
2.8. Flow cytometry
Cells were harvested and fixed by 70% alcohol, and then stored for 2 hr at 4℃. Then the cells were washed by PBS and according to the instructions exposed to PI (50mg/ml, BD Pharmingen, San Jose, CA) 1 hr and avoid light. Last we quantified the distribution of the cells through FCM (ModFit software).
2.9. Experimental mouse model
Six‐week old nude mice (BALB/c) were inoculated subcutaneously with 5 × 106 Caki‐1 cells transfected with or without miR‐ mimic or miR‐ inhibitor. The tumors were measured once a week with microcalipers and the tumors were collected and weighed at the end of the experiment, and then tumors were fixed in 4% of paraformaldehyde for further analyses. The tumor volume was calculated through the following formula: Tumor volumes (mm3) = 1/2 × (length × width2).
2.10. Terminal deoxynucleotidyltransferase dUTP nick end labeling assay
According to the protocol of Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay, cell apoptosis was detected in tumor tissues and staining was performed. Apoptosis was evaluated by counting the positive cells as well as the total number of cells at five random fields.
2.11. Statistical analysis
The software of Statistical Package for the Social Sciences Version 16 was used to statistical analysis. All data were calculated as means values ± SD (standard deviations). The Student t‐test was utilized to analyze the results between treated and control groups. The value of P < 0.05 was considered to have statistical significance.
3. RESULTS
3.1. miR‐33a is decreased in RCC clinical tissues and cells
In this study, the level of miR‐33a was detected by RT‐qPCR in 30 pairs of RCC specimens and adjacent normal renal tissues (Figure 1a). We found that the level of miR‐33a was obviously decreased in the RCC tissues group than the adjacent normal tissue group. Moreover, compared with HK‐2 cell line, the level of miR‐33a was also significantly reduced in the RCC cells (Caki‐1, ACHN, and 786‐O) (Figure 1b).
Figure 1.
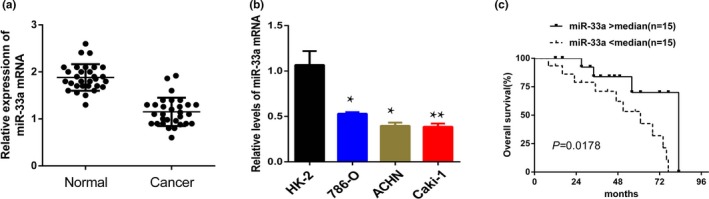
Expression of miR‐33a in renal cell cancer (RCC) tissues and cell lines. (A, B) RT‐qPCR analysis of miR‐33a expression in RCC tissues and cell lines. *p < 0.05, **p < 0.01 versus adjacent normal renal tissues or HK‐2 cells; (C) Kaplan–Meier analysis of overall survival in 30 RCC patients with low median (n = 15) and high median (n = 15) expression levels of miR‐33a. **p < 0.01 versus HK‐2 cell
The relationship between patient prognosis and the level of miR‐33a was analyzed. Patients with RCC were divided into low (n = 15) and high (n = 15) miR‐33a expression groups following the median value of expression levels of miR‐33a among RCC specimens, Kaplan–Meier curve was plotted for overall survival (OS). Statistical analysis showed that RCC patients with higher expression level of miR‐33a had significantly longer OS than patients with lower expression level of miR‐33a (p = 0.0178) (Figure 1c).
3.2. The effect of miR‐33a on cell growth in RCC cell
When transfected miR‐33a inhibitor and mimics into Caki‐1 and 786‐O cells, the results showed high‐transfection efficiency (Figure 2a). The RCC cell viability was measured by CCK‐8 assay, and the results indicated that miR‐33a mimics significantly inhibited the proliferation rate of RCC cells (Caki‐1 and 786‐O) compared with the control group (Figure 2b; p < 0.05).
Figure 2.
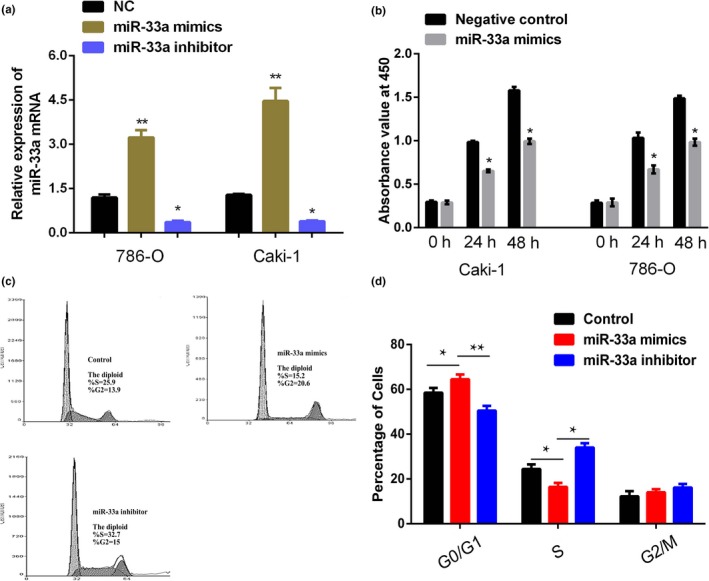
Inhibitory effects of miR‐33a on cell proliferation and cell cycle in renal cell cancer (RCC) cell lines. (a) Expression of miR‐33a in Caki‐1 and 786‐O cells after transfection with miR‐33a mimics and miR‐33a inhibitor or NC. NC represents negative control of miRNA. Results were expressed as of three independent experiments, with at least three replicates in each independent experiment, **p < 0.01 versus NC. (b) CCK‐8 assays indicated that the effects of miR‐33a on the growth of RCC cell lines. Results were expressed as of three independent experiments, with at least three replicates in each independent experiment, *p < 0.05 versus NC. (c, d) The results of flow cytometry showed that upregulation of miR‐33a significantly increased the percentages of cells in the G0/G1 phase, which showed that the upregulation of miR‐33a could suppress RCC cells proliferation. *p < 0.05 versus Control
And then, FCM results showed that the miR‐33a mimics significantly increased the percentages of cells in the G0/G1 phase, which showed that the overexpression of miR‐33a could suppress RCC cells proliferation while miR‐33a inhibitor could promote RCC cell proliferation (Figure 2c and d).
3.3. MDM4 is a direct target of miR‐33a in RCC cells
Bioinformatic algorithms were employed for target prediction, including miRsystem, miRanda, miRBase, and TargetScan which predicted the potential targets of miR‐33a. The results revealed that MDM4 was the potential direct target of miR‐33a (Figure 3a). Dual‐Luciferase reporter system indicated that miR‐33a mimics notably reduced the luciferase activity of MDM4‐wt, while miR‐33a failed to repress the expression of luciferase containing a mutant binding site (Figure 3b and c). The above results indicated that miR‐33a negatively regulated the expression level of MDM4 via directly targeting its 3′ UTR regions. Moreover, miR‐33a overexpression resulted in decreased expression levels of MDM4 protein, while miR‐33a knockdown increased the expression of MDM4 protein in Caki‐1 cells (Figure 3d and e).
Figure 3.
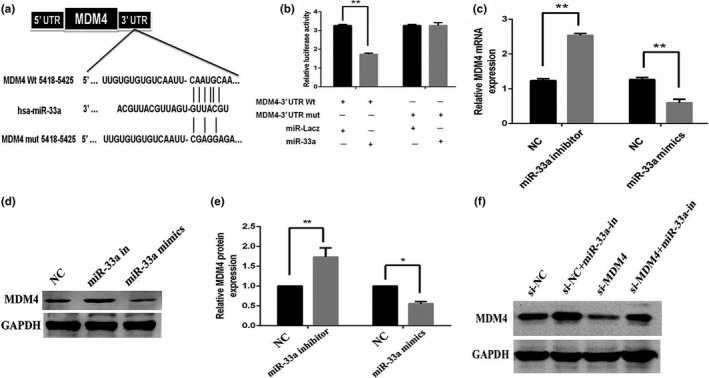
miR‐33a directly targets Mouse double minute 4 (MDM4). (a) The predicted miR‐33a binding site within MDM4 3′ UTR and miR‐33a mutated version by site mutagenesis; (b) Caki‐1 cells were transfected with reporter constructs containing either wild type (WT) MDM4, or MDM4 3′ UTR with mutation (MUT), along with miR‐33a mimics, or negative control, respectively. Relative luciferase activity was measured. (c) qPCR analysis of MDM4 expression in renal cell cancer (RCC) Caki‐1 cells after overexpression or knockdown of miR‐33a. (d, e) Western blotting analysis of MDM4 protein expression in RCC Caki‐1 cells after overexpression or knockdown of miR‐33a. (f) Western blot analysis revealed that transfection of MDM4 siRNA into Caki‐1 cells resulted in decreased MDM4 expression compared to the cells transfected with scrambled siRNA. These effects of siRNA were attenuated by anti‐miR‐33a inhibitor transfection. NC represents normal control, *p < 0.05, **p < 0.01 versus NC
siRNA‐targeting MDM4 was compounded and transfected into RCC Caki‐1 cells. In our study, the results indicated that siRNA‐MDM4 transfection downregulated the protein expression level of MDM4 (Figure 3f). Moreover, miR‐33a inhibitor transfection increased MDM4 protein expression, whereas MDM4 siRNA transfection partially reversed the effect of miR‐33a inhibitor on the expression of MDM4 protein (Figure 3f).
3.4. The effect of MDM4 on cell growth in RCC
RT‐qPCR results indicated that the expression level of MDM4 was significantly upregulated in RCC cells (Caki‐1, ACHN, and 786‐O) compared to that in HK‐2 cells (Figure 4a). Besides, western blot analysis also further corroborated the observation in RT‐qPCR results (Figure 4b and c). The relationship between OS and the level of MDM4 was analyzed. Patients with RCC were divided into low (n = 15) and high (n = 15) MDM4 expression groups following the median value of expression levels of MDM4 among RCC specimens, Kaplan–Meier curve was plotted for OS. Statistical analysis showed that patients with a higher expression level of MDM4 had significantly shorter OS than patients with lower expression level of MDM4 (p = 0.0372) (Figure 4d).
Figure 4.
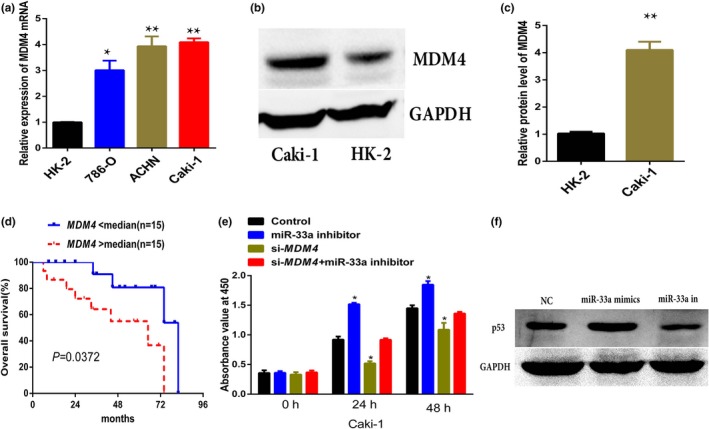
The effect of mouse double minute 4 (MDM4) on cell growth in renal cell cancer (RCC). (a, b, c) RT‐qPCR and western blot analysis of MDM4 expression in RCC cells. (d) Kaplan–Meier analysis of overall survival in 30 RCC patients with low median (n = 15) and high median (n = 15) expression levels of MDM4. (e) CCK‐8 assays indicated that the effects of MDM4 on growth of RCC cell lines. Results were expressed as ±s of three independent experiments, with at least three replicates in each independent experiment, *p < 0.05 versus NC. (f) Western blot analysis of p53 expression after miR‐33a mimics and ‐inhibitor transfection in RCC cells
CCK‐8 assay showed that miR‐33a inhibitor significantly promotes the proliferation rate of RCC cells (Caki‐1) compared with the control group (Figure 4e; p < 0.05), whereas si‐MDM4 transfection can partially reverse this effect in Caki‐1 cells (Figure 4e).Western blot detected the status of p53 after the transfection of miR‐33a mimics and ‐inhibitor, and the results showed that miR‐33a mimics transfection upregulated the expression of p53 and miR‐33a inhibitor inhibited the expression of p53 in Caki‐1 cells (Figure 4f).
3.5. mi‐33a inhibits renal cancer growth in vivo
In vivo, nude mice were subcutaneously injected with RCC Caki‐1 cells or cells transfected with miR‐33a mimics or inhibitor to form RCC. We found that tumor volumes in the miR‐33a inhibitor group were significantly larger than the control group (Figure 5a), and miR‐33a mimics could partially inhibit the growth of RCC (Figure 4a). Similarly, miR‐33a mimics or inhibitor had the similar effect on tumor weight (Figure 5b and c). Furthermore, we found that cells apoptosis induced by miR‐33a mimics were more than the control group (Figure 5d). In summary, the above results showed that miR‐33a mimic could inhibit RCC growth in vivo.
Figure 5.
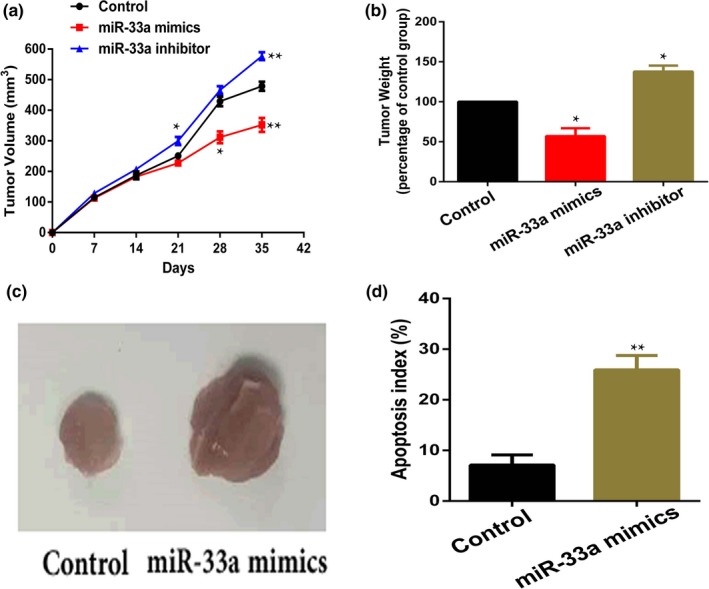
miR‐33a mimics inhibited renal cell cancer tumor growth in vivo. (a, b) miR‐33a mimics could suppress the tumor growth and reduced tumor weight compared with the control group, *p < 0.05, **p < 0.01 versus Control. (c) Images of tumor tissues from different groups on day 35. (d) TUNEL assay was performed to detect cell apoptosis in tumor tissues, **p < 0.01 versus Control
4. DISCUSSION
It is well known that miRNAs play a pivotal role in all kinds of processes of cell biology (Bartel, 2009; Ratert et al., 2013; Trujillo, Yue, Yue, Tang, O'Gorman, & Chen, 2010). Many researchers have found that miR‐33a plays the role of anticancer in various human cancers, and miR‐33a high expression could impede the cancer cell proliferation, invasion, migration, and regulate the chemoresistance in vivo (Chang et al., 2017; Du et al., 2017; Huang et al., 2018; Kang et al., 2018; Karatas et al., 2017; Li et al., 2018; Tian, Wei, Wei, Tian, Qiu, & Xu, 2016). In our study, we found that the level of miR‐33a was remarkably reduced in RCC samples and cells than the adjacent normal renal tissues and HK‐2 cells, respectively. Similarly, our study also showed that miR‐33a overexpression impeded the cell growth of RCC, while miR‐33a lower expression had an opposite effect. All the above results demonstrate that miR‐33a downregulation might be involved in the etiology and pathogenesis of RCC.
MDM4 can inhibit the function of P53 during a number of types of cancer for MDM4 shares an N‐terminal p53‐binding domain with MDM2 (OMIM 164,785) (Gansmo et al., 2015; Xu et al., 2016). The overexpression of MDM4 in a variety of tumors may be related to inhibition of the function of P53 and result in tumorigenesis, tumor progression, and metastasis (Bao et al., 2016; Cao et al., 2015; Marine & Jochemsen, 2016). Many studies reported the overexpression of MDM4 in prostate cancer, gastric cancer, hematologic malignancies, etc (Gansmo et al., 2015; Marine & Jochemsen, 2016; Miranda et al., 2017; Wang et al., 2016). All those reports indicate that MDM4 may be closely involved in tumorigenesis, tumor progression, and metastasis.
Dysregulation of miRNA was closely associated with kinds of cancers, and the microRNA's function was related to negatively regulate target gene expression for translational suppression or mRNA degradation (Bartel, 2009; Li et al., 2014; Sandbothe et al., 2017). Recently, many studies demonstrated that miR‐33a is widely involved in tumorigenesis by directly regulating its targeted genes. Zhang et al demonstrated that miR‐33a suppresses gallbladder cancer progression by targeted Twist (Zhang et al., 2016). Zhou et al demonstrated that miR‐33a could inhibit cell proliferation, invasion and metastasis in melanoma by binding HIF‐1α (OMIM 606,615) (Zhou et al., 2015). Kang et al indicated that miR‐33a inhibits the growth of lung cancer cells by negatively regulating the expression of CAND1 (OMIM 607,727; Kang et al., 2018). In addition, the experts found that miR‐33a, as a negative regulator, inhibit cells growth, invasion, and metastasis in breast cancer (Zhang et al., 2015). In our investigation, Targetscan predicted that MDM4 was the direct target of miR‐33a, and then our results demonstrated that the MDM4 3′UTR was the direct target of miR‐33a by luciferase assays. CCK‐8 assays demonstrated that miR‐33a mimics could impede RCC cell growth. Moreover, RT‐qPCR and western blotting suggested that miR‐33a overexpression remarkably reduced the expression level of MDM4. However, the expression of MDM4 could partially rescue by miR‐33a inhibitor, suggesting that MDM4 is a direct target of miR‐33a in RCC.
In conclusion, we demonstrated that miR‐33a directly targeted the expression of MDM4 in RCC cells. On the other hand, our study provided evidence that miR‐33a overexpression could inhibit cells growth in RCC. All above results indicated that miR‐33a inhibits cell growth by downregulating MDM4 in RCC. Taken together, miR‐33a may be served as a potential target for the patients with RCC.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
JKH wrote the manuscript and operated the experiments; SF involved in study supervision and edited the manuscript; BY and LGH performed the molecular experiments; ZP and ZJG performed the animal experiments. All the authors reviewed the manuscript.
ETHICAL APPROVAL
The ethics committee approved the study and all patients were informed about the study and a signed written consent was obtained.
ACKNOWLEDGMENTS
This study was funded by National Natural Science Cultivate Foundation of Guizhou Provincial People's Hospital (Number: [2018]5764‐01) and Doctoral Foundation of Guizhou Provincial People's Hospital (GZSYBS[2018]02), and Natural Science Foundation of Hubei Province of China (Number: 2017CFB516).
Jiang K, Sun F, Zhu J, Luo G, Ban Y, Zhang P. miR‐33a inhibits cell growth in renal cancer by downregulation of MDM4 expression. Mol Genet Genomic Med. 2019;7:e833 10.1002/mgg3.833
REFERENCES
- Aj, L. (1997). p53 the cellular gatekeeper for gatekeeper for growth and division. Cell, 88, 323–331. [DOI] [PubMed] [Google Scholar]
- Akhavan, A. , Richards, M. , Shnorhavorian, M. , Goldin, A. , Gow, K. , & Merguerian, P. A. (2015). Renal cell carcinoma in children, adolescents and young adults: A National Cancer Database study. The Journal of Urology, 193, 1336–1341. 10.1016/j.juro.2014.10.108 [DOI] [PubMed] [Google Scholar]
- Bao, J. N. A. , Song, H. , Xu, R. , Qu, G. , & Xue, Y. (2016). The overexpression of MDM4 an effective and novel predictor of gastric adenocarcinoma lymph node. Oncotarget, 7, 67212–67222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel, D. P. (2009). MicroRNAs: Target recognition and regulatory functions. Cell, 136, 215–233. 10.1016/j.cell.2009.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, L. F. L. , Xu, W. , & Li, J. Y. (2015). Targeting MDM4 as a Novel therapeutic approach for hematologic malignancies. Current Cancer Drug Targets, 15, 769–790. [DOI] [PubMed] [Google Scholar]
- Chang, M. , Qiao, L. , Li, B. , Wang, J. , Zhang, G. , Shi, W. , … Tian, Y. E. (2017). Suppression of SIRT6 by miR‐33a facilitates tumor growth of glioma through apoptosis and oxidative stress resistance. Clinical & Translational Oncology, 38, 1251–1258. 10.3892/or.2017.5780 [DOI] [PubMed] [Google Scholar]
- Chen et al., 2016 Chen, W. , Zheng, R. , Baade, P. D. , Zhang, S. , Zeng, H. , Bray, F. , … He, J . (2016). Cancer statistics in China, 2015. CA, 66(2), 115–132. 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- Dallavalle, C. , Albino, D. , Civenni, G. , Merulla, J. , Ostano, P. , Mello‐Grand, M. , … Carbone, G. M. (2016). MicroRNA‐424 impairs ubiquitination to activate STAT3 and promote prostate tumor progression. The Journal of Clinical Investigation, 126, 4585–4602. 10.1172/JCI86505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, M. , Shi, D. , Yuan, L. , Li, P. , Chu, H. , Qin, C. , … Wang, M. (2015). Circulating miR‐497 and miR‐663b in plasma are potential novel biomarkers for bladder cancer. Scientific Reports, 5, 10437 10.1038/srep10437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, M. , Zhang, Y. , Mao, Y. , Mou, J. , Zhao, J. , Xue, Q. I. , … Gao, Y. (2017). MiR‐33a suppresses proliferation of NSCLC cells via targeting METTL3 mRNA. Biochemical and Biophysical Research Communications, 482, 582–589. 10.1016/j.bbrc.2016.11.077 [DOI] [PubMed] [Google Scholar]
- Gansmo, L. B. , Romundstad, P. , Birkeland, E. , Hveem, K. , Vatten, L. , Knappskog, S. , & Lønning, P. E. (2015). MDM4 SNP34091 (rs4245739) and its effect on breast‐, colon‐, lung‐, and prostate cancer risk. Cancer Medicine, 4, 1901–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrique, R. , & Jeronimo, C. (2017). Testicular germ cell tumors go epigenetics: Will miR‐371a‐3p replace classical serum biomarkers? European Urology, 71, 221–222. 10.1016/j.eururo.2016.08.013 [DOI] [PubMed] [Google Scholar]
- Huang, Y. , Zhang, J. , Shao, H. , Liu, J. , Jin, M. , Chen, J. , & Zhao, H. (2018). miR‐33a mediates the anti‐tumor effect of lovastatin in osteosarcoma by targeting CYR61. Cellular Physiology and Biochemistry, 51, 938–948. 10.1159/000495396 [DOI] [PubMed] [Google Scholar]
- Kang, M. , Li, Y. , Zhao, Y. , He, S. , & Shi, J. (2018). miR‐33a inhibits cell proliferation and invasion by targeting CAND1 in lung cancer. Clinical and Translational Oncology, 20, 457–466. 10.1007/s12094-017-1730-2 [DOI] [PubMed] [Google Scholar]
- Karatas, O. F. , Wang, J. , Shao, L. , Ozen, M. , Zhang, Y. , Creighton, C. J. , & Ittmann, M. (2017). miR‐33a is a tumor suppressor microRNA that is decreased in prostate cancer. Oncotarget, 8, 60243–60256. 10.18632/oncotarget.19521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Q. , & Lozano, G. (2013). Molecular pathways: targeting Mdm2 and Mdm4 in cancer therapy. Clinical Cancer Research, 19, 34–41. 10.1158/1078-0432.CCR-12-0053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, S. , Wu, X. , Xu, Y. , Wu, S. , Li, Z. , Chen, R. , … Xu, X. (2016). miR‐145 suppresses colorectal cancer cell migration and invasion by targeting an ETS‐related gene. Oncology Reports, 36, 1917–1926. 10.3892/or.2016.5042 [DOI] [PubMed] [Google Scholar]
- Li, W.‐Y. , Chen, X.‐M. , Xiong, W. , Guo, D.‐M. , Lu, L. I. , & Li, H.‐Y. (2014). Detection of microvesicle miRNA expression in ALL subtypes and analysis of their functional roles. Journal of Huazhong University of Science and Technology Medical sciences = Hua zhong ke ji da xue xue bao Yi xue Ying De wen ban = Huazhong keji daxue xuebao. Yixue Yingdewen Ban, 34, 640–645. 10.1007/s11596-014-1330-0 [DOI] [PubMed] [Google Scholar]
- Li, Y. J. , Sun, Y. X. , Hao, R. M. , Wu, P. , Zhang, L. J. , & Chen, W. (2018). miR‐33a‐5p enhances the sensitivity of lung adenocarcinoma cells to celastrol by regulating mTOR signaling. International Journal of Oncology, 52, 1328–1338. [DOI] [PubMed] [Google Scholar]
- Li, Y. , Tang, X. , He, Q. , Yang, X. , Ren, X. , Wen, X. , … Ma, J. (2016). Overexpression of Mitochondria Mediator Gene TRIAP1 by miR‐320b Loss Is Associated with Progression in Nasopharyngeal Carcinoma. PLoS Genetics, 12, e1006183 10.1371/journal.pgen.1006183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marine, J.‐C. , & Jochemsen, A. G. (2016). MDMX (MDM4), a promising target for p53 reactivation therapy and beyond. Cold Spring Harbor Perspectives in Medicine, 6(7), a026237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda, P. J. , Buckley, D. , Raghu, D. , Pang, J.‐M. , Takano, E. A. , Vijayakumaran, R. , … Haupt, Y. (2017). MDM4 is a rational target for treating breast cancers with mutant p53. The Journal of Pathology, 241(5), 661–670. 10.1002/path.4877 [DOI] [PubMed] [Google Scholar]
- Pantuck, A. J. , Zisman, A. , & Belldegrun, A. S. (2001). The changing natural history of renal cell carcinoma. The Journal of Urology, 166, 1611–1623. 10.1016/S0022-5347(05)65640-6 [DOI] [PubMed] [Google Scholar]
- Ratert, N. , Meyer, H.‐A. , Jung, M. , Lioudmer, P. , Mollenkopf, H.‐J. , Wagner, I. , … Jung, K. (2013). miRNA profiling identifies candidate mirnas for bladder cancer diagnosis and clinical outcome. The Journal of Molecular Diagnostics, 15, 695–705. 10.1016/j.jmoldx.2013.05.008 [DOI] [PubMed] [Google Scholar]
- Sandbothe, M. , Buurman, R. , Reich, N. , Greiwe, L. , Vajen, B. , Gürlevik, E. , & Skawran, B. (2017). The microRNA‐449 family inhibits TGF‐beta‐mediated liver cancer cell migration by targeting SOX4. Journal of Hepatology, 66, 1012–1021. [DOI] [PubMed] [Google Scholar]
- Siegel, R. L. , Miller, K. D. , & Jemal, A. (2017). Cancer statistics, 2017. CA, 67(1), 7–30. 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- Tian, F. , Wei, H. , Tian, H. , Qiu, Y. , & Xu, J. (2016). miR‐33a is downregulated in melanoma cells and modulates cell proliferation by targeting PCTAIRE1. Oncology Letters, 11, 2741–2746. 10.3892/ol.2016.4321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo, R. D. , Yue, S.‐B. , Tang, Y. , O'Gorman, W. E. , & Chen, C.‐Z. (2010). The potential functions of primary microRNAs in target recognition and repression. The EMBO Journal, 29, 3272–3285. 10.1038/emboj.2010.208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein, B. , Lane, D. , & Levine, A. J. (2000). Surfing the p53 network. Nature, 408, 307–310. 10.1038/35042675 [DOI] [PubMed] [Google Scholar]
- Wang, M.‐J. , Luo, Y.‐J. , Shi, Z.‐Y. , Xu, X.‐L. , Yao, G.‐L. , Liu, R.‐P. , & Zhao, H. (2016). The associations between MDM4 gene polymorphisms and cancer risk. Oncotarget, 7, 55611–55623. 10.18632/oncotarget.10877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, C. , Wang, S. , Ye, Z.‐Q. , & Chen, Z.‐Q. (2016). miR‐206 inhibits renal cell cancer growth by targeting GAK. Journal of Huazhong University of Science and Technology [Medical Sciences], 36, 852–858. 10.1007/s11596-016-1674-8 [DOI] [PubMed] [Google Scholar]
- Xiong, S. , Pant, V. , Zhang, Y. , Aryal, N. K. , You, M. J. , Kusewitt, D. , & Lozano, G. (2016). The p53 inhibitor Mdm4 cooperates with multiple genetic lesions in tumourigenesis. The Journal of Pathology, 241(4), 501–510. 10.1002/path.4854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, C. Z. J. , Fu, W. , Liang, Z. , Song, S. , Zhao, Y. , Lyu, L. , … Duan, P. (2016). MDM4 rs4245739 A>C polymorphism correlates with reduced overall cancer risk in a meta‐analysis. Oncotarget, 7, 71718–71726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, C. , Zhang, Y. , Ding, W. , Lin, Y. , Huang, Z. , & Luo, Q. I. (2015). MiR‐33a suppresses breast cancer cell proliferation and metastasis by targeting ADAM9 and ROS1. Protein & Cell, 6, 881–889. 10.1007/s13238-015-0223-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, M. , Gong, W. , Zuo, B. , Chu, B. , Tang, Z. , Zhang, Y. , … Quan, Z. (2016). The microRNA miR‐33a suppresses IL‐6‐induced tumor progression by binding Twist in gallbladder cancer. Oncotarget, 7, 78640–78652. 10.18632/oncotarget.12693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, J. , Xu, D. , Xie, H. , Tang, J. , Liu, R. , Li, J. , … Cao, K. (2015). miR‐33a functions as a tumor suppressor in melanoma by targeting HIF‐1alpha. Cancer Biology & Therapy, 16, 846–855. [DOI] [PMC free article] [PubMed] [Google Scholar]


