Abstract
How taste buds detect NaCl remains poorly understood. Among other problems, applying taste-relevant concentrations of NaCl (50–500 mm) onto isolated taste buds or cells exposes them to unphysiological (hypo/hypertonic) conditions. To overcome these limitations, we used the anterior tongue of male and female mice to implement a slice preparation in which fungiform taste buds are in a relatively intact tissue environment and stimuli are limited to the taste pore. Taste-evoked responses were monitored using confocal Ca2+ imaging via GCaMP3 expressed in Type 2 and Type 3 taste bud cells. NaCl evoked intracellular mobilization of Ca2+ in the apical tips of a subset of taste cells. The concentration dependence and rapid adaptation of NaCl-evoked cellular responses closely resembled behavioral and afferent nerve responses to NaCl. Importantly, taste cell responses were not inhibited by the diuretic, amiloride. Post hoc immunostaining revealed that >80% of NaCl-responsive taste bud cells were of Type 2. Many NaCl-responsive cells were also sensitive to stimuli that activate Type 2 cells but never to stimuli for Type 3 cells. Ion substitutions revealed that amiloride-insensitive NaCl responses depended on Cl− rather than Na+. Moreover, choline chloride, an established salt taste enhancer, was equally effective a stimulus as sodium chloride. Although the apical transducer for Cl− remains unknown, blocking known chloride channels and cotransporters had little effect on NaCl responses. Together, our data suggest that chloride, an essential nutrient, is a key determinant of taste transduction for amiloride-insensitive salt taste.
SIGNIFICANCE STATEMENT Sodium and chloride are essential nutrients and must be regularly consumed to replace excreted NaCl. Thus, understanding salt taste, which informs salt appetite, is important from a fundamental sensory perspective and forms the basis for interventions to replace/reduce excess Na+ consumption. This study examines responses to NaCl in a semi-intact preparation of mouse taste buds. We identify taste cells that respond to NaCl in the presence of amiloride, which is significant because much of human salt taste also is amiloride-insensitive. Further, we demonstrate that Cl−, not Na+, generates these amiloride-insensitive salt taste responses. Intriguingly, choline chloride, a commercial salt taste enhancer, is also a highly effective stimulus for these cells.
Keywords: amiloride, Ca2+ imaging, fungiform taste bud, NaCl, sensory transduction, taste
Introduction
The mechanisms for detecting sweet, bitter, umami, and sour are now well understood (Liman et al., 2014; Roper and Chaudhari, 2017; Tu et al., 2018). However, understanding of salt taste lags. NaCl stimulates taste bud cells (Avenet and Lindemann, 1991; Yoshida et al., 2009a) and evokes responses in afferent nerves (Pfaffmann, 1941; Beidler, 1953). The diuretic, amiloride, selectively reduces salt responses in cellular, afferent nerve, and behavioral assays in rodents (Heck et al., 1984; Ninomiya and Funakoshi, 1988; Béhé et al., 1990; Avenet and Lindemann, 1991; McCaughey and Scott, 1998; Yoshida et al., 2009a). Amiloride-blocked epithelial sodium channels (ENaC) are thought to underlie amiloride-sensitive salt taste detection (Kretz et al., 1999; Chandrashekar et al., 2010). A second, amiloride-insensitive, component to salt taste transduction is also present across the concentration-response range of NaCl (Heck et al., 1984; Elliott and Simon, 1990; Ye et al., 1993; Ninomiya, 1998) and may encode aversive taste at high salt concentrations (Oka et al., 2013). Interestingly, amiloride has a more limited effect on salt taste in humans (Desor and Finn, 1989; Ossebaard and Smith, 1995; Halpern, 1998), emphasizing the importance of understanding the cells and mechanisms for amiloride-insensitive NaCl taste transduction.
Taste buds contain three cell types with distinct morphological and functional properties: Type 1 cells are “glial-like”; Type 2 cells express GPCRs for sweet, bitter, and umami stimuli; and Type 3 cells are responsible for detecting acid (sour) taste (Chaudhari and Roper, 2010; Liman et al., 2014; Roper and Chaudhari, 2017). The taste cell type(s) responsible for detecting NaCl remains uncertain. Amiloride-sensitive cation currents or Ca2+ responses have been recorded in rodent taste bud cells that resembled Type 2 cells (Bigiani and Cuoghi, 2007), or cells that were neither Type 2 nor Type 3 (Vandenbeuch et al., 2008; Chandrashekar et al., 2010). As for amiloride-insensitive NaCl taste, Types 2 and 3 have both been implicated (Oka et al., 2013; Lewandowski et al., 2016), and a consensus has yet to emerge.
The contribution of anions to salt taste (the “anion effect”) was first described by Beidler (1953). In gustatory nerve recordings, responses to sodium salts were decreased when large anions were substituted for chloride. The effect has been attributed either to retarded paracellular Na+ permeation into the taste bud (Elliott and Simon, 1990; Ye et al., 1991, 1993, 1994) or, conversely, to a direct inhibition of salt transduction (Beidler, 1967; Lewandowski et al., 2016). The mechanism of such inhibition has not been explored.
To address these open questions, we performed Ca2+ imaging on fungiform taste buds from mice. In vivo, only the apical tips of taste bud cells encounter wide fluctuations in salt concentration and tonicity, whereas the majority of the cell body is protected inside the taste bud, surrounded by interstitial fluid. Thus, we adapted a lingual slice preparation (Caicedo et al., 2002) to record from fungiform taste buds. This allowed us to stimulate focally at the apical taste pore, mimicking in vivo stimulation, while maintaining a constant ionic and osmotic milieu surrounding taste bud cells. We imaged responses to NaCl using mice expressing GCaMP3 selectively in Type 2 and 3 taste bud cells. Our findings indicate that NaCl elicits amiloride-insensitive responses in Type 2 cells. Furthermore, Cl− appears to play a critical role in amiloride-insensitive salt taste.
Materials and Methods
Mice.
All procedures were approved by the Institutional Animal Care and Use Committee of the University of Miami and conducted according to the National Institutes of Health's Guide for the care and use of laboratory animals. For functional assays, mice were killed by CO2 asphyxiation followed by cervical dislocation. Pirt-GCaMP3 heterozygous mice have previously been reported to express GCaMP in all sensory neurons of the dorsal root, trigeminal, and geniculate ganglia (Kim et al., 2014; Wu et al., 2015; Dvoryanchikov et al., 2017). Both sexes, 2–6 months of age, were used. We observed no significant differences between male and female mice.
Reagents.
All chemicals for buffers and taste stimuli were purchased from Sigma-Aldrich, except for citric acid (Thermo Fisher Scientific, #BP339). For recording, lingual slices were maintained in Tyrode's buffer with elevated Ca2+ (130 mm NaCl, 5 mm KCl, 8 mm CaCl2(H2O)2, 1 mm MgCl2(H2O)5, 10 mm HEPES, 10 mm glucose, 10 mm Na pyruvate, 5 mm NaHCO3, pH 7.32). All tastants were dissolved in Tyrode's buffer or artificial saliva (AS: 14.8 mm NaCl, 22.1 mm KCl, 3.1 mm CaCl2, 0.6 mm MgCl2). For Ca2+-free buffer, 8 mm NaCl was substituted for 8 mm CaCl2. For K+-free buffer, 5 mm K+ was removed without substitution. Amiloride hydrochloride, 4,4′-diisothiocyano-2,2′-stilbenedisulfonic acid (DIDS), and U73122 were from Tocris Bioscience (catalog #0890, #4523, and #1268, respectively). Solutions of amiloride and DIDS were prepared immediately before use; U73122 was made as a 2.6 mm stock solution in 5 mm DMSO, and stored at −20°C or dissolved in dichloromethane, evaporated under nitrogen gas, and reconstituted to 10 mm in DMSO.
Lingual slice preparation.
We used fungiform papillae from the anterior tongue, known to be optimally sensitive for detecting both amiloride-sensitive and -insensitive forms of NaCl taste (Ninomiya and Funakoshi, 1988; Spector and Grill, 1992). Tongues were rapidly dissected after death and placed in NMDG Tyrode's buffer at 37°C (130 mm NMDG-Cl replaces 130 mm NaCl) to prevent Na+-evoked responses during preparation (Gilbertson et al., 1992); ∼1 cm of the anterior tip of the tongue was excised and embedded in 4% low-melting temperature agarose (Sigma-Aldrich, #A9918) and cut into sagittal slices, 100 μm thick, using a vibratome (Leica, VT100s). Slices were placed onto coverslips precoated with Cell-Tak tissue adhesive (Corning, #354240), transferred to a recording chamber and continuously perfused with room temperature, high Ca2+ Tyrode's buffer at 2 ml/min under gravity. Imaging was on a Fluoview FV1000 laser scanning confocal microscope as previously detailed (Caicedo and Roper, 2001; Tomchik et al., 2007).
Focal application of tastants.
Slices were positioned such that a fungiform taste bud was centered under a 40× water-immersion objective. Tastants were pressure-ejected onto the apical pore via puffer pipettes (Caicedo and Roper, 2001) created from filamented borosilicate or theta borosilicate capillaries (WPI, #TST150-6, 1B120F-6) drawn to a tip diameter of ∼8 μm. Pipettes were back-filled with taste stimuli containing a tracer dye, Alexa-568 (1 μm), Alexa-647 (1 μm), or fluorescein (0.3 μm), to track the bulk flow and concentration of stimuli, and ensure that stimuli were focused at the taste pore. Separate dyes were used to track each stimulus. Each pipette was connected via a 34G Microfil (WPI, #MF34G-5) onto a Picospritzer (Medical Systems, PLI-100) set to deliver pressure pulses (3 s, ∼0.1–2 psi). Typically, stimuli reached the taste pore in ≤1 s following ejection. Lingual slices were continuously perfused with buffer with ≥30 s rinse intervals between stimuli.
In some experiments, we mimicked in vivo taste stimulation, where saliva (typically containing 15 mm NaCl) bathes the apical tips of taste cells and basolateral membranes are surrounded by interstitial fluid (130 mm NaCl). To this end, we positioned a secondary pipette that delivered a continuous local flow of AS across the edge of the slice along the mucosal surface. By monitoring a dye tracer, we verified that bulk flow of AS over the surface was continuous and limited to the mucosal edge of the slice. An extracellular barrier at the mucosal surface, around the bud, and between taste cells limits permeation of many ions into the taste bud (Michlig et al., 2007; Dando et al., 2015). Taste pores were adapted to AS for at least 5 min before applying taste stimuli (dissolved in AS). AS flow was controlled by a second picospritzer independent of stimulus delivery.
Imaging.
Confocal scanning images were captured at 1 frame/s, except where otherwise noted. Correction for photobleaching over time was applied when necessary (Caicedo et al., 2000). Taste bud cells responded repeatedly (>10 times) throughout a recording. Confocal scans were digitized and stabilized using ImageJ2 (Rueden et al., 2017). Fluorescence changes over baseline (ΔF/F0) were visualized, and responsive cells were identified (Ackman et al., 2012) using a custom plugin (dFoF, downloaded from https://github.com/ackmanlab). Fluorescence intensity data were exported to Excel, and responses were quantified as the maximum change in fluorescence, ΔF, in the 10 frames following stimulation, divided by baseline fluorescence, F0 (average of 10 frames before stimulus), that is, peak ΔF/F0. Cells were considered healthy and were included in the analysis only if responses were >3 times the SD of F0 and repeatable at least twice.
Because of subtle variations in stimulus ejection, flow, and dilution, it was difficult to deliver exactly the same concentration of taste stimuli repeatedly. Thus, when examining the effects of drugs, we recorded replicate NaCl responses before and after the drug treatment. We then rejected replicate responses for which the stimulus concentration deviated by >20% from the mean stimulus concentration before drug exposure.
Experimental design and statistical analysis.
In all instances, responses were measured independently, multiple times. Biological replicates are reported throughout as number of cells from number of mice (each mouse representing an independent experiment). Control and experimental conditions (e.g., ion substitutions or drug effects) were tested sequentially on the same cell, and ΔF/F0 for each cell was normalized to its maximum response in the control condition. These data were then compared using two-way paired Student's t tests. For concentration-response curves, response amplitude for each point was normalized to that cell's response at ∼160 mm NaCl, as this concentration was common to all cells. Curves were fit using nonlinear regression with variable slope and unconstrained for four parameters: top and bottom of curve, EC50, and Hill slope. Statistical analyses were performed using Prism 6 (GraphPad).
Immunostaining.
Mice were killed with a combination of ketamine (240 mg/kg) and xylazine (20 mg/kg) and perfused with PBS followed by 4% PFA in PBS through intracardiac cannulation. Segments of anterior tongue were postfixed with 4% PFA, cryoprotected, and cryosectioned at 25 μm, as previously reported (Dvoryanchikov et al., 2017). Sections were incubated with rabbit anti-PLCβ2 (1:1000, Santa Cruz Biotechnology, SC206), goat anti-Car4 (1:500, R&D Systems, AF2414), and chicken anti-GFP (1:1000, Aves, 1020) overnight, followed by fluorescent secondary antibodies (Thermo Fisher Scientific, A21207, A21447; Jackson ImmunoResearch Laboratories, 703-485-155), all diluted 1:1000.
For post hoc immunostaining, 100 μm lingual slices that had been imaged for taste responses were immersion-fixed in 4% PFA for 1 h at 4°C. Slices were permeabilized in 0.3% Triton overnight at room temperature, blocked for 3 h in 5% normal donkey serum, and incubated with primary antibodies (above) for 3 d at room temperature. After washing, sections were incubated with secondary antibodies for 24 h.
Results
Type 2 and 3 taste bud cells express GCaMP3
Pirt-GCaMP3 mice express the Ca2+ indicator, GCaMP3, in many sensory neurons (Kim et al., 2014). Immunostaining on tongue sections revealed that GCaMP3 could also be detected in fungiform taste buds. Triple immunostaining for PLCβ2, a marker for Type 2 cells, Car4, a marker for Type 3 cells (Liman et al., 2014; Roper and Chaudhari, 2017), and GFP, demonstrated that GCaMP3 is present in all Type 2 and Type 3 taste bud cells and is limited to these two cell types (Fig. 1A–D). Specifically, in 15 taste buds from 2 mice, we scored 103 Type 2 cells and 18 Type 3 cells. All 121 cells also expressed GCaMP, whereas only 1 cell expressed GCaMP without either marker. Although GCaMP is also in sensory fibers in and around taste buds, such fibers were readily distinguished by size from taste bud cells.
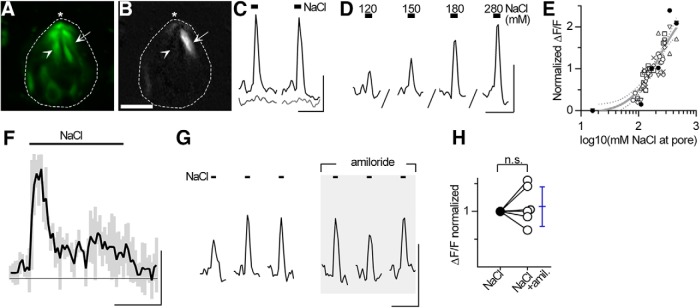
Figure 1.
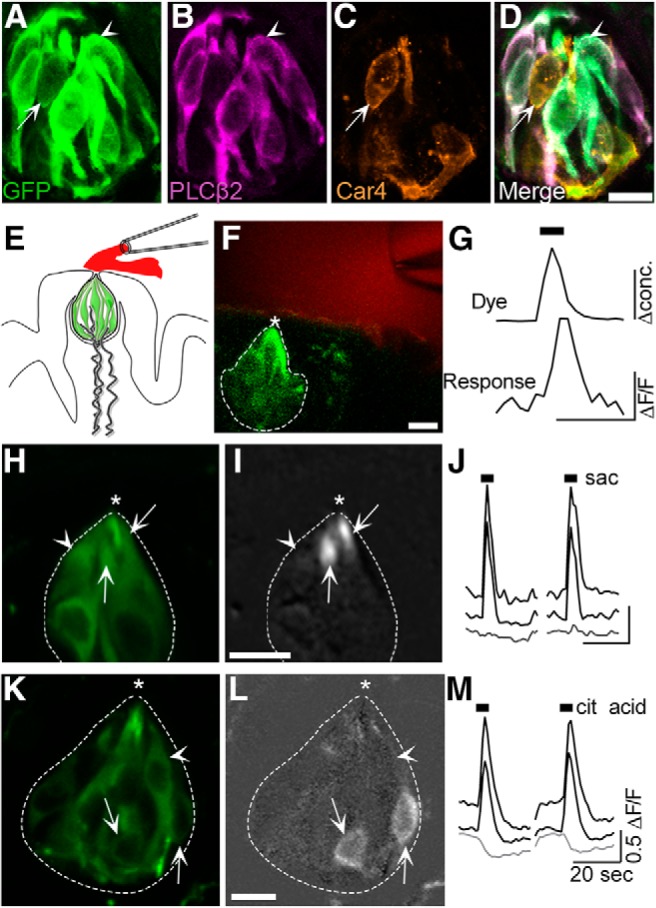
Type 2 and Type 3 fungiform taste bud cells in lingual slices respond to focal taste stimulation. A–D, GCaMP3 is selectively expressed in Type 2 and Type 3 taste bud cells. Cryosection of a fungiform papilla from a Pirt-GCaMP3 mouse, immunostained with anti-GFP to mark GCaMP3+ taste bud cells (A, green). Anti-PLCβ2 (B, magenta) to display Type 2 taste cells and anti-Car4 (C, orange) to visualize Type 3 taste cells. D, The merged image shows co-expression of GCaMP with PLCβ2+ (arrowhead) and Car4+ (arrow) cells. E, Diagram of a fungiform taste bud (green cells) in a lingual slice with focal taste stimulation tracked with a fluorescent dye (red). F, Micrograph showing living lingual slice, imaged for GCaMP (green), where the taste pore (*) is focally stimulated with a tastant (including a tracking dye, red). Dashed lines outline the taste bud. G, Focally applied taste stimuli at the taste pore (top) display rapid onset and washout (calibration bars, 2-fold change in concentration, top; 0.25 ΔF/F, bottom; 10 s for both). Responses are similarly rapid and closely track the change in stimulus concentration at the pore. H–J, Another fungiform taste bud showing baseline GCaMP fluorescence (H) and GCaMP fluorescence at peak of response to focally applied saccharin (50 mm) (I). Two cells that responded to saccharin (arrows) and one unresponsive cell (arrowhead) are indicated. I, Image is baseline-subtracted fluorescence, using a grayscale to show intensity. J, Black traces (top) represent responses to saccharin of two cells indicated by arrows in I. Gray trace (bottom) represents ΔF/F0 for the unresponsive cell (arrowhead). Black bars represent stimulus application. K–M, A different taste bud, showing responses of taste cells to 10 mm citric acid, displayed as in H–J. Scale bars: D, F, I, L, 10 μm. Brightness of image in A was enhanced for cell visibility.
Taste-evoked Ca2+ responses to NaCl in fungiform taste buds
To examine taste-evoked responses in fungiform taste bud cells, we adapted a slice preparation previously used for circumvallate taste buds (Caicedo and Roper, 2001). We validated the preparation using taste stimuli known to elicit responses in Type 2 cells (saccharin, 50 mm) and Type 3 cells (citric acid, 20 mm). Stimuli, applied briefly and focally at the taste pore, evoked responses in individual cells (typically up to 3 cells/bud) (Fig. 1E–G). Taste-evoked responses were repeatable and robust (ΔF/F0 = 15%–150%). Responses to saccharin, which represent intracellular Ca2+ mobilization, were mostly limited to the apical tips of fungiform taste cells (Fig. 1H–J, arrows), whereas responses to citric acid, generated by global depolarization and Ca2+ influx, were observed throughout the cell body (Fig. 1K–M, arrows).
NaCl-evoked Ca2+ responses mimic known properties of salt taste
Focal stimulation with NaCl (∼80–1000 mm) also elicited responses in taste bud cells (Fig. 2A–C). NaCl-evoked responses occurred in the apical processes of the cells, similar to those observed for saccharin. In total, we recorded 118 NaCl-responsive cells in 69 taste buds from 33 mice across this study. Individual taste buds had 0–3 NaCl-responsive cells.
Figure 2.
NaCl-evoked responses in the lingual slice preparation show properties of salt taste. A–C, Response to focal stimulation with 500 mm NaCl (as in Fig. 1H–J). A, Resting GCaMP3 fluorescence (F0, green). B, Baseline-subtracted peak fluorescence (ΔF/F0, grayscale) during focal stimulation with 500 mm NaCl. One NaCl-responsive (arrow) and one unresponsive cell (arrowhead) are indicated. Scale bar, 10 μm. C, ΔF/F for the cells indicated in A, B (arrow, black trace; arrowhead, gray trace), showing two successive applications of NaCl. D, Responses from a taste cell in another experiment, focally stimulated with increasing concentrations of NaCl. D–F, The apical tips of taste buds and surrounding mucosal surface were bathed in a continuous flow of AS for ≥5 min before focal stimulation with NaCl. E, Concentration-response relations for NaCl in fungiform taste cells. Each cell is represented by a different symbol. Responses of each cell were normalized to the same cell's response at ∼160 mm (n = 7 cells from 3 mice, 71 data points). Data are fit by a sigmoidal curve (R2 = 0.84). Estimated EC50 is 236 mm NaCl. Dotted lines indicate 95% CI. F, With prolonged stimulation, NaCl responses rapidly adapted. Responses were recorded using a relatively fast capture rate (0.4–0.5 s/frame) during a 20 s focal application of NaCl (black bar above trace). NaCl was maintained at 160 or 240 mm for each of 3 cells (2 separate taste buds, 1 mouse). Responses were aligned at stimulus onsets, normalized to the maximum amplitude for each cell, and then the responses were averaged. Black line indicates mean. Gray bars represent SD. A prominent phasic response is followed by a plateau that lasts for the duration of the stimulus. G, NaCl responses are not blocked by amiloride. Responses of a taste cell to 6 sequential applications of NaCl (500 mm) in the absence (left) and presence (right, shaded) of 100 μm amiloride. Before responses shown in shaded area, the preparation was additionally incubated in 100 μm amiloride for 5 min. H, Summary of amiloride data. Filled symbols represent responses before amiloride. Open symbols represent responses during amiloride. Each symbol represents the average of 3 successive NaCl responses from one cell. Responses were normalized to the mean control response (filled symbol) for each cell. Mean and 95% CI in blue. There was no significant effect of amiloride on NaCl responses (paired t test, t(5) = 0.76, p = 0.48, n = 6 cells from 2 mice). n.s. = not significant. Calibration: C, D, G, 20 s, 0.5 ΔF/F; F, 10 s, 0.5 ΔF/F.
Next, we determined concentration-response relations for NaCl-evoked responses. To mimic the in vivo physiological milieu, the taste pore was superfused with AS for 5 min, after which test concentrations of NaCl (in AS) were focally applied. Increasing concentrations of NaCl (80–460 mm, total) yielded responses of increasing amplitude (Fig. 2D,E). The concentration-response relation for NaCl (curve fit estimates EC50 ≈ 236 mm, saturation ≈ 490 mm) was in the expected range for salt taste (Beidler, 1953; Breslin et al., 1993; Halpern, 1998). The finding that taste bud cells consistently and progressively responded over the range of hypotonic, isotonic, and hypertonic concentrations of NaCl suggests that the responses were not caused by cell swelling or shrinking.
A notable feature of salt responses recorded from taste nerves is their brief latency to peak followed by rapid adaptation. Hence, we stimulated with prolonged (20 s) applications of NaCl (160–240 mm) while confirming with dye tracers that the NaCl concentration remained constant throughout the stimulation. NaCl-evoked responses rapidly reached a peak and adapted in <5 s, maintaining a plateau slightly above baseline (Fig. 2F). This closely resembles electrophysiological recordings of chorda tympani nerve responses to lingual stimulation with NaCl (Pfaffmann, 1941; Beidler, 1953; Brand et al., 1985).
NaCl-evoked responses are insensitive to amiloride
Loose-patch and afferent nerve recordings have identified both amiloride-sensitive and -insensitive NaCl responses originating in fungiform taste buds (Heck et al., 1984; Ninomiya and Funakoshi, 1988; Yoshida et al., 2009a). Thus, we measured responses to focally applied NaCl before and while bathing lingual slices in 100 μm amiloride (a supramaximal concentration). Amiloride was included in the stimulus and bathing buffer. NaCl-evoked responses were undiminished in the presence of amiloride (Fig. 2G,H). Separately, we also tested the high-potency amiloride analog, benzamil (1 μm; data not shown). Benzamil did not significantly alter the amplitude of responses to NaCl (p = 0.81, 1 mouse, 5 cells). In summary, NaCl-evoked Ca2+ responses in GCaMP-expressing fungiform taste bud cells were insensitive to amiloride or benzamil.
Are NaCl-responsive cells tuned to a single taste quality?
Salts and acid tastants are ionic stimuli and are proposed to be transduced in overlapping cell populations (Lindemann, 2001; Lewandowski et al., 2016). Hence, we tested whether NaCl responses were in Type 3 cells by sequentially stimulating with NaCl and citric acid (a Type 3 cell stimulus), applied to the same taste pore. None of the cells that responded to NaCl also responded to citric acid (Fig. 3A). In a separate series, we focally stimulated taste buds sequentially with NaCl followed by saccharin. We selected saccharin because it is known to stimulate both sweet and bitter taste receptors (Kuhn et al., 2004), and thus would activate a large subset of Type 2 cells. We did not attempt to distinguish among Type 2 cells selective for sweet versus bitter taste stimuli. The majority of cells (8 of 12 cells; 5 mice) that responded to NaCl also displayed responses to saccharin (Fig. 3B). Parenthetically, although saccharin was presented as the sodium salt, the concentration of Na+ was <50 mm (i.e., below the threshold for NaCl responses; Fig. 2E). We further tested a mix of cycloheximide and denatonium, stimuli that also are selective for Type 2 cells. Of 8 NaCl-responsive cells, 3 (from 1 mouse) also responded to this mix (Fig. 3C). In summary, we detected NaCl responses in cells that responded to prototypic stimuli for Type 2, but not Type 3, cells. Taste bud cells sensitive to both NaCl and saccharin often displayed small saccharin responses (Fig. 3B). We did not explore this point further.
Figure 3.
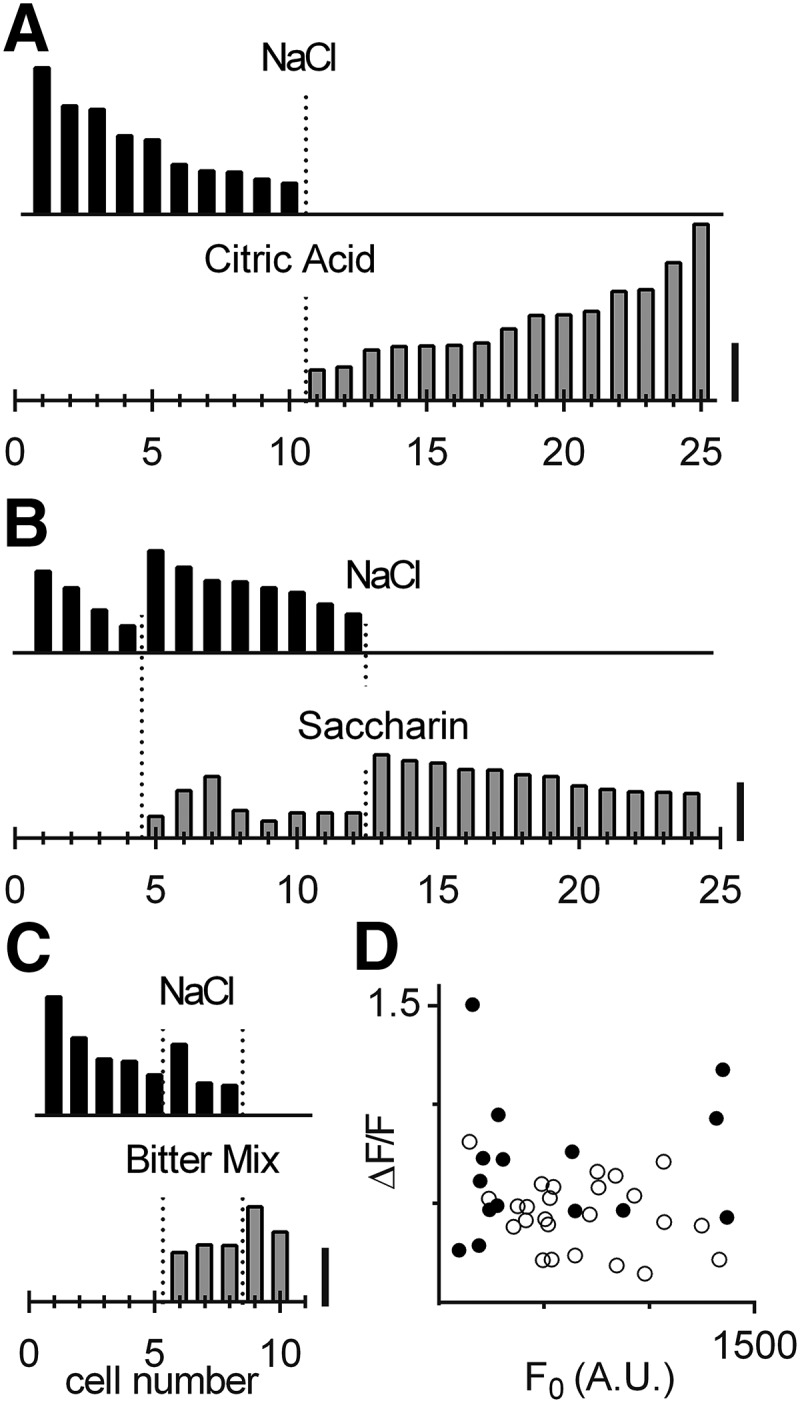
Many NaCl-responsive cells respond to prototypic stimuli for Type 2, but not Type 3, cells. A, Taste buds were focally stimulated sequentially with two stimuli: NaCl (130–850 mm, black bars) and citric acid (8–19 mm, gray bars). All 25 cells that responded to at least one of the stimuli are arranged sequentially from left to right along the x axis, according to peak response to NaCl (black) or citric acid (gray). Of 25 individual taste cells (from 4 mice), none responded to both test stimuli. B, A different set of taste buds was sequentially stimulated with NaCl (220–500 mm, black bars) and saccharin (31–49 mm, gray bars). Of 24 taste cells recorded (8 mice), 4 responded only to NaCl, 12 only to saccharin, and 8 to both. Parenthetically, some of the NaCl-saccharin dual-responsive cells may have been T2R-expressing bitter-sensing taste cells. The fraction of NaCl-responsive cells that also responded to citric acid (0 of 25) is significantly different from the fraction (8 of 24) that also responded to saccharin (p = 0.0070, Fisher's exact test). C, Yet another set of taste buds was sequentially stimulated with NaCl (260–480 mm, black bars) and a “bitter mix” of cycloheximide (10–83 μm) plus denatonium (0.1–8.3 mm, gray bars). Of 10 taste cells recorded (5 mice), 5 responded only to NaCl, 2 responded only to the mix, and 3 responded to both. D, Responses of the taste cells from A to citric acid (filled circles) or B, C to saccharin or bitter mix (open circles) did not correlate to their resting (baseline) fluorescence (r2 = 0.025, p = 0.57, n = 15; Type 3 cells from 4 mice; r2 = 0.050, p = 0.27, n = 26; Type 2 cells from 10 mice). Calibration: A–C, 0.5 ΔF/F.
In some instances, the expression level (Fig. 1A–C) and resting fluorescence (i.e., F0) (Fig. 1H,K) of GCaMP appeared lower in Type 3 relative to Type 2 cells. Nevertheless, cells with low F0 produced robust responses (Fig. 1K–M). Further, we found no correlation between baseline F0 and the amplitude of taste-evoked responses (measured as the peak response averaged across 2 or 3 trials of the same cell) across 26 Type 2 cells and 15 Type 3 cells (Fig. 3D). That is, the ability of GCaMP to measure intracellular Ca2+ changes evoked by taste stimulation was not limited by its expression level.
Type 2 cells respond to NaCl stimulation
For an independent verification of the cell type in which NaCl responses were observed, we used a histological criterion. First, we recorded NaCl-evoked responses as above. The lingual slice was then fixed and whole-mount immunostained post hoc with anti-PLCβ2 and anti-Car4 to identify Type 2 and 3 cells. We were able to align and reidentify responding cells in 9 of 10 mice; 24 of the 29 NaCl-responsive cells were immunopositive for PLCβ2 (Fig. 4D,H); 2 were immunopositive for Car4; 3 cells could not be definitely typed. That is, NaCl-evoked Ca2+ responses in fungiform taste buds occur predominantly in Type 2 taste bud cells.
Figure 4.
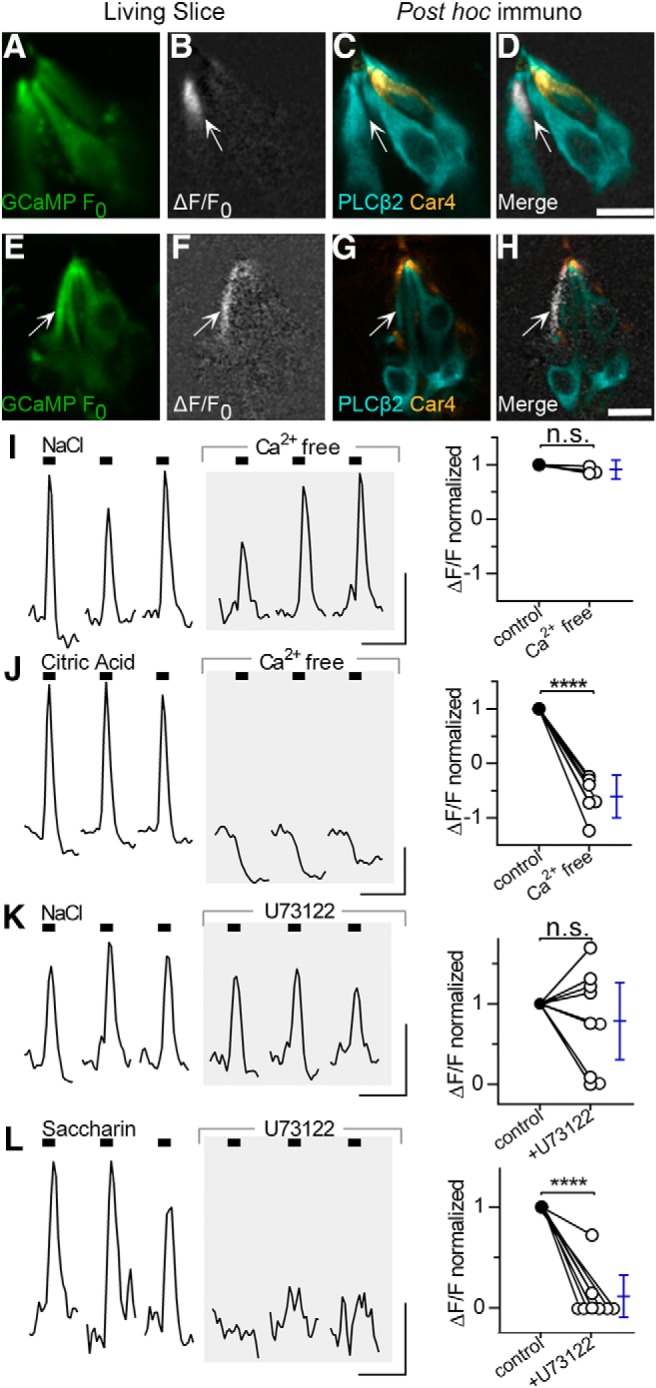
NaCl-responsive cells are a subset of Type 2 taste bud cells. A–H, Post hoc immunostaining to classify NaCl-responsive cells. A, Baseline GCaMP fluorescence (F0, green) of a taste bud before stimulation. B, ΔF/F0 for the same taste bud during peak response to NaCl (white). C, The lingual slice was then fixed and post hoc immunostained for PLCβ2 (cyan) and Car4 (orange). D, ΔF/F0 image (B) was overlaid onto the immunostained image (C) to reidentify the responsive cell as a PLCβ2+ (i.e., Type 2 cell). E–H, Another taste bud as in A–D. In both examples, NaCl-responsive cells overlap with PLCβ2 immunoreactivity. Scale bars, 10 μm. H, Contrast in the gray channel (ΔF/F0) was increased to improve the visibility of the overlay. I, NaCl responses are unaffected by removal of extracellular Ca2+. Traces represent responses of one cell to focal applications of matched concentrations of NaCl (210–320 mm NaCl) in Tyrode's buffer (left) and Ca2+-free Tyrode's (middle, gray shaded). Graph (right) represents the summary of data. Ca2+ removal had no significant effect on NaCl-responsive cells (t(2) = 2.54, p = 0.13, n = 3 cells, 3 mice). Blue error bars indicate mean and 95% CI. n.s. = not significant. J, In contrast, removing extracellular Ca2+ fully eliminated responses to 10–20 mm citric acid (t(5) = 10.5, p = 0.0001, n = 6 cells, 3 mice). The drop in baseline after citric acid application is attributed to the pH sensitivity of GCaMP to cytoplasmic acidification. K, NaCl responses before (left) and after incubation for 15–30 min in 10 μm U73122, a nonselective PLC inhibitor (middle, shaded). On average, no significant effect of the blocker was seen on responses to NaCl (210–500 mm; t(8) = 0.1.1, p = 0.31, 9 cells, 6 mice). n.s. = not significant. L, Responses to saccharin (25–50 mm) or a mix of denatonium (4–8 mm) and cycloheximide (40–80 μm) were significantly diminished after U73122 application (t(7) = 1.0, p < 0.0001, n = 8 cells, 4 mice). Significantly, the traces in K and L are from a single taste cell that responded to both NaCl and saccharin in the control condition, but only to NaCl after U73122 treatment. Calibration: I–L, 20 s, 0.5 ΔF/F. ****p < 0.0001.
Another criterion that distinguishes among Type 2 and 3 cells is the source of Ca2+ for taste-evoked responses. In Type 2 taste cells, the main source of Ca2+ is intracellular stores; Type 3 taste cells rely on Ca2+ entry through voltage-gated Ca2+ channels (Medler et al., 2003; DeFazio et al., 2006). We bathed lingual slices in Ca2+-free Tyrode's buffer and focally stimulated taste buds with NaCl (also in Ca2+ free buffer). NaCl-evoked responses were not significantly altered by removing extracellular Ca2+ (Fig. 4I). In contrast and as expected (Huang et al., 2008), citric acid-evoked responses were eliminated in Ca2+-free buffer (Fig. 4J). That is, NaCl-evoked responses do not rely on Ca entry but are likely produced by mobilizing intracellular Ca stores.
To investigate whether NaCl responses mobilized intracellular Ca2+ via PLCβ2, the canonical taste transduction pathway in Type 2 taste cells, we tested the effects of the nonselective PLC inhibitor, U73122 (Bleasdale et al., 1990). First, we stimulated taste buds with saccharin or a “bitter mix” of denatonium and cycloheximide, as a positive control for PLCβ2-dependent transduction, and subsequently with NaCl. We then incubated the lingual slice in 10 μm U73122 for 15–30 min and retested the same cells to NaCl and saccharin. As expected, responses to saccharin or bitter mix (8 cells, 4 mice) were inhibited by the PLC blocker (Fig. 4L). However, U73122 had a highly variable effect on NaCl responses (9 cells, 6 mice; Fig. 4K). The efficacy of the drug was verified on saccharin-evoked responses. Importantly, in a taste cell that was dually sensitive (i.e., responded to both NaCl and saccharin), U73122 completely inhibited saccharin-evoked responses while leaving NaCl responses unchanged (Fig. 4K,L).
NaCl-evoked Ca2+ responses are evoked by Cl−, not Na+
Next, we considered the relative roles of Na+ versus Cl− in generating NaCl responses in taste cells. Although salt taste is generally believed to occur through the action of Na+, it has long been recognized that the anion alters the efficacy of Na+ salts in taste nerve recordings (Elliott and Simon, 1990; Ye et al., 1991). We substituted Na+ and Cl− in turn with other cations or anions, respectively. All taste cells that responded to NaCl (70–350 mm) also responded to KCl (70–310 mm) (2 mice, 9 cells), although on average, responses to KCl were 46% smaller than those for matched concentrations of NaCl (Fig. 5A).
Figure 5.
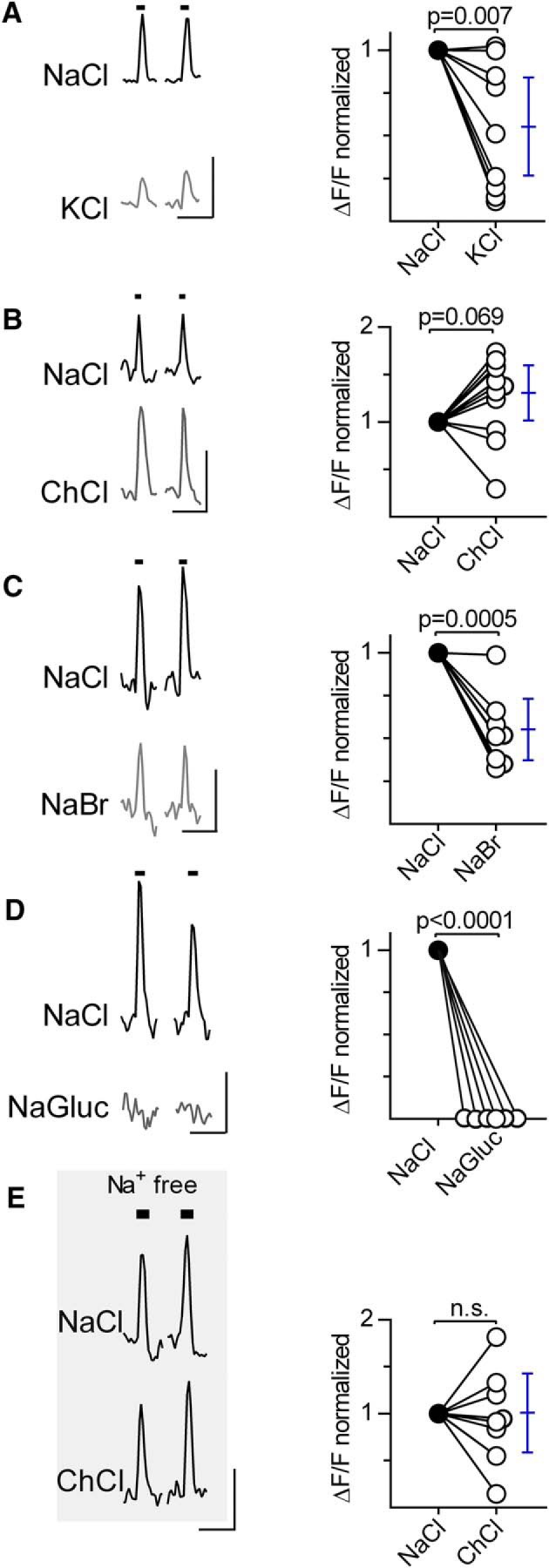
Ion substitution shows that responses to NaCl derive from the chloride ion. Taste buds were focally and sequentially stimulated with NaCl (black traces) and matched concentrations of one other salt (gray traces). Traces represent an example from each type of ion substitution. Plots represent response amplitudes to NaCl stimulation (filled circles) and to the other salt (open circles). Error bars indicate means and 95% CI. A, KCl (70–310 mm) elicited responses in all cells that responded to NaCl (70–350 mm). Amplitudes were significantly different between NaCl and KCl (t(8) = 3.6, p = 0.007, n = 9 cells, 2 mice) with a mean decrease of 46%. B, Choline chloride (ChCl, 170–340 mm) elicited responses in all cells that responded to NaCl (110–360 mm). Response amplitudes were not significantly different between matched concentrations of NaCl and ChCl (t(10) = 2.0, p = 0.069, n = 11 cells, 2 mice). C, Taste buds were stimulated with NaCl (90–340 mm) and NaBr (100–370 mm). All cells responded to both salts; responses to NaBr were smaller, a significant difference (t(7) = 6.1, p = 0.0005, n = 8 cells, 2 mice). D, Sodium gluconate (NaGluc, 150–370 mm) did not elicit responses in any of the NaCl (120–370 mm)-responsive cells (t(5) > 7, p < 0.0001, n = 6 cells, 2 mice). In all plots, the data for NaCl represent the mean of 2 or 3 replicate responses from each cell. In this series of experiments, the salt concentrations are noted as the concentration above that already present in Tyrode's buffer. E, Lingual slices were maintained in Na+-free NMDG-Tyrode's buffer (shaded) and stimulated with NaCl (90–290 mm; left), followed by matched concentrations of choline Cl (ChCl, middle). Responses are refractory to the complete elimination of permeable Na+, and response amplitudes to NaCl or choline chloride showed no significant difference (t(7) = 0.17, p = 0.87, n = 8 cells, 2 mice). n.s. = not significant. Calibration: A–E, 20 s, 0.5 ΔF/F.
We next tested whether substituting choline+ for Na+ altered salt-evoked responses. When lingual epithelium is mounted in Ussing chambers, choline chloride, like NaCl, is transported across the tissue but much less efficiently, suggesting that it may not be as effective a salt taste stimulus as NaCl (Mierson et al., 1985). Surprisingly, taste cells produced similar Ca2+ signals to matched concentrations of NaCl (110–360 mm) and choline chloride (170–340 mm) (Fig. 5B) (2 mice, 11 taste cells). The result suggests that Na+ may not be the principal driver of NaCl-evoked responses in fungiform taste bud cells.
Further, we tested the importance of the anion by substituting Cl− with Br−, a halide anion that shares physiochemical properties with Cl−. Taste cells that responded to NaCl also responded to NaBr (Fig. 5C) (2 mice, 8 taste cells). NaBr-evoked responses were, on average, 54% smaller than those to matched concentrations of NaCl. In contrast, when Cl− was substituted with the organic anion, gluconate, NaCl-responsive cells produced no responses to matched concentrations of Na gluconate (Fig. 5D; 2 mice, 6 taste cells), emphasizing the importance of Cl−.
Finally, to test whether Na+ in the surrounding medium is necessary for taste cells to generate salt-evoked responses, we bathed lingual tissue in Na+-free NMDG-Tyrode's buffer, continuously from preparing slices to recording responses. Even in the complete absence of extracellular Na+, taste cells continued to respond to focal stimulation with choline chloride (Fig. 5E) (2 mice, 8 cells). That is, Na+ appears to play no role, and Cl− is essential for these amiloride-insensitive NaCl-evoked Ca2+ responses in taste bud cells.
The role of Cl− in NaCl-evoked Ca2+ responses
We considered whether Cl− influx through channels or transporters plays a role for these NaCl-evoked Ca2+ signals in taste bud cells. To test this, we focally stimulated taste cells with NaCl in the presence of DIDS, a fast-acting, broad-spectrum inhibitor of many Cl− channels. Saturating concentrations of DIDS (500 μm) did not affect the amplitude of responses to either NaCl (Fig. 6A) or saccharin (data not shown).
Figure 6.
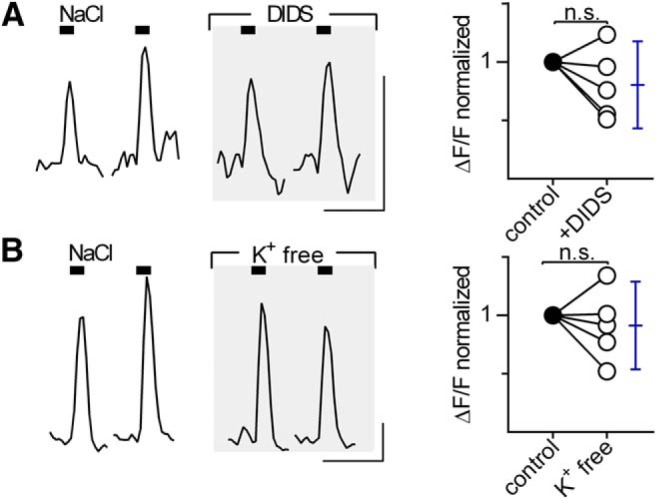
Responses to NaCl are not inhibited by blockers of Cl− channels or Cl− cotransporters. A, Taste buds were focally stimulated with NaCl (350–480 mm) in the absence (left) and presence (middle, shaded) of the broad-spectrum Cl− channel blocker, DIDS (500 μm). Right, The plot summarizes these data. Responses of each cell in the presence of DIDS were normalized to the mean control response amplitude of the same cell. There was no significant effect of DIDS treatment on NaCl response amplitudes (t(4) = 1.47, p = 0.21, n = 5 cells, 2 mice). Blue error bars indicate mean and 95% CI. n.s. = not significant. B, Taste buds were stimulated with NaCl (480–490 mm) in Tyrode's buffer before (left) and after (middle, shaded) the bath was exchanged for K+-free Tyrode's buffer. K+ removal had no effect on response amplitudes to NaCl (t(4) = 0.64, p = 0.56, n = 5 cells, 1 mouse). n.s. = not significant. All points on plots are means of two replicate stimulations. Calibration: A, B, 20 s, 0.5 ΔF/F.
We also considered the possible involvement of Cl− transporters. To test for the involvement of a K-Cl− cotransporter, we bathed lingual slices in K+-free Tyrode's buffer. Responses to NaCl were unaffected by K+ removal (Fig. 6B). As expected, K+ removal did not affect responses to saccharin either (data not shown). Because bathing slices in Na+-free buffer did not affect responses to Cl− salts (Fig. 5E), Na-Cl− cotransporters also must be eliminated as candidates.
Discussion
We used a semi-intact lingual slice preparation and confocal Ca2+ imaging to study salt-evoked responses in mouse fungiform taste bud cells. NaCl applied focally and briefly onto the chemosensitive apical tips of taste cells produced transient and local increases in intracellular Ca2+ in a subpopulation of cells. NaCl-evoked Ca2+ responses exhibited known and expected characteristics of salt taste they were concentration-dependent over a range similar to that reported for salt taste in rodents, and they rapidly adapted in the presence of a maintained NaCl stimulus (Pfaffmann, 1941; Beidler, 1953; Brand et al., 1985). Our data indicate that, in fungiform taste buds, amiloride-insensitive NaCl-evoked Ca2+ responses are limited to Type 2 cells, based on post hoc immunostaining, the Ca2+ mobilization underlying the responses, and the taste specificity of responsive cells.
Salt taste in the anterior tongue (fungiform taste buds) includes amiloride-sensitive and amiloride-insensitive components (Ninomiya and Funakoshi, 1988; Béhé et al., 1990), which may arise in separate populations of taste bud cells (Yoshida et al., 2009a; Chandrashekar et al., 2010). Although both types of taste cell responses have been shown to evoke Ca2+ transients (Chandrashekar et al., 2010; Oka et al., 2013), we observed only amiloride-insensitive responses in the present study. A possible explanation for the difference is that Chandrashekar et al. (2010) and Oka et al. (2013) loaded Ca2+ indicator iontophoretically into taste cells without targeting specific cell types, whereas in our study, GCaMP was genetically expressed only in Type 2 and 3 taste bud cells (Fig. 1). If amiloride-sensitive NaCl responses occur in taste cells other than Type 2 or Type 3 (Vandenbeuch et al., 2008; Chandrashekar et al., 2010), they would not be visible in the transgenic strain we used.
Our findings offer a resolution to a discrepancy in the literature regarding the NaCl concentration range of amiloride-insensitive responses. Previous Ca2+ imaging studies by Zuker and colleagues of amiloride-insensitive salt responses suggested that they were limited to the “high-salt” (≥300 mm) range (Chandrashekar et al., 2010; Oka et al., 2013), a finding at odds with recordings from several laboratories (Heck et al., 1984; Elliott and Simon, 1990; Ye et al., 1993; Ninomiya, 1998; Yoshida et al., 2009a; Wu et al., 2015). We show that amiloride-insensitive Ca2+ responses in taste bud cells are localized to the apical tips of cells. Because Zuker and colleagues imaged taste buds en face (i.e., parallel to and below the lingual surface), they likely would not have detected Ca2+ transients limited to the apical tips, or may have required higher concentrations of NaCl to visualize them.
Our assignment of amiloride-insensitive responses to Type 2 cells is consistent with other reports on fungiform taste buds, which showed these responses in either bitter only (Oka et al., 2013) or bitter and sweet cells (Yoshida et al., 2006, 2009b). Because anterior (fungiform) and posterior (circumvallate) taste fields differ functionally (Ninomiya, 1998; Danilova and Hellekant, 2003; Yasumatsu et al., 2015), this may account for the detection of amiloride-insensitive responses in only Type 3 circumvallate taste cells (Lewandowski et al., 2016).
Taste responses to Na+ salts are smaller with anions, such as benzoate, gluconate, or acetate relative to chloride. This “anion effect” was interpreted as direct inhibition of Na+ transduction by the large anions (Beidler, 1967). However, later studies of transepithelial transport in Ussing chambers reached a different conclusion. Because large anions cannot readily cross apical tight junctions between taste cells, the paracellular penetration of Na+ into the taste bud is retarded, resulting in decreased stimulation of basolateral receptors for Na+ (Elliott and Simon, 1990; Ye et al., 1991, 1993, 1994). However, a recent study on dissociated taste bud cells, which lack tight junctions and transepithelial potentials, still observed an anion effect on salt responses (Lewandowski et al., 2016). This observation renewed the hypothesis that anions directly inhibit Na+ transduction, albeit via an unknown mechanism.
In our study, NaCl-sensitive cells also responded to KCl (Fig. 5A), as seen in electrophysiological recordings of amiloride-insensitive salt responses (Ninomiya and Funakoshi, 1988; Breza and Contreras, 2012). Indeed, all chloride salts that we tested produced responses of similar magnitude and subcellular location, and only in the same cells as NaCl. The only nonchloride salt that yielded these responses was NaBr, which includes a closely related halide ion. Conversely, choline chloride produced similar responses in NaCl-sensing cells, even in the complete absence of Na+ in the bathing solution (Fig. 5B,E). In combination, the results suggest that Cl− is both necessary and sufficient for amiloride-insensitive NaCl responses in fungiform taste cells. Formaker and Hill (1988) reached a similar conclusion based on cross-adaptation studies on afferent nerve responses, that amiloride-insensitive salt taste includes a halide anion component. Our findings shed light on a cellular component of the anion effect by illustrating that large anions do not necessarily inhibit Na+ taste transduction (Beidler, 1967; Lewandowski et al., 2016) but instead, that they displace the Cl− ion that is essential for amiloride-insensitive salt responses. In the intact animal, the anion effect on salt taste likely includes both a direct transduction component and reduced transepithelial Na+ permeability.
We tested whether Cl− channels or cotransporters play a role in producing amiloride-insensitive NaCl responses. A broad-spectrum inhibitor of Cl− channels, DIDS, did not affect responses (Fig. 6A). Removing extracellular Na+ or K+ also did not alter responses, suggesting that the mechanism does not involve Na+- or K+-dependent Cl− transport (Figs. 5E, 6B). These findings agree with previous studies of inhibitors of chloride channels and transporters on NaCl responses in taste nerves (Elliott and Simon, 1990; Rehnberg et al., 1993).
How NaCl stimulation leads to release of Ca2+ from intracellular stores in Type 2 cells remains an open question, given that a PLC inhibitor did not consistently change NaCl-evoked Ca2+ responses (Fig. 4K). In agreement with this, previous studies found there is only a slight reduction in responses to high concentrations of NaCl in PLCβ2 KO relative to WT mice in both behavioral assays (Dotson et al., 2005) and nerve recordings (Oka et al., 2013). Together, our and others' findings suggest that PLCβ2 is not critical for salt taste. Pathways for mobilizing intracellular Ca2+ other than via PLCβ2 have been described. These include release from acidic stores or from vesicles tethered to the plasma membrane. In these cases, release is effected via second messengers, such as NAADP or cADPR, or via stimuli, such as localized membrane deformation (Patel and Docampo, 2010; Lam and Galione, 2013).
Both Na+ and Cl− are essential dietary minerals. Salt taste in humans is predominantly unaffected by amiloride (Desor and Finn, 1989; Breslin and Beauchamp, 1995; Ossebaard and Smith, 1995), which underscores the importance of understanding the cellular mechanisms of amiloride-insensitive NaCl taste. One possible interpretation of the data presented here is that there are two parallel taste transduction pathways in salt taste: one for Na+ and a separate, amiloride-insensitive one for Cl−. These two pathways might function as a coincidence detector for identifying NaCl. In short, the combined transduction of both ions might be critical for salt taste.
Footnotes
J.K.R. was supported in part by the Lois Pope LIFE Fellows Program. This work was supported by National Institutes of Health/National Institute on Deafness and Other Communication Disorders Grant R01 DC006308 to N.C. and Grant R01 DC007630 to S.D.R. We thank Peter Larsson for helpful discussions.
The authors declare no competing financial interests.
References
- Ackman JB, Burbridge TJ, Crair MC (2012) Retinal waves coordinate patterned activity throughout the developing visual system. Nature 490:219–225. 10.1038/nature11529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avenet P, Lindemann B (1991) Noninvasive recording of receptor cell action potentials and sustained currents from single taste buds maintained in the tongue: the response to mucosal NaCl and amiloride. J Membr Biol 124:33–41. 10.1007/BF01871362 [DOI] [PubMed] [Google Scholar]
- Béhé P, DeSimone JA, Avenet P, Lindemann B (1990) Membrane currents in taste cells of the rat fungiform papilla: evidence for two types of Ca currents and inhibition of K currents by saccharin. J Gen Physiol 96:1061–1084. 10.1085/jgp.96.5.1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beidler LM. (1953) Properties of chemoreceptors of tongue of rat. J Neurophysiol 16:595–607. 10.1152/jn.1953.16.6.595 [DOI] [PubMed] [Google Scholar]
- Beidler LM. (1967) Anion influences in taste receptor response. In: Olfaction and taste, Vol II (Hayashi T, ed), pp 509–534. Oxford: Pergamon. [Google Scholar]
- Bigiani A, Cuoghi V (2007) Localization of amiloride-sensitive sodium current and voltage-gated calcium currents in rat fungiform taste cells. J Neurophysiol 98:2483–2487. 10.1152/jn.00716.2007 [DOI] [PubMed] [Google Scholar]
- Bleasdale JE, Thakur NR, Gremban RS, Bundy GL, Fitzpatrick FA, Smith RJ, Bunting S (1990) Selective inhibition of receptor-coupled phospholipase C-dependent processes in human platelets and polymorphonuclear neutrophils. J Pharmacol Exp Ther 255:756–768. [PubMed] [Google Scholar]
- Brand JG, Teeter JH, Silver WL (1985) Inhibition by amiloride of chorda tympani responses evoked by monovalent salts. Brain Res 334:207–214. 10.1016/0006-8993(85)90212-4 [DOI] [PubMed] [Google Scholar]
- Breslin PA, Beauchamp GK (1995) Suppression of bitterness by sodium: variation among bitter taste stimuli. Chem Senses 20:609–623. 10.1093/chemse/20.6.609 [DOI] [PubMed] [Google Scholar]
- Breslin PA, Kaplan JM, Spector AC, Zambito CM, Grill HJ (1993) Lick rate analysis of sodium taste-state combinations. Am J Physiol 264:R312–R318. 10.1152/ajpregu.1993.264.2.R312 [DOI] [PubMed] [Google Scholar]
- Breza JM, Contreras RJ (2012) Anion size modulates salt taste in rats. J Neurophysiol 107:1632–1648. 10.1152/jn.00621.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caicedo A, Roper SD (2001) Taste receptor cells that discriminate between bitter stimuli. Science 291:1557–1560. 10.1126/science.1056670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caicedo A, Jafri MS, Roper SD (2000) In situ Ca2+ imaging reveals neurotransmitter receptors for glutamate in taste receptor cells. J Neurosci 20:7978–7985. 10.1523/JNEUROSCI.20-21-07978.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caicedo A, Kim KN, Roper SD (2002) Individual mouse taste cells respond to multiple chemical stimuli. J Physiol 544:501–509. 10.1113/jphysiol.2002.027862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekar J, Kuhn C, Oka Y, Yarmolinsky DA, Hummler E, Ryba NJ, Zuker CS (2010) The cells and peripheral representation of sodium taste in mice. Nature 464:297–301. 10.1038/nature08783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhari N, Roper SD (2010) The cell biology of taste. J Cell Biol 190:285–296. 10.1083/jcb.201003144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dando R, Pereira E, Kurian M, Barro-Soria R, Chaudhari N, Roper SD (2015) A permeability barrier surrounds taste buds in lingual epithelia. Am J Physiol Cell Physiol 308:C21–C32. 10.1152/ajpcell.00157.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilova V, Hellekant G (2003) Comparison of the responses of the chorda tympani and glossopharyngeal nerves to taste stimuli in C57BL/6J mice. BMC Neurosci 4:5. 10.1186/1471-2202-4-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFazio RA, Dvoryanchikov G, Maruyama Y, Kim JW, Pereira E, Roper SD, Chaudhari N (2006) Separate populations of receptor cells and presynaptic cells in mouse taste buds. J Neurosci 26:3971–3980. 10.1523/JNEUROSCI.0515-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desor JA, Finn J (1989) Effects of amiloride on salt taste in humans. Chem Senses 14:793–803. 10.1093/chemse/14.6.793 [DOI] [Google Scholar]
- Dotson CD, Roper SD, Spector AC (2005) PLCbeta2-independent behavioral avoidance of prototypical bitter-tasting ligands. Chem Senses 30:593–600. 10.1093/chemse/bji053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvoryanchikov G, Hernandez D, Roebber JK, Hill DL, Roper SD, Chaudhari N (2017) Transcriptomes and neurotransmitter profiles of classes of gustatory and somatosensory neurons in the geniculate ganglion. Nat Commun 8:760. 10.1038/s41467-017-01095-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott EJ, Simon SA (1990) The anion in salt taste: a possible role for paracellular pathways. Brain Res 535:9–17. 10.1016/0006-8993(90)91817-Z [DOI] [PubMed] [Google Scholar]
- Formaker BK, Hill DL (1988) An analysis of residual NaCl taste response after amiloride. Am J Physiol 255:R1002–R1007. 10.1152/ajpregu.1988.255.6.R1002 [DOI] [PubMed] [Google Scholar]
- Gilbertson TA, Avenet P, Kinnamon SC, Roper SD (1992) Proton currents through amiloride-sensitive Na channels in hamster taste cells: role in acid transduction. J Gen Physiol 100:803–824. 10.1085/jgp.100.5.803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern BP. (1998) Amiloride and vertebrate gustatory responses to NaCl. Neurosci Biobehav Rev 23:5–47. 10.1016/S0149-7634(97)00063-8 [DOI] [PubMed] [Google Scholar]
- Heck GL, Mierson S, DeSimone JA (1984) Salt taste transduction occurs through an amiloride-sensitive sodium transport pathway. Science 223:403–405. 10.1126/science.6691151 [DOI] [PubMed] [Google Scholar]
- Huang YA, Maruyama Y, Stimac R, Roper SD (2008) Presynaptic (Type III) cells in mouse taste buds sense sour (acid) taste. J Physiol 586:2903–2912. 10.1113/jphysiol.2008.151233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Chu Y, Han L, Li M, Li Z, LaVinka PC, Sun S, Tang Z, Park K, Caterina MJ, Ren K, Dubner R, Wei F, Dong X (2014) Central terminal sensitization of TRPV1 by descending serotonergic facilitation modulates chronic pain. Neuron 81:873–887. 10.1016/j.neuron.2013.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretz O, Barbry P, Bock R, Lindemann B (1999) Differential expression of RNA and protein of the three pore-forming subunits of the amiloride-sensitive epithelial sodium channel in taste buds of the rat. J Histochem Cytochem 47:51–64. 10.1177/002215549904700106 [DOI] [PubMed] [Google Scholar]
- Kuhn C, Bufe B, Winnig M, Hofmann T, Frank O, Behrens M, Lewtschenko T, Slack JP, Ward CD, Meyerhof W (2004) Bitter taste receptors for saccharin and acesulfame K. J Neurosci 24:10260–10265. 10.1523/JNEUROSCI.1225-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam AK, Galione A (2013) The endoplasmic reticulum and junctional membrane communication during calcium signaling. Biochim Biophys Acta 1833:2542–2559. 10.1016/j.bbamcr.2013.06.004 [DOI] [PubMed] [Google Scholar]
- Lewandowski BC, Sukumaran SK, Margolskee RF, Bachmanov AA (2016) Amiloride-insensitive salt taste is mediated by two populations of type III taste cells with distinct transduction mechanisms. J Neurosci 36:1942–1953. 10.1523/JNEUROSCI.2947-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liman ER, Zhang YV, Montell C (2014) Peripheral coding of taste. Neuron 81:984–1000. 10.1016/j.neuron.2014.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann B. (2001) Receptors and transduction in taste. Nature 413:219–225. 10.1038/35093032 [DOI] [PubMed] [Google Scholar]
- McCaughey SA, Scott TR (1998) The taste of sodium. Neurosci Biobehav Rev 22:663–676. 10.1016/S0149-7634(97)00067-5 [DOI] [PubMed] [Google Scholar]
- Medler KF, Margolskee RF, Kinnamon SC (2003) Electrophysiological characterization of voltage-gated currents in defined taste cell types of mice. J Neurosci 23:2608–2617. 10.1523/JNEUROSCI.23-07-02608.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michlig S, Damak S, Le Coutre J (2007) Claudin-based permeability barriers in taste buds. J Comp Neurol 502:1003–1011. 10.1002/cne.21354 [DOI] [PubMed] [Google Scholar]
- Mierson S, Heck GL, DeSimone SK, Biber TU, DeSimone JA (1985) The identity of the current carriers in canine lingual epithelium in vitro. Biochim Biophys Acta 816:283–293. 10.1016/0005-2736(85)90496-1 [DOI] [PubMed] [Google Scholar]
- Ninomiya Y. (1998) Reinnervation of cross-regenerated gustatory nerve fibers into amiloride-sensitive and amiloride-insensitive taste receptor cells. Proc Natl Acad Sci U S A 95:5347–5350. 10.1073/pnas.95.9.5347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninomiya Y, Funakoshi M (1988) Amiloride inhibition of responses of rat single chorda tympani fibers to chemical and electrical tongue stimulations. Brain Res 451:319–325. 10.1016/0006-8993(88)90777-9 [DOI] [PubMed] [Google Scholar]
- Oka Y, Butnaru M, von Buchholtz L, Ryba NJ, Zuker CS (2013) High salt recruits aversive taste pathways. Nature 494:472–475. 10.1038/nature11905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossebaard CA, Smith DV (1995) Effect of amiloride on the taste of NaCl, Na-gluconate and KCl in humans: implications for Na+ receptor mechanisms. Chem Senses 20:37–46. 10.1093/chemse/20.1.37 [DOI] [PubMed] [Google Scholar]
- Patel S, Docampo R (2010) Acidic calcium stores open for business: expanding the potential for intracellular Ca2+ signaling. Trends Cell Biol 20:277–286. 10.1016/j.tcb.2010.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffmann C. (1941) Gustatory afferent impulses. J Cell Comp Physiol 17:243–258. 10.1002/jcp.1030170209 [DOI] [Google Scholar]
- Rehnberg BG, MacKinnon BI, Hettinger TP, Frank ME (1993) Anion modulation of taste responses in sodium-sensitive neurons of the hamster chorda tympani nerve. J Gen Physiol 101:453–465. 10.1085/jgp.101.3.453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper SD, Chaudhari N (2017) Taste buds: cells, signals and synapses. Nat Rev Neurosci 18:485–497. 10.1038/nrn.2017.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueden CT, Schindelin J, Hiner MC, DeZonia BE, Walter AE, Arena ET, Eliceiri KW (2017) ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics 18:529. 10.1186/s12859-017-1934-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector AC, Grill HJ (1992) Salt taste discrimination after bilateral section of the chorda tympani or glossopharyngeal nerves. Am J Physiol Regul Integr Comp Physiol 263:R169–R176. 10.1152/ajpregu.1992.263.1.R169 [DOI] [PubMed] [Google Scholar]
- Tomchik SM, Berg S, Kim JW, Chaudhari N, Roper SD (2007) Breadth of tuning and taste coding in mammalian taste buds. J Neurosci 27:10840–10848. 10.1523/JNEUROSCI.1863-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu YH, Cooper AJ, Teng B, Chang RB, Artiga DJ, Turner HN, Mulhall EM, Ye W, Smith AD, Liman ER (2018) An evolutionarily conserved gene family encodes proton-selective ion channels. Science 359:1047–1050. 10.1126/science.aao3264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbeuch A, Clapp TR, Kinnamon SC (2008) Amiloride-sensitive channels in type I fungiform taste cells in mouse. BMC Neurosci 9:1. 10.1186/1471-2202-9-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD (2015) Breadth of tuning in taste afferent neurons varies with stimulus strength. Nat Commun 6:8171. 10.1038/ncomms9171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasumatsu K, Manabe T, Yoshida R, Iwatsuki K, Uneyama H, Takahashi I, Ninomiya Y (2015) Involvement of multiple taste receptors in umami taste: analysis of gustatory nerve responses in metabotropic glutamate receptor 4 knockout mice. J Physiol 593:1021–1034. 10.1113/jphysiol.2014.284703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q, Heck GL, DeSimone JA (1991) The anion paradox in sodium taste reception: resolution by voltage-clamp studies. Science 254:724–726. 10.1126/science.1948054 [DOI] [PubMed] [Google Scholar]
- Ye Q, Heck GL, DeSimone JA (1993) Voltage dependence of the rat chorda tympani response to Na+ salts: implications for the functional organization of taste receptor cells. J Neurophysiol 70:167–178. 10.1152/jn.1993.70.1.167 [DOI] [PubMed] [Google Scholar]
- Ye Q, Heck GL, DeSimone JA (1994) Effects of voltage perturbation of the lingual receptive field on chorda tympani responses to Na+ and K+ salts in the rat: implications for gustatory transduction. J Gen Physiol 104:885–907. 10.1085/jgp.104.5.885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida R, Shigemura N, Sanematsu K, Yasumatsu K, Ishizuka S, Ninomiya Y (2006) Taste responsiveness of fungiform taste cells with action potentials. J Neurophysiol 96:3088–3095. 10.1152/jn.00409.2006 [DOI] [PubMed] [Google Scholar]
- Yoshida R, Horio N, Murata Y, Yasumatsu K, Shigemura N, Ninomiya Y (2009a) NaCl responsive taste cells in the mouse fungiform taste buds. Neuroscience 159:795–803. 10.1016/j.neuroscience.2008.12.052 [DOI] [PubMed] [Google Scholar]
- Yoshida R, Miyauchi A, Yasuo T, Jyotaki M, Murata Y, Yasumatsu K, Shigemura N, Yanagawa Y, Obata K, Ueno H, Margolskee RF, Ninomiya Y (2009b) Discrimination of taste qualities among mouse fungiform taste bud cells. J Physiol 587:4425–4439. 10.1113/jphysiol.2009.175075 [DOI] [PMC free article] [PubMed] [Google Scholar]