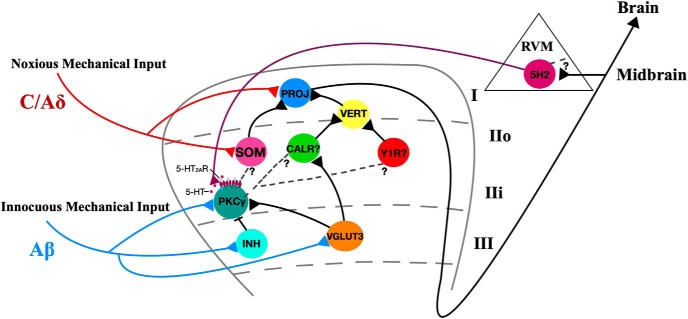
Nociceptors function to protect tissue from potential damage by thermal, mechanical, and chemical stimuli. The central terminals of primary nociceptive mechanical and thermal afferents (C/Aδ fibers) converge in superficial laminae I and II in the dorsal horn of the spinal cord. Nociceptive information is processed by excitatory and inhibitory interneurons in the dorsal horn of the spinal cord before being relayed to projection neurons in lamina I that transmit the information to higher brain centers that mediate the experience of pain (Koch et al., 2018) (Fig. 1).
Figure 1.
A dorsal horn model for circuits mediating mechanical allodynia. 5-HT, serotonergic descending projections; PROJ, lamina I pain projection neurons; VERT, vertical cells; SOM, somatostatin; CALR, calretinin; Y1R, neuropeptide Y1-receptor expressing; PKCγ, protein kinase C γ; INH, inhibitory interneurons; VGLUT3, vesicular glutamate transporter 3; RVM, rostral ventromedial medulla.
Light touch does not normally evoke pain, but after nerve injury, innocuous light touch can evoke a pain-like response called allodynia. Information about light touch is carried by low-threshold mechanical primary afferents (Aβ fibers) that synapse in laminae II-IV in the dorsal horn (Fig. 1). In inner lamina II, Aβ fibers synapse directly onto excitatory interneurons that express the γ isoform of protein kinase C (PKCγ) (Neumann et al., 2008; Lu et al., 2013). Although PKCγ interneurons do not receive direct input from mechanical nociceptors, they are strongly implicated in mediating mechanical allodynia (Lu et al., 2013; Petitjean et al., 2015). Allodynia is thought to stem from the loss of strong feedforward inhibition by inhibitory interneurons that prevent innocuous input from being transmitted as painful (Fig. 1). After nerve injury, these inhibitory synapses onto PKCγ interneurons are lost, and normally innocuous mechanical input from deep ventral laminae is transmitted to superficial lamina I to evoke pain (Miraucourt et al., 2007; Lu et al., 2013; Petitjean et al., 2015). Thus, the merging of the innocuous and noxious pathways promotes mechanical allodynia. Spinal nociceptive transmission is also modulated via descending supraspinal projections, which are responsible for the top-down processing of pain. Many of these descending projections contain the neuromodulator serotonin, which may play a role in mechanical allodynia as epidural 5HT2AR antagonists dose-dependently attenuate mechanical allodynia after nerve injury (Van Steenwinckel et al., 2008).
Mechanical allodynia is a hallmark of inflammatory as well as neuropathic pain, but the underlying circuitry remains incompletely understood. In particular, whether inflammatory pain also involves disinhibition of PKCγ interneurons and 5HT2ARs has not been clearly shown. Nonetheless, intrathecal administration of a PKCγ inhibitor attenuates capsaicin-induced inflammatory mechanical allodynia in mice, suggesting that PKCγ interneurons contribute to inflammatory allodynia (Petitjean et al., 2015). Because both PKCγ (Neumann et al., 2008) and 5HT2ARs (Fay and Kubin, 2000) are found predominately in excitatory interneurons of inner lamina II of the dorsal horn, Alba-Delgado et al. (2018) hypothesized that PKCγ and 5HT2ARs interact in PKCγ interneurons to facilitate inflammation-induced mechanical allodynia.
To test this hypothesis, the authors first tested the effects of 5HT2AR agonists and antagonists on mechanical withdrawal thresholds in rats treated with complete Freund's adjuvant (CFA) to induce inflammation. Pharmacological blockade of 5HT2ARs prevented CFA-induced mechanical facial allodynia in rats, and activation of 5HT2ARs was sufficient to induce facial mechanical allodynia in naive rats. In addition, the authors showed that PKCγ interneurons coexpress 5HT2ARs and that activation of 5HT2ARs increased levels of phosphorylated extracellular signal-regulated kinases 1/2 (pERK1/2), a marker of neuronal activation, in PKCγ-expressing neurons. These results suggest that activation of dorsal horn 5HT2ARs on PKCγ interneurons leads to mechanical facial allodynia.
Alba-Delgado et al. (2018) also probed the electrophysiological and morphological effects of CFA-induced inflammation and 5HT2AR blockade on lamina II interneurons. Intrinsic electrophysiological properties (resting membrane potential and slope in current–voltage plots) of lamina II excitatory interneurons differed between CFA-treated and sham animals, but these changes occurred independently of 5-HT2AR activation. In contrast morphological changes (reduction in the number of tertiary branches of the dendritic arbor, increase in spine density) induced by CFA occurred selectively in lamina II PKCγ-expressing interneurons, and these changes were partially prevented by blocking 5-HT2ARs. Finally, specific activation of 5-HT2ARs in naive rats replicated CFA-induced morphological changes in PKCγ interneurons. Together, these results indicate that activation of 5-HT2ARs on medullary dorsal horn PKCγ interneurons induces rapid morphological remodeling of the dendritic arbor, which may lead to the development of facial mechanical allodynia.
The 5-HT2AR-dependent morphological reorganization of PKCγ interneuron dendrites is a key finding that expands our understanding of the circuit underlying mechanical allodynia (Fig. 1). PKCγ interneurons lose inhibitory connections after neuropathic injury (Lu et al., 2013; Petitjean et al., 2015), and this loss might result from apoptosis of inhibitory interneurons or simply a loss of inhibitory contacts onto the PKCγ soma (Petitjean et al., 2015; Inquimbert et al., 2018). The results of Alba-Delgado et al. (2018) suggest that 5-HT2AR-mediated morphological reorganization reduces the dendritic arbor of PKCγ interneurons during inflammation. This reduced dendritic arbor might lead to decreases in the number of inhibitory synapses onto these neurons, causing a loss of the feedforward inhibition that normally prevents innocuous touch stimuli from exciting PKCγ interneurons. PKCγ interneurons would then be able to excite yet unknown postsynaptic neurons, allowing innocuous stimuli to reach pain projection neurons in the superficial lamina I of the dorsal horn (Fig. 1).
Alba-Delgado et al. (2018) raise an important question: what are the postsynaptic targets of the PKCγ interneurons that transmit innocuous mechanical input to superficial pain projection neurons? PKCγ interneurons synapse directly onto excitatory transient central cells in lamina II, and these synapse onto vertical cells, which then target lamina I pain projection neurons (Lu and Perl, 2005; Todd, 2017). The identity of transient central cells remains unknown, and researchers are trying to uncover these neural populations (Peirs and Seal, 2016). The most likely candidate is a subset of somatostatin-expressing excitatory interneurons in outer lamina II, as these neurons are necessary for mechanical pain (Duan et al., 2014) (Fig. 1). Calretinin interneurons are another probable candidate, as they are implicated in the development of mechanical allodynia (Peirs et al., 2015) (Fig. 1). The excitatory interneurons expressing neuropeptide Y1 receptors (Y1R), which are involved in both mechanical and thermal allodynia that arises after inflammatory and neuropathic injury, may also be a target of PKCγ interneurons as Y1Rs do not colocalize with PKCγ and are found superficial to PKCγ interneurons (Diaz-delCastillo et al., 2018; Nelson et al., 2019) (Fig. 1).
While Alba-Delgado et al. (2018) focus on PKCγ interneurons in mechanical allodynia, this is only a piece of the circuit. In deeper lamina III, interneurons that transiently express vesicular glutamate transporter 3 (VGLUT3) are upstream of PKCγ interneurons and also receive information about innocuous touch from Aβ fibers (Peirs and Seal, 2016) (Fig. 1). Most importantly, VGLUT3 interneurons in the spinal dorsal horn are both necessary and sufficient for mechanical allodynia (Peirs et al., 2015). Perhaps 5-HT2AR activation in PKCγ interneurons and the subsequent reorganization of the dendritic arbor permits them to be excited by VGLUT3 interneurons to induce mechanical allodynia.
The final questions Alba-Delgado et al. (2018) raise concerning the inflammation-induced mechanical allodynia circuitry pertain to the origin and cause of 5-HT release into the dorsal horn to act on PKCγ interneurons. Existing anatomical evidence indicates that 5-HT in the dorsal horn originates almost entirely from descending projections from the rostral ventromedial medulla (RVM) and is not released from local dorsal horn interneurons (Bannister and Dickenson, 2016). Inflammation may drive ascending pain signals from projection neurons that monosynaptically activate neurons in the RVM. The RVM's descending nociceptive projections, which likely include 5-HT fibers, release 5-HT to act on 5-HT2AR-expressing PKCγ interneurons and drive morphological reorganization and subsequent mechanical allodynia. Another possible circuit involves ascending pain projection neurons that activate neurons in the periaqueductal gray, which in turn can activate RVM descending nociceptive 5-HT fibers to the dorsal horn (Ossipov et al., 2014). In summary, the mechanical allodynia circuit includes not only projections from local dorsal horn excitatory and inhibitory interneurons but also descending 5-HT fibers from the RVM (Fig. 1).
Alba-Delgado et al. (2018) implicate descending 5-HT projections as mediators of morphological rearrangement in PKCγ interneurons that are both necessary and sufficient for inflammation-induced facial mechanical allodynia. This work is an important addition to the mechanical allodynia circuit summarized in Figure 1. In future studies, it will be important to uncover the cause of 5-HT release into the dorsal horn and the postsynaptic targets of the PKCγ interneurons that lead to the development of mechanical allodynia.
Footnotes
Editor's Note: These short reviews of recent JNeurosci articles, written exclusively by students or postdoctoral fellows, summarize the important findings of the paper and provide additional insight and commentary. If the authors of the highlighted article have written a response to the Journal Club, the response can be found by viewing the Journal Club at www.jneurosci.org. For more information on the format, review process, and purpose of Journal Club articles, please see http://www.jneurosci.org/content/jneurosci-journal-club.
I thank Heather Allen for critical review of the manuscript and thorough feedback.
The author declares no competing financial interests.
References
- Alba-Delgado C, Mountadem S, Mermet-Joret N, Monconduit L, Dallel R, Artola A, Antri M (2018) 5-HT2A receptor-induced morphological reorganization of PKCγ-expressing interneurons gates inflammatory mechanical allodynia in rat. J Neurosci 38:10489–10504. 10.1523/JNEUROSCI.1294-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister K, Dickenson AH (2016) What do monoamines do in pain modulation? Curr Opin Support Palliat Care 10:143–148. 10.1097/SPC.0000000000000207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-delCastillo M, Woldbye DP, Heegaard AM (2018) Neuropeptide Y and its involvement in chronic pain. Neuroscience 387:162–169. 10.1016/j.neuroscience.2017.08.050 [DOI] [PubMed] [Google Scholar]
- Duan B, Cheng L, Bourane S, Britz O, Padilla C, Garcia-Campmany L, Krashes M, Knowlton W, Velasquez T, Ren X, Ross S, Lowell BB, Wang Y, Goulding M, Ma Q (2014) Identification of spinal circuits transmitting and gating mechanical pain. Cell 159:1417–1432. 10.1016/j.cell.2014.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay R, Kubin L (2000) Pontomedullary distribution of 5-HT2A receptor-like protein in the rat. J Comp Neurol 418:323–345. [DOI] [PubMed] [Google Scholar]
- Inquimbert P, Moll M, Latremoliere A, Tong CK, Whang J, Sheehan GF, Smith BM, Korb E, Athié MC, Babaniyi O, Ghasemlou N, Yanagawa Y, Allis CD, Hof PR, Scholz J (2018) NMDA receptor activation underlies the loss of spinal dorsal horn neurons and the transition to persistent pain after peripheral nerve injury. Cell Rep 23:2678–2689. 10.1016/j.celrep.2018.04.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch SC, Acton D, Goulding M (2018) Spinal circuits for touch, pain, and itch. Annu Rev Physiol 80:189–217. 10.1146/annurev-physiol-022516-034303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Perl ER (2005) Modular organization of excitatory circuits between neurons of the spinal superficial dorsal horn (Laminae I and II). J Neurosci 25:3900–3907. 10.1523/JNEUROSCI.0102-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Dong H, Gao Y, Gong Y, Ren Y, Gu N, Zhou S, Xia N, Sun YY, Ji RR, Xiong L (2013) A feed-forward spinal cord glycinergic neural circuit gates mechanical allodynia. J Clin Invest 123:4050–4062. 10.1172/JCI70026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miraucourt LS, Dallel R, Voisin DL (2007) Glycine inhibitory dysfunction turns touch into pain through PKCgamma interneurons. PLoS One 2:e1116. 10.1371/journal.pone.0001116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson TS, Fu W, Donahue RR, Corder GF, Hökfelt T, Wiley RG, Taylor BK (2019) Facilitation of neuropathic pain by the NPY Y1 receptor subpopulation of excitatory interneurons in the dorsal horn. Sci Rep 9:7248. 10.1038/s41598-019-43493-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann S, Braz JM, Skinner K, Llewellyn-Smith IJ, Basbaum AI (2008) Innocuous, not noxious, input activates PKC interneurons of the spinal dorsal horn via myelinated afferent fibers. J Neurosci 28:7936–7944. 10.1523/JNEUROSCI.1259-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossipov MH, Morimura K, Porreca F (2014) Descending pain modulation and chronification of pain. Curr Opin Support Palliat Care 8:143–151. 10.1097/SPC.0000000000000055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirs C, Seal RP (2016) Neural circuits for pain: recent advances and current views. Science 354:578–584. 10.1126/science.aaf8933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirs C, Williams SP, Zhao X, Walsh CE, Gedeon JY, Cagle NE, Goldring AC, Hioki H, Liu Z, Marell PS, Seal RP (2015) Dorsal horn circuits for persistent mechanical pain. Neuron 87:797–812. 10.1016/j.neuron.2015.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitjean H, Pawlowski SA, Fraine SL, Sharif B, Hamad D, Fatima T, Berg J, Brown CM, Jan LY, Ribeiro-da-Silva A, Braz JM, Basbaum AI, Sharif-Naeini R (2015) Dorsal horn parvalbumin neurons are gate-keepers of touch-evoked pain after nerve injury. Cell Rep 13:1246–1257. 10.1016/j.celrep.2015.09.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd AJ. (2017) Identifying functional populations among the interneurons in laminae I-III of the spinal dorsal horn. Mol Pain 13:1–19. 10.1177/1744806917693003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Steenwinckel J, Brisorgueil MJ, Fischer J, Vergé D, Gingrich JA, Bourgoin S, Hamon M, Bernard R, Conrath M (2008) Role of spinal serotonin 5-HT2A receptor in 2′,3′-dideoxycytidine-induced neuropathic pain in the rat and the mouse. Pain 137:66–80. 10.1016/j.pain.2007.08.014 [DOI] [PubMed] [Google Scholar]