Abstract
Background
To comprehensively evaluate the etiology of chronic cough and the value of clinical feature in school age children in Suzhou, China.
Methods
School-age (6–14 years) children newly referred with chronic cough (>4 weeks) were prospectively evaluated by utilizing a diagnostic algorithm in this study. Clinical features of different etiologies of chronic cough were also investigated.
Results
In total, 118 patients were enrolled in the study. The cough duration ranged from 1 to 76 months. Upper airway cough syndrome (UACS) was found in 77 (65.3%) patients with chronic cough, cough-variant asthma (CVA) in 57 (48.3%) patients, protracted bronchitis (PB) in 15 (12.7%) patients, gastroesophageal reflux disease (GERD) in 7 (5.9%) patients, tic disorders (TD) in 3 (2.5%) patients and eosinophilic bronchitis (EB) in 2 (1.7%) patients. A single etiology was present in 75 patients and multiple etiologies were present in 43 patients. The three most common single etiologies were UACS (31.4%), CVA (14.4%), and PB (10.2%), followed by GERD (5.9%), and EB (1.7%). The most common multiple etiology was CVA + UACS (31.4%), followed by CVA + PB (2.5%), and TD + UACS (2.5%).
Conclusions
The common etiologies of chronic cough in school-age children were UACS, CVA, and PB, while EB and GERD were rare.
Keywords: Chronic cough, upper airway cough syndrome (UACS), cough-variant asthma (CVA), children
Introduction
Chronic cough, which is defined as a cough lasting for more than 4 weeks in children (1), is one of the most frequent symptoms for which children seek medical care in the clinical setting (2). A lot of children with chronic cough have not been diagnosed and treated appropriately. Chronic cough usually leads to substantial stress and heavy economic burdens. Quality of life of children and families of children are seriously impaired (3).
The identification of etiologies of chronic cough is the key to successful therapy. During the last three decades, there are a great number of studies focused on etiologies of chronic cough in adults, the common etiologies of chronic cough were cough-variant asthma (CVA), upper airway cough syndrome (UACS) and eosinophilic bronchitis (EB) (4-6). The etiologies of chronic cough in children should be different from adults (7,8)..Chang reported that the most common etiology of children with chronic cough was protracted bronchitis (PB) (7). Asthma and CVA seem the common etiologies in school-age children (8). Unfortunately, limited clinical data of chronic cough in children are available because many investigations such as the bronchial provocation test, esophageal 24-hour pH monitoring, and the cough provocation test are difficult to be conducted in children (9). Induced-sputum test for differential cells was seldom used in previous studies, it is not certain that whether the nonasthmatic EB is the common etiology of chronic cough like adults. Therefore, we conducted a prospective study to determine the etiologies of chronic cough and characteristics in school-age children.
Methods
Patients enrolled
This prospective single-center cohort study was conducted in Children’s Hospital of Soochow University from March 2012 to December 2013. Children’s Hospital of Soochow University is a tertiary hospital that provides service for most children in the Suzhou area. Children aged 6–14 years who were sequentially referred to our hospital for evaluation of chronic cough (>4 weeks) were eligible for the study (10). The exclusion criteria were previous known chronic respiratory illnesses such as bronchopulmonary dysplasia, immotile cilia syndrome, tuberculosis, asthma, and bronchiectasis. Patients with serious cardiac or systemic diseases were also excluded.
Informed consent
This study was conducted in accordance with the amended Declaration of Helsinki. The Ethical Committee of Suzhou University approved the protocol (SZU2012-C133), and written informed consent was obtained from the parents of all patients.
Study design
A systematic, step-by-step diagnostic algorithm was used, with uniform diagnostic criteria (Table 1) according to the American College of Chest Physicians (ACCP) pediatric guidelines (10). At enrollment, all patients underwent a detailed physical examination which included complete ear, nose, throat (examination of the ear with reflected light from a headlight, inspection of the external nose, and visualization of the larynx), respiratory, and cardiovascular examinations looking particularly for clubbing, chest deformity, cardiac abnormality, or auscultatory abnormality. Data regarding demographic and clinical characteristics, medical history, and family history were obtained using a standardized data collection form. Corticosteroids, bronchodilators, leukotriene receptor antagonists, and histamine antagonists were inhibited 2 weeks prior to the beginning of the study. Clinical tests including pulmonary function test, the bronchial provocation test, skin prick tests, esophageal pH monitoring and induced sputum test were performed. Other tests such as routine blood tests, serum Mycoplasma pneumoniae-specific antibody measurement, and posteroanterior chest X-rays were also performed. The cough symptom score was routinely documented as described in the established guidelines (11). Briefly, daytime scores were as follows: 5 = cannot perform most usual activities due to severe coughing; 4 = frequent coughing which interferes with school or other activities; 3 = frequent coughing but does not interfere with school or other activities; 2 = cough for more than two short periods; 1 = cough for one or two short periods only and 0 = no cough. Nighttime scores were as follows: 5 = distressing cough; 4 = frequent coughs most of the night; 3 = frequent waking due to coughing; 2 = awoken once or awoken early due to coughing; 1 = cough on waking or on going to sleep only and 0 = no cough at night. Cough resolution (considered to be a cough-free status) was defined as ≥75% improvement or total resolution of cough for ≥3 consecutive days according to the parental reports and cough diary data (7).
Table 1. Diagnostic criteria of chronic cough.
| Causes | Diagnostic criteria |
|---|---|
| Upper airway cough syndrome | (I) Presence of postnasal discharge, nasal mucosal edema, hyperemia, and faintness; (II) response to an antihistamine, nasal saline, and/or nasal steroid therapy in 2 to 4 weeks |
| Cough-variant asthma | (I) An isolated chronic, nonproductive cough lasting for more than 4 weeks; (II) airflow limitation demonstrated by bronchodilator responsiveness and/or response to inhaled steroid (budesonide, 400 µg/d) within 4 weeks |
| Gastroesophageal reflux cough | (I) Esophageal pH monitoring showed that the esophageal pH was <4 for more than 5% of the total monitoring time; (II) response to treatment with a proton pump inhibitor within 2 to 4 weeks |
| Eosinophilic bronchitis | (I) Normal spirometry with normal airway responsiveness; (II) eosinophil count >3% in non-squamous cell sputum; (III) response to glucocorticosteroids |
| Protracted bronchitis | (I) A history of chronic moist cough; (II) response to antibiotic therapy (clarithromycin, 15 mg/kg/d for 10 days) with resolution of cough within 2 to 4 weeks |
| Tic disorders | (I) A neuropsychiatric disorder characterized by a waxing and waning pattern of motor and vocal tics which occur several times a day, nearly every day or intermittently, over the span of more than a year |
Pulmonary function test
Pulmonary function tests were performed by spirometry (MasterScreen IOS; Jäger, Höchberg, Germany) and interpreted by a pediatric pulmonologist. Lung volume was measured according to standard criteria (12). The parameters measured were the forced expiratory flow volume in 1 second (FEV1), forced vital capacity (FVC) and forced expiratory flow (FEF) 25–75%. The patients performed three times of forced expiratory maneuvers from the total lung capacity to the residual volume, and the best of the three measurements was used in the final analysis.
Bronchial provocation test
A bronchial provocation test was performed when the baseline post-salbutamol FEV1 was >70% predicted. In each test, a nose clip was worn and an aerosol was inhaled through the mouth by tidal breathing for 2 min. Phosphate-buffered saline was inhaled first, followed at 2-min intervals by 2-fold increasing concentrations of histamine acid phosphate from 0.01 to 1.28 mg. The response was measured by FEV1 at the start of the test and at 2 min after each inhalation. The inhalations were continued until the FEV1 decreased by 20% or the highest cumulative dose of 2.4 mg was reached. The results were expressed as the concentration required to etiology a 20% fall in the FEV1 (PD20-FEV1).
Induced sputum test for differential cells
When the baseline post-salbutamol FEV1 was >70% predicted, a 3% hypertonic saline solution was inhaled via an ultrasonic nebulizer for 10 min as previously described (13). Inhalations were discontinued when sputum was obtained or when the FEV1 decreased by >20%. The collected sputum was treated with dithioerythritol, vortexed for 15 s, and incubated for 15 min in a 37 °C water bath. The suspension was then filtered through a 48-lm nylon filter and centrifuged at 3,000 ×g for 10 min. Cytospin slides were prepared and stained with eosin-hematoxylin, and a differential cell count was obtained from 400 non-squamous cells. Samples with cell viability of >70% and squamous cell contamination of <20% were considered as qualified samples (14).
Skin prick test
Thirteen kinds (6 groups) of common aeroallergens was tested, including mites (Dermatophagoides pteronyssinus, D. farinae, and Blomia tropicalis), cockroaches (Periplaneta americana and Blattella germanica), pollens (Artemisia vulgaris, Ambrosia artemisiifolia, mixed grasses, and mixed trees), cats (Felis domesticus), dogs (Canis familiaris), and molds (mold mixes I and IV). Allergens and negative control solutions were supplied by ALK (Hørsholm, Denmark). Atopy was defined as the presence of at least one positive skin reaction to any allergen tested by skin prick tests. We considered an allergen-specific skin test response to be positive if the difference between the wheal size of the allergen-specific test and the negative control was at least 3 mm.
Twenty-four-hour esophageal pH
The 24-hour esophageal pH values were recorded on Digitrapper MK III pH recorder (Swedish CTD-SYECTICS Ltd., Stockholm, Sweden) with bipolar antimony electrodes. The results are transferred to the computer for processing, and described as the DeMeester score and symptom association probability (15).
Chest X-ray
Plain posteroanterior chest radiographs were obtained in all the patients at the time of the first visit to hospital. The films were reviewed by a physician and a radiologist.
Statistical analysis
SPSS 17.0 version (IBM Corp., Armonk, NY, USA) was used for the statistical analysis. The numerical parameters are described as mean ± standard deviation or number with percentage as appropriate. Parametric and nonparametric comparative tests for continuous data and the chi-square test for categorical data were used to compare variables between groups. Sensitivity, specificity, and positive and negative predictive values for the presence of symptoms and signs and for each accessory examination were calculated. A two-tailed P value of <0.05 was considered statistically significant.
Results
Sample demographics
In total, 118 patients were enrolled in the study (mean age, 9.3±1.1 years). Of all the 118 patients, 68 (57.6%) were males and 50 (42.4%) were females; 53 patients (44.9%) were passive smokers. The cough duration ranged from 1 to 76 months. Thirty-five (29.7%) patients were enrolled in spring, 40 (33.9%) in summer, 35 (29.7%) in autumn, and 8 (6.8%) in winter.
Etiologies associated with chronic cough
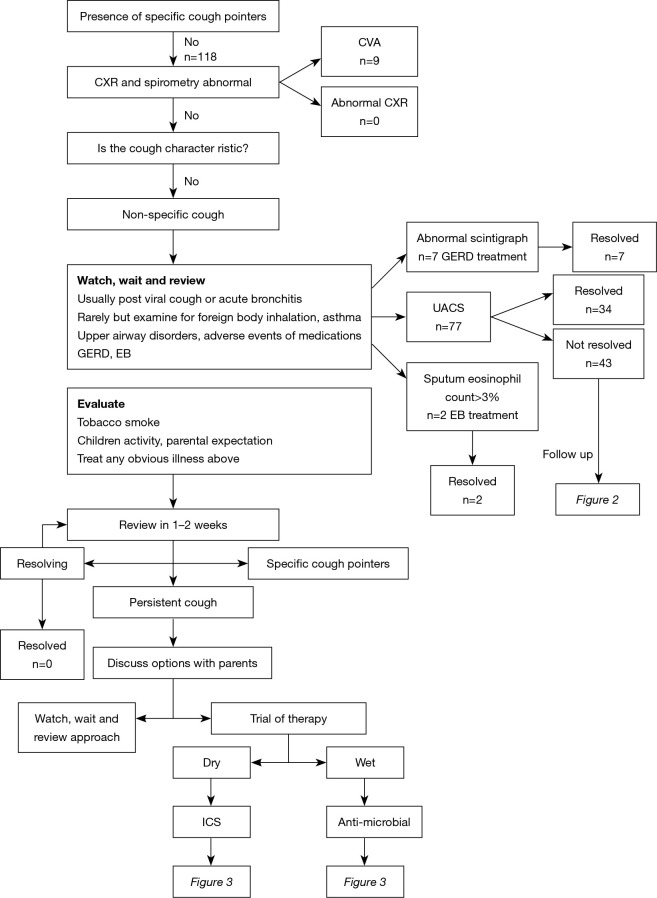
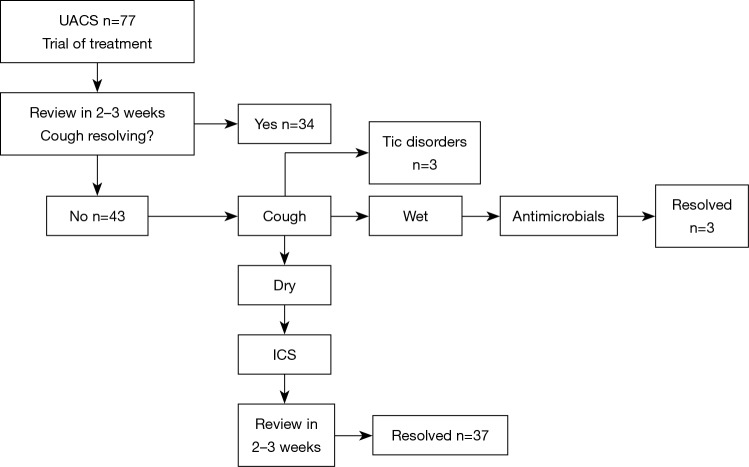
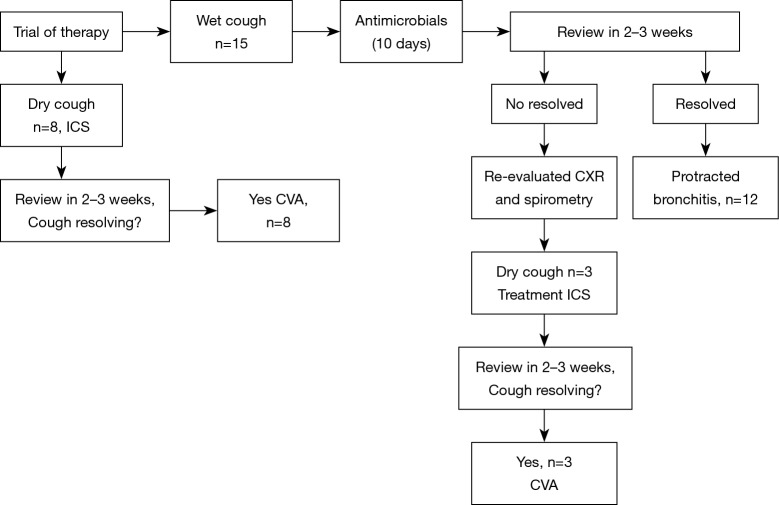
Algorithmic monitoring of our patients is shown in Figure 1. An oral antihistamine, a nasal saline solution and a steroid were administered to 77 patients in whom UACS was considered to be present after a medical history obtained and a physical examination. The evaluation of our patients with UACS is shown in Figure 2. The evaluation of our patients with productive and non-productive cough is shown in Figure 3.
Figure 1.
Monitoring algorithm for the management of our patients. Wet cough indicates of productive cough, while dry cough indicates of non-productive cough. CXR, chest radiograph; ICS, inhaled corticosteroid therapy; CVA, cough-variant asthma; UACS, upper airway cough syndrome; EB, eosinophilic bronchitis; GERD, gastroesophageal reflux disease; TD, tic disorders.
Figure 2.
Evaluation of our patients with UACS. Wet cough indicates of productive cough, while dry cough indicates of non-productive cough. UACS, upper airway cough syndrome; ICS, inhaled corticosteroid therapy.
Figure 3.
Evaluation of our patients with productive and non-productive cough. Wet cough indicates of productive cough, while dry cough indicates of non-productive cough. CXR, chest radiograph; ICS, inhaled corticosteroid therapy; CVA, cough-variant asthma.
Finally, UACS was found in 77 (65.3%) patients with chronic cough, CVA in 57 (48.3%) patients, PB in 15 (12.7%) patients, gastroesophageal reflux disease (GERD) in 7 (5.9%) patients, TD in 3 (2.5%) patients and EB in 2 (1.7%) patients. A single etiology was present in 75 patients and multiple etiologies were present in 43 patients (Table 2). The three most common single etiologies were UACS (31.4%), CVA (14.4%), and PB (10.2%), followed by GERD (5.9%), and EB (1.7%). The most common multiple etiology was CVA + UACS (31.4%), followed by CVA + PB (2.5%), and TD + UACS (2.5%).
Table 2. Final diagnosis of all patients.
| Final diagnosis | No of patients, n (%) |
|---|---|
| UACS | 37 (31.4) |
| CVA | 17 (14.4) |
| PB | 12 (10.2) |
| GERD | 7 (5.9) |
| EB | 2 (1.7) |
| CVA + UACS | 37 (31.4) |
| CVA + PB | 3 (2.5) |
| TD + UACS | 3 (2.5) |
UACS, upper airway cough syndrome; CVA, cough-variant asthma; PB, protracted bronchitis; GERD, gastroesophageal reflux disease; EB, eosinophilic bronchitis; TD, tic disorders.
Clinical features of different etiologies of chronic cough
The clinical features of chronic cough were analyzed in patients with different etiologies (CVA, CVA + UACS, UACS) (Table 3). The median cough durations of patients diagnosed with CVA, CVA + UACS and UACS were 20.1±22.2, 18.6±18.2 and 7.9±9.8 months, respectively. Nocturnal cough was a typical feature of CVA and had a high incidence of 64.7% (P<0.01). Productive cough was a typical feature of UACS and had a high incidence of 54.1% (P<0.01). The sensitivity, specificity, and positive and negative predictive values of each clinical feature are shown in Table 4.
Table 3. Clinical features of different causes of chronic cough.
| Feature | CVA | CVA + UACS | UACS | P value |
|---|---|---|---|---|
| Cases | 17 | 37 | 37 | – |
| Female (%) | 52.9 | 43.2 | 51.3 | 0.53 |
| Age (years) | 8±2 | 8±2 | 9±3 | 0.09 |
| Duration (months) | 20.1±22.2 | 18.6±18.2 | 7.9±9.8 | <0.01 |
| Cough features, n (%) | ||||
| Non-productive cough | 14 (82.4) | 24 (64.9) | 17 (45.9) | 0.07 |
| Productive cough | 3 (17.6) | 16 (43.2) | 20 (54.1) | 0.08 |
| Nocturnal cough | 11 (64.7) | 27 (73.0) | 4 (10.8) | <0.01 |
| Cough in the morning | 4 (23.5) | 26 (70.3) | 26 (70.3) | <0.01 |
| Cough before sleep | 2 (11.8) | 16 (43.2) | 18 (48.6) | 0.04 |
| No difference in cough time frame | 2 (11.8) | 4 (10.8) | 3 (8.1) | <0.01 |
| Sports-related | 16 (94.1) | 31 (83.8) | 23 (62.2) | 0.02 |
| Infection-related | 2 (11.8) | 11 (29.7) | 7 (18.9) | <0.01 |
| Cold air-related | 8 (47.1) | 14 (37.8) | 8 (21.6) | 0.26 |
| Season-related | 6 (35.3) | 18 (48.6) | 14 (37.8) | 0.55 |
| Pharyngeal positive signa, n (%) | 1 (5.9) | 15 (40.5) | 18 (48.6) | 0.02 |
| Passive smoke, n (%) | 12 (70.6) | 13 (35.1) | 16 (43.2) | 0.10 |
| Atopy, n (%) | 11 (64.7) | 21 (56.8) | 14 (37.8) | 0.12 |
a, pharyngeal positive sign means presence of secretions in posterior pharyngeal wall or cobble stone appearance of the pharyngeal wall mucosa. UACS, upper airway cough syndrome; CVA, cough-variant asthma.
Table 4. Sensitivity, specificity, PPV and NPV of each pointer in patients with different diagnosis.
| Final diagnosis | Clinical characteristics | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|
| CVA | Sports-related cough | 94.1 | 38.8 | 60.6 | 86.8 |
| Non-productive cough | 82.4 | 48.0 | 61.3 | 73.2 | |
| Nocturnal cough | 64.7 | 85.4 | 81.5 | 70.7 | |
| Atopy | 64.7 | 64.6 | 64.6 | 64.6 | |
| Season-related cough | 35.3 | 64.6 | 49.9 | 50.0 | |
| Cough in the morning | 23.5 | 35.4 | 26.7 | 31.6 | |
| Cough before sleep | 11.8 | 56.2 | 21.2 | 38.9 | |
| Productive cough | 17.6 | 52.1 | 26.9 | 38.7 | |
| UACS | Cough before sleep | 48.6 | 82.1 | 73.1 | 61.5 |
| pharyngeal positive signs | 81.1 | 57.1 | 65.4 | 75.1 | |
| Cough in the morning | 70.3 | 67.9 | 68.7 | 69.6 | |
| Productive cough | 54.1 | 78.6 | 71.7 | 63.1 | |
| Sports-related cough | 62.2 | 17.9 | 43.1 | 32.1 | |
| Atopy | 37.8 | 50 | 43.1 | 44.6 | |
| Nocturnal cough | 10.8 | 50.0 | 17.8 | 35.9 | |
| Non-productive cough | 45.9 | 21.4 | 36.9 | 28.3 |
CVA, cough variant asthma; UACS, upper airway cough syndrome; PPV, positive predictive value; NPV, negative predictive value.
Accessory examination features of different etiologies of chronic cough
No specific X-ray abnormality was found in any patients. Skin prick tests showed that 49 patients had positive outcomes. Among the 118 patients, esophageal pH monitoring was conducted in 84 patients, 7 were finally diagnosed with GERD. Induced sputum test was conducted in 93 patients, 2 were finally diagnosed with EB.
Sputum eosinophil percentage was associated with blood eosinophil percentage as shown in Figure 4. Sputum eosinophil percentage did not differ between patients with CVA and CVA + UACS, while sputum eosinophil percentages were higher in patients with CVA and CVA + UACS than in patients with UACS (both P<0.01). The PD20-FEV1 was higher in patients with UCAS than in those with CVA and CVA + UACS (both P<0.01). With respect to pulmonary function test parameters, we found that FEV1%Pred, FEF25%, FEF50%, and FEF75% were higher in patients with CVA and CVA + UACS than in those with UACS (all P<0.01).
Figure 4.
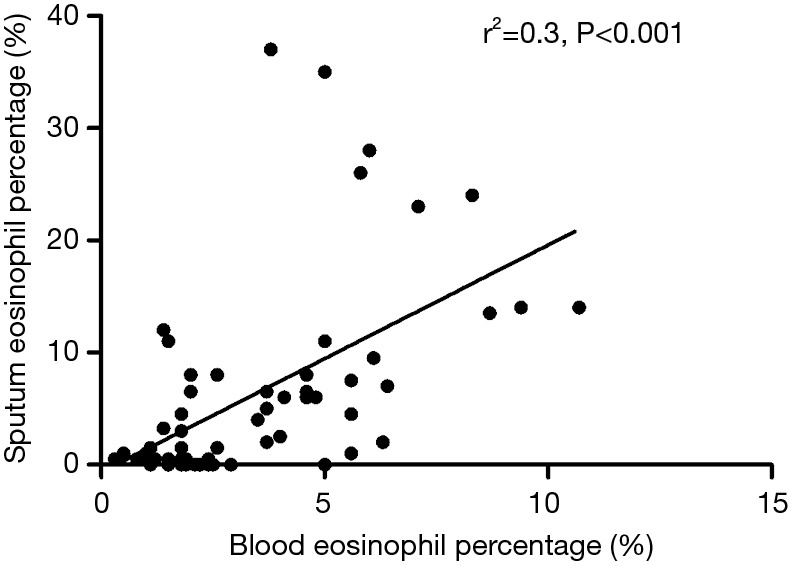
The correlation between serum and sputum eosinophils in patients with chronic cough.
Discussion
To our knowledge, this prospective study is the first to comprehensively evaluate the etiology of chronic cough and the value of clinical feature in school age children in China.
In 2012, a multicenter study on chronic cough in China revealed that CVA was the most common etiology (41.95%) followed by UACS (24.71%) and postinfectious cough (21.73%), which is in line with our study (16). When compared with other studies in Western countries, the proportions of etiologies were quite different. Asilsoy et al. also investigated 108 school-age (6–14 years) children with chronic cough and reported that asthma, asthma-like symptoms, and protracted bacterial bronchitis were responsible for almost half of all cases (6–14 years) of chronic cough (8). These results were quite similar to those obtained by Gedik et al. (9). Protracted bacterial bronchitis was considered the most common etiology of chronic cough (>3 weeks), affecting 41% of all patients in a multicenter study by Chang et al. (17). We attribute this difference to different study populations and diagnostic criteria. Moreover, various environmental factors in different countries may also contribute to the differences in study findings.
The strength of our study is that 24-hour esophageal pH and induced sputum test were performed in most of the patients (71.2% and 78.8%, respectively) with chronic cough. GERD was found in 5.9% of patients in our study. Whereas only 0.7% of patients with GERD was found in the previous Chinese study, in whose study 24-hour esophageal pH was only performed in the suspicious GERD cases (16). In Asilsoy’s study, GERD was also reported to affect 4.6% of the school-age patients with chronic cough (8). In Marchant et al.’s study, GERD was found in 14.8% of the patients, which is higher than our study. However, they did not further compare the GERD percentage between different age group (7). GERD is considered to be an uncommon etiology in children with chronic cough but listed in the top 3 etiologies in adult patients in Europe and America (18,19). However, in China, GERD was only found in 4.6% of the adult patients in a recent multicenter study (20). The disparity of GERD incidence between children and adults is not seen in China.
In adults, EB was one of the common etiologies of chronic cough (20). While, our results showed that EB was an uncommon etiology of chronic cough in children. Although previous study had found the low incidence of EB in China and other countries (7,16), they only performed induced sputum tests in suspected patients and some cases with EB may be omitted or might be diagnosed as asthma because of a good response to corticosteroid. Thus, our results can truly reflect the low incidence of EB in children with chronic cough.
The concept of cough hypersensitivity syndrome, defined as a clinical syndrome characterized by troublesome coughing often triggered by low levels of thermal, mechanical, or chemical exposure, has progressed as the mechanistic basis of chronic cough and is accepted by physicians to encompass idiopathic or unexplained cough in adults. However, in children, the role of cough hypersensitivity syndrome in chronic cough is still not fully understand. For children with unexplained chronic cough, whether cough hypersensitivity syndrome explains still needs further investigation.
The highlight of the present study was the analysis of the patients’ clinical characteristics and prediction of chronic cough based on these characteristics. CVA is characterized by non-productive cough and nighttime cough. We found that nighttime cough was an important indicator of CVA with a specificity of 85.4%. Conversely, the sensitivity of cough after sports and atopy were higher in patients with CVA, indicating their contribution to the diagnosis of CVA when a misdiagnosis might exist because of the low specificity. We showed 54.1% of patients with UACS had a productive cough. As previously reported, patients with UACS characterized by a non-productive cough potentially have high airway responsiveness (21). The high rate of positive pharyngeal signs in patients with UACS emphasizes the importance of the physical examination. Because of the limited numbers of patients with EB, TD, and GERD, we did not analyze their clinical characteristics for prediction of the etiologies of chronic cough.
The present study has some limitations. Although children of different ages have different etiologies of chronic cough (9), we only included school-age children in the present study. Second, our study was based on a single center. We anticipate the performance of multicenter studies to ensure precision, reduce selection bias, and increase generalizability. Third, the international experts of the ERS recommend the assessment of cough via subjective and objective measures, while this study only considers subjective methods for the assessment of cough and cough quality. Fourth, the concept of cough hypersensitivity syndrome, defined as a clinical syndrome characterized by troublesome coughing often triggered by low levels of thermal, mechanical, or chemical exposure, has progressed as the mechanistic basis of chronic cough and is accepted by physicians to encompass idiopathic or unexplained cough in adults (22). However, in children, especially those with unexplained cough, the role of cough hypersensitivity syndrome in chronic cough is still not fully understand and had not been evaluated in our study.
Conclusions
The common etiologies of chronic cough in school-age children were UACS, CVA, and PB, while EB and GERD were rare.
Acknowledgments
We thank Angela Morben, DVM, ELS, from Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Funding: This study was funded by Provincial Key Research and Development Program of Jiangsu (No. BE2016676, Chunagli Hao), Key Projects for Social Development of Jiangsu Province (No. BE2017657, Yuqing Wang) and the Science and Technology Program of Suzhou (SYS201641, Wujun Jiang and SS201765, Li Huang).
Ethical Statement: The study protocol was approved by the Ethics Committee of Suzhou University (SZU2012-C133; Suzhou, China). Written informed consent was obtained from all parents and children recruited into the study. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Chang AB, Robertson CF, Asperen PPV, et al. A Cough Algorithm for Chronic Cough in Children: A Multicenter, Randomized Controlled Study. Pediatrics 2013;131:e1576. 10.1542/peds.2012-3318 [DOI] [PubMed] [Google Scholar]
- 2.Clinical Research Coordination Group of Chronic Cough, The Subspecialty Group of Respiratory Diseases, The Society of Pediatrics, et al Guideline for diagnosis and treatment of chronic cough in Chinese children. Zhonghua Er Ke Za Zhi 2014;52:184-8. [PubMed] [Google Scholar]
- 3.Chang AB, Van Asperen PP, Glasgow N, et al. Children with chronic cough: when is watchful waiting appropriate? development of likelihood ratios for assessing children with chronic cough. Chest 2015;147:745-53. 10.1378/chest.14-2155 [DOI] [PubMed] [Google Scholar]
- 4.Morice AH, Kastelik JA. Cough. 1: Chronic cough in adults. Thorax 2003;58:901-7. 10.1136/thorax.58.10.901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song WJ, Chang YS, Faruqi S, et al. The global epidemiology of chronic cough in adults: a systematic review and meta-analysis. Eur Respir J 2015;45:1479-81. 10.1183/09031936.00218714 [DOI] [PubMed] [Google Scholar]
- 6.Irwin RS, Baumann MH, Bolser DC, et al. Diagnosis and management of cough executive summary: ACCP evidence-based clinical practice guidelines. Chest 2006;129:1S-23S. 10.1378/chest.129.1_suppl.1S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marchant JM, Masters IB, Taylor SM, et al. Evaluation and outcome of young children with chronic cough. Chest 2006;129:1132-41. 10.1378/chest.129.5.1132 [DOI] [PubMed] [Google Scholar]
- 8.Asilsoy S, Bayram E, Agin H, et al. Evaluation of chronic cough in children. Chest 2008;134:1122-8. 10.1378/chest.08-0885 [DOI] [PubMed] [Google Scholar]
- 9.Gedik AH, Cakir E, Torun E, et al. Evaluation of 563 children with chronic cough accompanied by a new clinical algorithm. Ital J Pediatr 2015;41:73. 10.1186/s13052-015-0180-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang AB, Glomb WB. Guidelines for evaluating chronic cough in pediatrics: ACCP evidence-based clinical practice guidelines. Chest 2006;129:260S-283S. 10.1378/chest.129.1_suppl.260S [DOI] [PubMed] [Google Scholar]
- 11.Chang AB, Newman RG, Carlin JB, et al. Subjective scoring of cough in children: parent-completed vs child-completed diary cards vs an objective method. Eur Respir J 1998;11:462-6. 10.1183/09031936.98.11020462 [DOI] [PubMed] [Google Scholar]
- 12.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26:319-38. 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 13.Gibson PG, Wlodarczyk JW, Hensley MJ, et al. Epidemiological association of airway inflammation with asthma symptoms and airway hyperresponsiveness in childhood. Am J Respir Crit Care Med 1998;158:36-41. 10.1164/ajrccm.158.1.9705031 [DOI] [PubMed] [Google Scholar]
- 14.Pavord ID, Pizzichini MM, Pizzichini E, et al. The use of induced sputum to investigate airway inflammation. Thorax 1997;52:498-501. 10.1136/thx.52.6.498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson LF, DeMeester TR. Development of the 24-hour intraesophageal pH monitoring composite scoring system. J Clin Gastroenterol 1986;8 Suppl 1:52-8. 10.1097/00004836-198606001-00008 [DOI] [PubMed] [Google Scholar]
- 16.Clinical Research Coordination Group of the Causes Constituents Ratio of Chronic Cough in Chinese Children Prospective multicenter clinical study on the causes constituents ratio of chronic cough in Chinese children. Zhonghua Er Ke Za Zhi 2012;50:83-92. [PubMed] [Google Scholar]
- 17.Chang AB, Robertson CF, Van Asperen PP, et al. A multicenter study on chronic cough in children: burden and etiologies based on a standardized management pathway. Chest 2012;142:943-50. 10.1378/chest.11-2725 [DOI] [PubMed] [Google Scholar]
- 18.Mohammed I, Cherkas LF, Riley SA, et al. Genetic influences in gastro-oesophageal reflux disease: a twin study. Gut 2003;52:1085-9. 10.1136/gut.52.8.1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Serag HB, Petersen NJ, Carter J, et al. Gastroesophageal reflux among different racial groups in the United States. Gastroenterology 2004;126:1692-9. 10.1053/j.gastro.2004.03.077 [DOI] [PubMed] [Google Scholar]
- 20.Lai K, Chen R, Lin J, et al. A prospective, multicenter survey on etiologies of chronic cough in China. Chest 2013;143:613-20. 10.1378/chest.12-0441 [DOI] [PubMed] [Google Scholar]
- 21.Bucca C, Rolla G, Scappaticci E, et al. Extrathoracic and intrathoracic airway responsiveness in sinusitis. J Allergy Clin Immun 1995;95:52-9. 10.1016/S0091-6749(95)70152-4 [DOI] [PubMed] [Google Scholar]
- 22.Chung KF, McGarvey L, Mazzone S. Chronic cough and cough hypersensitivity syndrome. Lancet Respir Med 2016;4:934-5. 10.1016/S2213-2600(16)30373-3 [DOI] [PubMed] [Google Scholar]