Abstract
Exudative pleural effusions, such as malignant and tuberculous pleural effusions, are associated with notable morbidity and mortality. Unfortunately, a significant number of these effusions will remain undiagnosed despite thoracentesis. Traditionally, closed pleural biopsies have been the next best diagnostic step, but the diagnostic yield of blind closed pleural biopsies for malignant pleural effusions is insufficient. When image-guided targeted biopsies are not possible, both pleuroscopy and video-assisted thoracoscopic surgery are reasonable options for obtaining pleural biopsies, but the decision to select one procedure over the other continues to raise much debate. Pleuroscopy (aka. medical thoracoscopy, local anaesthetic thoracoscopy) is a relatively common procedure performed by interventional pulmonologists in the bronchoscopy suite with local anesthesia, often as an outpatient procedure, on spontaneously breathing patients. Video-assisted thoracoscopic surgery, on the other hand, is performed by thoracic surgeons in the operating room, on mechanically ventilated patients under general anesthesia, though admittedly considerable overlap exists in practice. Both pleuroscopy and video-assisted thoracoscopic surgery have reported diagnostic yields of over 90%, although pleuroscopy more often leads to the unsatisfactory diagnosis of ‘non-specific’ pleuritis. These cases of ‘non-specific’ pleuritis need to be followed up for at least one year, as 10–15% of them will eventually lead to the diagnosis of cancer, typically malignant pleural mesothelioma. Both procedures have their pros and cons, and it is therefore of paramount importance that all cases be discussed as part of a multidisciplinary approach to diagnosis within a “pleural team” that should ideally include interventional pulmonologists and thoracic surgeons.
Keywords: Pleuroscopy, medical thoracoscopy, local anesthetic thoracoscopy, video-assisted thoracic surgery (VATS), malignant pleural effusion
Introduction
Pleural disease affects more than 300 per 100,000 individuals each year, worldwide (1). In the United States alone, 1.5 million pleural effusions are diagnosed annually, and represent about a quarter of diagnoses seen by pulmonologists (2). While history, physical exam and pleural fluid analysis will reveal the diagnosis in a majority of the cases, an estimated 26% of pleural effusions remain undiagnosed and warrant further work-up (3). Many of these undiagnosed effusions are exudative in nature and are especially worrisome, since in low tuberculosis (TB) prevalence areas, more than half of these will be malignant (4). In TB endemic areas, on the other hand, 84.5% of undiagnosed exudative pleural effusions were eventually found to be secondary to pleural TB (5).
Malignant pleural effusion (MPE) is a devastating illness which portends a generally poor prognosis. Median survival after the diagnosis of MPE is about 6 months but varies considerably, and can now be estimated via several prediction models (6-8). MPE leads to 100,000 hospital admissions in the US every year, with lung and breast cancers, and lymphoma being the most common etiologies (9). The challenge for chest physicians is three-fold: First, the diagnosis should be established promptly so that appropriate therapies may be instituted as soon as possible. Second, adequate tissue must be obtained, which allows for advanced contemporary genetic and molecular testing. Third, interventions to palliate the symptoms of MPE must be offered.
The procedures currently available for the diagnosis and treatment of pleural effusions include thoracentesis, closed pleural biopsies, image-guided pleural biopsies (ultrasound or CT-guided), pleuroscopy and video-assisted thoracic surgery (VATS). We will briefly discuss the utility of thoracentesis and closed pleural biopsies, before presenting a more detailed comparative overview of pleuroscopy and VATS.
Pleural procedures
Thoracentesis
Thoracentesis is typically the initial invasive procedure performed by pulmonologists to diagnose pleural effusions, as many laboratory tests can be run on the pleural fluid. When done under ultrasound guidance the risk of complications is very low (10). Thoracentesis can establish the etiology of pleural effusion in approximately 75% of cases (3). The diagnostic yield for MPE with the first pleural tap is 60%, and the second pleural tap increases the diagnostic yield by up to 27%, with little increase in diagnostic yield with further taps, although there is considerable variation (11). Interestingly, the yields vary considerably based on the tumor type: less than 40% in renal cell carcinomas, sarcomas and head and neck cancers, and almost 100% in breast and pancreatic cancers (12). In the same study by Grosu et al. the diagnostic yields for MPEs associated with small cell and non-small cell lung cancers were 78% and 90%, respectively. Nonetheless, a quarter of all MPEs will remain undiagnosed despite thoracentesis (13). Therefore, further testing is typically warranted after a negative thoracentesis.
Thoracentesis also provides symptom relief and guides potential future therapeutic interventions by answering three fundamental questions: (I) is the breathlessness relieved after fluid drainage, (II) is the lung reexpandable, which would allow consideration of pleurodesis and, perhaps more importantly, (III) will the effusion recur and how quickly? The 2018 American Thoracic Society (ATS) guidelines recommend large volume taps for all cases of proven and suspected MPEs for precisely these reasons (14). If patients do not report significant symptom relief with a large volume thoracentesis, it is unlikely that they will benefit from further therapeutic interventions such as indwelling pleural catheters (IPCs) or pleurodesis. If lung re-expansion is noted after the thoracentesis, patients may be candidates for both IPC and pleurodesis, however, if the lung fails to re-expand ATS guidelines recommend IPC for long-term symptom management (14).
Closed pleural biopsies
If the pleural effusion cannot be diagnosed with pleural fluid analysis, percutaneous closed pleural biopsies (CPB) with a reverse beveled needle have traditionally been the next step (15). The use of such needles was first reported in 1958 by Abrams et al., and one of the commonly used needles bears his name (16). The diagnostic yield of blind Abrams needle biopsy for MPE is only 40%, with a risk of pneumothorax as high as 11% (17). Others have reported that blind pleural biopsies add only 7–27% additional diagnostic yield on top of clinical evaluation and thoracentesis for MPE (11). The poor diagnostic yield of blind CPBs is in part due to the heterogeneous pleural involvement in MPEs.
Using image guidance to target focal areas of pleural abnormalities has been shown to dramatically increase the diagnostic yield of pleural biopsies. Maskell et al. conducted a randomized controlled trial (RCT) comparing CPB with Abrams needle to CT-guided cutting needle biopsy for the diagnosis of MPE (18). CT-guided biopsies had a significantly higher sensitivity at 87% compared to only 47% for blind Abrams needle biopsy. Similarly, ultrasound guided cutting needle biopsies have also been shown to have a high diagnostic yield of 76–85% for undiagnosed exudative pleural effusions (1). Perhaps the last remaining indication for CPB is for the diagnosis of exudative effusions in areas endemic for tuberculosis. When combined with pleural fluid adenosine deaminase (ADA) and lymphocyte to neutrophil ratio of >0.75, blind CPB have been reported to have a sensitivity of 93% and specificity of 100% for TB pleuritis. This is likely due to the more diffuse nature of pleural involvement in TB (5).
Investigators have also compared CPBs with pleuroscopy. Metintas et al. prospectively compared CPB with pleuroscopy. The site of CPB was determined based on the CT scan, however, no real-time image guidance was employed. The diagnostic sensitivities were 94.1% and 87.5% for pleuroscopy and CT-assisted CPB, respectively (15). No difference was noted in the diagnostic yield for MPE or TB pleuritis. These findings led to the British Thoracic Society (BTS) recommendation that in cases of suspected MPE, discrete pleural lesions may be targeted by image guided pleural biopsies. However, if no discrete areas of abnormalities are seen then the preferred next step is pleuroscopy (11).
Pleuroscopy
Background
Pleuroscopy is gaining popularity as the procedure of choice for diagnosing and treating exudative pleural effusions, which remain undiagnosed after thoracentesis. Pleuroscopy has variably been referred to as medical thoracoscopy (MT) or local anaesthetic thoracoscopy (LAT). Pleuroscopy is not a novel procedure: it was first performed by a Swedish internist, Hans Christian Jacobaeus in the early 20th century (19,20). Interestingly at that time its main application was adhesiolysis for creation of pneumothorax, the treatment of choice for TB in the pre-streptomycin era. With the advent of effective anti-TB medications, pleuroscopy fell out of favor. However, over the last two decades, with the development of high quality videoscopes and other innovations in the field of interventional pulmonology, pleuroscopy is being increasingly recognized as a safe, effective and low-cost alternative to VATS. The first report of pleuroscopy with a semi-rigid scope was published by Ernst et al. in 2002, and since then dozens of reports from around the world have corroborated its safety and efficacy (4,21).
Indications
The primary indication for pleuroscopy is obtaining parietal pleural biopsies in patients with exudative pleural effusions, when the diagnosis has remained elusive despite one or two thoracenteses (4). It is especially the modality of choice if no discrete pleural lesions are seen that could be targeted by percutaneous needle biopsies or if MPE is strongly suspected. In cases of recurrent symptomatic pleural effusions, where prior pleural taps have demonstrated the lung’s ability to re-expand with good apposition of visceral and parietal pleura, pleuroscopy can also be considered for pleurodesis (4,14). Talc poudrage is administered during pleuroscopy to achieve this.
The British Thoracic Society (BTS) recommends that pleuroscopy should be performed in individuals who are World Health Organization functional class 0, 1 or 2 (4). However, functional limitation due to a symptomatic pleural effusion should not preclude a patient’s candidacy for pleuroscopy. Some investigators have even used pleuroscopy for visceral pleural and lung parenchymal biopsies, however, these require advanced expertise and adequate literature is not available to back these practices (22).
Contra-indications
Absolute and relative contra-indications to pleuroscopy are summarized in Table 1 (2,23).
Table 1. Contraindications for pleuroscopy.
| Absolute |
| 1. Loculation or obliteration of the pleural space, as may be seen in cases of adhesions between the visceral and parietal pleura, or adhesions between lung and chest wall |
| 2. Severe respiratory distress |
| 3. Uncontrollable cough, that makes entry in to the pleural space and maneuverability of the thoracoscope hard |
| 4. Severe uncorrectable coagulopathy |
| 5. Limited cardiopulmonary reserves that might make it difficult for the patient to tolerate pneumothorax |
| 6. Lack of multi-disciplinary collaboration with cardiothoracic surgery |
| 7. Inability to tolerate sedation |
| 8. Lack of informed consent |
| Relative |
| 1. Acute coronary syndrome or cerebrovascular accident in the preceding 6 weeks |
| 2. Morbid obesity |
| 3. Bleeding diathesis |
| 4. Moderate to severe sleep apnea |
Procedure
Pleuroscopy is typically performed under conscious sedation in a spontaneously breathing patient. It can be conveniently performed in the bronchoscopy suite and as an outpatient procedure (13,24). These characteristics may contribute to cost-effectiveness and patient convenience, hence making it an attractive alternative to VATS.
Sedation
Propofol and midazolam are the most commonly used agents for sedation, along with opioids for analgesia in preparation for pleuroscopy. Grendelmeier et al. performed a randomized trial comparing propofol and midazolam for sedation (25). Patients in the propofol arm had significantly lower ‘mean lowest oxygen saturation’. Patients in the propofol group also had significantly more episodes of hypoxemia and hypotension. Other investigators have reported different anesthesia protocols. Rusch et al. performed thoracoscopies after patients had received oral diazepam and intramuscular morphine. Patients were administered intercostal nerve blocks and local lidocaine at the point of entry (26). Al-Abdullatief et al. reported satisfactory results with thoracic epidural analgesia and stellate ganglion nerve block for intractable cough (27). Most cases are performed with local anesthesia and a combination of low doses of midazolam and fentanyl in monitored setting with anesthesia support or back-up.
Point of entry
It is of paramount importance that the point of entry for the thoracoscope on the chest wall allows for access to free and open pleural space. At times, inadvertently the pleural space is accessed at a point where there are adhesions or loculations. In these circumstances not only is there a risk of injuring the lung, but adhesions between the visceral and parietal pleura complicate pleural inspection. Often, an inappropriate site of entry will lead to a failed or aborted procedure. Breakdown of adhesions should be cautious, as some adhesions are vascularized which may lead to unexpected bleeding.
In the absence of pleural fluid (common with patients in the lateral decubitus position), the proceduralist creates an artificial pneumothorax by introducing a small trocar (the Boutin trocar, Novatech, France) and letting the patient breathe spontaneously, entraining air through the trocar into the pleural space (28). Even a partial lung collapse typically allows for adequate pleural examination, especially with a semi-rigid pleuroscopy that can easily be maneuvered in the pleural space. An ultrasound sliding sign is a predictor of successful pneumothorax, and if absent, should lead to an alternative location (29). The thoracic US can additionally provide information on the amount and depth of pleural fluid, and presence of blood vessels.
Macha et al. first reported the use of thoracic ultrasonography (TU) to determine the point of entry (30). They reported their experience with 687 pleuroscopies performed with TU guidance. They reported a very low risk of complications. More recently, Huang et al. compared the two techniques for establishing the point of entry (artificial pneumothorax vs. TU), using a propensity score matching analysis (31). No significant differences in major and minor complications were noted between the two groups.
Rigid vs. semi-rigid scope
Pleuroscopy can be safely performed with both rigid and semi-rigid scopes (32). The semi-rigid (or flex-rigid) thoracoscope has a 2.8 mm working channel, which accommodates all flexible biopsy forceps. The rigid thoracoscope on the other hand, has a diameter of 9 mm and allows for the 5 mm rigid forceps to be deployed through it, though multiple models and sizes have been used (23,33).
Some experts believe that a semi-rigid scope is better tolerated (2). Moreover, given the similarities of controls on a semi-rigid thoracoscope and a flexible bronchoscope, interventional pulmonologists are likely to be more familiar with its handling. A RCT comparing rigid and semi-rigid thoracoscopes found that the diagnostic yield for rigid scopes was significantly higher than semi-rigid scopes (97.8% vs. 73.3%, P=0.002) (34). Rigid scopes also allowed for retrieval of significantly larger specimens compared to the semi-rigid scopes. Interestingly, the yield of semi-rigid scopes in this study was lower than that reported in several other studies. Agarwal et al. performed a meta-analysis of 17 pleuroscopy studies where semi-rigid scope had been used, the overall sensitivity and specificity were 91% and 100%, respectively (35). The overall complication rate was 1.5%. Other experts suggest that even though semi-rigid scope allows for greater maneuverability in the pleural space, the rigid scope is better suited for biopsying a thickened parietal pleura, particularly when mesothelioma is suspected, for which larger biopsies are desirable (23).
Practical steps
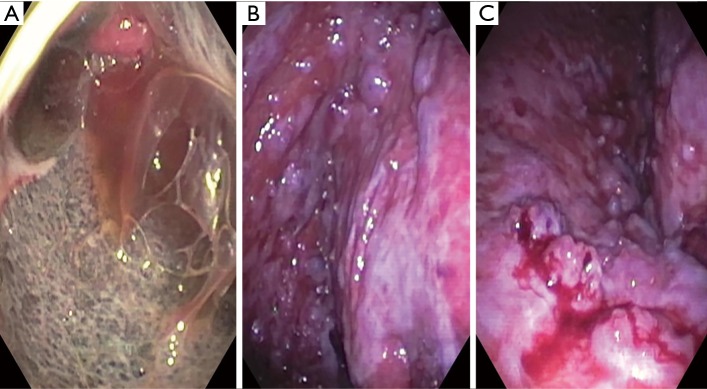
Table 2 shows our suggested practical steps for performing pleuroscopy. Similar, techniques have been reported by multiple other authors as well (5,13,29). Figure 1 shows images obtained during pleuroscopies. Panel a. was taken immediately after entering the pleural space in a patient with mesothelioma. Panel b. shows the costophrenic recess in a patient with metastatic thyroid cancer and panel c. shows minimal bleeding after forceps biopsies in the patient with metastatic thyroid cancer.
Table 2. Suggested step-by-step approach to pleuroscopy.
| a) Patient is placed in a lateral decubitus position with the procedure site up |
| b) We start examining the pleural space with TU starting at the 5th or 6th intercostal space in the mid-axillary line. If we don’t see the sliding lung and seashore signs, or if adhesions are appreciated, we move to adjacent areas |
| c) Once the point of entry has been determined, it is liberally anesthetized with 1% lidocaine under direct ultrasound guidance. Care must be taken to ensure that the lidocaine has infiltrated the subcutaneous space, muscle layer and the parietal pleura |
| d) Boutin blunt tip trochar is inserted in to the pleural space. This allows air to enter the pleural space, creating and artificial pneumothorax and collapsing the lung. (In patients with copious amount of pleural effusion this step can be skipped) (28) |
| e) A 1–2 cm incision is made, and blunt dissection using artery forceps is performed through the subcutaneous tissue, intercostal muscles and in to the pleural space |
| f) An 8 mm disposable trochar is then introduced in to the pleural space, which allows the passage of the thoracoscope |
| g) The pleural space is examined, and 6–8 biopsies are taken using forceps, with the ‘lift and peel technique’ |
| h) At the end of the procedure a 10–14 Fr pigtail catheter is placed in to the pleural space, which allows for the evacuation of the pneumothorax. Conversely, if the plan is to deploy an indwelling pleural catheter (IPC) for long term drainage, then the same IPC can also allow for the drainage of the air |
| i) Post-procedure a portable chest X-ray is obtained |
| j) Once the lung has re-expanded, the pigtail catheter can be removed |
Figure 1.
Images from pleuroscopy using a semi-rigid scope. (A) Image taken immediately after entering the pleural space in a patient with mesothelioma; (B) costophrenic recess in a patient with metastatic thyroid cancer; (C) minimal bleeding after forceps biopsies in the patient with metastatic thyroid cancer.
Pleurodesis vs. IPC
Freeman et al. performed a propensity-matched comparison of talc pleurodesis and IPC, after pleuroscopy in patients undergoing the procedure for suspected MPE (36). Talc pleurodesis was performed by insufflating 3 grams of sterile talc powder, with the patient in Trendelenburg, so that it would subsequently disseminate throughout the pleural space. Post-procedure a chest tube was left in place connected to suction. Overall, the degree of palliation was very similar in both groups. However, patients in talc pleurodesis group experienced significantly more complications, including one death that was attributed to talc related respiratory failure. Moreover, the hospital-stay and the interval to initiation of systemic therapy were significantly shorter in the IPC group.
In the above-mentioned study decision to proceed with talc pleurodesis vs. IPC was primarily based on patient preference. This is in line with other expert recommendations. However, the ATS guidelines do advise against talc pleurodesis if the patient has a trapped lung (14). Interestingly, the results of this study are similar to other studies where pleurodesis with talc slurry through a chest tube has been compared with IPC (37,38).
Potential caveats with talc pleurodesis include, the risk of jeopardizing future thoracoscopic examinations if biopsies turn out benign, and the difficulty to predict expandable lung during the procedure in a non-intubated patient. Even pleural experts are notoriously unreliable in predicting MPE and lung expandability during thoracoscopy (39). Accordingly, talc pleurodesis should be reserved for cases histologically confirmed malignancy during the procedure, in patients with documented reexpandable lung.
Performance
The sensitivity of pleuroscopy in the setting of MPE is >90% (13). Rahman et al. pooled data from 22 studies: the diagnostic sensitivity for malignant pleural disease was 92.6% (95% CI, 91.1% to 94.0%) (4). Interestingly, when the results of 8 studies where prior non-guided CPB had been negative, pleuroscopy still maintained a high sensitivity of 90.1% (95% CI, 86.6% to 92.9%). Even when specifically assessing mesothelioma, the diagnostic yield is 98% (40).
Minor complications have been reported in 7.3% of the cases. Major complications have been reported in 1.8% of the cases and include empyema, hemorrhage, tumor seeding and bronchopleural fistula (4). Additional significant complications reported in other studies include prolonged air-leak, subcutaneous emphysema, arrythmias, hypotension and worsening hypoxia (2).
Rahman et al. combined data from 47 studies and reported an overall mortality of 0.34% (16/4,736, 95% CI, 0.19% to 0.54%) (4). It is worth noting that all these deaths occurred in studies where talc poudrage had been performed and no deaths were noted with diagnostic pleuroscopy. Furthermore, 9 out of the 16 deaths, were from just one RCT, which had compared talc poudrage via pleuroscopy vs. talc slurry via chest tube (41). This study had used non-graded talc, a practice that has since changed.
Another, important consideration with regards to the performance of pleuroscopy is the 30–40% chance of biopsy results returning as non-specific pleuritis (13,42). Non-specific pleuritis denotes fibrinous, acute or chronic inflammatory changes which do not yield any specific diagnosis. These cases need to be followed up for 1–2 years, since about 14% patients will eventually be diagnosed with a malignancy (mostly malignant mesothelioma) (42,43). This rate is interestingly much smaller in the surgical literature, which may suggest better pleural space inspection with VATS, although this remains speculative (13).
Benign diseases
Pleuroscopy has also been extensively employed as part of work-up of benign diseases. TB pleuritis and empyema are two of the most common benign conditions which may warrant pleuroscopy (44). In most of the developing and some developed nations, TB remains the most common cause of exudative pleural effusions, that remain undiagnosed despite thoracentesis. Thomas et al. reported a review of 407 patients undergoing pleuroscopy for undiagnosed exudative pleural effusions, in the Arabian gulf state of Qatar (5). 84.5% of the patients were diagnosed with TB, while MPE accounted for only 5.2% of the cases. Overall, the diagnostic yield for TB was 91.4%. Minor bleeding was seen in only 1.2% of cases. Despite pleuroscopy being the gold standard for the diagnosis of TB, BTS only recommends it as a second line procedure for suspected cases, if CPB has been negative (4,45). This is because unlike MPE, blind CPB retains adequate diagnostic yield for suspected TB pleuritis.
Since the publication of MIST-2 trial, most cases of empyema are managed with a chest tube and intrapleural administration of tissue plasminogen activator and DNase (46). However, cases refractory to these interventions can potentially be amenable to pleuroscopy. In these cases pleuroscopy allows for lysis of adhesions and loculations, which expedites drainage and reduces the risk of fibrothorax (44). Ravaglia et al. reported their experience with 41 patients undergoing pleuroscopy for empyema (47). They divided cases of empyema in to three categories: free-flowing, septated (based on septations seen on CT scan or TU) and organized (based on pleural thickening seen on CT scan or TU). While the results of pleuroscopy were excellent for free flowing and septated empyemas, this practice remains anecdotal and is not yet recommended aside from selected cases. A multicenter study is currently attempting to clarify the role of pleuroscopy in empyema (ClinicalTrials.gov Identifier: NCT03468933).
Inpatient vs. outpatient
The last decade has seen a movement towards, making procedures minimally invasive and performing them as “outpatient” or “same-day” interventions. Performing procedures on outpatients not only minimizes the inconvenience for the patients, but also reduces the complications associated with hospitalization such as venous thromboembolic disease and hospital-acquired infections. Moreover, there may be significant cost reductions as well.
Many studies have shown that pleuroscopy can be safely performed as an outpatient procedure. DePew et al. reported their experience with 51 pleuroscopies attempted as outpatient procedures (13). In this study the average duration of the procedure was 40 mins and on average patients spent less than 5 hours in the hospital. No major complications were reported. One patient developed pneumothorax ex-vacuo. Three patients had to be admitted after the procedure, two for incision site pain control and one for post-sedation confusion. In fact, being able to discharge the patient the same day, is one of the reasons why IPCs as opposed to pleurodesis with talc poudrage, are the preferred method for management of recurrent symptomatic effusions in patients undergoing pleuroscopy. If talc poudrage pleurodesis is performed the average duration of hospitalization is about 4.6 days (4,24).
Video-assisted thoracoscopic surgery
Since its introduction in 1990s, VATS has replaced open thoracotomy as the preferred procedure for a multitude of thoracic pathologies (48). While lung resection is the most commonly performed procedure using VATS, it is also regularly utilized for pleural biopsies, pleurodesis, mediastinal resections, esophagectomies and sympathectomies (49). The main advantages of VATS over traditional open thoracotomy are reduced procedural morbidity and mortality, and faster recovery without compromising the effectiveness (48).
VATS has traditionally been performed using three chest ports (incisions to allow for instrument insertion), while the patient is under general anesthesia and undergoing single (contralateral) lung ventilation (2). Recent advances in minimally invasive thoracic surgery include development of robotic-assisted thoracic surgery (RATS), uniportal-VATS (U-VATS) and performance of VATS under conscious sedation (48-50). While these advances are blurring the boundaries between VATS and pleuroscopy, these innovative minimally invasive thoracic surgeries are only available at a few centers. The diagnostic yield for VATS is over 90% and is considered the gold standard for the diagnosis of MPE (51). Overall risk of complications is 4–6% (52). Most common complications include bleeding (0.5–1.9%), pneumonia (3%), empyema (1.4%), surgical wound infection (1.7%), cancer recurrence at port sites (0.2–0.5%) and post-operative pain. Post-operative mortality has been reported at 2% (53). However, in a different series of 86 patients from the United Kingdom, no deaths were reported.
VATS vs. pleuroscopy
Choosing between VATS and pleuroscopy for undiagnosed exudative effusions has been one of the most important pleural controversies and a source of debate between interventional pulmonologists and cardiothoracic surgeons. While VATS is considered the gold standard, pleuroscopy has been reported to be a less invasive, simpler and cost-effective alternative, without significantly compromising the diagnostic yield.
Performance
Diagnostic yield
The only study directly comparing pleuroscopy and VATS was recently published by McDonald et al. (54). They retrospectively compared pleuroscopy (78 cases) and VATS (99 cases). The authors reported an overall diagnostic yield for pleuroscopy at 93.6%, while 96% for VATS. The small difference was not statistically significant (P=0.591). 43.8% of the pleuroscopy cases showed non-specific inflammation, as compared to only 24.2% of the VATS cases. Of the 30 pleuroscopy cases with non-specific inflammation for which follow-up was available, 4 cases were either confirmed malignant or suspected to be malignant. Of note if only the cases where a definitive diagnosis was established at the time of the procedure are considered, the diagnostic yields for pleuroscopy and VATS were 52% and 72%, respectively.
Complications
Major complications were seen in 2.6% and 4.0% of pleuroscopy and VATS cases, respectively. Minor complications were seen in 17.9% and 16.2% of pleuroscopy and VATS cases, respectively. Again, the differences were not significant. Two deaths were reported in the study both in the VATS group.
Cost
The median length of stay was significantly longer in the VATS group [3 days (IQR: 1–4) vs. 0 days (IQR: 0–1), z =6.08, P<0.001] (54). While all patients in the VATS group required patient controlled intravenous analgesia (PCIA), none of the patients in the pleuroscopy group required it. The authors of the above study used the hospital administrative database to perform a cost-effectiveness analysis as well. The cost was adjusted for inflation. Not surprisingly, the per-procedure cost of VATS was significantly more than pleuroscopy (CAD 7,962 vs. CAD 2,815, P<0.001). Need for hospitalization and an operating room are likely the most important contributors to the higher costs associated with VATS.
In conclusion, the results presented by McDonald et al. are in line with the multiple prior studies that have individually looked at pleuroscopy and VATS. Overall, the diagnostic yields of the two procedures are comparable. Pleuroscopy achieved this diagnostic yield with less patient discomfort (as indicated by the lack of need for PCIA), significantly shorter hospital stays and at almost one-third the cost. However, the higher proportion of patients with non-specific biopsy results in the pleuroscopy group, means that a relatively higher number of patients will require long term follow-up and potentially additional thoracic procedures. In another study almost 15% patients with non-specific results were found to have pleural malignancy (43). Therefore, most experts recommend at least 1–2 years follow-up for cases of non-specific pleuritis, to exclude malignancy.
Future directions
Our understanding of pleural procedures continues to evolve, with a growing number of studies assessing the performance characteristics of commonly performed pleural interventions. At least two RCTs are underway to compare the efficacy of fibrinolytic therapy administered via chest tube with early medical thoracoscopy (ClinicalTrials.gov Identifier: NCT03213834 and NCT02973139). Another trial is ongoing in South Korea to assess the efficacy of intrapleural administration of Docetaxel in MPE (ClinicalTrials.gov Identifier: NCT03394105). Finally, Dhooria et al. are conducting a RCT to compare the conventional rigid thoracoscopy (outer diameter 10 mm) with “mini-thoracoscopy” using a mini-thoracoscope (outer diameter 5.5 mm). It is hypothesized that the smaller thoracoscope may be better tolerated by the patients with comparable efficacy (ClinicalTrials.gov Identifier: NCT03449602).
Conclusions
Pleuroscopy and VATS are complementary procedures for the diagnosis and treatment of pleural diseases. While the available evidence suggests that pleuroscopy is more cost-effective, associated with shorter hospital stays and better tolerated than VATS, roughly 12% patients will still need VATS after pleuroscopy (13). Therefore, it is of paramount importance that patients are appropriate for pleuroscopy based on a multi-disciplinary discussion in a pleural team comprised of interventional pulmonologists, cardiothoracic surgeons and anesthesiologists (55,56). Such, multidisciplinary collaboration also ensures adequate back-up for the interventional pulmonologists in case of serious complications. Ongoing clinical trials will not only further our understanding of the role of pleuroscopy for MPE, but also better define its place in the treatment algorithm of empyema.
Acknowledgments
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Hallifax RJ, Corcoran JP, Ahmed A, et al. Physician-based ultrasound-guided biopsy for diagnosing pleural disease. Chest 2014;146:1001-6. 10.1378/chest.14-0299 [DOI] [PubMed] [Google Scholar]
- 2.Kern RM, DePew ZS, Maldonado F. Outpatient thoracoscopy: safety and practical considerations. Curr Opin Pulm Med 2015;21:357-62. 10.1097/MCP.0000000000000168 [DOI] [PubMed] [Google Scholar]
- 3.Collins TR, Sahn SA. Thoracocentesis. Clinical value, complications, technical problems, and patient experience. Chest 1987;91:817-22. 10.1378/chest.91.6.817 [DOI] [PubMed] [Google Scholar]
- 4.Rahman NM, Ali NJ, Brown G, et al. Local anaesthetic thoracoscopy: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65 Suppl 2:ii54-60. 10.1136/thx.2010.137018 [DOI] [PubMed] [Google Scholar]
- 5.Thomas M, Ibrahim WH, Raza T, et al. Medical thoracoscopy for exudative pleural effusion: an eight-year experience from a country with a young population. BMC Pulm Med 2017;17:151. 10.1186/s12890-017-0499-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porcel JM, Gasol A, Bielsa S, et al. Clinical features and survival of lung cancer patients with pleural effusions. Respirology 2015;20:654-9. 10.1111/resp.12496 [DOI] [PubMed] [Google Scholar]
- 7.Psallidas I, Kanellakis NI, Gerry S, et al. Development and validation of response markers to predict survival and pleurodesis success in patients with malignant pleural effusion (PROMISE): a multicohort analysis. Lancet Oncol 2018;19:930-9. 10.1016/S1470-2045(18)30294-8 [DOI] [PubMed] [Google Scholar]
- 8.Clive AO, Kahan BC, Hooper CE, et al. Predicting survival in malignant pleural effusion: development and validation of the LENT prognostic score. Thorax 2014;69:1098-104. 10.1136/thoraxjnl-2014-205285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fortin M, Taghizadeh N, Tremblay A. Procedures Performed during Hospitalizations for Malignant Pleural Effusions: Data from the 2012 National Inpatient Sample. Respiration 2018;95:228-34. 10.1159/000485934 [DOI] [PubMed] [Google Scholar]
- 10.Krackov R, Rizzolo D. Real-time ultrasound-guided thoracentesis. JAAPA 2017;30:32-7. 10.1097/01.JAA.0000508210.40675.09 [DOI] [PubMed] [Google Scholar]
- 11.Hooper C, Lee YCG, Maskell N, BTS Pleural Guideline Group Investigation of a unilateral pleural effusion in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65 Suppl 2:ii4-17. 10.1136/thx.2010.136978 [DOI] [PubMed] [Google Scholar]
- 12.Grosu HB, Kazzaz F, Vakil E, et al. Sensitivity of Initial Thoracentesis for Malignant Pleural Effusion Stratified by Tumor Type in Patients with Strong Evidence of Metastatic Disease. Respiration 2018;96:363-9. 10.1159/000490732 [DOI] [PubMed] [Google Scholar]
- 13.DePew ZS, Wigle D, Mullon JJ, et al. Feasibility and safety of outpatient medical thoracoscopy at a large tertiary medical center: a collaborative medical-surgical initiative. Chest 2014;146:398-405. 10.1378/chest.13-2113 [DOI] [PubMed] [Google Scholar]
- 14.Feller-Kopman DJ, Reddy CB, DeCamp MM, et al. Management of Malignant Pleural Effusions. An Official ATS/STS/STR Clinical Practice Guideline. Am J Respir Crit Care Med 2018;198:839-49. 10.1164/rccm.201807-1415ST [DOI] [PubMed] [Google Scholar]
- 15.Metintas M, Ak G, Dundar E, et al. Medical thoracoscopy vs CT scan-guided Abrams pleural needle biopsy for diagnosis of patients with pleural effusions: a randomized, controlled trial. Chest 2010;137:1362-8. 10.1378/chest.09-0884 [DOI] [PubMed] [Google Scholar]
- 16.Abrams LD. A pleural-biopsy punch. Lancet 1958;1:30-1. 10.1016/S0140-6736(58)92521-2 [DOI] [PubMed] [Google Scholar]
- 17.Chakrabarti B, Ryland I, Sheard J, et al. The role of Abrams percutaneous pleural biopsy in the investigation of exudative pleural effusions. Chest 2006;129:1549-55. 10.1378/chest.129.6.1549 [DOI] [PubMed] [Google Scholar]
- 18.Maskell NA, Gleeson FV, Davies RJO. Standard pleural biopsy versus CT-guided cutting-needle biopsy for diagnosis of malignant disease in pleural effusions: a randomised controlled trial. Lancet 2003;361:1326-30. 10.1016/S0140-6736(03)13079-6 [DOI] [PubMed] [Google Scholar]
- 19.Hatzinger M, Kwon ST, Langbein S, et al. Hans Christian Jacobaeus: Inventor of human laparoscopy and thoracoscopy. J Endourol 2006;20:848-50. 10.1089/end.2006.20.848 [DOI] [PubMed] [Google Scholar]
- 20.Braimbridge MV. The history of thoracoscopic surgery. Ann Thorac Surg 1993;56:610-4. 10.1016/0003-4975(93)90929-C [DOI] [PubMed] [Google Scholar]
- 21.Ernst A, Hersh CP, Herth F, et al. A novel instrument for the evaluation of the pleural space: an experience in 34 patients. Chest 2002;122:1530-4. 10.1378/chest.122.5.1530 [DOI] [PubMed] [Google Scholar]
- 22.Tassi GF, Davies RJO, Noppen M. Advanced techniques in medical thoracoscopy. Eur Respir J 2006;28:1051-9. 10.1183/09031936.00014106 [DOI] [PubMed] [Google Scholar]
- 23.Murthy V, Bessich JL. Medical thoracoscopy and its evolving role in the diagnosis and treatment of pleural disease. J Thorac Dis 2017;9:S1011-21. 10.21037/jtd.2017.06.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Psallidas I, Corcoran JP, Fallon J, et al. Provision of Day-Case Local Anesthetic Thoracoscopy: A Multicenter Review of Practice. Chest 2017;151:511-2. 10.1016/j.chest.2016.11.002 [DOI] [PubMed] [Google Scholar]
- 25.Grendelmeier P, Tamm M, Jahn K, et al. Propofol versus midazolam in medical thoracoscopy: a randomized, noninferiority trial. Respiration 2014;88:126-36. 10.1159/000362797 [DOI] [PubMed] [Google Scholar]
- 26.Rusch VW, Mountain C. Thoracoscopy under regional anesthesia for the diagnosis and management of pleural disease. Am J Surg 1987;154:274-8. 10.1016/0002-9610(89)90609-0 [DOI] [PubMed] [Google Scholar]
- 27.Al-Abdullatief M, Wahood A, Al-Shirawi N, et al. Awake anaesthesia for major thoracic surgical procedures: an observational study. Eur J Cardiothorac Surg 2007;32:346-50. 10.1016/j.ejcts.2007.04.029 [DOI] [PubMed] [Google Scholar]
- 28.Astoul P, Tassi G, Tschopp JM. Thoracoscopy for Pulmonologists: A Didactic Approach. Springer Science & Business Media; 2013. 261 p. [Google Scholar]
- 29.Marchetti G, Valsecchi A, Indellicati D, et al. Ultrasound-guided medical thoracoscopy in the absence of pleural effusion. Chest 2015;147:1008-12. 10.1378/chest.14-0637 [DOI] [PubMed] [Google Scholar]
- 30.Macha HN, Reichle G, von Zwehl D, et al. The role of ultrasound assisted thoracoscopy in the diagnosis of pleural disease. Clinical experience in 687 cases. Eur J Cardiothorac Surg 1993;7:19-22. 10.1016/1010-7940(93)90142-X [DOI] [PubMed] [Google Scholar]
- 31.Huang J, Hu Y, Mu X, et al. Thoracic ultrasound versus artificial pneumothorax in complications of medical thoracoscopy-a propensity score matching analysis. J Thorac Dis 2018;10:5269-74. 10.21037/jtd.2018.08.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yap KH, Phillips MJ, Lee YCG. Medical thoracoscopy: rigid thoracoscopy or flexi-rigid pleuroscopy? Curr Opin Pulm Med 2014;20:358-65. 10.1097/MCP.0000000000000059 [DOI] [PubMed] [Google Scholar]
- 33.Loddenkemper R, Lee P, Noppen M, et al. Medical thoracoscopy/pleuroscopy: step by step. Breathe 2011;8:156-67. 10.1183/20734735.011611 [DOI] [Google Scholar]
- 34.Dhooria S, Singh N, Aggarwal AN, et al. A randomized trial comparing the diagnostic yield of rigid and semirigid thoracoscopy in undiagnosed pleural effusions. Respir Care 2014;59:756-64. 10.4187/respcare.02738 [DOI] [PubMed] [Google Scholar]
- 35.Agarwal R, Aggarwal AN, Gupta D. Diagnostic accuracy and safety of semirigid thoracoscopy in exudative pleural effusions: a meta-analysis. Chest 2013;144:1857-67. 10.1378/chest.13-1187 [DOI] [PubMed] [Google Scholar]
- 36.Freeman RK, Ascioti AJ, Mahidhara RS. A propensity-matched comparison of pleurodesis or tunneled pleural catheter in patients undergoing diagnostic thoracoscopy for malignancy. Ann Thorac Surg 2013;96:259-63: discussion 263-4. [DOI] [PubMed]
- 37.Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA 2012;307:2383-9. 10.1001/jama.2012.5535 [DOI] [PubMed] [Google Scholar]
- 38.Thomas R, Fysh ETH, Smith NA, et al. Effect of an Indwelling Pleural Catheter vs Talc Pleurodesis on Hospitalization Days in Patients With Malignant Pleural Effusion: The AMPLE Randomized Clinical Trial. JAMA 2017;318:1903-12. 10.1001/jama.2017.17426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hallifax RJ, Corcoran JP, Psallidas I, et al. Medical thoracoscopy: Survey of current practice-How successful are medical thoracoscopists at predicting malignancy? Respirology 2016;21:958-60. 10.1111/resp.12725 [DOI] [PubMed] [Google Scholar]
- 40.Boutin C, Rey F. Thoracoscopy in pleural malignant mesothelioma: a prospective study of 188 consecutive patients. Part 1: Diagnosis. Cancer 1993;72:389-93. [DOI] [PubMed] [Google Scholar]
- 41.Dresler CM, Olak J, Herndon JE, et al. Phase III intergroup study of talc poudrage vs talc slurry sclerosis for malignant pleural effusion. Chest 2005;127:909-15. 10.1378/chest.127.3.909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Janssen J, Maldonado F, Metintas M. What is the significance of non-specific pleuritis? A trick question. Clin Respir J 2018;12:2407-10. 10.1111/crj.12940 [DOI] [PubMed] [Google Scholar]
- 43.Yang Y, Wu YB, Wang Z, et al. Long-term outcome of patients with nonspecific pleurisy at medical thoracoscopy. Respir Med 2017;124:1-5. 10.1016/j.rmed.2017.01.005 [DOI] [PubMed] [Google Scholar]
- 44.Anevlavis S, Varga C, Nam TH, et al. Is there any role for Thoracoscopy in the diagnosis of benign pleural Effusions. Clin Respir J 2019;13:73-81. 10.1111/crj.12983 [DOI] [PubMed] [Google Scholar]
- 45.Diacon AH, Van de Wal BW, Wyser C, et al. Diagnostic tools in tuberculous pleurisy: a direct comparative study. Eur Respir J 2003;22:589-91. 10.1183/09031936.03.00017103a [DOI] [PubMed] [Google Scholar]
- 46.Rahman NM, Maskell NA, West A, et al. Intrapleural use of tissue plasminogen activator and DNase in pleural infection. N Engl J Med 2011;365:518-26. 10.1056/NEJMoa1012740 [DOI] [PubMed] [Google Scholar]
- 47.Ravaglia C, Gurioli C, Tomassetti S, et al. Is medical thoracoscopy efficient in the management of multiloculated and organized thoracic empyema? Respiration 2012;84:219-24. 10.1159/000339414 [DOI] [PubMed] [Google Scholar]
- 48.Ricciardi S, Davini F, Zirafa CC, et al. From “open” to robotic assisted thoracic surgery: why RATS and not VATS? J Vis Surg 2018;4:107. 10.21037/jovs.2018.05.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ghosh-Dastidar MB, Deshpande RP, Rajagopal K, et al. Day surgery unit thoracic surgery: the first UK experience. Eur J Cardiothorac Surg 2011;39:1047-50. 10.1016/j.ejcts.2010.09.032 [DOI] [PubMed] [Google Scholar]
- 50.Katlic MR, Facktor MA. Video-assisted thoracic surgery utilizing local anesthesia and sedation: 384 consecutive cases. Ann Thorac Surg 2010;90:240-5. 10.1016/j.athoracsur.2010.02.113 [DOI] [PubMed] [Google Scholar]
- 51.Dixon G, de Fonseka D, Maskell N. Pleural controversies: image guided biopsy vs. thoracoscopy for undiagnosed pleural effusions? J Thorac Dis 2015;7:1041-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Łochowski MP, Kozak J. Video-assisted thoracic surgery complications. Wideochir Inne Tech Maloinwazyjne 2014;9:495-500. 10.5114/wiitm.2014.44250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Downey RJ. Complications after video-assisted thoracic surgery. Chest Surg Clin N Am 1998;8:907-17, x. [PubMed] [Google Scholar]
- 54.McDonald CM, Pierre C, de Perrot M, et al. Efficacy and Cost of Awake Thoracoscopy and Video-Assisted Thoracoscopic Surgery in the Undiagnosed Pleural Effusion. Ann Thorac Surg 2018;106:361-7. 10.1016/j.athoracsur.2018.02.044 [DOI] [PubMed] [Google Scholar]
- 55.Maskell G. Commentary on: Does every patient need to be discussed at a multidisciplinary team meeting? Clin Radiol 2013;68:760-1. 10.1016/j.crad.2013.03.003 [DOI] [PubMed] [Google Scholar]
- 56.Bibby AC, Williams K, Smith S, et al. What is the role of a specialist regional mesothelioma multidisciplinary team meeting? A service evaluation of one tertiary referral centre in the UK. BMJ Open 2016 08;6:e012092. [DOI] [PMC free article] [PubMed]