Abstract
A high preoperative neutrophil-lymphocyte ratio (NLR) has been shown in several studies as a predictor of worse survival in many solid neoplasms, including esophageal cancer, but its impact remains unclear. The goal of this systematic review was to gain all the evidence about NLR in order to analyse its potential in predicting survival in esophageal cancer. Therefore, we conducted a systematic literature search of all relevant studies reporting data on NLR as prognostic marker in esophageal cancer patients. We considered overall survival (OS) as primary outcome, disease-free survival (DFS) and progression-free survival (PFS) as secondary outcomes. We included studies with a directly or indirectly available hazard ratio (HR), furthermore we used both fixed effect model and random effect model depending on heterogeneity. We included a total of 20 studies, published between 2011 and 2017, consisting of 6,457 patients. The NLR cut-off value ranges from 1.7 to 5. The HR for OS of all included studies was 1.60. The HR for DFS and PFS was 1.75 and 1.66 respectively. The survival sub-analysis about tumor characteristics, treatment modality, blood sample timing also confirmed NLR prognostic relevance with statistically significant results. The meta-analysis showed that high preoperative NLR is associated with worse survival in esophageal cancer, as shown in several solid tumors, but its use in the clinical practice is still underestimated. High-quality studies are needed to assess the most effective cut-off in survival prognostication and NLR relevance on postoperative complications.
Keywords: Neutrophil-lymphocyte ratio (NLR), esophageal cancer, prognosis, markers, meta-analysis
Introduction
Esophageal cancer is the seventh most frequent cancer worldwide, representing the 3.2% of all cancers, with a very high morality (5.3% of all deaths for cancer) (1). The 5-year survival in USA is 19.2%, showing that, despite the improvements in treatments and diagnostic tools, the prognosis remains poor. Therefore, the study and analysis of new prognostic factors is of paramount importance in order to provide the adequate treatment solution for each patient. As known, inflammatory response plays a key role in tumor growth (2) and a number of inflammation factors has been proposed as promising prognostic markers.
Measurable blood parameters that reflect the systemic inflammatory response includes C-reactive protein, cytokines, leucocytes and their subtypes, platelets (3). Recently, neutrophils-lymphocyte ratio (NLR) has been proposed as a prognostic indicator in several tumours. It is an easily measurable parameter consisting of the ratio of circulating blood neutrophils and lymphocytes. Neutrophils are a consistent part of peritumoral inflammatory cell infiltrate, named tumour-associated neutrophils (TANs). The infiltrate seems to be a direct result of cancer cell’s activity, suggesting that the presence of these neutrophils is related to tumour growth (4).
A meta-analysis of all available studies about NLR in all solid tumors, in 2014, showed that high NLR is associated with poor survival in many tumours, including gastroesophageal tumours, with a trend for the association of high NLR with worse OS to be greater for metastatic than non-metastatic disease (5). Small studies with cancer patients showed that chemotherapy can normalize elevated NLR early after the introduction of treatment and that patients with normalized NLR may have improved outcome (6,7).
The role of NLR in esophageal cancer has been investigated in several studies (Table 1), but its clinical relevance remains unclear (8). Most of the studies focus on preoperative NLR, and did not evaluate its evolution in postoperative period. The links between recurrence, immunosuppression and postoperative complications and infections remain unclear, as well as the role played by chemotherapy-related immunosuppression in the ratio normalization. A recent meta-analysis suggested that a lower preoperative NLR is associated with an improved survival in esophageal cancer patients, but their subgroup analysis about treatment modality and disease-free survival were inconclusive, obtaining non–statistically significant results (HR 1.20, P=0.27 and HR 1.54, P=0.20 respectively) (9).
Table 1. Studies characteristics.
| Author | Year | Country | Years | Patients | Design | Cut-off | Main treatment | NOS |
|---|---|---|---|---|---|---|---|---|
| Yuan D | 2014 | China | 2009–2012 | 327 | Retrospective | 5 | Surgery | 6 |
| Yoo EJ | 2014 | South Korea | 2005–2010 | 138 | Retrospective | 2 | DCRT | 6 |
| Sharaiha R | 2011 | USA | 1996–2009 | 295 | Retrospective | 5 | Surgery | 6 |
| Noble F | 2013 | UK | 2005–2010 | 246 | Retrospective | 2.5 | NCRT + surgery | 6 |
| Miyata H | 2011 | Japan | 2000–2008 | 152 | Retrospective | 4 | NCRT + surgery | 6 |
| Hirahara N | 2015 | Japan | 2006–2014 | 141 | Retrospective | 2.5 | Surgery | 5 |
| Grenader T | 2016 | UK | 2000–2005 | 908 | Prospective | 3 | DCRT | 6 |
| Feng JF | 2014 | China | 2005–2008 | 483 | Retrospective | 3.5 | Surgery | 5 |
| Duan H | 2015 | China | 2000–2007 | 371 | Retrospective | 3 | Surgery | 7 |
| Xie X | 2014 | China | 2008–2010 | 317 | Retrospective | 2.1 | Surgery | 6 |
| Han LH | 2015 | China | 2007–2008 | 218 | Retrospective | 2.6 | Surgery | 5 |
| Ji WH | 2016 | China | 2009–2012 | 41 | Retrospective | 5 | Surgery | 5 |
| Shao Y | 2015 | China | 2002–2012 | 916 | Retrospective | 1.7 | NCRT + surgery | 5 |
| Jung J | 2016 | South Korea | 2004–2012 | 119 | Retrospective | 2.97 | Surgery | 5 |
| Arigami T | 2015 | Japan | 1998–2012 | 238 | Retrospective | 3 | Surgery | 5 |
| Nakamura K | 2017 | Japan | 2005–2016 | 245 | Retrospective | 2.42 | Surgery | 5 |
| Zhou XL | 2017 | China | 2006–2010 | 517 | Retrospective | 5 | DCRT | 6 |
| Toyokawa | 2016 | Japan | 2000–2014 | 185 | Retrospective | 3.612 | Surgery | 6 |
| He YF | 2017 | China | 2000–2010 | 317 | Retrospective | 3.3 | Surgery | 5 |
| Kosumi K | 2016 | Japan | 2005–2011 | 283 | Retrospective | 1.94 | Surgery | 5 |
NCRT, neoadjuvant chemoradiation; DCRT, definitive chemoradiation.
In this context, the aim of our systematic review and meta-analysis is to assess the predictive value of pre-treatment NLR in forecasting the outcome of esophageal cancer patients. Furthermore, we have considered some sub-analyses in order to identify the differences in predicting the outcome of different treatment modalities and histological types.
Methods
We performed the analysis in according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines (10,11).
Search strategy
We searched PubMed, Embase, Web of Science and Cochrane online databases with the following keywords: (“NLR”[All Fields] OR ((“neutrophils”[MeSH Terms] OR “neutrophils”[All Fields] OR “neutrophil”[All Fields]) AND (“lymphocytes”[MeSH Terms] OR “lymphocytes”[All Fields] OR “lymphocyte”[All Fields]))) AND (“oesophageal neoplasms”[All Fields] OR “esophageal neoplasms”[MeSH Terms] OR (“esophageal”[All Fields] AND “neoplasms”[All Fields]) OR “esophageal neoplasms”[All Fields]) in February 2017. Reference list of original articles and review articles were considered as additional source of information. We also searched the PROSPERO database. The literature search was restricted to articles published in English language, without restrictions about the year of publication.
Inclusion criteria
The inclusion criteria we used are adapted from the REporting recommendations for tumour MARKer prognostic studies criteria (REMARK) (12). Inclusion criteria for the primary analysis were: (I) both prospective and observational studies reporting prognostic data on NLR in esophageal cancer patients without gender or anagraphic limitation; every stage of disease, histotype and treatment were considered; (II) studies reporting dichotomized data: comparison of low NLR and high NLR; (III) availability of HR including 95% CIs and P values (preferred) or Kaplan-Meier curves about overall survival (OS) or progression free survival (PFS) or disease free survival (DFS).
Data extraction
Two investigators extracted the data independently (G Pirozzolo, M Scarpa). To avoid systematic biases two authors independently reviewed all eligible studies until a complete concordance was reached for all assessed variables. Disagreements were resolved by discussion, with the participation of a third author (MI van Berge Henegouwen). Extracted data were: demographic data, patient’s characteristics, methodological data, OS HR, DFS HR, PFS HR, and postoperative complications. HR were extracted both from multivariate and univariate analysis, preferring data from multivariate analysis when available. When HR was not declared, it has been extracted from Kaplan-Meyer curves following the method described by Parmar (13).
Quality assessment
Two investigators assessed retrieved articles quality according with the Newcastle-Ottawa scale for assessing the quality of non-randomized studies in meta-analysis. Studies with less than 5 stars in the Newcastle-Ottawa assessment were not included in our meta-analysis. We assessed the possibility of publication bias by graphical evaluation of symmetry in a funnel plot.
Statistical analysis
Extracted data were analysed using RevMan 4.3 analysis software. Generic inverse variance was used to pool hazard ratios. We used both Fixed-Effect model and Random-Effect model, depending on heterogeneity. Heterogeneity, assessed using I2 statistic, was considered relevant when >30%. Statistical significance was considered relevant when P<0.001.
Results
Study selection
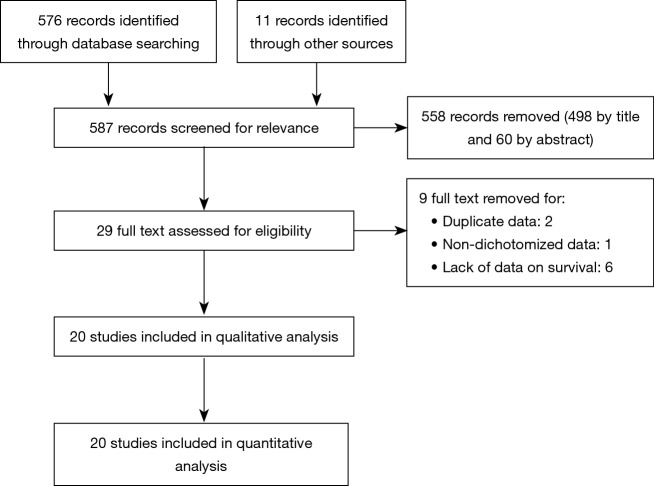
After combined search, we identified 587 studies. After evaluation of titles and abstracts, we identified 29 full text. One study was excluded because it showed NLR as a continuous data (14). Two studies were excluded because they showed duplicate data (15,16). Six studies were excluded because of the lack of data on survival (HR and related cut-off) (14,17-21) and one of these did not report any survival data (20). The remaining 20 studies, including 6,457 patients, were all included in the analysis. None of the considered studies were excluded after qualitative assessment. The study selection diagram is reported in Figure 1. All studies were published between 2011 and 2017. Included studies characteristics are reported in Table 1. One study considered NLR both as a continuous data and as a dichotomized data but, in the second case, did not report HR (22). For three studies, including this one, we extracted HR from Kaplan-Meier curves using the method described by Parmar (23,24).
Figure 1.
Selection of studies included in the analysis.
Nineteen studies had a retrospective design or reported a retrospective analysis of prospectively collected data. Only one study had a prospective design, reporting data from a phase III study (Real-2 study) (18,25).
In fourteen studies the main treatment was surgery (8,15,23,24,26-34) in three was neoadjuvant chemoradiation (NCRT) followed by surgery (22,24,35); in three was definitive chemoradiation (DCRT) (18,36,37). In one study the time of sampling was post-treatment (8). Chemotherapy treatment schemes, as reported in Table 1, are mainly 5-FU based or Cisplatin based. In 9 studies, having surgery as main treatment, the presence of preoperative or postoperative treatments was an exclusion criterion (8,15,23,24,28,31,33,38). Quality assessment is reported in Table 1: all selected studies were considered adequate, following our inclusion criteria. The funnel plot also showed substantial symmetry between the included studies.
Patients characteristics
Patients characteristics are reported in Table 2. Based on available data both adenocarcinoma (AC) and squamous cell carcinoma (SCC) are represented. One study included only AC patients (26); 13 studies included only SCC patients (8,15,23,24,28-30,32-34,37-39); the remaining studies considered both.
Table 2. Patients characteristics.
| Author | Patients | Male | Age | Histology | Site | Stage | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AC | SCC | Other | Mid-upper | GE-lower | Gastric | 1 | 2 | 3 | 4 | ||||||
| Yuan D | 327 | 282 | – | 327 | 0 | 0 | 0 | 327 | 0 | 150 | 177 | ||||
| Yoo EJ | 138 | 125 | 67.6 | 5 | 133 | 0 | 78 | 59 | 0 | 0 | 138 | 0 | |||
| Sharaiha R | 295 | 237 | 62.8 | 214 | 75 | 4 | 56 | 239 | 0 | 50 | 92 | 129 | 24 | ||
| Noble F | 246 | 195 | 67.0 | 211 | 32 | 0 | 18 | 228 | 0 | – | |||||
| Miyata H | 152 | 132 | 62.5 | 0 | 140 | 12 | 18 | 63 | 71 | 0 | 30 | 77 | 45 | ||
| Hirahara N | 141 | 127 | – | 0 | 141 | 0 | 76 | 64 | 0 | 55 | 34 | 52 | 0 | ||
| Grenader T | 908 | 735 | – | 800 | 88 | 0 | 0 | 550 | 358 | 0 | 0 | 908 | |||
| Feng JF | 483 | 411 | 59.1 | 0 | 483 | 0 | 274 | 249 | 0 | – | |||||
| Duan H | 371 | 276 | 57.0 | 0 | 371 | 0 | 279 | 92 | 0 | 20 | 206 | 145 | 0 | ||
| Xie X | 317 | 244 | 58.1 | 0 | 317 | 0 | 226 | 92 | 0 | 43 | 132 | 142 | 0 | ||
| Han LH | 218 | 177 | – | 0 | 218 | 0 | 145 | 73 | 0 | 133 | 85 | ||||
| Ji WH | 41 | 38 | 56.6 | 0 | 41 | 0 | 24 | 17 | 0 | 2 | 16 | 23 | 0 | ||
| Shao Y | 916 | 694 | – | 0 | 916 | 0 | 656 | 260 | 0 | 175 | 426 | 325 | 0 | ||
| Jung J | 119 | 112 | 63.6 | 0 | 119 | 0 | 77 | 42 | 0 | 37 | 33 | 49 | 0 | ||
| Arigami T | 238 | 210 | – | 0 | 238 | 0 | 157 | 81 | 0 | 86 | 70 | 82 | 0 | ||
| Nakamura K | 245 | 219 | – | 18 | 209 | 18 | 166 | 79 | 0 | – | |||||
| Zhou XL | 517 | 407 | 65.0 | 0 | 517 | 0 | 428 | 89 | 0 | 0 | 83 | 377 | 57 | ||
| Toyokawa | 185 | 152 | 64.0 | 0 | 185 | 0 | 133 | 52 | 0 | 67 | 78 | 40 | 0 | ||
| He YF | 317 | 268 | – | 0 | 317 | 0 | 189 | 128 | 0 | 217 | 100 | ||||
| Kosumi K | 283 | 248 | – | 0 | 283 | 0 | Nr | nr | 0 | 111 | 72 | 68 | 32 | ||
AC, adenocarcinoma; SCC, squamous cell carcinoma.
Mid-upper esophagus and lower esophagus-gastroesophageal junction were both well represented (3,000 and 2,784 patients respectively), but a sub-analysis based on tumor location was unfeasible for the lack of data.
NLR cut-off values
The NLR cut-off value ranges from 1.7 to 5 (mean 3.2). Different cut-offs were reported in the included studies: in 6 papers the cut-off was determined using receiver operating characteristic curves (C-index) (18,28,29,32,36,38), in other cases they mostly referred to literature, but the method of selection was unclear.
Overall survival
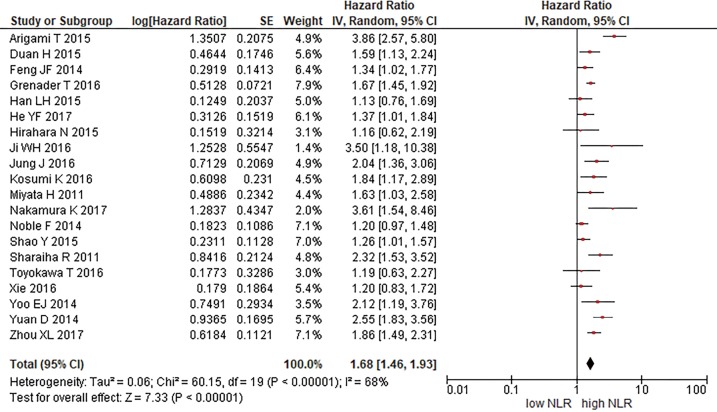
As previously reported three included study did not showed the OS HR as a dichotomized data, in this case HR has been extracted from Kaplan-Meier curves (22,24). All remaining OS HR are taken from multivariate analysis with the exception of one study (8) which OS HR is taken from univariate analysis. We pooled all OS HR, as showed in Figure 2. The comparison about overall survival of all included studies showed: HR 1.78 (95% CI: 1.46–1.93, P<0.00001, I2 =68%). We also performed sub-analysis considering the following parameters: main treatment modality, histotype, blood sample timing, cut-off method (ROC curve or obtained from existing literature). The results of quantitative synthesis, summarized in Table 3, did not show significant differences with the previous analysis, confirming the NLR predictive relevance.
Figure 2.
Overall survival.
Table 3. Sub-analysis summary.
| Variable | Studies | Patients | Model | HR (95% CI) | P value | I2 (%) |
|---|---|---|---|---|---|---|
| Treatment | ||||||
| Surgery +/− chemo | 14 | 4,455 | Random | 1.69 (1.39–2.06) | <0.001 | 73 |
| Neo + surgery | 3 | 477 | Random | 1.52 (1.00–2.31) | <0.001 | 57 |
| DCRT | 3 | 1,563 | Fixed | 1.74 (1.55–1.95) | <0.001 | 0 |
| Histotype | ||||||
| SCC | 15 | 4,419 | Random | 1.61 (1.36–1.90) | <0.001 | 64 |
| Timing | ||||||
| Preoperative | 13 | 4,313 | Random | 1.72 (1.40–2.12) | <0.001 | 75 |
| Pretreatment | 5 | 1,756 | Fixed | 1.74 (1.56–1.95) | <0.001 | 0 |
| Cut-off method | ||||||
| ROC curves | 6 | 2,137 | Fixed | 1.56 (1.39–1.74) | <0.001 | 30 |
| literature/other | 14 | 4,320 | Random | 1.81 (1.49–2.20) | <0.001 | 75 |
Progression-free survival and disease-free survival
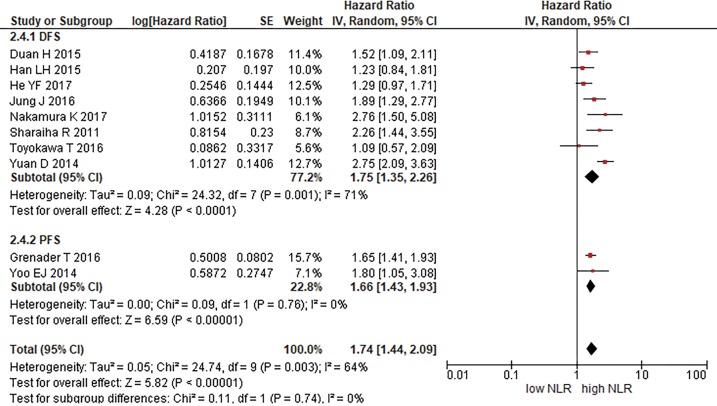
Ten studies reported data on PFS or DFS (18,23,26-28,31,36,38). We pooled these studies to obtain a sub-analysis for PFS and DFS (Figure 3). About DFS our analysis showed a 1.75 HR (95% CI: 1.35–2.26, P<0.00001, I2 =71%). The PFS HR is 1.66 (95% CI: 1.43–1.93, P<0.00001, I2 =0%).
Figure 3.
Disease-free survival and progression-free survival.
Discussion
The emerging role of systemic inflammation in postoperative complications and survival is a topic of research in several solid tumours (5,40,41). The present meta-analysis results showed a significant association between survival and systemic inflammation in esophageal cancer, considering NLR as an independent predictive marker. This parameter is relatively cheap, easy to evaluate in most medical centres and, being a ratio, the different ranges between laboratories have a limited impact.
If compared with the previous meta-analysis on the same argument (9) our study, including more data, reached more conclusive results. Furthermore, we considered as many variables as possible in performing a sub-analysis, such as treatment modality and histotype. Our analysis showed higher NLR is a significant prognostic marker of worse survival (Figure 2). This data is confirmed both in overall and in sub-analysis results. Interestingly NLR predicts survival independently of histotype (Table 3). Similarly, the treatment modality sub-analysis showed almost analogue results for the considered subgroups. The cut-off sub-analysis showed significant result for both considered methods, however some studies did not report the source of the cut-off threshold. A sub-analysis which considered tumor location and patients’ origin was unfeasible for lack of data, but it would have clarified possible geographic and anatomic differences. Similarly, a sub-analysis which considered NRL and survival rates per stage of disease was unfeasible, but would have been very useful in order to stratify the risk of worst prognosis per stage.
The mechanisms by which NLR is related to survival in esophageal cancer and other solid tumours are still unclear. The increased number of neutrophils in the peritumoral inflammatory cell infiltrate (TANs) can inhibit the antitumor activity of natural killer (NK) and activated T cells (4,42). Moreover, many cytokines (TNF, IL-1, IL-6) and VEGF, produced as result of neutrophils activation, may enhance tumour growth (43). The complexity of this relationship, which may seem paradoxical, could be summarized as follows: tumours produces inflammatory cytokines and chemokines and are infiltrated by leukocytes, but advanced neoplasms are associated with a defective systemic immune response (44). The role of neutrophil infiltration in tumor growth is controversial. Increasing experimental evidence indicate that neutrophils may directly or indirectly influence the tumor fate through the release of a wide array of molecules able to exert either pro-tumor or anti-tumor functions depending on the microenvironment milieu, including cytokines (45). Both human and murine activated neutrophils can produce and release is TRAIL, a trans-membrane/soluble molecule involved in tumor cell killing and autoimmunity (46,47). On the other hand, TANs have been shown to chemoattractants Tregs in a mouse model of cancer, mainly via CCL17 (47,48). Because neutrophil depletion, in this model, was shown to reduce Tregs recruitment and, consequently, tumor growth, data provide, for the first time, a clear link between TANs and Tregs, acting together to impair antitumor immunity (47,48). In fact, cancer immunoediting, mainly mediated by CD8 and CD4 T cells, macrophages, and NK cells, may lead to cancer cell destruction (cancer immunosurveillance) with complete cancer cells elimination, to an equilibrium phase or to an escape phase when cancer cells overcome immune defences and spread within the whole organism (49). A prevalence of neutrophils granulocytes over lymphocytes might be interpreted as a rough marker of immunosurveillance failure.
Few studies focused on the NLR variation through the neoadjuvant treatment. They are more difficult to interpreter because, even if there is not enough data within the included studies, there might be a direct effect of chemotherapy on the level of neutrophils and on lymphocytes ones. On the other hand, we analysed preoperative NLR and a time frame of at least 6–8 weeks should have provided the patients’ bone marrow time enough to recover. In any case, the effect of neoadjuvant systemic treatment on lowering NLR, improving, at the same time, survival has been shown in solid tumors (6,7,50,51). We found three studies which consider this effect in esophageal cancer (14,22,35). Noble showed that neutrophils count is statistically significantly lower in those patients who underwent neoadjuvant treatment, and that increasing NLR is an independent prognostic factor for reduced OS at multivariate analysis (22). Hyder et al., excluded from the qualitative analysis for the lack of data, showed that NLR increase is associated with a worse OS, but, on the other hand, with an improvement of PFS (14). In the third study, the survival analysis on NLR variations is incomplete due to the low number of patients with a NLR higher than the cut-off after the neoadjuvant treatment (35). These data are still largely unclear and, considering the relevance of neoadjuvant treatment on esophageal cancer, such as rectal cancer, this correlation should be evaluated in further research. In fact, there are not enough evidences in the included studies to confirm a decrease of NLR during neoadjuvant treatment is related to the decline of tumor load. It is even possible the opposite, a rise in NLR due to enhanced cell apoptosis during chemotherapy.
There are a number of limitations in our study. The major bias is the retrospective design of almost all included studies. The heterogeneity about treatment modalities, patients’ characteristics, duration of follow-up, samples timing and cut-off threshold are other sources of bias. Besides, several studies investigated NLR relevance as a prognostic marker for non-oncological diseases, mostly heart disease (52,53). Finally, neutrophils and lymphocytes are, by their very nature, easily influenced by inflammatory and infectious phenomena. All these conditions could have a confounding effect on NLR prognostic interpretation. In fact, one of the strengths of NLR is the fact that is a ratio, so different ranges between laboratories should have limited impact. On the contrary, we observed a widespread in cut-off values. These divergent cut off values may be due to heterogeneity on cut-off definition or heterogeneity in patient populations (age, sex, geographical origin, exposition) or heterogeneity in stage and histology of the disease or a mix of them.
In conclusion, our meta-analysis showed high NLR to be a predictor of adverse survival in esophageal cancer, both in SCC and in adenocarcinoma and therefore it could be promising as a factor predicting outcome. Further studies are needed to better define NLR role in therapeutic and diagnostic scenarios and use it to predict survival in preoperative setting. However, our analysis, suggests an actual use of this marker in clinical practice, also considering its negligible cost. NLR should be evaluated at the time of diagnosis and before surgery.
Acknowledgments
None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature 2008;454:436-44. 10.1038/nature07205 [DOI] [PubMed] [Google Scholar]
- 3.McCormick Matthews LH, Noble F, Tod J, et al. Systematic review and meta-analysis of immunohistochemical prognostic biomarkers in resected oesophageal adenocarcinoma. Br J Cancer 2015;113:1746. 10.1038/bjc.2015.460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gregory AD, Houghton AM. Tumor-associated neutrophils: New targets for cancer therapy. Cancer Res 2011;71:2411-6. 10.1158/0008-5472.CAN-10-2583 [DOI] [PubMed] [Google Scholar]
- 5.Templeton AJ, Mcnamara MG, Šeruga B, et al. Prognostic Role of Neutrophil-to-Lymphocyte Ratio in Solid Tumors: A Systematic Review and Meta-Analysis. J Natl Cancer Inst 2014;106:dju124. 10.1093/jnci/dju124 [DOI] [PubMed] [Google Scholar]
- 6.Kao SCH, Pavlakis N, Harvie R, et al. High blood neutrophil-to-lymphocyte ratio is an indicator of poor prognosis in malignant mesothelioma patients undergoing systemic therapy. Clin Cancer Res 2010;16:5805-13. 10.1158/1078-0432.CCR-10-2245 [DOI] [PubMed] [Google Scholar]
- 7.Chua W, Charles KA, Baracos VE, et al. Neutrophil/lymphocyte ratio predicts chemotherapy outcomes in patients with advanced colorectal cancer. Br J Cancer 2011;104:1288-95. 10.1038/bjc.2011.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirahara N, Matsubara T, Hayashi H, et al. Impact of inflammation-based prognostic score on survival after curative thoracoscopic esophagectomy for esophageal cancer. Eur J Surg Oncol 2015;41:1308-15. 10.1016/j.ejso.2015.07.008 [DOI] [PubMed] [Google Scholar]
- 9.Yodying H, Matsuda A, Miyashita M, et al. Prognostic Significance of Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio in Oncologic Outcomes of Esophageal Cancer: A Systematic Review and Meta-analysis. Ann Surg Oncol 2016;23:646-54. 10.1245/s10434-015-4869-5 [DOI] [PubMed] [Google Scholar]
- 10.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009;62:e1-34. 10.1016/j.jclinepi.2009.06.006 [DOI] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med 2009;6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McShane LM, Altman DG, Sauerbrei W, et al. REporting recommendations for tumor MARKer prognostic studies (REMARK). Breast Cancer Res Treat 2006;100:229-35. 10.1007/s10549-006-9242-8 [DOI] [PubMed] [Google Scholar]
- 13.Parmar MKB, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815-34. [DOI] [PubMed] [Google Scholar]
- 14.Hyder J, Boggs DH, Hanna A, et al. Changes in neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios during chemoradiation predict for survival and pathologic complete response in trimodality esophageal cancer patients. J Gastrointest Oncol 2016;7:189-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng JF, Huang Y, Chen QX. Preoperative platelet lymphocyte ratio (PLR) is superior to neutrophil lymphocyte ratio (NLR) as a predictive factor in patients with esophageal squamous cell carcinoma. World J Surg Oncol 2014;12:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu JS, Huang Y, Yang X, et al. A nomogram to predict prognostic values of various inflammatory biomarkers in patients with esophageal squamous cell carcinoma. Am J Cancer Res 2015;5:2180-9. [PMC free article] [PubMed] [Google Scholar]
- 17.Dutta S, Crumley ABC, Fullarton GM, et al. Comparison of the Prognostic Value of Tumour- and Patient-Related Factors in Patients Undergoing Potentially Curative Resection of Oesophageal Cancer. World J Surg 2011;35:1861-6. 10.1007/s00268-011-1130-7 [DOI] [PubMed] [Google Scholar]
- 18.Grenader T, Waddell T, Peckitt C, et al. Prognostic value of neutrophil-to-lymphocyte ratio in advanced oesophago-gastric cancer: Exploratory analysis of the REAL-2 trial. Ann Oncol 2016;27:687-92. 10.1093/annonc/mdw012 [DOI] [PubMed] [Google Scholar]
- 19.Sato H, Tsubosa Y, Kawano T. Correlation between the pretherapeutic neutrophil to lymphocyte ratio and the pathologic response to neoadjuvant chemotherapy in patients with advanced esophageal cancer. World J Surg 2012;36:617-22. 10.1007/s00268-011-1411-1 [DOI] [PubMed] [Google Scholar]
- 20.Rashid F, Waraich N, Bhatti I, et al. A pre-operative elevated neutrophil: Lymphocyte ratio does not predict survival from oesophageal cancer resection. World J Surg Oncol 2010;8:1. 10.1186/1477-7819-8-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ikeguchi M, Kouno Y, Kihara K, et al. Evaluation of prognostic markers for patients with curatively resected thoracic esophageal squamous cell carcinomas. Mol Clin Oncol 2016;5:767-72. 10.3892/mco.2016.1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noble F, Hopkins J, Curtis N, et al. The role of systemic inflammatory and nutritional blood-borne markers in predicting response to neoadjuvant chemotherapy and survival in oesophagogastric cancer. Med Oncol 2013;30:596. 10.1007/s12032-013-0596-6 [DOI] [PubMed] [Google Scholar]
- 23.Jung J, Park YP, Park S, et al. Prognostic value of the neutrophil-to-lymphocyte ratio for overall and disease-free survival in patients with surgically treated esophageal squamous cell carcinoma. Tumour Biol 2016;37:7149-54. 10.1007/s13277-015-4596-3 [DOI] [PubMed] [Google Scholar]
- 24.Arigami T, Okumura H, Matsumoto M, et al. Analysis of the fibrinogen and neutrophil-lymphocyte ratio in esophageal squamous cell carcinoma. Medicine (Baltimore) 2015;94:e1702. 10.1097/MD.0000000000001702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cunningham D, Starling N, Rao S, et al. Capecitabine and Oxaliplatin for Advanced Esophagogastric Cancer. N Engl J Med 2008;358:36-46. 10.1056/NEJMoa073149 [DOI] [PubMed] [Google Scholar]
- 26.Yuan D, Zhu K, Li K, et al. The preoperative neutrophil-lymphocyte ratio predicts recurrence and survival among patients undergoing R0 resections of adenocarcinomas of the esophagogastric junction. J Surg Oncol 2014;110:333-40. 10.1002/jso.23651 [DOI] [PubMed] [Google Scholar]
- 27.Sharaiha RZ, Halazun KJ, Mirza F, et al. Elevated preoperative neutrophil: Lymphocyte ratio as a predictor of postoperative disease recurrence in esophageal cancer. Ann Surg Oncol 2011;18:3362-9. 10.1245/s10434-011-1754-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duan H, Zhang X, Wang FX, et al. Prognostic role of neutrophil-lymphocyte ratio in operable esophageal squamous cell carcinoma. World J Gastroenterol 2015;21:5591-7. 10.3748/wjg.v21.i18.5591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie X, Luo KJ, Hu Y, et al. Prognostic value of preoperative platelet-lymphocyte and neutrophil-lymphocyte ratio in patients undergoing surgery for esophageal squamous cell cancer. Dis Esophagus 2016;29:79-85. 10.1111/dote.12296 [DOI] [PubMed] [Google Scholar]
- 30.Shao Y, Ning Z, Chen J, et al. Prognostic nomogram integrated systemic inflammation score for patients with esophageal squamouscell carcinoma undergoing radical esophagectomy. Sci Rep 2015;5:18811. 10.1038/srep18811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakamura K, Yoshida N, Baba Y, et al. Elevated preoperative neutrophil-to-lymphocytes ratio predicts poor prognosis after esophagectomy in T1 esophageal cancer. Int J Clin Oncol 2017;22:469-75. 10.1007/s10147-017-1090-5 [DOI] [PubMed] [Google Scholar]
- 32.Toyokawa T, Kubo N, Tamura T, et al. The pretreatment Controlling Nutritional Status (CONUT) score is an independent prognostic factor in patients with resectable thoracic esophageal squamous cell carcinoma: Results from a retrospective study. BMC Cancer 2016;16:722. 10.1186/s12885-016-2696-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He YF, Luo HQ, Wang W, et al. Preoperative NLR and PLR in the middle or lower ESCC patients with radical operation. Eur J Cancer Care (Engl) 2017;26. doi: . 10.1111/ecc.12445 [DOI] [PubMed] [Google Scholar]
- 34.Kosumi K, Baba Y, Ishimoto T, et al. Neutrophil/lymphocyte ratio predicts the prognosis in esophageal squamous cell carcinoma patients. Surg Today 2016;46:405-13. 10.1007/s00595-015-1197-0 [DOI] [PubMed] [Google Scholar]
- 35.Ji WH, Jiang YH, Ji YL, et al. Prechemotherapy neutrophil : lymphocyte ratio is superior to the platelet : lymphocyte ratio as a prognostic indicator for locally advanced esophageal squamous cell cancer treated with neoadjuvant chemotherapy. Dis Esophagus 2016;29:403-11. 10.1111/dote.12322 [DOI] [PubMed] [Google Scholar]
- 36.Yoo EJ, Park JC, Kim EH, et al. Prognostic value of neutrophil-to-lymphocyte ratio in patients treated with concurrent chemoradiotherapy for locally advanced oesophageal cancer. Dig Liver Dis 2014;46:846-53. 10.1016/j.dld.2014.05.009 [DOI] [PubMed] [Google Scholar]
- 37.Zhou XL, Li YQ, Zhu WG, et al. Neutrophil-to-lymphocyte ratio as a prognostic biomarker for patients with locally advanced esophageal squamous cell carcinoma treated with definitive chemoradiotherapy. Sci Rep 2017;7:42581. 10.1038/srep42581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han LH, Jia YB, Song QX, et al. Prognostic Significance of Preoperative Lymphocyte-Monocyte Ratio in Patients with Resectable Esophageal Squamous Cell Carcinoma. Asian Pac J Cancer Prev 2015;16:2245-50. 10.7314/APJCP.2015.16.6.2245 [DOI] [PubMed] [Google Scholar]
- 39.Miyata H, Yamasaki M, Kurokawa Y, et al. Prognostic value of an inflammation-based score in patients undergoing pre-operative chemotherapy followed by surgery for esophageal cancer. Exp Ther Med 2011;2:879-85. 10.3892/etm.2011.308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moyes LH, Leitch EF, McKee RF, et al. Preoperative systemic inflammation predicts postoperative infectious complications in patients undergoing curative resection for colorectal cancer. Br J Cancer 2009;100:1236-9. 10.1038/sj.bjc.6604997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Templeton AJ, Ace O, McNamara MG, et al. Prognostic role of platelet to lymphocyte ratio in solid tumors: A systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 2014;23:1204-12. 10.1158/1055-9965.EPI-14-0146 [DOI] [PubMed] [Google Scholar]
- 42.Shau HY, Kim A. Suppression of lymphokine-activated killer induction by neutrophils. J Immunol 1988;141:4395-402. [PubMed] [Google Scholar]
- 43.An X, Ding PR, Li YH, et al. Elevated neutrophil to lymphocyte ratio predicts survival in advanced pancreatic cancer. Biomarkers 2010;15:516-22. 10.3109/1354750X.2010.491557 [DOI] [PubMed] [Google Scholar]
- 44.Balkwill F, Mantovani A. Inflammation and cancer: Back to Virchow? Lancet 2001;357:539-45. 10.1016/S0140-6736(00)04046-0 [DOI] [PubMed] [Google Scholar]
- 45.Tecchio C, Scapini P, Pizzolo G, Cassatella MA. On the cytokines produced by human neutrophils in tumors Semin Cancer Biol 2013;23:159-70. 10.1016/j.semcancer.2013.02.004 [DOI] [PubMed] [Google Scholar]
- 46.Cassatella MA. On the production of TNF-related apoptosis-inducing ligand (TRAIL/Apo-2L) by human neutrophils. J Leukoc Biol 2006;79:1140-9. 10.1189/jlb.1005558 [DOI] [PubMed] [Google Scholar]
- 47.Tecchio C, Micheletti A, Cassatella MA. Neutrophil-Derived Cytokines: Facts Beyond Expression. Front Immunol 2014;5:508. 10.3389/fimmu.2014.00508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mishalian I, Bayuh R, Eruslanov E, et al. Neutrophils recruit regulatory T-cells into tumors via secretion of CCL17--a new mechanism of impaired antitumor immunity. Int J Cancer 2014;135:1178-86. 10.1002/ijc.28770 [DOI] [PubMed] [Google Scholar]
- 49.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting Nat Rev Immunol 2006;6:836-48. 10.1038/nri1961 [DOI] [PubMed] [Google Scholar]
- 50.Lee S, Oh SY, Kim SH, et al. Prognostic significance of neutrophil lymphocyte ratio and platelet lymphocyte ratio in advanced gastric cancer patients treated with FOLFOX chemotherapy. BMC Cancer 2013;13:350. 10.1186/1471-2407-13-350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jin H, Zhang G, Liu X, et al. Blood neutrophil-lymphocyte ratio predicts survival for stages III-IV gastric cancer treated with neoadjuvant chemotherapy. World J Surg Oncol 2013;11:112. 10.1186/1477-7819-11-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang X, Zhang G, Jiang X, et al. Neutrophil to lymphocyte ratio in relation to risk of all-cause mortality and cardiovascular events among patients undergoing angiography or cardiac revascularization: A meta-analysis of observational studies. Atherosclerosis 2014;234:206-13. 10.1016/j.atherosclerosis.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 53.Afari ME, Bhat T. Neutrophil to lymphocyte ratio (NLR) and cardiovascular diseases: An update. Expert Rev Cardiovasc Ther 2016;14:573-7. 10.1586/14779072.2016.1154788 [DOI] [PubMed] [Google Scholar]