Abstract
Background
Malaria “hotspots” have been proposed as potential intervention units for targeted malaria elimination. Little is known about hotspot formation and stability in settings outside sub-Saharan Africa.
Methods
Clustering of Plasmodium infections at the household and hotspot level was assessed over 2 years in 3 villages in eastern Cambodia. Social and spatial autocorrelation statistics were calculated to assess clustering of malaria risk, and logistic regression was used to assess the effect of living in a malaria hotspot compared to living in a malaria-positive household in the first year of the study on risk of malaria infection in the second year.
Results
The crude prevalence of Plasmodium infection was 8.4% in 2016 and 3.6% in 2017. Living in a hotspot in 2016 did not predict Plasmodium risk at the individual or household level in 2017 overall, but living in a Plasmodium-positive household in 2016 strongly predicted living in a Plasmodium-positive household in 2017 (Risk Ratio, 5.00 [95% confidence interval, 2.09–11.96], P < .0001). There was no consistent evidence that malaria risk clustered in groups of socially connected individuals from different households.
Conclusions
Malaria risk clustered more clearly in households than in hotspots over 2 years. Household-based strategies should be prioritized in malaria elimination programs in this region.
Keywords: malaria, hotspot, malaria elimination, Greater Mekong Subregion, epidemiology
In a low-transmission setting in eastern Cambodia, malaria risk clustered more clearly in households than in hotspots over 2 years. Household-based strategies should be prioritized in malaria elimination programs in this region.
Malaria elimination targets are focusing attention on the total Plasmodium infection burden, including asymptomatic infections [1–3], which are thought to contribute to ongoing transmission [1, 4]. Though the probability of onward transmission is lower [1, 5], asymptomatic infections represent a key challenge for malaria elimination, as they are not systematically detected in routine care [6]. In addition to standard vector control (long-lasting insecticidal nets and indoor residual spraying) and prompt case detection and treatment, there are 2 broad strategies promoted to reduce the parasite reservoir in malaria elimination settings: mass drug administration (MDA), in which entire populations are treated with antimalarial drugs; and targeted approaches, which aim to focus on “high risk” subgroups. MDA requires substantial resources and intensive community participation strategies to be effective [7–10]. MDA also raises ethical concerns in settings where the vast majority of individuals asked to comply with MDA are uninfected, and therefore unnecessarily exposed to drugs [11]. Alternatively, targeted approaches restrict screening and/or treatment to the population(s) most likely to be infected. Targeted strategies include reactive household-based screening and treatment [12] as well as proactive screening and treatment of at-risk populations [13]. The effectiveness of targeted strategies varies in different epidemiological contexts [14]. The parasite reservoir has also been indirectly targeted through widespread vector control and case detection and treatment, strategies that have historically supported malaria elimination in many countries [15].
“Hotspots” have also been proposed as a unit for targeted intervention [16]. Hotspots are geographical areas of higher-than-expected infection prevalence compared to surrounding areas and can be defined at various spatial scales, from the provincial to subvillage levels [16]. Hotspots may contribute to maintaining the parasite reservoir by individuals residing in hotspots harboring low-density infections that seed ongoing transmission [16]. However, evidence for the utility of hotspots as an intervention target remains scant; the first trial of its kind showed no effectiveness of hotspot targeting in Kenya [17]. In general, the apparent stability of hotspots varies according to the spatial scale at which they are defined; spatially and temporally stable hotspots at the village or district level have been reported in sub-Saharan African (SSA) [18–20], South Asian [21], and East Asian [22–24] settings. However, there was no consistent evidence for temporal stability of hotspots across sites in SSA [25]. Evidence of stable malaria hotspots in the Greater Mekong Subregion (GMS) that would support hotspot targeting into malaria elimination programs at the subvillage level is limited, despite this region having high heterogeneity of malaria risk [26], and being an international priority region for malaria elimination due to the emergence and spread of artemisinin-resistant Plasmodium falciparum malaria [27]. A previous study in 3 villages in western Cambodia found only transient spatial clustering of Plasmodium infections at the subvillage level, which the authors partly attributed to transmission occurring mostly away from the village [28]. Other studies in the GMS have either investigated spatial clustering at one time point only [29], or pooled cases from multiple time points into a single analysis [30].
Defining hotspots does pose challenges. Hotspots have mostly been defined based on static house locations in SSA where transmission mostly occurs indoors and at night. This does not reflect the malaria ecology in the GMS, where outdoor evening vector biting predominates, populations frequently move between their houses in the village and at farms, and people may congregate in areas other than their own houses for social events at peak evening biting times [31]. The effect of local-scale mobility on malaria risk has previously been analyzed in terms of spatial clustering of malaria risk at different residence locations [29]. However, this fails to capture effects of individuals from different households regularly spending time together in locations other than their own residences, such as outdoor evening social gatherings at which individuals from multiple households convene [32]. Any effect of this type of local-scale mobility would imply that spatial clustering of malaria would be more accurately detected based on clusters of people who frequently spend time together in the same locations, not only residence location.
To address these research gaps, this study aims to assess the stability of hotspots over 2 dry seasons in 3 villages in eastern Cambodia, and analyze whether incorporating fine-scale mobility data could improve identification and characterization of malaria hotspots.
METHODS
Study Design and Setting
A 2-year repeated population-based survey was conducted in 3 villages (Chamkar San, Tun, Phi) in Ratanakiri Province, eastern Cambodia. The population mostly comprises ethnic minority (including Jarai, Kreung, and Tompuon) populations that practice slash-and-burn agriculture at forest farms and fields. There is a growing Khmer (ethnic majority) population as well as increasing participation in labor markets [33, 34]. Local vector species include Anopheles dirus, Anopheles minimus, and Anopheles barbirostris [35]. Malaria control activities include long-lasting insecticidal net distributions and free testing and treatment of malaria through the village malaria worker program and at district and provincial health centers.
The study comprised 2 blood screening surveys in the dry seasons of 2016 and 2017, and an individual cross-sectional survey at the midpoint between the 2 blood screening surveys, which included questionnaires on individual and household-level risk factors for Plasmodium infection, as well as an individual questionnaire about mobility and contact patterns with other individuals in the same village. Details of the surveys have been published previously [29].
Participants
All individuals residing in the 3 study villages were eligible to participate in the blood screening surveys. All resident individuals aged ≥12 years were eligible to respond to the mobility and contact pattern questionnaire.
Data Collection
Fingerprick blood samples were analyzed by species-specific polymerase chain reaction (PCR) for Plasmodium infection (P. falciparum, Plasmodium vivax, Plasmodium malariae, and Plasmodium ovale), as previously described [22]. Farm and village house locations were collected by Global Positioning System (GPS) as previously published [29]. Mobility data were collected by taking unique random samples of 10 individuals (“contacts”) from the study census lists of each village, and asking each respondent questions about the locations (their own house, in the village, at farms, in the forest) and frequency of meeting the contacts from their village. Prompts were used to assist interviewers and respondents to correctly identify the randomly selected contact, including the selected contact’s name, age, and sex, as well as the name of the contact’s household head and spouse of the household head.
Data Analysis
Spatial Analysis
The primary outcome was the proportion of household members who were Plasmodium positive in each year. Moran I was used to test for global spatial autocorrelation in the distribution of Plasmodium infections in each village, separately for each year, each Plasmodium species and house location type, that is, village house, farm house, and self-reported main residence. For each village, year, and residence location, Moran I was calculated based on distance matrices for spatial adjacency between households, where a value of 1 was assigned to pairs of houses within a predefined distance cut-point, or 0 otherwise. Three distance cut-points were used (0.5 km, 1 km, and 2 km) to explore global spatial autocorrelation at different spatial scales. Local clustering detection was performed using Kulldorf spatial scan statistic implemented in SaTScan software [36]. Spatial analysis of the 2016 data has been published [29]; the analysis was repeated for the 2017 data.
The effects of individual Plasmodium infection, living in a household with at least one Plasmodium-infected individual, or living in a hotspot in 2016, on risk of Plasmodium infection in 2017 were investigated using binomial regression with a log-link function and robust variance estimates to account for within-household clustering.
Social Contact Analysis
For each respondent, “contact” was considered to occur if the respondent reported that they met the selected individual at least weekly. To test whether contact with a Plasmodium-infected individual increased risk of Plasmodium infection, a 2-level logistic regression model was fitted with the respondent’s Plasmodium infection status as the binary outcome, and the primary exposure was modeled as an interaction between the contact’s Plasmodium infection status and the occurrence of weekly contact, with secondary analyses for contact specifically at the respondents’ house(s) and at the farms and fields. Separate analyses were run for the contact’s individual infection status and a contact’s household infection status (ie, any household member who was Plasmodium positive). A random intercept was included for each respondent to account for the nested data structure of 10 contacts per respondent, with village adjusted for as a fixed effect. Additionally, Moran I was used to test for clustering of infection risk at household level, separately for each village and each year [37]. The cut-point of “at least weekly” contact was used to indicate regular co-location and thus shared risk of exposure to biting vectors. Other cut-points were also investigated, including any contact, monthly contact, and daily contact, but did not substantially change the results.
Regression modeling was conducted in Stata IC/13. Robust variance estimates were calculated using the vce (cluster) option in Stata. Moran I was calculated in R using the DCluster package. Maps were produced in R using Google Earth satellite images.
Ethical Analysis
Study protocols were approved by the institutional review board (IRB) of the National Ethical Committee on Health Research of Cambodia (approval number 309NECHR), the IRB of the Institute of Tropical Medicine Antwerp (approval number IRB/AB/ac/119), and the Ethical Committee of the University of Antwerp (approval number B300201525582). Written informed consent was obtained to participate in the malariometric survey. Verbal informed consent was obtained to additionally participate in the social contact survey. Minors assented to participate with the informed consent and in the presence of their parent or guardian. All individuals were free to refuse or withdraw participation at any time.
RESULTS
Participants
The study population comprised 1792 individuals in 343 households in 2016, of whom 85.9% had blood samples collected [29] and in 2017, 1117 individuals were reached in 269 households, of whom Plasmodium infection data were available for 1102 individuals (Table 1). More than 50% of the population was aged <20 years in both years. There were fewer individuals who resided primarily at their farm houses in 2017 (22%) compared to 2016 (44.6%), reflecting incomplete sampling of the resident population in 2017 (Table 1).
Table 1.
Demographic Characteristics and Prevalence of Any Plasmodium Infection in the Study Population in 2016 and 2017
| Characteristic | 2016a | 2017b | ||||||
|---|---|---|---|---|---|---|---|---|
| Population | Plasmodium Positive | Population | Plasmodium Positive | |||||
| Village | ||||||||
| Chamkar San | 619 | (34.54) | 31 | (5.79) | 446 | (39.93) | 12 | (2.69) |
| Phi | 717 | (40.01) | 53 | (8.98) | 289 | (25.87) | 11 | (3.94) |
| Tun | 456 | (25.45) | 45 | (10.90) | 382 | (34.20) | 17 | (4.51) |
| Age group, y | ||||||||
| 0–4 | 285 | (15.98) | 10 | (4.07) | 154 | (13.79) | 0 | (0.00) |
| 5–9 | 254 | (14.24) | 18 | (7.93) | 182 | (16.29) | 4 | (2.22) |
| 10–14 | 230 | (12.89) | 25 | (11.85) | 174 | (15.58) | 11 | (6.32) |
| 15–19 | 214 | (12.00) | 20 | (11.83) | 124 | (11.10) | 5 | (4.03) |
| 20–29 | 299 | (16.76) | 19 | (7.42) | 178 | (15.94) | 3 | (1.74) |
| 30–39 | 211 | (11.83) | 14 | (7.87) | 123 | (11.01) | 4 | (3.31) |
| 40–49 | 138 | (7.74) | 7 | (5.93) | 69 | (6.18) | 5 | (7.35) |
| ≥50 | 153 | (8.58) | 16 | (12.50) | 113 | (10.12) | 8 | (7.08) |
| Sex | ||||||||
| Male | 903 | (50.79) | 68 | (8.85) | 552 | (49.42) | 25 | (4.60) |
| Female | 875 | (49.21) | 61 | (8.05) | 565 | (50.58) | 15 | (2.69) |
| Main residence | ||||||||
| Village | 977 | (55.39) | 57 | (6.84) | 868 | (77.99) | 22 | (2.56) |
| Farm | 787 | (44.61) | 72 | (10.53) | 245 | (22.01) | 18 | (7.59) |
Data are presented as No. (%).
aThe population in 2016 reflects the resident population that was included in the census in January 2016, of whom 85.9% had blood collected for malaria testing.
bThe population in 2017 reflects the population that was reached in the follow-up survey, of whom 1102 (98.7%) had blood collected for malaria testing. Of the 1792 individuals in 2016, 1012 were reached again in 2017, and 105 new individuals were included who were not in the census in 2016, including births and migrants.
The overall prevalence of malaria in the 3 villages was 3.6% in 2017 and 8.4% in 2016 (Table 2). Plasmodium vivax monoinfections accounted for the majority of infections in both surveys (Table 2). Plasmodium malariae was more common than P. falciparum in Chamkar San and Phi, but was absent in Tun. Mixed infections accounted for 9.3% of infections (0.8% population prevalence) in 2016 and 7.5% of infections (0.3% population prevalence) in 2017.
Table 2.
Plasmodium Infection by Village, Species, and Year
| Plasmodium Species | Plasmodium Infection | |||
|---|---|---|---|---|
| 2016 | 2017 | |||
| Overall | ||||
| All species | 129 | (8.4) | 40 | (3.6) |
| P. falciparum | 16 | (1.0) | 4 | (0.4) |
| P. vivax | 75 | (4.9) | 26 | (2.4) |
| P. malariae | 26 | (1.7) | 7 | (0.6) |
| Mixed | 12 | (0.8) | 3 | (0.3) |
| Chamkar San | ||||
| All species | 31 | (5.8) | 12 | (2.7) |
| P. falciparum | 2 | (0.4) | 3 | (0.7) |
| P. vivax | 18 | (3.4) | 3 | (0.7) |
| P. malariae | 6 | (1.1) | 4 | (0.9) |
| Mixed | 5 | (0.9) | 2 | (0.4) |
| Phi | ||||
| All species | 53 | (9.0) | 11 | (3.9) |
| P. falciparum | 9 | (1.5) | 1 | (0.4) |
| P. vivax | 21 | (3.6) | 6 | (2.2) |
| P. malariae | 20 | (3.4) | 3 | (1.1) |
| Mixed | 3 | (0.5) | 1 | (0.4) |
| Tun | ||||
| All species | 45 | (10.9) | 17 | (4.5) |
| P. falciparum | 5 | (1.2) | 0 | (0) |
| P. vivax | 36 | (8.7) | 17 | (4.5) |
| P. malariae | 0 | (0) | 0 | (0) |
| Mixed | 4 | (1.0) | 0 | (0) |
Data are presented as No. (%). Missing data were as follows: Chamkar San, n = 86 (13.9%) in 2016, n = 135 (23.3%) in 2017; Phi, n = 127 (17.7%) in 2016, n = 469 (62.7%) in 2017; Tun, n = 44 (9.7%) in 2016, n = 40 (9.6%) in 2017.
At the individual level, among participants for whom data was available for both years, 25% (n = 19) of those who were Plasmodium positive in 2016 were Plasmodium positive in 2017, mostly individuals who had P. vivax infections in both years (Supplementary Table 1). Conversely, 51% of individuals who were Plasmodium positive in 2017 were Plasmodium negative in 2016, mostly with newly detected P. vivax infections (38%) (Supplementary Table 1).
At the household level, there were 217 (13.9%) individuals living in 35 households in which at least one person with Plasmodium infection was detected in 2017, compared to 679 (37.4%) individuals living in 101 households with at least one person with Plasmodium infection in 2016, including 25 households that had Plasmodium-positive members in both years.
Spatial Analyses
Several species-specific hotspots and coldspots of Plasmodium infection were detected in 2016 [29]. In 2017, only one statistically significant hotspot was detected, which was a cluster of 13 households in an area of radius 0.53 km based on village residence, in which 6 Plasmodium infections were detected (Supplementary Table 2). This hotspot had approximately the same location as a Plasmodium species hotspot detected in 2016, but in 2016 the hotspot had an 8-fold smaller radius (0.064 km). No Plasmodium species–specific hotspots were detected at P < .15 (Supplementary Table 2). There was no evidence for global spatial autocorrelation in the distribution of Plasmodium infections in either year (Supplementary Table 3).
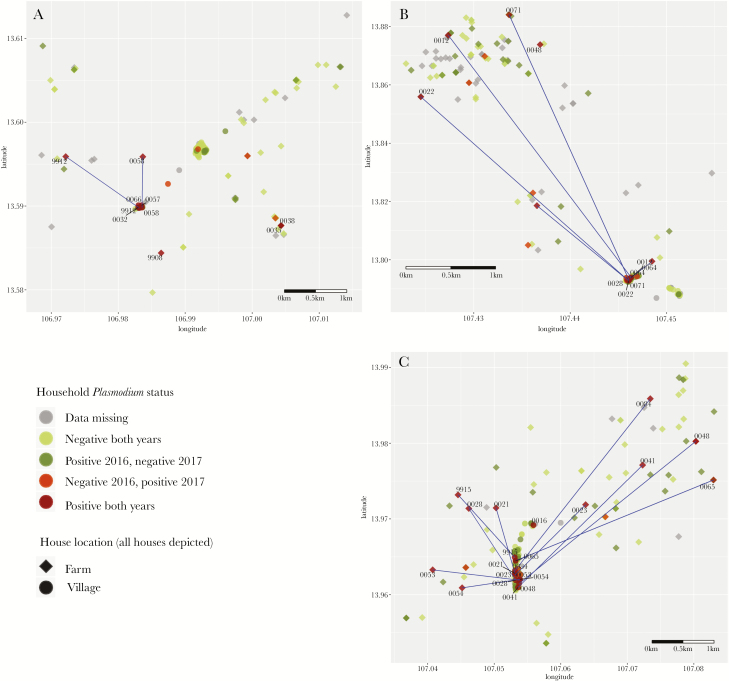
Descriptively, the spatial distribution of Plasmodium infections in 2016 and 2017 combined was scattered, and there was no overt clustering of households that had Plasmodium-positive members in both years (Figure 1). Of the 40 Plasmodium infections detected in 2017, 22 (55%) were in households identified as being within hotspots in 2016, compared to 30 (75%) in households that had at least 1 Plasmodium-infected individual in 2016. There were 9 households that had a Plasmodium-positive member in both years but were not located in a hotspot in 2016. There were 10 individuals in 10 households who were Plasmodium positive in 2017 who were not living in Plasmodium-positive households in 2016, of whom 5 were located inside hotspot areas and 5 outside hotspot areas.
Figure 1.
Distribution of Plasmodium infections at the household level in 2016 and 2017, in Chamkar San (A), Phi (B), and Tun (C), Cambodia.
After adjusting for individual and household infection status, living in a hotspot in 2016 did not predict Plasmodium risk at the individual level (Risk Ratio [RR], 1.43 [95% confidence interval {CI}, .67–3.05], P = .36) or household level (RR, 1.81 [95% CI, .85–3.85], P = .13) overall (Table 3). However, living in a hotspot in 2016 was a risk factor for living in a Plasmodium-positive household in Chamkar San in 2017 (RR, 3.45 [95% CI, 1.18–10.08], P = .024). In Chamkar San and Phi, individual infection in 2016 was the strongest predictor of individual infection risk in 2017, but in Tun, household-level (RR, 4.29 [95% CI, 1.04–17.64], P = .04) but not individual-level (RR, 2.51 [95% CI, .76–8.27], P = .13) infection in 2016 predicted individual-level infection risk in 2017. Living in a Plasmodium-positive household in 2016 was the strongest predictor of living in a Plasmodium-positive household in 2017 overall (RR, 5.00 [95% CI, 2.09–11.96], P < .0001) and was more than twice the magnitude of the hotspot effect in Chamkar San and Tun. There were no significant predictors of 2017 household-level Plasmodium status in Phi (Table 3).
Table 3.
Determinants of Plasmodium Infection Status at Individual and Household Level, 2017
| 2016 Status | 2017 Plasmodium-Positive Individual | 2017 Plasmodium-Positive Household | ||||
|---|---|---|---|---|---|---|
| RR | (95% CI) | P Value | RR | (95% CI) | P Value | |
| All villages | ||||||
| Individual Plasmodium negative | Ref. | … | Ref. | … | ||
| Individual Plasmodium positive | 6.46 | (2.85–14.65) | < .0001 | 1.12 | (.86–1.46) | .39 |
| Household Plasmodium negative | Ref. | … | Ref. | … | ||
| Household Plasmodium positive | 2.00 | (.73–5.50) | .18 | 5.00 | (2.09–11.96) | < .0001 |
| Outside hotspot | Ref. | … | Ref. | … | ||
| Inside hotspot | 1.43 | (.67–3.05) | .36 | 1.81 | (.85–3.85) | .13 |
| Chamkar San | ||||||
| Individual Plasmodium negative | Ref. | … | Ref. | … | ||
| Individual Plasmodium positive | 13.90 | (3.40–56.90) | < .0001 | 0.89 | (.71–1.11) | .30 |
| Household Plasmodium negative | Ref. | … | Ref. | … | ||
| Household Plasmodium positive | 1.95 | (.26–14.15) | .51 | 8.95 | (2.55–31.36) | .001 |
| Outside hotspot | Ref. | … | Ref. | … | ||
| Inside hotspot | 1.81 | (.55–5.90) | .33 | 3.45 | (1.18–10.08) | .024 |
| Phi | ||||||
| Individual Plasmodium negative | Ref. | … | Ref. | … | ||
| Individual Plasmodium positive | 21.75 | (2.84–166.61) | .003 | 0.96 | (.56–1.66) | .89 |
| Household Plasmodium negative | Ref. | … | Ref. | … | ||
| Household Plasmodium positive | 0.30 | (.04–2.55) | .27 | 2.81 | (.50–15.88) | .24 |
| Outside hotspot | Ref. | … | Ref. | … | ||
| Inside hotspot | 1.16 | (.23–5.83) | .85 | 0.83 | (.16–4.42) | .83 |
| Tun | ||||||
| Individual Plasmodium negative | Ref. | … | Ref. | … | ||
| Individual Plasmodium positive | 2.51 | (.76–8.27) | .13 | 1.24 | (.88–1.74) | .21 |
| Household Plasmodium negative | Ref. | … | Ref. | … | ||
| Household Plasmodium positive | 4.29 | (1.04–17.65) | .044 | 5.50 | (1.32–22.84) | .019 |
| Outside hotspot | Ref. | … | Ref. | … | ||
| Inside hotspot | 1.52 | (.48–4.84) | .47 | 2.36 | (.81–6.85) | .12 |
Abbreviations: CI, confidence interval; RR, Risk Ratio.
Social Contact Analyses
Social contact data were available for 712 respondents aged 12 years in 267 households, who were asked about 1445 unique contacts in 349 households, representing 97% of households in the study. Each respondent reported knowing a median of 7 of 10 contacts (interquartile range [IQR], 0.5–0.9), and met with a median of 4 of 10 contacts (IQR, 2–6) on at least a weekly basis. A median of 1 in 10 weekly contacts (IQR, 0–2) occurred at the respondent’s house(s), and a median of 1 (IQR, 0–3) at the farms and fields. There was some variation in contact patterns between villages, with more weekly contacts in Tun (median, 6 [IQR, 4–8]) than in Phi (median, 3 [IQR, 2–6]) or Chamkar San (median, 3 [IQR, 1–5]).
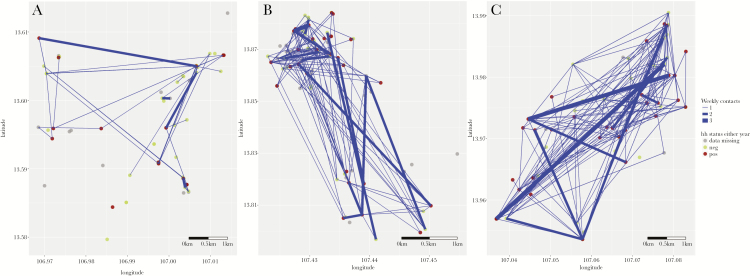
When aggregated at household level, there was considerable contact between members from different households (Figure 2). Overall, there was no evidence for an effect of weekly contact with Plasmodium-infected individuals (model A1, Table 4), nor when restricting to contact that occurred at the respondent’s house(s) or at the farms and fields (models A2 and A3, Table 4). There was no evidence for household-level increased Plasmodium infection risk due to contact with Plasmodium-positive households in the regression analyses (models B1, B2, and B3, Table 4). When analyzing clustering of Plasmodium infection risk among socially connected households, there was no evidence of social clustering in Chamkar San or Phi. In Tun, there was evidence of social clustering of Plasmodium infection in 2016, but not 2017 (Table 5).
Figure 2.
Spatial location, contact patterns, and Plasmodium status at the household level, in Chamkar San (A), Phi (B), and Tun (C), Cambodia. Figure shows weekly contacts that occur between individuals in different houses that were reported to occur at either village or farm houses. Farm house locations only are displayed. Blue lines indicate at least weekly contact between members of 2 different households; lines are weighted by number of contacts between 2 households. Abbreviations: hh, household; neg, negative; pos, positive.
Table 4.
Association Between Respondent Plasmodium Infection and at Least Weekly Contact With Plasmodium-Infected Individual (Model A), and Respondent’s Household Plasmodium Infection Status and at Least Weekly Contact With Any Member of a Plasmodium-Positive Household (Model B), by Location of Contact
| Individual Contact Models | ORa | (95% CI) | P Value | Household Contact Models | ORa | (95% CI) | P Value |
|---|---|---|---|---|---|---|---|
| A1. Contact’s Plasmodium status, weekly contact | B1. Contact’s household status, weekly contact | ||||||
| Plasmodium negative, <weekly contact | Ref | … | HH Plasmodium negative, <weekly contact | Ref | … | ||
| Plasmodium negative, ≥weekly contact | 1.16 | (.39–3.43) | .79 | HH Plasmodium negative, ≥weekly contact | 1.28 | (.34–4.8) | .72 |
| Plasmodium positive, <weekly contact | 1.05 | (.14–7.73) | .96 | HH Plasmodium positive, <weekly contact | 1.01 | (.26–4.01) | .99 |
| Plasmodium positive, ≥weekly contact | 1.06 | (.15–7.38) | .95 | HH Plasmodium positive, ≥weekly contact | 1.36 | (.35–5.36) | .66 |
| A2. Contact’s Plasmodium status, weekly contact at respondent’s household | B2. Contact’s household status, weekly contact at respondent’s household | ||||||
| Plasmodium negative, <weekly hh contact | Ref | … | HH Plasmodium negative, <weekly hh contact | Ref | … | ||
| Plasmodium negative, ≥weekly hh contact | 0.85 | (.19–3.82) | .84 | HH Plasmodium negative, ≥weekly hh contact | 0.77 | (.14–4.33) | .77 |
| Plasmodium positive, <weekly hh contact | 0.96 | (.22–4.06) | .95 | HH Plasmodium positive, <weekly hh contact | 0.97 | (.42–2.25) | .95 |
| Plasmodium positive, ≥weekly hh contact | 1.06 | (.03–35.62) | .97 | HH Plasmodium positive, ≥weekly hh contact | 0.94 | (.15–5.86) | .95 |
| A3. Contact’s Plasmodium status, weekly contact at farms/fields | B3. Contact’s household status, weekly contact at farms/fields | ||||||
| Plasmodium negative, <weekly ff contact | Ref | … | HH Plasmodium negative, <weekly ff contact | Ref | … | ||
| Plasmodium negative, ≥weekly ff contact | 1.38 | (.42–4.53) | .59 | HH Plasmodium negative, ≥weekly ff contact | 1.23 | (.28–5.49) | .79 |
| Plasmodium positive, <weekly ff contact | 0.97 | (.23–4.04) | .97 | HH Plasmodium positive, <weekly ff contact | 0.95 | (.37–2.47) | .92 |
| Plasmodium positive, ≥weekly ff contact | 1.18 | (.07–19.15) | .91 | HH Plasmodium positive, ≥weekly ff contact | 1.47 | (.32–6.73) | .62 |
Abbreviations: CI, confidence interval; ff, farm and field; hh, respondent’s household; HH, contact’s household; OR, odds ratio.
aORs adjusted for village to control for differences in malaria prevalence between the 3 villages. Effect of social contact with a Plasmodium-infected individual on respondent’s risk of Plasmodium infection should be apparent as an OR significantly greater than 1 for exposure to a Plasmodium-positive individual with whom contact occurs at least weekly. All other ORs should approximately equal 1.
Table 5.
Autocorrelation Statistics for Clustering of Plasmodium Species Infections in Socially Connected Households
| Location | P Values for Social Contact, Moran I | |
|---|---|---|
| 2016 | 2017 | |
| Chamkar San | .41 | .88 |
| Phi | .40 | .85 |
| Tun | .001 | .52 |
DISCUSSION
This study combined spatial and social contact patterns to investigate fine-scale heterogeneity in malaria risk at the subvillage level. This study found minimal evidence of stable spatial clustering of Plasmodium infection in “hotspots” over 2 dry seasons in 3 villages in Ratanakiri Province, eastern Cambodia, and there was no evidence that incorporating social contact patterns, which could indicate shared exposures, improves understanding of clustering of malaria risk at the subvillage level. A salient finding is that in the second year of the study, when Plasmodium infection prevalence halved, the spatial distribution of infections was more scattered, and hotspots became less apparent than in the first year. Other authors have cautioned that statistical power for cluster detection is weak in low-prevalence settings, and thus recommended a low threshold for acting on any apparent clustering despite lack of clear statistical evidence [25]. However, this presumes that apparent spatial clustering of malaria cases is a meaningful intervention target, even in the absence of statistical evidence [16, 38]. While the temporal stability of hotspots has been observed at larger spatial scales in SSA settings [20], the findings of our study do not support the spatial focalization of malaria risk to subvillage level in this low-prevalence setting. Compared to SSA settings, the lack of stable hotspots in general may be explained by differences in vector ecology or other environmental characteristics. Despite the negative findings of the social contact analysis, there may be an influence of small-scale human mobility on hotspot instability given that vector exposure is linked to early evening outdoor activities conducted in the vicinity of houses [35]; any shifts in the composition of groups of people who regularly spend time in the same outdoor locations in the evenings may explain the lack of temporal stability of hotspots.
These findings have implications for the key operational research question of whether the parasite reservoir can be identified and targeted at the village and subvillage levels. Malaria risk did appear to become more focal over time, but clustering occurred within households, not hotspots, with 75% of Plasmodium infections detected in the second year of the study arising in households in which at least one infection was detected in the first year. This is supported by a previous analysis on the same population showing that the strongest determinants of Plasmodium infection were household characteristics, rather than individual or village characteristics [39], and a study in low-transmission settings in Uganda that also indicated the importance of household-level heterogeneity in malaria risk [40]. A well-implemented household-based strategy based on active case detection and presumptive treatment of all household members, coupled with sustained and improved provision of vector control tools especially for higher-risk households, may achieve further reductions and even local interruption of transmission, and may be more feasible to sustain than MDA. Though households appear to be a relatively stable unit of clustering of infection, we are unable to directly infer whether clustering occurs due to indoor transmission within houses, or is due to shared evening activities or other exposures among household members.
There are several limitations to this study. This analysis was based on infections detected by PCR only. Serology markers may provide more stable measures of hotspots in endemic settings [41], though the utility of serology for estimating exposure in settings with rapidly declining transmission is unclear. A possible explanation for the lack of spatial or social contact-related clustering of malaria risk is that the extent to which asymptomatic infections contribute to transmission remains unclear [5]. The spatial and social contact patterns of the proportionally few symptomatic infections may be more important for understanding the distribution of the parasite reservoir. Additionally, the spatial analyses were based on Euclidean distances and did not take local topographical features into account, which may have affected vector dispersion. As most infections were P. vivax infections, for which it was not possible to separate new infections from relapsing infections, and as a considerable proportion of infections were due to P. malariae, which can persist for several years, the lack of detection of social or spatial clustering may also relate to the elapsed time since infections were acquired. The response rate in the second year of the survey was lower than in the first year, substantially so in Phi. This was in large part caused by a low willingness of the inhabitants of Phi to participate in the second survey. The data from Phi are included for completeness but only the findings from Chamkar San and Tun should be considered reliable. The small number of infections detected in the second year, particularly for P. falciparum, precluded species-specific statistical analyses. The decline in prevalence between the first and second year remains unexplained. As per national guidelines, no treatment was given for PCR-positive, rapid diagnostic test–negative (asymptomatic) malaria detected in the first survey. No change in the provision of malaria testing or treatment, or general health services occurred during the study period that could account for the observed change in prevalence. Finally, as there was no temporal dimension to the social contact data, it was not possible to directly investigate whether spending time with a Plasmodium-infected individual increased the risk of a new infection. More comprehensive assessment of fine-scale mobility patterns could be conducted with GPS tracking or comprehensive mobility diaries, but either approach was logistically too intensive to administer in the scope of this study.
CONCLUSIONS
In this study in a low-transmission setting in eastern Cambodia, there was no clear evidence for clustering of malaria risk based on spatial location of residence or social contact patterns that could be used to identify the malaria parasite reservoir. Instead, Plasmodium infection clustered most strongly within households. Household-based malaria prevention, including vector control as well as diagnosis and treatment interventions, should be prioritized as part of regional malaria control and elimination strategies.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We gratefully acknowledge the support of several colleagues who contributed to this study, including Ren Sophorn, Polin Sroy, Meas Rith, Myrthe Pareyn, Nimol Khim, and Mathilda Mushiete, for assisting with data collection and/or blood sample processing. We acknowledge the support of the Belgian cooperation (Directorate-General for Development Co-operation) for the institutional collaboration between National Center for Parasitology, Entomology and Malaria Control, Phnom Penh, Cambodia and the Institute of Tropical Medicine, Belgium.
Disclaimer. The funding sources were not involved in the design, implementation, or interpretation of the results of the study.
Financial support. This work was supported by the Department of Economy, Science and Innovation of the Flemish government. M. B. T. was supported by a 2014 Erasmus Mundus Joint Doctorate Fellowship (specific grant agreement 2014-0681), and by a Les Amis des Instituts Pasteur à Bruxelles 2017 research fellowship.
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
REFERENCES
- 1. Lin JT, Saunders DL, Meshnick SR. The role of submicroscopic parasitemia in malaria transmission: what is the evidence? Trends Parasitol 2014; 30:183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sturrock HJ, Hsiang MS, Cohen JM, et al. Targeting asymptomatic malaria infections: active surveillance in control and elimination. PLoS Med 2013; 10:e1001467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vinetz JM, Gilman RH. Asymptomatic Plasmodium parasitemia and the ecology of malaria transmission. Am J Trop Med Hyg 2002; 66:639–40. [DOI] [PubMed] [Google Scholar]
- 4. Canier L, Khim N, Kim S, et al. An innovative tool for moving malaria PCR detection of parasite reservoir into the field. Malar J 2013; 12:405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vantaux A, Samreth R, Piv E, et al. Contribution to malaria transmission of symptomatic and asymptomatic parasite carriers in Cambodia. J Infect Dis 2018; 217:1561–8. [DOI] [PubMed] [Google Scholar]
- 6. Bousema T, Okell L, Felger I, Drakeley C. Asymptomatic malaria infections: detectability, transmissibility and public health relevance. Nat Rev Microbiol 2014; 12:833–40. [DOI] [PubMed] [Google Scholar]
- 7. Tripura R, Peto TJ, Chea N, et al. A controlled trial of mass drug administration to interrupt transmission of multidrug-resistant falciparum malaria in Cambodian villages. Clin Infect Dis 2018; 67:817–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Landier J, Kajeechiwa L, Thwin MM, et al. Safety and effectiveness of mass drug administration to accelerate elimination of artemisinin-resistant falciparum malaria: a pilot trial in four villages of eastern Myanmar. Wellcome Open Res 2017; 2:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Newby G, Hwang J, Koita K, et al. Review of mass drug administration for malaria and its operational challenges. Am J Trop Med Hyg 2015; 93:125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Poirot E, Skarbinski J, Sinclair D, Kachur SP, Slutsker L, Hwang J. Mass drug administration for malaria. Cochrane Database Syst Rev 2013: 1–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cheah PY, White NJ. Antimalarial mass drug administration: ethical considerations. Intern Health 2016; 8:235–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gueye CS, Sanders KC, Galappaththy GN, et al. Active case detection for malaria elimination: a survey among Asia Pacific countries. Malar J 2013; 12:358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rossi G, Vernaeve L, Van den Bergh R, et al. Closing in on the reservoir: proactive case detection in high-risk groups as a strategy to detect Plasmodium falciparum asymptomatic carriers in Cambodia. Clin Infect Dis 2018; 66:1610–7. [DOI] [PubMed] [Google Scholar]
- 14. Hustedt J, Canavati SE, Rang C, et al. Reactive case-detection of malaria in Pailin Province, western Cambodia: lessons from a year-long evaluation in a pre-elimination setting. Malar J 2016; 15:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moonen B, Cohen JM, Snow RW, Slutsker L, Drakeley C, Smith DL. Operational strategies to achieve and maintain malaria elimination. Lancet 2010; 376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bousema T, Griffin JT, Sauerwein RW, et al. Hitting hotspots: spatial targeting of malaria for control and elimination. PLoS Med 2012; 9:e1001165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bousema T, Stresman G, Baidjoe AY, Bradley J, Knight P, Stone W. The impact of hotspot-targeted interventions on malaria transmission in Rachuonyo South District in the western Kenyan highlands: a cluster-randomized controlled trial. PLoS Med 2016; 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yeshiwondim AK, Gopal S, Hailemariam AT, Dengela DO, Patel HP. Spatial analysis of malaria incidence at the village level in areas with unstable transmission in Ethiopia. Int J Health Geogr 2009; 8:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ernst KC, Adoka SO, Kowuor DO, Wilson ML, John CC. Malaria hotspot areas in a highland Kenya site are consistent in epidemic and non-epidemic years and are associated with ecological factors. Malar J 2006; 5:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bejon P, Williams TN, Nyundo C, et al. A micro-epidemiological analysis of febrile malaria in coastal Kenya showing hotspots within hotspots. eLife 2014; 3: e02130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ahmed S, Galagan S, Scobie H, et al. Malaria hotspots drive hypoendemic transmission in the Chittagong Hill Districts of Bangladesh. PLoS One 2013; 8:e69713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sluydts V, Heng S, Coosemans M, et al. Spatial clustering and risk factors of malaria infections in Ratanakiri Province, Cambodia. Malar J 2014; 13:387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Heng S, Durnez L, Mao S, et al. Passive case detection of malaria in Ratanakiri Province (Cambodia) to detect villages at higher risk for malaria. Malar J 2017; 16:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hui F- M, Xu B, Chen Z- W, et al. Spatio-temporal distribution of malaria in Yunnan Province, China. Am J Trop Med Hyg 2009; 81:503–9. [PubMed] [Google Scholar]
- 25. Mogeni P, Omedo I, Nyundo C, Kamau A, Noor A, Bejon P. Effect of transmission intensity on hotspots and micro-epidemiology of malaria in sub-Saharan Africa. BMC Medicine 2017; 15:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cui L, Yan G, Sattabongkot J, et al. Malaria in the Greater Mekong Subregion: heterogeneity and complexity. Acta Tropica 2012; 121:227– 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. World Health Organization. Strategy for malaria elimination in the Greater Mekong Subregion: 2015–2030. Manila: WHO Regional Office for the Western Pacific, 2015. [Google Scholar]
- 28. Parker DM, Tripura R, Peto TJ, et al. A multi-level spatial analysis of clinical malaria and subclinical Plasmodium infections in Pailin Province, Cambodia. Heliyon 2017; 3:e00447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Durnez L, Pareyn M, Mean V, et al. Identification and characterization of areas of high and low risk for asymptomatic malaria infections at sub-village level in Ratanakiri, Cambodia. Malar J 2018; 17:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hu Y, Zhou G, Ruan Y, et al. Seasonal dynamics and microgeographical spatial heterogeneity of malaria along the China-Myanmar border. Acta Trop 2016; 157:12–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Durnez L, Coosemans. M. Residual transmission of malaria: an old issue for new approaches. In: Manguin S, ed. Anopheles mosquitoes—new insights into malaria vectors. InTech 2013. doi:10.5772/3392. [Google Scholar]
- 32. Bannister-Tyrrell M, Xa NX, Kattenberg JH, et al. Micro-epidemiology of malaria in an elimination setting in central Vietnam. Malar J 2018; 17:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Peeters Grietens K, Gryseels C, Dierickx S, et al. Characterizing types of human mobility to inform differential and targeted malaria elimination strategies in northeast Cambodia. Sci Rep 2015; 5:16837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gryseels C, Peeters Grietens K, Dierickx S, et al. High mobility and low use of malaria preventive measures among the Jarai male youth along the Cambodia-Vietnam border. Am J Trop Med Hyg 2015; 93:810–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Durnez L, Mao S, Denis L, Roelants P, Sochantha T, Coosemans M. Outdoor malaria transmission in forested villages of Cambodia. Malar J 2013; 12:329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kulldorff M. SaTScan—software for the spatial, temporal, and space-time scan statistics. Boston, MA: Harvard Medical School and Harvard Pilgrim Health Care, 2010. [Google Scholar]
- 37. Emch M, Root ED, Giebultowicz S, Ali M, Perez-Heydrich C, Yunus M. Integration of spatial and social network analysis in disease transmission studies. Ann Am Assoc Geogr 2012; 105:1004–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cotter C, Sturrock HJ, Hsiang MS, et al. The changing epidemiology of malaria elimination: new strategies for new challenges. Lancet 2013; 382:900–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bannister-Tyrrell M, Srun S, Sluydts V, et al. Importance of household-level risk factors in explaining micro-epidemiology of asymptomatic malaria infections in Ratanakiri Province, Cambodia. Sci Rep 2018; 8:11643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mugenyi L, Abrams S, Hens N. Estimating age-time-dependent malaria force of infection accounting for unobserved heterogeneity. Epidemiol Infec 2017; 145:2545–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mosha JF, Sturrock HJ, Greenwood B, et al. Hot spot or not: a comparison of spatial statistical methods to predict prospective malaria infections. Malar J 2014; 13:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.