Abstract
We evaluated immune biomarker profiles in human immunodeficiency virus (HIV)–infected adults (n = 398) from 5 African countries. Although all biomarkers decreased after antiretroviral therapy (ART) initiation, levels of C-X-C chemokine ligand 10 (CXCL10), lipopolysaccharide-binding protein, C-reactive protein, soluble CD163, and soluble scavenger receptor CD14 were significantly higher during ART than in an HIV-uninfected reference group (n = 90), indicating persistent monocyte/macrophage activation, inflammation, and microbial translocation. Before ART initiation, high HIV viral load was associated with elevated CXCL10 and tuberculosis coinfection was associated with elevated soluble CD14. High pre-ART levels of each biomarker strongly predicted residual immune activation during ART. Chemokine (C-C motif) ligand 2, lipopolysaccharide-binding protein, C-reactive protein, and interleukin 6 were differentially expressed between countries. Further research is needed on the clinical implications of residual immune dysregulation.
Keywords: HIV, biomarkers, immune activation, inflammation, sub-Saharan Africa, antiretroviral therapy
In a cohort of HIV-1-infected adults, HIV-induced systemic immune activation was only partially mitigated during suppressive ART, with evidence of persistent inflammation, monocyte/macrophage activation and microbial translocation. Elevated plasma biomarkers before ART predicted residual immune dysregulation during ART.
The introduction of combination antiretroviral therapy (ART) has dramatically increased the life expectancy of persons living with human immunodeficiency virus (HIV). However, despite sustained ART-mediated suppression of viral replication, HIV-infected persons still have higher risks of serious non-AIDS diseases (eg, cardiovascular disease) and AIDS-defining cancers and higher all-cause mortality rates, compared with HIV-uninfected individuals [1]. Persistent immune dysregulation through systemic inflammation and immune activation is thought to be a key contributor, driven by multiple factors, including microbial translocation, residual low-level viral replication, and coinfections [1].
Evidence from Europe and North America suggests that suppressive ART may reduce some plasma biomarkers of HIV-related immune activation, such as interleukin 6 (IL-6), C-reactive protein (CRP), C-X-C chemokine ligand 10 (CXCL10), and soluble scavenger receptor CD14 (sCD14), but not necessarily to levels present in HIV-uninfected individuals, especially when ART is initiated during severe immunodeficiency (CD4+ T-cell count <200/μL) [2]. Data from sub-Saharan Africa, where ART is generally started late and pathogen exposure is high, are limited [3–5].
The current study sought to investigate (1) the biomarker profiles of HIV-related immune activation in a well-characterized multicountry African adult cohort with advanced HIV infection, (2) the extent to which fully suppressive ART mitigates immune activation, and (3) factors associated with residual immune activation.
METHODS
Study Population
Pan-African Studies to Evaluate Resistance Monitoring is a multicountry cohort of HIV-1–infected adults (aged ≥18 years) starting first-line ART (2007–2014), as profiled elsewhere [6]. For the present retrospective analysis, 398 participants were selected from 8 study sites in Kenya, Nigeria, South Africa, Uganda, and Zambia; they had an undetectable (<50 copies/mL) plasma viral load at month 12 and had paired cryopreserved plasma samples available before ART initiation and after 12 months of first-line nonnucleoside reverse-transcriptase inhibitor–based ART. Stored plasma samples from 90 HIV-uninfected blood bank donors from Nigeria, South, Africa, and Uganda served as a reference group. The protocol was approved by the research ethics committees at all collaborating sites. All participants provided written informed consent, including for sample storage for future testing.
Biomarker Assays
A panel of 8 plasma biomarkers was selected based on their known associations with diminished CD4+ T-cell restoration [7], HIV disease progression and/or non-AIDS disease [1]. Levels of sCD14 and soluble CD163 (sCD163), CRP, CXCL10, IL-6, chemokine (C-C motif) ligand 2 (CCL2), C-X-C chemokine ligand 9 (CXCL9) were measured using 2 Luminex assays (Luminex; R&D Systems) and analyzed using BioPlex 200 apparatus and software (Bio-Rad Laboratories). Lipopolysaccharide (LPS)–binding protein (LBP) levels were determined using a DuoSet enzyme-linked immunosorbent assay (R&D Systems). A single lot of assay kits was used, to eliminate interlot variability. All samples were tested in duplicate on the same plate to minimize variability.
Statistical Analysis
We compared biomarker values in the same HIV-infected participants before and during ART (percentage change), and in the HIV-uninfected reference group, using Wilcoxon signed rank and Mann-Whitney U tests. We used multivariable linear regression to compare biomarker values between the 3 groups and to explore factors associated with biomarkers before and during ART. Associations were expressed using coefficients (95% confidence intervals and P values) and partial eta squared (ηp2), to estimate small (0.01), medium (0.06) or large (0.14) effect sizes [8]. To explore biomarker differences between countries, we performed post hoc pairwise comparisons, adjusted for participant group, age, and sex, with Šidák correction for multiple testing, and hierarchical cluster analysis (heat map) (see Supplementary Data). For values below or above the assay detection limits we used the respective detection limit in the analyses. All analyses were performed using Stata 12 software (StataCorp).
RESULTS
Participant Characteristics
Of 398 HIV-infected participants, 57.5% were women, with a median (interquartile range [IQR]) age of 36 (32–42) years; 92 (23.1%) were from Kenya, 57 (14.3%) from Nigeria, 65 (16.3%) from South Africa, 121 (30.4%) from Uganda, and 63 (15.8%) from Zambia. The median (IQR) CD4+ T-cell count was 137/µL (75–205/µL) before ART and 291/µL (216–395/µL) after 12 months of ART. The median (IQR) pre-ART viral load was 5.0 (4.4–5.5) log10 copies/mL. At enrollment, 50 (12.6%) participants had active tuberculosis, and 29 (7.3%) were coinfected with hepatitis B virus. Of the 90 HIV-uninfected reference persons, 24.4% were women, with a median (IQR) age of 36 (30–44) years; 29 (32.2%) were from Nigeria, 30 (33.3%) from South Africa, and 31 (34.4%) from Uganda (Supplementary Table 1).
Biomarker Values
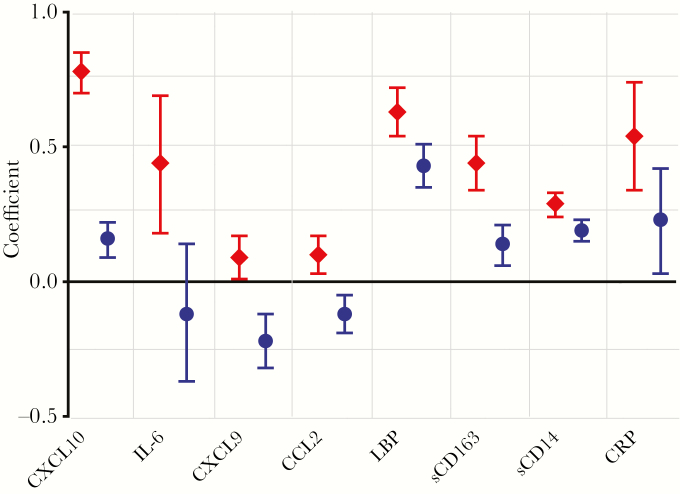
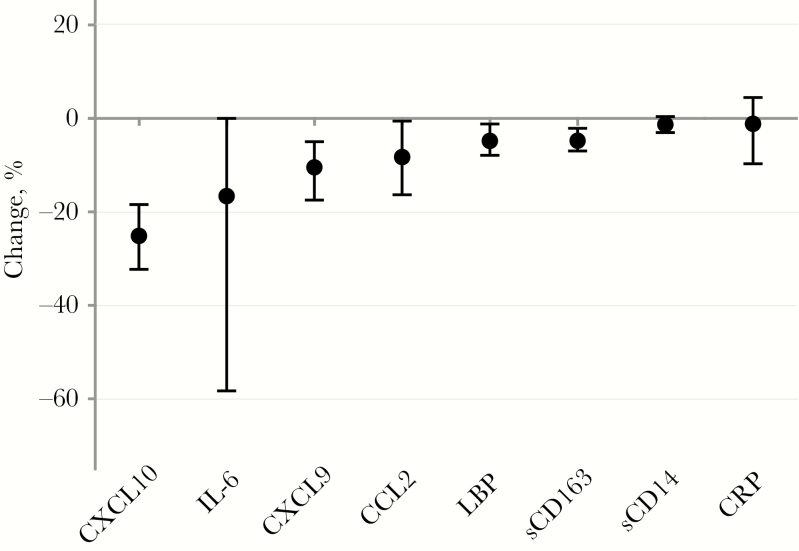
The levels of all 8 measured biomarkers were significantly higher in HIV-infected participants before ART than in the HIV-uninfected reference group, in unadjusted (Supplementary Figure 1 and Supplementary Table 2) and adjusted (Figure 1 and Supplementary Table 3) analyses. The median (IQR) decrease in biomarker value, from before to during ART, was greatest for CXCL10 (−25% [−32% to −18%]), followed by IL-6 (−17% [−58% to 0%]), CXCL9 (−10% [−17% to −5%]), CCL2 (−8% [−16% to −1%]), LBP (−5% [−8% to −1%]), sCD163 (−5% [−7% to −2%]), sCD14 (−1% [−3% to 0%]), and CRP (−1% [−10% to 4%) (Figure 2). These biomarker changes were all statistically significant (P < .001; Supplementary Table 2). In HIV-infected participants during ART, compared with the HIV-uninfected reference group, levels of CXCL10, LBP, CRP, sCD163, and sCD14 were significantly higher, levels of CXCL9 and CCL2 lower, and levels of IL-6 similar, in both unadjusted and adjusted analyses (Figure 1, Supplementary Figure 1, and Supplementary Table 2).
Figure 1.
Comparison of each of the immune biomarkers in human immunodeficiency virus (HIV)–infected participants before and during suppressive antiretroviral therapy (ART), relative to the HIV-uninfected reference group. Figure shows the multivariable linear regression analysis, expressed as regression coefficient (dots) with the 95% confidence limits (error bars), adjusted for sex, age, and country, with HIV-uninfected persons as the reference group. For each biomarker (x-axis), the regression coefficient is shown for HIV-infected participants before (diamonds) and during (dots) ART. Units of measure are as follows: log10 picograms per milliliter for C-X-C chemokine ligand 10 (CXCL10), interleukin 6 (IL-6), C-X-C chemokine ligand 9 (CXCL9), and chemokine (C-C motif) ligand 2 (CCL2); log10 nanograms per milliliter for lipopolysaccharide-binding protein (LBP), soluble CD163 (sCD163), and soluble CD14 (sCD14); and log10 milligrams per milliliter for C-reactive protein (CRP).
Figure 2.
Changes in biomarkers levels within the same human immunodeficiency virus–infected participants before and during suppressive antiretroviral therapy (ART), expressed as median percentage (dots) and interquartile range (error bars). Percentage change was calculated as follows: (Value during ART – Value before ART)/Value before ART × 100%. Abbreviations: CCL2, chemokine (C-C motif) ligand 2; CRP, C-reactive protein; CXCL9, C-X-C chemokine ligand 9; CXCL10, C-X-C chemokine ligand 10; IL-6, interleukin 6; LBP, lipopolysaccharide-binding protein; sCD14, soluble CD14; sCD163, soluble CD163.
The heat map suggested that CCL2 levels were elevated in participants from South Africa, and LBP levels elevated in participants from Zambia irrespective of HIV and ART status (both relative to the mean level) (Supplementary Figure 2). Post hoc pairwise comparisons found CCL2 levels to be higher in South Africa and LBP levels to be higher in Zambia than in the other countries, which was consistent with the heat map, CRP levels to be lower in Zambia than in Uganda and Nigeria, and IL-6 to be lower in South Africa than in Uganda, Nigeria and Kenya (all P < .001) (Supplementary Table 4).
Factors Associated With Residual Immune Activation During Suppressive ART
The set of independent variables tested explained 24%–50% of the variance of each of the biomarkers during ART (R2) (Supplementary Table 5). The pre-ART value of each biomarker was found to be strongly associated with the same biomarker during suppressive ART: IL-6 (coefficient 0.63 log10 pg/mL per unit increase; 95% confidence interval, .37–.88; P = .005), CXCL9 (0.55 log10 pg/mL per unit increase; .18–.92; P = .02), sCD14 (0.56 log10 ng/mL per unit increase; .14–.98; P = .02), LBP (0.53 log10 ng/mL per unit increase; .27–.78; P = .008), sCD163 (0.50 log10 ng/mL per unit increase; .28–.71; P = .006), CCL2 (0.36 log10 pg/mL per unit increase; .19–.52; P = .007), CRP (0.37 log10 mg/L per unit increase; .22–.55; P = .007), and CXCL10 (0.35 log10 pg/mL per unit increase; .31–.39; P < .001) (Supplementary Table 5). The effect size (ηp2) was estimated to be large for sCD163 (0.431), IL-6 (0.424), LBP (0.374), sCD14 (0.299), CXCL9 (0.213), CXCL10 (0.167), and CCL2 (0.151) and medium for CRP (0.117). The association was found to be statistically significant (P < .05) for some other factors, but all had small estimated effect sizes (ηp2 < 0.06) (Supplementary Tables 5 and 6).
Factors Associated With Immune Activation Before ART Initiation
The set of independent variables tested explained 4%–28% of the variance of each of the biomarkers before ART (R2) (Supplementary Table 7). High pre-ART viral load was associated with elevated CXCL10 levels (coefficient, 0.13 log10 pg/mL per unit increase; 95% CI, .06–.21; P = .01), with an estimated medium effect size (ηp2 = 0.107). Tuberculosis coinfection was associated with elevated sCD14 levels (coefficient, 0.11 log10 ng/mL per unit increase; 95% CI, .04–.18; P = .02), with an estimated medium effect size (ηp2 = 0.062). The association was found to be statistically significant (P < .05) for some other factors, but all had small estimated effect sizes (ηp2 < 0.06) (Supplementary Tables 7 and 8).
Discussion
In this large African HIV cohort, we found that all of the 8 measured plasma biomarkers were significantly elevated in untreated adults and decreased during ART-mediated viral suppression. Whereas 3 biomarkers, IL-6, CCL2, and CXCL9, normalized during suppressive ART, 5 biomarkers, CXCL10, LBP, CRP, sCD163, and sCD14, were still elevated, compared with the HIV-uninfected reference group. The pre-ART levels of each of the biomarkers were the strongest predictors of residual immune activation during ART. Before ART initiation, high viral load was associated with elevated CXCL10, and tuberculosis coinfection was associated with elevated sCD14. This study provides evidence of persistent monocyte/macrophage activation, inflammation, and microbial translocation in HIV-infected African adults during suppressive ART, extending data from Western countries. Residual immune dysregulation may have serious long-term health implications for the millions of Africans living with HIV, in terms of accelerated immune senescence, cardiovascular disease, and other serious non-AIDS diseases.
Residual CXCL10 elevation during ART could be driven by known or unmeasured coinfections, including tuberculosis, hepatitis C virus [9], and hepatitis B virus [10], and low-level HIV replication [11]. Increased plasma sCD14 and sCD163 levels, markers of monocyte activation, during durable control of viremia have been reported elsewhere [1]. LBP, known to accelerate binding of LPS, a cell wall component of gram-negative bacteria, to CD14 [12], was also elevated during ART. These findings concur with a previous study in South Africa that found a limited effect of ART on reducing sCD14, tumor necrosis factor and plasma LPS, suggesting that persistent microbial translocation is a major force driving chronic inflammation in HIV-infected Africans receiving ART [4]. Also in line with our findings, a recent study in Uganda found higher sCD14, intestinal fatty-acid binding protein and CRP levels in HIV-infected persons on ART, although, discordant with our study, they found women to have higher inflammation levels than men [5]. In addition, we found that active tuberculosis coinfection at ART initiation was associated with elevated pre-ART levels of sCD14, which was largely mitigated during ART, probably due to the effect of concomitant anti-tuberculosis treatment. This extends on earlier findings in South Africa where HIV-infected individuals with tuberculosis coinfection were found to have elevated monocyte activation (sCD14) and inflammation (CRP, IL-6, and CXCL10) [13].
CCL2, LBP, IL-6 and CRP were found to be differentially expressed in HIV-infected participants from the 5 countries during ART. Another recent study also found CCL2 and the interleukin 2 receptor alpha to be differentially expressed in HIV-infected participants across Uganda, Kenya, Tanzania, and Nigeria [14]. This suggests that differences between populations and geographic locations,, such as host genetics, environmental factors, smoking status and other lifestyle factors, coinfections, and hygiene standards, could play a major role in patterns of immune activation in sub-Saharan Africa.
The main strengths of the study were the measurement of a broad panel of the most clinically relevant immune biomarkers [1, 7] in a large cohort with broad geographic coverage, while mitigating the effect of HIV replication. There are several study limitations. First, our study was not able to fully assess some factors that could affect immune dysregulation, particularly coinfections (eg, cytomegalovirus, hepatitis C virus, and helminths), smoking status, and host genetics. Similarly, information on the HIV-uninfected blood donors was limited to age, sex, and country, with potential for uncontrolled confounding factors [15]. Second, we did not measure biomarkers beyond the first year of ART. However, studies have suggested that the main effect of ART on immune activation occurs in the first year, with possibly no further decrease during sustained viral suppression thereafter [2].
In conclusion, suppressive ART only partially mitigated HIV-related immune activation in African adults, with persistent monocyte/macrophage activation, inflammation, and microbial translocation. These findings provide further support to earlier ART initiation and also highlight the urgency for further research in African populations to elucidate the critical immune pathways and their clinical implications and to explore opportunities for adjunct host-directed therapies to improve long-term prognosis.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the study participants zand the staff at the collaborating clinical sites and reference laboratories. The Pan-African Studies to Evaluate Resistance (PASER) was part of the Linking African and Asian Societies for an Enhanced Response to HIV/AIDS program, a partnership of Stichting Aids Fonds, the Foundation for AIDS Research–TREAT Asia, PharmAccess Foundation, and International Civil Society Support.
Pan-African Studies to Evaluate Resistance Monitoring (PASER-M) collaborators. Collaborators included the following: Lusaka Trust Hospital (M. S.), Coptic Hospital (M. Labib), and KARA Clinic and Laboratory (J. Menke), Lusaka, Zambia; Muelmed Hospital, Pretoria (M. E. Botes [deceased], M. d. J.), Themba Lethu Clinic, Clinical HIV Research Unit (P. Ive, I. Sanne), and Department of Molecular Medicine and Haematology (E. Letsoalo, W. S. Stevens, K. Steegen), University of the Witwatersrand, Johannesburg, and Acts Clinic, White River (M. Hardman), South Africa; Newlands Clinic, Harare, Zimbabwe (M. Wellington, R. Luthy); Coast Province General Hospital, International Centre for Reproductive Health Kenya, Mombasa (K. M.), and Mater Misericordiae Hospital, Nairobi (M. Dolan), Kenya; Joint Clinical Research Centre, Fort Portal, Mbale and Kampala (C. M. K., S. Balinda, W. Namala, H. Namata, F. Senono, R. Nakanjako, M. Mutebi, I. Nankya, P. Mugyenyi), and Amsterdam Institute for Global Health and Development, Kampala (C. Nalubwama, H. Kakooza, M. Nakitto, M. O’Mello) Uganda; Lagos University Teaching Hospital, Nigeria (A. Osibogun, S. Akanmu, T. Adeyemo, T. Rodoye, H. Adelabu); and Department of Global Health, Amsterdam UMC of the University of Amsterdam, Amsterdam Institute for Global Health and Development, the Netherlands (R. L. H., K. C. E. Sigaloff, T. S. Boender, P. O., C. Manting-de Vries, N. Pakker, F. W. W., J. M. Lange [deceased], T. F. R. d. W.).
Author contributions. T. F. R.. d. W. is principal investigator of PASER-M, and R. L. H. is principal investigator of the Markers of Persistent immune ACTivation immune substudy. S. K. and R. L. H. conceived the immune substudy. C. M. K., M. S., S. A., K. M., M. d. J., T. F. R. d. W., and R. L. H. established the cohort and supervised data collection. S. K. and H. C. S. performed the laboratory testing, supervised by T. M. R. and N. K.; S. K. performed the statistical analyses, supervised by F. W. W. and R. L. H.; and S. K. and R. L. H. drafted the manuscript. All authors provided valuable input to interpretation of the data and critically reviewed the manuscript for important intellectual content. All authors reviewed and approved the final version of the manuscript.
Financial support. PASER is an initiative of the Amsterdam Institute for Global Health and Development, with major support provided by the Ministry of Foreign Affairs of the Netherlands through a partnership with Stichting Aids Fonds (grant 12454), and with additional support from De Grote Onderneming, the Embassy of the Kingdom of the Netherlands, Heineken Africa Foundation, Jura Foundation, and the Netherlands Organization for Scientific Research through the Netherlands-African Partnership for Capacity Development Clinical Interventions against Poverty-Related Diseases (NWO-WOTRO grants W07.10.101 and W07.10.106) and the Innovational Research Incentives Scheme Veni (grant 91615036 to R. L. H.).
Potential conflicts of interest. T. M. R. received travel support from Merck and payment for lectures from Merck and Abbott, all unrelated to the current work. P. R. through his institution has received independent scientific grant support from Gilead Sciences, Janssen Pharmaceuticals, Merck & Co, and ViiV Healthcare; he has served on scientific advisory boards for Gilead Sciences, ViiV Healthcare, Merck & Co, and Teva Pharmaceutical Industries and on a data safety monitoring committee for Janssen Pharmaceuticals, for which his institution has received remuneration, all unrelated to the current work. R. L. H. received research support from Gilead Sciences, unrelated to the current work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Utay NS, Hunt PW. Role of immune activation in progression to AIDS. Curr Opin HIV AIDS 2016; 11:131–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wada NI, Jacobson LP, Margolick JB, et al. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS 2015; 29:463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Malherbe G, Steel HC, Cassol S, et al. Circulating biomarkers of immune activation distinguish viral suppression from nonsuppression in HAART-treated patients with advanced HIV-1 subtype C infection. Mediators Inflamm 2014; 2014:198413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cassol E, Malfeld S, Mahasha P, et al. Persistent microbial translocation and immune activation in HIV-1-infected South Africans receiving combination antiretroviral therapy. J Infect Dis 2010; 202:723–33. [DOI] [PubMed] [Google Scholar]
- 5. Siedner MJ, Zanni M, Tracy RP, et al. Increased systemic inflammation and gut permeability among women with treated HIV infection in rural Uganda. J Infect Dis 2018; 218:922–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kroeze S, Ondoa P, Kityo CM, et al. Suboptimal immune recovery during antiretroviral therapy with sustained HIV suppression in sub-Saharan Africa. AIDS 2018; 32:1043–51. [DOI] [PubMed] [Google Scholar]
- 7. Liovat AS, Rey-Cuillé MA, Lécuroux C, et al. Acute plasma biomarkers of T cell activation set-point levels and of disease progression in HIV-1 infection. PLoS One 2012; 7:e46143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Erlbaum, 1988. http://www.utstat.toronto.edu/~brunner/oldclass/378f16/readings/CohenPower.pdf. Accessed 29 March 2019. [Google Scholar]
- 9. Zeremski M, Dimova R, Astemborski J, Thomas DL, Talal AH. CXCL9 and CXCL10 chemokines as predictors of liver fibrosis in a cohort of primarily African-American injection drug users with chronic hepatitis C. J Infect Dis 2011; 204:832–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhao K, Yang T, Sun M, et al. IP-10 Expression in patients with chronic HBV infection and its ability to predict the decrease in HBsAg levels after treatment with entecavir. Mol Cells 2017; 40:418–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pastor L, Casellas A, Rupérez M, et al. Interferon-γ-inducible protein 10 (IP-10) as a screening tool to optimize human immunodeficiency virus RNA monitoring in resource-limited settings. Clin Infect Dis 2017; 65:1670–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hailman E, Lichenstein HS, Wurfel MM, et al. Lipopolysaccharide (LPS)-binding protein accelerates the binding of LPS to CD14. J Exp Med 1994; 179:269–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sullivan ZA, Wong EB, Ndung’u T, Kasprowicz VO, Bishai WR. Latent and active tuberculosis infection increase immune activation in individuals co-infected with HIV. EBioMedicine 2015; 2:334–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Streeck H, Son G, Habermann D, et al. Immune activation parameters are differentially expressed across four countries in sub-Saharan Africa and are associated with comorbidities in HIV+ and HIV- individuals. In: The International AIDS Conference; Amsterdam, the Netherlands; 2018:WEPDA0104. [Google Scholar]
- 15. Aneke JC, Okocha CE. Blood transfusion safety: current status and challenges in Nigeria. Asian J Transfus Sci 2017; 11:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.