Abstract
Background
While the 2015–2016 Zika epidemics prompted accelerated vaccine development, decision makers need to know the potential economic value of vaccination strategies.
Methods
We developed models of Honduras, Brazil, and Puerto Rico, simulated targeting different populations for Zika vaccination (women of childbearing age, school-aged children, young adults, and everyone) and then introduced various Zika outbreaks. Sensitivity analyses varied vaccine characteristics.
Results
With a 2% attack rate ($5 vaccination), compared to no vaccination, vaccinating women of childbearing age cost $314–$1664 per case averted ($790–$4221/disability-adjusted life-year [DALY] averted) in Honduras, and saved $847–$1644/case averted in Brazil, and $3648–$4177/case averted in Puerto Rico, varying with vaccination coverage and efficacy (societal perspective). Vaccinating school-aged children cost $718–$1849/case averted (≤$5002/DALY averted) in Honduras, saved $819–$1609/case averted in Brazil, and saved $3823–$4360/case averted in Puerto Rico. Vaccinating young adults cost $310–$1666/case averted ($731–$4017/DALY averted) in Honduras, saved $953–$1703/case averted in Brazil, and saved $3857–$4372/case averted in Puerto Rico. Vaccinating everyone averted more cases but cost more, decreasing cost savings per case averted. Vaccination resulted in more cost savings and better outcomes at higher attack rates.
Conclusions
When considering transmission, while vaccinating everyone naturally averted the most cases, specifically targeting women of childbearing age or young adults was the most cost-effective.
Keywords: Zika, cost-effectiveness, vaccine
We simulated various vaccination strategies to mitigate Zika outbreaks in Honduras, Brazil, and Puerto Rico. While vaccinating everyone naturally averted the most cases, specifically targeting women of childbearing age or young adults was the most cost-effective and even provided cost savings.
(See the Editorial commentary by Clapham et al, on pages 917–9.)
The 2015–2016 Zika epidemics prompted accelerated development of a Zika vaccine as one potential strategy to prevent and mitigate future outbreaks [1–3]. Decision makers need to know the potential value of different vaccination strategies. Given limited resources, there is a balance between various mitigation strategies to prevent morbidity and costs associated with Zika-related complications, and the difficulty and cost of implementation. For vaccination strategies, possibilities range from vaccinating everyone to specific target populations. Previous studies have shown the potential cost of a Zika epidemic [4, 5]. Knowing the value of vaccination prior to rollout of the vaccine can help policy makers decide on vaccination as a prevention strategy, whom to vaccinate, and guide program planning. Since multiple candidates are currently being developed and it is unclear which vaccines will reach the market, it is also important to understand how these decisions may change based on differing vaccine characteristics, such as cost and efficacy [6]. Therefore, we developed a model of Honduras, Brazil, and Puerto Rico, represented various vaccination approaches, and simulated different Zika outbreaks to determine the resulting economic value of vaccination to prevent and mitigate outbreaks.
METHODS
We developed a Zika clinical and economic outcomes model coupled with a transmission model that translated the number of Zika infections into clinical and health outcomes and their associated costs from the healthcare system and societal perspectives. We evaluated Zika vaccination scenarios in Brazil, Honduras, and Puerto Rico, as these countries were significantly affected by the 2015–2016 Zika epidemic [7, 8] and are representative of other Latin American countries based on income group (defined by the World Bank [9, 10]) and country characteristics (eg, life expectancy, population age distribution, mortality rate).
Model Structure
We used a previously described transmission model [11] to quantify the number of people infected under various scenarios. Supplementary Appendix Figure 1 outlines the model. In brief, this model represents individuals aged 0–99 years and includes population demographics, age-specific fertility, and mortality. Mosquito vectors could be susceptible, exposed, or Zika-infected, where vector population prevalence was modeled with a seasonally varying carrying capacity defined according to country-specific female mosquito indices [12–14], to reflect seasonal variation in mosquito populations. Humans could be susceptible, exposed, infected, recovered, or vaccinated. Human infectiousness to vectors lasted 4–7 days [15] and was assumed to confer lifelong immunity [16]. Transmission between humans and vectors was frequency-dependent with a constant daily vector biting rate (which varied indirectly with seasonal carrying capacity). Transmission was based on the force of infection, which accounts for the transmission rate (ie, mosquito to humans and humans to mosquitos), the biting rate, and the proportion of infections in the population. To calibrate the model to specific attack rates, we ran the model for each country over 5 years with no vaccination, while varying the vector transmission rate, and computed the population fraction infected for each rate. We then recorded the transmission rate corresponding to each attack rate scenario.
The number of infected persons over the course of the outbreak and the number of vaccinated persons were input to the economic model (adapted from our previously published model [4]). Each Zika infection had a probability of developing symptoms, with a corresponding probability of seeking care. Those with Zika infection (symptomatic and asymptomatic) had a probability of developing Guillain-Barré syndrome (GBS). All GBS cases were hospitalized and had a probability of mortality. GBS patients had probabilities of developing mild, moderate, or severe motor deficits, each associated with a given duration of symptoms and disability. To determine the number of congenital Zika infections, we multiplied the number of live births to Zika-infected women by the probability of congenital infection given a live birth.
The healthcare system perspective included direct costs (ie, outpatient visits and hospitalization), while the societal perspective included direct and indirect costs (ie, productivity losses for absenteeism and mortality). Hospitalization costs were calculated as the bed-day cost for each hospitalization day. Productivity losses were estimated using the gross national income (GNI) per capita as a proxy for daily wages. We assumed symptomatic Zika cases only accrued losses if they sought ambulatory care. GBS cases accrued losses for the duration of their hospitalization or motor impairment, whichever was longer. Those with microcephaly accrued productivity losses attenuated by the disability weight for severe intellectual disability for the duration of their lifetime. Death resulted in the accrual of lifetime productivity losses using the median age of the population and discounted GNI over the remainder of the person’s life expectancy.
For each scenario, the following formula determined the incremental cost-effectiveness ratio (ICER):
where health effects were measured in disability-adjusted life-years (DALYs), cases, and deaths. DALYs equaled the sum of the years lived with disability (YLD) and the years of life lost (YLL) due to premature mortality, calculated as:
ICERs were considered highly cost-effective if they were less than or equal to the gross domestic product (GDP) per capita, cost-effective if between 1 and 3 times the GDP per capita, and not cost-effective if >3 times the GDP per capita. The 2017 GDP per capita of Puerto Rico, Brazil, and Honduras was $32307, $8910, and $2432, respectively. Vaccination strategies were economically dominant if they saved costs and provided health benefits (ie, averted DALYs) compared to no vaccination. All future costs and DALYs were discounted to net present value using a 3% discount rate.
Data Sources
Supplementary Appendix Table 1 summarizes the models’ input parameters, values, and sources. All inputs were country-specific when available. Population demographics, age-specific fertility, and age-specific mortality came from the United Nations [17]. We utilized care-seeking rates for fever of all causes as a proxy for care-seeking rates for symptomatic Zika infection. Cost of outpatient and hospital beds came from the World Health Organization’s Choosing Interventions That Are Cost Effective initiative (WHO-CHOICE) [18]. The direct lifetime medical and nonmedical cost for microcephaly came from a study using congenital cytomegalovirus as a proxy for Zika-associated microcephaly [19] and converted this cost for each country. GNI per capita emanated from the World Bank and life expectancy from the WHO’s Global Health Observatory [10, 20]. Disability weights came from the Global Burden of Disease Study [21] and assumed acute, mild episode of an infectious disease, motor impairment (mild, moderate, and severe), and severe intellectual disability as proxies for Zika infection, GBS, and congenital Zika infection, respectively. All costs were discounted to 2017 US dollars using a 3% rate.
Modeled Scenarios and Sensitivity Analyses
Our baseline scenario simulated the course of a Zika outbreak with no vaccination. Vaccination scenarios evaluated the impact of immunization of different target populations: women of childbearing age (women 15–49 years old), school-aged children (5–14 years old), young adults (15–20 years old), and everyone. We implemented large-scale vaccination of the target population at the start of the simulation (ie, year 1) followed by annual coverage of the youngest age in the target group in subsequent years. We choose these target populations as they are ideal to prevent congenital Zika infection (ie, women of childbearing age, young adults), to easily reach for heavy vaccination campaigns (ie, school-aged children), and to represent large campaigns to show the full impact of vaccination (ie, everyone).
Monte Carlo simulation (ie, probabilistic sensitivity analysis) consisted of 1000 trials and varied each parameter in the economic model throughout the ranges listed in Supplementary Appendix Table 1. Sensitivity analysis varied the vaccination cost per vaccinee ($5–$50), vaccine efficacy (25%–75%), vaccination coverage of the target population (25%–75%), lag time between vaccination and outbreak onset (1–5 years), and Zika attack rate (2%–50%, at 5 years). Additional scenarios evaluated the use of a booster (after the vaccine protection duration elapsed, varied from 1 to 5 years) to maintain the specified coverage level over the duration of the simulation. For each scenario, we estimated costs and health effects for the entire duration of the outbreak compared to no vaccination (as simulated in the transmission model).
RESULTS
No Vaccination
Tables 1–4 and Supplementary Appendix Tables 2–13 show the epidemiologic (ie, number of symptomatic cases and deaths) and economic (ie, direct costs, productivity losses, and total societal costs) outcomes of Zika outbreaks with attack rates of 2% and 20% occurring in year 6. An outbreak with a 50% attack rate resulted in $347.8 million in total societal costs in Honduras (551841 symptomatic cases; 215493 DALYs), $11.8 billion in Brazil (16.0 million cases; 775213 DALYs), and $1.2 billion in Puerto Rico (294573 cases; 82833 DALYs).
Table 1.
Epidemiologic, Clinical, and Economic Outcomes for Zika Vaccination When Vaccinating Women of Childbearing Age 5 Years Before the Start of an Outbreak With a 2% Attack Rate
| Country and Model | Total Vaccinateda, Millions | Symptomatic Zika Cases, No. | DALYs, Median (95% UI) |
Vaccination Costs | Total Direct Costs, Median (95% UI) | Total Societal Costs, Median (95% UI) | Cost per Case Avertedb |
|---|---|---|---|---|---|---|---|
| Honduras | |||||||
| No vaccinec | … | 9229 | 3535 (1074–8208) | … | 2.0 (1.4–3.5) | 5.8 (3.1–11.1) | |
| $5 vaccine | |||||||
| 25% efficacy | |||||||
| 25% coveragec | 0.7 | 7359 | 2757 (790–6630) | 3.5 | 5.0 (4.5–6.3) | 8.0 (5.9–11.8) | 1171 |
| 75% coveraged | 2.1 | 4844 | 1806 (510–4354) | 10.3 | 11.2 (10.9–12) | 13.1 (11.7–15.6) | 1664 |
| 75% efficacy | |||||||
| 25% coveraged | 0.7 | 5724 | 2141 (610–5152) | 3.5 | 4.6 (4.2–5.6) | 6.9 (5.3–9.8) | 314 |
| 75% coveragee | 2.1 | 3009 | 1112 (308–2695) | 10.3 | 10.7 (10.6–11.2) | 11.9 (11.0–13.5) | 979 |
| $25 vaccine | |||||||
| 25% efficacy | |||||||
| 25% coveragec | 0.7 | 7359 | 2812 (851–6539) | 23.7 | 19.0 (18.5–20.2) | 22.0 (19.9–26.3) | 8671 |
| 75% coveraged | 2.1 | 4844 | 1840 (551–4296) | 70.7 | 52.5 (52.3–53.2) | 54.5 (53.1–57.3) | 11102 |
| 75% efficacy | |||||||
| 25% coveraged | 0.7 | 5724 | 2182 (657–5083) | 23.7 | 18.6 (18.2–19.5) | 20.9 (19.3–24.2) | 4315 |
| 75% coveragee | 2.1 | 3009 | 1133 (334–2661) | 70.7 | 52.0 (51.9–52.5) | 53.3 (52.4–55) | 7630 |
| Brazil | |||||||
| No vaccine | … | 346206 | 106455 (32764–257059) | … | 193.9 (130.8–264.9) | 732.7 (366.0–1441.8) | … |
| $5 vaccine | |||||||
| 25% efficacy | |||||||
| 25% coverage | 17.8 | 261055 | 81754 (23135–193630) | 86.5 | 222.7 (177.9–278.7) | 634.6 (356.9–1195.1) | –1152 |
| 75% coverage | 52.7 | 156680 | 48932 (13710–116041) | 256.4 | 324.2 (302.1–354.7) | 572.2 (405.4–907.8) | –847 |
| 75% efficacy | |||||||
| 25% coverage | 17.8 | 184802 | 57803 (16278–136974) | 86.5 | 174.9 (145.9–212.5) | 467.3 (270.6–863.6) | –1644 |
| 75% coveraged | 52.7 | 83413 | 25914 (7135–61617) | 256.4 | 279.4 (271.9–294.6) | 411.8 (320.3–591.3) | –1221 |
| $25 vaccine | |||||||
| 25% efficacy | |||||||
| 25% coverage | 17.8 | 261055 | 80168 (24629–193713) | 23.7 | 570.0 (525.0–622.0) | 978.5 (702.5–1513.4) | 2886 |
| 75% coverage | 52.7 | 156680 | 47980 (14663–116074) | 70.7 | 1350.6 (1328.2–1379.1) | 1596.1 (1430.1–1914.9) | 4555 |
| 75% efficacy | |||||||
| 25% coverage | 17.8 | 184802 | 56667 (17367–137023) | 23.7 | 521.8 (492.8–556.3) | 811.6 (615.9–1190.2) | 489 |
| 75% coveraged | 52.7 | 83413 | 25418 (7695–61620) | 70.7 | 1305.3 (1297.7–1318.2) | 1436.2 (1348.6–1607.0) | 2677 |
| Puerto Rico | |||||||
| No vaccine | … | 7860 | 2337 (656–5390) | … | 9.2 (6.1–26.3) | 35.3 (16.2–70.9) | … |
| $5 vaccine | |||||||
| 25% efficacy | |||||||
| 25% coverage | 0.3 | 5792 | 1723 (460–3971) | 1.4 | 8.0 (5.6–19.7) | 27.5 (13.2–53.7) | –3769 |
| 75% coverage | 0.8 | 3392 | 1007 (267–2324) | 4.1 | 7.5 (6.2–14.4) | 19.0 (10.6–34.3) | –3648 |
| 75% efficacy | |||||||
| 25% coverage | 0.3 | 3936 | 1170 (311–2698) | 1.4 | 5.6 (4.1–13.5) | 18.9 (9.2–36.7) | –4177 |
| 75% coveraged | 0.8 | 1667 | 493 (130–1140) | 4.1 | 5.3 (4.8–8.7) | 11.0 (6.9–18.7) | –3921 |
| $25 vaccine | |||||||
| 25% efficacy | |||||||
| 25% coverage | 0.3 | 5792 | 1721 (481–3970) | 23.7 | 13.4 (11.2–25.9) | 32.7 (18.6–59.0) | –1249 |
| 75% coverage | 0.8 | 3392 | 1005 (279–2323) | 70.7 | 23.7 (22.6–31.0) | 35.1 (26.9–50.6) | –42 |
| 75% efficacy | |||||||
| 25% coverage | 0.3 | 3936 | 1168 (325–2697) | 23.7 | 11.0 (9.6–19.5) | 24.2 (14.6–42.2) | –2829 |
| 75% coveraged | 0.8 | 1667 | 491 (134–1139) | 70.7 | 21.6 (21.2–25.2) | 27.2 (23.2–34.9) | –1304 |
Costs are shown as million 2017 US dollars. Simulated outbreak occurs 5 years after the start of vaccination; all outbreaks lasted 5 years unless otherwise noted.
Abbreviations: DALY, disability-adjusted life-year; GBS, Guillain-Barré syndrome; UI, uncertainty interval.
aTransmission model result.
bFrom the societal perspective; US dollars; negative values imply savings.
cOutbreak duration 4 years.
dOutbreak duration 3 years.
eOutbreak duration 2 years.
Table 2.
Epidemiologic, Clinical, and Economic Outcomes for Zika Vaccination When Vaccinating School-Aged Children 5 Years Before the Start of an Outbreak With a 2% Attack Rate
| Country and Model | Total Vaccinateda, Millions | Symptomatic Zika Cases | DALYs, Median (95% UI) |
Vaccination Costs | Total Direct Costs, Median (95% UI) | Total Societal Costs, Median (95% UI) | Cost per Case Avertedb |
|---|---|---|---|---|---|---|---|
| Honduras | |||||||
| No vaccinec | … | 9229 | 3552 (1050–8461) | … | 2.1 (1.4–3.5) | 5.8 (3.1–10.8) | … |
| $5 vaccine | |||||||
| 25% efficacy | |||||||
| 25% coveragec | 0.6 | 7785 | 2861 (834–6971) | 2.8 | 4.5 (4.0–5.9) | 7.6 (5.5–11.9) | 1237 |
| 75% coveraged | 1.8 | 5747 | 2108 (611–5141) | 8.2 | 9.3 (9.0–10.3) | 11.6 (10.0–14.8) | 1666 |
| 75% efficacy | |||||||
| 25% coveraged | 0.6 | 6375 | 2341 (681–5706) | 2.8 | 4.2 (3.8–5.3) | 6.7 (5.0–10.3) | 310 |
| 75% coveragee | 1.8 | 3799 | 1390 (400–3394) | 8.2 | 8.9 (8.7–9.5) | 10.4 (9.3–12.5) | 836 |
| $25 vaccine | |||||||
| 25% efficacy | |||||||
| 25% coveragec | 0.6 | 7785 | 2994 (883–7135) | 23.7 | 15.9 (15.3–17) | 19.0 (16.7–23.2) | 9138 |
| 75% coveraged | 1.8 | 5747 | 2206 (647–5263) | 70.7 | 42.0 (41.6–42.9) | 44.4 (42.7–47.5) | 11066 |
| 75% efficacy | |||||||
| 25% coveraged | 0.6 | 6375 | 2449 (721–5841) | 23.7 | 15.5 (15.0–16.5) | 18.1 (16.2–21.6) | 4301 |
| 75% coveragee | 1.8 | 3799 | 1454 (423–3475) | 70.7 | 41.6 (41.3–42.1) | 43.1 (42.0–45.2) | 6860 |
| Brazil | |||||||
| No vaccine | … | 538546 | 159241 (49707–395540) | … | 308.2 (207.3–421.9) | 1106.5 (550.1–2270.3) | … |
| $5 vaccine | |||||||
| 25% efficacy | |||||||
| 25% coverage | 12.5 | 291471 | 92864 (26468–226681) | 57.6 | 214.4 (166.6–275.7) | 686.5 (364.2–1341.7) | –1133 |
| 75% coverage | 36.3 | 215403 | 68531 (19454–167444) | 166.7 | 274.6 (241.6–317.2) | 623.9 (386.1–1109.1) | –953 |
| 75% efficacy | |||||||
| 25% coverage | 12.5 | 232027 | 73863 (21002–180401) | 57.6 | 177.4 (140.8–224.4) | 554.1 (296.9–1076.2) | –1703 |
| 75% coveragec | 36.2 | 131899 | 41853 (11789–102442) | 166.6 | 223.5 (206.1–248.6) | 437.4 (291.8–738) | –1452 |
| $25 vaccine | |||||||
| 25% efficacy | |||||||
| 25% coverage | 12.5 | 291471 | 93652 (24903–217176) | 23.7 | 445.2 (392.5–502.7) | 914.2 (573.9–1524.1) | 3027 |
| 75% coverage | 36.3 | 215403 | 69133 (18341–160432) | 70.7 | 941.3 (905.1–981.4) | 1288 (1037.3–1739.5) | 4124 |
| 75% efficacy | |||||||
| 25% coverage | 12.5 | 232027 | 74503 (19784–172842) | 23.7 | 407.9 (367.8–451.8) | 781.7 (511.2–1268.1) | 291 |
| 75% coveragec | 36.2 | 131899 | 42241 (11157–98164) | 70.7 | 889.8 (870.8–912.4) | 110.02 (949.9–1378.7) | 1649 |
| Puerto Rico | |||||||
| No vaccine | … | 7860 | 2388 (686–5865) | … | 9.5 (6.2–25.4) | 35.6 (16.8–75.4) | … |
| $5 vaccine | |||||||
| 25% efficacy | |||||||
| 25% coverage | 0.2 | 6581 | 1959 (512–4527) | 0.9 | 8.6 (5.9–20.5) | 30.3 (15.0–60.8) | –4204 |
| 75% coverage | 0.6 | 4837 | 1439 (375–3327) | 2.5 | 8.0 (6.1–16.7) | 24.0 (12.7–46.5) | –3857 |
| 75% efficacy | |||||||
| 25% coverage | 0.2 | 5138 | 1529 (399–3534) | 0.9 | 6.7 (4.7–16.0) | 23.7 (11.8–47.6) | –4372 |
| 75% coveraged | 0.6 | 2838 | 843 (218–1951) | 2.5 | 5.4 (4.4–10.5) | 14.9 (8.2–28.1) | –4139 |
| $25 vaccine | |||||||
| 25% efficacy | |||||||
| 25% coverage | 0.2 | 6581 | 1999 (574–4910) | 23.7 | 12.1 (9.4–25.4) | 34.1 (18.4–67.4) | –1231 |
| 75% coverage | 0.6 | 4837 | 1468 (421–3608) | 70.7 | 18.1 (16.2–27.9) | 34.2 (22.7–58.8) | –469 |
| 75% efficacy | |||||||
| 25% coverage | 0.2 | 5138 | 1560 (448–3833) | 23.7 | 10.3 (8.2–20.7) | 27.4 (15.2–53.6) | –3019 |
| 75% coveraged | 0.6 | 2838 | 860 (246–2115) | 70.7 | 15.5 (14.5–21.3) | 25.0 (18.3–39.5) | –2114 |
Costs are shown as million 2017 US dollars. Simulated outbreak occurs 5 years after the start of vaccination; all outbreaks lasted 5 years unless otherwise noted.
Abbreviations: DALY, disability-adjusted life-year; GBS, Guillain-Barré syndrome; UI, uncertainty interval.
aTransmission model result.
bFrom the societal perspective; US dollars; negative values imply savings.
cOutbreak duration 4 years.
dOutbreak duration 3 years.
eOutbreak duration 2 years.
Table 3.
Epidemiologic, Clinical, and Economic Outcomes for Zika Vaccination When Vaccinating Young Adults 5 Years Before the Start of an Outbreak With a 2% Attack Rate
| Country and Model | Total Vaccinateda, Millions | Symptomatic Zika Cases | DALYs, Median (95% UI) | Vaccination Costs | Total Direct Costs, Median (95% UI) | Total Societal Costs, Median (95% UI) | Cost per Case Avertedb |
|---|---|---|---|---|---|---|---|
| Honduras | |||||||
| No vaccinec | … | 9229 | 3506 (1015–7703) | … | 2.1 (1.4–3.4) | 5.8 (3.2–10.4) | … |
| $5 vaccine | |||||||
| 25% efficacy | |||||||
| 25% coveragec | 0.8 | 7398 | 2873 (818–6432) | 3.6 | 5.2 (4.7–6.2) | 8.3 (6.0–12.4) | 1381 |
| 75% coveraged | 2.2 | 4968 | 1931 (551–4322) | 10.4 | 11.6 (11.2–12.2) | 13.6 (12.1–16.4) | 1849 |
| 75% efficacy | |||||||
| 25% coveraged | 0.8 | 5779 | 2246 (640–5026) | 3.6 | 4.9 (4.5–5.7) | 7.3 (5.5–10.5) | 442 |
| 75% coveraged | 2.2 | 3110 | 1211 (347–2707) | 10.4 | 11.2 (10.9–11.6) | 12.5 (11.5–14.2) | 1097 |
| $25 vaccine | |||||||
| 25% efficacy | |||||||
| 25% coveragec | 0.8 | 7398 | 2812 (815–6176) | 23.7 | 19.6 (19.0–20.7) | 22.6 (20.5–26.3) | 9172 |
| 75% coveraged | 2.2 | 4968 | 1891 (550–4150) | 70.7 | 53.4 (53.0–54.1) | 55.3 (53.9–57.9) | 11637 |
| 75% efficacy | |||||||
| 25% coveraged | 0.8 | 5779 | 2198 (638–4826) | 23.7 | 19.2 (18.8–20.1) | 21.6 (19.9–24.5) | 4579 |
| 75% coveraged | 2.2 | 3110 | 1185 (346–2600) | 70.7 | 52.9 (52.7–53.4) | 54.2 (53.3–55.8) | 7915 |
| Brazil | |||||||
| No vaccine | … | 346206 | 107296 (28385–255941) | … | 193.4 (132.4–266.2) | 737.5 (357.0–1443.0) | … |
| $5 vaccine | |||||||
| 25% efficacy | |||||||
| 25% coverage | 14.8 | 279544 | 85716 (27720–204492) | 69.3 | 225.4 (176.2–282.9) | 663.1 (358.2–1252.4) | –1115 |
| 75% coverage | 43.2 | 191468 | 58716 (18994–140072) | 202.5 | 310.3 (276.2–350.0) | 610.7 (401.5–1014.9) | –819 |
| 75% efficacy | |||||||
| 25% coverage | 14.8 | 212635 | 65206 (21093–155556) | 69.3 | 188.8 (151.1–233.1) | 522.5 (290.2–971.6) | –1609 |
| 75% coveragec | 43.2 | 109844 | 33692 (10905–80368) | 202.4 | 265.1 (245.3–288.1) | 437.9 (317.8–670.2) | –1268 |
| $25 vaccine | |||||||
| 25% efficacy | |||||||
| 25% coverage | 14.8 | 279544 | 86644 (22925–206669) | 23.7 | 503.5 (454–562.6) | 943.5 (635.8–1514.2) | 3090 |
| 75% coverage | 43.2 | 191468 | 59355 (15708–141564) | 70.7 | 1120.6 (1086.5–1161.3) | 1422.6 (1211.4–1814.4) | 4427 |
| 75% efficacy | |||||||
| 25% coverage | 14.8 | 212635 | 65915 (17444–157212) | 23.7 | 466.7 (428.8–511.9) | 802.0 (567.6–1237.2) | 483 |
| 75% coveragec | 43.2 | 109844 | 34061 (9018–81225) | 70.7 | 1075.1 (1055.2–1098.4) | 1248.6 (1127.3–1474.1) | 2163 |
| Puerto Rico | |||||||
| No vaccine | … | 7860 | 2421 (701–5659) | … | 9.4 (6.2–23.1) | 36.5 (17.3–71.7) | … |
| $5 vaccine | |||||||
| 25% efficacy | |||||||
| 25% coverage | 0.2 | 6369 | 1881 (549–4504) | 1.0 | 8.8 (6.1–21.5) | 30.2 (15.0–61.4) | –4180 |
| 75% coverage | 0.6 | 4407 | 1302 (380–3117) | 2.9 | 8.4 (6.5–17.2) | 23.3 (12.7–44.9) | –3823 |
| 75% efficacy | |||||||
| 25% coverage | 0.2 | 4787 | 1414 (413–3386) | 1.0 | 6.9 (4.9–16.5) | 23.1 (11.6–46.6) | –4360 |
| 75% coveragec | 0.6 | 2435 | 720 (210–1722) | 2.9 | 6.0 (5.0–10.9) | 14.3 (8.4–26.2) | –4093 |
| $25 vaccine | |||||||
| 25% efficacy | |||||||
| 25% coverage | 0.2 | 6369 | 1962 (568–4585) | 23.7 | 12.7 (10.1–23.8) | 34.7 (19.1–63.3) | –1195 |
| 75% coverage | 0.6 | 4407 | 1358 (393–3173) | 70.7 | 20.1 (18.3–27.8) | 35.3 (24.5–55.2) | –327 |
| 75% efficacy | |||||||
| 25% coverage | 0.2 | 4787 | 1475 (427–3447) | 23.7 | 10.9 (8.9–19.2) | 27.4 (15.7–49.0) | –2944 |
| 75% coveragec | 0.6 | 2435 | 751 (218–1753) | 70.7 | 17.8 (16.7–22.0) | 26.2 (20.2–37.2) | –1894 |
Costs are shown as million 2017 US dollars. Simulated outbreak occurs 5 years after the start of vaccination; all outbreaks lasted 5 years unless otherwise noted.
Abbreviations: DALY, disability-adjusted life-year; GBS, Guillain-Barré syndrome; UI, uncertainty interval.
aTransmission model result.
bFrom the societal perspective; US dollars; negative values imply savings.
cOutbreak duration 4 years.
dOutbreak duration 3 years.
Table 4.
Epidemiologic, Clinical, and Economic Outcomes for Zika Vaccination When Vaccinating Everyone 5 Years Before the Start of an Outbreak With a 2% Attack Rate
| Country and Model | Total Vaccinateda, Millions | Symptomatic Zika Cases | DALYs, Median (95% UI) |
Vaccination Costs | Total Direct Costs, Median (95% UI) | Total Societal Costs, Median (95% UI) | Cost per Case Avertedb |
|---|---|---|---|---|---|---|---|
| Honduras | |||||||
| No vaccinec | … | 9229 | 3395 (956–8158) | … | 2.0 (1.4–3.8) | 5.7 (3.1–11.0) | … |
| $5 vaccine | |||||||
| 25% efficacy | |||||||
| 25% coveraged | 2.4 | 4473 | 1672 (474–3778) | 11.6 | 12.6 (12.3–13.4) | 14.4 (13.1–17.1) | 1828 |
| 75% coveragee | 7.1 | 905 | 339 (96–765) | 34.6 | 34.8 (34.8–35.0) | 35.2 (34.9–35.7) | 3542 |
| 75% efficacy | |||||||
| 25% coveragee | 2.4 | 2725 | 1019 (289–2302) | 11.6 | 12.3 (12.1–12.7) | 13.3 (12.6–15.0) | 1171 |
| 75% coveragef | 7.1 | 536 | 200 (57–453) | 34.6 | 34.8 (34.7–34.8) | 35.0 (34.8–35.3) | 3365 |
| $25 vaccine | |||||||
| 25% efficacy | |||||||
| 25% coveraged | 2.4 | 4473 | 1645 (463–3954) | 58.2 | 59.2 (58.9–60.0) | 61.0 (59.7–63.5) | 11618 |
| 75% coveragee | 7.1 | 905 | 333 (94–801) | 173.2 | 173.4 (173.3–173.5) | 173.7 (173.5–174.2) | 20184 |
| 75% efficacy | |||||||
| 25% coveragee | 2.4 | 2725 | 1002 (282–2409) | 58.2 | 58.8 (58.6–59.3) | 59.9 (59.1–61.4) | 8329 |
| 75% coveragef | 7.1 | 536 | 197 (56–474) | 173.2 | 173.3 (173.2–173.4) | 173.5 (173.3–173.8) | 19301 |
| Brazil | |||||||
| No vaccine | … | 346206 | 105830 (29821–258523) | … | 191.5 (129.8–261.7) | 728.8 (368.1–1462.4) | … |
| $5 vaccine | |||||||
| 25% efficacy | |||||||
| 25% coverage | 58.6 | 142928 | 45350 (12796–102918) | 288.4 | 369.0 (342.7–399.6) | 599.7 (440.6–879.6) | –635 |
| 75% coveraged | 174.7 | 24829 | 7879 (2224–17879) | 859.8 | 873.9 (869.3–879.3) | 914.1 (886.4–962.8) | 577 |
| 75% efficacy | |||||||
| 25% coveraged | 58.6 | 74466 | 23630 (6669–53622) | 288.4 | 330.6 (316.8–346.5) | 450.9 (367.9–597.1) | –1022 |
| 75% coveragef | 174.7 | 13524 | 4292 (1212–9739) | 859.8 | 867.6 (865.0–870.5) | 889.4 (874.3–916.0) | 483 |
| $25 vaccine | |||||||
| 25% efficacy | |||||||
| 25% coverage | 58.6 | 142928 | 43697 (12319–106735) | 1441.9 | 1521.5 (1495.8–1550.7) | 1744.2 (1594.8–2048.4) | 4995 |
| 75% coveraged | 174.7 | 24829 | 7592 (2141–18542) | 4299.2 | 4313.1 (4308.6–4318.2) | 4351.9 (4325.9–4404.8) | 11274 |
| 75% efficacy | |||||||
| 25% coveraged | 58.6 | 74466 | 22768 (6421–55611) | 1441.9 | 1483.5 (1470.1–1498.8) | 1599.7 (1521.7–1758.5) | 3205 |
| 75% coveragef | 174.7 | 13524 | 4135 (1167–10100) | 4299.2 | 4306.8 (4304.4–4309.6) | 4327.9 (4313.7–4356.7) | 10819 |
| Puerto Rico | |||||||
| No vaccine | … | 7860 | 2224 (700–5648) | … | 9.3 (6.0–25.5) | 34.7 (16.9–71.4) | … |
| $5 vaccine | |||||||
| 25% efficacy | |||||||
| 25% coveragec | 1.0 | 2892 | 886 (244–2017) | 5.0 | 8.5 (7.3–15.5) | 18.5 (11.4–32.0) | –3262 |
| 75% coveragee | 3.0 | 456 | 140 (39–318) | 15.0 | 15.5 (15.3–16.6) | 17.1 (16.0–19.2) | –2381 |
| 75% efficacy | |||||||
| 25% coveraged | 1.0 | 1367 | 419 (116–954) | 5.0 | 6.7 (6.1–10.0) | 11.4 (8.0–17.8) | –3590 |
| 75% coveragef | 3.0 | 237 | 73 (20–165) | 15.0 | 15.2 (15.1–15.8) | 16.1 (15.5–17.2) | –2447 |
| $25 vaccine | |||||||
| 25% efficacy | |||||||
| 25% coveragec | 1.0 | 2892 | 818 (258–2078) | 25.1 | 28.5 (27.3–34.5) | 37.9 (31.3–51.5) | 646 |
| 75% coveragee | 3.0 | 456 | 129 (41–328) | 74.8 | 75.3 (75.1–76.3) | 76.8 (75.8–79.0) | 5684 |
| 75% efficacy | |||||||
| 25% coveraged | 1.0 | 1367 | 387 (122–982) | 25.1 | 26.7 (26.1–29.5) | 31.2 (28.0–37.6) | –549 |
| 75% coveragef | 3.0 | 237 | 67 (21–170) | 74.8 | 75.0 (74.9–75.5) | 75.8 (75.3–76.9) | 5392 |
Costs are shown as million 2017 US dollars. Simulated outbreak occurs 5 years after the start of vaccination; all outbreaks lasted 5 years unless otherwise noted.
Abbreviations: DALY, disability-adjusted life-year; GBS, Guillain-Barré syndrome; UI, uncertainty interval.
aTransmission model result.
bFrom the societal perspective; US dollars; negative values imply savings.
cOutbreak duration 4 years.
dOutbreak duration 3 years.
eOutbreak duration 2 years.
fOutbreak duration 1 year.
Vaccinating Women of Childbearing Age
Table 1 and Supplementary Appendix Tables 2–4 show the impact of vaccinating women of childbearing age on the resulting clinical and economic outcomes for each country when an outbreak occurred 5 years after vaccination start (ie, year 6). In some circumstances, the additional vaccination costs outweighed the cost savings generated by averted cases, resulting in higher overall costs. For example, in Honduras the cost of vaccination increased direct costs by at least an additional $2.5 million, but only saved as much as $2.5 million in productivity losses ($5 vaccination, 2% attack rate; Table 1). However, total cost savings were generated in many scenarios and could save thousands of dollars per case averted (Table 1). Vaccination resulted in even higher cost savings with a 50% attack rate (not shown).
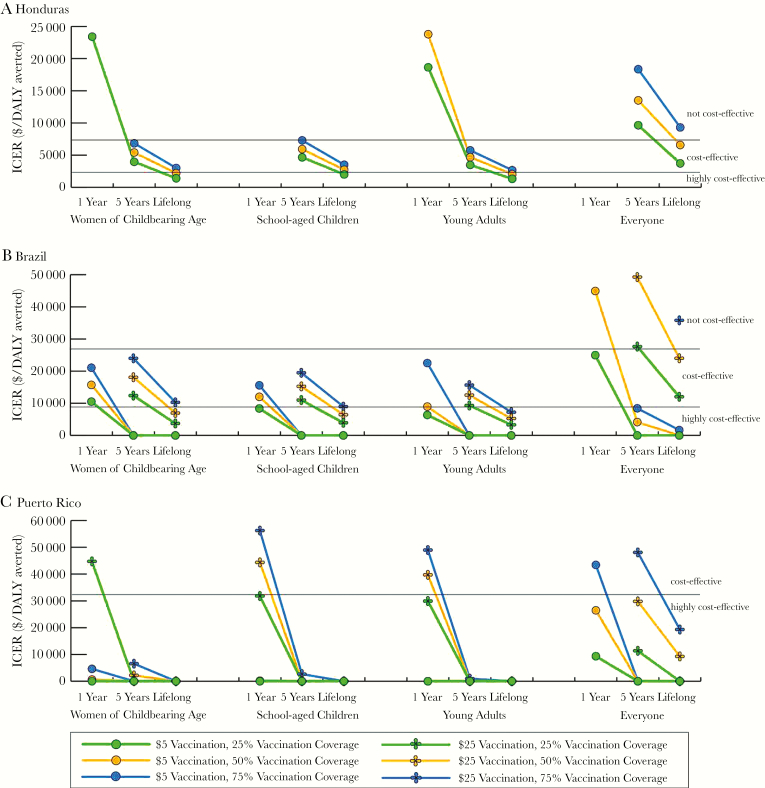
Figure 1 shows the ICER compared to no vaccination and how it changes with various vaccine characteristics under different Zika outbreak scenarios (an ICER of 0 indicates the strategy would be economically dominant, ie, saves cost and provides health benefits). Increases in both coverage and efficacy tended to have a linear effect on the ICER, while increasing vaccination costs had a nonlinear effect. Additionally, changes in vaccination coverage had a larger impact than efficacy changes. These trends also held for higher outbreak attack rates. In Honduras, a $5 vaccination would be cost-effective (ICERs: $790–$5270/DALY averted from both perspectives) compared to no vaccination with a 2% attack rate. For an outbreak with a ≥20% attack rate in Brazil, a ≤$50 vaccination was dominant, except with a vaccine efficacy of 25% from the healthcare system perspective (ICERs: $255–$390/DALY averted), while in Puerto Rico vaccination was dominant from both perspectives.
Figure 1.
Impact of varying vaccine characteristics on the incremental cost-effectiveness ratio (ICER) from the healthcare system perspective when vaccinating each target population (women of childbearing age, school-aged children, young adults, and everyone) for a Zika outbreak starting 5 years after vaccination in Honduras with a 20% attack rate (A), Brazil with a 2% attack rate (B), and Puerto Rico with a 2% attack rate (C). An ICER of 0 indicates the strategy would be economically dominant (provides cost savings and health benefits). ICERs greater than $50000 per disability-adjusted life-year (DALY) averted and $35000 per DALY averted are not shown for Brazil and Puerto Rico, respectively.
The tables can be used to determine the marginal cost of changing vaccine characteristics, especially for economically dominant scenarios. For example, in Puerto Rico a $5, 50% efficacious vaccination would be dominant for coverage rates of 25% and 50%, saving $2.6 and $3.4 million in total direct costs, respectively (Supplementary Appendix Table 6). It cost a marginal $1.3 million to increase coverage from 25% to 50% but would avert 472 more DALYs.
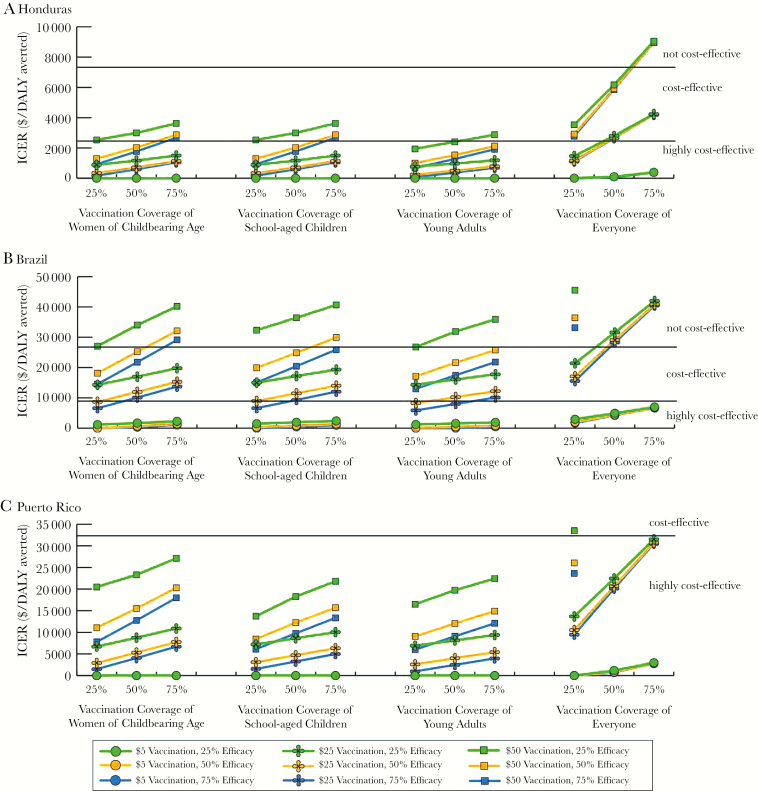
Figure 2 shows how the ICER changes when using a booster following various durations of vaccine protection. In many circumstances, an annual booster would not be cost-effective. However, $5 vaccination given every 5 years was cost-effective and even dominant compared to no vaccination under many conditions.
Figure 2.
Impact of using a booster with varying vaccine characteristics on the incremental cost-effectiveness ratio (ICER) from the societal perspective when vaccinating women of childbearing age, school-aged children, young adults, and everyone for a Zika outbreak starting 5 years after vaccination in Honduras (A), Brazil (B), and Puerto Rico (C) with a 2% attack rate. An ICER of 0 indicates the strategy would be economically dominant (provides cost savings and health benefits). Vaccine efficacy held at 50%. ICERs greater than $25000 per disability-adjusted life-year (DALY) averted, $50000 per DALY averted, and $60000 per DALY averted are not shown for Honduras, Brazil, and Puerto Rico, respectively.
When the outbreak occurred 1 year after the start of vaccination (ie, in year 2; data not shown), vaccinating women of childbearing age resulted in more cost savings and better outcomes, as more cases would be averted over the course of the outbreak. Vaccination saved $636–$684 per case averted in Honduras ($5 vaccination, 20% attack rate) and $1642–$2169 per case averted in Brazil ($5 vaccination, 2% attack rate, varying with efficacy and coverage).
Vaccinating School-Aged Children
Vaccinating school-aged children resulted in a similar epidemiologic impact as vaccinating women of childbearing age and therefore resulted in similar clinical outcomes and costs (Table 2 and Supplementary Appendix Tables 5–7). In Honduras, vaccination resulted in higher total direct and societal costs compared to no vaccination with an attack rate <20%. However, with a ≥20% attack rate, vaccination generated total direct cost savings with a $5 vaccination and societal cost savings with a ≤$25 vaccination. In Brazil, the averted productivity losses were always enough to overcome the higher total direct costs due to vaccination (saving ≤$15.3 billion in total costs for attack rates ≤50%), except with a ≥$25 vaccination and 2% attack rate (Supplementary Appendix Table 6). Total cost savings in Puerto Rico exceeded $1.1 million (≤$0.5 billion) for outbreaks with attack rates ≤50%.
Cost-effectiveness trends followed a similar pattern to that of vaccinating women of childbearing age (Figures 1 and 2) and under some conditions vaccinating school-aged children was more cost-effective (eg, $50 vaccination in Puerto Rico; Figure 1C). For a 2% outbreak, a $5 vaccination was cost-effective in Honduras (ICERs: $1210–$6052/DALY averted from both perspectives). In Brazil, vaccination was dominant compared to no vaccination for attack rates ≥20%, except for a $50 vaccination and 20% attack rate, but vaccination remained highly cost-effective. Although an annual booster was not cost-effective compared to no vaccination in Honduras (Figure 2A), it was cost-effective in Brazil (Figure 2B), and was dominant in Puerto Rico (Figure 2C). Supplementary Appendix Tables 5–7 can be used to calculate the marginal cost of increasing various vaccine characteristics. When the outbreak occurred 1 year after vaccine availability, vaccination resulted in more cost savings and better outcomes (results not shown).
Vaccinating Young Adults
Vaccinating young adults resulted in a similar epidemiologic impact as vaccinating women of childbearing age and school-aged children, thus yielding similar clinical outcomes and costs (Table 3 and Supplementary Appendix Tables 8–10). While vaccinating young adults averted thousands of Zika cases in Honduras and Puerto Rico and millions in Brazil (for an outbreak occurring 5 years after vaccine availability), overall cost savings were not always generated due to the additional vaccination cost (eg, in Honduras the vaccination cost increased direct costs by an additional $2.4–$80.5 million, and societal costs by an additional $0.9–$78.1 million with a 2% attack rate; Supplementary Appendix Table 8). However, vaccination garnered cost savings of ≤$214.0 million, ≤$13.3 billion, and ≤$471.7 million in Honduras, Brazil, and Puerto Rico, respectively, from the societal perspective (attack rates ≤50%).
ICERs showed similar trends as when vaccinating women of childbearing age (Figures 1 and 2). Vaccination was not cost-effective in Honduras with a 2% attack rate and ≥$25 vaccination, 25% efficacious, ≥50% coverage. In Puerto Rico, vaccination was dominant compared to no vaccination under all conditions tested, except at a 2% attack rate (Figure 1C). Even a vaccine that required an annual booster (ie, 1-year protection duration) was highly cost-effective under many circumstances (Figure 2). Again, the tables can be used to determine the marginal cost of increasing various vaccine characteristics.
When the outbreak occurred 1 year after the start of vaccination (ie, in year 2), vaccinating young adults resulted in more cost savings and better outcomes (results not shown). The cost per case averted, while still resulting in savings, was less than it was when vaccinating women of childbearing age. For example, in Honduras it saved $13–$38 less (20% attack rate) and in Brazil, vaccination saved $1482–$1912 per case averted ($5 vaccination, 2% attack rate, varying with efficacy and coverage).
Vaccinating Everyone
Vaccinating the entire population averted ≤5.7 million cases and ≤38158 GBS deaths (Table 4 and Supplementary Appendix Tables 11–13). While vaccinating everyone averted direct healthcare costs and productivity losses, vaccination costs were higher, and vaccination did not result in overall cost savings in Honduras or Brazil (except with a $5 vaccination, ≤50% coverage) for a 2% attack rate (Supplementary Appendix Tables 11 and 12). However, a $5 vaccination saved $29.7–$46.1 million in societal costs (all coverages) in Honduras and $0.4–$2.7 billion in direct costs and $3.6–$11.7 billion in societal costs in Brazil for a 20% attack rate. Vaccination usually generated cost savings in Puerto Rico (Supplementary Appendix Table 13), saving $0.1–$126.2 million in direct costs and $1.5–$499.5 million in societal costs, depending on vaccine characteristics. Vaccinating everyone decreased the cost savings per case averted (Table 4 and Supplementary Appendix Tables 11–13).
Increases in coverage resulted in larger increases in ICER values compared to the other target populations (Figures 1 and 2). Vaccination was cost-effective compared to no vaccination, except with a 2% attack rate in Honduras (>$5 vaccination, ≥75% coverage, ≤75% efficacious from both perspectives) and some conditions in Brazil (Figure 1B). In Puerto Rico, vaccinating everyone was dominant compared to no vaccination under all conditions tested, except when the attack rate was 2% (Figure 1C) and 20% with a ≥$50 vaccine and ≥75% coverage (ICERs: $429–$459/DALY averted, healthcare system perspective). Use of a booster was highly cost-effective under many conditions (Figure 2C). Supplementary Appendix Tables 11–13 can be used to determine the marginal cost to change vaccine characteristics. When the outbreak occurred in year 2, vaccinating everyone resulted in fewer cases, yielding higher cost savings.
DISCUSSION
Vaccinating different target populations to mitigate Zika outbreaks provided economic value in a wide range of epidemiologic and economic conditions. Our results show that vaccinating women of childbearing age, school-aged children, young adults, and everyone would result in cost savings (≥$1.0 million in total direct costs and ≥$1.8 million in productivity losses) under many conditions. Vaccination was cost-effective at high costs ($50 per vaccinee) and fairly low efficacies (25%) and Zika attack rates (2%). Assuming that the cost of vaccine delivery and administration is less than the cost savings generated from cases averted, Zika vaccination could actually pay for itself.
Cost-effectiveness varied the most with increasing vaccine coverage. Thus, there is a trade-off between the additional reduction in cases and increased vaccination costs, which is more prominent for lower attack rates. Our study can help guide decisions on coverage level needed, given this trade-off.
Our results show that women of childbearing age or young adults would be an ideal target group for vaccination. While vaccinating the entire population had the largest impact on mitigating an outbreak, it cost more. Short of vaccinating everyone, vaccinating women of childbearing age prevented the largest number of negative outcomes, while vaccinating young adults typically resulted in the greatest cost savings per case averted. Benefits of vaccinating these populations varied with outbreak timing and vaccine coverage. While vaccinating women averted more Zika cases, as young adults are a smaller population and include a part of the key vaccination target (ie, young women of childbearing age) similar gains were accrued for vaccinating fewer individuals. Thus, if vaccine availability was limited, women of childbearing age and young adults provided the best overall benefits (cases and deaths averted, while providing economic gains).
Better understanding the potential economic value of Zika vaccination in the context of preventing and mitigating a Zika outbreak can assist public health officials and other decision makers. Our results can aid in program planning, guide decisions on target populations for vaccination, and determine necessary coverage levels given different vaccine characteristics. Our results also show that under many conditions, third-party payers can save costs; therefore, they should consider providing coverage and reimbursements for such a vaccine. Considerable benefits of Zika vaccine in the event of a future outbreak support further investment into its development. One potential area of future work is to develop a tool for decision makers to generate other result comparisons and to evaluate vaccination in other countries and under different epidemiologic, economic, and vaccination conditions and other target groups to aid in policy decisions.
We attempted to be conservative in our analysis. We did not include any costs associated with additional testing or prenatal care for Zika-infected pregnant women. Nor did we include any potential costs or health effects associated with prenatal infection (eg, abortion or fetal death). Inclusion of these costs would further increase the value of vaccinating women of childbearing age. We did not include sexual transmission of the Zika virus, which would increase the number of women with infection, thereby increasing the number of perinatal infections; thus, we may underestimate the number of congenital Zika infection cases and subsequently their costs. We evaluated a 25% vaccine efficacy to show the range of possibilities, as a less efficacious vaccine may be better than no vaccination. Additionally, some vaccines have low efficacies, for example, the influenza vaccine has a variable efficacy of 19%–60% over the past 10 years [22], the BCG vaccine has a low efficacy (10%–66%) against pulmonary tuberculosis [23, 24], and the RTS,S vaccine for malaria has an efficacy of approximately 30% for first episodes and wanes to <10% over time [25–28]. We also used the disability weight for severe intellectual disability for congenital Zika infection, rather than profound disability, which may underestimate the burden.
There are limitations to this study. By definition, models are simplifications that aim to distill systems down to the most pertinent relationships and key factors without including extraneous detail. We assumed equal mixing between humans and vectors [29], when in reality, transmission is heterogeneous and driven by local neighborhood-level factors [30]. We assumed that Zika infection confers lifelong immunity, but this has not yet been established. Should recovered individuals be susceptible to reinfection, our results may underestimate vaccination’s benefit. While our data-driven analyses drew from a variety of sources and locations, the current literature on Zika is limited and new data continue to emerge. For instance, there are discrepancies in the reported microcephaly risk. The exact duration and impact of GBS may vary from person to person and have differing impacts on productivity losses; additionally, some patients may experience further productivity losses beyond the duration of hospitalization.
While vaccinating everyone will naturally avert the most cases, specifically vaccinating women of childbearing age or young adults garnered the highest economic value, often resulting in cost savings, even when considering transmission.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Disclaimer. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, and approval of the manuscript.
Financial support. This work was supported by the Bill & Melinda Gates Foundation (Award Number OPP1139051); Instituto Carlos Slim de la Salud; the National Institutes of Health (grant numbers U01 GM087719, U01 GM105627, U54HD070725, U01 HD086861); the Agency for Healthcare Research and Quality (grant number R01HS023317); and the US Agency for International Development (grant number AID-OAA-A-15-00064).
Potential conflicts of interest. All authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Durbin AP. Vaccine development for Zika virus-timelines and strategies. Semin Reprod Med 2016; 34:299–304. [DOI] [PubMed] [Google Scholar]
- 2. Morabito KM, Graham BS. Zika virus vaccine development. J Infect Dis 2017; 216:957–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Scherwitzl I, Mongkolsapaja J, Screaton G. Recent advances in human flavivirus vaccines. Curr Opin Virol 2017; 23:95–101. [DOI] [PubMed] [Google Scholar]
- 4. Lee BY, Alfaro-Murillo JA, Parpia AS, et al. . The potential economic burden of Zika in the continental United States. PLoS Negl Trop Dis 2017; 11:e0005531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Macciocchi D, Lanini S, Vairo F, et al. . Short-term economic impact of the Zika virus outbreak. New Microbiol 2016; 39:287–9. [PubMed] [Google Scholar]
- 6. Lee BY, Burke DS. Constructing target product profiles (TPPs) to help vaccines overcome post-approval obstacles. Vaccine 2010; 28:2806–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hennessey M, Fischer M, Staples JE. Zika virus spreads to new areas—region of the Americas, May 2015–January 2016. MMWR Morb Mortal Wkly Rep 2016; 65:55–8. [DOI] [PubMed] [Google Scholar]
- 8. Kindhauser MK, Allen T, Frank V, Santhana RS, Dye C. Zika: the origin and spread of a mosquito-borne virus. Bull World Health Organ 2016; 94:675–86C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. World Bank. Updated income classifications. 2015 https://blogs.worldbank.org/opendata/new-country-classifications. Accessed 17 June 2016. [Google Scholar]
- 10. World Bank. World development indicators, 1960–2015. 2016. http://datatopics.worldbank.org/world-development-indicators/. Accessed August 2017. [Google Scholar]
- 11. Durham DP, Fitzpatrick M, Ndeffo-Mbah ML, Parpia AS, Galvani AP. Evaluating Zika vaccination strategies in the Americas. Ann Intern Med 2018; 168:621–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Quintero J, Brochero H, Manrique-Saide P, et al. . Ecological, biological and social dimensions of dengue vector breeding in five urban settings of Latin America: a multi-country study. BMC Infect Dis 2014; 14:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rodrigues Mde M, Marques GR, Serpa LL, et al. . Density of Aedes aegypti and Aedes albopictus and its association with number of residents and meteorological variables in the home environment of dengue endemic area, São Paulo, Brazil. Parasit Vectors 2015; 8:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Focks DA, Brenner RJ, Hayes J, Daniels E. Transmission thresholds for dengue in terms of Aedes aegypti pupae per person with discussion of their utility in source reduction efforts. Am J Trop Med Hyg 2000; 62:11–8. [PubMed] [Google Scholar]
- 15. Kucharski AJ, Funk S, Eggo RM, Mallet HP, Edmunds WJ, Nilles EJ. Transmission dynamics of Zika virus in island populations: a modelling analysis of the 2013-14 French Polynesia outbreak. PLoS Negl Trop Dis 2016; 10:e0004726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fagbami AH. Zika virus infections in Nigeria: virological and seroepidemiological investigations in Oyo State. J Hyg (Lond) 1979; 83:213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Population Division, Department of Economic and Social Affairs, United Nations. World population prospects: 2015 revision, DVD ed. New York: United Nations. [Google Scholar]
- 18. World Health Organization. Choosing Interventions That Are Cost Effective (WHO-CHOICE): WHO-CHOICE unit cost estimates for service delivery http://www.who.int/choice/country/country_specific/en/index.html. Accessed 15 May 2016. [DOI] [PMC free article] [PubMed]
- 19. Li R, Simmons KB, Bertolli J, et al. . Cost-effectiveness of increasing access to contraception during the Zika virus outbreak, Puerto Rico, 2016. Emerg Infect Dis 2017; 23:74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. World Health Organization. Global health observatory: country statistics http://www.who.int/gho/countries/en/#M. Accessed 12 May 2016.
- 21. Alomon JA, Haagsma JA, Davis A, et al. . Disability weights for the Global Burden of Disease 2013 study. Lancet Glob Health 2015; 3:e712–23. [DOI] [PubMed] [Google Scholar]
- 22. Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases. Seasonal influenza vaccine effectiveness, 2005–2018 https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm. Accessed 30 August 2018.
- 23. Roy A, Eisenhut M, Harris RJ, et al. . Effect of BCG vaccination against Mycobacterium tuberculosis infection in children: systematic review and meta-analysis. BMJ 2014; 349:g4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barreto ML, Pereira SM, Ferreira AA. BCG vaccine: efficacy and indications for vaccination and revaccination. J Pediatr (Rio J) 2006; 82:S45–54. [DOI] [PubMed] [Google Scholar]
- 25. Olotu A, Fegan G, Wambua J, et al. . Seven-year efficacy of RTS,S/AS01 malaria vaccine among young African children. N Engl J Med 2016; 374:2519–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alonso PL, Sacarlal J, Aponte JJ, et al. . Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet 2004; 364:1411–20. [DOI] [PubMed] [Google Scholar]
- 27. RTS,S Clinical Trials Partnership Efficacy and safety of the RTS,S/AS01 malaria vaccine during 18 months after vaccination: a phase 3 randomized, controlled trial in children and young infants at 11 African sites. PLoS Med 2014; 11:e1001685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Agnandji ST, Lell B, Fernandes JF, et al. . RTS,S Clinical Trials Partnership A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N Engl J Med 2012; 367:2284–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Durham DP, Fitzpatrick MC, Ndeffo-Mbah ML, Parpia AS, Michael NL, Galvani AP. Evaluating vaccination strategies for Zika virus in the Americas. Ann Intern Med 2018; 168:621–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stoddard ST, Forshey BM, Morrison AC, et al. . House-to-house human movement drives dengue virus transmission. Proc Natl Acad Sci U S A 2013; 110:994–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.