Abstract
Background
It is recommended that needle and syringe programmes (NSP) distribute low dead space syringes (LDSS) to reduce blood-borne virus transmission. We explored the acceptability of detachable LDSS among people who inject drugs (PWID) and staff who work to support them.
Methods
Semi-structured interviews were performed with 23 PWID (15 men and 8 women) and 13 NSP staff members (6 men and 7 women) in Bath and Bristol, England. Recruited PWID reflected varying demographic characteristics, drug use and injecting preferences. Interviews explored experiences of different types of injecting equipment, facilitators and barriers of changing this equipment and attitudes towards detachable LDSS. Interviews were audio recorded, transcribed verbatim and analysed using the Framework Method.
Results
Decisions about injecting practices were underpinned by several factors, including early experiences and peer initiation; awareness and availability of alternatives; and the ability to inject successfully. Rinsing and re-using syringes represented a quandary where rinsing could encourage re-use, but not rinsing could result in the re-use of unclean equipment. Most PWID were reluctant to change equipment particularly in the absence of any problems injecting. Prioritising getting a ‘hit’ over the prevention of potential problems was an important barrier to change. Overall detachable LDSS are likely to be acceptable. Lower risk of transferring infections and reduced drug wastage were valued benefits of detachable LDSS. There was a preference for a gradual introduction of detachable LDSS in which PWID are given an opportunity to try the new equipment alongside their usual equipment.
Conclusion
Detachable LDSS are likely to be acceptable and should therefore be offered to those using detachable high dead space syringes and/or fixed 1ml LDSS syringes to inject into deeper femoral veins. An intervention is needed to support their introduction with ‘training’, ‘education’, ‘persuasion’ and eventual ‘restriction’ components.
Keywords: acceptability, low dead space syringes, qualitative research
Introduction
There are two main types of injecting equipment used by people who inject drugs (PWID), those where the needle is fixed or integral to the syringe and those where the needle can be detached and replaced. Replacing needles is important if they become blunt or blocked (Zule, Cross, Stover, & Pretorius, 2013), and when dividing drugs between users (WHO, 2012a). Detachable, longer needles are also needed for femoral vein injecting (Zule et al., 2013).
Injecting equipment contains either low or high amounts of dead space, hereafter referred to as Low Dead Space Syringes (LDSS) and High Dead Space Syringes (HDSS). Dead space is “the volume of fluid that is drawn up but not injected” (p4877) due to the design of the syringe (Strauss, van Zundert, Frid, & Costigliola, 2006). Standard injecting equipment with detachable needles contain ten times more “dead space” (Vickerman, Martin, & Hickman, 2013a) and transfer more blood if re-used (even if rinsed) than equipment with fixed needles (Gaughwin, Gowans, Ali, & Burrell, 1991; Zule et al., 2013).
HDSS use is hypothesised to increase the risk of human immunodeficiency virus (HIV) and Hepatitis C virus (HCV) transmission compared to LDSS (Abdala, Stephens, Griffith, & Heimer, 1999; Binka, Paintsil, Patel, Lindenbach, & Heimer, 2015). HCV (Paintsil, He, Peters, Lindenbach, & Heimer, 2010) and HIV (Abdala et al., 1999) survive longer in HDSS than LDSS (Paintsil et al., 2010). Weak evidence suggests HDSS compared to LDSS use is positively associated with blood borne virus (BBV) prevalence (Bobashev & Zule, 2010; Gyarmathy et al., 2010; Gyarmathy, Neaigus, Mitchell, & Ujhelyi, 2009; Zule & Bobashev, 2009; Zule, Desmond, & Neff, 2002). Modelling suggests increasing LDSS circulation could help reduce HIV prevalence (Bobashev & Zule, 2010; Vickerman et al., 2013a) and transmission (Boily & Shubber, 2014) and indicates that if all PWID changed from HDSS to LDSS 39,000–43,000 HIV infections would be prevented over 5 years (Zule et al., 2013). However, more robust evidence is needed (Ambekar & Pawar, 2013; Jacka, 2013). The World Health Organization (WHO) and the National Institute for Health and Clinical Excellence (NICE) recommend needle and syringe programme (NSP) supply LDSS to reduce BBV transmission risk (NICE, 2014; WHO, 2012b; WHO, UNAIDS, & United Nations Office on Drugs and Crime, 2007).
Previous research recommends interventions to promote LDSS: increase availability in specialist and pharmacy NSP (Gray, Nguyen, & Neukom, 2012; Huong et al., 2015; NICE, 2014; Rafful et al., 2015; WHO, 2012a); inform NSP providers about the dead space in syringes (Vickerman, Martin, & Hickman, 2013b), encourage the use of LDSS (Bobashev & Zule, 2010; Paintsil et al., 2010; WHO, 2012b; WHO et al., 2007) and discourage HDSS use (Gaughwin et al., 1991; Grund & Stern, 1991) through education (Rafful et al., 2015; Walsh, Verster, Rodolph, & Akl, 2014), social marketing (Zule, 2012), behaviour change techniques (Gray et al., 2012; Huong et al., 2015) and emphasising the benefits to users (Grund & Stern, 1991) such as the reduced drug waste (Zule et al., 2013). Peer based interventions have been highlighted as critical to the success of such initiatives previously (WHO, 2012a). One social marketing intervention attempted to increase the availability and distribution of LDSS through pharmacy and non-pharmacy settings (e.g. tea stalls, coffee shops and cigarette sellers) in Vietnam (Huong et al., 2015). This intervention achieved increased sales and reported use of LDSS, and an association between the use of LDSS and exposure to the intervention. Further research is needed to determine the effectiveness of interventions to change PWID preferences for HDSS (Vickerman et al., 2013b). In addition, the impact of interventions seeking to switch PWID to LDSS on BBV incidence needs to be evaluated (Gray et al., 2012; Zule et al., 2013).
Early work shows mixed acceptability of changing from HDSS to LDSS (Gray et al., 2012; WHO, 2012a; Zule & Cross, 2012; Zule et al., 2015). Satisfaction with LDSS has been primarily attributed to the syringe design which wastes less drug (Zule et al., 2002), rather than associated health benefits (Gray et al., 2012). Concerns from PWID are the lack of widely available LDSS with detachable needles and syringe volumes larger than 1ml (Albers, 2013; Ibragimov & Latypov, 2012; Jacka, 2013; WHO, 2012a; Zule, 2012; Zule & Cross, 2012; Zule et al., 2013; Zule et al., 2015).
Sharing injecting equipment, the key risk factor for transferring BBV (Palmateer et al., 2013), remains common – nearly 1 in 4 PWID in Bristol (England) report using equipment in the previous year that had already been used by someone else (Centre for Infectious Disease Surveillance and Control and Microbiology Services, 2015). Self-reported rinsing also appears low; approximately a third of those surveyed report rinsing used equipment with water, bleach or detergent prior to reuse (Centre for Infectious Disease Surveillance and Control and Microbiology Services, 2015; Public Health England, Health Protection Scotland, Public Health Wales, & Public Health Agency Northern Ireland, 2015).
The development of detachable LDSS (Figure 1) has the potential to increase the proportion of LDSS syringes in circulation and reduce BBV transmission risk. An understanding of the acceptability of detachable LDSS among PWID should inform their introduction (Albers, 2013; Ambekar & Pawar, 2013; Ciccarone, 2013; Gyarmathy et al., 2009; Zule et al., 2013) and PWID should contribute to the design of interventions (Zule et al., 2013). Therefore, testing the acceptability of detachable LDSS to PWID, designing interventions to promote them and evaluating their use is needed (Craig et al., 2008).
Figure 1.
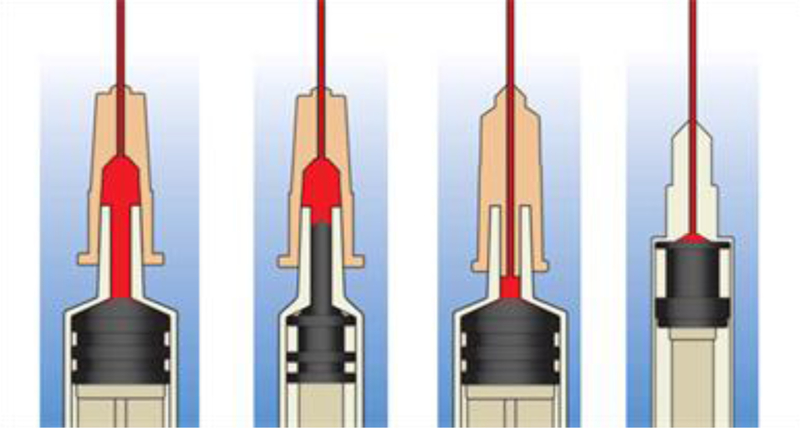
Illustrations of dead space in common needle and syringe designs (artwork licensed by Creative Commons) Reproduced with permission from William Zule. From left to right: Standard detachable needle on standard syringe, Standard detachable needle on low dead space syringe, Low dead space detachable needle on standard syringe, Low dead space syringe with fixed needle
Aim
This study explored the views of PWID and NSP staff in West of England (WE) on the use of detachable LDSS to determine whether (1) they are acceptable, (2) an intervention is required to promote their use, and (3) the findings could help develop evidence-based recommendations for their introduction.
Methods
Research design
A qualitative approach, using semi-structured interviews was adopted to capture the perspectives of both PWID and NSP staff. PWID received £10 as reimbursement for their time. Two sites were selected - Bristol and Bath (WE) where NSP are provided by specialist agencies and community pharmacies. Bristol is estimated to have the highest number of PWID in WE, while Bath and North East Somerset has the fourth lowest (Public Health England, 2013). Bristol is the eighth largest city in England (Bristol City Council, 2016) with a relatively large and mature population of PWID – with prevalence close to 1% in adults (Jones et al., 2015) and chronic HCV prevalence slightly above national average (Martin et al., 2015). Recent estimates of PWID in Bristol in 2011/12 were approximately 2770 or 0.9% of the 15–64 year old population (Jones et al., 2015).
In Bristol, interviews were conducted at Bristol Drugs Project (BDP) an independent agency with NSP open six days a week providing free injecting equipment to approximately 1156 PWID (1011 men and 145 women), and a range of safer injecting interventions [13] and in Bath, at Developing Health and Independence (DHI), which runs NSP twice a week (DHI Online, 2015). Detachable LDSS were not in use in either city at the time of the study.
The intention was to conduct the majority of interviews with PWID and professionals and volunteers in Bristol and to supplement these interviews with a smaller number of interviews from Bath to understand whether experiences are comparable to those from a different setting where drug service provision may differ. This is important because the acceptability of detachable LDSS may vary in different contexts. For example, unlike BDP, DHI supply fixed needle, colour coded ‘Nevershares’ (LDSS) aimed at decreasing the risk of accidental sharing. Preferences for colour coded equipment may influence how favourably non-colour coded detachable LDSS are viewed.
Recruitment of PWID through community pharmacies was also planned; however after the first few interviews it was apparent that most PWID also accessed injecting equipment from pharmacies as well as BDP or DHI. Therefore we were able to explore differences between advice and information provided in pharmacies by sampling through BDP and DHI.
Participant recruitment
Adults injecting heroin, crack cocaine and other opioids, and NSP staff who work with PWID were eligible to participate. BDP and DHI staff facilitated recruitment of PWID and staff via their NSP by distributing information leaflets and discussing the project. The advantage of this approach is that BDP and DHI have a rapport and trusting relationship with PWID who use their services.
Sampling of PWID occurred in waves to support the targeted recruitment of a diverse group with varying drug and injecting preferences and demographic characteristics. In relation to gender, we tried to reflect the profile of BDP users (~ 90 male: 10 female) and we sought to interview those using different injecting equipment (detachable needles of various sizes vs fixed equipment) and drug type as we wanted to understand the reasons for equipment choices among those with different preferences. We aimed to interview approximately 10–15 staff and 25–35 PWID.
Prior to conducting the interviews, JK had been through a process of familiarisation with the services offered by BDP, in particular the NSP, different injecting practices, language used by PWID and staff and the equipment offered by NSP.
Interview topic guide
Topic guides for PWID and staff (Supplementary files) explored how choices were made between available injecting equipment and the facilitators of, barriers to and processes of changing equipment type, focusing on attitudes towards detachable LDSS. In addition, a sorting task was devised where participants ordered ten features of detachable LDSS, derived from published literature, according to perceived importance while describing the reasons for their decisions. Through the ordering process and accompanying discussion we aimed to understand the likely acceptability of the new syringes. For example, if the equipment features were disliked or not viewed as important then this was likely to indicate limited acceptability. The sorting task was also intended to elicit the most promising way to promote the use of detachable LDSS. Participants were then asked about the willingness to use detachable LDSS (Appendix A). A question about rinsing equipment was added after 10 interviews as recent evidence suggested detachable LDSS may require several rinses to eliminate HCV (Binka et al., 2015).
Analysis
Interviews were digitally recorded, transcribed verbatim, checked for accuracy and anonymised. The Framework Method of analysis (Gale, Heath, Cameron, Rashid, & Redwood, 2013) was used with the support of QSR NVivo10. Transcripts were coded systematically and inductively, producing initial themes. These were discussed with the study team and developed to produce an agreed coding framework applied to all transcripts. During the coding process, revisions were made to the framework in response to new or redundant information. Themes were summarised in the framework matrix for each participant and PWID and staff responses were triangulated (Farmer, Robinson, Elliott, & Eyles, 2006). Frequent de-briefing meetings between study team members ensured different interpretations of the data were considered. This method was selected because the condensing and summarising process of completing the framework facilitates reflections on meaningful, salient themes as well as connecting or divergent perspectives between sets of participants.
The Faculty of Medicine and Dentistry Committee for Research Ethics, University of Bristol (Reference 19861) granted ethical permission. Written informed consent was sought prior to interview.
Results
Participant characteristics
23 PWID (15 men and 8 women) were interviewed through BDP (n=19) and DHI (n=4). The majority were housed and the average age was 40 years (range 28–53). The participants injected either heroine and or crack cocaine, using fixed 1ml syringes (n=18) and or detachable syringes (n=16). Participants used a range of injection sites: groin (n=12), arm (n=11), hands, wrists and fingers (n=6), legs (n=5) and feet (n=4). 19 PWID accessed injecting equipment through BDP, 2 from DHI, 16 from pharmacy NSP and, 2 were recruited through DHI services but used pharmacy NSP exclusively. ‘Other users’ were a reported source of equipment by two PWID. 13 staff (6 men and 7 women) were interviewed (BDP n=10 and DHI n=3) with a range of roles represented including NSP trainees (n=3), volunteers (n=3), engagement workers (n=3), managers (n=2) and pharmacists (n=2). The mean interview length was 29 minutes (range 16 to 50 minutes) for PWID and 42 minutes (range 27 to 66 minutes) for staff.
Factors informing injection practice decision making
Decisions about equipment type, injection site and syringe rinsing were informed by several factors: early experiences and peer initiation; awareness and availability of alternatives; and the ability to inject successfully. These factors are explored in-depth below.
Early experiences of injecting strongly influenced injecting practices. It was common for PWID to be shown how to inject or to be injected by their more experienced peers, particularly when starting to groin inject. Thus peer initiation was powerful in shaping equipment and injection site choices. PWID and staff also reported the importance of information sharing and problem-solving among peers, although a limited number of PWID described becoming isolated from peers. Staff highlighted the risks of peer initiation, such as the sharing of inaccurate information (e.g. advising the use of inappropriate equipment for the injection site), leading to risky behaviours and reluctance to try alternative approaches.
The first time I injected in my groin, an acquaintance done it for me, because I didn’t know how to do it.
A lot of people might be initiated into injecting by another person and will do what that person has shown them and we have to (…) give advice differently sometimes from what their friends have given them and that can be quite difficult. (…). You trust what your friends say, don’t you?
In addition to peers, NSP staff, and healthcare providers were identified as sources of information and support for maintaining and/or changing injecting practices. Specialist NSP staff in BDP and DHI provided harm reduction advice to PWID, this included advising on the most suitable equipment.
It’s about (…) trying to make sure people are using the right equipment. That can be about them using small enough needles or sometimes big enough needles, because the other bit of risk is needle snapping or people (…) not using a big enough gauge needle for intramuscular or for deep vein or groin vein injecting.
Researcher: Where d’you get sort of information and advice about different types of syringes and things?
Mainly here, on the [NSP] van.
Researcher: What kind of advice do you get here?
Well, anything you could ask for, really. They give you advice on how to use it, y’know.
Some participants were unaware of alternative more suitable injecting equipment. This was more common in PWID using pharmacy NSP where the range of equipment and specialist advice is limited. For instance, a minority of PWID were unaware that detachable needles were available for 1ml barrel sizes or that barrel sizes smaller than 1mls were available.
I think if people don’t ask [for advice in a pharmacy], quite often they won’t … it will often just literally be an exchange
I think that’s a good thing, that is, detachables. And I said I think they shoulda done it on the one mils they got now.
Instead of using the most suitable injecting equipment for their site, some PWID used needles which were either too long or short.
A chap who came in (…) was picking up long oranges. He was injecting in his arms which was a little bit concerning but because his peers had introduced him to injecting, they were using long oranges; he had no awareness around the availability of (…) the different gauges of the needles and the harm reduction message of using a smallest pin possible for your veins.
Even in my groin I will not do a two mil, because (…) if I can’t get it with a one mil after pushing it in as far as I can (…), that’s enough, that’s got to stop, not exchange different needles for me.
PWID injection practices were also influenced by the advice given by NSP staff. The decision for staff to give advice about rinsing equipment and provide cleaning tablets was problematic because historically reuse was strongly discouraged. However, a more pragmatic approach acknowledged the need to modify the advice.
The best we can achieve for someone (…) [is] that they use a clean pin every time they pierce their skin. That’s not always gonna be realistic for a whole variety of reasons. Some of those reasons we can change, but intransigence means that sometimes isn’t gonna be, and so at three o’clock in the morning, when we’re shut, and someone’s living in a hostel, they’re gonna re-use a dirty pin. (…) Now we talk more about safer re-using almost, so cleaning and talking to people about how to do that has become part of our intervention.
While every PWID who was asked about rinsing was aware of it, not everyone reported rinsing themselves. The main motivation for rinsing was to reduce the harms of re-use. Previous infections motivated PWID to not rinse or re-use. The relationship between rinsing and re-using equipment was also complex from PWID’s perspective: not rinsing risked re-use of unclean equipment in the event of shortages, while rinsing could encourage re-use.
Most people do [rinse syringes]. (…) I am trying to rinse them out, but then I think, well if I am rinsing them out, that means I am going to re-use them and that goes against the whole looking after your veins.
I actually don’t [rinse syringes], I am quite lazy, I just tend to take them out and put them straight in the bin, which in a way I think is good because then later on if I need to use one, I can’t, because I can clean it out, but it will be more hard work, I watch my boyfriend and he actually rinses his out after [use], but yeah I’m lazy but again there’s pros and cons to both.
Researcher: Can you tell me a bit about your reasons for why you were cleaning them and why you’re not cleaning them now?
‘Cause I got hepatitis now and I don’t wanna catch that again, ‘cause it was hard enough going through it the first time.
The injection site influenced the choice of equipment, for example vein depth and size are suited to different needle gauge and length. PWID attributed equipment and injection site choices to the ability to inject successfully whilst avoiding or minimising problems such as difficulties finding a vein, vein damage and collapse, pain due to abscesses and infections. As a result of such problems some PWID described themselves as having limited options for injection sites.
I haven’t had no (…) serious problems to go to the next needle up stage.
Obviously with the crack, cos obviously it’s quite (…) bitty and obviously when you suck it up it gets stuck in the pin and it’s hard to push in, so I tend to just use the blue [needle] cos it’s a lot easier. But I haven’t resorted to the long (…) I know people’s on long blues, but that’s after years and years, and I think the veins have shrunk or sunk back more so they obviously needs a longer one to reach it.
I’m not gonna sit there and mess about in my arms for 15, 20 minutes when I knows I can just go there [in the groin] and it’s in and it’s done straight away.
The [fixed] one mils, I think that’s the only ones really I could sort of use in the arms.
Others described preferences for discrete sites such as the groin which are hidden by clothing. Conversely, several PWID described reluctance to inject in the groin due to associated health risks.
I wouldn’t like to do my arms, cos obviously it shows and (…) you get track marks everywhere. So in that place [groin] it’s quite out the way and no one really knows.
I am kind of a bit scared, not scared, but I’m wary about using deeper veins. I’ve seen too many people, amputees and what have you, so I am very wary about using deeper veins.
Factors affecting willingness to change
Most PWID were reluctant to change injecting equipment and several barriers to change were identified by staff and PWID: length of time injecting; familiarity and routine; absence of problems injecting; prioritisation of getting a hit quickly over the prevention of future problems; mental state/withdrawing, and wariness about being able to successfully inject with different equipment.
The length of time PWID have been injecting was seen by staff as influencing whether PWID were open to equipment change advice. Similarly, the credibility of staff advising change may be questioned more strongly by PWID who have been injecting for a long time. Some PWID described themselves as very knowledgeable about injecting equipment and as not needing advice from staff.
Long term users they have been doing it for years they know what they’re doing – well they think they know what they’re doing. And, yeah, they’re not really open to suggestion to change.
I generally don’t take advice from the (name of specialist NSP) unless I’m asking for it, (…) It’s easier to ask somebody that I use with because I don’t feel any embarrassment about showing them my sites (…). So it’s easier to discuss with them because obviously they’ve got hands-on experience and they know, I’m not saying they don’t know what they’re talking about but it’s more theoretical.
Most PWID were reluctant to consider changing injecting equipment if they experienced no problems. Their injecting equipment had become an important component of a familiar routine. Indeed, several PWID and staff described PWID feeling attached to their equipment, describing it as what they are most “comfortable” and “familiar” with. For those with little control over their lives, equipment was depicted as part of an injecting routine which provided continuity. Furthermore, PWID were described predominantly by staff as wary of changing equipment because it might not be as easy to use and could result in the loss of drugs.
It’s only when problems occur, you know, the vein goes shy or it disappears or (…) neuropathy happens in the lower leg or abscesses occur, and then they get really scared, that they’ll consider something else.
That’s what I am used to, if it ain’t broke, don’t fix it and (…) the truth is there is a lot of habit involved in this whole thing you know, so I don’t really wanna change it.
We don’t like change (…) we think a lot of us are our events that have happened in our life, so we don’t like good or bad change, we are not controlling the event and as addicts, it is all about control.
There is always gonna be a bit of fear around using something new - using something that they’re not used to using. (…) Just feeling like they’re not gonna know how to use it, that it’s gonna waste their hit, that they’re not gonna find a vein quite so easily, that’s it’s gonna break somehow.
Previous experience of unplanned equipment change in one NSP resulted in some complaints about the poor quality of new equipment (e.g. flimsy needles which blocked easily). Quality checks were made and feedback to the manufacturer led to improvements to the syringe and needle design, but staff felt that further complaints reflected resistance to change of familiar equipment and lack of choice.
People have said that they [new equipment] block, other people say that they’re bent (…). Every time we have a complaint we’ll sort of have a look through – it just seems more than anything like they just don’t like the fact it’s something different.
The prioritisation of getting a hit quickly over the prevention of future problems was identified explicitly by staff and more implicitly by PWID as an important barrier to change. This prioritisation could be explained by PWID not being in the right state of mind to consider harm reduction practices when injecting in part due to the symptoms of withdrawing and being in a hurry to inject. One staff member recognised that PWID want to minimise the risks of injecting but are not in the right frame of mind when injecting. Further barriers to harm reduction practices included not realising that an infection is developing, experiencing no immediate side effects from an infection or perceiving themselves to have no other options than to inject in harmful or riskier ways (e.g. re-using or injecting into riskier sites). A small number of staff and PWID reported that in their experience PWID see problems with injecting and associated health problems as an inevitable part of intravenous drug use. In staff members’ view, focusing on the benefits of equipment change in relation to drug use, rather than on the prevention of potential health risks was more likely to be effective.
When you’re ill [withdrawing], you’re like just trying to get it anywhere, do you know what I mean? Some people just tend to push it in and just makes the leg just comes up in all sorts of trouble.
Some people think of it’s just like it’s going to happen, (…) So I am not really interested you know and I am thinking about right now, today, not in a few months or in a few years. I am not really interested, I want something now.
My observations and my personal experience is [as a former PWID], it’s about getting the drug in and the health and safety comes afterwards, always. You know, if you get someone whose clucking [withdrawing], their first thought in their head isn’t gonna be, “I need to wipe that with a swab.” It’s gonna be, “I want that in my body.” And that’s what I get off clients as well. (…) Or, “I’ve got hep and I’ve had it for years and it’s not affecting me now,”
If PWID experienced problems with their equipment, they sought changes to resolve them as described above, for example progressing onto a longer needle, and if there was a perceived benefit they were more willing to consider change. PWID being in a hurry during needle exchanges, for example if they want to take drugs soon or are withdrawing from the last hit, presented a barrier to encouraging a behaviour change for staff.
I just thought well, I can’t get it with that, I need a longer one, I’ll go long oranges.
If there was something which (…) definitely had no problems with it and it would leave smaller track marks and was maybe sharper, I wouldn’t have a problem changing, but(…) I would have to be a hundred per cent sure on that.
There will be a whole host of reasons why somebody, even just for that particular hour, doesn’t wanna listen. They might have drugs in their pocket, they might be really feeling like they’re in pain and really need to go and use, and it’s just not the right time.
To support behaviour change, staff sought to raise awareness of alternative, more suitable equipment by explaining the benefits of change, referring to positive experiences of others, offering some needles/syringes to try alongside usual equipment and inviting feedback at the next visit. Building trusting relationships with PWID and supporting their autonomy to make decisions about their equipment were seen as important.
I always say to them ‘you are the expert on you, however, have you thought about? (…). I say ‘here are your options because I think it’s always good for you to know what is available, then for you to make that choice’, because I think it gives them a sense of self-empowerment and they are more in control.
Managing the introduction of detachable LDSS
There was a preference for a gradual introduction of detachable LDSS with information and opportunity to try them alongside usual equipment. This was expected to allow PWID to experience the benefits of detachable LDSS and for NSP to respond to problems if they arose. Replacing old equipment with detachable LDSS completely was suggested by a minority.
If you just did replace (…) all of them, (…) some people might not get on with them, (…) but if they’ve got a few and they’ve still got their normal ones then they would see a difference if they’re better.
In addition to conversations about the benefits of detachable LDSS, educational events, posters, leaflets, videos and presentations were also suggested as useful tools to raise awareness of detachable LDSS.
Talking to people about what the equipment … why it was different and how it was beneficial, (…) talking about high dead space and sort of the theory behind that, of carrying infection, and also what low dead space means so the fact that it means that more of the hit’s coming out. I don’t think people are gonna struggle with the idea that it’s a good thing but the real work (…) is after people have gone away and used the equipment, is if there were any issues around it – that’s when we would then have to sort of have a conversation about tackling that.
Training for staff about the features of detachable LDSS was expected to be valuable, especially for less experienced staff, and peer encouragement to use detachable LDSS was thought to be helpful, although this may happen unprompted.
I think that [information about new equipment] would automatically get spread about as well. Like I said, me I don’t really bother with a lot of people, but most people talks. They’re always talking about drugs and the stuff they’re using or whatever. I guarantee it will get about, no problem.
In terms of cost, staff thought that detachable LDSS equipment may be more expensive initially. In contrast, there were assumptions among PWID that new equipment is introduced because it is cheaper or recycled. In Bristol, approximately 35% of the detachable HDS needles issued through the NSP have LDS equivalents. Replacing these would incur a 19% cost increase at current prices. This may vary in other parts of the UK and internationally with locally agreed suppliers’ contracts. Given the beneficial effect of NSP on BBV incidence and prevalence (Turner et al., 2011; Vickerman, Martin, Turner, & Hickman, 2012) any impact on the transmission of BBV is likely to be cost-effective (Zoe Ward, personal communication, April 14th, 2016).
Factors influencing the acceptability of detachable LDSS
Despite the anticipated initial irritation about the removal of familiar equipment, most PWID were expected to be willing to try detachable LDSS and to continue using them if they worked as well as the original equipment.
If it’s gonna make a difference for us in catching infections then its good.
As long as they got their hit, I don’t think they’d care. I don’t think they would care, as long as they got what they want out of it, (…) I can’t see no issue.
The value ascribed to ten detachable LDSS features (presented below) determined through the sorting task (described in the Methods) was indicative of the acceptability of detachable LDSS. The lower risk of BBV transmission and reduced drug wastage were particularly valued features.
1). “Lower risk of transferring infection if shared”
Staffs expected ‘getting a hit’ to be prioritised over harm reduction practices and were sceptical about the value PWID would place on this feature because PWID were expected to value the feature ‘less wasted drugs’ more. They were also concerned about explaining the lower risk of transferring infections to PWID in case this led to sharing/re-using equipment. However, the majority of PWID valued the reduced risk of BBV because they did not want to acquire or transfer infections.
If they think they’re not wasting any drug, that is gonna be ultimately … the worry about BBVs and all that is gonna be secondary.
I don’t want to get infections … Because I can be quite lax on thinking this stuff at the time, so I think that is quite important. Yeah definitely, lower the risk of transferring infections. (…)
You get the thinking of I will deal with it later, if I get an illness I will deal with it later.
2). “Less wasted drug” and 3) “better value for money”
The majority of PWID and staff valued the feature ‘less wasted drug’ and felt that it would encourage the use of detachable LDSS. Conversely, eight PWID commented that this feature was not important or was less important than “lower risk of transferring infections” due to the small volume of drug wasted. While the majority of PWID and staff valued the importance of detachable LDSS representing better value for money, a few PWID commented that the waste of drug was a greater concern than the contemporaneous waste of money.
Less waste is obvious isn’t it, no-one wants to waste anything in life, but drugs since it is our obsession it’s the most important thing.
It’s a really helpful intervention (…) I don’t have to talk about diseases and viruses and stuff, but these syringes here, you get absolutely all your drug.
4). “Easier to share drugs accurately”
Those who divided drugs in groups acknowledged that arguments about unfair drug distribution do occur with all equipment types although not everyone viewed this as an important issue.
5). “No need for ‘flushing’” (pushing/pulling the plunger in and out while in the vein)
This feature was viewed positively by a few PWID. In contrast, a few PWID and roughly half the staff did not think that flushing would stop after the introduction of detachable LDSS due to its habitual nature.
[Flushing] is one of those things I have always done right from the start, so I am not going to stop doing it now and I feel like there is something missing if I don’t do it.
6). “Same experience of the drug”, 7) “look and feel the same”, 8) “as easy to find a vein”, 9) “detachable needles” and 10) “range of needle and syringe sizes”
Most staff felt that these features were important to PWID and could help reassure PWID that their experience would be unaffected by the new equipment. However, PWID tended to view these features as less important. Several PWID thought that the new equipment would not affect the experience of the drug or ease of finding a vein. Most PWID felt that equipment looking and feeling the same was not as important as whether it worked the same. Availability of detachable needles in different barrel and needle sizes enabled people to have the equipment suited to their needs.
The explanation that they’re not different from other ones, like in negative ways, would be quite important saying that, yeah, like, it doesn’t … has the same experiences, it’s easy to find the vein, as I say, the look and feel of the normal equipment.
Look and feel the same, I am not quite sure if that’s particularly important, if they work, they work and people might be a bit kind of curious or even a bit sceptical or hesitant about, but once they see that it works, they will be fine.
Discussion
This qualitative study explored the acceptability of detachable LDSS to PWID. Our findings suggest that an important motivation of PWID is to inject successfully and without problems and therefore changes to equipment were considered only when injecting problems necessitated them. Change was also considered by PWID in this sample if there was a perceived benefit of optimising drug use. Generally, PWID preferred to avoid changing equipment because routines and rituals were experienced as sources of control and comfort. Despite this reluctance, the findings indicate that detachable LDSS are likely to be acceptable to PWID if they work as well as the original equipment because of the perceived benefits of a reduction in wasted drug, and a lower risk of transferring BBV. These findings have been used locally to develop recommendations for a targeted intervention within NSP with ‘training’, ‘education’, ‘persuasion’ and eventual ‘restriction’ components.
We corroborate the WHO ‘Values and preferences’ report on interviews with PWID and service providers which found no preference for or against LDSS. However, the WHO study did not focus on detachable LDSS (WHO, 2012a). Our participants valued the lower risk of BBV transmission. This differs from previous research which identified the main benefit of LDSS as reduced drug wastage (Gray et al., 2012). Therefore, while it may be prudent to focus on the immediate benefit of detachable LDSS (i.e. reduced drug wastage) because PWID appear to focus on the present rather than future problems, the importance attached to the lower risk of transferring infections suggests that this information should also be given to PWID, especially those known to share or re-use equipment. However, some staff were reluctant to tell PWID about the lower risk of BBV transmission because they were concerned that it may inadvertently encourage sharing or re-use of LDSS (Bobashev & Zule, 2010). Indeed, motivation for immediate, individual-level benefits of detachable LDSS, such as reduced drug wastage, is expected to be greater than reduced population-level risk of future BBV infection (Rose, 2001).
Our work and that of others, contradict assumptions that PWID are not concerned with their health (Olsen, Banwell, Dance, & Maher, 2012) and illustrate that a set of complex factors inform injecting decisions. Despite concern for their health and valuing harm reduction advice, we found that PWID prioritise getting a hit quickly over the prevention of future problems when they are not in the right state of mind when they are in drug withdrawal and in a hurry to inject. This finding also reflects the work of others (Bonar & Rosenberg, 2014; Gleghorn & Corby, 1996). ‘Crisis moment’ narratives in which equipment is shared during exceptional circumstances (e.g. drug withdrawal) provide an illustration of situations when risks are temporarily denied and there is a conflict between ‘caring’ about harm reduction practices but ‘not caring when in crisis’ p228 (Rhodes, Prodanović, et al., 2008). In our interviews we also found that participants experienced tensions between rinsing injecting equipment and the risks of re-use. Furthermore, in our study, a sense of fatalism about the development of injecting problems (McBride, Pates, Arnold, & Ball, 2001) and infections contributed to difficulties focusing on future health risks (Wilton, 1997) and or taking preventative actions (Rhodes, Žikić, Prodanović, Kuneski, & Bernays, 2008). Olsen and colleagues also found that HCV was viewed as “inevitable, or at least an accepted risk of injecting drugs” p313 (Olsen et al., 2012).
Peer initiation and on-going information sharing was found to be powerful in shaping equipment choices. Staff supported these accounts, but also highlighted the risks of peer initiation of distributing inaccurate information, leading to risky behaviours and reluctance to try alternative approaches. Other studies report that peer injecting is also a common way to overcome initial fear of needles and injections (McBride et al., 2001). Peer encouragement to use detachable LDSS was thought to be helpful, although this may happen anyway. This finding is echoed by the WHO which highlights peer based interventions as critical to the success of initiatives (WHO, 2012a). The benefits of PWID-led safer injecting education include PWID’s ability to relate to their peers and use language in more appropriate and accessible ways than professional or other staff. There are also psychological benefits for peer educators (Callon, Charles, Alexander, Small, & Kerr, 2013). Such peer education could help to shift norms of acceptable injecting equipment to reduce harms among PWID (Rose, 2001).
Our findings relating to the resistance to change syringes are consistent with previous research (Zule & Cross, 2012) and may be explained in part by equipment becoming part of important injecting rituals. Such rituals may reflect conditioned responses to the reward of injecting (McBride et al., 2001). Fear can also trigger change towards more positive health behaviours among PWID (Stajduhar, Funk, Shaw, Bottorff, & Johnson, 2009); for example, in our study PWID were afraid of injecting into their groin because of the health risks associated with this practice. Furthermore, experiences of problems associated with injecting caused fear which prompted PWID to consider change. However, in our study fear of being unable to inject successfully with unfamiliar equipment contributed to resistance to changing equipment.
Strengths and limitations
Sampling participants from two sites generated evidence about the acceptability of PWID from different contexts, enhancing the findings’ credibility and transferability. Although we conducted fewer interviews in Bath compared to Bristol we did not find any notable difference in the attitudes towards detachable LDSS between both settings. PWID were positioned as the expert and reminded of the researcher’s independence from the NSP at the start of the interview to encourage honest responses. Triangulation of staff and PWID perspectives generated a rich understanding of the acceptability of detachable LDSS. Frequent debriefing sessions with BDP staff and service user input during the analysis process ensured that our findings were firmly grounded in the reality of PWID experiences.
A limitation of this study is that participants were responding to a hypothetical change to equipment which they had not used or seen. The sorting task helped overcome this limitation as it enabled participants to appraise the features of detachable LDSS.
Recommendations for the development of an intervention to support the introduction of detachable LDSS
Using the COM-B (Capability, Opportunity, Motivation – Behaviour) model (Michie, Atkins, & West, 2014), our findings suggest that a targeted intervention within NSP with ‘training’, ‘education’, ‘persuasion’ and eventual ‘restriction’ is likely to enhance the acceptability of detachable LDSS. The components of the model are relevant to managing a switch from HDSS to LDSS: ‘Psychological capability’ (understanding why changing to detachable LDSS is important and overcoming reluctance to change), ‘Physical and Social opportunity’ (ensuring detachable LDSS are available to try, and providing support in NSP), ‘Reflective motivation’ (encouraging PWID to want detachable LDSS by understanding their benefits) and ‘Automatic motivation’ (developing new habitual patterns) (Michie et al., 2014).
Training for NSP staff
Training for NSP staff about the benefits of detachable LDSS, how to identify PWID who may benefit from them, and how to encourage behaviour change is needed. Where PWID mainly use pharmacy NSP, an increase in provision of “advice and information on how to reduce the harms caused by injecting drugs” (NICE, 2014) is required.
Education for PWID
Verbal and written information for PWID will raise awareness of the benefits of detachable LDSS and provide reassurance that the injecting process and experience would not be affected. Education should focus on the benefits of detachable LDSS (i.e. less wasted drug and lower risk of transferring infections) and should be tailored to the needs and preferences of service users. The spread of information via peer PWID networks should be promoted within NSP. Encouraging appropriate syringe rinsing methods for PWID known to re-use or share equipment is also required. This is underscored by the recent finding that while HCV is undetectable after one rinse in the fixed LDSS, several rinses are required for detachable LDSS (Binka et al., 2015).
Persuasion and restriction
Gradual introduction of detachable LDSS was preferred over replacing equipment without warning or support for PWID autonomy. When introducing an intervention into routine practice, monitoring is required to identify adverse events and long term outcomes(Craig et al., 2008), therefore NSP should value and report negative feedback to manufacturers to facilitate improvements in syringe design (Jacka, 2013; WHO, 2012b). Once the majority of NSP users have received information and new equipment to try, old HDSS can be phased out.
Conclusion
This is the first qualitative study assessing the acceptability of detachable LDSS to PWID. While changing equipment is difficult for PWID, the benefits of detachable LDSS were viewed favourably and gradual change in equipment, supported by verbal and written information from NSP which empowers PWID, is expected to enhance their acceptability.
Supplementary Material
Acknowledgements
The research is supported by CLAHRC West at University Hospitals Bristol NHS Foundation Trust. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Jo Kesten is partly funded by CLAHRC West at University Hospitals Bristol NHS Foundation Trust and NIHR Health Protection Research Unit in Evaluation of Interventions at University of Bristol in partnership with Public Health England.
Matthew Hickman and Peter Vickerman are funded by a NIHR Health Protection Research Unit in Evaluation of Interventions at University of Bristol in partnership with Public Health England. This work was additionally supported by the National Institute on Drug Abuse (grant number R01 DA037773–01A1).
Sabi Redwood is funded by a NIHR CLAHRC West.
A steering group comprising researchers, public health managers, Bristol Drugs Project (BDP) NSP staff and a current NSP service user managed the study.
References
- Abdala N, Stephens PC, Griffith BP, & Heimer R (1999). Survival of HIV-1 in syringes. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology, 20(1), 73–80. [DOI] [PubMed] [Google Scholar]
- Albers ER (2013). Implementation of low dead-space syringes needs consultation and engagement with drug users. International Journal of Drug Policy, 24(1), 15–22. [DOI] [PubMed] [Google Scholar]
- Ambekar A, & Pawar A (2013). Low Dead-Space Syringes for HIV prevention among people who inject drugs: interesting, but a much stronger case is required. Int J Drug Policy, 24(1), 16–18. [DOI] [PubMed] [Google Scholar]
- Binka M, Paintsil E, Patel A, Lindenbach BD, & Heimer R (2015). Survival of Hepatitis C Virus in Syringes Is Dependent on the Design of the Syringe-Needle and Dead Space Volume. PLoS ONE, 10(11), e0139737. doi: 10.1371/journal.pone.0139737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobashev GV, & Zule WA (2010). Modeling the effect of high dead-space syringes on the human immunodeficiency virus (HIV) epidemic among injecting drug users. Addiction, 105(8), 1439–1447. doi: 10.1111/j.1360-0443.2010.02976.x [DOI] [PubMed] [Google Scholar]
- Boily MC, & Shubber Z (2014). Modelling in concentrated epidemics: Informing epidemic trajectories and assessing prevention approaches. Current Opinion in HIV and AIDS, 9(2), 134–149. [DOI] [PubMed] [Google Scholar]
- Bonar EE, & Rosenberg H (2014). Injection Drug Users’ Perceived Barriers to Using Self-Initiated Harm Reduction Strategies. Addiction research & theory, 22(4), 271–278. doi: 10.3109/16066359.2013.838225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristol City Council. (2016). The Population of Bristol July 2016. Retrieved from https://www.bristol.gov.uk/statistics-census-information/the-population-of-bristol:
- Callon C, Charles G, Alexander R, Small W, & Kerr T (2013). ‘On the same level’: facilitators’ experiences running a drug user-led safer injecting education campaign. Harm Reduct J, 10, 4. doi: 10.1186/1477-7517-10-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centre for Infectious Disease Surveillance and Control and Microbiology Services, C., Public Health England. (2015). Unlinked Anonymous Monitoring Survey of People Who Inject Drugs. The report on the results obtained from the samples and questionnairesreceived during 2014 from Bristol Drugs Project. [Google Scholar]
- Ciccarone D (2013). Saying goodbye to high-dead-space syringes. International Journal of Drug Policy, 24(1), 15–16. doi: 10.1016/j.drugpo.2012.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, & Petticrew M (2008). Developing and evaluating complex interventions: new guidance. Retrieved from London: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison C, Smith GD, & Frankel S (1991). Lay epidemiology and the prevention paradox: the implications of coronary candidacy for health education. Sociology of Health & Illness, 13(1), 1–19. doi: 10.1111/j.1467-9566.1991.tb00085.x [DOI] [Google Scholar]
- DHI Online. (2015). Engagement. Retrieved 28t July 2016 from http://www.dhi-online.org.uk/do/bath/engagement/
- Farmer T, Robinson K, Elliott SJ, & Eyles J (2006). Developing and Implementing a Triangulation Protocol for Qualitative Health Research. Qualitative Health Research, 16(3), 377–394. doi: 10.1177/1049732305285708 [DOI] [PubMed] [Google Scholar]
- Gale N, Heath G, Cameron E, Rashid S, & Redwood S (2013). Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Medical Research Methodology, 13(1), 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaughwin MD, Gowans E, Ali R, & Burrell C (1991). Bloody needles: The volumes of blood transferred in simulations of needlestick injuries and shared use of syringes for injection of intravenous drugs. AIDS, 5(8), 1025–1027. [PubMed] [Google Scholar]
- Gleghorn AA, & Corby NH (1996). Injection Drug Users’ Reactions to Guidelines for Bleach Disinfection of Needles and Syringes: Implications for HIV Prevention. Journal of Drug Issues, 26(4), 865–881. doi: 10.1177/002204269602600408 [DOI] [Google Scholar]
- Gray R, Nguyen MT, & Neukom J (2012). Rapid assessment of needle and syringe types used by people who inject drugs in Hanoi and Ho Chi Minh City, Vietnam. Retrieved from Vietnam: [Google Scholar]
- Grund JP, & Stern LS (1991). Residual blood in syringes: size and type of syringe are important. AIDS, 5(12), 1532–1533. [DOI] [PubMed] [Google Scholar]
- Gyarmathy VA, Neaigus A, Li N, Ujhelyi E, Caplinskiene I, Caplinskas S, & Latkin CA (2010). Liquid drugs and high dead space syringes may keep HIV and HCV prevalence high - a comparison of Hungary and Lithuania. European Addiction Research, 16(4), 220–228. doi: 10.1159/000320287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyarmathy VA, Neaigus A, Mitchell MM, & Ujhelyi E (2009). The association of syringe type and syringe cleaning with HCV infection among IDUs in Budapest, Hungary. Drug and Alcohol Dependence, 100(3), 240–247. doi: 10.1016/j.drugalcdep.2008.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huong NT, Mundy G, Neukom J, Zule W, Tuan NM, & Tam NM (2015). Social marketing of low dead space syringes in Vietnam: findings from a 1-year pilot program in Hanoi, Thai Nguyen, and Ho Chi Minh City. Harm Reduct J, 12, 15. doi: 10.1186/s12954-015-0049-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibragimov U, & Latypov A (2012). Needle and syringe types used by people who inject drugs in Eastern Europe and Central Asia: Key findings from a rapid situation assessment. Retrieved from Vilnius: [Google Scholar]
- Jacka. (2013). How to encourage use of low dead space syringes? The Viet Nam experience. Int J Drug Policy, 24(1), 18–19. [DOI] [PubMed] [Google Scholar]
- Jones HE, Welton NJ, Ades AE, Pierce M, Davies W, Coleman B, … Hickman M (2015). Problem drug use prevalence estimation revisited: heterogeneity in capture–recapture and the role of external evidence. Addiction, n/a-n/a. doi: 10.1111/add.13222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin NK, Foster GR, Vilar J, Ryder S, Cramp ME, Gordon F, … Hickman M (2015). HCV treatment rates and sustained viral response among people who inject drugs in seven UK sites: real world results and modelling of treatment impact. J Viral Hepat, 22(4), 399–408. doi: 10.1111/jvh.12338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride AJ, Pates RM, Arnold K, & Ball N (2001). Needle fixation, the drug user’s perspective: a qualitative study. Addiction, 96(7), 1049–1058. doi: 10.1046/j.1360-0443.2001.967104914.x [DOI] [PubMed] [Google Scholar]
- Michie S, Atkins L, & West R (2014). The behaviour change wheel. A guide to designing interventions. Great Britain: Silverback Publishing. [Google Scholar]
- NICE. (2014). Needle and syringe programmes. NICE public health guidance 52. Retrieved from London: [Google Scholar]
- Olsen A, Banwell C, Dance P, & Maher L (2012). Positive health beliefs and behaviours in the midst of difficult lives: women who inject drugs. Int J Drug Policy, 23(4), 312–318. doi: 10.1016/j.drugpo.2012.01.003 [DOI] [PubMed] [Google Scholar]
- Paintsil E, He H, Peters C, Lindenbach BD, & Heimer R (2010). Survival of hepatitis C virus in syringes: implication for transmission among injection drug users. J Infect Dis, 202(7), 984–990. doi: 10.1086/656212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmateer NE, Hutchinson SJ, Innes H, Schnier C, Wu O, Goldberg DJ, & Hickman M (2013). Review and meta-analysis of the association between self-reported sharing of needles/syringes and hepatitis C virus prevalence and incidence among people who inject drugs in Europe. Int J Drug Policy, 24(2), 85–100. doi: 10.1016/j.drugpo.2012.08.006 [DOI] [PubMed] [Google Scholar]
- Public Health England. (2013). Facts & figures. Prevalence Data. Estimates of the Prevalence of Opiate Use and/or Crack Cocaine Use, 201½012. Prevalence estimates by local authority. Retrieved from http://www.nta.nhs.uk/facts-prevalence.aspx
- Public Health England, Health Protection Scotland, Public Health Wales, & Public Health Agency Northern Ireland. (2015). Shooting up. Infections among people who inject drugs in the UK, 2014. An update, November 2015. Retrieved from London: [Google Scholar]
- Rafful C, Zule W, Gonzalez-Zniga PE, Medina-Mora ME, Magis-Rodriguez C, & Strathdee S (2015). High dead-space syringe use among people who inject drugs in Tijuana, Mexico. Drug and Alcohol Dependence, 146, e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes T, Prodanović A, Žikić B, Kuneski E, Pavićević T, Karadžić D, & Bernays S (2008). Trust, disruption and responsibility in accounts of injecting equipment sharing and hepatitis C risk. Health, Risk & Society, 10(3), 221–240. doi: 10.1080/13698570802160921 [DOI] [Google Scholar]
- Rhodes T, Žikic B, Prodanovic A, Kuneski E, & Bernays S (2008). Hygiene and uncertainty in qualitative accounts of hepatitis C transmission among drug injectors in Serbia. Social Science & Medicine, 66(6), 1437–1447. doi: 10.1016/j.socscimed.2007.11.009 [DOI] [PubMed] [Google Scholar]
- Rose G (2001). Sick individuals and sick populations. Int J Epidemiol, 30(3), 427–432. doi: 10.1093/ije/30.3.427 [DOI] [PubMed] [Google Scholar]
- Stajduhar KI, Funk L, Shaw AL, Bottorff JL, & Johnson J (2009). Resilience from the perspective of the illicit injection drug user: An exploratory descriptive study. International Journal of Drug Policy, 20(4), 309–316. doi: 10.1016/j.drugpo.2008.07.003 [DOI] [PubMed] [Google Scholar]
- Strauss K, van Zundert A, Frid A, & Costigliola V (2006). Pandemic influenza preparedness: The critical role of the syringe. Vaccine, 24(22), 4874–4882. doi: 10.1016/j.vaccine.2006.02.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner, Hutchinson S, Vickerman P, Hope V, Craine N, Palmateer N, … Hickman M (2011). The impact of needle and syringe provision and opiate substitution therapy on the incidence of hepatitis C virus in injecting drug users: pooling of UK evidence. Addiction, 106(11), 1978–1988. doi: 10.1111/j.1360-0443.2011.03515.x [DOI] [PubMed] [Google Scholar]
- Vickerman P, Martin N, Turner K, & Hickman M (2012). Can needle and syringe programmes and opiate substitution therapy achieve substantial reductions in hepatitis C virus prevalence? Model projections for different epidemic settings. Addiction, 107(11), 1984–1995. doi: 10.1111/j.1360-0443.2012.03932.x [DOI] [PubMed] [Google Scholar]
- Vickerman P, Martin NK, & Hickman M (2013a). Could low dead-space syringes really reduce HIV transmission to low levels? Int J Drug Policy, 24(1), 8–14. doi: 10.1016/j.drugpo.2012.10.006 [DOI] [PubMed] [Google Scholar]
- Vickerman P, Martin NK, & Hickman M (2013b). Could low dead-space syringes really reduce HIV transmission to low levels? International Journal of Drug Policy, 24(1), 8–14. doi: 10.1016/j.drugpo.2012.10.006 [DOI] [PubMed] [Google Scholar]
- Walsh N, Verster A, Rodolph M, & Akl EA (2014). WHO guidance on the prevention of viral hepatitis B and C among people who inject drugs. International Journal of Drug Policy, 25(3), 363–371. doi: 10.1016/j.drugpo.2014.01.009 [DOI] [PubMed] [Google Scholar]
- WHO. (2012a). Annex 8: Values and Preferences report. Based on interviews with community members affected by and providers working on viral hepatitis. Retrieved from Geneva, Switzerland: [Google Scholar]
- WHO. (2012b). Guidance on prevention of viral hepatitis B and C among people who inject drugs. Retrieved from Geneva, Switzerland: [PubMed] [Google Scholar]
- WHO, UNAIDS, & United Nations Office on Drugs and Crime. (2007). Guide to starting and managing needle and syringe programmes. Retrieved from Geneva, Switzerland: [Google Scholar]
- Wilton T (1997). Look after yourself: HIV/AIDS health education and promotion Engendering AIDS Deconstructing Sex, Text and Epidemic (pp. 48): Sage Publications. [Google Scholar]
- Zule. (2012). Low dead-space syringes for preventing HIV among people who inject drugs: Promise and barriers. Current Opinion in HIV and AIDS, 7(4), 369–375. doi: 10.1097/COH.0b013e328354a276 [DOI] [PubMed] [Google Scholar]
- Zule, & Bobashev G (2009). High dead-space syringes and the risk of HIV and HCV infection among injecting drug users. Drug and Alcohol Dependence, 100(3), 204–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zule, & Cross H (2012). Switching people who inject drugs from high dead space to low dead space syringes as a structural intervention to prevent injection-related HIV epidemics. Journal of the International AIDS Society, 15, 151. [Google Scholar]
- Zule, Cross HE, Stover J, & Pretorius C (2013). Are major reductions in new HIV infections possible with people who inject drugs? The case for low dead-space syringes in highly affected countries. International Journal of Drug Policy, 24(1), 1–7. doi: 10.1016/j.drugpo.2012.07.002 [DOI] [PubMed] [Google Scholar]
- Zule, Desmond DP, & Neff JA (2002). Syringe type and drug injector risk for HIV infection: A case study in Texas. Social Science and Medicine, 55(7), 1103–1113. [DOI] [PubMed] [Google Scholar]
- Zule, Latypov A, Otiashvili D, Kirtadze I, Ibragimov U, & Bobashev G (2015). Acceptability and preferences for low dead space needles and syringes among needle and syringe program clients. Paper presented at the Submitted to 24th International Harm Reduction Conference, Kuala Lumpur. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


