Abstract
AMD11070 binds to the chemokine receptor CXCR4, with anti-HIV-1 activity in vitro and in vivo. We conducted a phase IB/IIA proof-of-concept dose-escalating, open-label study to determine safety and antiviral activity of AMD11070 administered over 10 days to HIV-1-infected participants who harbored CXCR4-tropic virus. Primary endpoints were ≥1 log10 rlu (relative luminescence units) reduction in CXCR4-tropic virus during 10 days of AMD11070 treatment or in the 7 days following treatment discontinuation, rlu changes over 10 days of treatment, and safety. Plasma pharmacokinetic parameters, HIV-1 RNA, and safety labs were obtained over 90 days of study. The study was stopped early due to emerging AMD11070 animal toxicity data. Six HIV-infected participants with plasma HIV-1 RNA ≥5,000 copies/mL on no antiretroviral therapy for 14 days before entry were treated. AMD11070 was well-tolerated when administered at 200 mg orally every 12 h for 10 days. All enrolled participants had dual/mixed (D/M) viruses. Reductions of almost 1 log10 rlu or more in CXCR4 virus were seen in three of six participants after 10 days of treatment. No participants had ≥1 log10 decline in plasma HIV-1 RNA from baseline at day 10. No clear relationship between pharmacokinetic parameters and response to therapy (X4 log rlu reduction) was observed. AMD11070 demonstrated in vivo activity as measured by reductions in CXCR4 rlu signal. Despite the finding of discordant rlu and plasma HIV RNA responses in these participants with D/M viruses, exploration of other HIV-1 CXCR4 antagonist therapies is possible.
Keywords: HIV-1 entry inhibitors, CXCR4 inhibitor, AMD11070
Introduction
HIV-1 entry into permissive CD4+ T cells requires that the virus initially binds to envelope gp120 and subsequently utilizes either the cellular CC chemokine receptor 5 (CCR5 or R5 virus), the CXC chemokine receptor 4 (CXCR4 or X4 virus), or both receptors (termed dual-tropic or mixed-population [D/M] viruses).1–5 Antagonists to CCR5 or CXCR4 have been identified with activity in vitro and in vivo.6–20 Maraviroc, a CCR5 antagonist, is now approved for HIV-1 infection in antiretroviral (ARV) treatment-experienced patients.21
AMD11070 exhibits potent and selective inhibition of HIV-1 replication by binding to the chemokine receptor CXCR4, with activity observed in vitro and in HIV-infected human participants.11,13,17,20 The starting dose, based on the determination in A519117 of plasma concentrations that approached the no-observed-adverse-event level in dogs, was 200 mg orally every 12 h. In the absence of dose-limiting toxicities by day 17, additional cohorts were to be opened in dose increments of 200 mg per cohort. Plasma AMD11070 concentrations observed 12 h postdose fell within the range or exceeded IC90 values (adjusted for protein binding) for the inhibition of replication of CXCR4-dependent virus, suggesting that antiviral activity may be observed in HIV-infected patients using these dose regimens.
We conducted a phase IB/IIA proof-of-concept, dose-escalating, open-label study to determine the safety and antiviral activity of AMD11070 administered orally over 10 days to HIV-1-infected participants who harbored CXCR4-tropic virus. By design, the study would examine eight cohorts; however, only the first cohort was studied due to animal toxicity data that emerged during the trial, leading to termination of the development of AMD11070. Results from this first cohort are presented here.
Materials and Methods
Eight cohorts were planned, six participants per AMD11070 dose level for a total of 48 participants. Doses planned ranged from 200 mg every 12 h to 2,000 mg once a day. Treatment would continue for 10 consecutive days. Entry criteria included: HIV-infected men and women ≥18 years, no active opportunistic infection, plasma HIV-1 RNA ≥5,000 copies/mL, may have failed any number of prior ARV regimens or had no prior ARV therapy, no ARV drugs for at least 14 days at study entry, and presence of CXCR4-tropic virus or D/M CXCR4/CCR5 virus, as measured by a screening HIV-1 co-receptor tropism assay (Trofile; Monogram Biosciences, Inc., South San Francisco, CA) and defined as CXCR4 luciferase signal activity ≥2,000 relative luminescence units (rlu).22 The parent clinical trial protocol was approved by the local institutional review boards.
Written informed consent was obtained from all participants. Primary endpoints were ≥1 log10 rlu reduction in CXCR4-tropic virus (a direct reflection of CXCR4 receptor inhibition) during 10 days of AMD11070 treatment or in the 7 days following treatment discontinuation, rlu changes over 10 days of CXCR4 treatment, and safety. Baseline AMD11070 susceptibility was also determined (HIV-1 PhenoSense Entry assay; Monogram Biosciences, Inc.).23 Participants could restart ARV therapy after study day 11 based on screening HIV-1 drug resistance results. Plasma HIV-1 RNA levels and safety labs were obtained over 90 days of study. HIV-1 RT and protease genotyping was performed to demonstrate that AMD11070 therapy did not lead to viral resistance evolution in other regions of HIV-1 genes (HIV-1 TruGene; Siemens Healthcare Diagnostics).24
Whole blood for AMD11070 concentrations in plasma was collected at baseline and 0.5, 1.0, 1.5, 2.0, 4, 6, 8, 10, 12, 24, and 30–38 h postdose at steady-state (after last dose on day 10) under fasting conditions. AMD11070 concentrations were assayed at AnorMED, Inc. (Langley, British Columbia) using liquid chromatography with mass spectrometry detection. Participants in cohort A received AMD11070 200 mg orally twice daily for 10 days.25,26 As-treated statistical analyses were conducted for participants who remained on study drug through day 10; these analyses may be considered intent-to-treat as well, as all participants remained on treatment through day 10.
Based on long-term animal toxicity seen in rats (eyes), an ophthalmology exam was done locally after 2–3 years of follow-up on available participants. The evaluation included a visual acuity assessment, color vision assessment, slit lamp examination, retinal examination with photographs, and an electroretinogram. Given true probabilities of adverse events of 3%, 10%, 40%, and 50%, a sample size of six per cohort gave probabilities of escalating to the next highest-dose cohort of 99%, 89%, 23% and 11%, respectively.
Results
Screening
Seventy-eight participants were screened at eight sites. Six participants were enrolled (8%). Screen failures occurred in 72 participants (92%). The most common reason for screen failure was the detection of CCR5-only tropism or detection of CXCR4 using virus that had <2,000 CXCR4 rlu; 76% of screen failures had a CXCR4 rlu level that was too low.
Activity, safety, and pharmacokinetic findings
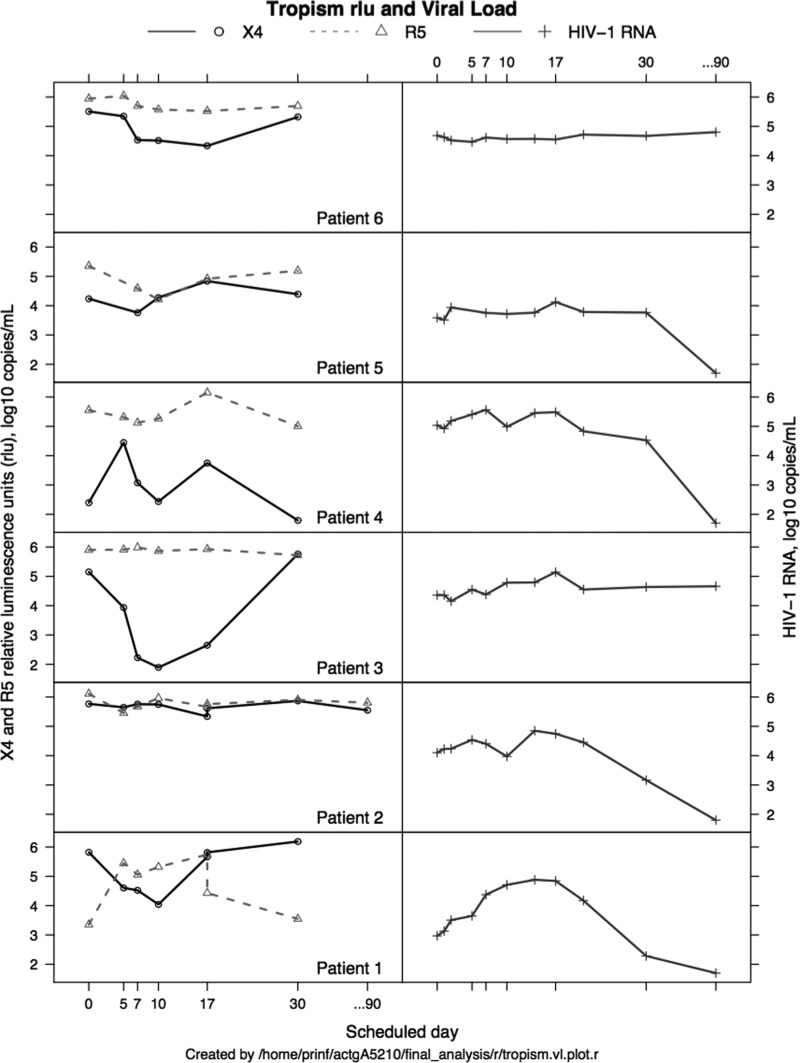
Six participants enrolled: one Caucasian, five African Americans; three male and three female, with a mean age of 41 years. Four participants were ARV-naïve, two were ARV-experienced. All six treated participants were entered in the first-dose group and received 200 mg orally every 12 h for 10 days. The mean baseline CD4+ T cell count was 196/mm3 (range 25–531). The median baseline log10 plasma HIV-1 RNA was 4.23 copies/mL (range 2.97–5.03). All six participants' viruses were dual/mixed X4/R5 at entry: median log10 rlu 5.33 (X4) and 5.73 (R5). At day 10, five of six participants' viruses were dual/mixed X4/R5, and one of six was R5 transiently (Fig. 1; participant 3, X4 fell <2,000 rlu). At day 30, one of six participants had undetectable X4 virus [Fig. 1; participant 4 had log10 (X4) of 1.792 rlu].
FIG. 1.
Log10 X4 and R5 rlu and log10 plasma HIV-1 RNA (copies/mL) viral load results for six study participants during a 10-day treatment with AMD11070 and following treatment discontinuation. rlu, relative luminescence units.
Two participants had a ≥1 log10 reduction in CXCR4 rlu signal at day 10, with changes from baseline of −1.78 and −3.26 log10 rlu, respectively. A third participant exhibited a change of −0.99 log10 rlu (Fig. 1; tropism rlu; participants 1, 3, and 6). The remaining three participants had changes in CXCR4 rlu signal of −0.02, 0.04, and 0.04 log10 rlu, respectively (Fig. 1; tropism rlu: participants 2, 4, and 5). Changes in log10 CCR5 rlu ranged from −1.14 to 1.97 (median −0.22). No participant had 1 log10 decline in plasma HIV-1 RNA from baseline at day 10 (Fig. 1; viral load; participants 1–6). Changes in CD4+ T cell counts ranged from −21 to +93 cells/mm3 (median −3.5 cells/mm3). No grade 3 or worse toxicities were seen during 10 days of treatment or 7 days after AMD11070 therapy. At steady state, the AMD11070 pharmacokinetic parameters were similar to previously reported data.17
Following 200 mg twice-daily dosing (n = 6), the mean 24-h area-under-the-curve (AUC24), maximum plasma concentration (Cmax), trough concentration (C24h), and elimination half-life (t1/2) were 4,472, 1,069, 49 ng/mL, and 7.9 h, respectively. No relationships between AMD11070 pharmacokinetic parameters and treatment response (as measured by X4 log10 reduction) were observed.
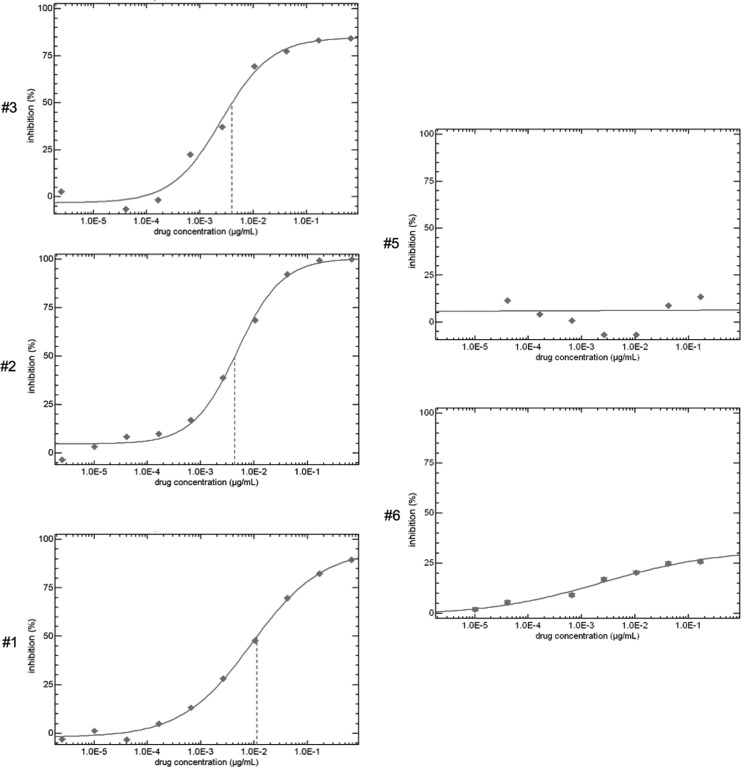
Baseline AMD11070 viral susceptibility was performed in five of six participants.23 In three participants' isolates, a dose–response curve was achievable (Fig. 2; participants 1–3). In contrast, a marked plateau effect in two other participants (Fig. 2; patients 5 and 6) demonstrated a failure to achieve a dose–response effect across all AMD11070 doses tested in vitro. Specimens from participants #1 and #3 also demonstrated more modest reductions in susceptibility, with failure to fully extinguish luciferase activity at the highest AMD11070 doses tested in vitro (i.e., reductions in the percent maximal inhibition [PMI]). No new HIV-1 drug RT or protease resistance mutations were detected in isolates derived from treated participants between screening/entry and day 30 (after 10 days of AMD11070 dosing) (data not shown).
FIG. 2.
Baseline AMD11070 susceptibility shown as percent inhibition versus percent AMD11070 drug concentration as determined by the HIV-1 PhenoSense Entry assay (Monogram Biosciences, Inc., South San Francisco, CA) for five of six evaluable study participants.
This study was stopped early due to emerging AMD11070 animal toxicity data. An FDA clinical hold was placed on AMD11070 due to animal liver (dogs, rats) and eye (rat) toxicity findings that emerged during the A5210 study.20 No further cohorts (B–H) were enrolled and the AIDS Clinical Trials Group (ACTG) closed the study. Genzyme also acquired AnorMED after completion of the cohort A study treatment and did not pursue further AMD11070 drug development for HIV infection. Based on animal toxicity findings that emerged during the A5210 study, a follow-up ophthalmology exam was arranged for A5210-treated participants. This exam was completed 2–3 years after AMD11070 therapy in four of the six available participants later off study; two other participants could not be located. The ophthalmology exams revealed no evidence of long-term retinal toxicity after short-term AMD11070 exposure in available participants (data not shown).
Discussion
AMD11070 was well tolerated when administered at 200 mg orally every 12 h for 10 days in A5210 participants. Reductions of almost 1 log10 rlu or more in CXCR4 virus were seen in three of six participants after 10 days of treatment, which was the primary endpoint of this study. However, no participants had ≥1 log10 decline in plasma HIV-1 RNA from baseline at day 10. These results provide evidence and proof-of-concept support that an oral CXCR4 antagonist such as AMD11070 can selectively inhibit CXCR4-tropic virus in HIV-1-infected participants.
These results extend observations seen with AMD11070 therapy in a parallel clinical trial conducted by AnorMED, Inc.20 In that study, participants were similarly required to demonstrate CXCR4-tropic virus or dual-tropic virus with a CXCR4 viral population ≥2,000 rlu in luciferase activity using the Trofile assay at screening.22 The median baseline CD4+ T cell count was 160 cells/mm3, and the median HIV-1 RNA level was 91,447 copies/mL. Among the 10 participants who received AMD11070 monotherapy (200 mg to eight participants and 100 mg to two participants) orally twice daily for 10 days, four of nine evaluable participants achieved a reduction in CXCR4 virus population ≥1 log10 rlu. The median change in CXCR4 virus population at the end of treatment was −0.22 log10 rlu (range, −1.90 to +0.23 log10 rlu). No significant changes in plasma HIV-1 RNA level, CD4+ T cell count, CCR5 virus levels (i.e., changes in the luciferase activity in the Trofile assay), or WBCs were observed during the treatment period.
No drug-related serious adverse events, adverse events above grade 2, or laboratory abnormalities were observed. The authors concluded that AMD11070 was active against X4-tropic HIV-1 populations, well tolerated, and without safety concerns over a 10-day course of therapy. These results mirrored findings from our study and an earlier proof-of-concept study with another CXCR4 antagonist, AMD3100,18 where no significant plasma HIV-1 RNA changes occurred despite demonstration of reductions of CXCR4 viral populations in 10 of 14 AMD3100-treated participants.18
The question arises as to why plasma HIV-1 RNA reductions were not seen in AMD11070-treated participants despite indications that AMD11070 treatment in vivo resulted in reductions of near 1 log10 or more rlu in CXCR4 viral populations after 10 days of therapy in 50% of these participants. One possible reason is related to the study design, specifically enrolling participants screened for the presence of CXCR4 or dual/mixed CXCR4/CCR5 by a HIV-1 co-receptor tropism assay (Trofile; Monogram Biosciences, Inc.). Even though screening samples in all enrolled participants indicated the presence of CXCR4-using viruses, all subjects who enrolled into A5210 harbored virus that used both CXCR4 and CCR5 receptors at screening. No subject harbored pure CXCR4-using viruses exclusively.
The activity of any co-receptor tropism antagonist is most readily demonstrated in purely monotropic viral populations, that is, R5 tropic viruses for CCR5 antagonist and X4 tropic viruses for CXCR4 antagonist. While pure R5 tropism is relatively common (i.e., ∼82% of treatment-naïve individuals with subtype clade B HIV-1 infection),27–29 pure X4 tropism is relatively rare, 0.3% in one large treatment-naïve dataset.28 Given the relative rarity of pure X4 tropism, studies of CXCR4 antagonists have included individuals with D/M tropism. In such populations, defining the activity of any co-receptor tropism antagonist may be complex, and compensatory use of the R5 receptor may have occurred. In our study, we indeed saw two participants who exhibited “phenotypic switches” (i.e., D/M viruses became predominantly R5 when tested during the study); this is analogous to the “phenotypic switch” described in some CCR5-treated participants experiencing virologic failure, with viruses displaying switches from predominantly R5 to X4 or D/M during therapy.30
Some participants exhibited significant pre-existing phenotypic resistance to AMD11070 (“plateaus”) before therapy. This was evident in the flat dose–response curves in vitro, wherein higher concentrations of the drug did not result in higher degrees of viral suppression, particularly in participants #5 and #6, whose viruses demonstrated phenotypic resistance with a marked plateau effect in vitro (Fig. 2). Specimens from participants #1 and #3 also demonstrated attenuated susceptibility, with failure to fully extinguish luciferase activity at the highest drug concentrations tested in vitro (i.e., reductions in the PMI). This reduction in PMI in dose–response curves during in vitro testing of clinical isolates has also been described for CCR5 co-receptor antagonists,31 which possibly relates to the allosteric mechanism of co-receptor antagonist inhibition of HIV-1 replication. Although primary phenotypic resistance to CXCR4 antagonists has been described previously, it has not been definitively linked to attenuated clinical response; so caution is advised before defining phenotypic plateaus as a primary cause of failure to suppress rlu activity in these AMD11070-treated participants.
The absence of significant reductions in plasma HIV-1 RNA levels during AMD11070 therapy was not due to failure to achieve sufficient drug concentrations. All six treated HIV-infected A5210 participants achieved plasma concentration parameters for AMD11070 similar to healthy volunteers,25 and no clear relationship between response to therapy and pharmacokinetic parameters was evident.
A limitation to this study was the absence of a placebo group, which would have provided some contrast to the intervention group for both safety, rlu activity, and reduction in HIV RNA.
Due to the therapeutic promise of this approach, further studies of CXCR4 inhibitors are still possible to determine the optimal agents, dosing, long-term safety, and efficacy. As more people receive combined therapy for HIV-1 infection for longer periods of time, there remains a continuing need to further explore and develop ARVs with novel mechanisms of action on other viral targets within the cellular HIV-1 replicative cycle. A possible approach yet to be studied to achieve maximal virus suppression is combining several HIV-1 entry inhibitors that act against either CXCR4 or CCR5 together, along with other clinically available ARVs that target other virus-specific steps within the HIV-1 life-cycle.32–34 Significant theoretical concerns exist regarding adverse immunologic consequences from simultaneous blockade of both the CCR5 and CXCR4 pathways.
The impact of CXCR4 blockade, from both an activity and safety standpoint, remains important to address despite lingering questions about discordant responses (e.g., CXCR4 rlu reductions without net impact on plasma HIV-1 viremia) as the development of novel drugs for HIV infection continues awaiting HIV-1 curative therapies.
Acknowledgments
The project described was supported by award number U01AI068636 from the National Institute of Allergy and Infectious Diseases and supported by the National Institute of Mental Health, National Institute of Dental and Craniofacial Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the NIH.
The present work was supported in part by the National Institute of Allergy and Infectious Diseases grants to the ACTG (AI38858 and A168636) and Statistical and Data Management Center/Harvard School of Public Health (SDMC Grant No. AI68634). The National Clinical Trial number is NCT00033163. This work was also supported by grants from the NIH: MH50421, P30 AI27767, U01 AI38858, MO1 RR00032, T32 AI07493; and the Birmingham VA Medical Center and UAB CFAR core clinic and laboratory facilities.
Other members of the A5210 Protocol Team included Roger Bedimo, MD (University of Texas–Southwestern Medical Center); Susan Owens, RN, MS (Frontier Science and Technology Research Foundation, Inc., Amherst, NY); Elaine Ferguson, RPh, MS (Pharmaceutical Affairs Branch, DAIDS, NIAID, NIH); Lisa Kessels, RN, ACRN (Washington University School of Medicine, St. Louis, MO); David Shugarts, MA (University of Colorado Health Sciences, Denver, CO); Vincent Parrillo (Beth Israel Medical Center, New York, NY). The A5210 team acknowledges study participants. The A5210 site staff who screened and enrolled subjects included: Kerry Upton, BSN, and Heather V. White, BA, CTU grant number U01 AI069452 and GCRC grant number M01 RR-00032—University of Alabama at Birmingham (Site 5801); Mitchell Goldman, MD, and Beth W. Zwickl, FNP, CCRC, CTU grant number U01 AI025859 and Indiana CTSI ICRC, UL RR025761—Indiana University ACTU (Site 2601); Carlos del Rio, MD, and Akaki Turkia, MD, MPH, CTU grant number UO1 AI069418, ACTSI award number UL1 RR025008 (from the Clinical and Translational Science Award Program, NIH/NCRR)—Emory University CTU (Site 5802). Other sites who screened patients included the University of Rochester Medical Center, Vanderbilt University, University of Colorado, University of Texas–Galveston, and Cornell Clinical Trials Unit. The A5210 team acknowledges the following technical support: Amanda Zadzilk (Data Management Center, Frontier Science & Technology Research Foundation, Inc., Amherst, NY); virology laboratory support (HIV-1 drug resistance) by J. Darren Hazelwood, BS (University of Alabama at Birmingham School of Medicine Virology Specialty Laboratory 54, Birmingham, AL); and the graphical assistance of Darlene Lu, MS (Statistical and Data Analysis Center, Harvard School of Public Health, Boston, MA).
Contributor Information
Collaborators: for the NIH/NIAID AIDS Clinical Trials Group A5210 Protocol Team, Roger Bedimo, Susan Owens, Elaine Ferguson, Lisa Kessels, David Shugarts, Vincent Parrillo, Kerry Upton, Heather V. White, Mitchell Goldman, Beth W. Zwickl, Carlos del Rio, Akaki Turkia, Amanda Zadzilk, J. Darren Hazelwood, and Darlene Lu
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Deng H, Liu R, Ellmeier W, et al. : Identification of a major co-receptor for primary isolates of HIV-1. Nature 1996;381:661–666 [DOI] [PubMed] [Google Scholar]
- 2. Feng Y, Broder CC, Kennedy PE, Berger EA: HIV-1 entry cofactor: Functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science 1996;272:872–877 [DOI] [PubMed] [Google Scholar]
- 3. Dittmar MT, McKnight A, Simmons G, Clapham PR, Weiss RA, Simmonds P: HIV-1 tropism and co-receptor use. Nature 1997;385:495–496 [DOI] [PubMed] [Google Scholar]
- 4. Berger EA, Murphy PM, Farber JM: Chemokine receptors as HIV-1 coreceptors: Roles in viral entry, tropism, and disease. Annu Rev Immunol 1999;17:657–700 [DOI] [PubMed] [Google Scholar]
- 5. Gallo SA, Finnegan CM, Viard M, et al. : The HIV Env-mediated fusion reaction. Biochem Biophys Acta 2003;1614:36–50 [DOI] [PubMed] [Google Scholar]
- 6. Hendrix CW, Flexner C, MacFarland RT, et al. : Pharmacokinetics and safety of AMD-3100, a novel antagonist of the CXCR-4 chemokine receptor, in human volunteers. Antimicrob Agents Chemother 2000;44:1667–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Doms RW, Moore JP: HIV-1 membrane fusion: Targets of opportunity. J Cell Biol 2000;151:F9–F14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moore JP, Doms RW: The entry of entry inhibitors: A fusion of science and medicine. Proc Natl Acad Sci U S A 2003;100:10598–10602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schols D, Claes S, Hatse K, et al. : Anti-HIV activity profile of AMD070, an orally bioavailable CXCR4 antagonist [abstract 563]. In: Program and abstracts of the 10th Conference on Retroviruses and Opportunistic Infections Boston, 2003 [Google Scholar]
- 10. Markovic I, Clouse KA: Recent advances in understanding the molecular mechanisms of HIV-1 entry and fusion: Revisiting current targets and considering new options for therapeutic intervention. Curr HIV Res 2004;2:223–234 [DOI] [PubMed] [Google Scholar]
- 11. Hendrix CW, Collier AC, Lederman MM, et al. : Safety, pharmacokinetics, and antiviral activity of AMD3100, a selective CXCR4 receptor inhibitor, in HIV-1 infection. J Acquir Immune Defic Syndr 2004;37:1253–1262 [DOI] [PubMed] [Google Scholar]
- 12. Fatkenheuer G, Pozniak AL, Johnson MA, et al. : Efficacy of short-term monotherapy with maraviroc, a new CCR5 antagonist, in patients infected with HIV-1. Nature Med 2005;11:1170–1172 [DOI] [PubMed] [Google Scholar]
- 13. Mosi R, Anastassova V, Bodart V, et al. : AMD070: A new CXCR4 entry inhibitor. In: Program and abstracts of the Keystone Symposia Conference on Cell Biology of Virus Entry, Replication and Pathogenesis Sante Fe, NM, 2006 [Google Scholar]
- 14. Lalezari J, Goodrich J, DeJesus E, et al. : Efficacy and safety of maraviroc plus optimized background therapy in viremic ART-experienced patients infected with CCR5-tropic HIV-1: 24-week results of a phase 2b/3 study in the US and Canada (abstract 104bLB). In: Program and abstracts of the 14th Conference on Retroviruses and Opportunistic Infections Los Angeles, 2007 [Google Scholar]
- 15. Schurmann D, Fatkenheuer G, Reynes J, et al. : Antiviral activity, pharmacokinetics and safety of vicriviroc, an oral CCR5 antagonist, during 14-day monotherapy in HIV-infected adults. AIDS 2007;21:1293–1299 [DOI] [PubMed] [Google Scholar]
- 16. Gulick RM, Su Z, Flexner C, et al. : Phase 2 study of the safety and efficacy of vicriviroc, a CCR5 inhibitor, in HIV-1–infected, treatment experienced patients: AIDS Clinical Trials Group 5211. J Infect Dis 2007;196:304–312 [DOI] [PubMed] [Google Scholar]
- 17. Stone ND, Dunaway SB, Flexner C, et al. : Multiple-dose escalation study of the safety, pharmacokinetics, and biologic activity of oral AMD070, a selective CXCR4 receptor inhibitor, in human subjects. Antimicrob Agents Chemother 2007;51:2351–2358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fransen S, Bridger G, Whitcomb JM, et al. : Suppression of dual tropic human immunodeficiency virus type 1 by the CXCR4 antagonist AMD3100 is associated with efficiency of CXCR4 use and baseline virus composition. Antimicrob Agents Chemother 2008;52:2608–2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gulick R, Lalezari J, Goodrich J, et al. : Maraviroc for previously treated patients with R5 HIV-1 infection. N Engl J Med 2008;359:1429–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moyle G, DeJesus E, Boffito M, et al. : Proof of activity with AMD11070, an orally bioavailable inhibitor of CXCR4-tropic HIV type 1. Clin Infect Dis 2009;48:798–805 [DOI] [PubMed] [Google Scholar]
- 21. Selzentry (maraviroc) [package insert]. Research Triangle Park, NC: ViiV Healthcare; 2018 [Google Scholar]
- 22. Whitcomb JM, Huang W, Fransen S, et al. : Development and characterization of a novel single-cycle recombinant-virus assay to determine human immunodeficiency virus type 1 coreceptor tropism. Antimicrob Agents Chemother 2007;51:566–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Westby M, Smith-Burchnell C, Mori J, et al. : Reduced maximal inhibition in phenotypic susceptibility assays indicates that viral strains resistant to the CCR5 antagonist maraviroc utilize inhibitor-bound receptor for entry. J Virol 2007;81:2359–2371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grant RM, Kuritzkes DR, Johnson VA, et al. : Accuracy of the TRUGENE HIV-1 genotyping kit. J Clin Microbiol 2003;41:1586–1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nyunt M, Becker S, MacFarland R, et al. : Pharmacokinetic effect of AMD070, an oral CXCR4 antagonist, on CYP3A4 and CYP2D6 substrates midazolam and dextromethorphan in healthy volunteers. J Acquir Immune Defic Syndr 2008;47:559–565 [DOI] [PubMed] [Google Scholar]
- 26. Cao Y, Flexner C, Dunaway S, et al. : Effect of low-dose ritonavir on the pharmacokinetics of the CXCR4 antagonist AMD070 in healthy volunteers. Antimicrob Agents Chemother 2008;52:1630–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moyle GJ, Wildfire A, Mandalia S, et al. : Epidemiology and predictive factors for chemokine receptor use in HIV-1 infection. J Infect Dis 2005;191:866–872 [DOI] [PubMed] [Google Scholar]
- 28. Coakley EP, Benhamida J, Chappey C, et al. : An evaluation of tropism profiles and other characteristics among 3988 individuals screened for the A4001026, A4001027 (Motivate 1) and A4001028 (Motivate 2) studies for Maraviroc (abstract 8). In: Proceedings of the 2nd International Workshop on Targeting HIV Entry Boston MA, USA, October20–21, 2006 [Google Scholar]
- 29. Wilkin TJ, Su Z, Kuritzkes DR, et al. : HIV type 1 chemokine coreceptor use among antiretroviral-experienced patients screened for a clinical trial of a CCR5 inhibitor: AIDS Clinical Trial Group A5211. Clin Infect Dis 2007;44:591–595 [DOI] [PubMed] [Google Scholar]
- 30. Westby M, Lewis M, Whitcomb J, et al. : Emergence of CXCR4-using human immunodeficiency virus type 1 (HIV-1) variants in a minority of HIV-1–infected patients following treatment with the CCR5 antagonist maraviroc is from a pretreatment CXCR4-using virus reservoir. J Virol 2006;80:4909–4920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Petropoulos CJ, Huan W, Toma J, Fransen S, Bonhoeffer S, Whitcomb JM: Resistance to HIV-1 entry inhibitors may occur by multiple molecular mechanisms. In: Proceedings of the XXII International Drug Resistance Workshop Tenerife, Canary Islands, June2004 [Google Scholar]
- 32. Tremblay CL, Giguel F, Guan Y, Chou T-C, Takashima K, Hirsch MS: TAK-220, a novel small-molecule CCR5 antagonist, has favorable anti–human immunodeficiency virus interactions with other antiretrovirals in vitro. Antimicrob Agents Chemother 2005;49:3483–3485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tremblay CL, Giguel F, Kollmann C, et al. : Anti–human immunodeficiency virus interactions of SCH-C (SCH 351125), a CCR5 antagonist, with other antiretroviral agents in vitro. Antimicrob Agents Chemother 2002;46:1336–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nakata H, Steinberg SM, Koh Y, et al. : Potent synergistic anti-human immunodeficiency virus (HIV) effects using combinations of the CCR5 inhibitor aplaviroc with other anti-HIV drugs. Antimicrob Agents Chemother 2008;52:2111–2119 [DOI] [PMC free article] [PubMed] [Google Scholar]