Abstract
Coronary heart disease (CHD) remains a major cause of mortality with a huge economic burden on healthcare worldwide. Here, we conducted a systematic review to investigate the efficacy and safety of Chinese herbal medicine (CHM) for CHD based on high-quality randomized controlled trials (RCTs) and summarized its possible mechanisms according to animal-based researches. 27 eligible studies were identified in eight database searches from inception to June 2018. The methodological quality was assessed using seven-item checklist recommended by Cochrane Collaboration. All the data were analyzed using Rev-Man 5.3 software. As a result, the score of study quality ranged from 4 to 7 points. Meta-analyses showed CHM can significantly reduce the incidence of myocardial infarction and percutaneous coronary intervention, and cardiovascular mortality (P < 0.05), and increase systolic function of heart, the ST-segment depression, and clinical efficacy (P < 0.05). Adverse events were reported in 11 studies, and CHMs were well tolerated in patients with CHD. In addition, CHM exerted cardioprotection for CHD, possibly altering multiple signal pathways through anti-inflammatory, anti-oxidation, anti-apoptosis, improving the circulation, and regulating energy metabolism. In conclusion, the evidence available from present study revealed that CHMs are beneficial for CHD and are generally safe.
Keywords: Traditional Chinese medicine, coronary heart disease, high-quality randomized controlled trials, clinical evidence, possible mechanisms, systematic review
Introduction
Coronary heart disease (CHD) incurs a huge economic burden on healthcare and society (Dunbar et al., 2018). According to the epidemiological data from 1990 to 2013, 92.94 million people were suffering from this disease, which eventually led to 8.1 million deaths (Murray et al., 2015; Roth et al., 2015). Current treatments for CHD include coronary revascularization, drug intervention, risk factor control, cardiac rehabilitation, and lifestyle improvement (Arslan et al., 2018). Among them, percutaneous coronary intervention (PCI) and coronary artery bypass grafting are the most effective (Roffi et al., 2016). However, PCI is mainly for the treatment of locally severe stenotic vessels and has limited therapeutic effect on extensive coronary stenosis and microcirculation lesions (Heusch and Gersh, 2017). Meanwhile, the prognosis of patients treated with PCI is sometimes not ideal because myocardial ischemia/reperfusion injury, no reflow, coronary dissection, stent thrombosis, and acute coronary occlusion still exist (Hausenloy and Yellon, 2013; Arslan et al., 2018). Although the technology of coronary intervention is still improving and conventional medicine is constantly updating, novel treatments that can stabilize arterial plaque, improve microcirculation, and angina symptoms; prevent acute myocardial infarction; delay the development of ischemic cardiomyopathy; ultimately reduce PCI; and improve prognosis are urgently needed.
Traditional Chinese medicine (TCM) includes herbal medicine (CHM), acupuncture, and other non-pharmacological therapies, which is a holistic approach to health and healing (Xu et al., 2013). CHM has been used to treat CHD for thousands of years, and in modern time, many claimed randomized controlled trials (RCT) have reported some TCM Fufang exerted the cardioprotective function (Han et al., 2008; Gao et al., 2010; Chung et al., 2013; Liu et al., 2013). However, most of these studies are poor methodological quality, leading that there is still insufficient evidence to support routine use of CHMs for CHD. Thus, the Cochrane group guidelines for clinical reviews may exclude the ‘‘not-so-good’’ studies (Chan et al., 2012). In addition, in a TCM reviewing process, researchers may need to include such high-quality studies about a medical certain issue to identify current problems and areas worthy of improvement for its future development (Chan et al., 2012). Thus, we performed a systematic review to assess the efficacy and safety of CHM for CHD according to high-quality studies with at least four domains of “yes” in Cochrane risk of bias (RoB) tool (Li et al., 2015).
Methods
Search Strategy and Study Selection
Studies estimating the efficacy of CHMs in patients with CHD were systematically searched from EMBASE, PubMed, Cochrane Library, Wangfang database, China National Knowledge Infrastructure (CNKI), VIP database (VIP), and China Biology Medicine disc (CBM) from inception to the end of June 2018. The key words were used as follows: “coronary disease OR acute coronary syndrome OR myocardial infarction OR myocardial ischemia” AND “herb OR traditional Chinese medicine OR Chinese Materia Medica.” Moreover, reference lists of potential articles were searched for relevant studies.
Inclusion and Exclusion Criteria
The inclusion criteria were prespecified as follows: (1) RCTs that investigated the efficacy and safety of CHM for CHD were included. Quasi-randomized trials, such as those in which patients were allocated according to date of birth and order of admission number, were excluded. If a three-arm design was used in a study, we extracted data only for the group(s) involving CHM and the control group(s). (2) All participants were patients with a diagnosis of CHD based on one of the following criteria: (1) The guideline of unstable or stable angina from Chinese cardiovascular association in different years, (2) the guideline of unstable or stable angina from American College of Cardiology (ACC) or American Heart Association (AHA) or European Society of Cardiology (ESC) or World Health Organization (WHO) in different years, (3) be diagnosed by coronary angiography, (4) patients after PCI, and (5) diagnostic criteria made by other authors with comparable definitions were also used. (3) The treatment interventions included CHMs used as monotherapies or adjunct with conventional medicine (i.e., antiplatelet, stable plaque, control ventricular rate) or supportive treatment (i.e., nutrition support, exercise therapy, psychotherapy). Interventions for control group were restricted to no intervention, placebo, conventional medicine, and supportive treatment. Studies comparing a CHM agent with another CHM agent were excluded. (4) The primary outcome measures were the incidence of myocardial infarction and/or the incidence of PCI and/or cardiovascular mortality and/or the level of ST-segment depression and/or indicators which represent systolic and diastolic function of the heart in cardiac ultrasound. The secondary outcome measures were clinical effective rating, and the safety of co-administration of CHM. The exclusion criteria were prespecified as follows: (1) no predetermined outcome index; (2) compared or combined with other Chinese herb medicine; (3) not randomized, double-blind, placebo-controlled designed; (4) no control group; and (5) double publication.
Data Extraction
Two authors independently reviewed each included study and extracted following aspects of details: (1) name of first author, year of publication; (2) diagnostic criteria; (3) detail information of participants for each study, including sample size, gender composition, and mean age; (4) detail information of treatment and control group, including therapeutic drug dosage, method of administration, and duration of treatment; and (5) outcome measures and intergroup differences. The data of predetermined primary and secondary outcomes were extracted for further qualitative and quantitative syntheses. We made efforts to contact authors for further information when some records’ published data were only in graphical format or not in the publication. And the numerical values were measured from the graphs by digital ruler software when response was not received from authors.
Risk of Bias in Individual Studies
The methodological quality of each included study was evaluated by two authors with the seven-item checklist recommended by Cochrane Collaboration (Higgins and Green, 2012). Only RCTs with a cumulative score of at least four points were included in our systematic review. Any disagreements from two authors were dealt with through discussion with the corresponding author (GQZ).
Statistical Analysis
The statistical analysis was conducted via RevMan version 5.3. A fixed-effects model (FEM) or random-effects model (REM) was conducted to analyze pooled effects. When the outcome measurements in all included studies in meta-analysis were based on the same scale, weighted mean difference (WMD) with 95% confidence intervals was calculated as a summary statistic, otherwise standard mean difference (SMD) was calculated. Heterogeneity between study results was investigated based on a standard chi-square test and I2 statistic. A fixed-effects model (I2 < 50%) or a random-effects model (I2 > 50%) was used depending on the value of I2. Funnel plots were used to visually estimate publication bias. A probability value 0.05 was considered statistically significant.
CHM Composition and Possible Mechanisms of Active Ingredients
Specific herbs in the CHM formulae were recorded. The frequency of use for particular herb was calculated and those used at a high frequency that are described in detail. Animal-based mechanism studies of active ingredients from frequently used herbs were searched. The following information was recorded for such studies: identity of active ingredients and their herbal sources, suggested mechanisms and implicated signaling pathways, first author’s name and publication year of the citation, and structure of active ingredients.
Results
Study Selection
A total of 2,158 studies were retrieved after systematical searches from the database, of which 287 were reduplicated and irrelevant studies. After screening title and abstract, 180 were excluded because they were: (1) animal trial, (2) case report, (3) review article, and (4) meeting abstract. After reviewing the full text of the remaining 87 articles, 60 studies were excluded if: (1) no predetermined outcome index; (2) compared or combined with other CHM; (3) not randomized, double-blind, and placebo-controlled designed; (4) no control group; (5) double publication; and (6) data of result was not available. Ultimately, 27 studies with Cochrane RoB score ≧4 (Lu et al., 2006; Qiao et al., 2006; Lu et al., 2008; Cheng et al., 2009; Chu et al., 2010; Qiu et al., 2009; Wang et al., 2009; Zhang et al., 2010; Shang et al., 2011; Mo et al., 2012; Wang S. H. et al., 2012; Wang Y. G. et al., 2012; Chen et al., 2013; Shang et al., 2013; Hu et al., 2014; Liu et al., 2014; Lu et al., 2014; Sun, 2014; Xu et al., 2014; Xu et al., 2015; Zhang et al., 2015; Duan et al., 2016; Mao et al., 2016; Wang et al., 2016; Zhu et al., 2016; Wang et al., 2017; Yang et al., 2017) were selected ( Figure 1 ).
Figure 1.
Summary of the process for identifying candidate studies.
Characteristics of Included Studies
17 studies (Lu et al., 2006; Qiao et al., 2006; Cheng et al., 2009; Chu et al., 2010; Qiu et al., 2009; Mo et al., 2012; Wang S. H. et al., 2012; Wang Y. G. et al., 2012; Chen et al., 2013; Hu et al., 2014; Liu et al., 2014; Lu et al., 2014; Sun, 2014; Xu et al., 2014; Xu et al., 2015; Duan et al., 2016; Zhu et al., 2016; Wang et al., 2017; Yang et al., 2017) were published in Chinese and 10 studies (Lu et al., 2008; Wang et al., 2009; Zhang et al., 2010; Chu et al., 2010; Shang et al., 2011; Shang et al., 2013; Xu et al., 2015; Zhang et al., 2015; Mao et al., 2016; Wang et al., 2016) in English between 2006 and 2017. All studies were conducted in China. The sample size of the included studies ranged from 57 to 4,870 with a total of 11,732 participants, including 5,916 patients in treatment groups and 5,816 patients serving as controls. Of 27 included studies, 18 studies (Cheng et al., 2009; Qiu et al., 2009; Wang et al., 2009; Shang et al., 2011; Mo et al., 2012; Wang S. H. et al., 2012; Shang et al., 2013; Hu et al., 2014; Liu et al., 2014; Sun, 2014; Xu et al., 2014; Zhang et al., 2015; Duan et al., 2016; Wang et al., 2016; Zhu et al., 2016; Wang et al., 2017; Yang et al., 2017) were based on patients with angina pectoris of CHD and nine studies (Lu et al., 2006; Qiao et al., 2006; Lu et al., 2008; Chu et al., 2010; Zhang et al., 2010; Chen et al., 2013; Lu et al., 2014; Xu et al., 2015; Mao et al., 2016) were based on patients with acute coronary syndrome. Comparisons of CHM plus a conventional treatment (i.e., antiplatelet, stable plaque, control ventricular rate) versus a conventional treatment were conducted in 26 trials, and comparisons of CHM versus a placebo were performed in one trial (Hu et al., 2014). The CHMs were administered orally (i.e., tablets, capsules, granules, or decoction). The duration of follow-up was varied from 4 weeks to 4.5 years. All studies accounted for baseline comparability. The incidence of myocardial infarction (MI) was utilized as outcome measure in 10 studies (Lu et al., 2006; Lu et al., 2008; Wang et al., 2009; Shang et al., 2011; Shang et al., 2013; Lu et al., 2014; Sun, 2014; Xu et al., 2015; Mao et al., 2016; Wang et al., 2016), the incidence of PCI in five studies (Lu et al., 2006; Lu et al., 2008; Wang et al., 2009; Shang et al., 2013; Wang et al., 2016), cardiovascular mortality in seven studies (Lu et al., 2008; Wang et al., 2009; Shang et al., 2013; Sun, 2014; Xu et al., 2015; Mao et al., 2016; Wang et al., 2016), left ventricular ejection fraction (LVEF) in five studies (Qiao et al., 2006; Qiu et al., 2009; Chen et al., 2013; Sun, 2014; Mao et al., 2016), the ventricular wall motion score in two studies (Qiao et al., 2006; Chen et al., 2013), and the level of ST-segment elevation in three studies (Chu et al., 2010; Hu et al., 2014; Sun, 2014). The efficiency of angina improved was reported in 12 studies (Lu et al., 2006; Chu et al., 2010; Shang et al., 2011; Mo et al., 2012; Wang S. H. et al., 2012; Hu et al., 2014; Lu et al., 2014; Sun, 2014; Zhang et al., 2015; Duan et al., 2016; Zhu et al., 2016; Yang et al., 2017), the usage of nitroglycerin in two studies (Sun, 2014; Xu et al., 2014), low-density lipoprotein (LDL) in four studies (Lu et al., 2008; Wang Y. G. et al., 2012; Zhu et al., 2016; Wang et al., 2017), hypersensitive C-reactive protein (hsCRP) in two studies (Mo et al., 2012; Wang S. H. et al., 2012), the degree of coronary artery stenosis in two studies (Lu et al., 2006; Yang et al., 2017), and the rate of coronary restenosis in two studies (Shang et al., 2011; Lu et al., 2014). The overall characteristics of included studies are shown in Table 1 .
Table 1.
Characteristics of the 27 included studies.
| Study (years) | Diagnostic criteria | Number of participants (male/female), mean age (years) | Interventions | Conventional medicine or basic treatment | Duration of treatment | Outcome index | Intergroup differences | ||
|---|---|---|---|---|---|---|---|---|---|
| Trial | Control | Trial | Control | ||||||
| Lu et al., 2006 | After PCI | 60 58.94 ± 10.79 |
58 57.1 ± 9.81 |
Xiongshao capsule (0.5g, tid, p.o). | Placebo | Clopidogrel, aspirin; atorvastatin, low molecular weight heparin | 6 months | 1. Cardiovascular mortality 2. The rate of coronary restenosis 3. The degree of coronary artery stenosis 4. The efficiency of angina pectoris 5. Myocardial infarction rate 6. The incidence of PCI |
1. P < 0.05 2. P < 0.05 3. P < 0.05 4. P < 0.05 5. P < 0.05 6. P < 0.05 |
| Qiao et al., 2006 | After PCI | 30 (17/13) 64.0 ± 11.2 |
29 (18/11) 65.7 ± 12.2 |
Tongguan capsule (three doses, tid, p.o). | Placebo | Anticoagulant, antiplatelet, anti-infection | 1 month | 1. LVEF 2. The ventricular wall motion score 3. Survey of angina pectoris in Seattle |
1. P < 0.05 2. P < 0.05 3. P < 0.05 |
| Lu et al., 2008 | Documented previous myocardial infarction | 2,429 58.35 ± 9.02 |
2,441 58.35 ± 9.02 |
Xuezhikang capsule (0.6g, bid, p.o). | Placebo | Other drugs that do not affect blood lipids | 4.5 years | 1. Myocardial infarction rate 2. Cardiovascular mortality 3. The incidence of PCI 4. TC 5. TG 6. HDL-C 7. LDL-C |
1. P < 0.05 2. P < 0.05 3. P < 0.05 4. P < 0.01 5. P < 0.01 6. P < 0.01 7. P < 0.01 |
| Cheng et al., 2009 | The guideline of chronic stable angina from China, 2007 | 41 (37/4) 49.97 ± 6.19 |
41 (39/2) 51.12 ± 7.33 |
Qingre Quyu granule (6g, bid, p.o). | Placebo | Aspirin, bisoprolol fumarate, isosorbide dinitrate sustained release tablets | 25 weeks | 1. Number of atherosclerotic plaques 2. Arterial plaque score 3. Intima thickness of carotid artery 4. hsCRP |
1. P < 0.05 2. P < 0.05 3. P < 0.05 4. P < 0.05 |
| Qiu et al., 2009 | The guideline of acute myocardial infarction from China, 2001 | 51 (45/6) 57.82 ± 10.23 |
52 (44/8) 55.79 ± 11.06 |
Compound Salvia tablet and Xinyue capsule | Placebo | Antiplatelet agents, anticoagulant, β blocker, angiotensin converting enzyme inhibitor, nitrates, and lipid-regulating drugs | 3 months | 1. LVEF | 1. P < 0.05 |
| Wang et al., 2009 | The guideline of unstable angina pectoris from ACC/AHA USA, 2002 | 32 (12/20) 61.65 ± 8.15 |
31 (13/18) 64.47 ± 9.21 |
Shenshao tablet (0.3g, qd, p.o). | Placebo | Aspirin, isosorbide, mononitrate, simvastatin, Benner Pury, amlodipine, metoprolol |
4 weeks | 1. Frequency of angina pectoris 2. Seattle score 3. The incidence of PCI 4. Acute myocardial infarction rate |
1. P < 0.05 2. P < 0.05 3. P > 0.05 4. P > 0.05 |
| Chu et al., 2010 | After PCI | 28 (18/10); 61.7 ± 9.6 |
29 (20/9); 58.8 ± 8.9 |
Xuefu Zhuyu capsule | Placebo | Clopidogrel, aspirin, low molecular weight heparin, metoprolol tartrate, atorvastatin | 4 weeks | 1. The efficiency of angina pectoris 2. Electrocardiogram curative effect 3. Survey of angina pectoris in Seattle |
1. P < 0.05 2. P < 0.05 3. P < 0.05 |
| Zhang et al., 2010 | The guideline of segment elevation myocardial infarction from WHO | 108 (92/16) 58.5 ± 10.6 |
111 (96/15) 57.6 ± 11.2 |
Tongxinluo (2.08g, qd, p.o). | Placebo | Aspirin, clopidogrel | 180 days | 1. The incidence of no reflow of myocardium | 1. P = 0.0031 |
| Shang et al., 2011 | The guideline of stable angina pectoris from WHO | 73 (50/23) 67.79 ± 4.77 |
79 (52/27) 66.7 ± 4.16 |
Chuangxiongol (250mg, tid, p.o). | Placebo | Aspirin, tilopidine, diltiazem, nitroglycerin, heparin | 6 months | 1. Restenosis rate 2. The efficiency of angina pectoris 3. Cardiovascular mortality 4. Acute myocardial infarction rate 5. The incidence of PCI |
1. P > 0.05 2. P < 0.01 3. P > 0.05 4. P > 0.05 5. P > 0.05 |
| Mo et al., 2012 | The guideline of criteria for the naming and diagnosis of ischemic heart disease | 60 (43/17) 67.15 ± 4.87 |
60 (42/18) 66.22 ± 5.12 |
Yixin Mai granule (one dose, tid, p.o). | Placebo | Isosorbide, metoprolol, fosinopril, aspirin | 4 weeks | 1. hsCRP 2. IL-6 3. IL-18 4. The efficiency of angina pectoris |
1. P < 0.01 2. P < 0.01 3. P < 0.01 4. P = 0.037 |
| Wang S. H. et al., 2012 | The guideline of unstable angina pectoris from ACC/AHA USA, 2002 | 33 (26/7) 60.2 ± 9 |
33 (25/8) 62.7 ± 7.1 |
Tablets of betel (1.5g, bid, p.o). | Placebo | Aspirin, simvastatin, isosorbide, dinitrate | 28 days | 1. The efficiency of angina pectoris 2. Electrocardiogram efficiency 3. Nitroglycerin consumption 4. hsCRP 5. sCD40L |
1. P < 0.05 2. P > 0.05 3. P < 0.05 4. P < 0.05 5. P < 0.05 |
| Wang Y. G. et al., 2012 | The guideline of chronic stable angina from China, 2007 | 76 | 72 | Double ginseng capsule and Tongguan capsule (four doses, tid, p.o). | Placebo | Original treatment | 6 months | 1. TG 2. TC 3. HDL-C 4. LDL-C |
1. P < 0.05 2. P > 0.05 3. P < 0.05 4. P < 0.05 |
| Chen et al., 2013 | After PCI | 30 (17/13) 65.5 ± 7.5 |
30 (16/14) 63.8 ± 6.3 |
Tongguan capsule (three doses, tid, p.o). | Placebo | Clopidogrel, aspirin, low molecular weight heparin | 3 months | 1. LVEF 2. The ventricular wall motion score 3. The number of endothelial progenitor cell in peripheral blood |
1. P < 0.05 2. P < 0.05 3. P < 0.05 |
| Shang et al., 2013 | The guideline of chronic stable angina from China, 2004 | 1,746 (1,191/555) 58.35 ± 9.02 |
1,759 (1,260/499) 58.28 ± 8.99 |
QSYQ (0.5g, tid, p.o). | Placebo | Antihypertensive drugs, hypoglycemic agent, lipid-lowering medicine | 12 months | 1. Cardiovascular mortality 2. Myocardial infarction rate 3. The incidence of PCI |
1. P > 0.05 2. P > 0.05 3. P > 0.05 |
| Hu et al., 2014 | The guideline of chronic stable angina from China, 2007 | 192 57.82 ± 10.23 |
99 57.82 ± 10.23 |
Reachable film (three doses, tid, p.o). | Placebo | NM | 4 weeks | 1. The efficiency of angina pectoris 2. The total curative effect of TCM Syndrome 3. Electrocardiogram efficiency |
1. P < 0.05 2. P < 0.05 3. P < 0.05 |
| Liu et al., 2014 | The guideline of chronic stable angina from China, 2007 | 120 59.21 ± 7.92 |
120 60.64 ± 7. 69 |
Chek Shincen Tongxin granule | Placebo | Aspirin, atorvastatin | 4 weeks | 1. Body limitation 2. Stable state of angina pectoris 3. Episodes of angina pectoris 4. Satisfaction with treatment 5. The degree of understanding of disease 6. Electrocardiogram efficiency |
1. P < 0.05 2. P < 0.05 3. P < 0.05 4. P < 0.05 5. P < 0.05 6. P < 0.05 |
| Lu et al., 2014 | After PCI | 90 (48/42) 60.2 ± 6.9 |
90 (46/44) 61.8 ± 7.2 |
Tongxinluo capsule (three doses, tid, p.o). | Placebo | Original treatment | 12 months | 1. The incidence of coronary restenosis 2. The degree of coronary restenosis 3. The efficiency of angina pectoris 4. Myocardial infarction rate 5. Cardiovascular mortality |
1. P < 0.05 2. P < 0.01 3. P = 0.04 4. P = 0.19 5. P > 0.05 |
| Sun, 2014 | The guideline of chronic unstable angina from China, 2007 | 64 (42/22) 70.81 ± 10.76 |
64 (44/20) 69.8 ± 10.98 |
Musk Baoxin pill (two doses, tid, p.o). | Placebo | Isosorbide dinitrate tablets, atorvastatin, thiazepine, enteric aspirin | 6 months | 1. The efficiency of angina pectoris 2. Myocardial infarction rate 3. Cardiovascular mortality 4. Nitroglycerin consumption 5. Electrocardiogram efficiency 6. LVEF |
1. P < 0.05 2. P < 0.05 3. P > 0.05 4. P < 0.01 5. P < 0.05 6. P < 0.05 |
| Xu et al., 2014 | The guideline of unstable angina pectoris from ACC/AHA USA, 2002 | 55 (29/26) 69.47 ± 8 |
59 (33/26) 70.41 ± 8.6 |
Shenzhu Guanxin recipe (12g, qd, p.o). | Placebo | Conventional western medicine (unspecified) | 12 weeks | 1. The efficiency of angina pectoris 2. The duration of angina pectoris 3. Total use of nitroglycerin 4. The degree of physical activity induced by angina pectoris 5. The degree of angina pectoris |
1. P < 0.05 2. P < 0.05 3. P < 0.05 4. P < 0.05 5. P < 0.01 |
| Xu et al., 2015 | After PCI | 113 (86/27) 70.35 ± 9.61 |
74 (51/23) 68.08 ± 10.38 |
Shenzhu Guanxin recipe | Placebo | Aspirin, ticplopidine, diltiazem, glyceryl, trinitrate, heparin | 3 months | 1. Angina pectoris score 2. Cardiovascular mortality 3. Myocardial ischemia rate |
1. P = 0.66 2. P = 0.33 3. P = 0.63 |
| Zhang et al., 2015 | The guideline of unstable angina pectoris from ACC/AHA USA, 2002 | 119 (56/63) 59.46 ± 6.524 |
120 (52/68) 58.82 ± 7.061 |
Wufuxinnaoqing capsules | Placebo | Antiplatelet, aggregation, ACEI or ARB, statin two hydrogen arsenide | 12 weeks | 1. The efficiency of angina pectoris 2. Nitroglycerin consumption |
1. P < 0.01 2. P < 0.01 |
| Duan et al., 2016 | The guideline of chronic stable angina from China, 2007 | 64 (38/26) 59.7 ± 6.34 |
67 (47/20) 60.7 ± 6.44 |
Live heart pill (two doses, tid, p.o). | Placebo | Conventional western medicine (unspecified) | 8 weeks | 1. Symptom score of angina pectoris 2. Nitroglycerin consumption 3. Electrocardiogram plate movement 4. Seattle scale 5. Syndromes of traditional Chinese Medicine 6. hsCRP 7. Blood lipid |
1. P < 0.01 2. P < 0.01 3. P < 0.01 4. P < 0.01 5. P < 0.01 6. P > 0.05 7. P > 0.05 |
| Mao et al., 2016 | After PCI | 42 67.54 ± 8.39 |
41 68.38 ± 10.41 |
Danlou tablet | Placebo | Conventional western medicine (unspecified) | 90 days | 1. Left ventricular end diastolic volume index 2. End systolic volume index of left ventricle 3. LVEF 4. Cardiovascular mortality 5. Myocardial infarction rate |
1. P < 0.001 2. P < 0.001 3. P < 0.001 4. P < 0.05 5. P < 0.05 |
| Wang et al., 2016 | The guideline of unstable angina pectoris from ACCF/AHA USA, 2007 | 109 (72/37) 62.89 ± 9.23 |
110 (74/36) 63.89 ± 10.03 |
Danlou tablet (4.5g, qd, p.o). | Placebo | Antiplatelet, aggregation, anticoagulant, lipid-lowering, improvement of myocardial, remodeling, step-down | 90 days | 1. Cardiovascular mortality 2. Myocardial infarction rate 3. Reconstructive rate of blood vessels 4. Troponin 5. hsCRP |
1. P > 0.05 2. P = 0.04 3. P > 0.05 4. P > 0.05 5. P > 0.05 |
| Zhu et al., 2016 | The guideline of chronic stable angina from China, 2007 | 76 (48/28) 51.8 ± 1.6 |
74 (46/28) 51. 5 ± 1. 4 |
Traditional Chinese medicine prescription (10 mg, tid, p.o). | Placebo | Isosorbide, aspirin, atorvastatin | 4 weeks | 1. The efficiency of angina pectoris 2. Electrocardiogram efficiency 3. TG 4. TC 5. HDL-C 6. LDL-C 7. TCM syndrome score |
1. P < 0.05 2. P < 0.05 3. P < 0.05 4. P < 0.05 5. P < 0.05 6. P < 0.05 7. P < 0.05 |
| Wang et al., 2017 | The guideline of unstable angina pectoris from ACC/AHA USA, 2011 | 40 (17/23) 70.68 ± 6.87 |
40 (21/19) 71.65 ± 4.32 |
Xuesaitong soft capsule (0.66g, bid, p.o). | Placebo | Conventional western medicine (unspecified) | 4 weeks | 1. TC 2. TG 3. HDL 4. LDL 5. Survey of angina pectoris in Seattle |
1. P < 0.05 2. P < 0.05 3. P < 0.05 4. P < 0.05 5. P < 0.05 |
| Yang et al., 2017 | The guideline of chronic stable angina from China, 2014 | 33 (20/13) 61.18 ± 6.61 |
33 (21/12) 61.03 ± 7.51 |
Coronary Ningtong prescription | Placebo | Aspirin enteric-coated tablets, simvastatin tablets, isosorbide mononitrate, metoprolol | 24 weeks | 1. Coronary stenosis 2. The efficiency of angina pectoris |
1. P < 0.05 2. P < 0.05 |
PCI, percutaneous coronary intervention; LVEF, left ventricular ejection fraction; hsCRP, high sensitive C reactive protein; TC, total cholesterol; TG, total glycerol three fat; HDL, high density lipoprotein; LDL, low density lipoprotein; STEMI, segment elevation myocardial infarction; WHO, world health organization; NM, not mention; QSYQ, Qi-Shen-Yi-Qi dripping pills.
Study Quality
The quality score of study ranged from 4 to 7 in a total of 7 points. Of which, six studies (Lu et al., 2006; Chu et al., 2010; Wang et al., 2009; Liu et al., 2014; Xu et al., 2015; Wang et al., 2016) got 7 points, three studies (Zhang et al., 2015; Duan et al., 2016; Mao et al., 2016) got 6 points, 15 studies (Qiao et al., 2006; Lu et al., 2008; Cheng et al., 2009; Qiu et al., 2009; Zhang et al., 2010; Shang et al., 2011; Mo et al., 2012; Wang S. H. et al., 2012; Chen et al., 2013; Shang et al., 2013; Hu et al., 2014; Xu et al., 2014; Zhu et al., 2016; Wang et al., 2017) got 5 points, and three studies (Lu et al., 2014; Sun, 2014; Yang et al., 2017) got 4 points. All 27 included studies had random allocation, including 10 (Qiao et al., 2006; Cheng et al., 2009; Qiu et al., 2009; Mo et al., 2012; Wang S. H. et al., 2012; Chen et al., 2013; Lu et al., 2014; Sun, 2014; Zhu et al., 2016) in which a random number table was used, eight (Lu et al., 2006; Chu et al., 2010; Wang et al., 2009; Shang et al., 2011; Wang Y. G. et al., 2012; Liu et al., 2014; Duan et al., 2016; Wang et al., 2017) that employed a computer generated random sample set, three (Xu et al., 2015; Zhang et al., 2015; Wang et al., 2016) that applied block randomization, and six (Lu et al., 2008; Zhang et al., 2010; Shang et al., 2013; Hu et al., 2014; Xu et al., 2014; Mao et al., 2016) that stated that randomization was used without providing methodological details. Of the 27 included studies, all studies reported blinding of participants and personnel and withdraw bias. Additionally, nine studies (Lu et al., 2006; Chu et al., 2010; Wang et al., 2009; Liu et al., 2014; Xu et al., 2015; Zhang et al., 2015; Duan et al., 2016; Mao et al., 2016; Wang et al., 2016) reported using allocation concealment; eight studies (Lu et al., 2006; Chu et al., 2010; Wang et al., 2009; Liu et al., 2014; Xu et al., 2015; Zhang et al., 2015; Duan et al., 2016; Wang et al., 2016) applied blinding specifically during outcome measure assessment, and 22 studies (Lu et al., 2006; Qiao et al., 2006; Lu et al., 2008; Cheng et al., 2009; Chu et al., 2010; Qiu et al., 2009; Wang et al., 2009; Zhang et al., 2010; Shang et al., 2011; Mo et al., 2012; Wang S. H. et al., 2012; Chen et al., 2013; Shang et al., 2013; Hu et al., 2014; Liu et al., 2014; Xu et al., 2014; Xu et al., 2015; Mao et al., 2016; Wang et al., 2016; Zhu et al., 2016; Wang et al., 2017) reported selective reporting. No study provided sample size estimation information. The methodological quality is concluded in Table 2 .
Table 2.
Quality assessment of included studies.
| Study | A | B | C | D | E | F | G | Total |
|---|---|---|---|---|---|---|---|---|
| Lu et al., 2006 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| Qiao et al., 2006 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 5 |
| Lu et al., 2008 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 5 |
| Chu et al., 2010 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| Cheng et al., 2009 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 5 |
| Qiu et al., 2009 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 5 |
| Wang et al., 2009 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| Zhang et al., 2010 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 5 |
| Shang et al., 2011 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 5 |
| Mo et al., 2012 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 5 |
| Wang S. H. et al., 2012 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 5 |
| Wang Y. G. et al., 2012 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 5 |
| Chen et al., 2013 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 5 |
| Shang et al., 2013 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 5 |
| Lu et al., 2014 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 4 |
| Hu et al., 2014 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 5 |
| Liu et al., 2014 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| Sun, 2014 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 4 |
| Xu et al., 2014 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 5 |
| Xu et al., 2015 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| Zhang et al., 2015 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 6 |
| Duan et al., 2016 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 6 |
| Mao et al., 2016 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 6 |
| Wang et al., 2016 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| Zhu et al., 2016 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 5 |
| Wang et al., 2017 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 5 |
| Yang et al., 2017 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 4 |
A, adequate sequence generation; B, concealment of allocation; C, blinding of participants and personnel; D, blinding of outcome assessment; E, incomplete out-come data; F, selective reporting; G, other bias; 1, low risk of bias, the information of the domain was adequate in the text; 0, high risk of bias, the information of the domain was inadequate in the text.
Effectiveness
The Incidence of MI and PCI
Meta-analysis of 10 studies (Lu et al., 2006; Lu et al., 2008; Wang et al., 2009; Shang et al., 2011; Shang et al., 2013; Lu et al., 2014; Sun, 2014; Xu et al., 2015; Mao et al., 2016; Wang et al., 2016) found a significant difference in favor of CHM for decreasing the incidence of MI compared with control group (n = 9510, OR = 0.50, 95% CI (0.41, 0.63), P < 0.00001, I2 = 0%) ( Figure 2 ). Meta-analysis of five studies (Lu et al., 2006; Lu et al., 2008; Wang et al., 2009; Shang et al., 2013; Wang et al., 2016) showed CHM existed significant effect for decreasing the incidence of PCI compared with control group (n = 8775, OR = 0.66, 95% CI (0.51, 0.86), P = 0.002, I2 = 0%) ( Figure 3 ).
Figure 2.
The forest plot: effects of Chinese herbal medicine for decreasing the incidence of myocardial infarction compared with control group.
Figure 3.
The forest plot: effects of Chinese herbal medicine for decreasing the incidence of percutaneous coronary intervention compared with control group.
Cardiovascular Mortality
Seven studies (Wang et al., 2009; Shang et al., 2013; Sun, 2014; Xu et al., 2015; Mao et al., 2016; Wang et al., 2016) reported cardiovascular mortality as the outcome measure. Of which, there were no deaths were found in three studies (Lu et al., 2008; Wang et al., 2009; Wang et al., 2016). Meta-analysis of remaining four studies (Shang et al., 2013; Sun, 2014; Xu et al., 2015; Mao et al., 2016) showed CHM existed significant effect for decreasing cardiovascular mortality compared with control group (n = 9,060, OR = 0.73, 95% CI: 0.58,0.93, P = 0.009, I2 = 15%) ( Figure 4 ).
Figure 4.
The forest plot: effects of Chinese herbal medicine for decreasing the cardiovascular mortality compared with control group.
Systolic and Diastolic Functions of the Heart in Cardiac Ultrasound and the Level of ST-Segment Depression in Electrocardiogram
For systolic function, five studies (Qiao et al., 2006; Qiu et al., 2009; Chen et al., 2013; Sun, 2014; Mao et al., 2016) showed CHM existed significant effect for increasing LVEF compared with control group (P < 0.05). For diastolic function, there was no study involving related indicators as outcome measure. Two studies (Qiao et al., 2006; Chen et al., 2013) showed that CHM could decrease the ventricular wall motion score compared with control (P < 0.05). In addition, meta-analysis of three studies (Chu et al., 2010; Hu et al., 2014; Sun, 2014) reported that CHM can increase degree of decline in the ST-segment compared with control (n = 473, OR = 2.51, 95% CI: 1.64∼3.83, P < 0.0001, I2 = 0%) ( Figure 5 ).
Figure 5.
The forest plot: effects of Chinese herbal medicine for increasing degree of decline in the ST segment compared with control group.
Clinical Efficacy
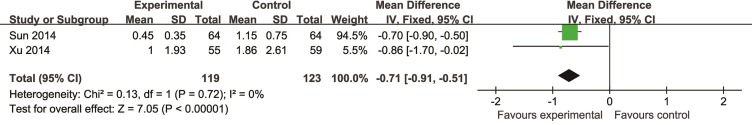
Compared with controls, meta-analysis of 12 studies (Lu et al., 2006; Chu et al., 2010; Shang et al., 2011; Mo et al., 2012; Wang S. H. et al., 2012; Hu et al., 2014; Lu et al., 2014; Sun, 2014; Zhang et al., 2015; Duan et al., 2016; Zhu et al., 2016; Yang et al., 2017) showed that the efficiency of angina improved more obviously in the TCM group than that in the control group (n = 1711, OR = 0.21, 95% CI: 0.17∼0.26, P = 0.09, I2 = 41%) ( Figure 6 ); two studies (Sun, 2014; Xu et al., 2014) for reducing the usage of nitroglycerin (n = 242, MD = -0.71, 95%CI: -0.91∼-0.51, P < 0.00001, I2 = 0%) ( Figure 7 ), four studies (Lu et al., 2008; Wang Y. G. et al., 2012; Zhu et al., 2016; Wang et al., 2017) for reducing LDL (n = 5,248, SMD = -0.67, 95%CI: -0.73∼-0.61, P < 0.00001,I2 = 0%) ( Figure 8 ), two studies (Mo et al., 2012; Wang S. H. et al., 2012) for reducing hsCRP (n = 182, OR = -0.95, 95% CI: -1.26∼0.64, P < 0.00001, I2 = 0%) ( Figure 9 ), two studies (Lu et al., 2006; Yang et al., 2017) for reducing the degree of coronary artery stenosis (P < 0.05), and two studies (Shang et al., 2011; Lu et al., 2014) for reducing the rate of coronary restenosis (P < 0.05).
Figure 6.
The forest plot: effects of Chinese herbal medicine for improving the efficiency of angina compared with control group.
Figure 7.
The forest plot: effects of Chinese herbal medicine for reducing the usage of nitroglycerin compared with control group.
Figure 8.
The forest plot: effects of Chinese herbal medicine for reducing low density lipoprotein.
Figure 9.
The forest plot: effects of Chinese herbal medicine for reducing hypersensitive C-reactive protein.
The Safety of Co-Administration of CHM
Adverse events were reported in 11 studies (Lu et al., 2006; Chu et al., 2010; Wang et al., 2009; Zhang et al., 2010; Shang et al., 2011; Shang et al., 2013; Sun, 2014; Zhang et al., 2015; Wang et al., 2016; Zhu et al., 2016; Yang et al., 2017), analyzed but not observed in four studies (Lu et al., 2008; Cheng et al., 2009; Chen et al., 2013; Liu et al., 2014), and not analyzed in 12 studies (Qiao et al., 2006; Qiu et al., 2009; Mo et al., 2012; Wang S. H. et al., 2012, Wang Y. G. et al., 2012; Lu et al., 2014; Hu et al., 2014; Xu et al., 2014; Xu et al., 2015; Duan et al., 2016; Mao et al., 2016; Wang et al., 2017). In the 11 studies with adequate information about adverse events, a total of 106/5,134 (2.06%) patients suffered adverse events in the treatment groups and 118/5,167 (2.28%) patients in control groups. Gastrointestinal discomfort symptoms, including nausea, stomachache, vomiting, diarrhea, anorexia, and constipation, were the most frequently occurring adverse events, affecting 74/106 (69.8%) patients in the treatment groups and 80/118 (67.8%) in control group patients. Allergy, hemorrhage, hepatic insufficiency, headache, and urinary tract infection were reported frequently also, affecting 20/106 (18.8%) patients in the treatment groups and 26/118 (22.0%) of patients in the control groups. The majority of above adverse events were mild and resolved by stopping related drugs and symptomatic treatment. Although some serious adverse events such as heart failure (1/106), cerebral hemorrhage (1/106), pericardial tamponade (1/106), coronary bypass surgery (1/106), and death (1/106) were reported in the two groups, there was no significant difference between the two groups.
Ingredients of CHM Formulae and Frequently Used Herbs
The ingredients of CHM in each RCT are listed in Table 3 . The most frequently used herbs across all formulae were Miltiorrhiza (nine formulae), pseudo-ginseng (seven formulae), ginseng (seven formulae), Radix Paeoniae rubra (six formulae), Astragalus membranaceus (five formulae), rhizome of Chuanxiong (five formulae), leech (five formulae), borneol (five formulae), and safflower (four formulae). Chinese Angelica, Achyranthes bidentata, Rehmannia glutinosa, peach kernel, liquorice, hawthorn, Trichosanthes, Cinnamomum, Poria, aloes, Rhizoma Corydalis, and ginkgo biloba were also frequently used.
Table 3.
Ingredients of Chinese herbal medicine formulae.
| Study (years) | Prescription | Ingredients of herb prescription | Usage of prescription | Preparations | Quality control |
|---|---|---|---|---|---|
| Chu et al., 2010 | Xuefu Zhuyu capsule | Peach kernel, Angelica sinensis, rhizome of Chuanxiong, safflower, Radix Paeoniae rubra, Radix Rehmanniae, Fructus aurantii, Radix Bupleuri, Platycodon grandiflorum, Radix Achyranthis bidentatae, and liquorice | 3#tid po | Capsule | Traditional Chinese patented medicine WY: Z12020223 |
|
Qiao et al., 2006 Chen et al., 2013 |
Tongguan capsule | Astragalus membranaceus, Miltiorrhiza, leech, etc. | 3#tid po | Capsule | Produced by The Second Affiliated Hospital Of Guangzhou University Of Traditional Chinese Medicine |
| Cheng et al., 2009 | Qingre Quyu granule | Fructus trichosanthis 15g, Miltiorrhiza 30g, hawthorn 30g, Fritillaria thunbergii 10g, pseudo-ginseng 3g, Lignum Millettiae 30g, and the seed of cowherb 15g | 1#bid po | Decoction | Produced by China Pharmaceutical Materials Group Company |
| Lu et al., 2006 Shang et al., 2011 | Xiongshao capsule | Rhizome of Chuanxiong and Radix Paeoniae rubra | 2#tid po | Capsule | Unreported |
| Wang et al., 2017 | Xuesaitong soft capsule | Pseudo-ginseng | 2#bid po | Capsule | Traditional Chinese patented medicine WY: Z19990022 |
| Mo et al., 2012 | Yixin Mai granule | Ginseng, cassia twig, Fructus trichosanthis, leech, and Poria cocos | 1#tid po | Decoction | Produced by Ruikang Hospital Affiliated to Guangxi College of Traditional Chinese Medicine |
| Liu et al., 2014 | Red ginseng Tongxin granule | Radix Paeoniae rubra 10g, Agilawood 1g, Angelica sinensis 10g, orange peel 10g, Rhizoma Corydalis 6g, rhizome of Chuanxiong 6g, Miltiorrhiza 10g, astragalus 6g, peach kernel 10g, and safflower 10g | Unreported | Decoction | Produced by Jiangyin Tianjiang Pharmaceutical Co., Ltd. |
| Zhu et al., 2016 | Traditional Chinese medicine prescription | Hawthorn, Miltiorrhiza, ginkgo leaf, lentil, Psoralea, sapanwood, Ganoderma, Polygonum multiflorum, Cornus officinalis, Alisma orientalis, Radix Paeoniae alba, cinnamon, Pericarpium Citri reticulatae, liquorice, Fructus cnidii, cicada slough, and Ramuli Umcariae Cumuncis | 1#tid po | Capsule | Unreported |
| Wang et al., 2009 | Shenshao tablet | Radix Paeoniae alba and ginseng | 4#tid po | Tablet | Traditional Chinese patented medicine WY: Z19990059 |
|
Xu et al., 2015 Xu et al., 2014 |
Shenzhu Guanxin recipe | Ginseng 5g, Rhizoma Atractylodis 10g, Radix Notoginseng 10g, Rhizoma Pinelliae 10g, leech 3g, Radix Panacis quinquefolium 5g, and Folium nelumbinis 15g | 50ml qd po | Decoction | Produced by Jiangxi Jiangyin Pharmaceutical Factory |
| Wang et al., 2012 Wang et al., 2016 Mao et al., 2016 |
Danlou tablet | Fructus trichosanthis, Allium macrostemon, the root of kudzu vine, rhizome of Chuanxiong, Miltiorrhiza, Radix Paeoniae rubra, Alisma orientalis, Astragalus membranaceus, Curcuma aromatica, and Drynaria rhizome | 4.5g qd po | Tablet | Traditional Chinese patented medicine WY: YBZ17382006 |
|
Zhang et al., 2010 Lu et al., 2014 |
Tongxinluo capsule | Ginseng, leech, scorpion, red peony root, cicada slough, soil turtle worm, centipede, sandalwood, Lignum acronychiae, frankincense, jujube nut, and borneol | Before PCI: 8# qd po After PCI: 4#tid po |
Capsule | Traditional Chinese patented medicine WY: Z19980015 |
| Qiu et al., 2009 | Compound Salvia tablet and Xinyue capsule | Miltiorrhiza 450mg, pseudo-ginseng 141mg, borneol 8mg, ginseng 50mg | Unreported | Capsule | Traditional Chinese patented medicine WY: Z44023372 and Z20030073 |
| Hu et al., 2014 | Kodaling tablet | Rhizoma Corydalis | 3#tid po | Tablet | Produced by Zhejiang KangEnbei Pharmaceutical Co., Ltd. |
| Duan et al., 2016 | Live heart pill | Ginseng, Radix Aconiti carmichaeli, Ganoderma lucidum, safflower, musk, bezoar, bear bile, pearl, toad venom, and borneol | 2#tid po | Tablet | Traditional Chinese patented medicine WY: Z44021835 |
| Zhang et al., 2015 | Wufuxinnaoqing | Safflower oil, borneol, vitamin E, and vitamin B6 | 2#tid po | Capsule | Produced by Shineway Pharmaceutical Group Co., Ltd. |
| Lu et al., 2008 | Xuezhikang capsule | Red yeast Chinese rice | 600mg bid po | Capsule | Produced by the Beijing WBL Peking University Biotech Co. Ltd. |
| Shang et al., 2013 | Qi-Shen-Yi-Qi dripping pills | Miltiorrhizae, pseudo-ginseng, Lignum Dalbergiae odoriferae, and Astragalus membranaceus | 0.5g tid po | Pill | Unreported in detail |
| Yang et al., 2017 | Coronary Ningtong prescription | Astragalus membranaceus 30g, Miltiorrhiza 30g, mulberry parasitism 30g, Gynostemma pentecox 30g, hawthorn 30g, the root of kudzu vine 30g, Herba Rhodiolae 30g, Fructus trichosanthis 15g, Allium macrostemon 15g, Rhizoma Pinellinae praeparata 15g, immature bitter orange 10g, safflower 10g, Rhizoma Sparganii 10g, Zedoaria 10g, Rhizoma coptidis 6g, pseudo-ginseng 3g, and leech 3g | 100ml bid po | Decoction | Unreported |
| Sun, 2014 | Musk Baoxin pill | artificial musk, ginseng, cinnamon, toad venom, storax, artificial bezoar, and borneol | 2#tid po | Pill | Produced by Shanghai and Huangyao Pharmaceutical Industry |
| Wang et al., 2012 | Double ginseng capsule And Tongguan capsule | Miltiorrhiza, ginseng, Herba Rhodiolae, pseudo-ginseng and Lignum Dalbergiae odoriferae | 4#tid po | Capsule | Produced by Shaanxi Pharmaceutical Group Shaanxi New Drug Technology Development Center |
d, day; #: tablet; PCI, Percutaneous Coronary Intervention; Co., Ltd, Company Limited.
Possible Mechanism of Herbal Benefits for CHD
A total of 45 experimental studies (Jormalainen et al., 2004; Liu et al., 2008; Chen et al., 2008; He et al., 2008; Nizamutdinova et al., 2008; Wang et al., 2008; Liu et al., 2010; Liu et al., 2010; Tang et al., 2010; Zhang et al., 2010; Liu and Niu, 2011; Liu et al., 2011; Pan et al., 2011; Wu et al., 2011; Zhai et al., 2011; Liu et al., 2012; Lv et al., 2012; Zhu et al., 2013; Lim et al., 2013; Tu et al., 2013; Wang et al., 2013; Yin et al., 2013; Zhang et al., 2013; He et al., 2014; Li et al., 2014; Liu et al., 2014; Park et al., 2014; Qian et al., 2014; Tang et al., 2014; Tao et al., 2014; Wei et al., 2014; Xue et al., 2014; Zhang et al., 2014; Deng et al., 2015; Lu et al., 2015; Wang et al., 2015; Xia et al., 2015; Yu et al., 2015; Chen et al., 2016; Fan et al., 2016; Hu et al., 2016; Leng et al., 2015; Ma et al., 2016; Meng et al., 2016; Yu et al., 2016) were identified in our electronic searches to investigate the effects and mechanisms of the main active components of single flavored Chinese medicine which were frequently used on I/R injury models ( Table 4 ). The possible mechanisms of them are summarized as follows: (1) oxidative stress is important reaction after myocardial ischemia. The function of free radical scavenging system is decreased in myocardial ischemia. Large amounts of free radicals was produced by the unbalanced endogenous antioxidant systems, which further leads to the peroxidation of lipids, proteins and nucleic acids, the biochemical alteration (reducing SOD, and GSH-Px, and increasing MDA), and further led to cardiomyocyte death (Yellon and Hausenloy, 2007). Based on these observations, antioxidant therapy is the key step considered to prevent I/R injury. In our study, Salvia miltiorrhiza, salvianolic acid B, tanshinone IIA, notoginsenoside R1, ginsenoside Rb1, ginsenoside Rb3, astragaloside IV, and ligustrazine could enhance SOD (Chan et al., 2012; Lv et al., 2012; Liu et al., 2014; Tang et al., 2014; Xue et al., 2014; Wang et al., 2015; Xia et al., 2015) and attenuate chondriokinesis to reduce the release of MDA (Liu et al., 2011; Chan et al., 2012; Liu et al., 2014; Tang et al., 2014; Xue et al., 2014; Xia et al., 2015); borneol, ginsenoside Rd, and hydroxysafflor yellow A could reduce ROS (He et al., 2008; Liu and Niu, 2011; Wang et al., 2013). S. miltiorrhiza and hydroxysafflor yellow A (Hu et al., 2016) exhibit antioxidant effects via PI3K/Akt signaling pathway; tanshinone IIA (Wei et al., 2014) increases NADPH oxidase via AMPK/Akt/PKC pathway; and astragaloside IV (Zhang et al., 2014) could reduce ROS via the PI3K/Akt/mTOR pathway. Our study showed TCM could improve the antioxidant function to reduce the damage of myocardial ischemia. (2) Apoptosis was an energy-requiring programmed cell death (Zhang and Xu, 2000). Apoptosis can be activated extrinsically by sarcolemmal receptors such as FAS: FAS(CD 95) and tumor necrosis factor alpha (TNF-α) (Kleinbongard et al., 2011), or intrinsically by cytochrome c which initiates the caspase cascade activation result in intracellular proteolysis. In addition, the opening of mitochondrial permeability transition pore (MPTP) conduces the mitochondrial matrix swelling, then leading to rupture of the outer membrane and release of cytochrome c, activating the caspase cascade, ultimately resulting in the apoptotic cell death (Heusch et al., 2010). Proapoptotic and antiapoptotic proteins of the Bcl family interact with the MPTP (Baines, 2009). In present study, S. miltiorrhiza, salvianolic acid A, salvianolic acid B, paeonol, paeoniflorin, ginsenoside Rb1, ginsenoside Rb3, ginsenoside Rd, ginsenoside Rg3, and ligustrazine could increase Bcl-2 expression (Nizamutdinova et al., 2008; Wang et al., 2008; Tang et al., 2010; Zhai et al., 2011; Wang et al., 2013; Liu et al., 2014; Wang et al., 2015; Fan et al., 2016) and the Bcl-2/Bax ratio (Nizamutdinova et al., 2008; Tang et al., 2010; Wu et al., 2011; Zhai et al., 2011; Wang et al., 2013; Liu et al., 2014; Wang et al., 2015; Chen et al., 2016; Fan et al., 2016). Three studies (Wang et al., 2008; Zhang et al., 2013; Chen et al., 2016) reported that salvianolic acid A, tanshinone IIA, and ginsenoside Rb1 exhibit anti-apoptotic effects via PI3K/Akt signaling pathway, and one study (Wang et al., 2013) reported that ginsenoside Rd could decrease caspase-3 and caspase-9 activities. (3) The inflammation during myocardial I/R injury was reviewed by previous studies (Marchant et al., 2012). The excessive inflammation can lead to cardiomyocyte damage. When the myocardium got reperfused, the NF-κB pathway was activated by pattern recognition receptors, culminating in promoted cytokine expression. S. miltiorrhiza, tanshinone I, tanshinone IIA, paeonol, notoginsenoside r1, ginsenoside Re, ginsenoside Rg1, ligustrazine, astragaloside IV, and astragalus polysaccharides were shown to exert anti-inflammatory effects by decreasing TNF-alpha (Zhang et al., 2010; Lim et al., 2013; Tu et al., 2013; Deng et al., 2015; Lu et al., 2015; Xia et al., 2015), IL-6 (Chen et al., 2008; Zhang et al., 2010), IL-8 (Li et al., 2014), and NF-κB (Tu et al., 2013; Qian et al., 2014; Deng et al., 2015; Lu et al., 2015). Two studies (Qian et al., 2014; Zhu et al., 2013) reported that ligustrazine and astragalus polysaccharides exhibit anti-inflammatory effects via inhibiting P38MAPK pathway, and one study (Zhang et al., 2010) reported that tanshinone IIA could decrease TNF-alpha and IL-6 via PI3K/Akt pathway. (4) Nitric oxide is an essential modulator of cardiovascular system. The NO can decrease intracellular calcium concentration in vascular smooth muscle cells, which further induces vasodilation (Schulz et al., 2004). Salvianolic acid B, tanshinone IIA, ginsenoside Rb1, ginsenoside Rg3, ligustrazine, astragaloside IV, and hydroxysafflor yellow A were shown to improve circulation by increasing NO expression (Liu et al., 2008; Liu et al., 2010; Pan et al., 2011; Lv et al., 2012; Leng et al., 2015; Wang et al., 2015) via up-regulating eNOS phosphorylation (Liu et al., 2008; Liu et al., 2010; Pan et al., 2011; Lv et al., 2012; Leng et al., 2015; Wang et al., 2015). (5) S. miltiorrhiza and notoginsenoside r1 were shown to regulate energy metabolism via p-JNK-NF-kappaB-TRPC6 pathway (Meng et al., 2016) and ROCK-dependent ATP5D modulation separately (He et al., 2014; Li et al., 2014). (6) Hirudin was shown to attenuate coagulation and enhance microvascular flow during reperfusion (Jormalainen et al., 2004). Thus, antioxidant, anti-apoptotic, circulation improvement, anti-inflammatory, and energy metabolism regulation actions have been promoted as important mechanisms of herbal compounds used to treat I/R injury.
Table 4.
Mechanisms of the main active components of single flavored Chinese Medicine on organic injury induced by ischemia/reperfusion.
| Active ingredients | Herb source | Possible mechanisms (signaling pathway) | Citation | Structure |
|---|---|---|---|---|
| Salvia miltiorrhiza | Miltiorrhiza | 1. Regulation of energy metabolism (p-JNK-NF-kappaB-TRPC6 pathway) 2. Attenuation of oxidative stress (Akt/Nrf2/HO-1 pathway) 3. Anti-inflammation 4. Anti-apoptosis (increase expression of Bcl-2 and increase Bcl-2/Bax ratio, affect Akt, and ERK1/2 phosphorylation) |
1. Meng et al., 2016 2. Hu et al., 2016 3. Yin et al., 2013 4. Yu et al., 2015; Fan et al., 2016 |
 |
| Salvianolic acid A | Miltiorrhiza | 1. Anti-apoptosis (increase Bcl-2/Bax ratio via JNK/PI3K/Akt signaling pathway) | 1. Chen et al., 2016 |  |
| Salvianolic acid B | Miltiorrhiza | 1. Improve circulation (increase expression of NO via up-regulating eNOS phosphorylation) 2. Attenuation of oxidative stress (increase SOD and decrease MDA) 3. Anti-apoptosis |
1. Pan et al., 2011 2. Xue et al., 2014 3. Xue et al., 2014 |
 |
| Tanshinone I | Miltiorrhiza | 1. Anti-inflammation | 1. Park et al., 2014 |  |
| Tanshinone IIA | Miltiorrhiza | 1. Improve circulation (increase expression of NO via up-regulating eNOS phosphorylation) 2. Attenuation of oxidative stress (increase SOD and HO-1, decrease MDA, increase NADPH oxidase via AMPK/Akt/PKC pathway) 3. Anti-inflammation (decrease TNF-alpha and IL-6 via PI3K/Akt-dependent pathway) 4. Anti-apoptosis (decrease caspase-3 activity via Akt/FOXO3A/Bim-mediated signal pathway) |
1. Pan et al., 2011 2. Tang et al., 2014; Wei et al., 2014 3. Zhang et al., 2010 4. Zhang et al., 2013 |
 |
| Paeonol | Radix Paeoniae rubra | 1. Anti-inflammation 2. Anti-apoptosis (increase expression of Bcl-2 and increase Bcl-2/Bax ratio) |
1. Ma et al., 2016 2. Nizamutdinova et al., 2008 |
 |
| Paeoniflorin | Radix Paeoniae rubra | 1. Anti-apoptosis (increase expression of Bcl-2 and increased Bcl-2/Bax ratio) | 1. Tang et al., 2010 |  |
| Notoginsenoside r1 | Pseudo-Ginseng | 1. Anti-inflammation (decrease IL-6, IL-8, and TNF-alpha) 2. Regulation of energy metabolism (ROCK-dependent ATP5D modulation) 3. Anti-apoptosis (increase Bcl-2 expression) 4. Attenuation of oxidative stress (increase SOD, and decrease MDA) 5. Attenuation of endoplasmic reticulum stress |
1. Chen et al., 2008; Li et al., 2014; Xia et al., 2015 2. He et al., 2014; Li et al., 2014 3. Liu et al., 2010 4. Xia et al., 2015 5. Yu et al., 2016 |
 |
| Ginsenoside Rb1 | Ginseng | 1. Anti-apoptosis (increase Bcl-2 expression and increased Bcl-2/Bax ratio via PI3K/Akt pathway) 2. Attenuation of oxidative stress (increase SOD, and decrease MDA) 3. Improve circulation (increase NO expression via up-regulating eNOS phosphorylation) |
1. Wang et al., 2008; Wu et al., 2011 2. Chan et al., 2012 3. Leng et al., 2015 |
 |
| Ginsenoside Rb3 | Ginseng | 1. Attenuation of oxidative stress (increase SOD, and decrease MDA) 2. Anti-apoptosis (increase Bcl-2 expression and increase Bcl-2/Bax ratio) |
1. Liu et al., 2014 2. Liu et al., 2014 |
 |
| Ginsenoside Rd | Ginseng | 1. Attenuation of oxidative stress (decrease ROS) 2. Anti-apoptosis (increase Bcl-2 expression and increase Bcl-2/Bax ratio, decrease caspase-3 and caspase-9 activity via mitochondrial-dependent apoptotic pathway) |
1. Wang et al., 2013 2. Wang et al., 2013 |
 |
| Ginsenoside Re | Ginseng | 1. Anti-inflammation (decrease TNF-alpha) | 1. Lim et al., 2013 |  |
| Ginsenoside Rg1 | Ginseng | 1. Anti-inflammation (decrease TNF-alpha and IL-1beta, in part via the NF-κB signaling pathway) | 1. Tao et al., 2014; Deng et al., 2015 |  |
| Ginsenoside Rg3 | Ginseng | 1. Attenuation of oxidative stress (increase SOD) 2. Anti-apoptosis (increase Bcl-2 expression and increase Bcl-2/Bax ratio) 3. Improve circulation (increase NO expression via up-regulating eNOS phosphorylation) |
1. Wang et al., 2015 2. Wang et al., 2015 3. Wang et al., 2015 |
 |
| Borneol | Borneol | 1. Attenuation of oxidative stress (decrease ROS) | 1. Liu and Niu, 2011 |  |
| Ligustrazine | Rhizome of Chuanxiong | 1. Attenuation of oxidative stress (increase SOD and decrease MDA) 2. Improve circulation (increase NO expression via up-regulating eNOS phosphorylation) 3. Anti-inflammation (decrease the expression of NF-κB via inhibiting P38MAPK pathway) 4. Anti-apoptosis (increase Bcl-2 expression and increase Bcl-2/Bax ratio) |
1. Liu et al., 2011; Lv et al., 2012 2. Lv et al., 2012 3. Qian et al., 2014 4. Zhai et al., 2011 |
 |
| Astragaloside IV | Astragalus membranaceus | 1. Promoting angiogenesis (increase the expression of VEGF) 2. Anti-apoptosis (increase Bcl-2 expression and increase Bcl-2/Bax ratio, decrease caspase-3) 3. Anti-inflammation (decrease the expression of TNF-alpha and NF-κB) 4. Improve circulation (increase NO expression via up-regulating eNOS phosphorylation) 5. Attenuation of oxidative stress (reduce ROS to via the PI3K/Akt/mTOR pathway) |
1. Yu et al., 2015 2. Tu et al., 2013; Lu et al., 2015 3. Tu et al., 2013; Lu et al., 2015 4. Liu et al., 2010 5. Zhang et al., 2014 |
 |
| Astragalus polysaccharides | Astragalus membranaceus | 1. Anti-inflammatory (via the p38 MAPK signaling pathway) | 1. Zhu et al., 2013 |  |
| Hirudin | Leech | 1. Attenuate coagulation and enhance microvascular flow during reperfusion | 1. Jormalainen et al., 2004 |  |
| Hydroxysafflor yellow A | Safflower | 1. Attenuation of oxidative stress (Akt/Nrf2/HO-1 pathway, decrease ROS) 2. Improve circulation (increase NO expression via up-regulating eNOS phosphorylation) |
1. He et al., 2008; Hu et al., 2016 2. Liu et al., 2008 |
 |
HO-1, heme oxygenase-1; SOD, super oxide dismutase; PI3K, phosphatidylinositol 3-kinase; ROS, reactive oxygen species; eNOS, endothelial nitric oxide synthase; MDA, malonaldehyde; NADPH, nicotinamide adenine dinucleotide phosphate; VEGF, vascular endothelial growth factor; NF-κB, nuclear factor κB.
Discussion
Summary of Evidence
This is the first clinical systematic review of 27 high-quality RCTs involving 11,732 participants to estimate the efficacy and safety of CHMs for CHD. The evidence available from present study revealed that CHMs are beneficial for CHD and are generally safe. In addition, CHM exerted cardioprotection for CHD, possibly altering multiple signal pathways through anti-inflammation, anti-oxidation, anti-apoptosis, circulation improvement, and energy metabolism regulation.
Limitations
First, there were still some methodological weaknesses in the primary studies although we included high-quality studies. Only nine of the 27 included studies (Lu et al., 2006; Chu et al., 2010; Wang et al., 2009; Liu et al., 2014; Xu et al., 2015; Zhang et al., 2015; Duan et al., 2016; Mao et al., 2016; Wang et al., 2016) reported allocation concealment, and eight included studies (Lu et al., 2006; Chu et al., 2010; Wang et al., 2009; Liu et al., 2014; Xu et al., 2015; Zhang et al., 2015; Duan et al., 2016; Wang et al., 2016) reported blinding during outcome assessment. It is worth noting that an average 18% more “beneficial” effect in trials with inadequate or unclear concealment of allocation compared with adequate concealment (Higgins and Green, 2012). And blinding during outcome assessment is an essential method to avoid systemic errors which existed in the outcome assessment of non-blinded studies (Higgins and Green, 2012). Second, English and Chinese literatures were included only in present study and the absence of studies written in other languages may generate selective bias in a certain degree. Third, no included trials were reported to have been registered, and negative findings were less likely to be published, which may lead to the efficacy being overestimated.
Implications
The findings from present study indicate that CHM paratherapy is beneficial for CHD and is well tolerated. Thus, we recommended, at least to an extent, to use CHMs for CHD, especially selected case. Further study should identify specific CHM and/or indications of CHM. In addition, the findings of the most frequently used herbs such as Miltiorrhiza, pseudo-ginseng, ginseng, Radix Paeoniae rubra, Astragalus membranaceus, rhizome of Chuanxiong, leech, borneol, and safflower and their main active components should be considered as further development of herbal prescriptions and component injection for CHD.
Some methodological weaknesses still existed in the primary studies. Recommendations for further research are as follows: (1) the CONSORT 2010 statement (Schulz et al., 2010), CONSORT for TCM (Bian et al., 2011), RCTs investigating CHM (Flower et al., 2012), and CONSORT Extension for Chinese Herbal Medicine Formulas 2017 (Cheng et al., 2017) should be abided by for the design. (2) Clinic trials should be registered in a generally accessible database (www.clinicaltrials.com) prior to first case inclusion. It allows verification of predefined study hypothesis and end-points of the study, which would help to the report of negative findings and reduce publication bias (Rongen and Wever, 2015). (3) In view of trials with insufficient statistical power that runs the risk of over estimating therapeutic efficacy (Kjaergard et al., 2001), the further studies are recommended to provide statistical information of sample size estimation. (4) In order to ensure the efficacy of TCM, the identity and quantity of the herbal preparations should be described clearly in further research. (5) The safety of TCM has been increasingly concerned by both medical workers and the public.
The frequency of use for particular herb was calculated and those used at a high frequency that are described in detail in the part 3.6 and Table 3 . The high-frequency herbs that we selected can ignite the treatment based on syndrome differentiation according to the herbal functions Table 5 . Ginseng and Astragalus membranaceus benefit qi; Miltiorrhiza, pseudo-ginseng, Radix Paeoniae rubra, rhizome of Chuanxiong, leech, and safflower promote blood circulation for removing blood stasis; and borneol has function of resuscitation with aromatics for relieving pain. Thus, we can also deduce that the main patterns of CHD are qi deficiency and blood stasis. The selected high-frequency herbs are composed of a herbal prescription for CHD, which can be used for clinic and as a candidate for RCT.
Table 5.
Different syndromes of coronary heart disease and the classification of herbs according to syndrome differentiation therapy for different syndromes.
| Syndrome | Syndrome differentiation therapy for different syndromes | Representative herbs in the theory of traditional Chinese medicine mentioned in present study |
|---|---|---|
| Syndrome of coagulation cold in heart vessel | 1. Dispelling cold 2. Dredging channel blockade and yang |
1. Cinnamon, Psoralea, Ramuli Umcariae Cumuncis 2. Angelica sinensis, radix Paeoniae rubra, pseudo-ginseng, rhizome Of Chuanxiong, frankincense, Miltiorrhiza, safflower, peach kernel, rhizoma Corydalis, leech, soil turtle worm, Lignum Millettiae, ginkgo leaf, sapanwood, red yeast Chinese, Allium macrostemon |
| Syndrome of qi stagnation in heart and chest | 1. Dispersing stagnated liver qi for regulating qi-flowing | 1. Hawthorn, Fructus aurantii, Lignum acronychiae, Agilawood, radix Bupleuri, rhizoma Corydalis |
| Syndrome of blockade of heart blood | 1. Promoting blood circulation for removing blood stasis | 1. Angelica sinensis, radix Paeoniae rubra, pseudo-ginseng, rhizome Of Chuanxiong, frankincense, Miltiorrhiza, safflower, peach kernel, rhizoma Corydalis, leech, soil turtle worm, Lignum Millettiae, ginkgo leaf, sapanwood, red yeast Chinese rice |
| Syndrome of turbid phlegm blocking heart | 1. Dredging yang for resolving turbidity 2. Eliminating phlegm for resolving masses |
1. Allium macrostemon 2. Rhizoma Pinelliae, liquorice, Platycodon grandiflorum, Fritillaria thunbergii, orange peel |
| Syndrome of deficiency of both qi and yin | 1. Benefiting qi and nourishing yin 2. Promoting blood circulation for dredging vessels |
1. Angelica sinensis, ginseng, Astragalus Membranaceus, radix Panacis quinquefolium, Rhizoma Atractylodis, liquorice 2. Radix Paeoniae Rubra, pseudo-ginseng, rhizome Of Chuanxiong, frankincense, Miltiorrhiza, safflower, peach kernel, rhizoma Corydalis, leech, soil turtle worm, Lignum Millettiae, ginkgo leaf, sapanwood, red yeast Chinese rice, musk |
| Syndrome of yin deficiency of heart and kidney | 1. Nourishing yin and clearing heat 2. Activating blood circulation for nourishing heart |
1. Angelica sinensis, borneol, Cassia twig, cicada slough, Fructus trichosanthis, Fritillaria thunbergii, orange peel, Folium nelumbinis, bezoar, bear bile, pearl, toad venom, Gynostemma pentecox, Rhizoma Coptidis, liquorice 2. Radix Paeoniae rubra, pseudo-ginseng, rhizome of Chuanxiong, frankincense, Miltiorrhiza, safflower, peach kernel, rhizoma Corydalis, leech, soil turtle worm, Lignum Millettiae, ginkgo leaf, sapanwood, red yeast Chinese rice |
| Syndrome of yang deficiency of heart and kidney | 1. Warmly tonifying yang qi and inspiring heart yang | 1. Ginseng, Astragalus Membranaceus, radix Panacis quinquefolium, Rhizoma Atractylodis, liquorice, Ganoderma, Herba Rhodiolae |
Cardioprotection by anti-inflammation, antioxidant, anti-apoptosis, and circulation improvement for myocardial I/R injury (Xu et al., 2014) was an innovative strategy for antagonizing the injurious biochemical and molecular events that eventually resulted in irreversible ischemic injury (Wu and He, 2010). The included preclinical trials presented the main active components of the most frequently used herbs that performed anti-inflammatory, anti-oxidation, anti-apoptosis, energy metabolism regulation, and circulation improvement mechanisms in multiple models of I/R injury through multiple signal pathways, including the PI3K/Akt signaling pathway, AMPK/Akt/PKC pathway, PI3K/Akt/mTOR pathway, mitochondrial-dependent apoptotic pathway, P38MAPK pathway, eNOS phosphorylation, and p-JNK-NF-kappaB-TRPC6 pathway. Further studies of CHM for CHD should explore the multi-drug, multi-target signal pathway using novel techniques such as network pharmacological approach.
Conclusion
The findings from present study indicate that CHMs are beneficial for CHD and are generally safe. In addition, CHM exerted cardioprotection for CHD, possibly altering multiple signal pathways through anti-inflammatory, anti-oxidation, anti-apoptosis, circulation improvement, and energy metabolism regulation mechanisms.
Author Contributions
Study conception and design: GQZ, YW, KJZ, and QZ. Acquisition, analysis and/or interpretation of data: QZ, KJZ, JZZ, XYB, QT, PCZ, ZZ, YYH, GQZ, YW. Final approval and overall responsibility for this published work: GQZ and YW.
Funding
This project was supported by the grant of National Natural Science Foundation of China (81573750/81473491/81173395/H2902).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Arslan F., Bongartz L., Ten Berg J. M., Jukema J. W., Appelman Y., Liem A. H., et al. (2018). ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: comments from the Dutch ACS working group. Neth. Heart J. 26 (9), 417–421. 10.1007/s12471-018-1134-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines C. P. (2009). The mitochondrial permeability transition pore and ischemia-reperfusion injury. Basic Res. Cardiol. 104 (2), 181–188. 10.1007/s00395-009-0004-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian Z., Liu B., Moher D., Wu T., Li Y., Shang H., et al. (2011). Consolidated standards of eporting trials (CONSORT) for traditional Chinese medicine: urrent situation and futured evelopment. Front. Med. 5 (2), 171–177. 10.1007/s11684-011-0132-z [DOI] [PubMed] [Google Scholar]
- Chan K., Shaw D., Simmonds M. S., Leon C. J., Xu Q., Lu A., et al. (2012). Good practice in reviewing and publishing studies on herbal medicine, with special emphasis on traditional Chinese medicine and Chinese materia medica. J. Ethnopharmacol. 140 (3), 469–475. 10.1016/j.jep.2012.01.038 [DOI] [PubMed] [Google Scholar]
- Chen P., Zhu C. L., Zhang M. Z. (2013). Effect of Tongguan capsule on the number of endothelial progenitor cells in the peripheral blood of patients with coronary artery disease after PCI. Zhongguo Zhong Xi Yi Jie He Za Zhi 33 (7), 873–876. 10.7661/CJIM.2013.07.0873 [DOI] [PubMed] [Google Scholar]
- Chen Q., Xu T., Li D., Pan D., Wu P., Luo Y., et al. (2016). JNK/PI3K/Akt signaling pathway is involved in myocardial ischemia/reperfusion injury in diabetic rats: effects of salvianolic acid A intervention. Am. J. Transl. Res. 8, 2534–2548. [PMC free article] [PubMed] [Google Scholar]
- Chen W. X., Wang F., Liu Y. Y., Zeng Q. J., Sun K., Xue X., et al. (2008). Effect of notoginsenoside R1 on hepatic microcirculation disturbance induced by gut ischemia and reperfusion. World J. Gastroenterol. 14, 29–37. 10.3748/wjg.14.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C. W., Wu T. X., Shang H. C., Li Y. P., Altman D. G., Moher D., et al. (2017). CONSORT extension for Chinese herbal medicine formulas 2017: recommendations, explanation, and elaboration. Ann. Intern. Med. 167 (2), W21–W34. 10.7326/M16-2977 [DOI] [PubMed] [Google Scholar]
- Cheng W. L., Wang Y., Cai Z., Ke Y. N., Liu X. F., Fan S. Y. (2009). Effect of Qingre Quyu G ranule on the vulnerable atherosclerotic plaque of carotid artery in patients with stable coronary artery disease. Zhongguo Zhong Xi Yi Jie He Za Zhi 29 (12), 1085–1088. 10.3321/j.issn:1003-5370.2009.12.007 [DOI] [PubMed] [Google Scholar]
- Chu F. Y., Wang J., Yao K. W., Li Z. Z. (2010). Effect of Xuefu Zhuyu Capsule on the symptoms and signs and health-related quality of life in the unstable angina patients with blood-stasis syndrome after percutaneous coronary intervention: a randomized controlled trial. Chin. J. Integr. Med. 16 (5), 399–405. 10.1007/s11655-010-9999-9 [DOI] [PubMed] [Google Scholar]
- Chung V. C., Chen M., Ying Q., Tam W. W., Wu X. Y., Ma P. H., et al. (2013). Add-on effect of chinese herbal medicine on mortality in myocardial infarction: systematic review and meta-analysis of randomized controlled trials. Evid. Based Complementary Altern. Med. 2013, 675906. 10.1155/2013/675906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y., Yang M., Xu F., Zhang Q., Zhao Q., Yu H., et al. (2015). Combined salvianolic Acid B and Ginsenoside Rg1 exerts cardioprotection against ischemia/reperfusion injury in rats. PLoS One 10, e0135435. 10.1371/journal.pone.0135435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan W. H., Xu H., Wang C. P., Gao H. C., Li Y. L., Chen Y. S. (2016). A multicenter, randomized, double-blind, placebo-controlled clinical study of HuoXin Wan (concentrated pill) for the treatment of stable angina pectoris with the syndrome of qi deficiency and blood stasis. Chin. J. Evid. Based Med. 8 (9), 1110–1115. 10.3969/j.issn.1674-4055.2016.09.29 [DOI] [Google Scholar]
- Dunbar S. B., Khavjou O. A., Bakas T., Hunt G., Kirch R. A., Leib A. R., et al. (2018). American Heart Association. Projected costs of informal caregiving for cardiovascular disease: 2015 to 2035: a policy statement from the American Heart Association. Circulation 137 (19), e558–e577. 10.1161/CIR.0000000000000570 [DOI] [PubMed] [Google Scholar]
- Fan G., Yu J., Asare P. F., Wang L., Zhang H., Zhang B., et al. (2016). Danshensu alleviates cardiac ischaemia/reperfusion injury by inhibiting autophagy and apoptosis via activation of mTOR signalling. J. Cell Mol. Med. 20 (10), 1908–1919. 10.1111/jcmm.12883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flower A., Witt C., Liu J. P., Ulrich-Merzenich G., Yu H., Lewith G. (2012). Guidelines for randomised controlled trials investigating Chinese herbal medicine. J. Ethnopharmacol. 140, 550–554. 10.1016/j.jep.2011.12.017 [DOI] [PubMed] [Google Scholar]
- Gao Z. Y., Zhang J. C., Xu H., Shi D. Z., Fu C. G., Qu D., et al. (2010). Analysis of relationships among syndrome, therapeutic treatment, and Chinese herbal medicine in patients with coronary artery disease based on complex networks. Zhong Xi Yi Jie He Xue Bao 8 (3), 238–243. 10.3736/jcim20100307 [DOI] [PubMed] [Google Scholar]
- Han J. Y., Fan J. Y., Horie Y., Miura S., Cui D. H., Ishii H., et al. (2008). Ameliorating effects of compounds derived from Salvia miltiorrhiza root extract on microcirculatory disturbance and target organ injury by ischemia and reperfusion. Pharmacol. Ther. 117 (2), 280–295. 10.1016/j.pharmthera.2007.09.008 [DOI] [PubMed] [Google Scholar]
- Hausenloy D. J., Yellon D. M. (2013). Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J. Clin. Invest. 123 (1), 92–100. 10.1172/JCI62874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H., Liu Q., Shi M., Zeng X., Yang J., Wu L., et al. (2008). Cardioprotective effects of hydroxysafflor yellow A on diabetic cardiac insufficiency attributed to up-regulation of the expression of intracellular calcium handling proteins of sarcoplasmic reticulum in rats. Phytother. Res. 22 (8), 1107–1114. 10.1002/ptr.2468 [DOI] [PubMed] [Google Scholar]
- He K., Yan L., Pan C. S., Liu Y. Y., Cui Y.C., Hu B.H., et al. (2014). ROCK-dependent ATP5D modulation contributes to the protection of notoginsenoside NR1 against ischemia-reperfusion-induced myocardial injury. Am. J. Physiol. Heart Circ. Physiol. 307, H1764–1776. 10.1152/ajpheart.00259.2014 [DOI] [PubMed] [Google Scholar]
- Heusch G., Gersh B. J. (2017). The pathophysiology of acute myocardial infarction and strategies of protection beyond reperfusion: a continual challenge. Eur. Heart J. 38 (11), 774–784. 10.1093/eurheartj/ehw224 [DOI] [PubMed] [Google Scholar]
- Heusch G., Boengler K., Schulz R. (2010). Inhibition of mitochondrial permeability transition pore opening: the Holy Grail of cardioprotection. Basic Res. Cardiol. 105 (2), 151–154. 10.1007/s00395-009-0080-9 [DOI] [PubMed] [Google Scholar]
- Higgins J. P. T., Green S. (2012). Cochrane Handbook for Systematic Reviews of Interventions. Version 5. 0. 1, Updated March 2011. Oxford, UK: the Cochrane Collaboration; http://www.cochrane-handbook.org/. [Google Scholar]
- Hu J. N., Qu W., Zhang Q. M., Jiang M. X., Ye W., Wang R. W. (2014). To observe the clinical efficacy and safety on treating and angina pectoris coronary heart disease with Kodaling Tablets. Clin. J. Chin. Med. 6 (5), 31–34. 10.3969/j.issn.1674-7860.2014.05.014 [DOI] [Google Scholar]
- Hu T., Wei G., Xi M., Yan J., Wu X., Wang Y., et al. (2016). Synergistic cardioprotective effects of Danshensu and hydroxysafflor yellow A against myocardial ischemia-reperfusion injury are mediated through the Akt/Nrf2/HO-1 pathway. Int. J. Mol. Med. 38, 83–94. 10.3892/ijmm.2016.2584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jormalainen M., Vento A. E., Wartiovaara-Kautto U., Suojaranta-Ylinen R., Rämö O. J., Petäjä J., et al. (2004). Recombinant hirudin enhances cardiac output and decreases systemic vascular resistance during reperfusion after cardiopulmonary bypass in a porcine model. J. Thorac. Cardiovasc. Surg. 128 (2), 189–196. 10.1016/j.jtcvs.2003.11.058 [DOI] [PubMed] [Google Scholar]
- Kjaergard L. L., Villumsen J., Gluud C. (2001). Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Ann. Intern. Med. 135, 982–989. 10.7326/0003-4819-135-11-200112040-00010 [DOI] [PubMed] [Google Scholar]
- Kleinbongard P., Schulz R., Heusch G. (2011). TNF-α in myocardial ischemia/reperfusion,remodeling and heart failure. Heart Fail. Rev. 16 (1), 49–69. 10.1007/s10741-010-9180-8 [DOI] [PubMed] [Google Scholar]
- Leng X., Zhang L. D., Jia L.Q., Zang A. Y., Cao J., Li Q. F., et al. (2015). Effect of ginsenoside Rb1 on isoproterenol-nduced acute myocardial ischemia in rats and its mechanism of action. Chin. J. Exp. Trad. Med. Formulae 21 (24), 104–108. 10.13422/j.cnki.syfjx.2015240104 [DOI] [Google Scholar]
- Li C., Li Q., Liu Y. Y., et al. (2014). Protective effects of Notoginsenoside R1 on intestinal ischemia-reperfusion injury in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 306, G111–122. 10.1152/ajpgi.00123.2013 [DOI] [PubMed] [Google Scholar]
- Li H. Q., Wei J. J., Xia W., Li J. H., Liu A. J., Yin S. B., et al. (2015). Promoting blood circulation for removing blood stasis therapy for acute intracerebral hemorrhage: a systematic review and meta-analysis. Acta Pharmacol. Sin. 36 (6), 659–675. 10.1038/aps.2014.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim K. H., Lim D. J., Kim J. H. (2013). Ginsenoside-Re ameliorates ischemia and reperfusion injury in the heart: a hemodynamics approach. J. Ginseng Res. 37 (3), 283–292. 10.5142/jgr.2013.37.283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Niu P. W. (2011). Protective effects of chuan xiong qin against ischemia/reperfusion injury in rats. China Prac. Med. 6, 29–30. 10.14163/j.cnki.11-5547/r.2011.02.021 [DOI] [Google Scholar]
- Liu C. E., Wu S. X., Ye G. (2012). Mechanism of ginsenoside Rb1 against myocardial apoptosis during ischemia-reperfusion injury in diabetic rats. J. Emerg. Trad. Chin. Med. 21 (7), 1080–1081. 10.3969/j.issn.1009-0959.2010.03.054 [DOI] [Google Scholar]
- Liu G. Y., Zhang Z., Li Z., Yang G. L. (2014). Integrative interventions stable angina syndrome of blood stasis due to qi deficiency clinical efficacy. Chin. Arch. Trad. Chin. Med. 32 (11), 2616–2618. 10.13193/j.issn.1673-7717.2014.11.016 [DOI] [Google Scholar]
- Liu J., Ding Y. J., Lin N., Shu B. (2010). Protective effect of astragaloside on cardiac function injury induced by myocardial ischemia in Beagles. China Prac. Med. 5 (33), 12–15. 10.3969/j.issn.1673-7555.2010.33.006 [DOI] [Google Scholar]
- Liu Q., Li J., Wang J., Li J., Janicki J. S., Fan D. (2013). Effects and mechanisms of Chinese herbal medicine in ameliorating myocardial ischemia-reperfusion injury. Evid. Based Complementary Altern. Med. 2013, 925625. 10.1155/2013/925625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R., Zhang L., Lan X., Li L., Zhang T.T., Sun J.H., et al. (2011). Protection by borneol on cortical neurons against oxygen-glucose deprivation/reperfusion: involvement of anti-oxidation and anti-inflammation through nuclear transcription factor KappaB signaling pathway. Neuroscience 176, 408–419. 10.1016/j.neuroscience.2010.11.029 [DOI] [PubMed] [Google Scholar]
- Liu W. J., Tang H. T., Jia Y. T., Ma B., Fu J. F., Wang Y., et al. (2010). Notoginsenoside R1 attenuates renal ischemia-reperfusion injury in rats. Shock 34, 314–320. 10.1097/SHK.0b013e3181ceede4 [DOI] [PubMed] [Google Scholar]
- Liu X., Jiang Y., Yu X., Fu W., Zhang H., Sui D. (2014). Ginsenoside-Rb3 protects the myocardium from ischemia-reperfusion injury via the inhibition of apoptosis in rats. Exp. Ther. Med. 8, 1751–1756. 10.3892/etm.2014.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. N., Zhou Z. M., Chen P. (2008). Evidence that hydroxysafflor yellow A protects the heart against ischaemia-reperfusion injury by inhibiting mitochondrial permeability transition pore opening. Clin. Exp. Pharmacol. Physiol. 35 (2), 211–216. 10.1111/j.1440-1681.2007.04814.x [DOI] [PubMed] [Google Scholar]
- Lu H. W., Zhang J., Chen X., Zheng C. (2014). Clinical observation on tongxinluo capsule combined with outine western medicine in the prevention of restenosis after percutaneous coronary intervention in 90 cases. J. Trad. Chin. Med. 55 (24), 2117–2120. 10.13288/j.11-2166/r.2014.24.013 [DOI] [Google Scholar]
- Lu M., Tang F., Zhang J., Luan A., Mei M., Xu C., et al. (2015). Astragaloside IV attenuates injury caused by myocardial ischemia/reperfusion in rats via regulation of toll-like receptor 4/Nuclear Factor-κB signaling pathway. Phytother. Res. 4, 599–606. 10.1002/ptr.5297 [DOI] [PubMed] [Google Scholar]
- Lu X. Y., Shi D. Z., Xu H., Chen K. Y., Lv S. Z. (2006). Clinical study on effect of Xiongshao Capsule on restenosis after percutaneous coronary intervention. Zhongguo Zhong Xi Yi Jie He Za Zhi 26 (1), 13–17. 10.3321/j.issn:1003-5370.2006.01.006 [DOI] [PubMed] [Google Scholar]
- Lu Z., Kou W., Du B., Wu Y., Zhao S., Brusco O. A., et al. (2008). Chinese Coronary Secondary Prevention Study Group, Li S. Effect of Xuezhikang, an extract from red yeast Chinese rice, on coronary events in a Chinese population with previous myocardial infarction. Am. J. Cardiol. 101 (12), 1689–1693. 10.1016/j.amjcard.2008.02.056 [DOI] [PubMed] [Google Scholar]
- Lv L., Jiang S. S., Xu J., Gong J. B., Cheng Y. (2012). Protective effect of ligustrazine against myocardial ischaemia reperfusion in rats: the role of endothelial nitric oxide synthase. Clin. Exp. Pharmacol. Physiol. 39, 20–27. 10.1111/j.1440-1681.2011.05628.x [DOI] [PubMed] [Google Scholar]
- Ma L., Chuang C. C., Weng W., Zhao L., Zheng Y., Zhang J., et al. (2016). Paeonol protects rat heart by improving regional blood perfusion during no-reflow. Front. Physiol. 7, 298. 10.3389/fphys.2016.00298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao S., Wang L., Ouyang W. W., Zhou Y. S., Qi J. Y., Guo L. H. (2016). Traditional Chinese medicine, Danlou tablets alleviate adverse left ventricular remodeling after myocardial infarction: results of a double-blind, randomized, placebo-controlled, pilot study. BMC Complement. Altern. Med. 16, 1–8. 10.1186/s12906-016-1406-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant D. J., Boyd J. H., Lin D. C., Granville D. J., Garmaroudi F. S., McManus B. M., et al. (2012). Inflammation in myocardial diseases. Circ. Res. 110 (1), 126–144. 10.1161/CIRCRESAHA.111.243170 [DOI] [PubMed] [Google Scholar]
- Meng Y., Li W. Z., Shi Y. W., Zhou B. F., Ma R., Li W. P. (2016). Danshensu protects against ischemia/reperfusion injury and inhibits the apoptosis of H9c2 cells by reducing the calcium overload through the p-JNK-NF-kappaB-TRPC6 pathway. Int. J. Mol. Med. 37, 258–266. 10.3892/ijmm.2015.2419 [DOI] [PubMed] [Google Scholar]
- Mo Q. Y., Fang X., Zhang C., Wu S. J., He J. S., Wang Q., et al. (2012). Effect of YIXINMAI granule on high-sensitivity c-reactive protein, interlenkin-6 and interlenkin-18 in patients with coronary artery disease. Guangxi Med. J. 34 (5), 537–539. 10.3969/j.issn.0253-4304.2012.05.007 [DOI] [Google Scholar]
- Murray C. J., Barber R. M., Foreman K. J., Abbasoglu Ozgoren A., Abd-Allah F., Abera S. F., et al. (2015). Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990-2013: quantifying the epidemiological transition. Lancet 386 (10009), 2145–2191. 10.1016/S0140-6736(15)61340-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizamutdinova I.T., Jin Y. C., Kim J. S., Yean M. H., Kang S. S., Kim Y. S., et al. (2008). Paeonol and paeoniflorin, the main active principles of Paeonia albiflora, protect the heart from myocardial ischemia/reperfusion injury in rats. Planta Med. 74, 14–18. 10.1055/s-2007-993775 [DOI] [PubMed] [Google Scholar]
- Pan H., Li D., Fang F., Chen D., Qi L., Zhang R., et al. (2011). Salvianolic acid A demonstrates cardioprotective effects in rat hearts and cardiomyocytes after ischemia/reperfusion injury. J. Cardiovasc. Pharmacol. 58, 535–542. 10.1097/FJC.0b013e31822de355 [DOI] [PubMed] [Google Scholar]
- Park J. H., Park O., Cho J. H., Chen B. H., Kim I. H., Ahn J. H., et al. (2014). Anti-inflammatory effect of tanshinone I in neuroprotection against cerebral ischemia-reperfusion injury in the gerbil hippocampus. Neurochem. Res. 39, 1300–1312. 10.1007/s11064-014-1312-4 [DOI] [PubMed] [Google Scholar]
- Qian W., Xiong X., Fang Z., Lu H., Wang Z. (2014). Protective effect of tetramethylpyrazine on myocardial ischemia-reperfusion injury. Evid. Based Complementary Altern. Med. 2014, 107501. 10.1155/2014/107501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao Z. Q., Zhang M. Z., Liu H., Cheng K. L., Li S. (2006). A randomized double-blind placebo-controlled clinical trial of Tongguan Capsule in improving cardiac function after percutaneous coronary intervention in patients with coronary heart disease. Chin. Arch. Trad. Chin. Med. 24 (9), 1667–1668. 10.7666/d.y738108 [DOI] [Google Scholar]
- Qiu S. L., Jin M., Zhu T. G., Quan X., Liang Y., Shi D. Z. (2009). Effect of replenishing Qiand nourishing yin to promote the blood circulationon 103 patients with acute myocardial infarction after reperfusion. J. Cap. Med. Univ. 30 (4), 426–429. 10.3969/j.issn.1006-7795.2009.04.005 [DOI] [Google Scholar]
- Roffi M., Patrono C., Collet J. P., Mueller C., Valgimigli M., Andreotti F., et al. (2016). 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). G. Ital. Cardiol. (Rome) 17 (10), 831–872. 10.1714/2464.25804 [DOI] [PubMed] [Google Scholar]
- Rongen G. A., Wever K. E. (2015). Cardiovascular pharmacotherapy: innovation stuck in translation. Eur. J. Pharmacol. 759, 200–204. 10.1016/j.ejphar.2015.03.035 [DOI] [PubMed] [Google Scholar]
- Roth G. A., Huffman M. D., Moran A. E., Feigin V., Mensah G.A., Naghavi M., et al. (2015). Global and regional patterns in cardiovascular mortality from 1990 to 2013. Circulation 132 (17), 1667–1678. 10.1161/CIRCULATIONAHA.114.008720 [DOI] [PubMed] [Google Scholar]
- Schulz K.F., Altman D.G., Moher D., CONSORT Group. (2010). CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. BMJ 340, c332. 10.1136/bmj.c332 [DOI] [PubMed] [Google Scholar]
- Schulz R., Kelm M., Heusch G. (2004). Nitric oxide in myocardial ischemia/reperfusion injury. Cardiovasc. Res. 61 (3), 402–413. 10.1016/j.cardiores.2003.09.019 [DOI] [PubMed] [Google Scholar]
- Shang H. C., Zhang J. H., Yao C., Liu B. Y., Gao X. M., Ren M. (2013). Qi-Shen-Yi-Qi dripping pills for the secondary prevention of myocardial infarction: a randomised clinical trial. Evid. Based Complementary Altern. Med. 2013, 738391. 10.1155/2013/738391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Q. H., Xu H., Lu X. Y., Wen C., Shi D. Z., Chen K. J. (2011). A multi-center randomized double-blind placebo-controlled trial of Xiongshao Capsule in preventing restenosis after percutaneous coronary intervention: a subgroup analysis of senile patients. Chin. J. Integr. Med. 17 (9), 669–674. 10.1007/s11655-011-0843-7 [DOI] [PubMed] [Google Scholar]
- Sun S. Z. (2014). Observation on efficacy and safety of Shexiang Baoxin Pills in the treatment of unstable angina pectoris of coronary heart disease in elderly patients. Mod. J. Integr. Trad. Chin. West. Med. 23 (4), 391–393. 10.3969/j.issn.1008-8849.2014.04.020 [DOI] [Google Scholar]
- Tang H., Pan C. S., Mao X. W., Liu Y. Y., Yan L., Zhou C. M., et al. (2014). Role of NADPH oxidase in total salvianolic acid injection attenuating ischemia-reperfusion impaired cerebral microcirculation and neurons: implication of AMPK/Akt/PKC. Microcirculation 21, 615–627. 10.1111/micc.12140 [DOI] [PubMed] [Google Scholar]
- Tang N. Y., Liu C. H., Hsieh C. T., Hsieh C. L. (2010). The anti-inflammatory effect of paeoniflorin on cerebral infarction induced by ischemia-reperfusion injury in Sprague-Dawley rats. Am. J. Chin. Med. 38, 51–64. 10.1142/S0192415X10007786 [DOI] [PubMed] [Google Scholar]
- Tao T., Chen F., Bo L., Xie Q., Yi W., Zou Y., et al. (2014). Ginsenoside Rg1 protects mouse liver against ischemia-reperfusion injury through anti-inflammatory and anti-apoptosis properties. J. Surg. Res. 191, 231–238. 10.1016/j.jss.2014.03.067 [DOI] [PubMed] [Google Scholar]
- Tu L., Pan C. S., Wei X. H., Yan L., Liu Y. Y., Fan J. Y., et al. (2013). Astragaloside IV protects heart from ischemia and reperfusion injury via energy regulation mechanisms. Microcircultion 20 (8), 736–747. 10.1111/micc.12074 [DOI] [PubMed] [Google Scholar]
- Wang J., He Q. Y., Zhang Y. L. (2009). Effect of shenshao tablet on the quality of life for coronary heart disease patients with stable angina pectoris. Chin. J. Integr. Med. 15 (5), 328–332. 10.1007/s11655-009-0328-0 [DOI] [PubMed] [Google Scholar]
- Wang J., Qiao L., Li Y., Yang G. (2008). Ginsenoside Rb1 attenuates intestinal ischemia-reperfusion- induced liver injury by inhibiting NF-kappaB activation. Exp. Mol. Med. 40, 686–698. 10.3858/emm.2008.40.6.686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Teng F., Liu Y. M., Chen G. (2017). Invention effect of xuesaitong for coronary heart disease unstable angina with blood stasis and relevant microRNA. Chin. J. Exp. Trad. Med. Formulae 23 (9), 11–16. 10.13422/j.cnki.syfjx.2017190011 [DOI] [Google Scholar]
- Wang L., Zhao X. J., Mao S., Liu S. N., Guo X. F., Guo L. H. (2016). Efficacy of danlou tablet in patients with non-st elevation acute coronary syndrome undergoing percutaneous coronary intervention: results from a multicentre, placebo-controlled, randomized trial. Evid. Based Complementary Altern. Med. 26, 7960503. 10.1155/2016/7960503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. H., Wang J., Li J., Xiong X. J., Ye Y., Zhu M. J. (2012). Efficacy assessment of treating patients with coronary heart disease angina of phlegm and stasis mutual obstruction syndrome by Danlou Tablet. Zhongguo Zhong Xi Yi Jie He Za Zhi 32 (8), 1051–1055. 10.7661/CJIM.2012.8.1051 [DOI] [PubMed] [Google Scholar]
- Wang Y. G., You J. Z., Qi J., Shang P. J., Wang Q. A., Zhong W. (2012). The influence on blood lipids, blood rheology and TCM syndromes by Shuangshen Tongguan capsule on patients with stable angina. Mod. Trad. Chin. Med. 32 (5), 4–7. [Google Scholar]
- Wang Y., Hu Z., Sun B., Xu J., Jiang J., Luo M. (2015). Ginsenoside Rg3 attenuates myocardial ischemia/reperfusion injury via Akt/endothelial nitric oxide synthase signaling and the Bcell lymphoma/Bcell lymphomaassociated X protein pathway. Mol. Med. Rep. 11, 4518–4524. 10.3892/mmr.2015.3336 [DOI] [PubMed] [Google Scholar]
- Wang Y., Li X., Wang X., Lau W., Wang Y., Xing Y., et al. (2013). Ginsenoside Rd attenuates myocardial ischemia/reperfusion injury via Akt/GSK-3beta signaling and inhibition of the mitochondria-dependent apoptotic pathway. PLoS One 8, e70956. 10.1371/journal.pone.0070956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei B., Li W. W., Ji J., Hu Q. H., Ji H. (2014). The cardioprotective effect of sodium tanshinone IIA sulfonate and the optimizing of therapeutic time window in myocardial ischemia/reperfusion injury in rats. Atherosclerosis 235, 318–327. 10.1016/j.atherosclerosis.2014.05.924 [DOI] [PubMed] [Google Scholar]
- Wu Y., He L. (2010). Advances in research on mechanisms of myocardial ischemia-reperfusion injury and related therapeutic drugs. Prog. Pharm. Sci. 34 (7), 305–312. 10.3969/j.issn.1001-5094.2010.07.003 [DOI] [Google Scholar]
- Wu Y., Xia Z. Y., Dou J., Zhang L., Xu J. J., Zhao B., et al. (2011). Protective effect of ginsenoside Rb1 against myocardial ischemia/reperfusion injury in streptozotocin-induced diabetic rats. Mol. Biol. Rep. 38, 4327–4335. 10.1007/s11033-010-0558-4 [DOI] [PubMed] [Google Scholar]
- Xia K. P., Ca H. M., Shao C. Z. (2015). Protective effect of notoginsenoside R1 in a rat model of myocardial ischemia reperfusion injury by regulation of vitamin D3 upregulated protein 1/NF-kappaB pathway. Pharmazie 70, 740–744. 10.1691/ph.2015.5694 [DOI] [PubMed] [Google Scholar]
- Xu M. (2014). Research on main mechanisms of myocardial ischemia reperfusion injury and related drug therapy. Pract. Pharm. Clin. Remed. 17 (8), 1052–1055. 10.14053/j.cnki.ppcr.2014.08.068 [DOI] [Google Scholar]
- Xu D. P., Wang X., Sheng X. G., Lin Y., Li S., Zheng Z. Y. (2014). Double-blind, randomized, controlled clinical trial of Shenzhu Guanxin prescription for treatment of stable angina due to coronary heart disease. J. Guangzhou Univ. Trad. Chin. Med. 31 (2), 173–177. 10.13359/j.cnki.gzxbtcm.2014.02.001 [DOI] [Google Scholar]
- Xu D. P., Wu H. L., Lan T. H., Wang X., Sheng X. G., Lin Y. (2015). Effect of Shenzhu Guanxin recipe on patients with angina pectoris after percutaneous coronary intervention: a prospective, randomized controlled trial. Chin. J. Integr. Med. 21 (6), 408–416. 10.1007/s11655-015-2040-6 [DOI] [PubMed] [Google Scholar]
- Xu Q., Bauer R., Hendry B. M., Fan T. P., Zhao Z., Duez P., et al. (2013). The quest for modernisation of traditional Chinese medicine. BMC Complement. Altern. Med. 13, 132. 10.1186/1472-6882-13-132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue L., Wu Z., Ji X. P., Gao X. Q., Guo Y. H., (2014). Effect and mechanism of salvianolic acid B on the myocardial ischemia-reperfusion injury in rats. Asian Pac. J. Trop. Med. 7 (4), 280–284. 10.1016/S1995-7645(14)60038-9 [DOI] [PubMed] [Google Scholar]
- Yang H., Yang B., Xiong Q. X. (2017). Effect of coronary Ningtong prescription combined with metoprolol on coronary heart disease and angina pectoris. Shaanxi J. Trad. Chin. Med. 38 (8), 1006–1007. 10.3969/j.issn.1000-7369.2017.08.010 [DOI] [Google Scholar]
- Yellon D. M., Hausenloy D. J. (2007). Myocardial reperfusion injury. N. Engl. J. Med. 357 (11), 1121–1135. 10.1056/NEJMra071667 [DOI] [PubMed] [Google Scholar]
- Yin Y., Guan Y., Duan J., Wei G., Zhu Y., Quan W., et al. (2013). Cardioprotective effect of Danshensu against myocardial ischemia/reperfusion injury and inhibits apoptosis of H9c2 cardiomyocytes via Akt and ERK1/2 phosphorylation. Eur. J. Pharmacol. 699, 219–226. 10.1016/j.ejphar.2012.11.005 [DOI] [PubMed] [Google Scholar]
- Yu J. M., Zhang X. B., Jiang W., Wang H. D., Zhang Y. N., (2015). Astragalosides promote angiogenesis via vascular endothelial growth factor and basic fbroblast growth factor in a rat model of myocardial infarction. Mol. Med. Rep. 12 (5), 6718–6726. 10.3892/mmr.2015.4307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Sun G., Luo Y., Wang M., Chen R., Zhang J., et al. (2016). Cardioprotective effects of Notoginsenoside R1 against ischemia/reperfusion injuries by regulating oxidative stress-and endoplasmic reticulum stress- related signaling pathways. Sci. Rep. 6 (5), 21730. 10.1038/srep21730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Z. Y., Yang J. H., Hang S.M., Wu B. H., Xin D., Zhou L. H., et al. (2011). Role of Janus kinase 2/signal transducer and activator of transcription 3 signaling pathway in attenuation of myocardial ischemia-reperfusion injury by teramethylpyrazine in rats. Chin. J. Anesthesiol. 31, 1005–1008. 10.3760/cma.j.issn.0254-1416.2011.08.029 [DOI] [Google Scholar]
- Zhang J. H., Xu M. (2000). DNA fragmentation in apoptosis. Cell Res. 10 (3), 205–211. 10.1038/sj.cr.7290049 [DOI] [PubMed] [Google Scholar]
- Zhang H. T., Jia Z. H., Zhang J., Ye Z. K., Yang W. X., Tian Y. Q. (2010). No-reflow protection and long-term efficacy for acute myocardial infarction with Tongxinluo: a randomized double-blind placebo-controlled multicenter clinical trial (ENLEAT Trial). Chin. Med. J. (Engl.) 123 (20), 2858–2864. [PubMed] [Google Scholar]
- Zhang J., Ma C. L., Wang G. Z. (2014). Improvement effects of astragaloside IV on myocardial focal ischemia-reperfusion injury and its influence in PI3K/Akt/mTOR signaling pathway. J. Jilin Univ. (Med. Ed.) 40 (5), 991–996. 10.13481/j.1671-587x.20140517 [DOI] [Google Scholar]
- Zhang M. Q., Zheng Y. L., Chen H., et al. (2013). Sodium tanshinone IIA sulfonate protects rat myocardium against ischemia-reperfusion injury via activation of PI3K/Akt/FOXO3A/Bim pathway. Acta Pharmacol. Sin. 34 (11), 1386–1396. 10.1038/aps.2013.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Wei L., Sun D., et al. (2010). Tanshinone IIA pretreatment protects myocardium against ischaemia/reperfusion injury through the phosphatidylinositol 3-kinase/Akt-dependent pathway in diabetic rats. Diabetes Obes. Metab. 12 (4), 316–322. 10.1111/j.1463-1326.2009.01166.x [DOI] [PubMed] [Google Scholar]
- Zhang Z.F., Xu F. Q., Liu H. X., Wang F. R., Zhao M. J., Sun L. J., et al. (2015). A multicenter, randomized, double-blind clinical study on wufuxinnaoping soft capsule in treatment of chronic stable angina patients with blood stasis syndrome. Chin. J. Integr. Med. 21 (8), 571–578. 10.1007/s11655-014-1953-9 [DOI] [PubMed] [Google Scholar]
- Zhu H. Y., Gao Y. H., Wang Z. Y., Xu B., Wu A. M., Wei Y. X., et al. (2013). Astragalus polysaccharide suppresses the expression of adhesion molecules through the regulation of the p38 mapk signaling pathway in human cardiac microvascular endothelial cells after ischemia-reperfusion injury. Evid. Based Complement Altern. Med. 2013:280493. 10.1155/2013/280493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q. H., Wang M. R., Qeng J. F., Chen S. J., Xiao Y. S., et al. (2016). Clinical effect of self-made traditional prescription on premature coronary heart disease diagnosed as tcm syndrome of phlegm turbidity and blood stasis. Zhongguo Zhong Xi Yi Jie He Za Zhi 8 (9), 92–95. 10.3969/j.issn.1008-5971.2016.08.025 [DOI] [Google Scholar]