Abstract
The focus of this study was to evaluate the antioxidants and antimycobacterial activities of extracts of Schkuhria pinnata. Serial exhaustive extraction procedure was employed using solvents of varying polarity to obtain the desired extracts. Thin layer chromatography and standard chemical tests were used to analyze phytochemicals constituents. Free radical scavenging 2,2-diphenyl-1-picrylhydrazyl (DPPH) methods were used to detect the presence of antioxidant compounds. Antimycobacterial activity was evaluated using microdilution and bioautography assays. A variety of secondary metabolites such as flavonoids, tannins, and alkaloids were detected in the extract. Ethyl acetate and acetone extracts had high antioxidant activity on chromatograms eluted in ethyl acetate/methanol/water while methanol extract at various concentrations had the best scavenging activity. The minimum inhibitory concentration (MIC) values ranged from 0.02 to 2.50 mg/mL. Total phenol content was 55.33 ± 3.51 mg of gallic acid equivalent (GAE)/g and higher when compared with flavonoids (4.00 ± 0.35 mg of quercetin equivalent [QE]/mg) and tannin content (28.00 ± 1.73 mg of GAE/g). The most effective antimycobacterial activity against Mycobacterium smegmatis was observed with the lowest inhibitory concentrations of acetone (0.27 mg/mL), dichloromethane (0.32 mg/mL), and ethyl acetate (0.32 mg/mL) in that order. In massive extraction, hexane and dichloromethane had the greatest inhibitory bands on benzene/ethanol/ammonium hydroxide bioautograms. Antimmycobacterial activity gives promising potential leads of S pinnata extracts to be used in the development of antimycobacterial drugs. The presence of antioxidant and antimycobacterial compounds requires further isolation and purification.
Keywords: medicinal plants, phytochemicals, antioxidants, antimycobacterial activity
Mycobacterium smegmatis is a Gram positive and acid fast bacterium that falls under the Mycobacteriaceae family, which includes Mycobacterium tuberculosis, Mycobacterium fortuitum, Mycobacterium abscessus, and Mycobacterium chelonae, that have shown resistance against many antibiotics due to their protective outer layer.1 M smegmatis lives in aggregated layers of community called biofilm and are commonly found on plants, soil, and in water.2
M smegmatis have been reported to cause diseases such as skin and soft tissue infections, and bone diseases.3 The organism can be transmitted as a results of contaminated materials during invasive procedures, which can result in infection.4 Best and Best3 reported that M smegmatis is resistant to isoniazid and rifampicin, 2 widely used antibiotics for treatment of tuberculosis.
Other debilitating effects resulting from the treatment of M smegmatis infection include prolonged antibiotic therapy, which is toxic to the patients, and surgical debridement of infected tissues, which is expensive to poor rural people.3 The rise in bacterial resistance against a broad spectrum of antibiotics as well as high cost of therapies is a major concern, especially to the rural poor. There is, thus a spike in the surge in trying to identify plant derived drugs that are nontoxic, cost-effective, and possess improved biological efficacy.
Plants have been used by humans for thousands of years for various purposes, including food transport and as medicine.5–7 Sofowora8 reported that about 80% of the population in developing countries use medicinal plants for their primary health care needs. Plants produce primary and secondary metabolites, which they use in their metabolisms and defense against invading pathogens. Various studies have indicated that theses metabolites possess healing power that can be used in treatment of chronic as well as infectious diseases.9,10 Bioactive metabolites have consistently provided a platform for new drug leads against a host of diseases.
Schkuhria pinnata is a herbaceous and exotic plant that belong to the family Asteraceae. The species within this family have distinctive phytochemicals that differentiate them. S pinnata is recorded to grow in some regions in South America and in some African countries namely Zimbabwe and South Africa. It grows in cultivated lands, along roadsides and fields.11 It has been employed as a herbal remedy for kidney, liver, renal problems, malaria, diabetes, allergies, yeast infections, prostate inflammation, digestive disorders, and intestinal gas.12,13 The plant has been reported to have therapeutic effects in the treatment of eye infections, pneumonia, heart water, diarrhea, wound infections, and retained placenta in livestock.14,15 Extracts of S pinnata have also been reported to be effective against the pathogens that cause mastitis in dairy cattle Mupfure et al.16
Medicinal plants are considered to have less or no side effects, affordable, and readily available to the community. However, clinical trials for the biological activities from medicinal plants are necessary to give a clear understanding of the safety and efficacy of medicinal plants by traditional healers and other herbalist.17 The focus of this study is to assess the phytochemical, antioxidant, and antimycobacterial activities of S pinnata extracts.
Methods
Plant Collection
Schkuhria pinnata (Lam.) Kuntze ex Thell was collected at the University of Limpopo, South Africa. Voucher specimen was identified at Larry Leach herbarium (UNIN 12298). Plant materials were dried at ambient temperature at the Microbiology Department, University of Limpopo. The roots of the plants were separated and discarded. The remaining plant materials were milled to fine powder using a grinding machine (Trf400) (animal ration shredder hammer mill foliage machine) at the school of Agricultural and Environmental Sciences (University of Limpopo). The powdered material was stored in the dark at room temperature in an air-tight container until further use.
Extraction Procedure
Serial Exhaustive Extraction
Finely ground plant material (5 g) was exhaustively extracted with 50 mL of n-hexane. The bottle was shaken for 1 hour at 200 rpm on a series 25 shaking machine (New Brunswick Scientific Co, Inc). The supernatant was filtered using the Whatman No. 1 filter paper into preweighed bottles and the process was repeated 3 times. The same procedure was followed, on the same plant residues with 50 mL of chloroform, dichloromethane, ethyl acetate, acetone, ethanol, and methanol to exhaustively extract compounds of varying polarities. The supernatants collected were dried under a stream of air, after which the mass was determined, and extracts reconstituted in acetone at a concentration of 10 mg/mL.
Qualitative Phytochemical Constituent Analysis
Phytochemical constituents were analyzed using thin layer chromatography (TLC). Briefly, 10 µL of extracts were loaded on aluminum-backed TLC plates. Three solvent systems of varying polarity, benzene/ethanol/ammonium hydroxide (BEA) (90:10:1) (nonpolar/basic); chloroform/ethyl acetate/formic acid (CEF) (5:4:1) (intermediate polarity/acidic); ethyl acetate/methanol/water (EMW) (40:5.4:5) (polar/neutral) were used to elute TLC plates in saturated tanks.18 Developed plates were observed under ultraviolet light at 254 and 365 nm for the presence of quenching and fluorescing compounds, respectively, and thereafter sprayed with vanillin sulfuric acid reagent (0.1 g vanillin [Sigma]:28 methanol:1 mL sulfuric acid). Plates were heated at 110° C for optimal color development.
Preliminary Biochemical Analysis of Phytochemicals
Acetone plants extracts were tested for the presence of saponin, phlobatannin, tannins, terpenes/terpenoids, steroids, cardiac glycosides, and flavonoids using the standard procedures as described by Borokini and Omotayo.19
Quantitative Analysis of Total Phenolic, Flavonoids, and Tannins Content
Total Phenol Content
Total phenolic contents (TPC) in the S pinnata extracts were estimated, following the method of Singleton et al.20 Aliquots of 1.0 mL of water or acetone extracts were mixed with 5 mL of 10-fold diluted Folin-Ciocalteu reagent and 4 mL of 7% sodium carbonate (Na2CO3) solution. The mixture was allowed to stand for 90 minutes at room temperature and the absorbance was measured at 550 nm. Results were expressed as milligrams of gallic acid equivalents per gram of extract (mg GAE/g).
Total Flavonoid Content
Total flavonoid contents were quantified using a modified colorimetric method as described by Tambe and Bhambar.21 Briefly, 5 mL of water or acetone extract was mixed with 0.3 mL of 5% sodium nitrite for 5 minutes in a test tube. Then 0.3 mL of 10% aluminum chloride was added. After 6 minutes, 2 mL sodium hydroxide was added to stop the reaction and the mixture was further diluted with distilled water up to 10 mL. The absorbance was immediately measured at 510 nm and results were expressed as milligrams of quercetin equivalents per gram of extract (mg of QE/g).
Total Tannin Content
Total tannin contents were measured following the procedure of Tambe and Bhambar.21 Briefly, 0.5 mL of Folin-Ciocalteu reagent and 1 mL of 35% Na2CO3 solutions were added in 10 mL of sample extract. The absorption was measured at 725 nm after 45 minutes of incubation at room temperature. Results were expressed as milligrams of gallic acid equivalents per gram of extract (mg of GAE/g).
Antioxidant Activity
Qualitative DPPH Assay
Aluminum-baked TLC plates coated with silica were used to detect the presence of antioxidant compounds from the plant extracts. Ten microliters of plant extracts were loaded on the plates and developed in 3 solvent systems of varying polarity, BEA (90:10:1) (nonpolar/basic); CEF (5:4:1) (intermediate polarity/acidic); and EMW (40:5.4:5) (polar/neutral). Thereafter the plates were dried under a stream of air at room temperature for about 1 minute and sprayed with 0.2% 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH; Sigma) in methanol. A positive result was indicated by the presence of yellow bands against the purple background.22
Quantitative Total Antioxidant Activity Assay
DPPH scavenging activity was designed following the method described formerly by Brand-Williams.23 Briefly, 2.0 mL of the extracts or standards was added to 5 mL of DPPH solution (0.1 mM in methanol), vortexed vigorously and incubated in dark for 30 minutes at room temperature. The decolorization of DPPH was measured against a blank at 517 nm. Percentage scavenging was calculated as
Ferric Reducing Power
Ferric ion reducing power from S pinnata extracts was determined. Various concentrations of plant extracts (1 to 0.0625 mg/mL) were prepared in test tubes. Ascorbic acid was used as a standard control and a blank solution was prepared without adding extracts. Two milliliters of 0.2 M sodium phosphate buffer and 2 mL of 1% potassium ferricyanide were added to the test tubes containing extracts of different concentrations. The solution was mixed well and incubated in a water bath at 50°C for 20 minutes. After incubation, 2.5 mL of 10% trichloroacetic acid was added into the test tubes and centrifuged at 650 rpm for 10 minutes. The supernatant was mixed with 10 mL of distilled water and 1 mL of freshly prepared ferric chloride solution (0.1%) and the solution was mixed. The absorbance of the solution was recorded at 700 nm against the blank solution.24
Bacterial Species
The test organism M smegmatis ATCC 1441 was obtained from the School of Molecular and Cell Biology, University of Witwatersrand. The bacterial specie was grown and maintained in Middlebrook 7H9 (Fluka M0178) broth with glycerol (Fluka 49769) or Tween 80 (Fluka 93780) and Middlebrook Oleic Albumin Dextrose Catalase (OADC) growth supplement (Fluka M0553).
Minimum Inhibitory Concentration Determination
The minimum inhibitory concentration (MIC) values were determined using the serial microplate method developed by Eloff.25 Minimum inhibitory concentration is described as the lowest concentration of the compounds inhibiting the growth of microorganisms. Dried extracts were redissolved in acetone to a concentration of 10 mg/mL of crude extracts. The plant extracts were serially diluted 50% with water in 96-well microtiter plates. Bacterial cultures were subcultured and transferred into fresh Middlebrook 7H9 broth and 100 μL of the culture was transferred into each well and appropriate acetone blanks were included. The microtitre plate was incubated at 37°C for 24 hours. After incubation, 20 μL of p-iodonitrotetrazolium violet (Sigma) (INT) dissolved in water was added to each of the microplate wells as an indicator of growth. The covered microplates were incubated for 30 minutes at 35°C and 100% relative humidity for color development. All determinations were carried out in triplicate. Microorganism growth led to the emergence of a purple-red color resulting from the reduction of INT to formazan. Clear wells indicate the presence of compound in the extracts that inhibited the growth of the microorganisms tested.
Qualitative Antibacterial Activity (Bioautography)
For bioautographic analysis 20 μL of each extract was loaded on the TLC plates. The plates were developed in mobile phases as previously mentioned. The chromatograms were dried at room temperature for about 4 days to remove the solvents used to develop chromatograms. The chromatograms were sprayed with overnight culture of M smegmatis until completely wet and were incubated at 37°C in a humidified chamber for 24 hours. The plates were sprayed with INT (Sigma) and incubated for a further 24 hours. The presence of clear bands on the plates against a purple background indicates growth inhibition.26
Results
Phytochemical Constituents
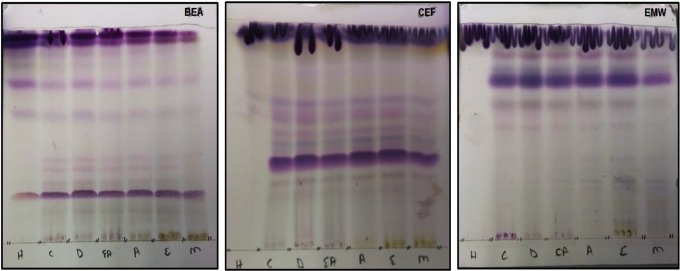
Phytochemical constituents from the crude extracts were analyzed using aluminum-backed TLC plates, which were developed in solvent systems of different polarity (BAE, CEF, and EMW) and sprayed with vanillin–sulfuric acid reagent for color development. Solvent system CEF followed by BEA separated more bands of phytochemical constituents that react with vanillin–sulfuric reagent while EMW separated fewer bands (Figure 1).
Figure 1.
The chromatograms of vanillin reactive phytochemical constituents of Schkuhria pinnata extracts extracted with solvents of varying polarity: H, hexane; C, chloroform; D, dichloromethane; EA, ethyl acetate; A, acetone; E, ethanol; and M, methanol, developed under 3 solvent systems.
Preliminary Biochemical Analysis of Phytochemicals
Various standard phytochemical tests were conducted to test for the presence of different compounds. S pinnata extracts tested positive for all tested phytoconstituents, namely: tannins, saponins, phlabotannins, terpenoids, alkaloids, steroids, and cardiac glycosides (Table 1). The presence of these secondary metabolites may be responsible in fighting against diseases.
Table 1.
Phytochemical Constituents From Schkuhria pinnata Extracts.
| Phytochemical Constituents | Reactiona |
|---|---|
| Tannins | + |
| Saponins | + |
| Phlabotannis | + |
| Flavonoids | + |
| Terpernoids | + |
| Alkaloids | + |
| Cardiac glycoside | + |
| Steroids | + |
a + indicates presence.
Quantitative Analysis of Total Phenolic, Flavonoids, and Tannins Content
The extracts had high concentrations of phenolic and tannin contents and low flavonoid contents (Table 2).
Table 2.
Determined Total Phenol, Flavonoid, and Tannin Content From Schkuhria pinnata Extracts.
| Sample | Total Phenol (mg of GAE/g) | Total Tannin (mg of GAE/g) | Total Flavonoid (mg of QE/mg) |
|---|---|---|---|
| Schkuhria pinnata | 55.33 ± 3.51 | 28.00 ± 1.73 | 4.00 ± 0.35 |
Abbreviations: GAE, gallic acid equivalent; QE, quercetin equivalent.
Qualitative DPPH Assay
S pinnata sample was extracted with solvents of varying polarities. The ethyl acetate and acetone extracts had strong antioxidant activity from all solvent systems. In BEA and CEF solvent systems, the compounds did not migrate; best separation was observed in EMW solvent system (Figure 2). It can be concluded that the extracted compounds that showed activity in this assay were polar.
Figure 2.
The chromatograms indicating the antioxidant compounds from the plant extracts extracted with the following: H, hexane; C, chloroform; D, dichloromethane; EA, ethyl acetate; A, acetone; E, ethanol; M, methanol. The plates were sprayed with 0.2% DPPH (2,2-diphenyl-1-picrylhydrazyl) in methanol.
Quantitative Total Antioxidant Activity Assay
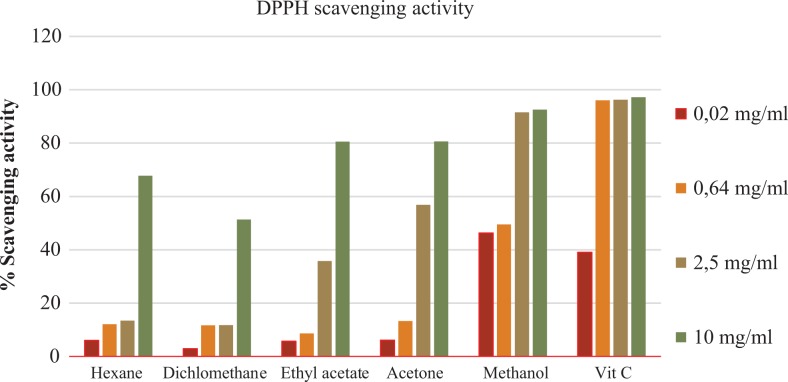
The quantitative antioxidant activity from S pinnata extracts was performed using the DPPH free radical scavenging activity assay. Methanol extracts had the greatest antioxidant activity when compared with other extracts at all concentrations, followed by ethyl acetate and acetone extracts. The lowest percentage scavenging activity was observed with the dichloromethane extracts (0.02 mg/mL) (Figure 3).
Figure 3.
Quantitative percentage scavenging activity of Schkuhria pinnata extracts at different concentrations.
Ferric Reducing Power
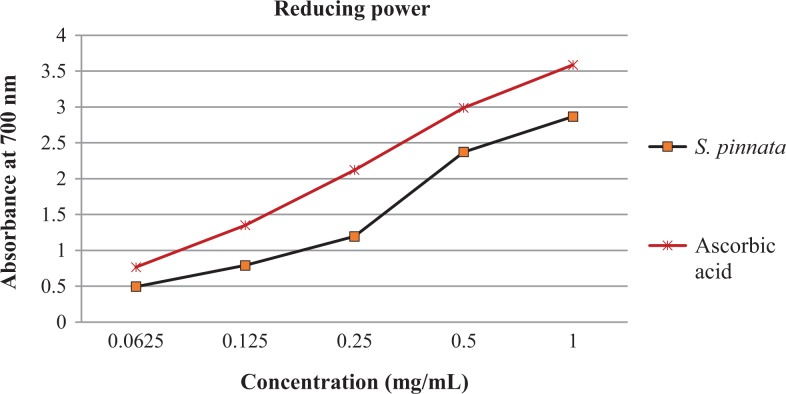
The ferric reducing power of S pinnata extracts was evaluated at different concentrations in comparison with ascorbic acid the positive control. The absorbance was observed to be increased as concentration increases; the same trend was observed with the positive control (Figure 4).
Figure 4.
The ferric reducing power of Schkuhria pinnata extracts at various concentrations.
Minimum Inhibitory Concentration Determination
Antimycobacterial activity of S pinnata extracts was evaluated using microdilution assay. The lowest MIC value was observed with acetone extracts (0.27 mg/mL) followed by ethyl acetate (0.32 mg/mL) and dichloromethane (0.32 mg/mL). The highest MIC value was observed with hexane extracts (2.5 mg/mL) (Table 3). The total activity is the values in which 1 g of dried plant material can be diluted and still inhibit the growth of microorganism. Acetone extracts indicated the highest total activity (Table 3).
Table 3.
The Minimum Inhibitory Concentration (MIC) of the Plant Extracts With Their Total Activity Values.
| H | C | D | EA | A | E | M | Rifampicin |
|---|---|---|---|---|---|---|---|
| MIC Values (mg/mL) | |||||||
| 2.5 | 0.43 | 0.32 | 0.32 | 0.27 | 0.37 | 0.53 | 0.08 |
| Total Activity (mL) | |||||||
| 26 | 337 | 478 | 353 | 521 | 368 | 306 | |
Abbreviations: H, hexane; C, chloroform; D, dichloromethane; EA, ethyl acetate; A, acetone; E, ethanol; M, methanol.
Qualitative Antibacterial Activity (Bioautography)
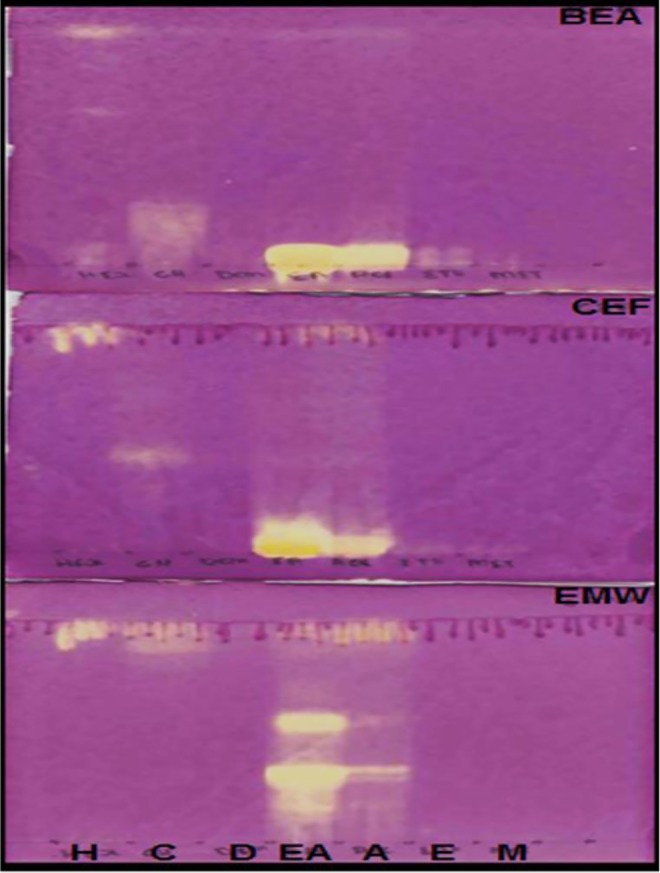
Bioautography assay was used to detect the presence of antimycobacterial compounds in the plant extracts on TLC plates. Figure 5 shows the presence of antimycobacterial compounds from chloroform and ethyl acetate extracts that were separated in CEF and EMW solvent systems.
Figure 5.
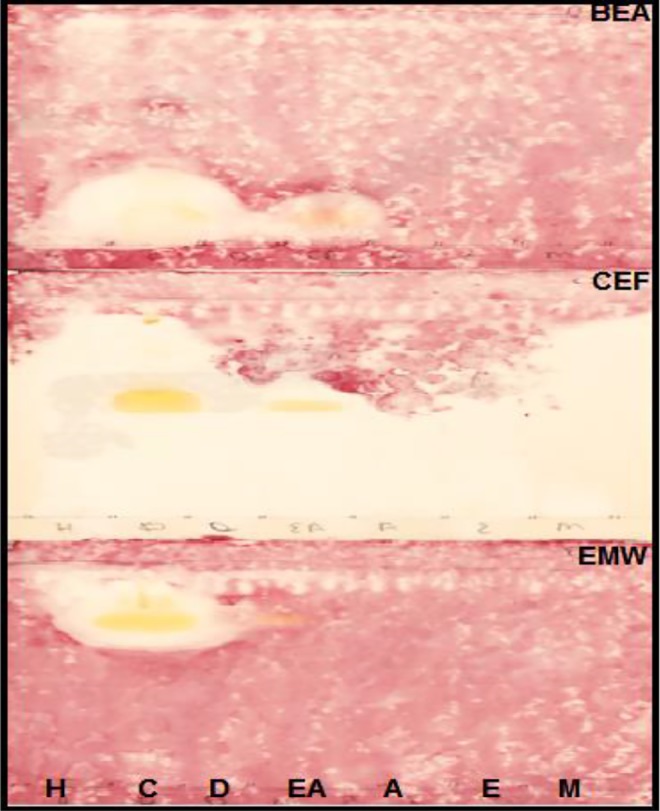
Bioautograms of Schkuhria pinnata extracts developed under 2 solvent systems (BEA, CEF, and EMW). The extracts were extracted with the following: H, hexane; C, chloroform; D, dichloromethane; EA, ethyl acetate; A, acetone; E, ethanol; M, methanol and sprayed with Mycobacterium smegmatis. White areas indicate where reduction of INT (p-iodonitrotetrazolium violet) to the colored formazan did not take place due to the presence of compounds that inhibited the growth of M smegmatis.
Discussion
Drugs derived from medicinal plants are developed from plant phytochemical constituents such as alkaloids, flavonoids, tannins, terpenoids, and saponins, which are of great importance in human’s health, veterinary, and agriculture. Analysis of plant phytochemical constituents is necessary for the synthesis of drugs and other therapeutic agents. After extraction, the plant extracts were reconstituted to a certain concentration with acetone based on reports by Eloff25 that it is nontoxic to microorganism and can dissolves compounds of varying polarities. TLC was employed in the screening of the phytochemical profiles of S pinnata, as it was considered to be highly efficient.27 CEF solvent system was observed to be the best mobile phase that separated most of vanillin reactive compounds, followed by BEA. And the least compounds were separated in EMW solvent system (Figure 1). Different colors observed on the chromatograms shows that S pinnata has different compounds of varying polarities. Since solvent system CEF separate compounds of intermediate polarity,15 this suggest that S pinnata has high amount of intermediate compounds. The bands that turn blue on the chromatogram when sprayed with vanillin reagent depict the presence of terpenes.28
Chemical quantitative tests were carried out on S pinnata extracts to detect the presence or absence of secondary metabolites. Metabolites present in S pinnata are known to have various pharmacological actions in human.29 The result of phytochemicals screening for S pinnata (Table 1) shows the presence of tannins, saponins, phlabotannins, flavonoids, terpernoids, alkaloids, cardiac glycoside, and steroid compounds. Oryema et al30 also detected the presence of alkaloids, steroids, and terpenoids in S pinnata extracts. Ethanol extracts of S pinnata have been reported to possess the sterol triterpenes and flavonoids compounds.31 Researches have reported the anticancer activity of S pinnata extracts of which triterpenes compounds are responsible for the activity.32–34 Sesquiterpene lactones and eucannabinolide are compounds that have been isolated from S pinnata extracts and the family Asteraceae have been reported to mostly possess these compounds.35
Phenolic compounds are the largest group of phytochemicals which have been recorded from every plant part36; the total phenol content was determined to be highest at 55.33 GAE/g. this may be due to the presence of flavonoids, tannins, and alkaloids, which are part of phenolic group. It has been reported that phenol compounds are responsible for biological activities such as antioxidants, antibacterial, antimalarial, and antidairearhea.37,38 Tawaha et al39 reported on the high phenolic content from plants falling under the family Asteraceae. This is the first study to report the total tannin and flavonoids content of S pinnata extracts. The tannin content of 28 GAE/g was observed to be higher than that of flavonoids (4 QE/mg). Natural antioxidants have been reported to protect against chronic diseases and oxidative stress. The presence of antioxidant compounds was indicated by the yellow bands against the purple background on the TLC plates. The intensity of the yellow color depends on the quantity and nature of compounds present in extracts at that area.40 The antioxidant activity observed might be due to phytochemical constituents which have been found to be present in S pinnata extracts. The qualitative antioxidant assay indicated that methanol extracts had the highest scavenging activity when compared with the positive control at all concentrations. Masevhe et al41 indicated that S pinnata had weak antioxidant activity. However, the methanol extracts have been reported to have high antioxidant activity.42,43 The results from qualitative analysis and quantitative analysis do not correlate as the ethyl acetate and acetone extracts were observed to have high antioxidants activity with methanol extract not showing activity. The lack of activity with the methanol extract might be due to synergistic mechanism of compounds and the evaporation of solvent systems. All plant extracts had high antioxidant activity at high concentration (10 mg/mL), and at low concentration, only methanol and hexane had high activity (Figure 3). There are different mechanisms by which antioxidants prevent oxidative stress and ferric reducing power falls under one of the mechanisms. The results indicated that S pinnata had high ferric reducing power when compared with the positive control (Figure 4). Tannins have been reported to have ion chelator activity, which might be responsible for the reducing power of the plant extracts.44
Medicinal plants are considered the greatest source of antimicrobial drugs.45 A white area against a pink color on bioautograms indicates that chloroform and ethyl acetate extracts have antimycobacterial compounds (Figure 5). Alkaloids and flavonoids were also reported for their antibacterial activity.46,47 Antibacterial activity has been reported from other Asteraceae species.48 The MIC was observed from acetone extracts followed by ethyl acetate and dichloromethane extracts. The activity might be due to the presence of saponins, glycosides, steroids, and polyphenols compounds.49 Antibacterial activity of the same plant have also been reported by Masevhe et al.41 The lower the MIC value, the higher the total activity volume. This was observed with acetone extracts (Table 3). Total activity is referred to as; the amounts in which the active compounds in dried plant material can be diluted and still inhibit the growth of the microorganism.50 The white area on bioautograms developed under solvent system CEF, could be explained by evaporation of solvent system, which might have not evaporated properly or the low concentration of the active compounds from the extracts under the tested condition or by disruption of synergistic mechanism between active compounds caused by TLC separation.51,52
Conclusion
The observed results indicated that S pinnata possess compounds of intermediate polarity. The plant has the potential biologically active compounds which can be used in the development of new drugs. The antioxidant and antimycobacterial compounds detected require isolation and characterization. Further studies should be conducted for the cytotoxic effects of the plant extracts to address the safety of the plant.
Acknowledgments
We would like to thank the National Research Foundation and University of Limpopo for financial support. We would like to thank Mr I. Njanje and Dr V. P. Bagla for proofreading.
Footnotes
Author Contributions: PM was involved with conception and design of the study. MVM carried out the experiments and analyzed the data.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Financial assistance was provided by the University of Limpopo and National Research Foundation.
ORCID iD: Peter Masoko, PhD  https://orcid.org/0000-0002-8076-083X
https://orcid.org/0000-0002-8076-083X
Ethical Approval: The study protocol was confirmed by University of Limpopo Ethics Committee (TREC/248/2017: IR).
References
- 1. Nash KA. Intrinsic macrolide resistance in Mycobacterium smegmatis is conferred by a novel erm gene, erm (38). Antimicrob Agents Chemother. 2003;47:3053–3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tsukamura M. Properties of Mycobacterium smegmatis freshly isolated from soil. Jpn J Microbiol. 1976;20:355–356. [DOI] [PubMed] [Google Scholar]
- 3. Best CA, Best TJ. Mycobacterium smegmatis infection of the hand. Hand (N Y). 2009;4:165–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wagner D, Young LS. Nontuberculous mycobacterial infections: a clinical review. Infection. 2004;32:257–270. [DOI] [PubMed] [Google Scholar]
- 5. Gurib-Fakim A. Medicinal plants: traditions of yesterday and drugs of tomorrow. Mol Aspects Med. 2006;27:1–93. [DOI] [PubMed] [Google Scholar]
- 6. Prakash MA, Sharifi-Rad M, Shariati MA. et al. Bioactive compounds and health benefits of edible Rumex species—a review. Cell Mol Biol (Noisy-le-grand). 2018;64:27–34. [PubMed] [Google Scholar]
- 7. Mishra AP, Saklani S, Salehi B. et al. Satyrium nepalense, a high altitude medicinal orchid of Indian Himalayan region: chemical profile and biological activities of tuber extracts. Cell Mol Biol (Noisy-le-grand). 2018;64:35–43. [PubMed] [Google Scholar]
- 8. Sofowora A. Medicinal plants and traditional medicine in Africa Ibadan, Nigeria: Spectrum Books Ltd; 1993:191–289. [Google Scholar]
- 9. Duraipandiyan V, Ayyanar M, Ignacimuthu S. Antimicrobial activity of some ethnomedicinal plants used by Paliyar tribe from Tamil Nadu, India. BMC Complement Altern Med. 2006;6:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sharifi-Rad J, Sharifi-Rad M, Salehi B. et al. In vitro and in vivo assessment of free radical scavenging and antioxidant activities of Veronica persica Poir. Cell Mol Biol (Noisy-le-grand). 2018;64:57–64. [PubMed] [Google Scholar]
- 11. Germplasm Resources Information Network (GRIN). Taxonomy for Plants: Schkuhria pinnata. Beltsville, MD: National Germplasm Resources Laboratory; 2012. [Google Scholar]
- 12. Deutschländer MS, van de Venter M, Roux S, Louw J, Lall N. Hypoglycaemic activity of four plant extracts traditionally used in South Africa for diabetes. J Ethnopharmacol. 2009;124:619–624. [DOI] [PubMed] [Google Scholar]
- 13. Njoroge GN, Bussmann RW, Gemmill B, Newton LE, Ngumi VW. Utilisation of weed species as sources of traditional medicines in central Kenya. Lyonia. 2004;7:71–87. [Google Scholar]
- 14. van der Merwe D, Swan GE, Botha CJ. Use of ethnoveterinary medicinal plants in cattle by Setswana-speaking people in the Madikwe area of the North West Province of South Africa. J S Afr Vet Assoc. 2001;72:189–196. [DOI] [PubMed] [Google Scholar]
- 15. Luseba D, Elgorashi EE, Ntloedibe DT, van Staden J. Antibacterial, anti-inflammatory and mutagenic effects of some medicinal plants used in South Africa for the treatment of wounds and retained placenta in livestock. S Afr J Bot. 2007;73:378–383. [Google Scholar]
- 16. Mupfure A, Matondi GHM, Imbayarwo-Chikosi VE, Nyamushamba GB, Marandure T, Masama E. Potential use of Schkuhria pinnata in the control of mastitis pathogens. Int J Innov Res Dev. 2014;3:415–420. [Google Scholar]
- 17. Sasidharan S, Chen Y, Saravanan D, Sundram KM, Latha LY. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr J Tradit Complement Altern Med. 2011;8:1–10. [PMC free article] [PubMed] [Google Scholar]
- 18. Kotze M, Eloff JN. Extraction of antibacterial compounds from Combretum spp (combretaceae). S Afr J Bot. 2002;68:62–67. [Google Scholar]
- 19. Borokini TI, Omotayo FO. Phytochemical and ethnobotanical study of some selected medicinal plants from Nigeria. J Med Plant Res. 2012;6:1106–1118. [Google Scholar]
- 20. Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteau reagent. Methods Enzymol. 1999;299:152–178. [Google Scholar]
- 21. Tambe VD, Bhambar RS. Estimation of total phenol, tannin, alkaloid and flavonoid in Hibiscus tiliaceus Linn. wood extracts. Res Rev. 2014;2:41–47. [Google Scholar]
- 22. Deby C, Magotteaux G. Relationship between essential fatty acids and tissue antioxidant levels in mice [in French]. C R Seances Soc Biol Fil. 1970;164:2675–2681. [PubMed] [Google Scholar]
- 23. Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28:25–30. doi:10.1016/S0023-6438(95)80008-5 [Google Scholar]
- 24. Oyaizu M. Studies on products of browning reactions: antioxidative activities of products of browning reaction prepared from glucosamine. Jpn J Nutr Diet. 1986;44:307–315. [Google Scholar]
- 25. Eloff JN. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med. 1998;64:711–713. [DOI] [PubMed] [Google Scholar]
- 26. Begue WJ, Kline RM. The use of tetrazolium salts in bioautographic procedures. J Chromatogr. 1972;64:182–184. [DOI] [PubMed] [Google Scholar]
- 27. McGaw LJ, Jäger AK, van Staden J, Eloff JN. Variation in antibacterial activity of Schotia species. S Afr J Bot. 2002;68:41–46. [Google Scholar]
- 28. Gibbons S, Gray AI. Isolation by planar chromatography. Nat Prod Isolation. 1998:209–245. doi:10.1007/978-1-59259-256-2_7 [Google Scholar]
- 29. Ndukwe IG, Bello AI, Habila JD, John C. Phytochemical and antimicrobial screening of the crude petroleum spirit and methanol extracts of the stem bark, leaves and roots of Ficus thoningii (blume). Afr J Biotechnol. 2007;6:2645–2649. [Google Scholar]
- 30. Oryema C, Ziraba RB, Odyek O, Omagor N, Opio A. Phytochemical properties and toxicity to brine shrimp of medicinal plants in Erute county, Lira district, Uganda. J Med Plant Res. 2011;5:5450–5457. [Google Scholar]
- 31. Rodrigo G, Almanza GR, Cheng Y. et al. Antiproliferative effects of curcuphenol, a sesquiterpene phenol. Fitoterapia. 2010;81:762–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dufour D, Pichette A, Mshvildadze V. et al. Antioxidant, anti-inflammatory and anticancer activities of methanolic extracts from Ledum groenlandicum Retzius. J Ethnopharmacol. 2007;111:22–28. [DOI] [PubMed] [Google Scholar]
- 33. He X, Liu RH. Triterpenoids isolated from apple peels have potent antiproliferative activity and may be partially responsible for apple’s anticancer activity. J Agric Food Chem. 2007;55:4366–4370. [DOI] [PubMed] [Google Scholar]
- 34. Fu L, Zhang S, Li N. et al. Three new triterpenes from Nerium oleander and biological activity of the isolated compounds. J Nat Prod. 2005;68:198–206. [DOI] [PubMed] [Google Scholar]
- 35. Khorombi TE, Fouché G, Kolesnikova NI, Maharaj VJ, Nthambeleni R, van der Merwe MR. Investigation of South African plants for anti-cancer properties. Pharmacologyonline. 2006;3:494–500. [Google Scholar]
- 36. Dai J, Mumper RJ. Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010;15:7313–7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tomás-Barberán FA, Espin JC. Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables. J Sci Food Agri. 2001;81:853–876. [Google Scholar]
- 38. Zheng W, Wang SY. Antioxidant activity and phenolic compounds in selected herbs. J Agric Food Chem. 2001;49:5165–5170. [DOI] [PubMed] [Google Scholar]
- 39. Tawaha K, Alali FQ, Gharaibeh M, Mohammad M, El-Elimat T. Antioxidant activity and total phenolic content of selected Jordanian plant species. Food Chem. 2007;104:1372–1378. [Google Scholar]
- 40. Scio E, Mendes RF, Motta EV. et al. Antimicrobial and antioxidant activities of some plant extracts In: Rao V, ed. Phytochemicals as Nutraceuticals—Global Approaches to Their Role in Nutrition and Health. London, England: InTechOpen; 2012:21–42. [Google Scholar]
- 41. Masevhe NA, Aroke A, McGaw LJ, Eloff JN. Evaluating antioxidant activity and cytotoxicity of the selected South African medicinal plant species. S Afr J Bot. 2012;79:197–199. [Google Scholar]
- 42. Candan F, Unlu M, Tepe B. et al. Antioxidant and antimicrobial activity of the essential oil and methanol extracts of Achillea millefolium subsp. millefolium Afan. (Asteraceae). J Ethnopharmacol. 2003;87:215–220. [DOI] [PubMed] [Google Scholar]
- 43. Stanojević L, Stanković M, Nikolić V. et al. Antioxidant activity and total phenolic and flavonoid contents of Hieracium pilosella L. extracts. Sensors (Basel). 2009;9:5702–5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. van der Watt E, Pretorius JC. Purification and identification of active antibacterial components in Carpobrotus edulis L. J Ethnopharmacol. 2001;76:87–91. [DOI] [PubMed] [Google Scholar]
- 45. Rosakutty PJ, Roslin AS. Isolation and characterization of an antimicrobial compound from the traditional medicinal plant Pittosporum tetraspermum Wight & Arn. Int J Med Aromatic Plants. 2012;2:141–150. [Google Scholar]
- 46. Cushnie TT, Lamb AJ. Antimicrobial activity of flavonoids. Int J Antimicrob Agents. 2005;26:343–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Karou D, Savadogo A, Canini A. et al. Antibacterial activity of alkaloids from Sida acuta . Afr J Biotechnol. 2006;5:195–200. [Google Scholar]
- 48. Borkataky M, Kakoty BB, Saikia LR. Proximate analysis and antimicrobial activity of Eclipta alba (L.) Hassk. a traditionally used herb. Int J Pharm Pharm Sci. 2013;5:149–154. [Google Scholar]
- 49. Amusan OO, Sukati NA, Dlamini PS, Sibandze FG. Some Swazi phytomedicines and their constituents. Afr J Biotechnol, 2007;6:267–272. [Google Scholar]
- 50. Eloff JN. On expressing the antibacterial activity of plant extracts—a small first step in applying scientific knowledge to rural primary health care. S Afr J Sci. 2000;96:116–118. [Google Scholar]
- 51. Mdee LK, Masoko P, Eloff JN. The activity of extracts of seven common invasive plant species on fungal phytopathogens. S Afr J Bot. 2009;75:375–379. [Google Scholar]
- 52. Masoko P, Eloff JN. Bioautography indicates the multiplicity of antifungal compounds from twenty-four southern African Combretum species (Combretaceae). Afr J Biotechnol. 2006;5:1625–1647. [Google Scholar]