Abstract
Background:
Patients diagnosed with chronic obstructive pulmonary disease (COPD) have increased risks for a series of physical and mental illnesses. Tumor necrosis factor-α (TNF-α) has been reported to participate in the development of COPD and its complications. However, the values of blood TNF-α level used in the diagnosis of COPD remains controversial. In view of this, we performed a systematic review and meta-analysis to evaluate the correlation between TNF-α level and COPD.
Methods:
We searched PubMed, Web of Science, Embase and CNKI up to May 2018. The selection criteria were set according to the PICOS framework. A random-effects model was then applied to evaluate the overall effect sizes by calculating standard mean difference (SMD) and its 95% confidence intervals (CIs).
Results:
A total of 40 articles containing 4189 COPD patients and 1676 healthy controls were included in this meta-analysis. The results indicated a significant increase in TNF-α level in the COPD group compared with the control group (SMD: 1.24, 95% CI: 0.78–1.71, p < 0.00001). According to the subgroup analyses, we noted that TNF-α level was associated with predicted first second of forced expiration (FEV1) (%) and study region. However, no association between TNF-α level and COPD was found when the participants were nonsmokers, and the mean age was less than 60 years.
Conclusions:
Our results indicated that TNF-α level was increased in COPD patients when compared with healthy controls. Illness progression and a diagnosis of COPD might contribute to higher TNF-α levels. However, the underlying mechanism still remains unknown and needs further investigation.
The reviews of this paper are available via the supplemental material section.
Keywords: biomarker, chronic obstructive pulmonary disease, meta-analysis, tumor necrosis factor-α
Introduction
Chronic obstructive pulmonary disease (COPD) kills more than 3 million people worldwide every year.1 Many factors have been reported to be associated with COPD, including systemic and local inflammation, air pollution and a sedentary lifestyle.2–4 However, the exact mechanisms underlying COPD still remain unclear. Since COPD is a chronic inflammatory disease, the relationship between inflammation and COPD has been widely evaluated. Tumor necrosis factor-α (TNF-α), one of the major inflammatory factors, is implicated in the pathogenesis of many disorders, including COPD.5,6 However, due to the small sample sizes, most studies lack adequate statistical power to clarify the relationship between TNF-α and COPD. Moreover, currently available studies have provided inconsistent, or even contrary, results. For example, Karadag and colleagues have pointed out that raised serum level of TNF-α can be used as a biomarker for the systemic inflammatory response in stable COPD patients.7 But Franciosi and colleagues showed that healthy people and COPD patients at different stages had no statistical difference in TNF-α concentrations.8 To comprehensively investigate the association between TNF-α and COPD, and evaluate the diagnostic value of TNF-α in COPD, we conducted this meta-analysis to systematically evaluate the relationship between them.
Materials and methods
Literature search
We systematically searched four electronic databases (PubMed, Web of Science, EMBASE, and Cochrane library database CENTRAL) up to May 2018. The search terms were [‘pulmonary disease, chronic obstructive’ (MeSH Terms) or ‘chronic obstructive pulmonary disease’ or ‘COPD’ or ‘COAD’ or ‘chronic obstructive airway disease’ or ‘chronic obstructive lung disease’ or ‘emphysema’ or ‘chronic bronchitis’] and [‘Tumor necrosis factor-a’ (MeSH Terms) or ‘Tumor necrosis factor-a ’or ‘TNF-α’] and (‘systemic inflammation’ or ‘biological markers’) (Supplementary Table S1). Only articles published in English were included. We also went through the references of eligible studies and review articles manually to identify possible relevant publications.
Study selection and inclusion and exclusion criteria
The inclusion criteria, set according to the PICOS framework (population, intervention, comparison, outcomes, study design), were as follows (Table 1): population, COPD patients; intervention, TNF-α; comparison, healthy control or non-COPD; outcomes, concentration of TNF-α; study design, case-control study.
Table 1.
PICOS table of included studies.
| Category | Description | Search strategy terms |
|---|---|---|
| Population | COPD | COPD OR Chronic obstructive pulmonary disease |
| Intervention | TNF-α | TNF-α OR Tumor necrosis factor-alpha |
| Control | Healthy control or non-COPD | Healthy control or non-COPD |
| Outcome | Concentration of TNF-α | TNF-α concentration OR TNF-α level |
| Study Design | Case-control study | Case-Control study OR Case-Comparison Studies OR Case-compare study OR case-referent study OR Matched case-control study NOT animals |
COPD, chronic obstructive pulmonary disease; PICOS, population, intervention, comparison, outcomes, study design; TNF-α, tumor necrosis factor-alpha
The eligible studies had to meet all of the following criteria: evaluation of the association between TNF-α and COPD was described; the specific concentration of TNF-α was provided; TNF-α level in both the control and COPD group was provided; sufficient patient data for calculating standard mean difference (SMD) and its 95% confidence intervals (CIs) were provided; COPD patients were diagnosed according to the criteria of the American Thoracic Society or Global Initiative for Chronic Obstructive Lung Disease; and healthy controls who had no medical illness or abnormalities in physical examination and laboratory date, and presented no symptoms of infection, were included. The exclusion criteria included: patients who received nutritional support with therapy; conference papers, reports, comments or review articles; studies without a control group; and patients with a history or diagnosis of asthma, allergy, or respiratory diseases other than COPD. The reasons for exclusion are shown in Table 2.
Table 2.
Exclusion criteria.
| Characteristics of excluded studies | Reasons |
|---|---|
| Patients who received nutritional support with therapy | Nutritional support is likely to affect the expression of TNF-α |
| Conference papers, reports, comments or review articles | Conference papers, reports, comments, or review articles do not have enough case-control studies. These paper cannot provide enough data about PICOS |
| Without control group | All included studies are case-control studies, in which patients with COPD are diagnosed as cases, and individuals who do not have the disease or non-COPD are comparable as controls |
| Patients with a history or diagnosis of asthma, allergy, or respiratory diseases other than COPD | The aim of this review was to investigate the relationship between TNF-α and COPD rather than other respiratory diseases |
COPD, chronic obstructive pulmonary disease; TNF-α, tumor necrosis factor-alpha
Quality assessment
Two reviewers (YY and ZJ) independently evaluated the quality of included studies according to the Newcastle-Ottawa Quality Assessment Scale (NOS). The NOS is a semiquantitative scale composed of three domains: selection, comparability, and exposure. The maximum NOS score is 9: a study with a total score of ⩽3 was considered as poor quality, those scoring 4–6 were of moderate quality, and a score of 7–9 was considered high quality.
Data extraction
Two investigators (DX and YY) independently extracted the following information from the original studies: first author’s name, year of publication, country, sample size, clinical characteristics [including sex ratio, mean ages, smoking status, COPD status, body mass index (BMI), and the predicted first second of forced expiration (FEV1)]. Disagreements between the two reviewers were resolved by consultation with a third reviewer (WSY).
Statistical analysis
The RevMan 5.3 software was used to perform the meta-analysis. The SMD and corresponding 95% CI were calculated to evaluate the relationships between TNF-α level and COPD. The Chi-squared test and I2 statistics were applied to detect the heterogeneity among studies. A p < 0.05 in Chi-squared test or I2 > 50% indicated the presence of significant heterogeneity. A random effect model or fixed model was then used based on the presence or absence of significant heterogeneity. A sensitivity analysis was performed to explore the origins of heterogeneity. Publication bias was assessed using funnel plots with standard error.
Results
Study selection
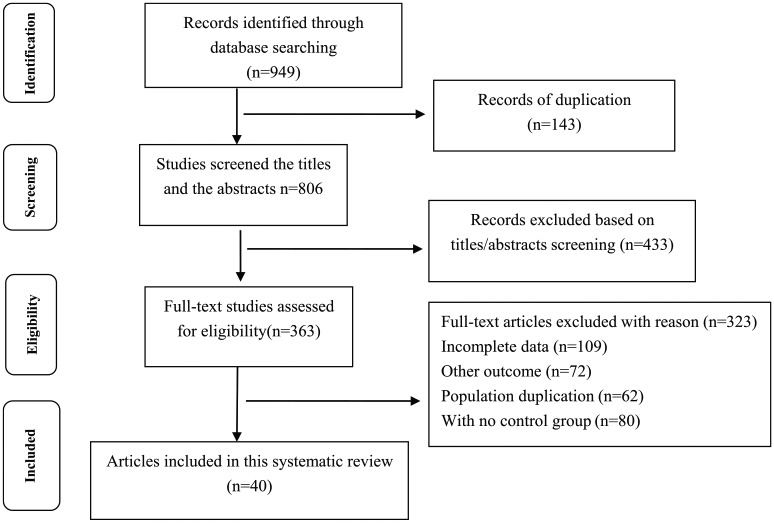
The initial literature search returned a total of 949 articles. We excluded 143 duplicated studies. After a careful review of the titles and abstracts of remaining studies, a further 433 articles were excluded, and another 323 articles were also excluded for various reasons. Finally, 40 studies involving 4189 COPD patients and 1676 healthy controls were included in this meta-analysis.9–45 The flowchart for the literature search is presented in Figure 1.
Figure 1.
Flow diagram of the literature search process.
The characteristics of the included studies are summarized in Table 3. Eight studies had a NOS score of 925,29,33,36,37,40,42,45; seven studies scored 813–15,17,18,22,44; nine studies scored 711,19,30,31,34,35,38,41,46; ten studies scored 69,10,12,24,27,28,32,43,47,48; four studies scored 516,20,26,39; and the last two studies scored 4.21,23 The NOS scores suggested that all included studies were of moderate or high quality. Regarding location, the majority of studies were from Europe,26 two studies were from the US,14,26 one study was from Africa,15 and eight studies were from Asia.22,29,32,34,35,38,43,45 Patients in 9 studies were treated with steroids, while patients in the remaining 24 studies were not treated with steroids. The mean age, smoking status, COPD status, gender, and BMI of the study participants in the included studies are also provided in Table 3.
Table 3.
Characteristics of the included studies.
| Study | Year | Country | Sample size | Mean age | Sex(male/Female) | Smoking status |
Reversibility test |
Treat with | COPD status |
NOS | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Control | Case | Control | Steroids | ||||||||
| Calikoglu9 | 2004 | Turkey | 41 | 62.18 ± 2.50 | 54.73 ± 2.23 | NR | NR | NR | No | NR | Exacerbated | 6 |
| Agusti10 | 2012 | Spain | 2409 | 63.5 ± 7.1 | 53.0 ± 8.6 | 1160/1004 | 76/169 | NR | Yes | NR | NR | 6 |
| Once11 | 2010 | Turkey | 73 | 62.8 ± 5.5 | 61.8 ± 7.4 | 38/2 | 31/2 | Ex-smokers | No | No | NR | 7 |
| Kleniewska12 | 2016 | Poland | 42 | 59.8 ± 6.7 | 43.7 ± 14.4 | 20/0 | 15/7 | NR | Yes | No | Stable | 6 |
| Rovina13 | 2007 | Greece | 30 | 54 ± 9 | 46 ± 11 | NR | NR | Current-smokers | Yes | No | NR | 8 |
| Gagnon14 | 2014 | Canada | 56 | 65 ± 6 | 62 ± 8 | 25/12 | 13/6 | Ex-smokers | No | NR | Mild | 8 |
| Ben Anes15 | 2017 | Tunisia | 285 | 61.58 ± 1.75 | 58.15 ± 0.7 | 50/6 | 203/26 | Ex-smokers | Yes | NR | Exacerbated | 8 |
| Perez-deLiano16 | 2017 | Spain | 109 | 65.6 ± 10.1 | 59.8 ± 10.5 | 18/26 | 53/12 | NR | Yes | NR | NR | 5 |
| FoschinoBarbaro17 | 2007 | UK | 42 | NR | NR | 24/3 | 12/3 | Ex-smokers | Yes | No | Stable | 8 |
| Barreiro18 | 2013 | Spain | 21 | 59 ± 8 | 58 ± 14 | 9 | 12 | Ex-smokers | No | No | NR | 8 |
| Beeh19 | 2003 | Germany | 26 | 59 ± 9.25 | 31 ± 8.75 | 8/4 | 8/6 | Ex-smokers | Yes | No | Stable | 7 |
| Di Stefano20 | 2018 | Italy | 41 | NR | NR | 19/4 | 8/10 | Ex-smokers | Yes | No | Exacerbated | 5 |
| Breyer21 | 2011 | Netherlands | 127 | NR | NR | NR | NR | Ex-smokers | No | No | Exacerbated | 4 |
| Zhang22 | 2016 | China | 89 | 61.14 ± 10.21 | 60.92 ± 9.62 | 30/20 | 23/16 | NR | Yes | No | Moderate | 8 |
| Dima23 | 2010 | Greece | 38 | 58.4 ± 2.0 | 41.5 ± 3.5 | NR | NR | Ex-smokers | Yes | No | NR | 4 |
| Kawayama24 | 2016 | UK | 20 | 62.2 ± 6.6 | 64.2 ± 6.6 | 7/3 | 5/5 | Ex-smokers | No | Inhaled | NR | 6 |
| Gaki25 | 2011 | Greece | 354 | 63 ± 1.86 | 60 ± 1.71 | 169/53 | 97/35 | Ex-smokers | No | Inhaled | Stable | 9 |
| Godoy26 | 2003 | Brazi | 24 | 62 ± 2.25 | 54 ± 1.5 | 14/0 | 5/5 | NR | No | No | NR | 5 |
| Hacievliyagil27 | 2012 | Turkey | 40 | 61.2 ± 1.7 | 59.1 ± 5.4 | 17/3 | 14/6 | NR | Yes | Oral | Stable | 6 |
| Huertas28 | 2010 | Italy | 33 | 69 ± 8 | 63 ± 7 | NR | NR | NR | No | No | Stable | 6 |
| Ju29 | 2011 | China | 130 | 65.17 ± 6.79 | 63.98 ± 5.77 | 54/16 | 21/39 | Ex-smokers | Yes | No | Stable | 9 |
| Karadag30 | 2007 | Turkey | 125 | 63.5 ± 7.59 | 61.10 ± 7.68 | NR | NR | NR | Yes | Inhaled | Stable | 7 |
| Karadag31 | 2008 | Turkey | 65 | 65.6 ± 7.8 | 63.2 ± 7.6 | NR | NR | Ex-smokers | Yes | No | Stable | 7 |
| Shin32 | 2007 | Korea | 105 | 63.6 ± 7.4 | 66.5 ± 8.9 | NR | NR | NR | No | No | Stable | 6 |
| Kythreotis33 | 2008 | Greece | 77 | 65.8 ± 8.3 | 65.9 ± 9.6 | 43/9 | 19/6 | Ex-smokers | No | No | Exacerbation | 9 |
| Liu34 | 2009 | China | 63 | 70 ± 7 | 70 ± 7 | NR | NR | No-smoker | No | No | Stable | 7 |
| Huang35 | 2016 | China | 67 | 60.2 ± 10.1 | 55.7 ± 10.3 | 21/11 | 19/16 | NR | Yes | NR | NR | 7 |
| Moermans36 | 2011 | Belgium | 128 | 62 ± 12 | 40 ± 12 | 73/21 | 24/10 | Ex-smokers | Yes | Yes | Stable | 9 |
| Piehl-Aulin37 | 2008 | Sweden | 40 | 64 ± 8.7 | 61.9 ± 7.9 | 11/15 | 7/7 | Ex-smokers | No | Stable | 9 | |
| Tan38 | 2016 | China | 20 | 65 ± 3 | 50 ± 5 | 6/4 | 4/6 | Ex-smokers | Yes | Yes | Stable | 7 |
| Guiot39 | 2017 | Belgium | 62 | 63 ± 9 | 55 ± 9 | 24/8 | 11/19 | NR | No | NR | NR | 5 |
| Sarioglu40 | 2015 | Turkey | 175 | 64.0 ± 8.9 | 61.5 ± 9.2 | 100/10 | 55/10 | Ex-smokers | Yes | No | Stable | 9 |
| Uzum 41 | 2013 | Turkey | 49 | 65.9 ± 10.0 | 50.2 ± 8.4 | NR | NR | No-smoker | No | No | Stable | 7 |
| Kosacka42 | 2015 | Poland | 210 | 62.2 ± 9.37 | 49.48 ± 13.68 | 121/60 | 18/11 | Ex-smokers | No | NR | Stable | 9 |
| Cheng43 | 2008 | China | 343 | 71.9 ± 8.0 | 74.7 ± 3.7 | 152/32 | 129/30 | Ex-smokers | Yes | NR | NR | 6 |
| Valipour44 | 2008 | Austria | 60 | 62 ± 9 | 59 ± 8 | 23/7 | 23/7 | NR | Yes | No | Exacerbation | 8 |
| Zhang45 | 2010 | China | 65 | 70.93 ± 5.58 | 69.16 ± 7.43 | 38/8 | 13/6 | Ex-smokers | Yes | No | Stable | 9 |
| Soler46 | 1999 | Spain | 21 | 68 ± 9 | 51 ± 11 | 13/0 | 5/3 | Ex-smokers | No | No | Stable | 7 |
| Vera47 | 1996 | UK | 30 | 62.5 ± 3.2 | 39.4 ± 3.1 | NR | NR | Ex-smokers | No | No | NR | 6 |
| De Godoy48 | 1996 | US | 23 | 67.0 ± 4.9 | 63.5 ± 5.8 | 6/4 | 11/2 | NR | No | No | NR | 6 |
COPD, chronic obstructive pulmonary disease; NOS, Newcastle-Ottawa Quality Assessment Scale; NR, not recorded
Meta-analysis
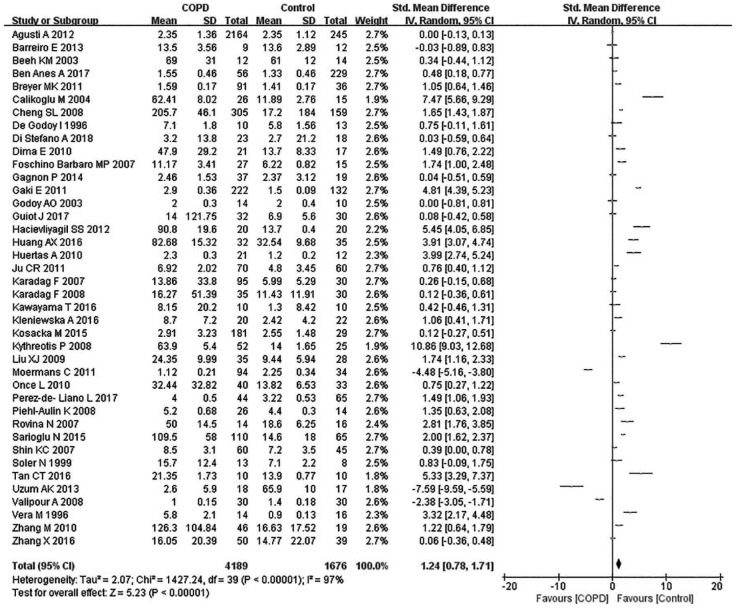
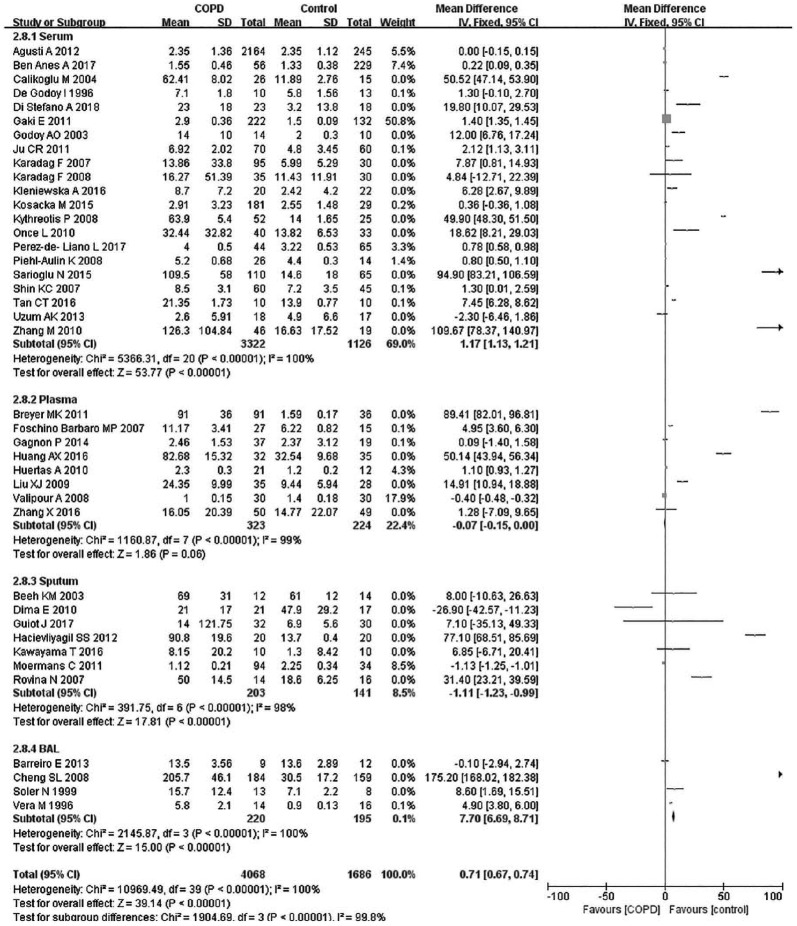
Due to the existence of significant heterogeneity (p < 0.00001, I2 = 98%), this meta-analysis used a random effect model. Compared with the control group, the COPD patients had a significantly elevated level of TNF-α (SMD: 1.45, 95% CI: 0.44–2.27, p < 0.00001) (Figure 2).
Figure 2.
Comparison of tumor necrosis factor-α level between COPD patients and controls in the included studies.
CI, Confidence interval; COPD, chronic obstructive pulmonary disease; SD, standard deviation.
Subgroup analysis
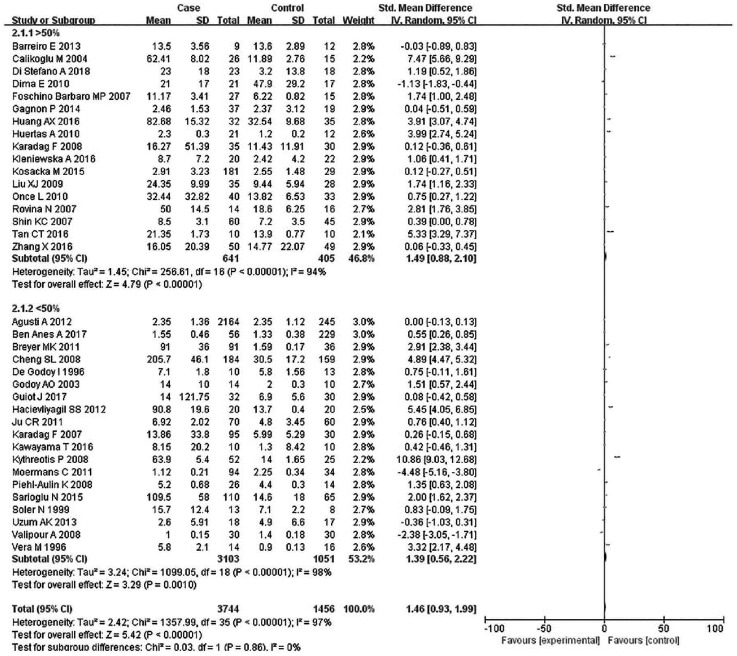
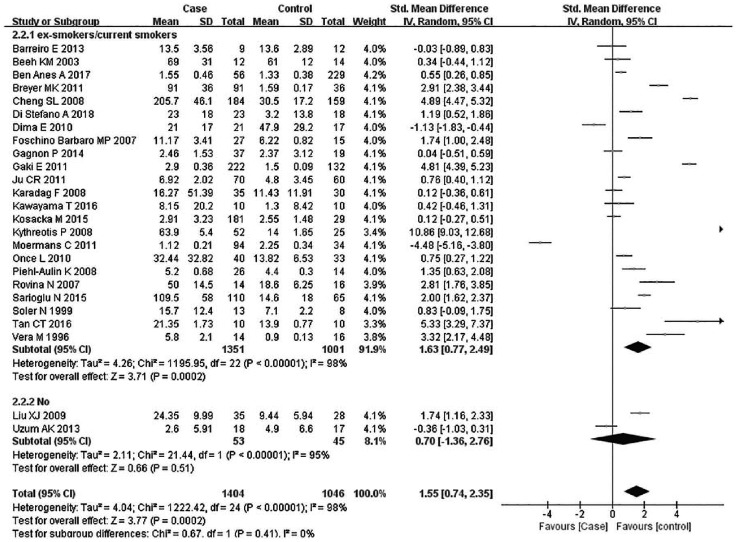
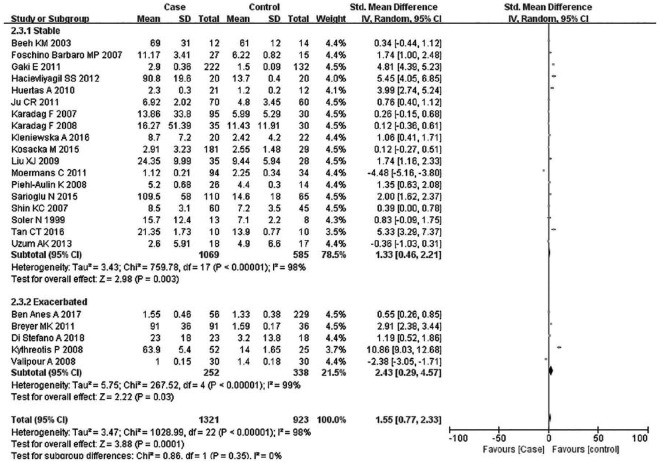
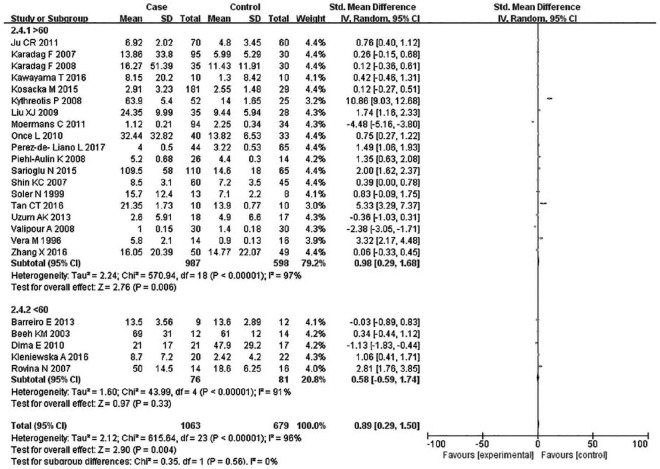
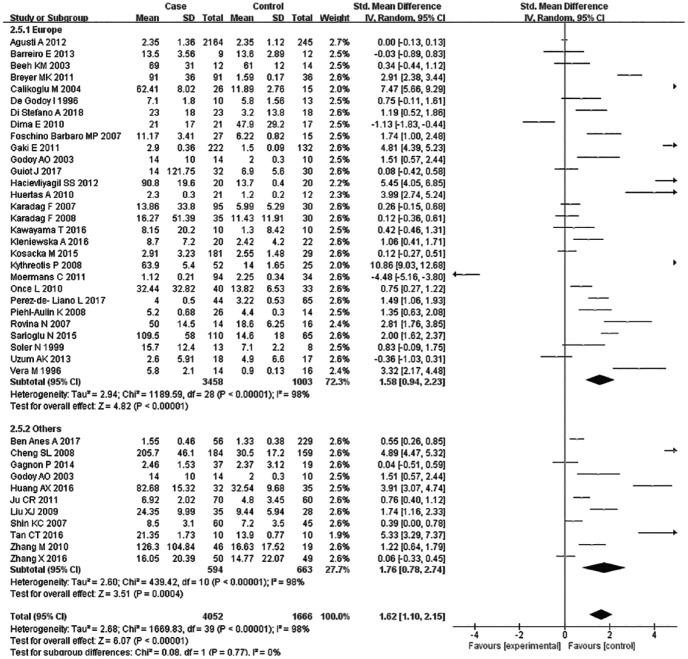
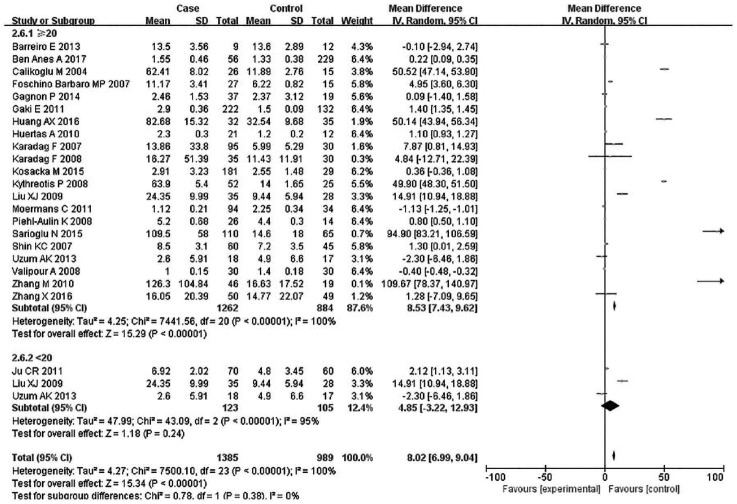
Subsequently, subgroup analyses stratified for FEV1%, smoking history, COPD status, country, mean age, and BMI were performed to further understand the association between TNF-α level and COPD, and discover the source of heterogeneity (Table 4). A total of 36 studies were included in the subgroup analysis based on FEV1%; the TNF-α level was 1.49 higher in COPD group compared with the control group (95% CI: 0.89–2.00, p < 0.00001) (Figure 3). The heterogeneity was still significant (>50%: p < 0.00001, I2 = 94%; <50%: p < 0.0001, I2 = 98%). In the subgroup analysis based on smoking status (Figure 4), the TNF-α level in the ex-smokers/current smoker group was higher than those in the control and case groups (SMD: 1.63, 95% CI: 0.77–2.49, p = 0.0002), but was not different for smoking patients (SMD: 0.70, 95% CI: –1.36 to 2.76, p = 0.51). The heterogeneity in both groups was still significant (ex-smokers/current smoker: p < 0.00001, I2 = 98%; No: p < 0.00001, I2 = 95%). A subgroup analysis was then performed according to COPD status (Figure 5). Patients with stable COPD and exacerbated COPD had higher TNF-α levels than the control group (stable: SMD: 1.33, 95% CI: 0.46–2.21, p = 0.003; exacerbated: SMD: 2.43, 95% CI: 0.29–4.57, p = 0.03), but the heterogeneity was still significant regardless of COPD status (stable: p < 0.00001, I2 = 98%; exacerbated: p < 0.00001, I2 = 99%). Moreover, a subgroup analysis was carried out based on mean age (Figure 6). The TNF-α level in age >60 groups was 0.98 higher than that of the control group (SMD: 0.98 95% CI: 0.29–1.68, p = 0.006). The heterogeneity was still significant in both groups (>60: p < 0.00001, I2 = 97%; <60: p < 0.00001, I2 = 91%) (Figure 6). In addition, in the country and BMI subgroup, the TNF-α level in the case group was significantly higher than that of the control group (Europe: SMD: 1.58, 95% CI: 0.94–2.23, p < 0.00001; others: SMD: 1.76, 95% CI: 0.78–2.74, p = 0.0004) (BMI ⩾20: SMD: 8.53, 95% CI: 7.43–9.62, p < 0.00001; BMI <20: SMD: 4.85, 95% CI: –3.22 to 12.93, p = 0.24). The heterogeneity was still obvious (Europe: p < 0.00001, I2 = 98%; others: p < 0.00001, I2 = 98%) (BMI ⩾ 20: p < 0.00001, I2 = 100%; BMI < 20: p < 0.00001, I2 = 95%). (Figures 7 and 8). Finally, subgroup analysis was performed based on sample source. The TNF-α level in the case group was significantly higher than that of the control group in serum and BAL; the difference has statistical significance (serum: p < 0.00001, I2 = 100%; BAL: p < 0.00001, I2 = 100%) (Figure 9).
Table 4.
Subgroup analysis of TNF-α level in COPD.
| Subgroups | N | SMD (95%CI) | p | Test of heterogeneity |
|
|---|---|---|---|---|---|
| I 2 | p | ||||
| COPD Status | |||||
| Stable | 1654 | 1.33 (0.46–2.21) | p = 0.003 | 98 | p < 0.00001 |
| Exacerbated | 590 | 2.43 (0.29–4.57) | p = 0.03 | 99 | p < 0.00001 |
| FEV1 % | |||||
| >50% | 1046 | 1.49 (0.88–2.10) | p < 0.00001 | 94 | p < 0.00001 |
| <50% | 4154 | 1.39 (0.56–2.22) | p = 0.0010 | 98 | p < 0.00001 |
| Current smoking status Ex-smokers/current smokers |
2352 |
1.63 (0.77–2.49) |
p = 0.0002 |
98 |
p < 0.00001 |
| No | 98 | 0.70 (–1.36 to 2.76) | p = 0.51 | 95 | p < 0.00001 |
| Country | |||||
| Europe | 4461 | 1.58 (0.94–2.23) | p < 0.00001 | 98 | p < 0.00001 |
| Others | 1190 | 1.76 (0.78–2.74) | p = 0.0004 | 98 | p < 0.00001 |
| Mean age | |||||
| >60 | 1585 | 0.98 (0.29–1.68) | p = 0.006 | 97 | p < 0.00001 |
| <60 | 157 | 0.58 (–0.59 to 1.74) | p = 0.33 | 91 | p < 0.00001 |
| BMI | |||||
| >20 | 2146 | 0.72 (0.69–0.76) | p < 0.00001 | 100 | p < 0.00001 |
| <20 | 228 | 2.61 (1.67–3.55) | p < 0.00001 | 95 | p < 0.00001 |
BMI, Body mass index; COPD, chronic obstructive pulmonary disease; FEV1, first second of forced expiration; TNF-α, tumor necrosis factor-alpha
Figure 3.
Subgroup analyses of the relationship between tumor necrosis factor-α and chronic obstructive pulmonary disease according to first second of forced expiration (%).
Figure 4.
Subgroup analyses of the relationship between tumor necrosis factor-α and chronic obstructive pulmonary disease according to smoking status.
Figure 5.
Subgroup analyses of the relationship between TNF-α and chronic obstructive pulmonary disease (COPD) according to COPD status.
Figure 6.
Subgroup analyses of the relationship between tumor necrosis factor-α and chronic obstructive pulmonary disease according to age.
Figure 7.
Subgroup analyses of the relationship between tumor necrosis factor-α and chronic obstructive pulmonary disease according to country.
Figure 8.
Subgroup analyses of the relationship between tumor necrosis factor-α and chronic obstructive pulmonary disease according to body mass index.
Figure 9.
Subgroup analyses of the relationship between tumor necrosis factor-α and chronic obstructive pulmonary disease according to sample source.
Meta-regression analysis
To further determine the source of heterogeneity, meta-regression analyses were conducted. The results indicated that publication year, region, BMI, NOS, study sample size, and smoking status were not potential sources of heterogeneity (Table 5).
Table 5.
Meta-regression analysis coefficients for TNF-α levels.
| Covariates | Coefficient | p | 95% confidence interval |
|---|---|---|---|
| Year | −0.44053 | 0.593 | (–0.21028 to 0.12217) |
| Region | −0.20124 | 0.843 | (–2.25307 to 1.85058) |
| BMI | −0.33315 | 0.724 | (–2.23490 to 1.56859) |
| Sample size | −0.000526 | 0.659 | (–0.00293 to 0.00187) |
| Smoking status | −0.203374 | 0.810 | (–1.91399 to 1.50724) |
| NOS | 0.556458 | 0.886 | (–7245434 to 0.83583) |
BMI, Body mass index; NOS, Newcastle-Ottawa Quality Assessment Scale; TNF-α, tumor necrosis factor-alpha
Sensitivity analysis and publication bias
The sensitivity analysis showed that removing each of the 40 included studies did not result in significant change in the pooled effect size, indicating that the results of the present meta-analysis were stable (Table 6). Potential publication bias in this meta-analysis was evaluated with a funnel plot. The result showed that the included studies were symmetrically distributed, excluding the presence of significant publication bias (Figure 10).
Table 6.
Sensitivity analysis.
| Study | SMD (95% CI) | p Heterogeneity | I 2 |
|---|---|---|---|
| Calikoglu 20049 | 0.69 (0.62–0.76) | p < 0.00001 | 97 |
| Agusti 201210 | 1.00 (0.91–1.08) | p < 0.00001 | 97 |
| Once 201011 | 0.70 (0.63–0.77) | p < 0.00001 | 98 |
| Kleniewska 201612 | 0.70 (0.63–0.77) | p < 0.00001 | 98 |
| Rovina 200713 | 0.69 (0.62–0.77) | p < 0.00001 | 97 |
| Gagnon 201414 | 0.71 (0.64–0.79) | p < 0.00001 | 98 |
| Ben Anes 201715 | 0.72 (0.64–0.79) | p < 0.00001 | 98 |
| Perez-de-Liano 201716 | 0.68 (0.61–0.75) | p < 0.00001 | 97 |
| Foschino Barbaro 200717 | 0.69 (0.62–0.77) | p < 0.00001 | 97 |
| Barreiro 201318 | 0.71 (0.64–0.78) | p < 0.00001 | 98 |
| Beeh 200319 | 0.71 (0.63–0.78) | p < 0.00001 | 98 |
| Di Stefano 201820 | 0.71 (0.64–0.78) | p < 0.00001 | 98 |
| Breyer 201121 | 0.69 (0.62–0.77) | p < 0.00001 | 98 |
| Zhang 201622 | 0.72 (0.65–0.80) | p < 0.00001 | 97 |
| Dima 201023 | 0.70 (0.62–0.77) | p < 0.00001 | 98 |
| Kawayama 201624 | 0.71 (0.63–0.78) | p < 0.00001 | 98 |
| Gaki 201125 | 0.58 (0.50–0.65) | p < 0.00001 | 97 |
| Godoy 200326 | 0.71 (0.64–0.78) | p < 0.00001 | 98 |
| Hacievliyagil 201227 | 0.69 (0.62–0.76) | p < 0.00001 | 97 |
| Huertas 201028 | 0.69 (0.62–0.76) | p < 0.00001 | 97 |
| Ju 201129 | 0.70 (0.63–0.77) | p < 0.00001 | 98 |
| Karadag 200730 | 0.72 (0.64–0.79) | p < 0.00001 | 98 |
| Karadag 200831 | 0.72 (0.64–0.79) | p < 0.00001 | 98 |
| Shin 200732 | 0.71 (0.64–0.79) | p < 0.00001 | 98 |
| Kythreotis 200833 | 0.69 (0.62–0.76) | p < 0.00001 | 97 |
| Liu 200934 | 0.69 (0.62–0.76) | p < 0.00001 | 97 |
| Huang 2016235 | 0.68 (0.61–0.75) | p < 0.00001 | 97 |
| Moermans 201136 | 0.76 (0.69–0.83) | p < 0.00001 | 97 |
| Piehl-Aulin 200837 | 0.70 (0.62–0.77) | p < 0.00001 | 98 |
| Tan 201638 | 0.70 (0.63–0.77) | p < 0.00001 | 97 |
| Guiot 201739 | 0.72 (0.64–0.79) | p < 0.00001 | 98 |
| Sarioglu 201540 | 0.65 (0.58–0.73) | p < 0.00001 | 97 |
| Uzum 201341 | 0.71 (0.64–0.79) | p < 0.00001 | 97 |
| Kosacka 201542 | 0.72 (0.64–0.80) | p < 0.00001 | 97 |
| Cheng 200843 | 0.59 (0.51–0.67) | p < 0.00001 | 97 |
| Valipour 200844 | 0.74 (0.64–0.81) | p < 0.00001 | 97 |
| Zhang 201045 | 0.70 (0.62–0.77) | p < 0.00001 | 98 |
| Vera 199646 | 1.19 (0.72–1.66) | p < 0.00001 | 97 |
| Soler 199947 | 1.25 (0.78–1.73) | p < 0.00001 | 97 |
| De Godoy 199648 | 1.25 (0.78–1.73) | p < 0.00001 | 97 |
SMD, Standard mean difference; CI, 95% confidence intervals
Figure 10.
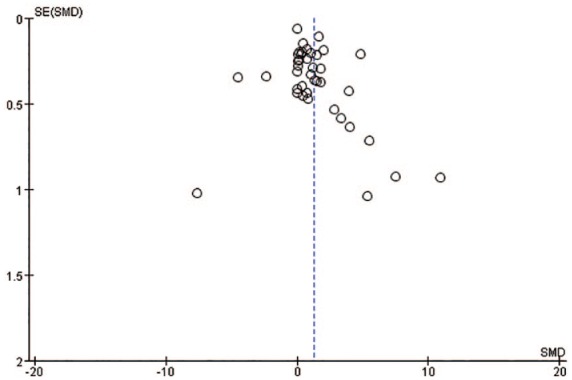
A funnel plot analysis of publication bias.
Discussion
COPDs induced by chronic bronchitis and emphysema are characterized by not fully reversible and progressive airflow limitation, and represent one of the most serious public health concerns in the world.49,50 As an inflammatory disease, inflammation of airways and lung parenchyma have been identified as one of the major pathogenic mechanisms of COPD.51 Inflammation is a complex process, in which a variety of cells and molecules are involved and a series of inflammatory signaling pathways are activated.
Previously, several meta-analyses have evaluated the association between TNF-α levels and COPD; however, the conclusions were conflicting. Gan and colleagues performed a meta-analysis including 14 studies and reported a significant correlation between systemic inflammatory markers, including TNF-α, and lung function.52 Bin and colleagues, however, indicated that there was no significant correlation between COPD and TNF-α level in a meta-analysis of 24 studies.53 The main limitation of the previous meta-analyses is the relatively small number of the included studies, which leads to a small size of participant cohort. To overcome this limitation, we conducted the updated meta-analysis presented here, which includes 40 articles with 4152 COPD patients and 1639 healthy controls, to better evaluate the potential associations between TNF-α level and COPD. We found that COPD patients had significantly higher TNF-α levels than healthy controls. To explain this result, the following factors need to be taken into account. First, common genetic or constitutional differences between COPD patients and controls probably exist, and these differences predispose COPD patients to both systemic and pulmonary inflammation.54 Second, during inflammation processes, activated inflammatory cells and a variety of released inflammatory mediators, such as IL-8, IL-6, and TNF-α,can destroy lung structure and promote the inflammatory response of neutrophils.55 Third, the elevated blood inflammatory factors might be explained by several previously proposed mechanisms, such as local pulmonary inflammation due to smoking, oxidative stress, and tissue hypoxia.56
Due to the high heterogeneity, a subgroup analysis was then performed to minimize heterogeneity among the included studies. FEV1 is the most widely used parameter for diagnosis and evaluation of treatment effect in severe COPD, and the current COPD staging system is based mainly on this parameter.8 Therefore, we subclassified the patients into two subgroups: FEV1% >50% and FEV1% <50% to perform subgroup analysis. The results showed that TNF-α level was elevated in COPD patients with both FEV1% >50% and FEV1% <50% compared with controls. Smoking is known to be one of the main causes of COPD; thus, a subgroup analysis based on smoking status was performed. We found a significant association between TNF-α level and COPD in participants with smoking history, but we failed to find this association in nonsmoking participants. This result was consistent with that of a study by Mosran and colleagues, who showed that, compared with non-COPD smokers, smokers with COPD had markedly higher levels of TNF-α,57 suggesting that smoking can further increase TNF-α levels. In addition, the level of TNF-α was also associated with COPD status, region of study, and BMI of participants. However, no association was found between TNF-α level and COPD if the mean age was less than 60 years.
Although our results reached the same conclusion as many studies, some other studies report different results. Schmidt-loanas and colleagues suggested there were no significant differences in the correlation between TNF-α levels and COPD exacerbation.58 Monika and colleagues also did not observe any obvious difference in serum TNF-α levels between COPD patients and controls.42 This inconsistency among studies could be explained by differences in study design; different COPD status of enrolled patients across the included studies, since early-stage COPD are insensitive to TNF-α; and the inclusion of studies with different baseline characteristics.
Before we draw any firm conclusions, there are several limitations of this study that need to be considered. First, the significant heterogeneity in the present meta-analysis may limit generalization of the pooled results. Second, the methods for measuring TNF-α level were inconsistent. Third, since we limited the language of publication to English, we may have missed other related studies published in other languages. For example, the literature search for CNKI found several related studies in Chinese, but we excluded them from this study according to the exclusion criteria. Finally, the association between TNF-α level and patient quality of life was not evaluated due to the limited information available.
Conclusion
In this meta-analysis, a significant association between COPD and elevated TNF-α level was identified. These results encourage further exploration of the roles of TNF-α in COPD formation and development, and the potential of TNF-α as a novel biomarker and therapeutic target for COPD.
Supplemental Material
Supplemental material, Author_Response_1_1 for Association between tumor necrosis factor-α and chronic obstructive pulmonary disease: a systematic review and meta-analysis by Yang Yao, Jing Zhou, Xin Diao and Shengyu Wang in Therapeutic Advances in Respiratory Disease
Supplemental Material
Supplemental material, PRISMA_2009_checklist for Association between tumor necrosis factor-α and chronic obstructive pulmonary disease: a systematic review and meta-analysis by Yang Yao, Jing Zhou, Xin Diao and Shengyu Wang in Therapeutic Advances in Respiratory Disease
Supplemental Material
Supplemental material, Reviewer_1_v.1 for Association between tumor necrosis factor-α and chronic obstructive pulmonary disease: a systematic review and meta-analysis by Yang Yao, Jing Zhou, Xin Diao and Shengyu Wang in Therapeutic Advances in Respiratory Disease
Supplemental Material
Supplemental material, Supplementary_Table_S1 for Association between tumor necrosis factor-α and chronic obstructive pulmonary disease: a systematic review and meta-analysis by Yang Yao, Jing Zhou, Xin Diao and Shengyu Wang in Therapeutic Advances in Respiratory Disease
Footnotes
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and publication of this article: This study was supported by funds from the Respiratory Prevention and Treatment Center of Shaanxi Provincial Government (2016HXKF09), Shaanxi Province Key Program (2017SF-256).
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Yang Yao  https://orcid.org/0000-0002-5437-1558
https://orcid.org/0000-0002-5437-1558
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Yang Yao, Department of Pulmonary and Critical Care Medicine, The First Affiliated Hospital of Xi’an Medical University, Xi’an, Shaanxi, PR China.
Jing Zhou, Department of Pulmonary and Critical Care Medicine, The First Affiliated Hospital of Xi’an Medical University, Xi’an, Shaanxi, PR China.
Xin Diao, Department of Pulmonary and Critical Care Medicine, The First Affiliated Hospital of Xi’an Medical University, Xi’an, Shaanxi, PR China.
Shengyu Wang, Department of Pulmonary and Critical Care Medicine, The First Affiliated Hospital of Xi’an Medical University, Xi’an, Shaanxi, 710002, PR China.
References
- 1. Rabe KF, Watz H. Chronic obstructive pulmonary disease. Lancet 2017; 389: 1931–1940. [DOI] [PubMed] [Google Scholar]
- 2. Dransfield M, Stolz D, Kleinert S, et al. Towards eradication of chronic obstructive pulmonary disease: a lancet commission. Lancet 2019; 393: 1786–1788. [DOI] [PubMed] [Google Scholar]
- 3. Cai C, Xu CQ, Jin HL, et al. Combined effects of chronic obstructive pulmonary disease and depression on spatial memory in old rats. Chin Med Sci J 2018; 30: 260–266. [DOI] [PubMed] [Google Scholar]
- 4. Yazdani R, Marefati H, Shahesmaeili A, et al. Effect of aerobic exercises on serum levels of apolipoprotein A1 and apolipoprotein B, and their ratio in patients with chronic obstructive pulmonary disease. Tanaffos 2018; 17: 82–89. [PMC free article] [PubMed] [Google Scholar]
- 5. Emami Ardestani M, Zaerin O. Role of serum interleukin 6, albumin and C-reactive protein in COPD patients. Tanaffos 2015; 14: 134–140. [PMC free article] [PubMed] [Google Scholar]
- 6. Crisafulli E, Menendez R, Huerta A, et al. Systemic inflammatory pattern of patients with community-acquired pneumonia with and without COPD. Chest 2013; 143: 1009–1017. [DOI] [PubMed] [Google Scholar]
- 7. Karadag F, Karul AB, Cildag O, et al. Biomarkers of systemic inflammation in stable and exacerbation phases of COPD. Lung 2008; 186: 403–409. [DOI] [PubMed] [Google Scholar]
- 8. Franciosi LG, Page CP, Celli BR, et al. Markers of disease severity in chronic obstructive pulmonary disease. Pulm Pharmacol Ther 2006; 19: 189–199. [DOI] [PubMed] [Google Scholar]
- 9. Calikoglu M, Sahin G, Unlu A, et al. Leptin and TNF-alpha levels in patients with chronic obstructive pulmonary disease and their relationship to nutritional parameters. Respiration 2004; 71: 45–50. [DOI] [PubMed] [Google Scholar]
- 10. Agusti A, Edwards LD, Rennard SI, et al. Persistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotype. PLos One 2012; 7: e37483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oncel C, Baser S, Cam M, et al. Peripheral neuropathy in chronic obstructive pulmonary disease. COPD 2010; 7: 11–16. [DOI] [PubMed] [Google Scholar]
- 12. Kleniewska A, Walusiak-Skorupa J, Piotrowski W, et al. Comparison of biomarkers in serum and induced sputum of patients with occupational asthma and chronic obstructive pulmonary disease. J Occup Health 2016; 58: 333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rovina N, Papapetropoulos A, Kollintza A, et al. Vascular endothelial growth factor: an angiogenic factor reflecting airway inflammation in healthy smilers and in patients with bronchitis type of chronic obstructive pulmonary disease? Respir Res 2007; 8: 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gagonon P, Lemire BB, Dube A, et al. Preserved function and reduced angiogenesis potential of the quadriceps in patients with mild COPD. Respir Res 2014; 15: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ben Anes A, Ben Nasr H, Garrouch A, et al. Alterations in acetylcholinesterase and butyrylcholinesterase activities in chronic obstructive pulmonary disease: relationships with oxidative and inflammatory markers. Mol Cell Biochem 2018; 445: 1–11. [DOI] [PubMed] [Google Scholar]
- 16. Pérez-de-Llano L, Cosio BG. and CHACOS Study Group. Asthma-COPD overlap is not a homogeneous disorder: further supporting data. Respir Res 2017; 18: 183–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Foschino Barbaro MP, Carpagnano GE, Spanevello A, et al. Inflammation, oxidative stress and systemic effects in mild chronic obstructive pulmonary disease. Int J Immunopathol Pharmacol 2007; 20: 753–763. [DOI] [PubMed] [Google Scholar]
- 18. Barreiro E, Fermoselle C, Mateu-Jimenez M, et al. Oxidative stress and inflammation in the normal airways and blood of patients with lung cancer and COPD. Free Radic Biol Med 2013; 65: 859–871. [DOI] [PubMed] [Google Scholar]
- 19. Beeh KM, Beier J, Kornmann O, et al. Sputum matrix metalloproteinase-9, tissue inhibitor of metalloprotinease-1, and their molar ratio in patients with chronic obstructive pulmonary disease, idiopathic pulmonary fibrosis and healthy subjects. Respir Med 2003; 97: 634–639. [DOI] [PubMed] [Google Scholar]
- 20. Di Stefano A, Coccini T, Roda E, et al. Blood MCP-1 levels are increased in chronic obstructive pulmonary disease patients with prevalent emphysema. Int J Chron Obstruct Pulmon Dis 2018; 24: 1691–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Breyer MK, Rutten EP, Vernooy JH, et al. Gender differences in the adipose secretome system in chronic obstructive pulmonary disease (COPD): a pivotal role of leptin. Respir Med 2011; 105: 1046–1053. [DOI] [PubMed] [Google Scholar]
- 22. Zhang X, Li D, Wang H, et al. Gender difference in plasma fatty-acid-binding protein 4 levels in patients with chronic obstructive pulmonary disease. Biosci Rep 2016; 36: e00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dima E, Rovina N, Gerassimou C, et al. Pulmonary function tests, sputum induction, and bronchial provocation tests: diagnostic tools in the challenge of distinguishing asthma and COPD phenotypes in clinical practice. Int J Chron Obstruct Pulmon Dis 2010; 7: 287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kawayama T, Kinoshita T, Matsunaga K, et al. Responsiveness of blood and sputum inflammatory cells in Japanese COPD patients, non-COPD smoking controls, and non-COPD nonsmoking controls. Int J Chron Obstruct Pulmon Dis 2016; 10: 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gaki E, Kontogianni K, Papaioannou AI, et al. Associations between BODE index and systemic inflammatory biomarkers in COPD. COPD 2011; 8: 408–413. [DOI] [PubMed] [Google Scholar]
- 26. Godoy I, Campana AO, Geraldo RR, et al. Cytokines and dietary energy restriction in stable chronic obstructive pulmonary disease patients. Eur Respir J 2003; 22: 920–925. [DOI] [PubMed] [Google Scholar]
- 27. Hacievliyaqil SS, Mutlu LC, Temel I, et al. Airway inflammatory markers in chronic obstructive pulmonary disease patients and healthy smokers. Niger J Clin Pract 2013; 16: 76–81. [DOI] [PubMed] [Google Scholar]
- 28. Huertas A, Testa U, Riccioni R, et al. Bone marrow-derived progenitors are greatly reduced in patients with severe COPD and low-BMI. Respir Physiol Neurobiol 2010; 31: 23–31. [DOI] [PubMed] [Google Scholar]
- 29. Ju CR, Chen RC. Serum myostatin levels and skeletal muscle wasting in chronic obstructive pulmonary disease. Respir Med 2012; 106: 102–108. [DOI] [PubMed] [Google Scholar]
- 30. Karadag F, Ozcan H, Karul AB, et al. Correlates of erectile dysfunction in moderate-to-severe chronic obstructive pulmonary disease patients. Respirology 2007; 12: 248–253. [DOI] [PubMed] [Google Scholar]
- 31. Karadag F, Kirdar S, Karul AB, et al. The value of C-reactive protein as a marker of systemic inflammation in stable chronic obstructive pulmonary disease. Eur J Intern Med 2008; 19: 104–108. [DOI] [PubMed] [Google Scholar]
- 32. Shin KC, Chung JH, Lee KH. Effects of TNF-αlpha and leptin on weight loss in patients with stable chronic obstructive pulmonary disease. Korean J Intern Med 2007; 22: 249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kythreotis P, Kokkini A, Avgeropoulou S, et al. Plasma leptin and insulin-like growth factor I levels during acute exacerbations of chronic obstructive pulmonary disease. BMC Pulm Med 2009; 5: 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu X, Ji Y, Chen J, et al. Circulating visfatin in chronic obstructive pulmonary disease. Nutrition 2009; 25: 373–378. [DOI] [PubMed] [Google Scholar]
- 35. Huang AX, Lu LW, Liu WJ, et al. Plasma Inflammatory Cytokine IL-4, IL-8, IL-10, and TNF-α levels correlate with pulmonary function in patients with asthma-chronic obstructive pulmonary disease (COPD) overlap syndrome. Med Sci Monit 2016; 9: 2800–2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Moermans C, Heinen V, Nguyen M, et al. Local and systemic cellular inflammation and cytokine release in chronic obstructive pulmonary disease. Cytokine 2011; 56: 298–304. [DOI] [PubMed] [Google Scholar]
- 37. Piehl-Aulin K, Jones I, Lindvall B, et al. Increased serum inflammatory markers in the absence of clinical and skeletal muscle inflammation in patients with chronic obstructive pulmonary disease. Respiration 2009; 78: 191–196. [DOI] [PubMed] [Google Scholar]
- 38. Tan C, Xuan L, Cao S, et al. Decreased histone deacetylase 2 (HDAC2) in peripheral blood monocytes (PBMCs) of COPD patients. PLoS One 2016; 11: e0147380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Guiot J, Henket M, Corhay JL, et al. Sputum biomarkers in IPF: evidence for raised gene expression and protein level of IGFBP-2, IL-8 and MMP-7. PLoS One 2017; 12: e0171344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sarioglu N, Hismiogullari AA, Bilen C, et al. Is the COPD assessment test (CAT) effective in demonstrating the systemic inflammation and other components in COPD? Rev Port Pneumol 2016; 22: 11–17. [DOI] [PubMed] [Google Scholar]
- 41. Uzum AK, Aydin MM, Tutuncu Y, et al. Serum ghrelin and adiponectin levels are increased but serum leptin level is unchanged in low weight chronic obstructive pulmonary disease patients. Eur J Intern Med 2014; 25: 363–369. [DOI] [PubMed] [Google Scholar]
- 42. Kosacka M, Porebska I, Korzeniewska A, et al. Serum levels of apoptosis-related markers (sFasL, TNF-α, p53 and bcl-2) in COPD patients. Pneumonol Alergol Pol 2016; 84: 11–15. [DOI] [PubMed] [Google Scholar]
- 43. Cheng SL, Wang HC, Yu CJ, et al. Increased expression of placenta growth factor in COPD. Thorax 2008; 63: 500–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Valipour A, Schreder M, Wolzt M, et al. Circulating vascular endothelial growth factor and systemic inflammatory markers in patients with stable and exacerbated chronic obstructive pulmonary disease. Clin Sci (Lond) 2008; 115: 225–232. [DOI] [PubMed] [Google Scholar]
- 45. Zhang M, Li Q, Zhang XY, et al. Relevance of lower airway bacterial colonization, airway inflammation, and pulmonary function in the stable stage of chronic obstructive pulmonary disease. Eur J Clin Microbiol Infec Dis 2010; 29: 1487–1493. [DOI] [PubMed] [Google Scholar]
- 46. Soler N, Ewig S, Torres A, et al. Airway inflammation and bronchial microbial patterns in patients with stable chronic obstructive pulmonary disease. Eur Respir J 1999; 14: 1015–1022. [DOI] [PubMed] [Google Scholar]
- 47. Keatings VM, Collins PD, Scott DM, et al. Differences in interleukin-8 and tumor necrosis factor-alpha in induced sputum from patients with chronic obstructive pulmonary disease or asthma. Am J Respir Crit Care Med 1996; 153: 530–534. [DOI] [PubMed] [Google Scholar]
- 48. De Godoy I, Donahoe M, Calhoun WJ, et al. Elevated TNF-alpha production by peripheral blood monocytes of weight-losing COPD patients. Am J Respir Crit Care Med 1996; 153: 633–637. [DOI] [PubMed] [Google Scholar]
- 49. Ostojic J, Pintaric H. Chronic obstructive pulmonary disease and heart failure: closer than close. Acta Clin Croat 2017; 56: 269–276. [DOI] [PubMed] [Google Scholar]
- 50. Chen H, Zhang L, He Z, et al. Vitamin D binding protein gene polymorphisms and chronic obstructive pulmonary disease: a meta-analysis. J Thorac Dis 2015; 7: 1423–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jiang DH, Wang X, Liu LS, et al. The effect of ventilator mask atomization inhalation of ipratropium bromide and budesonide suspension liquid in the treatment of COPD in acute exacerbation period on circulating levels of inflammation and prognosis. Eur Rev Med Pharmacol Sci 2017; 21: 5211–5216. [DOI] [PubMed] [Google Scholar]
- 52. Gan WQ, Man SFP, Senthilselvan A, et al. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a metaanalysis. Thorax 2004; 59: 547–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Su B, Liu T, Fan H, et al. Inflammatory markers and the risk of chronic obstructive pulmonary disease: a systematic review and meta-analysis. PLos One 2016; 11: e0150586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pauwels RA, Buist AS, Calverley PM, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO global initiative for chronic obstructive lung disease (GOLD) workshop summary. Am J Respir Crit Care Med 2001; 163: 1256–1276. [DOI] [PubMed] [Google Scholar]
- 55. Emami Ardestani M, Zaerin O. Role of serum interleukin 6, albumin and C-reactive protein in COPD patients. Tanaffos 2015; 14: 134–140. [PMC free article] [PubMed] [Google Scholar]
- 56. Vaguliene N, Zemaitis M, Lavinskiene S, et al. Local and systemic inflammation in chronic obstructive pulmonarydisease. BMC Immunol 2013; 14: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mosrane Y, Bougrida M, Alloui A, et al. Systemic inflammatory profile of smokers with and without COPD. Rev Pneumol Clin 2017; 73: 188–198. [DOI] [PubMed] [Google Scholar]
- 58. Schmidt-loanas M, Pletz MW, de Roux A, et al. Apoptosis of peripheral blood neutrophils in COPD exacerbation does not correlate with serum cytokines. Respir Med 2006; 100: 639–647. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Author_Response_1_1 for Association between tumor necrosis factor-α and chronic obstructive pulmonary disease: a systematic review and meta-analysis by Yang Yao, Jing Zhou, Xin Diao and Shengyu Wang in Therapeutic Advances in Respiratory Disease
Supplemental material, PRISMA_2009_checklist for Association between tumor necrosis factor-α and chronic obstructive pulmonary disease: a systematic review and meta-analysis by Yang Yao, Jing Zhou, Xin Diao and Shengyu Wang in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1 for Association between tumor necrosis factor-α and chronic obstructive pulmonary disease: a systematic review and meta-analysis by Yang Yao, Jing Zhou, Xin Diao and Shengyu Wang in Therapeutic Advances in Respiratory Disease
Supplemental material, Supplementary_Table_S1 for Association between tumor necrosis factor-α and chronic obstructive pulmonary disease: a systematic review and meta-analysis by Yang Yao, Jing Zhou, Xin Diao and Shengyu Wang in Therapeutic Advances in Respiratory Disease