Abstract
Management of pediatric pulmonary hypertension associated with congenital heart disease (PHT-CHD) is challenging. Some patients have persistently elevated pulmonary artery pressure (PAP) after cardiac surgery, an undesired condition that is difficult to predict. We investigated the value of clinical, hemodynamic, and histopathological data in predicting the outcome in a prospective cohort. Patients with PHT-CHD received sildenafil orally pre- and postoperatively for six months and then were subjected to a catheter study. Thirty-three patients were enrolled (age range = 4.6–37.0 months). Pulmonary vascular resistance (PVR) was 4.9 (range = 3.9–7.2) Wood units × m2 (median with IQR). Twenty-two patients had a ≥ 20% decrease in PVR and pulmonary-to-systemic vascular resistance ratio (PVR/SVR) in response to inhaled nitric oxide (NO). The response was directly related to the degree of medial hypertrophy of pulmonary arterioles (P < 0.05) (morphometric analysis, intraoperative lung biopsy). Subsequently, five of the non-responders had a ≥ 30% increase in pulmonary blood flow in response to sildenafil (3.0 [2.0–4.0] mg/kg/day). Six months after surgery, PAP and PVR were significantly lower (P < 0.001 vs. baseline), even in seven patients with Heath-Edwards grade III/IV pulmonary vascular lesions (P = 0.018), but still abnormal in 12 individuals (>25 mmHg and >3.0 U × m2, respectively). A preoperative PVR/SVR of ≥24% during NO inhalation and a wall thickness of arteries accompanying respiratory bronchioli of ≥4.7 (Z score) were identified, respectively, as risk and protection factors for abnormal postoperative hemodynamics (hazard ratio [95% CI] = 1.09 [1.01–1.18], P = 0.036; and 0.69 [0.49–0.98], P = 0.040, respectively). Thus, in PHT-CHD patients receiving oral sildenafil pre- and post-surgical repair of cardiac lesions, mid-term postoperative outcome is predictable to some extent.
Keywords: congenital heart disease, pulmonary hypertension, pediatric cardiac surgery, nitric oxide, sildenafil
Introduction
Pulmonary vascular disease may limit the successful treatment of congenital cardiac defects in pediatric patients. Pulmonary vascular changes pose a risk factor for immediate postoperative hemodynamic disturbances and residual pulmonary arterial hypertension (PAH) late after operation.1 If treatment is considered in a very timely fashion (early in life), patients at a high risk of persistent PAH make up to small percentage of individuals undergoing surgery. Nevertheless, severe postoperative pulmonary hypertensive crises are still associated with high mortality rates despite progress in terms of management.2–4 Furthermore, persistent postoperative pulmonary hypertension (PH) is associated with poorer survival estimates compared to other etiologies of pulmonary vascular disease in the pediatric population.5
There are no specific guidelines for the pre- and postoperative management of the pediatric patient at high risk for persistent PAH after cardiac surgery.6 Currently, most pediatric cardiologists and surgeons would not consider surgery based purely on a positive response to vasodilators during cardiac catheterization. The acute pulmonary vasodilator test is not standardized in the pediatric population, nor does the response clearly correlate with immediate postoperative outcomes in congenital heart disease.7–9 In fact, a “positive test” would actually unmask the potential for severe vasoconstriction in the early postoperative course. Thus, characterization of high-risk and low-risk patients and definition of operability are problems that have not yet been completely solved.
There have been attempts to prevent persistent PH in patients with congenital heart disease by using pulmonary vasodilators both in the pre- and postoperative periods. Following surgical correction, intravenous, inhaled, and oral drugs have been employed either singly or in combination with inhaled nitric oxide (NO) to prevent or stabilize pulmonary vascular reactivity.10–12 Benefits have been demonstrated in terms of facilitated weaning from NO and reduced time of mechanical ventilation and intensive care unit stay.13 Preoperatively, the use of vasodilator drugs has been proposed as an attempt to improve early postoperative outcomes.14 Alternatively, vasodilator agents have been administered to patients initially deemed inoperable as an attempt to reverse vascular changes. However, this strategy has often proven unsuccessful and there is only anecdotal evidence to support it.7,15
The present report is essentially descriptive. It contains data of a prospective cohort of young pediatric patients under consideration for surgical repair of congenital cardiac shunts, presenting with clinical features suggestive of moderate to severe pulmonary vascular abnormalities. In view of the potential risk of relevant hemodynamic complications, patients were managed pre- and postoperatively with the phosphodiesterase type 5 inhibitor sildenafil. Hemodynamic and histopathological parameters were examined as an attempt to describe the basic characteristics of this population and were tested for a possible association with outcomes. The study was not focused on immediate postoperative outcomes. The main objective of the study was to analyze factors with potential impact on pulmonary hemodynamics six months after surgery, in patients with PH who received oral sildenafil before and after repair of cardiac lesions. The study protocol was designed to include preoperative cardiac catheterization with acute pulmonary vasodilator testing, intraoperative lung biopsy with morphometric analysis of pulmonary arteries, and a second catheter study six months after surgery.
Patients and methods
Patients were from the Heart Institute (InCor), University of São Paulo School of Medicine, São Paulo, Brazil. They entered the study from 2012 to 2016. Screening criteria included: age ≥ 3 years; presence of unrestrictive cardiac communications; biventricular physiology; and clinical features indicative of the presence of PH with no signs of advanced pulmonary vascular disease (predominant right-to-left shunting). For patient inclusion as “high-risk” in terms of persistence of PH after surgery, at least three of the following criteria were necessary: (1) absence of clinical features indicative of pulmonary overcirculation and congestion; (2) bidirectional flow across the cardiac communication; (3) sustained or intermittent systemic oxygen saturation < 90%; (4) presence of Down syndrome; (5) age > 18 months. Patients with these features generally have post-tricuspid defects (ventricular septal defect, patent ductus arteriosus, aorto-pulmonary window) eventually associated with a pre-tricuspid communication, atrioventricular septal defect (A-V canal), or a conotruncal anomaly with biventricular physiology. Patients defined as “high-risk” according to the mentioned criteria correspond to 6–7% of all individuals with cardiac communications referred to our institution for surgical treatment. As a reference, we also included follow-up data of patients with unrestrictive cardiac septal defects not considered to be “high risk” who underwent surgery at the Heart Institute during the same period (2012–2016). Neonates, patients under intensive care, and those with any respiratory diseases or ventilator disturbances potentially affecting pulmonary hemodynamics were not included. Patients with extracardiac syndromes other than Down syndrome were not included either. A written informed consent from the parents was necessary for inclusion. The study protocol was approved by the Institutional Scientific and Ethics Committee (CAPPesq #0502/11) and registered as ClinicalTrials NCT01548950.
Non-invasive and invasive evaluation
Non-invasive evaluation consisted of detailed physical examination (including intensity of dyspnea and oxygen saturation in upper and lower extremities, presence or absence of a dynamic precordium, characteristics of second and third heart sounds and diastolic murmurs), chest radiography (heart size and pattern of pulmonary circulation), and echocardiography. In addition to providing anatomic data, transthoracic echocardiography was used to estimate pulmonary-to-systemic blood flow ratio (Qp/Qs) and the velocity-time integral of blood flow in pulmonary veins (VTIPV).16,17 Cardiac catheterization was performed under general anesthesia and mechanical ventilation. Pulmonary and systemic blood flow were calculated by the Fick method and used in the assessment of pulmonary and systemic vascular resistance (PVR and SVR, Wood units × m2). Measurements were made at baseline (oxygen concentration of 21–30%) and at the end of 10-min NO inhalation (40 ppm). Baseline PVR and PVR/SVR, the magnitude of pulmonary vasodilation on NO (percent change from baseline), and final PVR and PVR/SVR (levels obtained at 10 min on NO) were recorded. A ≥ 20% decrease in PVR and PVR/SVR was necessary for the response to be considered as effective. Final levels of < 6.0 U × m2 and < 0.3, respectively, were taken into consideration in patient selection for surgical treatment, although not exclusively.8
Sildenafil administration
Following cardiac catheterization, oral sildenafil was started at a dose of 1.0 mg/kg/day (every 6 h) and increased progressively to 5.0 mg/kg/day until a favorable or unfavorable response ensued. A favorable response was judged by an elevation in peripheral oxygen saturation and/or Qp/Qs by echocardiography. An unfavorable response was judged by absence of these effects at a maximum tolerated dose of 5.0 mg/kg/day; we noted a decrease of > 10% in mean systemic blood pressures or other clinically relevant adverse effects as indicating the need for dose reduction or discontinuation. Uptitration in increments of 0.5 mg/kg/day of sildenafil were carried out every 2–3 days. Escalation to higher doses (e.g. 4.0–5.0 mg/kg/day) required at least one week of observation. For patients using the highest tolerated doses to be considered non-responders (no increase in dyspnea, oxygen saturation, or Qp/Qs), at least four weeks of observation were considered necessary. Although we did not establish any specific parameter cut-off to define responders and non-responders, we attempted to correlate changes in Qp/Qs with the magnitude of the response to NO during cardiac catheterization. Ophthalmologic evaluation including fundoscopy was performed in all patients before initiation of sildenafil. Further evaluations during the course of sildenafil administration were planned on an individual basis if necessary.
Perioperative and postoperative assessments
Decision to operate was based on complete diagnostic evaluation including non-invasive and invasive procedures. In patients with PVR > 6.0 U × m2, additional data were taken into consideration to judge whether or not surgery should be offered. The following observations influenced positively the decision to offer surgical treatment: (1) a ≥ 20% decrease in PVR and PVR/SVR during NO inhalation, with respective final levels of < 6.0 U × m2 and < 0.3; (2) development of hyperdynamic circulation during sildenafil administration, with pulmonary congestion (dyspnea and chest radiographic changes) and need for initiation or intensification of diuretic therapy; (3) changes in echocardiographic flow parameters (for example, a 20% increase in Qp/Qs and/or VTIPV while on sildenafil); and (4) increase in peripheral oxygen saturation during sildenafil use (e.g. from < 93% to > 95%). Patient age and the type of cardiac anomaly were taken into consideration as well. For example, patients with conotruncal anomalies (truncus arteriosus or transposition of the great arteries) referred for treatment aged > 2 years would hardly ever be selected for surgery if they did not have congestive heart failure and failure to thrive in their clinical history.
In patients who were assigned to surgery, sildenafil was restarted via an enteral tube 4 h after weaning from cardiopulmonary bypass and given orally after discharge from the intensive care unit. Pulmonary hypertensive crises (usually life-threatening events) were defined as sustained systemic or suprasystemic elevations of PAP associated with hypoxemia (peripheral oxygen saturation < 90%), with a > 20% decrease in systemic pressure and/or bradycardia. NO and extracorporeal membrane oxygenation was available for all patients eventually requiring their use. After hospital discharge, patients were seen on a monthly basis and maintained on sildenafil therapy for at least six months. At that time, complete cardiac catheterization data were obtained (end of the study). The results were taken into consideration for decision about drug discontinuation. Normalization of pulmonary hemodynamics was defined by mean PAP (mPAP) ≤ 25 mmHg and PVR ≤ 3.0 U × m2.
Assessment of pulmonary vascular abnormalities
Patients were scheduled to have a lung biopsy specimen collected intraoperatively on an individual basis if the surgeon considered the procedure to be low risk. Specimens were collected with airways distended, fixed in buffered formalin, and subjected to routine histological processing. Four-micrometer-thick sections were obtained and stained with hematoxylin and eosin (H&E) and Miller’s elastic stain. Vascular abnormalities were graded according to the Heath–Edwards classification.18 Grades I and II correspond to medial hypertrophy of pulmonary arteries and intimal non-occlusive proliferation, respectively. Grades III–VI correspond to advanced vascular abnormalities, including occlusive intimal proliferation, plexiform, and dilatation lesions. For morphometric assessment of medial layer hypertrophy, arteries were landmarked by reference to the type of airway they accompanied: preacinar terminal bronchiolus; respiratory bronchiolus; and alveolar duct. The external diameter of an artery was measured between the external elastic laminae across the shorter axis of the vessel. Wall thickness was measured from the external to internal elastic lamina and computed as a percentage of the external diameter as follows:
In each patient, a mean value was calculated for each artery category and the final result was obtained after a Z-score transformation. For this purpose, normal values for age were obtained from Haworth and Hislop.19
Preoperative and postoperative assessments in patients not considered to be “high risk”
These patients were included in the study as a reference group and assigned to surgery on the basis of non-invasive evaluation only. They were not subjected to cardiac catheterization or intraoperative lung biopsy and they were not treated with sildenafil preoperatively or postoperatively. Echocardiographic flow parameters were recorded at baseline for comparison with the main group of patients. At six months postoperatively, systolic PAP was estimated using the peak velocity of tricuspid regurgitant flow and the groups were compared again.
Summary of the study protocol
Having met the inclusion criteria, all patients were subjected to non-invasive evaluation of blood flow patterns (echocardiography). Patients in the main group (i.e. high-risk individuals in terms of PH) were further evaluated by cardiac catheterization. All of them received oral sildenafil subsequently and the response was analyzed non-invasively. Decision to operate was based on non-invasive and invasive evaluation as well as response to sildenafil. Patients in the reference group (i.e. those not considered to be “high risk”) were assigned to surgery based on non-invasive evaluation. Whenever possible, intraoperative lung biopsy was performed in high-risk patients. This group was kept on sildenafil postoperatively, while patients in the reference group did not receive pulmonary vasodilators at any time. At six months postoperatively, all patients were re-evaluated non-invasively. All individuals in the main group were subjected to a second catheterization study and outcome predictors were determined.
Statistical analysis
Data analysis was carried out using the IBM-SPSS statistical software (version 25; Armonk, NY, USA). For the numerical variables, the results are presented as individual values and as medians with interquartile ranges. For the categorical variables, the results are presented as number of cases and percentage. Differences between individuals were tested using the Mann–Whitney test. Differences between conditions (e.g. measurements performed on NO vs. baseline, during sildenafil use vs. baseline, and at six months postoperatively vs. baseline) were tested using the Wilcoxon test. Regression analysis was used to test for possible associations between cardiac catheterization data and histopathological findings. Univariate and multivariate logistic regression analyses were used for identifying possible predictors of hemodynamic outcome (normal vs. abnormal PAP and PVR) six months after surgery. The corresponding hazard ratios (HR) with 95% confidence intervals (CI) are provided. Once a predictor was identified, a receiver operating characteristic (ROC) curve was constructed and the area under the curve was expressed as a 95% CI. In all assessments, 0.05 was considered as the level of significance.
Results
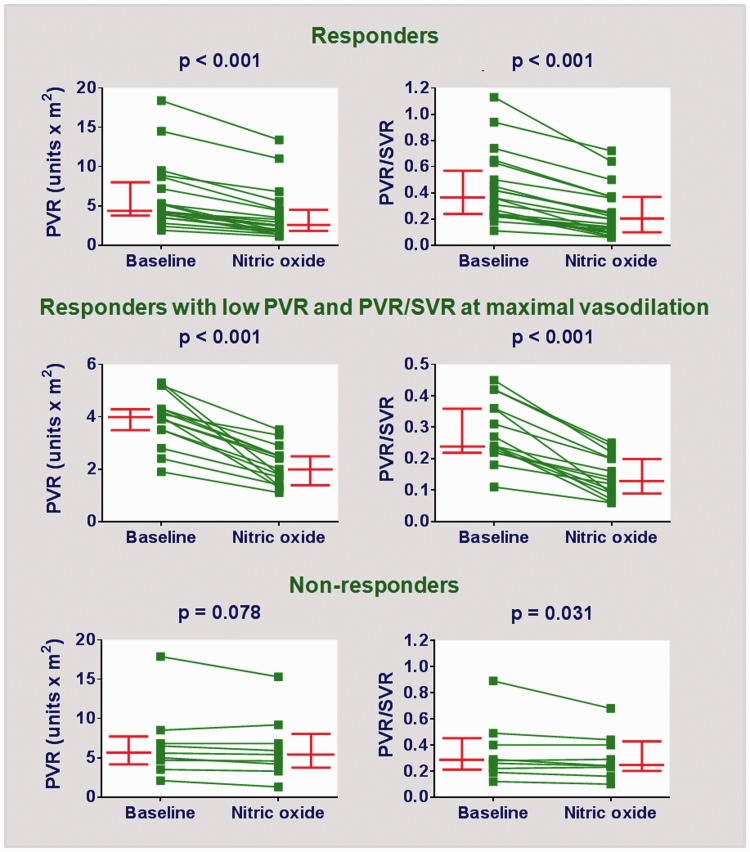
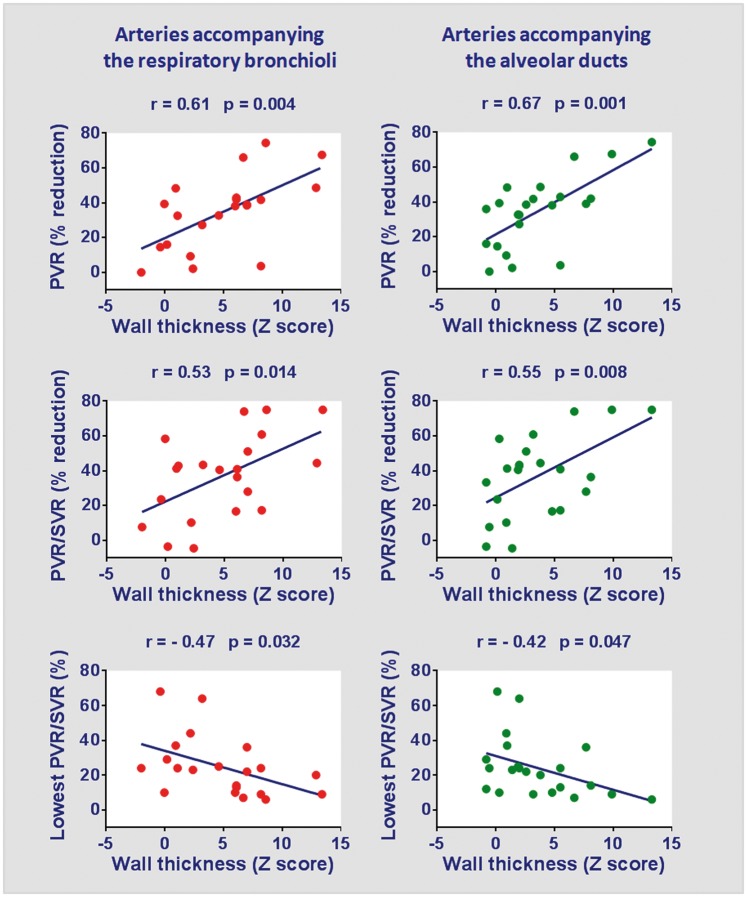
Thirty-three patients were enrolled with ages in the range of 4.6–37.0 months. Demographic and diagnostic data are depicted in Table 1. The median values of variables in the table indicate that several patients had an estimated Qp/Qs of <2.0 (echocardiography), a peripheral oxygen saturation of <93%, and baseline PVR > 4.7 U × m2. These patients had an age range similar to the ones in the reference group (n = 32) (11.0 [7.4–19.7] months and 8.9 [5.4–12.7] months, respectively; median with interquartile range, P = 0.138). However, they had lower pulmonary blood flow (Qp/Qs, 2.0 [1.4–2.2] and 2.8 [2.4–3.4], respectively; P < 0.001) and lower oxygen saturation (93% [90–95%] and 97% [94–98%] respectively; P = 0.002), compatible with heightened PVR. The percentage of individuals with Down syndrome was similar (73% and 66%, respectively; P = 0.725). For patients in the main group, individual hemodynamic assessments at baseline and during NO inhalation are shown in Fig. 1. It is fair to say that baseline PVR was probably reduced in some cases, due to the effects of general anesthesia. For example, 10 patients had baseline PVR < 4.0 U × m2 (Fig. 1). Seven of them had either baseline (i.e. bedside) oxygen saturation < 93% or Qp/Qs (echocardiography) < 1.5, suggesting that PVR levels were probably higher in physiological conditions. For the entire group, there was a significant decrease in PVR (4.9 [3.9–7.2] U × m2 to 3.3 [1.8–5.6] U × m2, P < 0.001) and PVR/SVR (0.30 [0.23–0.49] to 0.23 [0.12–0.37], P < 0.001) in response to NO. Twenty-two patients had a ≥ 20% decrease in both parameters, but only 15 of those had final levels of < 6.0 U × m2 and < 0.3, respectively (Fig. 1). Subsequently, lung biopsy specimens were collected intraoperatively in 24 patients; the material was adequate for morphometric analysis of pulmonary vessels in 22 patients. Medial hypertrophy was observed in preacinar and intra-acinar vessels. For the arteries accompanying the terminal bronchioli, respiratory bronchioli, and alveolar ducts, the wall thickness (Z score) was 8.1 (4.8–11.4), 6.0 (1.0–7.6), and 2.3 (0.8–5.8), respectively. In these patients, the magnitude of pulmonary vasodilation in response to inhaled NO (preoperative cardiac catheterization) was directly related to the degree of medial hypertrophy of intra-acinar arteries. This is shown in Fig. 2.
Table 1.
Demographic and diagnostic data in the main patient group.
| All patients (n = 33) | Patients selected for surgery (n = 30) | Patients not selected for surgery (n = 3) | |
|---|---|---|---|
| Age (months) | 11.0 (7.4–19.7) | 9.7 (6.9–16.8) | 31.8 (29.8–35.0) |
| Gender (M:F) | 13:20 | 12:18 | 1:2 |
| Down syndrome, n (%) | 24 (73) | 23 (77) | 1 (33) |
| Weight (kg) | 6.4 (5.5–8.4) | 6.2 (5.4–7.9) | 10.8 (9.7–12.5) |
| Peripheral oxygen saturation (%) | 93 (90–95) | 93 (90–95) | 93 (89–98) |
| Echocardiography | |||
| Type/localization of cardiac anomalies | |||
| Pre-tricuspid defects (n (%)) | 1 (3) | 0 (0) | 1 (33) |
| Post-tricuspid defects except for atrioventricular canal (n (%)) | 14 (42) | 13 (43) | 1 (33) |
| Atrioventricular canal (n (%)) | 17 (52) | 17 (57) | 0 (0) |
| Conotruncal defects (n (%)) | 1 (3) | 0 (0) | 1 (33) |
| Pulmonary-to-systemic blood flow ratio (Qp/Qs)(*) | 2.0 (1.4–2.2) | 2.0 (1.4–2.3) | 1.7 (1.1–2.2) |
| Velocity-time integral of blood flow in pulmonary veins (VTIPV, cm)( * ) | 21.9 (17.3–25.1) | 21.9 (17.4–24.8) | 19.0 (15.0–27.0) |
| Cardiac catheterization | |||
| Mean pulmonary arterial pressure (mmHg) | 45 (41–61) | 44 (40–57) | 62 (57–76) |
| Mean systemic arterial pressure (mmHg) | 56 (53–70) | 56 (52–66) | 73 (56–79) |
| Pulmonary-to-systemic blood flow ratio | 2.1 (1.5–2.9) | 2.4 (1.6–3.0) | 1.1 (0.9–2.0) |
| Pulmonary vascular resistance index (Wood units × m2) | 4.7 (3.7–7.5) | 4.5 (3.5–6.9) | 8.9 (4.6–14.5) |
| Pulmonary-to-systemic vascular resistance ratio | 0.31 (0.23–0.47) | 0.29 (0.22–0.43) | 0.74 (0.40–0.94) |
Numeric variables are expressed as medians (interquartile ranges; columns All patients, n = 33 and Patients selected for surgery, n = 30) or medians (range; column Patients not selected for surgery, n = 3).
Fig. 1.
Cardiac catheterization data showing PVR and PVR/SVR at baseline and 10 min of NO inhalation (40 ppm). The upper panels correspond to all patients meeting the response criteria: ≥ 20% decrease in PVR and PVR/SVR from baseline (n = 22). The middle panels represent the responders for whom final levels of PVR and PVR/SVR (at 10 min of NO administration) were < 6.0 Wood units × m2 and 0.30, respectively (n = 15). The lower panels correspond to patients who did not meet the response criteria (n = 11). Bars represent median values with interquartile ranges.
Fig. 2.
Relationship between medial hypertrophy of intra-acinar pulmonary arteries (intraoperative lung biopsy) and response to inhaled NO during preoperative cardiac catheterization. The mean wall thickness of arteries present in the biopsy specimen was calculated and expressed as Z score using normal values for age according to Haworth and Hislop.19 In the upper and middle panels, changes in PVR and PVR/SVR as a result of NO administration are expressed as percent reduction from baseline. The lower panels show absolute values for PVR/SVR registered at 10 min of NO inhalation.
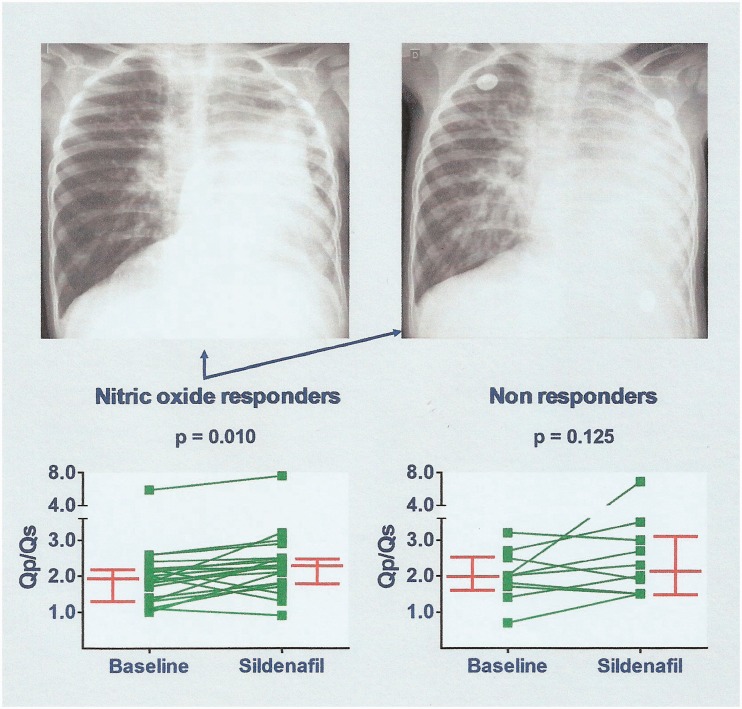
Sildenafil was administered preoperatively for an average length of time of 27 days. The maximum dose achieved per patient was 3.0 (2.0–4.0) mg/kg/day. No relevant side effects were observed during sildenafil administration. There was no need for drug discontinuation in any patients. In response to sildenafil, most patients displayed dyspnea and chest radiographic features indicating pulmonary overcirculation (Fig. 3) and were promptly assigned to surgery. For the entire group, sildenafil administration resulted in a significant increase in Qp/Qs assessed by echocardiography (2.0 [1.4–2.2] to 2.3 [1.8–2.8], P = 0.002) associated with an increase in peripheral oxygen saturation from baseline (93% [90–95%] to 95% [93–97%], P < 0.001). The majority of patients with a ≥ 20% decrease in PVR and PVR/SVR during NO inhalation (cardiac catheterization) had an increase in Qp/Qs during sildenafil use (P = 0.010; Fig. 3). It is noteworthy that five of the patients previously considered as non-responders had a ≥ 30% increase in Qp/Qs while on oral sildenafil (Fig. 3).
Fig. 3.
Effects of sildenafil administration. On the top, representative chest radiographs before (left) and at 12 days of sildenafil use (right). This 18-month-old patient with a ventricular septal defect and left lung hypoplasia had a baseline PVR of 8.7 Wood units × m2, decreasing to 4.5 units × m2 during NO inhalation (cardiac catheterization). Pulmonary vascular markings and cardiac size increased during sildenafil administration. The lower panels show changes in pulmonary-to-systemic blood flow ratio (Qp/Qs by echocardiography) as a result of sildenafil use. Increased Qp/Qs was seen for some patients who did not respond to NO. Bars represent median values with interquartile ranges.
Thirty patients in the main group were assigned to surgical repair of cardiac anomalies. Surgical treatment was not offered to three patients. They were older at presentation (29.8–35.0 months of age), had no history of congestive heart failure or failure to thrive, and no clinical evidence of pulmonary overcirculation. Two of them had an important elevation of PVR at baseline (8.9 and 14.5 U × m2) with inadequate levels during NO inhalation (6.8 and 11.0 U × m2, respectively. Furthermore, they had no relevant changes in clinical and echocardiographic parameters during sildenafil administration. Demographic and diagnostic data of patients selected for surgical treatment and those for whom surgery was not recommended are depicted in Table 1. Analysis of lung biopsy specimens collected intraoperatively confirmed the presence of moderate to severe pulmonary vasculopathy in the study population. Grade III or grade IV vascular lesions were seen in 7/24 specimens analyzed. There were three immediate postoperative deaths, which were related to severe pulmonary hypertensive crises in two cases. Another patient with severe crises had full recovery after several days on extracorporeal membrane oxygenation. All 32 patients in the reference group underwent surgical repair of cardiac lesions. There was one death in this group due to sepsis and other complications unrelated to PH.
In the main group of patients, those with fatal outcome (numbers 6, 16, and 26 in the series) and the one who required circulatory assistance to support life (number 27) did not have any preoperative features suggestive of more advanced disease. Rather, they have features pointing towards a reactive pulmonary circulation. All of them had mildly elevated PVR (3.5, 5.0, 5.2, and 4.3 U × m2, respectively). Three of them had peripheral oxygen saturation ≤93% suggesting some degree of right-to-left shunting. However, all four subjects had low PVR/SVR while on NO (0.20, 0.29, 0.06, and 0.14, respectively). Furthermore, three patients had increased wall thickness of small pulmonary arteries as judged by the results of morphometric analysis. The respective Z scores were: 15.1, 1.6, 5.5, and 18.3 (arteries accompanying the terminal bronchioli); 12.9, 0.2, 8.6, and 6.1 (arteries accompanying the respiratory bronchioli); and 3.8, −0.8, 13.3, and 8.1 (arteries accompanying the alveolar ducts). Qualitative analysis of pulmonary arteries showed that three patients had only mild to moderate abnormalities (grade II, grade I, grade II, and grade IV vasculopathy, respectively).
Twenty-seven patients in the main group were discharged from hospital on sildenafil therapy and re-evaluated at six months postoperatively. In the reference group, 31 patients not receiving sildenafil at any time were re-evaluated at six months as well. Peripheral oxygen saturation became normal in the main group, and not different from the reference group (97% [96–98%] and 98% [96–98%], respectively; P = 0.198). Systolic PAP (echocardiography) remained abnormal in some patients in the main group, but not much elevated compared to the reference group (41 [30–51] mmHg and 34 [29–40] mmHg, respectively; P = 0.077).
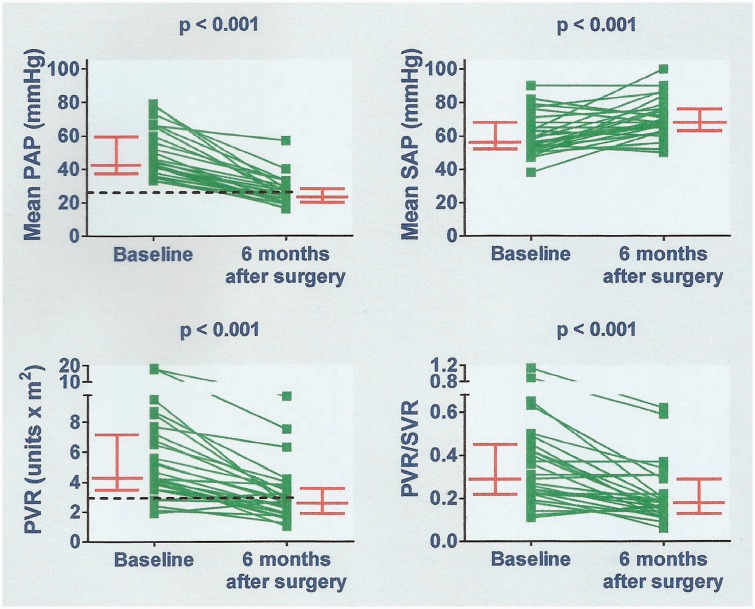
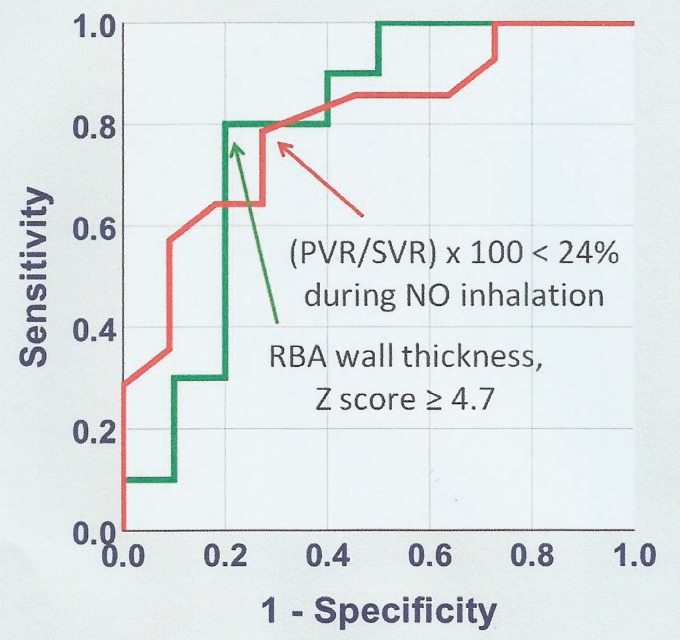
Cardiac catheterization data obtained at six months of follow-up (main group) are shown in Fig. 4. There was a significant decrease in PAP and PVR in comparison with baseline levels. The systemic PAP increased mildly. Improvement of pulmonary hemodynamics was observed even in patients with grade III/IV pulmonary vascular lesions (Fig. 5). In this subgroup (n = 7), preoperative and six-month postoperative PVR was 6.8 (4.3–8.7) U × m2 and 3.0 (2.6–6.3) U × m2, respectively (P = 0.018). In patients not judged to be reactive at preoperative cardiac catheterization, preoperative, and six-month postoperative PVR was 6.1 (3.8–8.1) U × m2 and 2.9 (2.0–3.9) U × m2, respectively (P = 0.012). Twelve patients had elevated mPAP and/or PVR six months after surgery. Clinical, echocardiographic, hemodynamic, treatment-related, and histopathological parameters obtained perioperatively were tested for their ability to differentiate between patients who remained with abnormal pulmonary hemodynamics and those who reached complete normalization. Logistic regression analysis identified two variables associated with hemodynamic outcome, namely, PVR/SVR during preoperative pulmonary vasodilator challenge with inhaled NO (P = 0.036), and the wall thickness of the arteries accompanying the respiratory bronchioli (P = 0.040). The results are shown in Table 2. ROC curves representing levels of sensitivity and specificity for these variables are shown in Fig. 6. A PVR/SVR of ≥24% during NO inhalation was identified as a risk factor for abnormal pulmonary hemodynamics six months after surgery (sensitivity = 73%; specificity = 79%). The HR associated with a PVR/SVR of ≥24% relative to lower values was 9.78 (95% CI = 1.55–61.65, P = 0.015). A mean wall thickness of arteries accompanying the respiratory bronchioli of ≥4.7 (Z score) was identified as a protection factor (sensitivity = 80%; specificity = 80%). The HR was 0.06 (95% CI = 0.01–0.56, P = 0.013).
Fig. 4.
Cardiac catheterization data obtained six months after cardiac surgery compared to baseline. All patients were on oral sildenafil. PAP, pulmonary arterial pressure; SAP, systemic arterial pressure; PVR, pulmonary vascular resistance index; PVR/SVR, pulmonary-to-systemic vascular resistance ratio. Bars represent median values with interquartile ranges. Dashed lines correspond to 25 mmHg for mPAP and 3.0 Wood units × m2 for PVR.
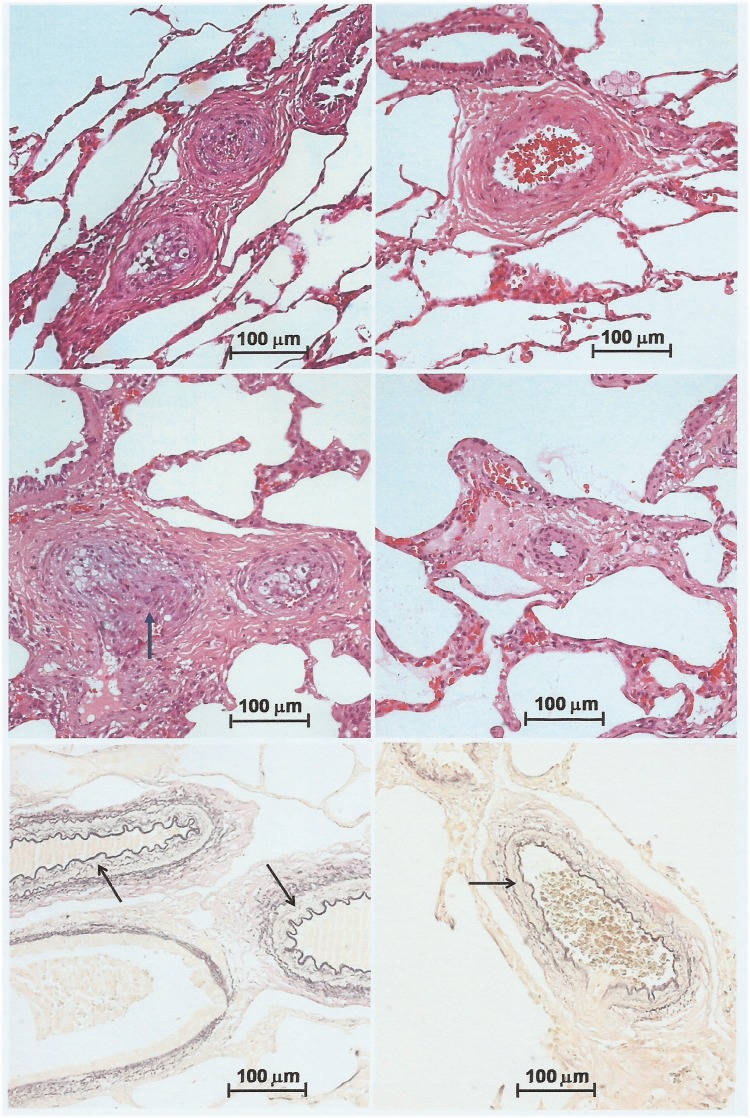
Fig. 5.
Severe and mild/moderate pulmonary arterial lesions coexisting in the same patient, thus explaining hemodynamic improvement after surgery. Top panels, 18-month-old patient with a preoperative and six-month postoperative PVR of 8.7 and 4.2 Wood units × m2, respectively. Upper left, intra-acinar arterioles showing severe occlusive intimal lesions (grade III). Upper right, preacinar artery showing moderate hypertrophy of the medial layer, with no intimal proliferation (grade I). Middle panels, 11-month-old patient with a preoperative and six-month postoperative PVR of 5.2 and 2.8 U × m2, respectively. Middle left, two preacinar arteries with intimal lesions, the bigger one showing a nodular proliferation of spindle cells, compatible with an initial plexiform lesion (grade IV, arrow). Middle right, intra-acinar arteriole with mildly occlusive intimal lesion and moderate medial hypertrophy (grade II). In upper and middle panels, H&E stain. Lower panels, Miller’s elastic stain used for quantifying medial hypertrophy of pulmonary arteries (arrows). Left and right, patients with wall thickness of arteries accompanying the terminal bronchioli of 8.1 and 7.5 (Z score), respectively. Both had normal PVR six months postoperatively (1.8 and 2.4 U × m2, respectively). In all panels, objective magnification 20×.
Table 2.
Potential predictors of abnormal pulmonary hemodynamics six months after surgery.
| Variables | n | Hazard ratio | 95% CI | P value |
|---|---|---|---|---|
| Age at operation | 27 | 1.05 | 0.95–1.17 | 0.350 |
| Peripheral oxygen saturation | 27 | 0.91 | 0.72–1.14 | 0.410 |
| Down syndrome | 27 | 0.50 | 0.09–2.86 | 0.436 |
| Pulmonary vascular resistance* | ||||
| Baseline | 27 | 1.49 | 0.99–2.25 | 0.059 |
| During NO inhalation | 27 | 1.43 | 0.96–2.13 | 0.080 |
| During NO inhalation (% baseline) | 27 | 1.03 | 0.98–1.07 | 0.215 |
| Pulmonary-to-systemic vascular resistance ratio | ||||
| Baseline | 27 | 1.06 | 1.00–1.12 | 0.055 |
| During NO inhalation | 27 | 1.09 | 1.01–1.18 | 0.036 |
| During NO inhalation (% baseline) | 27 | 1.02 | 0.98–1.06 | 0.392 |
| Pulmonary-to-systemic blood flow ratio† | ||||
| Baseline | 27 | 1.47 | 0.57–3.78 | 0.425 |
| During sildenafil administration | 27 | 1.32 | 0.71–2.45 | 0.386 |
| During sildenafil administration (% baseline) | 27 | 1.35 | 0.31–5.87 | 0.691 |
| Lung biopsy findings | ||||
| Grade of most severe lesions‡ | 21 | 1.52 | 0.67–3.44 | 0.320 |
| Percent wall thickness of arteries accompanying…§ | ||||
| Terminal bronchiole | 15 | 0.73 | 0.49–1.06 | 0.101 |
| Respiratory bronchiole | 20 | 0.69 | 0.49–0.98 | 0.040 |
| Alveolar ducts | 21 | 0.72 | 0.51–1.03 | 0.074 |
Fig. 6.
Parameters and respective levels in prediction of normal pulmonary hemodynamics six months after surgery. For PVR/SVR during NO administration and the wall thickness of arteries accompanying the respiratory bronchioli (RBA), respectively, the area under the curve was 0.80 (95% CI = 0.63–0.98, P = 0.011) and 0.79 (95% CI = 0.58–1.00, P = 0.028).
Patients with mPAP > 25 mmHg or PVR > 3.0 U × m2 six months after surgery were kept on sildenafil. Late drug discontinuation was considered in patients with consistent normalization of clinical and echocardiographic parameters. Unmedicated patients who presented with mild elevation of PAP (systolic pressure of 41–50 mmHg by echocardiography) were kept under observation. Those with higher pressure levels were considered for cardiac catheterization.
Discussion
The ability to demonstrate a pulmonary vascular response to vasodilators (oxygen, NO, or other agents) has no longer been considered as a primary criterion to recommend surgical correction in patients with congenital cardiac septal defects and PH. In lack of more evidence-based guidelines, expert consensus recommendations state that baseline PVR should guide the decision to consider surgery, at least in adults.9 The acute vasodilator response has not been shown to predict immediate postoperative outcomes so far.8,9 There have been suggestions to incorporate the acute vasodilator test in the assessment of operability for shunt lesions, but not to rely on the response as the primary and sole parameter.8,20 Medial hypertrophy of pulmonary arteries, although potentially reversible, constitutes a substrate for severe, sometimes life-threatening pulmonary vasoconstriction under certain postoperative stimuli. In the present study, baseline PVR as well as the acute response to inhaled NO were taken into consideration for decision about surgery, but not exclusively.
The patients with a reactive pulmonary vascular bed have a vascular pathology consistent with medial hypertrophy rather than widespread intimal occlusive lesions. Most important is our observation that even in the absence of demonstrable pulmonary vasoreactivity at the time of cardiac catheter study, reversal of hemodynamic abnormalities may occur with postoperative vasodilator therapy. Monitoring acute vasoreactivity in the catheter laboratory as a single criterion for operability may be problematic. In this study, we used two different approaches to demonstrate pulmonary vasoreactivity. We showed that pulmonary vasodilation could occur both acutely, during NO inhalation, and in a more protracted way during sildenafil administration. It was interesting that the response to NO was more pronounced in patients with the highest degree of medial hypertrophy of pulmonary arterioles. Both responses need to be considered. Using a ≥ 20% decrease in PVR and PVR/SVR ratio (with respective final levels of < 6.0 U × m2 and <0.3) as a criterion of response to NO,2,7,20 only 15 patients would be classified as full responders. Of the 15 patients who were operated on but did not meet these classical hemodynamic criteria for operability, six had completely normal PAP and PVR six months after surgery.
Long-term hemodynamic normalization is an obvious goal to be achieved. However, it is not expected to occur in all patients. There has been much discussion about reversibility of pulmonary vascular disease following repair of cardiac shunts. In particular, there remains controversy about the criteria used to predict or “measure” the biological process, especially in humans. The initial assumption that the disease should be deemed irreversible when response to vasodilators cannot be demonstrated in the catheterization laboratory does not sound reasonable on the basis of current knowledge. Some authors use histopathology data to define a priori the reversibility of pulmonary vasculopathy,21 while others use late postoperative hemodynamic data to characterize, in retrospect, reversible and irreversible disease.22 There have been attempts to examine the potential for reversibility of pulmonary vascular disease at the cellular and molecular levels. A number of biological markers have been studied in this way.21–23 An important concept is that the potential for regression of specific pulmonary vascular lesions and the potential for pulmonary arterial “deremodeling” as a general process are different things. Regression of pulmonary vascular disease depends not only on the presence of advanced vascular lesions like those seen in Fig. 3, upper and middle left panels, but particularly on the number and behavior of vessels with less severe structural changes, as shown in the same figure. What makes hemodynamic unloading relevant is the fact that it is probably required for initiation of vascular “deremodeling.” In experimental PH, hemodynamic unloading is associated with molecular reprogramming and phenotype switching of vascular cells, with regression of moderate to severe vascular lesions.24–26
In the present study, pulmonary hemodynamics normalized in more than half of the cases. Substantial improvement occurred even in patients with grade III/IV vascular lesions that coexisted with less severe vascular abnormalities. However, these individuals were aged < 19 months. It has been demonstrated that improvement is not expected to occur when grade II–IV lesions are detected above the age of two years.27 As far as we could see while analyzing a relatively small cohort, in patients kept on sildenafil, hemodynamic normalization six months after surgery was related to PVR/SVR level achieved preoperatively during NO inhalation. However, while predictive of a good outcome, this response should not be looked on as an absolute prerequisite to consider vasodilator therapy. In the study, medial hypertrophy of pulmonary arterioles was quantitatively relevant and related to favorable hemodynamic results as well. Thus, in addition to removing the excessive pulmonary flow, one might consider managing PVR in order to promote effective hemodynamic unloading in these patients.
Study limitations
The small number of patients in the study cohort was an obvious limitation. As mentioned in the description of the study population, it was difficult for us to recruit a substantial number of patients with unrestrictive cardiac shunts and absence of pulmonary overcirculation (thus indicating inappropriately elevated PVR), since these patients now represent ∼6–7% of all individuals with cardiac communications assisted in our institution. We believe that this is probably so for many tertiary centers at present. Furthermore, the fact that our institution is a reference center for cardiac surgery in the country explains the high referral rates of patients with Down syndrome. Our results should therefore be interpreted taking this context into consideration. Finally, because the study was not placebo-controlled, we cannot be absolutely sure about the real benefits of sildenafil administration. Despite the lack of evidence, we would not feel comfortable offering surgery to patients with clinical features like the ones mentioned in the study without using vasodilators as add-on therapy. The relatively satisfactory outcomes in subjects with demonstrated moderate to severe pulmonary vasculopathy suggests that sildenafil was effective to some extent. In treated patients, systolic PAP at six months after surgery was close to levels observed in patients considered to have a less severe disease.
Conclusions
Residual PH after surgical repair of congenital cardiac communications remains a problem, even in relatively young patients assisted on the basis of careful diagnostic evaluation. This was clearly seen at postoperative cardiac catheterization. In the study, patients with hypertrophic intra-acinar pulmonary arteries were the best responders to NO at preoperative catheter investigation. NO responders seemed to respond to sildenafil as well. Having been kept on sildenafil postoperatively, these patients had the best hemodynamic outcomes. Improvement was observed even for those with advanced pulmonary vascular lesions. The basis for such improvement seemed to be the coexistence of vessels (arteries) with less severe structural changes (e.g. medial hypertrophy eventually associated with non-occlusive intimal proliferation). Thus, pulmonary flow reduction, as a result of shunt repair, and pharmacological modulation of PVR may be looked on as important factors for achievement of hemodynamic unloading in this population. We speculate that these factors allow for initiation of biological events associated with vascular “deremodeling.”
Acknowledgments
The authors gratefully acknowledge Mrs. Roseli Polo for her technical assistance in the preparation of this manuscript.
Conflict of interest
The authors declare that there is no conflict of interest.
Funding
This work was supported by the Foundation for Research Support of the State of São Paulo (FAPESP), Brazil, grant no. 2011/09341-0.
References
- 1.Kozlik-Feldmann R, Hansmann G, Bonnet D, et al. Pulmonary hypertension in children with congenital heart disease (PAH-CHD, PPHVD-CHD). Expert consensus statement on the diagnosis and treatment of paediatric pulmonary hypertension. Heart 2016; 102(Suppl. 2): ii42–48. [DOI] [PubMed] [Google Scholar]
- 2.Balzer DT, Kort HW, Day RW, et al. Inhaled Nitric Oxide as a Preoperative Test (INOP Test I): the INOP Test Study Group. Circulation 2002; 106(Suppl. 1): I76–81. [PubMed] [Google Scholar]
- 3.Lindberg L, Olsson AK, Jögi P, et al. How common is severe pulmonary hypertension after pediatric cardiac surgery? J Thorac Cardiovasc Surg 2002; 123(6): 1155–1163. [DOI] [PubMed] [Google Scholar]
- 4.Kannan BR, Sivasankaran S, Tharakan JA, et al. Long-term outcome of patients operated for large ventricular septal defects with increased pulmonary vascular resistance. Indian Heart J 2003; 55(2): 161–166. [PubMed] [Google Scholar]
- 5.Haworth SG, Hislop AA. Treatment and survival in children with pulmonary arterial hypertension: the UK Pulmonary Hypertension Service for Children 2001–2006. Heart 2009; 95(4): 312–317. [DOI] [PubMed] [Google Scholar]
- 6.Giglia TM, Humpl T. Preoperative pulmonary hemodynamics and assessment of operability: is there a pulmonary vascular resistance that precludes cardiac operation? Pediatr Crit Care Med 2010; 11(Suppl. 2): S57–69. [DOI] [PubMed] [Google Scholar]
- 7.Myers PO, Tissot C, Beghetti M. Assessment of operability of patients with pulmonary arterial hypertension associated with congenital heart disease. Circ J 2014; 78(1): 4–11. [DOI] [PubMed] [Google Scholar]
- 8.Lopes AA, Barst RJ, Haworth SG, et al. Repair of congenital heart disease with associated pulmonary hypertension in children: what are the minimal investigative procedures? Consensus statement from the Congenital Heart Disease and Pediatric Task Forces, Pulmonary Vascular Research Institute (PVRI). Pulm Circ 2014; 4(2): 330–341. Erratum in: Pulm Circ 2014; 4(3): 531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37(1): 67–119. [DOI] [PubMed] [Google Scholar]
- 10.Nemoto S, Sasaki T, Ozawa H, et al. Oral sildenafil for persistent pulmonary hypertension early after congenital cardiac surgery in children. Eur J Cardiothorac Surg 2010; 38(1): 71–77. [DOI] [PubMed] [Google Scholar]
- 11.Fraisse A, Butrous G, Taylor MB, et al. Intravenous sildenafil for postoperative pulmonary hypertension in children with congenital heart disease. Intensive Care Med 2011; 37(3): 502–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loukanov T, Bucsenez D, Springer W, et al. Comparison of inhaled nitric oxide with aerosolized iloprost for treatment of pulmonary hypertension in children after cardiopulmonary bypass surgery. Clin Res Cardiol 2011; 100(7): 595–602. [DOI] [PubMed] [Google Scholar]
- 13.Lee JE, Hillier SC, Knoderer CA. Use of sildenafil to facilitate weaning from inhaled nitric oxide in children with pulmonary hypertension following surgery for congenital heart disease. J Intensive Care Med 2008; 23(5): 329–334. [DOI] [PubMed] [Google Scholar]
- 14.El Midany AA, Mostafa EA, Azab S, et al. Perioperative sildenafil therapy for pulmonary hypertension in infants undergoing congenital cardiac defect closure. Interact Cardiovasc Thorac Surg 2013; 17(6): 963–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beghetti M, Galiè N, Bonnet D. Can “inoperable” congenital heart defects become operable in patients with pulmonary arterial hypertension? Dream or reality? Congenit Heart Dis 2012; 7(1): 3–11. [DOI] [PubMed] [Google Scholar]
- 16.Laird TH, Stayer SA, Rivenes SM, et al. Pulmonary-to-systemic blood flow ratio effects of sevoflurane, isoflurane, halothane, and fentanyl/midazolam with 100% oxygen in children with congenital heart disease. Anesth Analg 2002; 95(5): 1200–1206. [DOI] [PubMed] [Google Scholar]
- 17.Rivera IR, Mendonça MA, Andrade JL, et al. Pulmonary venous flow index as a predictor of pulmonary vascular resistance variability in congenital heart disease with increased pulmonary flow: a comparative study before and after oxygen inhalation. Echocardiography 2013; 30(8): 952–960. [DOI] [PubMed] [Google Scholar]
- 18.Heath D, Edwards JE. The pathology of hypertensive pulmonary vascular disease; a description of six grades of structural changes in the pulmonary arteries with special reference to congenital cardiac septal defects. Circulation 1958; 18(4 Part 1): 533–547. [DOI] [PubMed] [Google Scholar]
- 19.Haworth SG, Hislop AA. Pulmonary vascular development: normal values of peripheral vascular structure. Am J Cardiol 1983; 52(5): 578–583. [DOI] [PubMed] [Google Scholar]
- 20.Abman SH, Hansmann G, Archer SL, et al. Pediatric pulmonary hypertension: Guidelines From the American Heart Association and American Thoracic Society. Circulation 2015; 132(21): 2037–2099. Erratum in: Circulation 2016; 133(4): e368. [DOI] [PubMed] [Google Scholar]
- 21.Smadja DM, Gaussem P, Mauge L, et al. Circulating endothelial cells: a new candidate biomarker of irreversible pulmonary hypertension secondary to congenital heart disease. Circulation 2009; 119(3): 374–381. [DOI] [PubMed] [Google Scholar]
- 22.Huang L, Li L, Hu E, et al. Potential biomarkers and targets in reversibility of pulmonary arterial hypertension secondary to congenital heart disease: an explorative study. Pulm Circ 2018; 8(2): 2045893218755987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Feen DE, Bartelds B, de Boer RA, et al. Pulmonary arterial hypertension in congenital heart disease: translational opportunities to study the reversibility of pulmonary vascular disease. Eur Heart J 2017; 38(26): 2034–2041. [DOI] [PubMed] [Google Scholar]
- 24.O’Blenes SB, Fischer S, McIntyre B, et al. Hemodynamic unloading leads to regression of pulmonary vascular disease in rats. J Thorac Cardiovasc Surg 2001; 121(2): 279–289. [DOI] [PubMed] [Google Scholar]
- 25.Abe K, Shinoda M, Tanaka M, et al. Haemodynamic unloading reverses occlusive vascular lesions in severe pulmonary hypertension. Cardiovasc Res 2016; 111(1): 16–25. [DOI] [PubMed] [Google Scholar]
- 26.Hsu CH, Roan JN, Chen JH, et al. Functional improvement and regression of medial hypertrophy in the remodeled pulmonary artery after correction of systemic left-to-right shunt. Sci Rep 2016; 6: 37684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rabinovitch M, Keane JF, Norwood WI, et al. Vascular structure in lung tissue obtained at biopsy correlated with pulmonary hemodynamic findings after repair of congenital heart defects. Circulation 1984; 69(4): 655–667. [DOI] [PubMed] [Google Scholar]