Abstract
Background
The purpose of the present study was to analyze and report the clinical outcomes following revision shoulder arthroplasty for failed humeral head resurfacing hemiarthroplasty (HHRH).
Methods
All patients who underwent revision shoulder arthroplasty for failed HHRH at our institution were retrospectively reviewed. Twenty-two shoulders in 20 patients were available for analysis. Mean age at the time of HHRH was 60 years (range 42 years to 75 years). The cohort consisted of 17 females and three males.
Results
The mean time from HHRH to revision was 5 years (range 1 year to 8 years). Mean age at the time of revision surgery was 62 years (range 44 years to 80 years). Patients were followed-up for a mean of 3.3 years (range 2 years to 4 years) after revision. Following revision surgery, there was an increase in forward elevation from 67° (range 0° to 130°) to 97° (range 40° to 160°) (p = 0.04). This was accompanied by an improvement in both the Oxford Shoulder Score and the subjective shoulder value, which increased from 13 (range 2 to 28) to 39 (range 24 to 48) (p = 0.000) and from 23 (range 0 to 65) to 79 (range 25 to 100) (p = 0.000) respectively.
Conclusions
Revision shoulder arthroplasty for failed HHRH improves functional outcome.
Keywords: hemiarthroplasty, resurfacing, revision surgery, shoulder arthritis, shoulder replacement, total shoulder replacement
Introduction
The National Joint Registry for England, Wales, Northern Ireland and the Isle of Man reported that 714 resurfacing total and hemiarthroplasty procedures were performed in 2014, accounting for 15% of all primary shoulder replacements.1 Humeral head resurfacing hemiarthroplasty (HHRH) is most commonly undertaken for osteoarthritis of the shoulder.1–3 Resurfacing arthroplasty requires limited bone resection and is frequently considered for young, active patients who are likely to undergo revision surgery at some point in their lives.4 Its advantages include the potential for accurate restoration of articular retroversion, neck-shaft angle, offset and centre of rotation.5,6 Revision surgery is facilitated because the prosthesis can be removed with almost no bone loss from the proximal humeral metaphysis and a glenoid prosthesis can be implanted if indicated.7 Technical difficulties associated with resurfacing arthroplasty are predominantly a result of incorrectly sizing and orienting the prosthesis, resulting in ‘over-stuffing’ of the joint.8 Few studies have evaluated the results following revision total shoulder arthroplasty (TSA) for failed HHRH.9,10 Those that do report variable outcomes that are often disappointing.9,10
Understanding the reasons for failure of HHRH and the outcome of subsequent revision is essential for patient counselling and future prosthetic design. The aim of the present retrospective cohort study was to analyse and report the clinical outcomes of a consecutive series of patients who underwent revision shoulder arthroplasty following failure of a resurfacing hemiarthroplasty prosthesis.
Materials and methods
Between September 2009 and January 2014, 20 consecutive patients underwent revision shoulder arthroplasty for failed HHRH at our study institution. Two patients had bilateral procedures allowing 22 shoulders to be available for analysis. All cases were identified using a computerized database and were performed by the senior authors (MF, DH and SML). The indication for HHRH was primary osteoarthritis in 16 shoulders, rheumatoid arthritis in four shoulders and rotator cuff tear arthropathy in two shoulders. Resurfacing components included 22 Copeland Surface Replacement Arthroplasty (CSRA) (Biomet, Swindon, UK) prostheses. All index procedures were performed elsewhere and referred to our complex shoulder unit for further evaluation. If there was a strong clinical suspicion of infection pre-operatively, intra-articular fluid and tissue samples were taken in the operating theatre before revision and evaluated for organisms such as Propionibacterium acnes.
Mean age at the time of HHRH was 60 years (range 42 years to 75 years). The cohort consisted of 17 females and three males. The dominant arm was affected in 12 cases. Two patients underwent other prior surgery, comprising two acromioclavicular joint excisions. Reasons for failure included glenoid erosion in 18 shoulders, rotator cuff tear arthropathy in two shoulders and painful stiffness without glenoid erosion in two shoulders. No cases of periprosthetic infection were noted in the cohort.
Surgical technique
Index surgery was carried out using a deltopectoral approach in 18 shoulders and an anterolateral (deltoid splitting) approach in four shoulders. The deltopectoral approach was used for revision in all cases. Subscapularis was detached from its insertion in external rotation and subsequently repaired directly to bone. The rotator cuff was examined to determine whether an anatomical or reverse anatomy replacement was most suitable. The following parameters were evaluated intra-operatively: prosthetic loosening, implant position, implant size, bone resorption under the implant, glenoid cartilage loss, articular bone loss and the presence of a rotator cuff tear.10 Glenoid bone loss was treated with morcelized humeral head autograft compressed beneath a metal-back glenoid.
Radiographic assessment
Pre- and post-revision radiographs were performed in all cases and included anteroposterior and axillary views. Plain radiographs were reviewed for the presence of glenohumeral subluxation, periprosthetic lucency and glenoid erosion.11,12 Computer tomography was used to evaluate glenoid bone stock to ensure that a glenoid component could be placed. Following revision surgery, all reverse anatomy prostheses were additionally assessed for scapular notching and classified according to the size of the defect on the anteroposterior radiograph using the four-part grading system devised by Sirveaux et al.13
Glenohumeral subluxation was assessed by evaluating the direction and the amount of translation of the centre of the prosthetic head relative to the centre of the glenoid or the glenoid component. It was graded as present if translation was greater than 25% and absent when translation was less than 25%.14 Periprosthetic loosening was evaluated by assessing the glenoid and humeral components for lucent lines and an alteration in position.12 For the glenoid, this was defined as migration/tilting of the component or a complete lucent line with part of it measuring at least 1.5 mm in width. Loosening of a humeral prosthesis was identified by a lucent line at least 2 mm in width or tilting/subsidence of the implant.
Glenoid erosion was graded as none, mild if there was erosion into subchondral bone, moderate if there was medialization of the glenoid subchondral bone with associated hemispheric deformation of the glenoid, or severe if there was complete hemispheric deformation of the glenoid with bone loss to the base of the coracoid.11
Clinical assessment
Clinical outcome measures examined pre- and post-revision surgery included active forward elevation and active external rotation. All patients were evaluated with the Oxford Shoulder Score (OSS). In addition, all patients were assessed using the subjective shoulder value (SSV), which uses a scale from 0 (worst score) to 100 (best score) to describe the affected shoulder.15 This can be used as a supplementary tool to traditional, more complex outcome measures and may be used in conjunction with other scores to assess patient outcome.
Statistical analysis
A paired t-test was used to compare range of motion, OSS and SSV before and after surgery. p < 0.05 was considered statistically significant. SPSS, version 23 (SPSS Inc., IBM Corp., Armonk, NY, USA) was used to analyze data.
Results
The mean interval from HHRH to revision shoulder arthroplasty was 5 years (range 1 year to 8 years). Mean age at the time of revision surgery was 62 years (range 44 years to 80 years). Patients were followed-up for a mean of 3.3 years (range 2 years to 4 years).
Intra-operative evaluation
Intra-operative assessment at the time of revision demonstrated loosening in eight shoulders, an excessively large implant in five shoulders, bone resorption in the proximal humerus in 11 shoulders, a rotator cuff tear in 10 shoulders, a deficient subscapularis in three shoulders, glenoid cartilage loss in 22 shoulders and glenoid bone loss in 12 shoulders. The coronal alignment of the implant was considered neutral in 17 shoulders, varus in four shoulders and valgus in one shoulder.
Choice of revision implant was determined by pre-operative radiological assessment and the aforementioned intra-operative findings. An ‘off the shelf’ reverse anatomy implant was used in the presence of a rotator cuff tear and a computer-assisted design/computer-assisted manufacturing (CAD/CAM) prosthesis was used in cases where bone loss precluded safe implantation of a conventional glenoid component. Anatomical TSA was used in all remaining cases. Revision surgery was undertaken using an Epoca (DePuySynthes, Leeds, UK) anatomical total shoulder replacement with a metal-backed glenoid in 11 cases (Figure 1), a fixed fulcrum fully constrained reverse anatomy prosthesis (Stanmore Implants, Elstree, UK) in six cases (Figure 2) and a CAD/CAM TSA (Stanmore Implants) in five cases (Figure 3). Impaction grafting using morcelized humeral head autograft was used to treat glenoid bone loss in six cases.
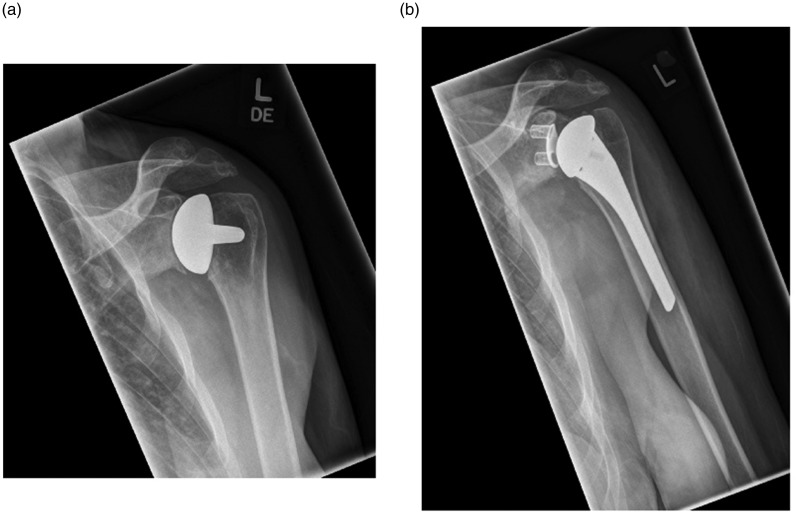
Figure 1.
(a) Anteroposterior radiograph of a 42-year-old right-hand dominant female with glenoid erosion following left humeral head resurfacing hemiarthroplasty. (b) Anteroposterior radiograph of a 42-year-old right-hand dominant female 2 months after left revision anatomical total shoulder arthroplasty.
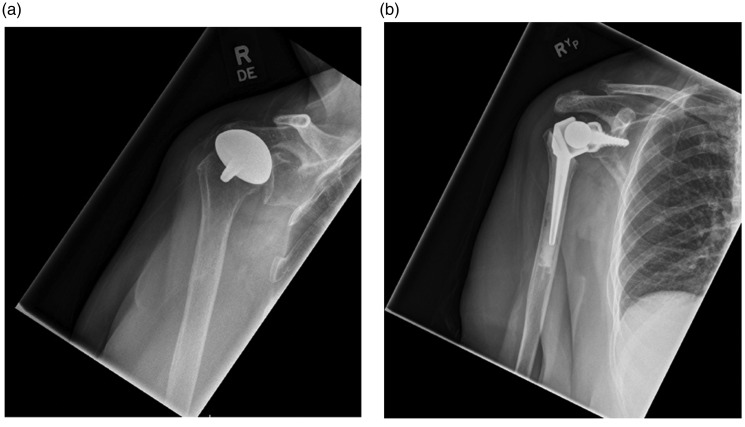
Figure 2.
(a) Anteroposterior radiograph of a 66-year-old right-hand dominant female with glenoid erosion and superior migration of the proximal humerus following right humeral head resurfacing hemiarthroplasty. (b) Anteroposterior radiograph of a 66-year-old right-hand dominant female 18 months after right revision fixed fulcrum fully constrained reverse anatomy arthroplasty.
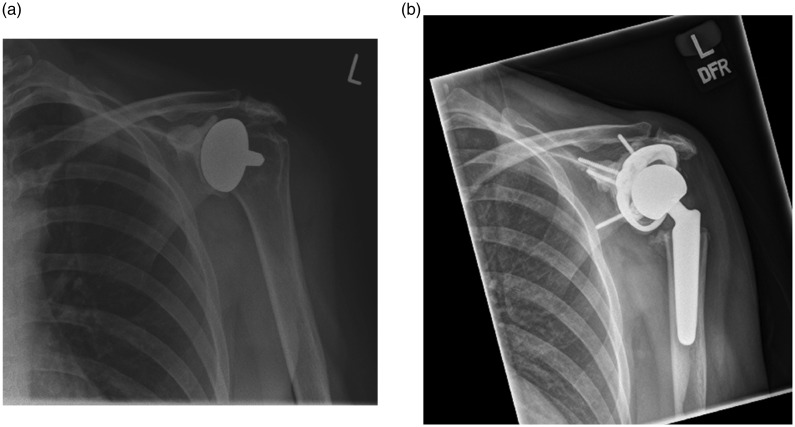
Figure 3.
(a) Anteroposterior radiograph of a 45-year-old right-hand dominant female with glenoid erosion and superior migration of the proximal humerus following left humeral head resurfacing hemiarthroplasty. (b) Anteroposterior radiograph of a 45-year-old right-hand dominant female 3 months after left revision computer-assisted design/computer-assisted manufacturing total shoulder arthroplasty.
Radiological assessment
Analysis of resurfacing prostheses before revision surgery demonstrated subluxation in 19 cases (superior and anterior in five cases, superior and posterior in five cases, superior in three cases, anterior in five cases and posterior in one case), loosening in three cases, moderate glenoid erosion in 10 cases and severe glenoid erosion in 16 cases. Following revision surgery, evaluation of all TSA implants revealed subluxation in six cases (anterior in four cases, posterior in one case and superior in one case) and loosening of the glenoid component in two cases. Scapular notching was not present in any of the reverse anatomy prostheses at the latest follow-up.
Clinical outcomes
Mean active forward elevation increased from 67° (range 0° to 130°) to 97° (range 40° to 160°) (p = 0.04) following revision surgery. An improvement was also noted in mean active external rotation, which increased from 25° (range 0° to 70°) to 34° (10° to 70°) (p = 0.111) following revision surgery.
The mean OSS improved from 13 pre-operatively (range 2 to 28) to 39 postoperatively (range 24 to 48) at the final follow-up (p = 0.000). An increase was also noted in the mean SSV, which improved from 23 (range 0 to 65) pre-operatively to 79 (range 25 to 100) postoperatively (p = 0.000).
Complications
Further revision surgery was required in one patient, with a fixed fulcrum fully constrained reverse anatomy prostheses, as a result of loosening of the glenoid component. In this case, an isolated glenoid replacement was undertaken, which resulted in an improvement in both the OSS and the SSV. No other complications were noted in the cohort.
Discussion
HHRH is a well-established treatment modality for osteoarthritis of the shoulder, although its use has been expanded to include cases of rheumatoid arthritis, isolated chondral defects, osteonecrosis and cuff tear arthropathy.2,10,16,17 Good clinical results have been reported in the short- and mid-term following resurfacing arthroplasty but registry data have demonstrated a cumulative five-year revision rate of approximately 10%, with reasons for failure infrequently discussed.9,10,18
Using the Danish Shoulder Arthroplasty Registry, Rasmussen et al.9 evaluated the results of revision shoulder arthroplasty after resurfacing hemiarthroplasty in patients with osteoarthritis. One hundred and seven cases were identified, of which 80 were followed up with postoperative functional outcome assessment only. Of these, 33 (41%) had an unacceptable outcome, defined as a Western Ontario Osteoarthritis of the Shoulder index of ≤50 points. Further revision surgery was required in 11 cases (10%). Streubel et al.10 reported the results of 11 patients who underwent revision of a HHR implant. After a mean follow-up of 3.5 years, an unsatisfactory outcome was noted in six cases and further surgery was required in two cases (one haematoma and one revision for instability).
Our results suggest that failed HHRH can be successfully revised with a range of implants. Revision surgery was carried out a mean of 5 years after the index procedure and the most common reason for failure was glenoid erosion causing pain. At short-term follow-up, there was an increase in external rotation, a significant improvement in forward elevation and a significant improvement in functional outcome. This is in contrast to other reports evaluating the results following revision shoulder arthroplasty for failed humeral resurfacing, where an unsatisfactory outcome was frequently noted.9,10 At the time of revision, eight implants were found to be loose, although only three of these were evident on pre-operative radiographs. One re-operation was undertaken for glenoid loosening in a patient with a reverse total shoulder replacement, although there was still an improvement in functional outcome.
Success of a cementless prosthesis (such as HHRH) is dependent upon firm contact between the implant and the bone, and bony ingrowth onto the implant surface.19–21 Resurfacing arthroplasty affects load transfer and induces stress shielding, leading to excessive bone resorption and loosening.19,22,23 Conventional radiographs are unable to accurately assess the bearing bone as it is covered by the radiopaque shell of the prosthesis.22 In a recent study examining osteointegration in two resurfacing shoulder implants (Copeland and Epoca) without clinical evidence of loosening, limited bone was observed around the central stem of the CSRA, in contrast to the Epoca Resurfacing Head prosthesis (Synthes, Oberdof, Switzerland) where there was uniform bone contact over the entire surface.24 In a similar study, Schmidutz et al.22 investigated the bone–implant interface in four different HHRH implants: CSRA (n = 5), Epoca (n = 7), Capica (Implantcast, Buxtehude, Germany) (n = 1) and Global CAP (DePuy, Raynham, MA, USA ) (n = 1). Stress shielding and reduced bone stock under the implant shell was observed in the majority of cases. For stemmed prostheses such as the CSRA, bone stock was reduced between the central stem and outer rim. Alternatively, in conical-crowned implants such as the Epoca, bone stock was predominantly reduced at the inner margin of the crown.
All implants examined in the present study were CSRA prostheses. Stress shielding could potentially be responsible for the bone resorption found in 50% of cases (11 out of 22 shoulders) in the present study because this has been demonstrated previously in a finite element analysis of CSRA.25 This did not manifest radiologically in all patients because it may have been preceded by failure for other reasons such as glenoid erosion. Radiological lucency in the medium-term has been demonstrated to occur in 18% of cases, although this may be an underestimation because the area of bone beneath a resurfacing arthroplasty is covered and therefore not visible on plain radiographs.3 Glenoid bone loss was observed in 55% (12 out of 22 shoulders) of patients and is an important consideration for revision surgery because the limited bone stock may preclude safe glenoid implantation. In some cases, this may require either glenoid reconstruction using bone graft or a custom-made prosthesis. At our study institution, a CAD/CAM shoulder (Stanmore Implants) is often used for these challenging cases because it secures the glenoid shell to the surrounding scapula, as well as the deficient glenoid.26
HHRH can be a technically demanding procedure especially in cases where exposure is compromised by body habitus or surgical approach, leading to inaccurate identification and sizing of the anatomical neck and placement of an implant that is either too large or mal-aligned.10 As reported by other studies evaluating the results of revision arthroplasty, all index procedures were undertaken at a different institution and subsequently referred to our high-volume unit.10 Although there is no evidence to suggest that surgical experience influences the outcome following resurfacing arthroplasty, it is likely to be a contributing factor because mal-aligned and/or inappropriately large prostheses were observed in 45% of cases (10 out of 22 shoulders) in the present study.5,6
Limitations of the present study included its retrospective design, the small sample size, the short follow-up and the different prostheses used during revision surgery. Nonetheless, the present study provides useful information to surgeons carrying out revision surgery for failed humeral head resurfacing.
In conclusion, we have reported the results of revision shoulder arthroplasty for failed humeral head resurfacing hemiarthroplasty. Glenoid erosion was the most common reason for failure and, at short-term follow-up, there was a significant improvement in both forward elevation and functional outcome. Given the popularity of resurfacing arthroplasty, larger long-term studies are needed to identify factors that increase the likelihood of failure and to establish the longevity of implants used in the revision setting.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Review and Patient Consent
Not required for this article.
Level of Evidence
Case series (Level IV)
References
- 1.National Joint Registry for England, Wales, Northern Ireland and Isle of Man. 12th Annual Report, 2015. Available at: www.njrcentre.org.uk.
- 2.Levy O, Copeland SA. Cementless surface replacement arthroplasty (Copeland CSRA) for osteoarthritis of the shoulder. J Shoulder Elbow Surg 2004; 13: 266–271. [DOI] [PubMed] [Google Scholar]
- 3.Levy O, Funk L, Sforza G, Copeland SA. Copeland surface replacement arthroplasty of the shoulder in rheumatoid arthritis. J Bone Joint Surgery 2004; 86A: 512–518. [DOI] [PubMed] [Google Scholar]
- 4.Bailie DS, Llinas PJ, Ellenbecker TS. Cementless humeral resurfacing arthroplasty in active patients less than fifty-five years of age. J Bone Joint Surgery 2008; 90A: 110–117. [DOI] [PubMed] [Google Scholar]
- 5.Burgess DL, McGrath MS, Bonutti PM, Marker DR, Delanois RE, Mont MA. Shoulder resurfacing. J Bone Joint Surgery 2009; 91A: 1228–1238. [DOI] [PubMed] [Google Scholar]
- 6.Thomas SR, Sforza G, Levy O, Copeland SA. Geometrical analysis of Copeland surface replacement shoulder arthroplasty in relation to normal anatomy. J Shoulder Elbow Surg 2005; 14: 186–192. [DOI] [PubMed] [Google Scholar]
- 7.Carroll RM, Izquierdo R, Vazquez M, Blaine TA, Levine WN, Bigliani LU. Conversion of painful hemiarthroplasty to total shoulder arthroplasty: long-term results. J Shoulder Elbow Surg 2004; 13: 599–603. [DOI] [PubMed] [Google Scholar]
- 8.Mechlenburg I, Amstrup A, Klebe T, Jacobsen SS, Teichert G, Stilling M. The Copeland resurfacing humeral head implant does not restore humeral head anatomy. A retrospective study. Arch Orthop Trauma Surg 2013; 133: 615–619. [DOI] [PubMed] [Google Scholar]
- 9.Rasmussen JV, Olsen BS, Al-Hamdani A, Brorson S. Outcome of revision shoulder arthroplasty after resurfacing hemiarthroplasty in patients with glenohumeral osteoarthritis. J Bone Joint Surg 2016; 98A: 1631–1637. [DOI] [PubMed] [Google Scholar]
- 10.Streubel PN, Simone JP, Cofield RH, Sperling JW. Revision of failed humeral head resurfacing arthroplasty. Int J Shoulder Surg 2016; 10: 21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartelt R, Sperling JW, Schleck CD, Cofield RH. Shoulder arthroplasty in patients aged fifty-five years or younger with osteoarthritis. J Shoulder Elbow Surg 2011; 20: 123–130. [DOI] [PubMed] [Google Scholar]
- 12.Sperling JW, Cofield RH, O'Driscoll SW, Torchia ME, Rowland CM. Radiographic assessment of ingrowth total shoulder arthroplasty. J Shoulder Elbow Surg 2000; 9: 507–513. [DOI] [PubMed] [Google Scholar]
- 13.Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Mole D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg 2004; 86B: 388–395. [DOI] [PubMed] [Google Scholar]
- 14.Sperling JW, Cofield RH, Rowland CM. Minimum fifteen-year follow-up of Neer hemiarthroplasty and total shoulder arthroplasty in patients aged fifty years or younger. J Shoulder Elbow Surg 2004; 13: 604–613. [DOI] [PubMed] [Google Scholar]
- 15.Gilbart MK, Gerber C. Comparison of the subjective shoulder value and the Constant score. J Shoulder Elbow Surg 2007; 16: 717–721. [DOI] [PubMed] [Google Scholar]
- 16.Jerosch J, Sokkar SM, Neuhaeuser C, Abdelkafy A. Humeral resurfacing arthroplasty in combination with latissimus dorsi tendon transfer in patients with rotator cuff tear arthropathy and preserved subscapularis muscle function: preliminary report and short-term results. Europ J Orthop Surgery Trauma 2014; 24: 1075–1083. [DOI] [PubMed] [Google Scholar]
- 17.Sweet SJ, Takara T, Ho L, Tibone JE. Primary partial humeral head resurfacing: outcomes with the HemiCAP implant. Am J Sports Med 2015; 43: 579–587. [DOI] [PubMed] [Google Scholar]
- 18.Rasmussen JV, Polk A, Sorensen AK, Olsen BS, Brorson S. Outcome, revision rate and indication for revision following resurfacing hemiarthroplasty for osteoarthritis of the shoulder: 837 operations reported to the Danish Shoulder Arthroplasty Registry. Bone Joint J 2014; 96B: 519–525. [DOI] [PubMed] [Google Scholar]
- 19.Ruben RB, Fernandes PR, Folgado J. On the optimal shape of hip implants. J Biomech 2012; 45: 239–246. [DOI] [PubMed] [Google Scholar]
- 20.Jasty M, Bragdon C, Burke D, O'Connor D, Lowenstein J, Harris WH. In vivo skeletal responses to porous-surfaced implants subjected to small induced motions. J Bone Joint Surg 1997; 79A: 707–714. [DOI] [PubMed] [Google Scholar]
- 21.Pilliar RM, Lee JM, Maniatopoulos C. Observations on the effect of movement on bone ingrowth into porous-surfaced implants. Clin Orthop Rel Res 1986; 208: 108–113. [PubMed] [Google Scholar]
- 22.Schmidutz F, Sprecher CM, Milz S, Gohlke F, Hertel R, Braunstein V. Resurfacing of the humeral head: an analysis of the bone stock and osseous integration under the implant. J Orthop Res 2015; 33: 1382–1390. [DOI] [PubMed] [Google Scholar]
- 23.Decking R, Puhl W, Simon U, Claes LE. Changes in strain distribution of loaded proximal femora caused by different types of cementless femoral stems. Clin Biomech 2006; 21: 495–501. [DOI] [PubMed] [Google Scholar]
- 24.Ajami S, Blunn GW, Lambert S, Alexander S, Foxall Smith M, Coathup MJ. Histological evaluation of two designs of shoulder surface replacement implants. Bone Joint J 2016; 98B: 504–511. [DOI] [PubMed] [Google Scholar]
- 25.Schmidutz F, Agarwal Y, Muller PE, Gueorguiev B, Richards RG, Sprecher CM. Stress-shielding induced bone remodeling in cementless shoulder resurfacing arthroplasty: a finite element analysis and in vivo results. J Biomech 2014; 47: 3509–3516. [DOI] [PubMed] [Google Scholar]
- 26.Uri O, Bayley I, Lambert S. Hip-inspired implant for revision of failed reverse shoulder arthroplasty with severe glenoid bone loss. Improved clinical outcome in 11 patients at 3-year follow-up. Acta Orthop 2014; 85: 171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]