Abstract
Background
Positioning of the glenoid component is one of the most challenging steps in shoulder arthroplasty, and prosthesis longevity as well as functional outcomes is considered highly dependent on accurate positioning. This review considers the evidence supporting surgical navigation and patient-specific instruments for glenoid implant positioning in anatomic and reverse total shoulder arthroplasty.
Methods
A systematic literature search was performed for studies assessing glenoid implant positioning accuracy as measured by cross-sectional imaging on live subjects or cadaver models. Meta-analysis of controlled studies was performed to estimate the primary effects of navigation and patient-specific instruments on glenoid implant positioning error. Meta-analysis of absolute positioning outcomes was also performed for each group incorporating data from controlled and uncontrolled studies.
Results
Nine studies, four controlled and five uncontrolled, with 258 total subjects were included in the analysis. Meta-analysis of controlled studies supported that both navigation and patient-specific instruments had a moderate statistically significant effect on improving glenoid implant positioning outcomes. Meta-analysis of absolute positioning outcomes demonstrates glenoid implant positioning with standard instrumentation results in a high rate of malposition.
Discussion
Navigation and patient-specific instruments improve glenoid positioning outcomes. Whether the improvement in positioning outcomes achieved translate to better clinical outcomes is unknown.
Keywords: total shoulder arthroplasty, reverse total shoulder arthroplasty, glenoid, patient-specific instruments, surgical navigation, image-guided surgery
Introduction
Glenoid component position is a critical factor for postoperative function and long-term implant survival in both anatomic and reverse total shoulder arthroplasty,1 defining shoulder motion, impingement points, and stresses at the bone–prosthesis interface.2 Glenoid component failure is one of the most common complications of total shoulder arthroplasty,3,4 and malposition of the glenoid component has been associated with instability, implant, loosening, early failure, and inferior clinical outcomes.5
Achieving adequate alignment and stable fixation of the glenoid component can be technically challenging3 owing to restricted visualization, limited bony landmarks, and the complex and variable scapular geometry. Abnormal glenoid morphology and bone loss is common in primary and revision procedures, which further complicates positioning the baseplate along the anatomic centerline and achieving stable fixation.6
Over the last decade, surgical navigation (NAV) and patient-specific instrument (PSI) techniques have been developed for improving glenoid implant positioning in total shoulder arthroplasty.7 The efficacy of these systems at improving radiographic outcomes has been investigated in a number of controlled and uncontrolled studies, with varying sample size, methodological quality, and results.7–30 Some studies have found significant improvement in glenoid positioning accuracy; however, glenoid positioning systems have not yet been widely adopted. There exist trade-offs between the potential benefits of these positioning systems and potential challenges relating to increased cost, increased operative time, system availability and reliability, operator training needs, and other barriers to adoption and implementation.7 A systematic review of the available literature and meta-analysis is necessary as a first step to quantitatively evaluate these trade-offs.
A meta-analysis of seven controlled glenoid NAV studies was conducted by Sadoghi et al.31 However, this previous review did not apply rigorous study-methodology selection criteria, include any PSI studies, or assess accuracy of the translational degrees of freedom in the glenoid implant position.
The purpose of this meta-analysis is to compare radiographic positioning outcomes obtained by standard instrumentation (SI), surgical NAV, and patient-specific instruments (PSI) for glenoid implant positioning in anatomic and reverse total shoulder arthroplasty. The null hypothesis is that radiographic outcomes are equivalent between positioning techniques.
Materials and methods
Eligibility criteria
This study was conducted and reported according to the PRISMA guidelines for systematic reviews and meta-analysis,32 and prospectively registered with PROSPERO (registration number: CRD42017080177).
Randomized controlled trials (RCTs), non-randomized controlled studies (NRSs), and cohort studies (retrospective and prospective) measuring glenoid implant positioning error with PSI, NAV, or SI and meeting the following eligibility criteria were included in this study: (1) the study was performed on human or human cadaver shoulders, (2) positioning error is measured using cross-sectional (CT or MRI) imaging, (3) mean positioning error magnitudes are reported.
Studies were excluded if (1) the procedure was performed on a sawbone or synthetic model; (2) the results were reported in terms of guide-pin position only; (3) multiple procedures were performed on the same cadaver model; or (4) the glenoid was adulterated prior to arthroplasty, such as to create a glenoid defect model.
Search strategy and study selection
PubMed and MEDLINE were searched from inception to 6 October 2017. The search strings and parameters used are detailed in Appendix 1 (supplementary material). Two independent reviewers (DMB, TF) screened the titles and abstracts of all retrieved studies for potential relevance. Full-text review was then conducted for final selection according to the eligibility criteria. Reference lists of the retrieved manuscripts were also hand searched for potentially relevant studies not identified by the database search. Disagreements were resolved by discussion between the two reviewers and the senior author. No blinding of author, journal, or institutional names was performed.
Data extraction
A single reviewer performed the data extraction on eligible papers. The primary outcome for this study was glenoid component positioning error, defined by version, inclination, and offset errors. Secondary outcomes extracted when available included number of outliers (defined as version or inclination error greater than 10° or offset error greater than 4 mm),2,19,26,33 number of aborted procedures, operative time increase, and cost increase. In addition to primary and secondary outcomes, the study design, type of subject (human or cadaver), type of implant (anatomic or reverse), and NAV or PSI system used was extracted.
Methodological quality assessment
Two independent investigators (DMB, TF) graded risk of bias and methodological quality of controlled trials using the Cochrane Collaboration's Risk of Bias Tool for Randomized Trials.34 Therapeutic level of evidence was graded according to Wright et al.35 Disagreements were resolved by discussion between the two investigators and the senior author.
Statistical analysis
Meta-analysis of positioning outcomes from controlled studies was performed with a mean difference (MD) random effects model, using the restricted maximum-likelihood estimator method. Heterogeneity of studies comparing the same treatments was quantified by calculation of the I2 statistic and assessed for statistical significance by calculation of the P-value for the heterogeneity statistic Q. Publication bias is assessed using Begg's Rank Correlation Test36 and Egger's Regression Test37 to detect funnel plot asymmetry.
Using the Begg and Pilote's38 method for incorporating historical controls into meta-analysis, a multivariate mixed effects model was implemented incorporating the full dataset of controlled and uncontrolled studies. The effect of subject type (human versus cadaver) and study type (case series versus controlled study) was assessed independently for significance as moderators in the multivariate model.
Pooled estimates (meta-means) for radiographic outcome performance were calculated for the NAV, PSI, and SI groups using a random effects model, with the restricted maximum-likelihood estimator method.
Statistical analyses were performed using R (R Foundation for Statistical Computing, Vienna, Austria) using the metafor package.39
Results
Search results
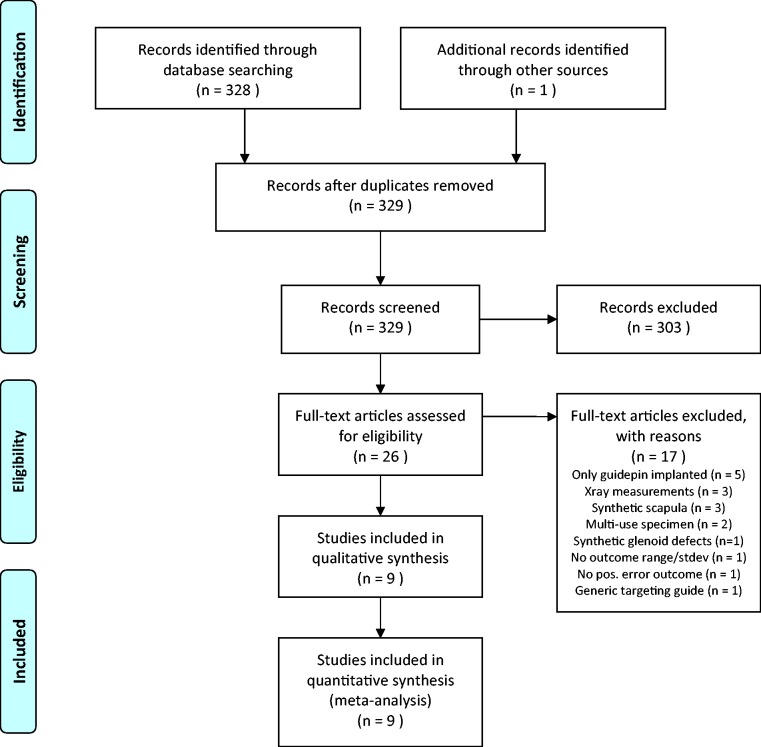
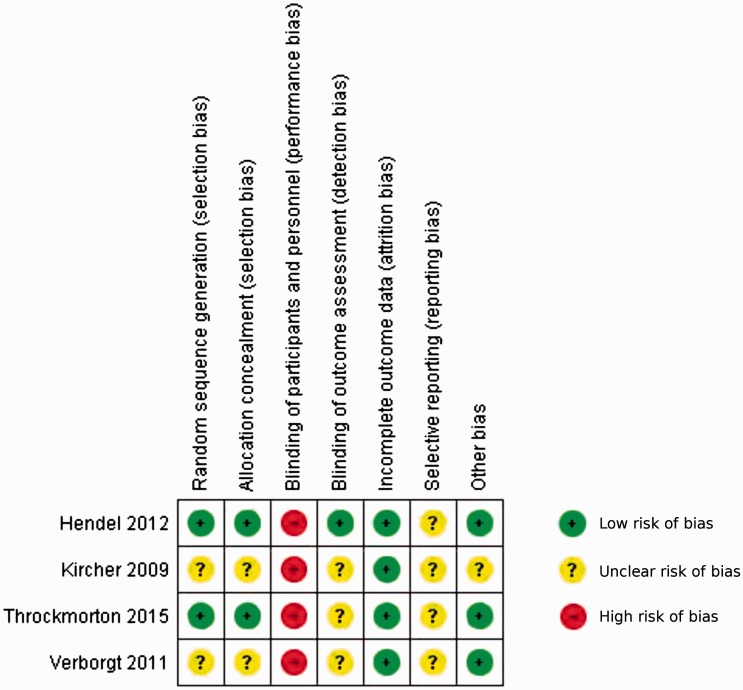
The results of the literature search, study screening, and selection are detailed in a flow diagram (Figure 1). The database and bibliography searches yielded 329 potentially relevant studies. Screening of the titles and abstracts yielded 26 relevant articles5,7–30,40 for full-text review and assessment for eligibility criteria. Nine studies met our inclusion criteria of which three were prospective randomized controlled studies,19,26,29 one was a prospective NRS,7 four were prospective uncontrolled cohort studies,8,9,12,25 and one was a retrospective uncontrolled cohort study.5 Study characteristics and level of evidence are reported in Table 1. Assessment of methodological quality and individual study risk of bias is summarized in Figure 2 for the controlled studies. The sum total sample size was 135 for the included controlled studies alone and 258 for all included studies.
Figure 1.
PRISMA flow diagram, depicting the flow of information through the stages of this systematic review.
Table 1.
Summary of study characteristics.
| Study | Design | Level of evidence | n (NAV) | n (SI) | Subject | Implant(s) | Error outcomes | Navigation system |
|---|---|---|---|---|---|---|---|---|
| Group 1: Surgical navigation (NAV) | ||||||||
| Verborgt et al.7 | NRS | II | 7 | 7 | Cadaver | rTSA | V, I | Praxim Nano Station |
| Kircher et al.29 | RCT | II | 10 | 10 | Human | aTSA | V | Praxim Nano Station |
NAV: navigation; NRS: nonrandomized controlled study; PC: prospective cohort; PSI: patient-specific instruments; RC: retrospective cohort; RCT: randomized controlled trial; SI: standard instrumentation; V,I,O: version,inclination,offset.
Figure 2.
Risk of bias assessment for controlled studies.
Radiographic outcome effects: Controlled studies
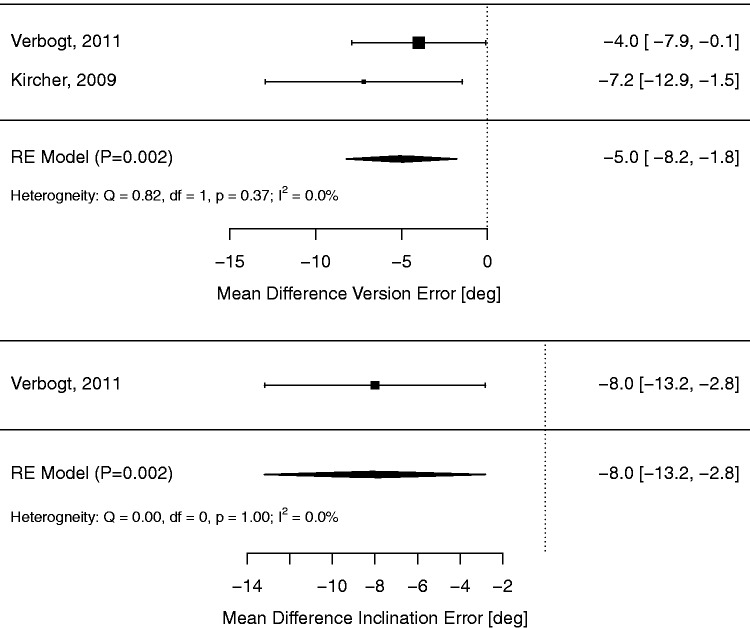
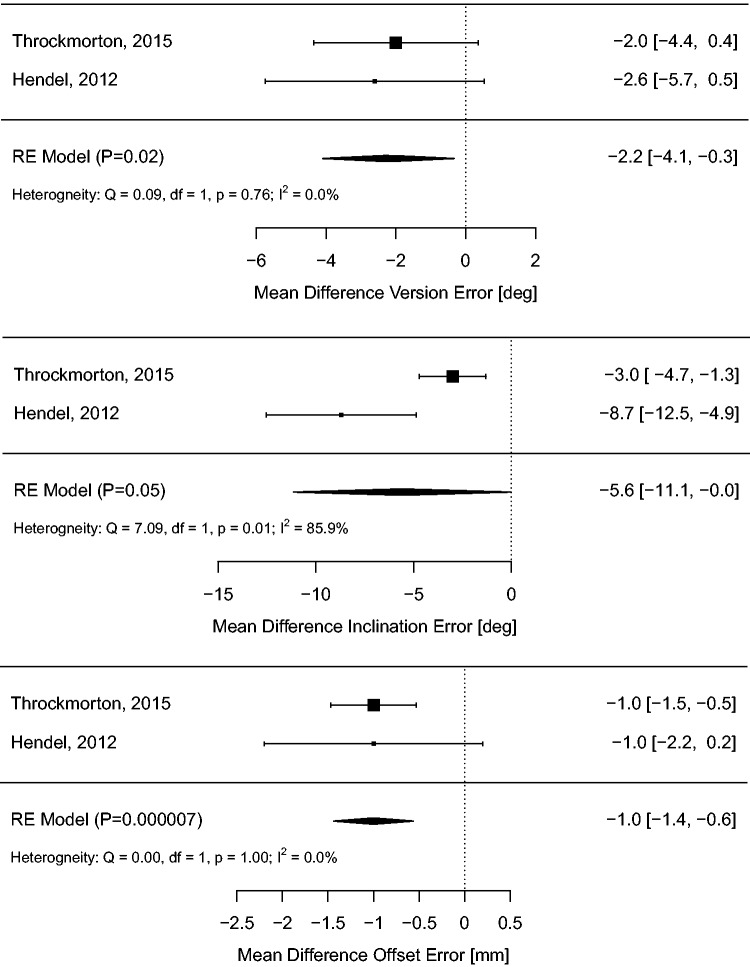
The radiographic outcome meta-analyses for controlled studies are summarized with forest plots in Figures 3 and 4 for NAV and PSI groups, respectively.
Figure 3.
Meta-analysis of glenoid positioning outcomes for controlled studies comparing NAV versus SI.
Figure 4.
Meta-analysis of glenoid positioning outcomes for controlled studies comparing PSI versus SI.
NAV use resulted in a statistically significant reduction in version error (MD −5.0 °; 95% CI −1.8 ° to −8.2 °; P = 0.002) with little heterogeneity (I2 = 0%, P = 0.37). Only a single NAV study reported inclination error,7 demonstrating a significant beneficial effect (MD −8.0 °; 95% CI −2.8 ° to −13.2 °; P = 0.002), and no NAV studies reported offset error.
PSI use resulted in modest but statistically significant reductions in version error (MD −2.2 °; 95% CI −4.1 ° to −0.3 °; P = 0.02), inclination error (MD −5.6 °; 95% CI −0.0 ° to −11.1 °; P = 0.05), and offset error (MD −1.0 °; 95% CI −0.6 ° to −1.4 °; P < 0.001). Heterogeneity was low for version error (I2 = 0%, P = 0.76) and offset error (I2 = 0%, P = 1.0), but large for inclination error (I2 = 86%, P = 0.01).
Radiographic outcome effects: All studies
Meta-analysis of radiographic outcomes using the full dataset of controlled and uncontrolled studies was performed using a mixed effects model. There was no significant effect of NAV on version error (MD −2.15 °; 95% CI 5.3 ° to −9.6 °; P = 0.57) or inclination error (MD −2.0 °; 95% CI 7.3 ° to −11.2 °; P = 0.68). There was a statistically significant effect of PSI on version error (MD −6.3 °; 95% CI −0.7 ° to −11.8 °; P = 0.03) and inclination error (MD −8.2 °; 95% CI −2.0 ° to −14.4 °; P < 0.01). However, there was no significant effect of PSI on offset error (MD −1.3 mm; 95% CI 0.7 to −3.3 °; P = 0.21).
Absolute radiographic outcomes
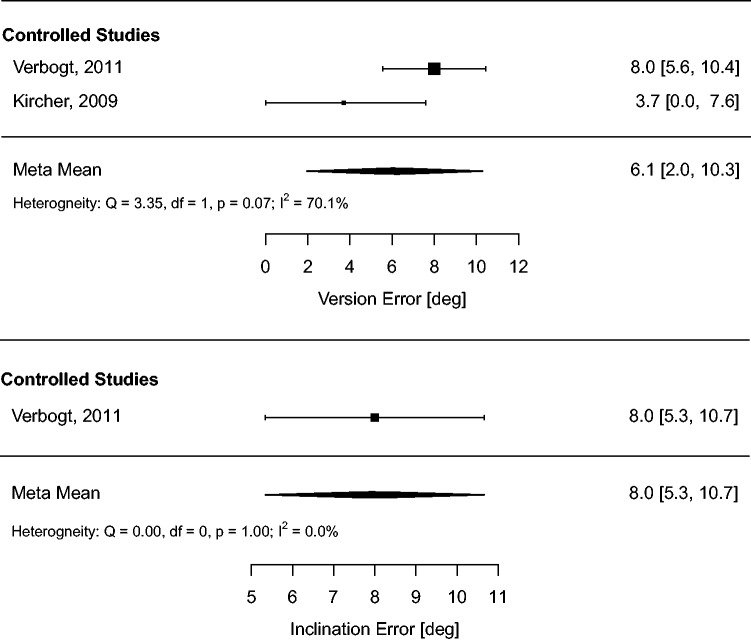
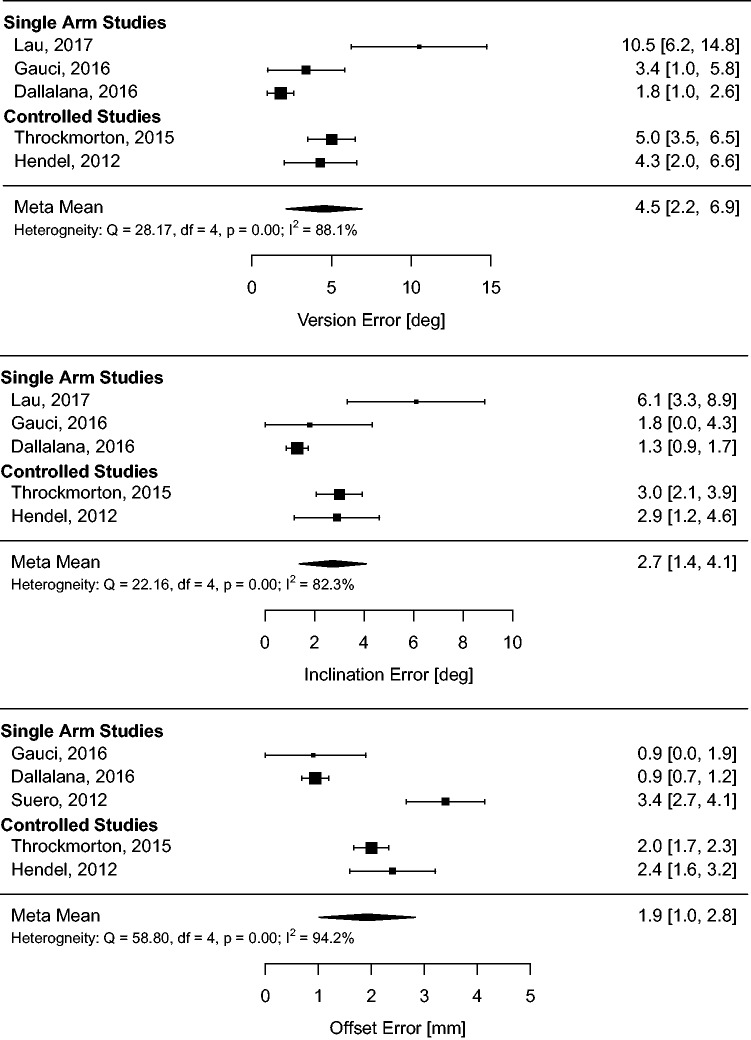
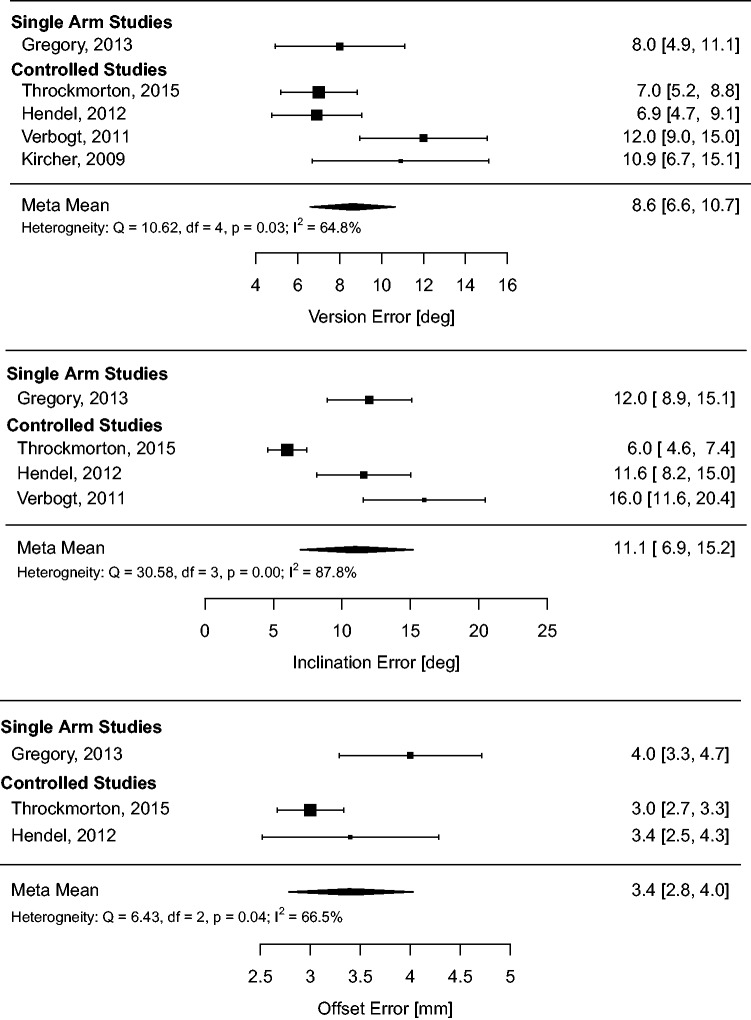
Meta-analysis of the radiographic outcome means (meta-means) on the full dataset was conducted to derive pooled estimates of performance and quantify heterogeneity in the full sample. The results of this analysis are summarized in Table 2 and forest plots (Figures 5 to 7).
Table 2.
Summary of absolute glenoid positioning outcomes (meta-means) and heterogeneity for each positioning method.
| Method | Version error (°) |
Inclination error (°) |
Offset error (mm) |
|||
|---|---|---|---|---|---|---|
| Mean (95% CI) | I2, p(Q) | Mean (95% CI) | I2, p(Q) | Mean (95% CI) | I2, p(Q) | |
| NAV | 6.1 (2.0, 10.3) | 70%, p = .07 | 8.0 (5.3, 10.7) | 0%, p = 1.0 | – | – |
| PSI | 4.5 (2.2, 6.9) | 88%, p<.01 | 2.7 (1.4, 4.1) | 82%, p<.01 | 1.9 (1.0, 2.8) | 94%, p<.01 |
| SI | 8.6 (6.6, 10.7) | 65%, p = .03 | 11.1 (6.9, 15.2) | 88%, p<.01 | 3.4 (2.8, 4.0) | 66%, p = .04 |
NAV: navigation; PSI: patient-specific instruments; SI: standard instrumentation.
Figure 5.
Meta-analysis of glenoid absolute positioning outcomes (meta-means) for NAV.
Figure 6.
Meta-analysis of glenoid absolute positioning outcomes (meta-means) for PSI.
Figure 7.
Meta-analysis of glenoid absolute positioning outcomes (meta-means) for SI.
Subject type and study type effects
Two multivariate meta-analysis models were fit to the full dataset (all studies) to assess the effect of subject type (cadaver versus patient) and study type (case series versus controlled study).
With positioning method and subject type as moderators, there was no significant effect of using human versus cadaver subjects for version error (P = 0.40), inclination error (P = 0.76), and offset error (P = 0.97).
With positioning method and study type as moderators, there was no significant difference in positioning outcome between single arm and controlled studies for version (P = 0.55), inclination (P = 0.54), and offset (P = 0.77).
Positioning outliers
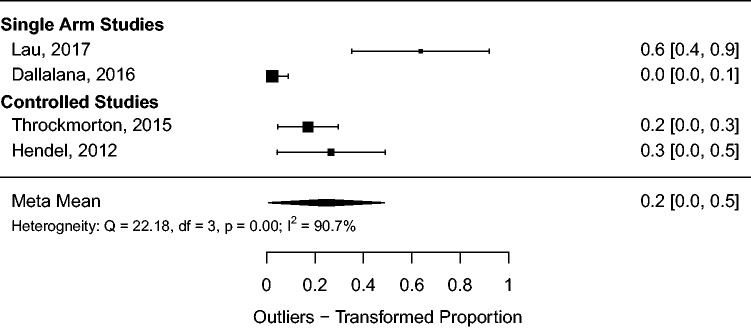
Outliers, defined as version or inclination errors greater than 10 ° or offset errors greater than 4 mm, were reported in only several studies in the PSI group.9,12,19,26 There was a statistically significant reduction in the number of outliers with PSI in comparison to SI in the controlled studies of Hendel et al.26 (27% versus 75%; P < 0.05) and Throckmorton et al.19 (17% versus 66%; P < 0.05). Meta-analysis of outlier raw proportion was performed for the PSI groups and is summarized in Figure 8 forest plot. The pooled estimate for outlier proportion is 20%, 95% CI (0%, 50%), with significant heterogeneity present (I2 = 91%, P < 0.01).
Figure 8.
Meta-analysis of malposition proportion for PSI.
Complications
There were no intraoperative complications for any NAV or PSI procedures in the included studies. The SI case series by Gregory et al.5 reported five (7%) glenoid vault perforations by the keel of the aTSA glenoid component on postoperative imaging, but did not assess long-term complications or revisions associated with this. Hendel et al.26 reported a postoperative axillary nerve palsy in their study's SI group. Long-term follow-up was only reported by Lau and Keith9 (to one year), with one case of scapular notching not requiring revision and one soft tissue infection in their PSI cohort of 11 patients.
Time, cost, and aborted procedures
Procedural time associated with NAV was only measured in the Kircher et al.29 study, with a mean 31 additional minutes for navigated procedures in comparison to controls. In addition, Kircher abandoned NAV in six of the 16 navigated procedures (37.5%) due to problems registering the glenoid anatomy. Verborgt et al.7 estimated that 20 min would be required for surgical NAV once a surgeon was accustomed to using the NAV system. No studies assessed the effect of PSI on surgical time, and no studies assessed the effect of NAV or PSI on procedure cost.
Effect on clinical outcomes
Of the studies included in this review, only Gregory et al.5 assessed the effect of malposition on clinical and radiographic outcomes in anatomic total shoulder arthroplasty. At one year postoperatively, glenoid malposition was associated with inferior range of motion. Retroversion and superior inclination of the glenoid was associated with an inferior Mole score (for radiolucent lines around the glenoid).
Bias across studies
The number of included studies permitted limited assessment of publication bias for the PSI intervention only. There was no evidence of funnel plot asymmetry with the Rank Correlation Test for version error (τ = 0.40, P = 0.48), inclination error (τ = 0.40, P = 0.48), or offset error (τ = 0.00, P = 1.0) outcomes; or the Egger Regression Test for version error (t = 2.8, P = 0.07), inclination error (t = 2.1, P = 0.12), or offset error (t = 1.1, P = 0.33). Note, however, the sensitivity of these tests to detect publication bias is limited by the sample size and number of studies included in this review.
Discussion
This systematic review and meta-analysis supports that both NAV and PSI improve positioning outcomes for the glenoid implant in total shoulder arthroplasty. This support is derived chiefly from a patient pool of 135 in four controlled studies (three RCTs, one NRS) that were evaluated as fair to good in quality but suffered from moderate to large heterogeneity for some positioning outcomes.
Heterogeneity of the glenoid positioning outcomes is particularly apparent when examining absolute outcomes across the full set of controlled and uncontrolled studies (see Figures 5 to 7). Heterogeneity is high in positioning system groups (NAV, PSI) and also in the SI group. The source of this heterogeneity is unknown; however, differences in surgical technique, implant, surgeon, radiographic image analysis technique, and positioning system are all potential significant factors. Although the multivariate meta-analysis demonstrated no significant effect of study type on outcome, the results of single arm studies should be interpreted with trepidation as there is little validity in comparing single arm positioning outcomes to such heterogeneous historical controls.
Twenty-six studies were identified that were relevant to our research question; however, only nine were ultimately included in the synthesis and analysis. The majority of exclusions resulted from study methodologies that were judged a priori to be noncomparable to the clinical setting; such as by using a synthetic scapula model without a soft tissue envelop, measuring accuracy from a guide-pin only (no prosthesis implantation), or using a single cadaver specimen for multiple procedures and data points. However, despite the measures taken to exclude what was considered nonrepresentative studies from analysis, significant heterogeneity still remains in the sample.
The pooled radiographic outcome meta-means calculated in this review corroborate the existing literature indicating glenoid positioning with SI is inaccurate.5,40 Pooling data from the positioning system controls and the SI series by Gregory et al.,5 we calculated meta-mean positioning errors (8.6 °, 11.1 °, 3.4 mm) very near to what is considered clinically significant positioning error values (10 °, 10 °, and 4 mm) for version, inclination, and offset, respectively.2,33 Pooled outlier proportion using these definitions remains high (20%) even for procedures guided with PSI.
In vitro and finite element studies have demonstrated that nonneutral anatomic TSA glenoid component version and inclination results in eccentric loading; increased stresses at the bone–prosthesis interface; and adverse effects on shoulder range of motion, kinematics, and stability.1,2,33,41–43 It has been inferred from the biomechanical data that malposition imparts a higher risk of fixation failure,1,2,44 with version or inclination errors greater than 10 °2,19,26,33 and offset errors greater than 4 mm19 considered clinically significant. Clinical studies have shown that malposition is common in failed TSA,45 and that malposition is associated with reduced range of motion,5 and increased radiolucent lines.5,46
The biomechanically optimal glenosphere baseplate position in reverse TSA is less clear. In vitro and finite element studies by Gutiérrez et al.47,48 indicate that neutral version and inferior inclination is desirable for concentric and laterally eccentric implants, but that neutral inclination is desirable for inferiorly eccentric implant designs. Malposition of the glenosphere (particularly superior offset and superior inclination) is associated with scapular notching, a radiographic finding commonly observed after rTSA with varying severity. Scapular notching in turn is associated with inferior clinical outcomes (range of motion, strength, pain) and the development of worrying radiolucent lines.1,49–51 A link between scapular notching and early glenoid component failure has been suggested,52,53 but not conclusively demonstrated.54
Little to no data was found to support NAV or PSI from a health care economics perspective. No study identified in this review estimated the costs associated with use of NAV or PSI. Kircher et al.29 reported a 31 min increase in operative time associated with successful NAV procedures and aborted use of NAV in 37.5% of cases due to technical challenges.
No studies identified in the literature search directly compare NAV to PSI for shoulder arthroplasty, and the large heterogeneity in observed outcomes within positioning groups preclude meaningful indirect comparison (network meta-analysis). It is apparent that there has been a trend away from NAV and toward PSI in terms of publication activity. This may reflect the notion that NAV can be more labor intensive, time consuming, costly, and unreliable than PSI and SI for total shoulder arthroplasty.7 The logistic challenges associated with PSI, chiefly the cost and time required to template, manufacture, and order a custom device may have better acceptability to clinicians than the disadvantages associated with NAV.
This study demonstrates that use of either NAV or PSI provides a statistically significant improvement in radiographic positioning outcomes in total shoulder arthroplasty. The magnitude of the improvement over SI is substantial; however, it is unclear to what extent these improvements will improve prosthesis longevity or patient functional outcomes. There is insufficient data to evaluate or compare the cost-effectiveness of these interventions, and therefore we cannot recommend widespread adoption at this time. However, this study should alert shoulder surgeons to the high rate of glenoid component malposition that can occur with use of SI and supports either NAV or PSI as potential options for reducing the incidence of glenoid malposition in total shoulder arthroplasty.
Conclusions
SI for glenoid positioning in total shoulder arthroplasty is inaccurate; however, the clinical significance of this has not been well established. NAV and PSIs improve glenoid positioning outcomes, but with significant heterogeneity of results across studies. Whether the improvement in positioning outcomes achieved with these technologies translate to better clinical outcomes for patients undergoing total shoulder arthroplasty is unknown.
Supplementary Material
Supplemental material for this article is available online.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Review and Patient Consent
Not required for this article.
References
- 1.Nyffeler RW, Werner CML, Gerber C. Biomechanical relevance of glenoid component positioning in the reverse Delta III total shoulder prosthesis. J Shoulder Elbow Surg 2005; 14: 524–528. [DOI] [PubMed] [Google Scholar]
- 2.Iannotti JP, Spencer EE, Jr, Winter U, et al. Prosthetic positioning in total shoulder arthroplasty. J Shoulder Elbow Surg 2005; 14: S111–S121. [DOI] [PubMed] [Google Scholar]
- 3.Matsen III FA. Glenoid component failure in total shoulder arthroplasty. J Bone Jt Surg Am 2008; 90: 885. [DOI] [PubMed] [Google Scholar]
- 4.Norris TR, Iannotti JP. Functional outcome after shoulder arthroplasty for primary osteoarthritis: a multicenter study. J Shoulder Elbow Surg 2002; 11: 130–135. [DOI] [PubMed] [Google Scholar]
- 5.Gregory TM, Sankey A, Augereau B, et al. Accuracy of glenoid component placement in total shoulder arthroplasty and its effect on clinical and radiological outcome in a retrospective, longitudinal, monocentric open study. PLoS One 2013; 8: e75791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iannotti JP, Greeson C, Downing D, et al. Effect of glenoid deformity on glenoid component placement in primary shoulder arthroplasty. J Shoulder Elbow Surg 2012; 21: 48–55. [DOI] [PubMed] [Google Scholar]
- 7.Verborgt O, Vanhees M, Heylen S, et al. Computer navigation and patient-specific instrumentation in shoulder arthroplasty. Sports Med Arthrosc Rev 2014; 22: e42–e49. [DOI] [PubMed] [Google Scholar]
- 8.Gauci MO, Boileau P, Baba M, et al. Patient-specific glenoid guides provide accuracy and reproducibility in total shoulder arthroplasty. Bone Jt J 2016; 98-B: 1080–1085. [DOI] [PubMed] [Google Scholar]
- 9.Lau SC, Keith PPA. Patient-specific instrumentation for total shoulder arthroplasty: not as accurate as it would seem. J Shoulder Elbow Surg 2018; 27: 90–95. [DOI] [PubMed] [Google Scholar]
- 10.Mulligan RP, Azar FM, Throckmorton TW. Generic targeting guides place revision glenoid components in more anatomic version than traditional techniques. J Shoulder Elbow Surg 2017; 26: 786–791. [DOI] [PubMed] [Google Scholar]
- 11.Theopold J, Pieroh P, Scharge ML, et al. Improved accuracy of K-wire positioning into the glenoid vault by intraoperative 3D image intensifier-based navigation for the glenoid component in shoulder arthroplasty. Orthop Traumatol Surg Res 2016; 102: 575–581. [DOI] [PubMed] [Google Scholar]
- 12.Dallalana RJ, McMahon RA, East B, et al. Accuracy of patient-specific instrumentation in anatomic and reverse total shoulder arthroplasty. Int J Shoulder Surg 2016; 10: 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eraly K, Stoffelen D, Vander Sloten J, et al. A patient-specific guide for optimizing custom-made glenoid implantation in cases of severe glenoid defects: an in vitro study. J Shoulder Elbow Surg 2016; 25: 837–845. [DOI] [PubMed] [Google Scholar]
- 14.Heylen S, Van Haver A, Vuylsteke K, et al. Patient-specific instrument guidance of glenoid component implantation reduces inclination variability in total and reverse shoulder arthroplasty. J Shoulder Elbow Surg 2016; 25: 186–192. [DOI] [PubMed] [Google Scholar]
- 15.Lewis GS, Stevens NM, Armstrong AD. Testing of a novel pin array guide for accurate three-dimensional glenoid component positioning. J Shoulder Elbow Surg 2015; 24: 1939–1947. [DOI] [PubMed] [Google Scholar]
- 16.Mulligan RP, Azar FM, Throckmorton TW. Is a generic targeting guide useful for glenoid component placement in shoulder arthroplasty?. J Shoulder Elbow Surg 2016; 25: e90–e95. [DOI] [PubMed] [Google Scholar]
- 17.Venne G, Rasquinha BJ, Pichora D, et al. Comparing conventional and computer-assisted surgery baseplate and screw placement in reverse shoulder arthroplasty. J Shoulder Elbow Surg 2015; 24: 1112–1119. [DOI] [PubMed] [Google Scholar]
- 18.Iannotti JP, Weiner S, Rodriguez E, et al. Three-dimensional imaging and templating improve glenoid implant positioning. J Bone Jt Surg Am 2015; 97: 651–658. [DOI] [PubMed] [Google Scholar]
- 19.Throckmorton TW, Gulotta LV, Bonnarens FO, et al. Patient-specific targeting guides compared with traditional instrumentation for glenoid component placement in shoulder arthroplasty: a multi-surgeon study in 70 arthritic cadaver specimens. J Shoulder Elbow Surg 2015; 24: 965–971. [DOI] [PubMed] [Google Scholar]
- 20.Verstraeten TRGM, Berghs B, Tongel AV, et al. Can an extracorporeal glenoid aiming device be used to optimize the position of the glenoid component in total shoulder arthroplasty?. Int J Shoulder Surg 2015; 9: 114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walch G, Vezeridis PS, Boileau P, et al. Three-dimensional planning and use of patient-specific guides improve glenoid component position: an in vitro study. J Shoulder Elbow Surg 2015; 24: 302–309. [DOI] [PubMed] [Google Scholar]
- 22.Iannotti J, Baker J, Rodriguez E, et al. Three-dimensional preoperative planning software and a novel information transfer technology improve glenoid component positioning. J Bone Jt Surg 2014; 96: e71. [DOI] [PubMed] [Google Scholar]
- 23.Levy JC, Everding NG, Frankle MA, et al. Accuracy of patient-specific guided glenoid baseplate positioning for reverse shoulder arthroplasty. J Shoulder Elbow Surg 2014; 23: 1563–1567. [DOI] [PubMed] [Google Scholar]
- 24.Stübig T, Petri M, Zeckey C, et al. 3D navigated implantation of the glenoid component in reversed shoulder arthroplasty. Feasibility and results in an anatomic study. Int J Med Robot 2013; 9: 480–485. [DOI] [PubMed] [Google Scholar]
- 25.Suero EM, Citak M, Lo D, et al. Use of a custom alignment guide to improve glenoid component position in total shoulder arthroplasty. Knee Surg Sports Traumatol Arthrosc 2013; 21: 2860–2866. [DOI] [PubMed] [Google Scholar]
- 26.Hendel MD, Bryan JA, Barsoum WK, et al. Comparison of patient-specific instruments with standard surgical instruments in determining glenoid component position. J Bone Jt Surg 2012; 94: 2167–2175. [DOI] [PubMed] [Google Scholar]
- 27.Briem D, Ruecker AH, Neumann J, et al. 3D fluoroscopic navigated reaming of the glenoid for total shoulder arthroplasty (TSA). Comput Aided Surg 2011; 16: 93–99. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen D, Ferreira LM, Brownhill JR, et al. Improved accuracy of computer assisted glenoid implantation in total shoulder arthroplasty: an in-vitro randomized controlled trial. J Shoulder Elbow Surg 2009; 18: 907–914. [DOI] [PubMed] [Google Scholar]
- 29.Kircher J, Wiedemann M, Magosch P, et al. Improved accuracy of glenoid positioning in total shoulder arthroplasty with intraoperative navigation: a prospective-randomized clinical study. J Shoulder Elbow Surg 2009; 18: 515–520. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen D, Ferreira LM, Brownhill JR, et al. Design and development of a computer assisted glenoid implantation technique for shoulder replacement surgery. Comput Aided Surg 2007; 12: 152–159. [DOI] [PubMed] [Google Scholar]
- 31.Sadoghi P, Vavken J, Leithner A, et al. Benefit of intraoperative navigation on glenoid component positioning during total shoulder arthroplasty. Arch Orthop Trauma Surg 2015; 135: 41–47. [DOI] [PubMed] [Google Scholar]
- 32.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farron A, Terrier A, Büchler P. Risks of loosening of a prosthetic glenoid implanted in retroversion. J Shoulder Elbow Surg 2006; 15: 521–526. [DOI] [PubMed] [Google Scholar]
- 34.Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wright JG, Swiontkowski MF, Heckman JD. Introducing levels of evidence to the journal. JBJS 2003; 85: 1. [PubMed] [Google Scholar]
- 36.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50: 1088–1101. [PubMed] [Google Scholar]
- 37.Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Begg CB, Pilote L. A model for incorporating historical controls into a meta-analysis. Biometrics 1991; 47: 899–906. [PubMed] [Google Scholar]
- 39.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw 2010; 36: 1–48. [Google Scholar]
- 40.Nyffeler RW, Jost B, Pfirrmann CW, et al. Measurement of glenoid version: conventional radiographs versus computed tomography scans. J Shoulder Elbow Surg 2003; 12: 493–496. [DOI] [PubMed] [Google Scholar]
- 41.Oosterom R, Rozing PM, Bersee HEN. Effect of glenoid component inclination on its fixation and humeral head subluxation in total shoulder arthroplasty. Clin Biomech 2004; 19: 1000–1008. [DOI] [PubMed] [Google Scholar]
- 42.Hopkins AR, Hansen UN, Amis AA, et al. The effects of glenoid component alignment variations on cement mantle stresses in total shoulder arthroplasty. J Shoulder Elbow Surg 2004; 13: 668–675. [DOI] [PubMed] [Google Scholar]
- 43.Favre P, Moor B, Snedeker JG, et al. Influence of component positioning on impingement in conventional total shoulder arthroplasty. Clin Biomech 2008; 23: 175–183. [DOI] [PubMed] [Google Scholar]
- 44.Strauss EJ, Roche C, Flurin P-H, et al. The glenoid in shoulder arthroplasty. J Shoulder Elbow Surg 2009; 18: 819–833. [DOI] [PubMed] [Google Scholar]
- 45.Franta AK, Lenters TR, Mounce D, et al. The complex characteristics of 282 unsatisfactory shoulder arthroplasties. J Shoulder Elbow Surg 2007; 16: 555–562. [DOI] [PubMed] [Google Scholar]
- 46.Ho JC, Sabesan VJ, Iannotti JP. Glenoid component retroversion is associated with osteolysis. J Bone Joint Surg Am 2013; 95: e82. [DOI] [PubMed] [Google Scholar]
- 47.Gutiérrez S, Walker M, Willis M, et al. Effects of tilt and glenosphere eccentricity on baseplate/bone interface forces in a computational model, validated by a mechanical model, of reverse shoulder arthroplasty. J Shoulder Elbow Surg 2011; 20: 732–739. [DOI] [PubMed] [Google Scholar]
- 48.Gutiérrez S, Greiwe RM, Frankle MA, et al. Biomechanical comparison of component position and hardware failure in the reverse shoulder prosthesis. J Shoulder Elbow Surg 2007; 16: S9–S12. [DOI] [PubMed] [Google Scholar]
- 49.Simovitch RW, Zumstein MA, Lohri E, et al. Predictors of scapular notching in patients managed with the Delta III reverse total shoulder replacement. J Bone Joint Surg Am 2007; 89: 588–600. [DOI] [PubMed] [Google Scholar]
- 50.Lévigne C, Garret J, Boileau P, et al. Scapular notching in reverse shoulder arthroplasty: is it important to avoid it and how?. Clin Orthop 2011; 469: 2512–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sadoghi P, Leithner A, Vavken P, et al. Infraglenoidal scapular notching in reverse total shoulder replacement: a prospective series of 60 cases and systematic review of the literature. BMC Musculoskelet Disord 2011; 12: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vanhove B, Beugnies A. Grammont's reverse shoulder prosthesis for rotator cuff arthropathy. A retrospective study of 32 cases. Acta Orthop Belg 2004; 70: 219–225. [PubMed] [Google Scholar]
- 53.Delloye C, Joris D, Colette A, et al. Complications mécaniques de la prothèse totale inversée de l'épaule. Rev Chir Orthop Reparatrice Appar Mot 2002; 88: 410–414. [PubMed] [Google Scholar]
- 54.Nicholson GP, Strauss EJ, Sherman SL. Scapular notching: recognition and strategies to minimize clinical impact. Clin Orthop 2011; 469: 2521–2530. [DOI] [PMC free article] [PubMed] [Google Scholar]