Abstract
Numerous studies have examined how alexithymia (difficulty identifying and describing one’s emotions and a preference for externally oriented thinking) relates to chronic pain and associated disability. We conducted a systematic review and meta-analysis to summarize individual studies that either assessed alexithymia in individuals with chronic pain vs controls or related alexithymia to pain intensity, physical interference, depression, and anxiety. We searched MEDLINE, Embase, and PsyclNFO from inception through June 2017; 77 studies met the criteria (valid assessment of alexithymia in adults or children with any chronic pain condition) and were included in analyses (n = 8019 individuals with chronic pain). Primary analyses indicated that chronic pain samples had significantly higher mean alexithymia scores compared with nonclinical (d = 0.81) and clinical nonpain (d = 0.55) controls. In chronic pain samples, alexithymia was significantly positively associated with pain intensity (d = 0.20), physical interference (d = 0.17), depression (d = 0.46), and anxiety (d = 0.43). Secondary meta-analyses of 14 studies that conducted partial correlations that controlled for negative affect-related measures revealed that alexithymia was no longer significantly related to pain intensity or interference. Meta-analysis findings demonstrated that alexithymia is elevated in individuals with chronic pain and related to greater pain intensity and physical interference, although the latter relationships may be accounted for by negative affect. Critical future work is needed that examines alexithymia assessed using non-self-report measures, develops a person-centered perspective on this construct, and identifies how alexithymia is relevant to the assessment and treatment of individuals with chronic pain.
Keywords: Chronic pain, Alexithymia, Pain intensity, Physical interference
1. Introduction
Chronic pain is common, occurring in approximately 11% to 31% of adults50,87 and children.46,55 Chronic pain affects physical, social, and emotional functioning13,93 and can be severely disabling.46,87 Chronic pain is also associated with heightened symptoms of depression and anxiety.2,89,102 Given the high costs and societal burden of chronic pain,30,37 identifying biopsychosocial treatment targets to improve functioning in this population is critical.
Individual differences in emotional awareness may relate to the experience of chronic pain, and various constructs within this domain have been studied and debated. One specific concept, labeled with the term alexithymia, has received considerable theoretical and empirical attention over the past several decades.118 Alexithymia refers to the inability to label and describe one’s emotions and a preference for externally oriented thinking (EOT).110 Alexithymia has been assessed primarily with self-report measures, the most common of which is the Toronto Alexithymia Scale-20 (TAS-206). Alexithymia has been found to be elevated in adults and youth with chronic pain compared with healthy samples22,31 and may be associated with greater pain intensity and disability.3,72 In addition, alexithymia is associated with greater depressive and anxiety symptoms in various populations63,64 including individuals with chronic pain.23 This may be because alexithymia relates to reduced ability to successfully regulate, or reduce, negative emotion.115 Although a number of factors—such as self-efficacy, stressor intensity, contextual cues, and cultural norms—influence an individual’s choice of emotion regulation strategy, increased awareness of one’s emotional states is thought to be key to taking active steps towards adaptive emotion regulation.39,54 In his extended process theory of emotion regulation, Gross39 explains that emotion identification enables an individual to take adaptive steps to regulate their emotions and is thus an essential first step in the emotion regulation process. Lieberman et al.65,66 show that the simple act of applying a verbal label to one’s emotional state results in reductions in negative affect both subjectively and neurologically, further demonstrating the emotion-regulating properties of emotional awareness.
Although most research examining the relationship between alexithymia and chronic pain has relied on cross-sectional methods, some prospective studies show that alexithymia may constitute a risk factor for pain outcomes.10,100 Recent research highlights the malleability of alexithymia with psychological interventions,11,14,79 which increases the relevance of alexithymia to chronic pain clinical practice. For example, in a recent single-arm trial, adults with chronic musculoskeletal pain undergoing group intervention targeting emotional awareness and expression demonstrated improvements in alexithymia, which were associated with reductions in psychological distress and improvements in pain intensity and interference.12 A better understanding of the relationship between alexithymia and chronic pain has potential to clarify aspects of emotion awareness that relate to chronic pain and lead to more specific psychological prevention or intervention programs to improve emotional functioning in individuals with chronic pain.
A great deal of research has examined alexithymia in individuals with chronic pain; however, no systematic reviews or meta-analyses of this literature have been conducted to summarize individual studies and provide estimates of the magnitude of the relationships between alexithymia and relevant pain variables. We conducted a systematic review of alexithymia in people with chronic pain and its relation to pain intensity, physical interference, anxiety, and depression. Specifically, we had 3 primary aims: (1) to determine levels of alexithymia in adults and youth with chronic pain; (2) to estimate the magnitude of the difference in alexithymia levels between samples with chronic pain and various comparison samples; and (3) to estimate the associations between alexithymia and pain intensity, physical function, and symptoms of depression and anxiety in people with chronic pain. There is ongoing debate in this field as to whether observed associations between measures of alexithymia, pain intensity, and functional impairment are confounded by depression or psychological distress.43,75 Thus, a secondary aim was to identify and summarize studies that covaried measures of negative affect when testing the relationship between alexithymia and pain intensity and interference. In conducting the present systematic review and meta-analysis, we hope to make the available evidence more accessible, to facilitate a critical examination of the application of this construct, and to generate broad conclusions that help pave the path for next steps in the area of emotions and pain.
2. Methods
The systematic review and meta-analysis protocol for the current study was registered on September 25, 2017, and can be found through PROSPERO (ID: CRD42017077551).
2.1. Search methods
We conducted systematic searches in MEDLINE, PsyclNFO, and EMBASE from the inception of each database through June 2017. We included general search terms of “pain,” “alexithymia,” and related search terms to capture all studies conducted in this area. See Appendix for the specific search criteria. Two authors (R.A. and E.F.) independently sorted through abstracts to determine possible relevance (available as supplemental digital content at http://links.lww.com/PAIN/A734). The 2 resulting abstract lists were then reviewed together, and inconsistencies were resolved. Where disagreements emerged, a third author (T.P.) was consulted until 100% agreement was achieved. Next, articles identified as possibly relevant were reviewed in full to determine their eligibility against inclusion/exclusion criteria. Once a final set of studies was generated from our search, we reviewed their reference lists to ensure that other relevant articles had not been overlooked. Studies identified in this manner were then reviewed and assessed for eligibility. In addition, as described below, we contacted authors of articles that were missing key data or analyses to request those data.
2.2. Inclusion criteria
Inclusion criteria for individual studies were the following: (1) included youth (age <18 years) or adults (age ≥18 years) with chronic pain conditions including, but not limited to, headache, abdominal pain, musculoskeletal pain, and autoimmune disease-related pain; (2) assessed alexithymia using an established, psychometrically sound measure; and (3) published in English in a peer-reviewed journal.
2.3. Exclusion criteria
We excluded studies that (1) included chronic pain participants with primary psychiatric diagnoses (eg, substance abuse); (2) combined individuals with acute and chronic pain without reporting data separately for these groups; (3) screened and recruited based on “high” or “low” alexithymia; and (4) had less than 20 participants.
2.4. Data extraction
Data were extracted by R.A. and R.V. We extracted only the baseline data from longitudinal studies. When data from the same study were published in different articles, we extracted data from the earliest published article that met our inclusion criteria, unless a subsequently published article reported a larger sample size. Some studies provided data on distinct pain samples (eg, people with fibromyalgia vs headache). Some studies also provided data on nonclinical comparison samples and/or nonpain clinical samples (eg, diabetes). These data were extracted separately.
2.4.1. Study characteristics
We extracted study characteristics including sample size, chronic pain characteristics (condition, location, and duration), and demographic information (age, sex, and country of study) from included studies. When studies included a comparison sample, we extracted the sample size and type of comparison.
2.4.2. Alexithymia
Measures of alexithymia typically yield a continuous alexithymia score and a clinical cutoff score. The most commonly used measure, the Toronto Alexithymia Scale-20 (TAS-206), which was adapted from the original 26-item TAS,5 yields a continuous score ranging from 20 to 100, with higher scores indicating greater alexithymia and a clinical cutoff score of ≥61 indicating elevated alexithymia.106 The TAS-20 has 3 subscales: difficulty identifying feelings (DIF), difficulty describing feelings (DDF), and EOT. The Alexithymia Questionnaire for Children (AQC)103 is parallel to the TAS-20 but is linguistically adapted for children. We extracted mean alexithymia total and subscale scores and percentage of people above clinical cutoff scores in both chronic pain and comparison samples. We also recorded information on how alexithymia was assessed (ie, assessment tool and scoring parameters).
2.4.3. Correlation analysis
We extracted correlation coefficients describing the relationship between alexithymia and pain intensity, physical interference, anxiety, and depression. Some studies have examined partial correlations between alexithymia and pain intensity and physical interference while controlling for an index of depression or psychological distress. In the present review, we identified studies that conducted such partial correlations and extracted the resulting correlation coefficients for use in a secondary meta-analysis of partial correlations. A number of studies reported correlations selectively based on significance. Where we were unable to achieve representative analyses, we applied a rule established a priori to exclude selectively reported findings to avoid biasing results towards statistically significant findings.
2.4.4. Study quality
For the purpose of better characterizing studies included in the meta-analysis, we extracted information related to study quality. We adapted the National Institutes of Health Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies,88 excluding items that were irrelevant or inconsistent with our study inclusion/exclusion criteria. Of the remaining items, some were adapted or reworded to more directly align with our criteria. Given the numerous international studies in this field, we evaluated whether authors included an alexithymia measure that had been validated in their country’s language. We assigned studies 1 point per each criterion met, which were summed for a total quality score of 0 to 8 (0 indicating lowest quality and 8 highest quality).
2.4.5. Missing data
Of studies that met our inclusion criteria, many were missing some or all data points relevant to our meta-analyses. In many cases, it was clear from the authors’ Methods section that relevant variables had been measured. Specifically, we requested (1) mean values and SDs of alexithymia total and subscale scores;(2) correlations between alexithymia and key variables (pain intensity, physical interference, depression, and anxiety); and (3) partial correlations of alexithymia with pain intensity and physical interference, controlling for negative affect (eg, explicit measures of negative, depression, or anxiety). We provided 1 follow-up email for those authors who did not initially respond.
2.5. Data analysis
For aim 1, we summarized alexithymia (total and subscales) scores and percentage of people exceeding clinical cutoff scores separately for adults and youth with chronic pain. All pooled mean values were calculated using the formula indicated in Higgins and Green42 (p. 177).
For aim 2, we meta-analyzed the effect sizes of group differences in alexithymia scores between chronic pain and comparison groups with random effects models using Review Manager 5.1. We calculated standardized mean differences and 95% confidence intervals (CIs). Some studies compared 2 chronic pain samples with a single nonclinical comparison sample. In such cases, we extracted each of these comparisons separately and halved the n of the nonclinical sample to prevent inflating the weight of the individual study. For total alexithymia scores, we included in our analyses any alexithymia measure and scoring convention. However, for alexithymia subscale analyses, we examined only the TAS-20, given that this was the most commonly used measure, and other measures have different factor structures.
For aim 3, we meta-analyzed correlations of alexithymia with pain intensity, physical interference, depression, and anxiety using random effects models. Finally, for our secondary analysis, we meta-analyzed partial correlations that examined the relationship between alexithymia and pain intensity and physical interference when controlling for measures of negative affect using random effects models. Meta-analyses of correlation coefficients were conducted in STATA using the DerSimonian-Laird random effects method. We examined the effect size and 95% CIs. When there were more than 8 studies in a meta-analysis, trim and fill analyses were used to detect publication bias using Duval and Tweedie’s25 method and Rosenthal’s fail-safe N (1979). Trim and fill analyses evaluate the overall effect and variance of each study included in a meta-analysis, identifies possible missing studies that may have resulted from publication bias (eg, selective publication of statistically significant results), and imputes values for these missing studies.25 We subsequently reran meta-analyses to ensure that significant findings were not the result of publication bias.
For all meta-analyses, we considered between-study heterogeneity (I2) and interpreted findings using Cochrane standards as a guide (Higgins and Green42). Specifically, we adopted the following cutoffs for describing heterogeneity: (0%−29% low heterogeneity; 30%−49% moderate heterogeneity; 50%−74% substantial heterogeneity; and 75%−100% considerable heterogeneity). To explore whether variability in study quality influenced study findings, we conducted correlations between effect size estimates and quality ratings for analyses of group differences in alexithymia, and for analyses of correlations of alexithymia with pain intensity, physical interference, depression, and anxiety.
3. Results
3.1. Study selection
Our systematic search resulted in 1240 abstracts. After screening these abstracts, we identified 157 full texts to review for eligibility. Through review, we excluded 80 studies (Fig. 1). This resulted in 77 full studies for analysis. Among these 77 studies, 10 reported data from distinct chronic pain samples (eg, a study reported data from 2 distinct samples of individuals with an autoimmune disease and headache). We preserved data from these distinct samples and therefore included a total of 87 chronic pain samples in our review. We use the terminology “study” to identify overarching studies, or articles, and the terminology “sample” to identify a distinct group of individuals with chronic pain.
Figure 1.
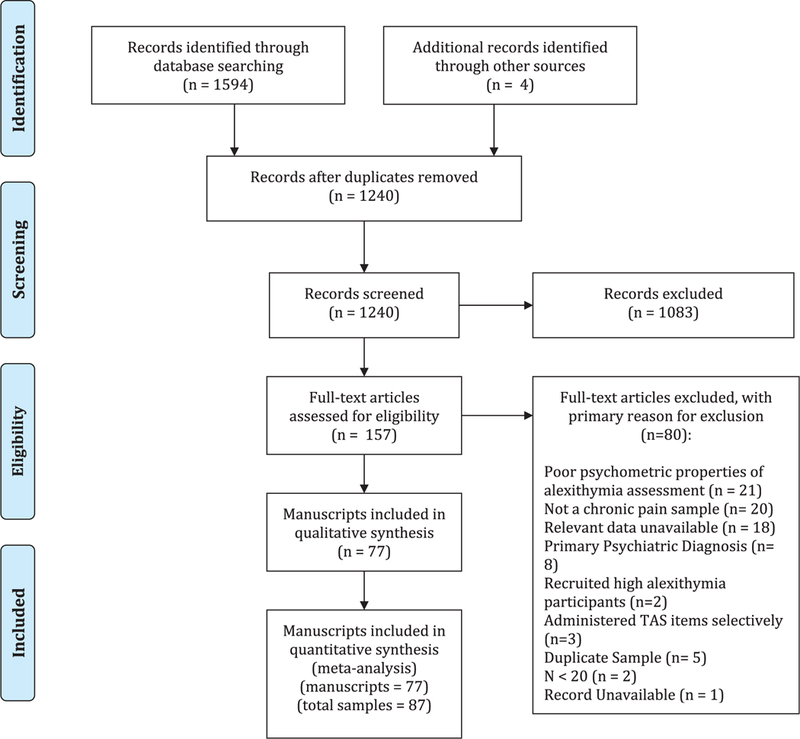
PRISMA flowchart diagramming studies Included In meta-analyses.
3.2. Missing data
We contacted 71 authors to request additional data and analyses. Additional data were obtained for 22 studies. Authors of an additional 17 studies responded but could not complete the request because they lacked access to the original data (n = 12) or their schedules were too busy (n = 5).
3.3. Study characteristics
From the 77 studies, there were data from 82 adult and 5 youth chronic pain samples (Table 1). Of note, 1 additional study examined a mixed sample of adolescents and young adults (aged 16–20 years) with inflammatory bowel disease or irritable bowel syndrome.44 Developmentally, alexithymia scores in individuals within this age range are more similar to adults,95 and therefore, we categorized this study as an adult sample.
Table 1.
Summary of studies included in meta-analysis.
| Study author and year | Pain condition | Study design |
Country of study |
Pain group (N) |
Age (mean and SD) |
Sex (% female) |
Alexithymia measure |
Quality assessment (0–8) |
|---|---|---|---|---|---|---|---|---|
| Adult studies | ||||||||
| Ak (2004)1 | Mixed chronic pain | G, M | Turkey | 30 | 40.6 (11.4) | 83% | TAS-20 | 5 |
| Atagun (2012)3 | Fibromyalgia | C, M | Turkey | 57 | 40.9 (6.9) | 100% | TAS-20 | 5 |
| Baeza-Velasco(2012)4,* | Mixed chronic pain | C, G, M | France | 39 | 52.2 (8.9) | 100% | TAS-20 | 3 |
| Balaban (2012)8 | Headache | M | Turkey | 31 | 20.9 (1.7) | 81% | TAS-20 | 5 |
| Burger (2012)12,* | MSK | C, M | United States | 72 | 49.3 (15.6) | 79% | TAS-20 | 8 |
| Castelli (2012)16 | TMD | C, M | Italy | 45 | 38.9 (11.6) | 100% | TAS-20 | 4 |
| Castelli (2012)15 | Fibromyalgia | G, M | Italy | 55 | 52.8 (10.5) | 100% | TAS-20 | 4 |
| Cerutti (2016)17,*,† | Headache | M | Italy | 53 | 41.8 (3.9) | 53% | TAS-20 | 4 |
| Chang (2017)18,† | Chronic pain | G, M | Taiwan | 121 | 54.0 (15.6) | 9% | TAS-20 | 6 |
| Chang (2017)18,† | Fibromyalgia | G, M | Taiwan | 58 | 57.5 (15.8) | 71% | TAS-20 | 6 |
| Cologno (2005)19 | Headache | M | Italy | 35 | 38.0 (9.3) | 80% | TAS-20 | 3 |
| Di Tella (2017)23,* | Fibromyalgia | C, M | Italy | 159 | 52.5 (10.2) | 100% | TAS-20 | 6 |
| Duruk (2015)24,* | Fibromyalgia | C, G, M | Turkey | 35 | 41 (9.7) | 100% | TAS-20 | 6 |
| Esin (2017)26 | Headache | M | Russia | 137 | 40.8 (6.3) | 61% | TAS-26 | 3 |
| Evren (2006)27 | Fibromyalgia | C, M | Turkey | 51 | 37.2 (9.3) | 100% | TAS-20 | 6 |
| Fernandez (1989)28 | Autoimmune | G, M | India | 40 | 36.1 (11.3) | 65% | TAS-26 | 3 |
| Galli (2017)29,* | Headache | G, M | Italy | 80 | 44.7 (8.6) | 74% | TAS-20 | 5 |
| Ghiggia (2017)33,* | Fibromyalgia | C, G, M | Italy | 181 | 51.7 (10.2) | 100% | TAS-20 | 5 |
| Glaros (2005)34 | TMD | C, G, M | United States | 49 | 39.9 (12) | NR | TAS-20 | 5 |
| Gregory (2000)35 | Chronic pain | G, M | United States | 140 | 44.7 (11.2) | 75% | TAS-20 | 5 |
| Gregory (2005)36,† | Chronic pain | G, M | United States | 46 | NR | NR | TAS-20 | 6 |
| Gregory (2005)36,† | MSK | G, M | United States | 49 | NR | NR | TAS-20 | 7 |
| Gulec (2008)40,* | Fibromyalgia | C, M | Turkey | 75 | 43.6 (10.6) | 100% | TAS-20 | 7 |
| Haas (2013)41 | TMD | G | Germany | 20 | 40.6 (14.7) | 90% | TAS-26 | 7 |
| Hosoi (2010)43 | Autoimmune | C | United States | 129 | 52 (12.4) | 56% | TAS-20 | 6 |
| Huang (2016)44 | Mixed chronic pain | G, M | United States | 20 | 17 (NR) | 55% | TAS-20 | 5 |
| Huber (2009)45,* | Fibromyalgia | C, M | Italy | 68 | 43.4 (11.1) | 100% | TAS-20 | 5 |
| Jasinski (2016)47 | MSK | M | United States | 95 | 46.9 (9.7) | 52% | TAS-20 | 4 |
| Jerjes (2007)48 | Headache | G, M | United Kingdom | 51 | 40 (13) | 67% | TAS-20 | 5 |
| Jerlang (1997)49 | BMS | M | Denmark | 20 | 67 (NR) | 100% | TAS-26 | 4 |
| Johannsen (2017)51,* | Cancer | C, M | Denmark | 129 | 56.8 (9.1) | 100% | TAS-20 | 4 |
| Karahan (2016)53 | Autoimmune | G, M | Turkey | 148 | 52.6 (12.0) | 78% | TAS-20 | 5 |
| Kojima (2014)57,* | Autoimmune | C, M | Japan | 213 | 60 (12) | 82% | TAS-20 | 7 |
| Kosturek (1998)59 | MSK | M | United States | 50 | 43.6 (10.9) | 46% | TAS-20 | 6 |
| Kugu (2009)60 | Fibromyalgia | G, M | Turkey | 54 | 48.1 (8.1) | 100% | TAS-20 | 4 |
| Lumley (1997)68 | Chronic pain | C, G | United States | 30 | 40.4 (NR) | 70% | TAS-26 | 5 |
| Lumley (20 02)74,* | Myofascial | C, M | United States | 80 | 48.7 (11.8) | 75% | TAS-20 | 6 |
| Lumley (2005)72,† | Autoimmune | C, M | United States | 155 | 55 (NR) | 88% | TAS-20 | 5 |
| Lumley (2005)72,† | Headache | C, M | United States | 160 | 31.8 (NR) | 84% | TAS-20 | 5 |
| Makino (2013)75 | Mixed chronic pain | C, M | Japan | 128 | 52.3 (16.3) | 74% | TAS-20 | 7 |
| Margalit (2014)76,† | CRPS | C, M | Israel | 30 | 38.3 (14.3) | 40% | TAS-20 | 5 |
| Margalit (2014)76,† | MSK | C, M | Israel | 30 | 38.2 (12.5) | 40% | TAS-20 | 5 |
| Marino (2015)77 | BMS | C, G, M | Italy | 58 | 65.6 (10.5) | 79% | TAS-20 | 4 |
| Martinez (2015)78,* | Fibromyalgia | C | Spain | 100 | 48.4 (7.5) | 100% | TAS-20 | 4 |
| Melin (2010)79,* | Chronic pain | C, M | Sweden | 59 | 46 (NR) | 88% | TAS-20 | 4 |
| Melis (2014)80,* | Pelvic pain | C, M | Italy | 41 | 31.5 (6.4) | 100% | TAS-20 | 5 |
| Millard (1992)81 | Mixed chronic pain | C, M | United States | 194 | 42.8 (11.2) | 64% | TAS-26 | 5 |
| Mingarelli (2013)82 | TMD | C, M | Italy | 132 | 39.2 (13.6) | 85% | TAS-20 | 7 |
| Miyaoka (1996)83 | BMS | G | Japan | 50 | 55.8 (8.7) | 100% | TAS-26 | 2 |
| Montoro (2016)84,* | Fibromyalgia | C, G, M | Spain | 55 | 51.9 (8.8) | 100% | TAS-20 | 6 |
| Muftuoglu (2004)86 | Headache | G | Turkey | 50 | 32.1 (NR) | 64% | TAS-26 | 4 |
| Ozturk (2015)92 | Mastalgia | M | Turkey | 88 | 29.6 (8.2) | 100% | TAS-20 | 6 |
| Pecukonis (2009)96 | MSK | G | United States | 59 | 33.3 (11.9) | 100% | TAS-26 | 6 |
| Periacoba (2013)97 | Fibromyalgia | G | Spain | 120 | 50.9 (9.8) | 100% | TAS-20 | 5 |
| Pepe (2014)98,* | MSK | C, M | Italy | 40 | 44.8 (9.7) | 30% | TAS-20 | 6 |
| Porcelli (2014)99,* | IBS | C, M | Italy | 177 | 34.5 (11.7) | 71% | TAS-20 | 5 |
| Portincasa (2003)101 | IBS | G, M | Italy | 100 | 48 (2) | 27% | TAS-20 | 4 |
| Saariaho (2016)105 | Chronic pain | C, M | Finland | 83 | 49.5 (7.19) | 41% | TAS-20 | 5 |
| Sayar (20 04)107,† | Fibromyalgia | C , M | Turkey | 50 | 40.5 (8.8) | 100% | TAS-20 | 4 |
| Sayar (2004)107,† | Autoimmune | C , M | Turkey | 20 | 45.6 (14.9) | 100% | TAS-20 | 4 |
| Shibata (2014)109,* | Chronic pain | C, M | Japan | 439 | 61.1 (11) | 65% | TAS-20 | 6 |
| Sinikallio (2006)111 | MSK | M | Finland | 100 | 61.6 (11.2) | 58% | TAS-20 | 4 |
| Slavin-Spenny (2003)112 | Headache | M | United States | 147 | 22.1 (6) | 88% | TAS-20 | 7 |
| Steinweg (2011)113,† | Autoimmune | G, M | United States | 43 | 59.8 (13.7) | 9% | TAS-20 | 7 |
| Steinweg (2011)113,† | Fibromyalgia | G, M | United States | 48 | 54.1 (13.4) | 92% | TAS-20 | 7 |
| Tuzer (2011)120,† | Fibromyalgia | G, M | Turkey | 70 | 39.0 (7.9) | 100% | TAS-20 | 6 |
| Tuzer (2011)120,† | MSK | G, M | Turkey | 56 | 44.2 (9.3) | 100% | TAS-20 | 6 |
| Vadacca (2014)121,*,† | Autoimmune | C, M | Italy | 25 | 46 (11) | 100% | TAS-20 | 5 |
| Vadacca (2014)121,*,† | Autoimmune | C, M | Italy | 24 | 64 (10) | 100% | TAS-20 | 5 |
| Valkamo (2001)122 | Chest pain | M | Finland | 200 | 58.1 (9.4) | 34% | TAS-20 | 4 |
| van Middendorp (2008)123 | Fibromyalgia | C, G, M | Netherlands | 403 | 46.5 (12.3) | 100% | TAS-20 | 6 |
| Veehof (2011)124 | Fibromyalgia | M | Netherlands | 141 | 43.1 (10.9) | 92% | TAS-20 | 5 |
| Vieira (2013)125,* | Headache | C, M | Brazil | 39 | 43.6 (10.7) | 100% | TAS-26 | 6 |
| Villani (2005)126 | Headache | M | Italy | 42 | 35.7 (10.6) | 83% | TAS-20 | 3 |
| Villani (2010)127 | Headache | M | Italy | 465 | 34.7 (10.9) | 82% | TAS-20 | 3 |
| White (2011)130 | Chest pain | M | United States/ Israel | 229 | 50 (10.3) | 56% | TAS-20 | 6 |
| Wise (1994)131,† | Headache | M | United States | 61 | 37.8 (13.6) | NR | TAS-26 | 5 |
| Wise (1994)131,† | Headache | M | United States | 39 | 37.8 (13.6) | NR | TAS-26 | 5 |
| Yalug (2010)132 | Headache | C, M | Turkey | 300 | 37.4 (13.8) | 83% | TAS-20 | 7 |
| Yucel (2002)133 | Headache | G | Turkey | 105 | 33 (10) | 78% | TAS-26 | 6 |
| Zeng (2016)134 | Chronic pain | C | China | 147 | 34.9 (11.3) | 100% | TAS-20 | 4 |
| Zincir (2014)137 | Chest pain | G, M | Turkey | 51 | 33 (7.3) | 73% | TAS-20 | 6 |
| Youth studies: | ||||||||
| Cerutti (2016)17,*,† | Headache | C, M | Italy | 53 | 13.4 (2.4) | 57% | TAS-20 | 4 |
| Gatta (2011)31 | Headache | M, G | Italy | 32 | 11.2 (2) | 81% | AQC | 6 |
| Gatta (2015)32,† | Headache | M | Italy | 42 | 13.1 (2.4) | 57% | TAS-20 | 5 |
| Gatta (2015)32,† | Headache | M | Italy | 47 | 12.4 (2.3) | 77% | TAS-20 | 5 |
| Sayin (2007)108 | Mixed chronic pain | C | Turkey | 21 | 14.4 (2.6) | 33% | TAS-26 | 3 |
The study design column refers to included analyses relevant for the present meta-analysis: C, correlational; G, group difference; M, mean or prevalence.
Additional data provided by the author on personal request.
Represents data from 1 of 2 samples reported in the same article.
AQC, Alexithymia Questionnaire for Children; BMS, burning mouth syndrome; CPRS, complex regional pain syndrome; IBS, irritable bowel syndrome; MSK, musculoskeletal pain; NR, not reported; TMD, temporomandibular disorder.
From 82 adult samples (across 77 studies), data were available on alexithymia mean scores or clinical cutoff scores from 74 (90%), comparison of alexithymia scores of a chronic pain sample to a comparison sample from 34 (41%), zero-order correlations between alexithymia and pain intensity, physical interference, depression and/or anxiety from 40 (49%), and partial correlations from 14 (17%). From 5 youth samples (across 5 studies), we obtained alexithymia mean scores or clinical cutoff scores from 4; correlations between alexithymia and key variables of interest from 2; and group differences between a chronic pain and comparison sample from 1. There were not enough extractable data in studies of youth to conduct meta-analyses. All included studies used some variation of the Toronto Alexithymia Scale, including the TAS-26 (k = 14), TAS-20 (k = 72), and AQC (k = 1). In this case, the AQC was scored using the same scale as the TAS-20 (ie, 20–100). Other psychometrically sound self-report measures (eg, Bermond-Vorst Alexithymia Questionnaire128) or clinician-administered measures (eg, Toronto Structured Interview for Alexithymia7) of alexithymia were not used in this body of literature.
3.3.1. Pooled mean values and prevalence of alexithymia in individuals with chronic pain
Among the 82 samples of adults with chronic pain, TAS-20 total scores were available for 61, and TAS-26 total scores were available for 9. The pooled mean values of TAS-26 total and TAS- 20 total and subscale scores are presented in Table 2. In addition to total scores, the percentage of individuals with a total alexithymia score beyond the clinical cutoff score for TAS-20 was available for 39 samples (n = 4092). Of these, 28% of adults with chronic pain exceeded this threshold. The percentage of individuals with a total alexithymia score beyond the clinical cutoff scores for TAS-20 was available for 5 samples(n = 460); of these, 36% of adults exceeded this threshold.
Table 2.
Pooled alexithymia total and subscale scores for the overall adult sample.
| Range | k | N | Mean | SD | |
|---|---|---|---|---|---|
| TAS-20 | |||||
| Total score | 20–100 | 60 | 5964 | 52.08 | 12.97 |
| Difficulty identifying feelings | 7–35 | 46 | 4654 | 17.79 | 6.75 |
| Difficulty describing feelings | 5–25 | 46 | 4654 | 13.73 | 5.05 |
| Externally oriented thinking | 8–40 | 45 | 4216 | 20.06 | 4.83 |
| TAS-26 | |||||
| Total score | 26–156 | 9 | 630 | 66.99 | 17.66 |
Range indicates minimum to maximum score.
Two studies including youth-reported mean alexithymia using a standard scoring convention: Gatta et al. (2011) measured alexithymia using the AQC in a sample of 32 youth with tension-type headache and reported a mean total score of 58.13 (SD = 10.64). Cerutti et al. (2016) assessed alexithymia with the TAS-20 in 53 youth with migraines and reported a mean total score of 56.3 (SD = 12.14); 31% of the youth exceeded clinical cutoffs for alexithymia. These data should be interpreted with caution, however, as the validity of applying adult-derived clinical cutoff scores to characterize alexithymia in youth has been questioned.95
3.3.2. Group differences in alexithymia in pain vs comparison groups
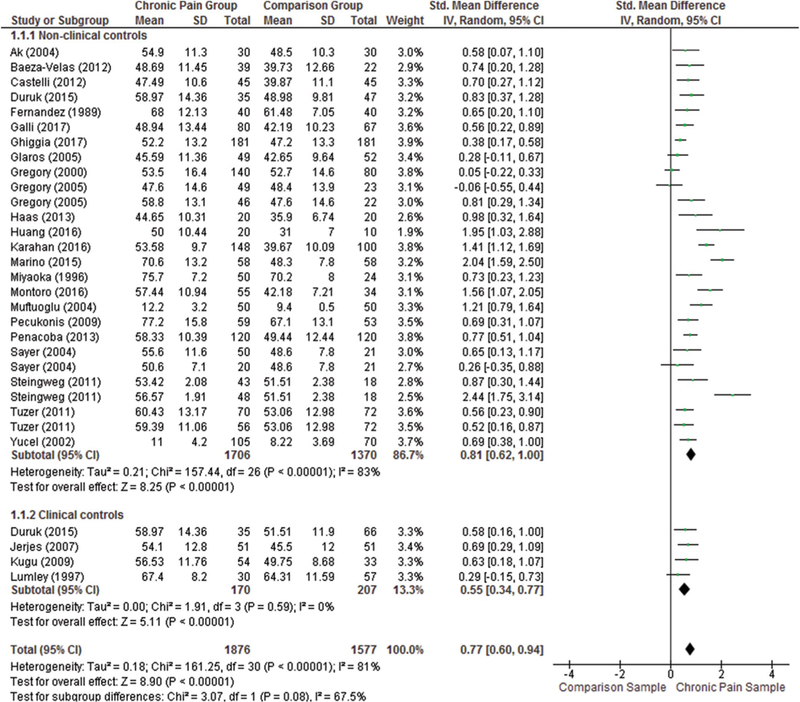
We examined group differences in alexithymia total scores in adults with chronic pain (n = 1706) compared with nonclinical comparison samples (n = 1370; k = 27). We also examined 4 studies that compared alexithymia total scores in adults with chronic pain (n = 170) compared with nonpain clinical comparison samples (n = 207). One study in the latter analysis was an outlier, reporting an unexpectedly large effect size difference in alexithy-mia, and was excluded from analyses.101 A significant and large group difference in total alexithymia between adults with chronic pain and nonclinical comparison samples remained (d = 0.81, 95% CI 0.62–1.00), although with substantial heterogeneity (I2 = 83%). Among studies that compared alexithymia total scores in adults with chronic pain with nonpain clinical comparison samples (k = 4), there was a statistically significant medium-sized effect (d = 0.55, 95% CI 0.34–0.77) with no observed heterogeneity (I2 = 0%). A forest plot depicting group differences in alexithymia total scores is presented in Figure 2.
Figure 2.
Summary of quality ratings across all samples (87) included in the manuscript.
We also examined group differences in alexithymia subscale scores (Table 3). Adults with chronic pain had greater DIF scores compared with nonclinical samples, and this was statistically significant with a large effect size. Adults with chronic pain had greater DDF compared with nonclinical samples with a medium effect size. Findings were similar for group differences between adults with chronic pain and nonpain clinical comparison samples. There was no statistically significant group difference in EOT between adults with chronic pain and nonclinical samples. There were too few studies to examine group differences in EOT between adults with chronic and nonpain clinical samples and group differences in alexithymia total and subscale scores in youth.
Table 3.
Meta-analysis of differences in alexithymia total and subscale scores between adult samples with chronic pain and comparison samples.
| Alexithymia subscale | Studies (k) | Chronic pain (n) | Comparison (n) | Overall effect and heterogeneity |
||
|---|---|---|---|---|---|---|
| Effect size | 95% CI | I2, % | ||||
| All comparisons | ||||||
| Total | 31 | 1876 | 1577 | 0.77 | 0.60 to 0.94 | 81 |
| DIF | 21 | 1506 | 995 | 0.86 | 0.63 to 1.10 | 85 |
| DDF | 21 | 1506 | 995 | 0.46 | 0.32 to 0.61 | 62 |
| EOT | 18 | 1068 | 692 | 0.18 | −0.07 to 0.44 | 83 |
| Nonclinical comparison | ||||||
| Total | 27 | 1706 | 1370 | 0.81 | 0.62 to 1.00 | 83 |
| DIF | 18 | 1401 | 845 | 0.92 | 0.66 to 1.19 | 86 |
| DDF | 18 | 1401 | 845 | 0.46 | 0.29 to 0.62 | 67 |
| EOT | 16 | 963 | 608 | 0.13 | −0.15 to 0.40 | 85 |
| Medical comparison | ||||||
| Total | 4 | 170 | 207 | 0.55 | 0.34 to 0.77 | 0 |
| DIF | 3 | 140 | 150 | 0.51 | 0.27 to 0.75 | 0 |
| DDF | 3 | 140 | 150 | 0.57 | 0.33 to 0.81 | 0 |
Insufficient datato meta-analyze differences in EOT in chronic pain vs medical comparison.
CI, confidence interval; DIF, difficulty identifying feelings; DDF, difficulty describing feelings; EOT, externally oriented thinking.
3.3.3. Relationships of alexithymia with pain intensity, physical interference, depression, and anxiety within pain samples
As shown in Table 4, we found significant, small-magnitude, positive correlations between alexithymia and pain intensity and pain interference and significant, medium-size magnitude, positive relationships between alexithymia and depression and anxiety. Across all analyses, DIF was the most strongly associated alexithymia subscale with these measures, followed by DDF. Small relationships were observed between EOT and all variables except pain interference. Trim and fill analyses identified missing studies in just 1 analysis (relationship between EOT and depression). When missing studies were subsequently filled, the pattern of significance remained the same.
Table 4.
Summary of meta-analyses of correlations between alexithymia total and subscale scores with variables of interest.
| Variable | Alexithymia subscale | Overall effect and heterogeneity | |||||
|---|---|---|---|---|---|---|---|
| Studies (k) | Participants (n) | Effect size | 95% CI | I2 | Trim and fill (Y or N) | ||
| Pain intensity | |||||||
| Total | 27 | 2749 | 0.20 | 13 to 0.28 | 70.94 | N | |
| Difficulty identifying feelings | 24 | 2718 | 0.18 | 0.14 to 0.23 | 30.94 | N | |
| Difficulty describing feelings | 23 | 2591 | 0.19 | 0.12 to 0.25 | 57.47 | N | |
| Externally oriented thinking | 22 | 2188 | 0.08 | 0.01 to 0.14 | 51.67 | N | |
| Physical interference | |||||||
| Total | 19 | 2049 | 0.17 | 0.10 to 0.24 | 57.22 | N | |
| Difficulty identifying feelings | 18 | 1880 | 0.21 | 0.12 to 0.30 | 70.44 | N | |
| Difficulty describing feelings | 17 | 1752 | 0.16 | 0.10 to 0.23 | 46.49 | N | |
| Externally oriented thinking | 17 | 1752 | 0.03 | −0.05 to 0.11 | 60.32 | N | |
| Depression | |||||||
| Total | 20 | 2678 | 0.46 | 0.41 to 0.51 | 48.27 | N | |
| Difficulty identifying feelings | 21 | 2260 | 0.42 | 0.34 to 0.49 | 74.81 | N | |
| Difficulty describing feelings | 20 | 2132 | 0.38 | 0.31 to 0.46 | 69.36 | N | |
| Externally oriented thinking | 20 | 2132 | 0.20 | 0.12 to 0.28 | 70.43 | Y | |
| Anxiety | |||||||
| Total | 22 | 2431 | 0.43 | 0.36 to 0.49 | 62.54 | N | |
| Difficulty identifying feelings | 19 | 2114 | 0.46 | 0.40 to 0.52 | 60.71 | N | |
| Difficulty describing feelings | 17 | 1933 | 0.38 | 0.31 to 0.46 | 45.10 | N | |
| Externally oriented thinking | 18 | 1986 | 0.12 | 0.04 to 0.20 | 68.01 | N | |
Small effect size = 0.2; moderate effect size = 0.5; large effect size = 0.8. Significant effects are bolded. I2:0% to 29% low heterogeneity; 30% to 49% moderate heterogeneity; 50% to 74% substantial heterogeneity; 75% to 100% considerable heterogeneity.
CI, confidence interval.
Only 2 studies examined these correlations in youth; thus, meta-analyses for youth could not be conducted. One study showed a significant positive relationship between alexithymia and anxiety and a nonsignificant relationship between alexithymia and depression108 in a small sample (n = 21) of youth with mixed headache and abdominal pain. The other study showed significant correlations between alexithymia and anxiety and depression in a sample of youth with migraine.17
3.3.4. Meta-analysis of partial correlations controlling for psychological distress
Partial correlations between alexithymia and pain measures (intensity and interference) were published in only 3 studies, and we obtained unpublished data from authors of 11 additional studies (thus, k = 14). We meta-analyzed partial correlations between alexithymia (total and subscale scores), pain intensity, and pain interference while controlling for negative affect. As shown in Table 5, there were no significant partial correlations of alexithymia total or subscale scores with pain intensity and pain interference.
Table 5.
Summary of meta-analyses of partial correlations between alexithymia total and subscale scores with variables of interest, while controlling for a measure of negative affect.
| Variable | Alexithymia subscale | Overall effect and heterogeneity | |||||
|---|---|---|---|---|---|---|---|
| Studies (k) | Participants (n) | Effect size | 95% CI | I2 | Trim and fill (Y or N) | ||
| Pain intensity | |||||||
| Total | 14 | 1621 | 0.09 | −0.04 to 0.21 | 83.28 | N | |
| Difficulty identifying feelings | 13 | 1582 | 0.06 | −0.03 to 0.15 | 65.23 | N | |
| Difficulty describing feelings | 11 | 1323 | 0.07 | −0.40 to 0.36 | 82.27 | N | |
| Externally oriented thinking | 12 | 1522 | 0.06 | −0.04 to 0.16 | 71.70 | N | |
| Physical interference | |||||||
| Total | 10 | 1101 | 0.06 | −0.06 to 0.16 | 63.25 | N | |
| Difficulty identifying feelings | 11 | 1176 | 0.03 | −0.05 to 0.11 | 36.42 | N | |
| Difficulty describing feelings | 11 | 1176 | 0.04 | −0.06 to 0.13 | 51.36 | N | |
| Externally oriented thinking | 11 | 1176 | −0.01 | −0.13 to 0.10 | 69.65 | N | |
Small effect size = 0.2; moderate effect size = 0.5; large effect size = 0.8. I2: 0% to 29% low heterogeneity; 30% to 49% moderate heterogeneity; 50% to 74% substantial heterogeneity; 75% to 100% considerable heterogeneity.
CI, confidence interval.
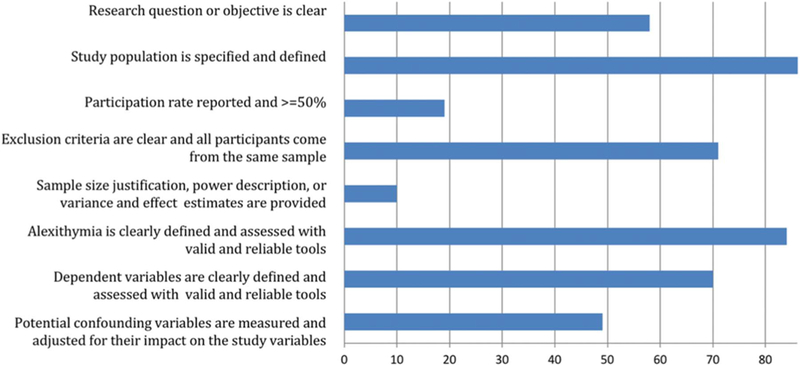
3.4. Quality assessment
Overall quality ratings are depicted in Figure 3, offering a relative assessment of the overall quality of included studies. For studies of adults, quality ratings ranged from 2 to 8 (mean = 5.13, SD = 1.21; median = 5). For youth studies, quality ranged from 3 through 6 (mean = 4.60, median = 5). Study quality was not significantly correlated with effect size for group differences in alexithymia (r = 0.13, P = 0.429) or correlations of alexithymia with pain intensity (r = −0.21, P = 0.282), physical interference (r = 0.20, P = 0.403), depression (r = 0.29, P = 0.138), or anxiety (r = 0.10, P = 0.650).
Figure 3.
Forest plot depicting group differences in the alexithymia score between adults with chronic pain and nonclinical and clinical comparison samples.
4. Discussion
This is the first systematic review and meta-analysis of alexithymia in individuals with chronic pain. We identified a large number of articles (k = 77), reflecting substantial interest in the assessment of alexithymia, primarily in adults, with a range of pain conditions. Overall, our findings demonstrated that alexithymia was higher in individuals with chronic pain compared with both nonclinical samples (large effect) and clinical samples without pain (medium effect). The latter finding suggests that the elevated alexithymia is not solely a bias associated with health care seeking or having a chronic illness. We found that 26% of adults with chronic pain met the threshold of a clinical cutoff score for alexithymia—an estimate that is higher than the 13% reported in the general population.106 In addition to elevated alexithymia in people with chronic pain, our meta-analyses indicated that among adults with chronic pain, alexithymia was associated with greater pain intensity and physical interference, with correlations that were small in magnitude. Alexithymia was also positively associated with depression and anxiety, with correlations that were medium in magnitude.
Among adults with chronic pain, greater alexithymia was associated with greater pain intensity and physical interference, with small-magnitude effect sizes. These findings are consistent with laboratory-based studies demonstrating altered pain processing in relation to heightened alexithymia in healthy adults.45,52,90 As expected, alexithymia was associated with greater depression and anxiety with large effect sizes. Emotional awareness is important for effective emotion regulation,38,54 and alexithymia is associated with reliance on ineffective approaches to emotion regulation including suppression and avoidance.94,115 The experience of chronic pain confers additional stress in many life domains (eg, social, emotional, physical)119; in the face of these stressors, alexithymia may be associated with greater difficulty regulating emotions, contributing to poor mental health outcomes.69
Specific subscales of alexithymia measures showed differential effects; in particular, effects were larger for DIF and DDF, but lower or nonsignificant for EOT. Contributing to this pattern may be the weak psychometric properties of the EOT subscale of the TAS-20,58 particularly in non-Western cultures.20 It is also possible that chronic pain is more tightly linked to the constructs of difficulty identifying and describing feelings. With the inability to identify or describe one’s emotions, it is possible that awareness of or attention to interoceptive cues is associated with misinterpreting emotional cues as harmful or as signs of illness.70,117 Our review highlights the important need for future research on the conceptualization and assessment of emotional awareness as it applies to chronic pain.
We meta-analyzed partial correlations from 14 studies that examined relationships of alexithymia to pain intensity and pain interference while controlling for psychological distress. These analyses revealed that alexithymia was no longer uniquely related to pain variables. There are several possible interpretations of these results. First, some scholars have questioned the validity of self-reported alexithymia, especially given its convergence with measures of negative affect,62,67 which could spuriously inflate alexithymia’s correlations with self-reported measures of pain and other symptoms. Relatedly, individuals with depressive symptoms are more likely to evaluate themselves and their abilities negatively on a self-report survey.62,67 Another intriguing possibility is that distress or negative affect may mediate the links between alexithymia and pain. Unfortunately, cross-sectional analyses such as those in the current review cannot differentiate these 2 possibilities. We also note that potential confounding by negative affect is by no means limited to studies of alexithymia; such bias complicates the assessment and interpretation of many or most self-reported psychological constructs that are related to pain.114,129 It should also be noted that only 3 of the 14 sets of partial correlations that we meta-analyzed had been peer reviewed and published; the other 11 were provided to us directly by the authors on our request. Given these considerations, we suggest caution in making conclusions about the role of negative affect in the relationship between alexithymia and pain based on the current findings. Of note, the fact that partial correlation analyses were available but rarely published raises the issue of a publication bias in scientific literature towards positive findings.104 Moving forward, we recommend that investigators examine and report the role of negative affect in the relationship between alexithymia or other psychological constructs and chronic pain and to test alternative models (eg, moderation and mediation) to better parse these relationships.
Results from the present systematic review and meta-analysis reveal many significant gaps in this large body of literature. For example, only 5 studies examined alexithymia in youth with chronic pain, and it is unclear whether this construct can be adequately measured in youth. These studies typically applied adult-derived alexithymia cutoff scores, not considered valid in youth populations.136 Data were insufficient to examine group differences and correlations in youth with chronic pain. Future studies are needed to clarify the role of emotional awareness in youth with pain, using age-appropriate assessment (cf.136), to determine how it relates to pain intensity, physical interference, depression, and anxiety. Adolescence is a critical window for the development of emotional insight and emotion regulation (Somerville, 2016). Clarifying the role of emotional awareness in youth with pain may have developmental implications and unique treatment considerations.
Another gap in the broad alexithymia and chronic pain literature is the preponderance of cross-sectional study designs. Very few studies in this field have examined temporal patterns or longitudinal relationships between alexithymia and chronic pain. Alexithymia may be a risk factor for subsequent pain where reduced ability to label and describe emotions leads to misperceptions of the physiological correlates of emotion as signs of illness.61,71,116 On the other hand, chronic pain may contribute to affective deficits observed in alexithymia. Constructivist theories of emotion argue that the subjective experience of emotion results in part from awareness of bodily cues.9 A recent systematic review concluded that individuals with chronic pain have reduced accuracy in detecting interoceptive cues compared with comparison samples.21 The presence of pain, a potentially threatening biological cue, may distract from awareness of relevant interoceptive cues, particularly those subtle cues involved in daily experiences and interactions with others. This is an empirical question for future research.
Addressing these gaps in the literature has potential implications for clinical intervention. It is possible that individuals with both chronic pain and elevated alexithymia may benefit from interventions that directly target processes related to emotional awareness, such as emotional awareness and expression training.73 On the other hand, more general psychological interventions may function to reduce depression and anxiety and in turn result in reduced alexithymia. Future research is needed to investigate ontological distinctions between alexithymia and negative affect, and if and how they relate temporally.
Current findings should be considered in light of additional limitations. There is substantial debate about the alexithymia construct more broadly. Alexithymia generally, and the TAS-20 specifically, has been criticized for relying on Western norms, which value individual emotional experience and verbal expression of emotion.56 Diverging from this norm may indicate psychopathology in Western culture, but not necessarily in others. Nonetheless, a strength of the current meta-analysis is representation from many different geographic regions and cultures, and the TAS-20 has been validated in a number of Eastern cultures.85,135 Future studies should examine whether ethnicity or culture moderates the relationship between alexithymia and chronic pain. Another limitation of the TAS-20, particularly the DIF subscale, is that it contains items pertaining to physiological sensations (eg, “I have physical sensations that even doctors don’t understand;” “I am often puzzled by sensations in my body.”). Such items likely reflect the experience of chronic pain, artificially inflating correlations between the TAS-20 and pain-related measures. A number of interview or observer-based measures of alexithymia have been developed, although, unfortunately, we did not identify studies that made use of these assessment tools in the current meta-analysis.
Meta-analyses in the current study were generally characterized by substantial heterogeneity and thus should be interpreted with caution. There may be relevant demographic or clinical differences that contribute to such heterogeneity. For example, the relationship of alexithymia to pain severity and interference may vary by sex or ethnicity20,91 and be a function of pain characteristics, such as duration of pain or specific pain condition. Examining demographic and clinical moderators was beyond the scope of the present metaanalysis and represent potential future avenues of research.
We examined study quality using general criteria and assigned a relative quality value to each included study. The overall mean and median quality scores of included studies were relatively high; however, a large subset of studies was characterized by low study quality. Future studies with rigorous methodology and reporting are needed to increase confidence in this body of work. Relative areas of weakness include failing to provide sample size justification and to report participation rates. In addition, very few studies tested the unique validity of alexithymia measures by controlling for various potential confounds: future studies should consider accounting for demographic (eg, education and age) and clinical factors (eg, psychological distress) that relate differentially to alexithymia scores.
In conclusion, this systematic review helps summarize a substantial literature examining alexithymia in chronic pain, finding that alexithymia is elevated in youth and adults with chronic pain conditions. In adults, alexithymia is associated with greater pain intensity, physical interference, depression, and anxiety. Alex-ithymia may be relevant to the presence and severity of chronic pain and associated mental health symptoms. Future empirical research is needed to better illuminate these relationships and clarify the potentially confounding role of negative affect. In particular, using prospective designs and non-self-repot alexithymia assessment tools would add greater clarity to this construct and inform development of targeted interventions.
Supplementary Material
Acknowledgments
The authors thank the many researchers who contributed additional data on our request, allowing for a more comprehensive review. These authors are indicated in Table 1. They also thank Mark Jensen for his assistance on this study’s protocol. R.V. Aaron was supported by NIH T32GM086270 awarded to T.M. Palermo. T.M. Palermo was supported by NIH K24HD060068.
Footnotes
Conflict of interest statement
The authors have no conflict of interest to declare.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/A734.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.painjournalonline.com).
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- [1].Ak I, Sayar K, Yontem T. Alexithymia, somatosensory amplification and counter-dependency in patients with chronic pain. Pain Clinic 2004;16:43–51. [Google Scholar]
- [2].Asmundson GJ, Katz J. Understanding the co-occurrence of anxiety disorders and chronic pain: state-of-the-art. Depress Anxiety 2009;26: 888–901. [DOI] [PubMed] [Google Scholar]
- [3].Atagun MI, Atagun Z, Evren C, Balaban OD, Yalcinkaya EY, Ones K. Mental symptoms are related with impact of the disease and impairment in quality of life in female patients with fibromyalgia. Dusunen Adam 2012;25:338–44. [Google Scholar]
- [4].Baeza-Velasco C, Carton S, Almohsen C, Blotman F, Gely-Nargeot MC. Alexithymia and emotional awareness in females with painful rheumatic conditions. J Psychosom Res 2012;73:398–400. [DOI] [PubMed] [Google Scholar]
- [5].Bagby M, Taylor GJ, Ryan DJP. Toronto alexithymia scale: relationship with personality and psychopathology measures. Psychother Psychosom 1986;45:207–15. [DOI] [PubMed] [Google Scholar]
- [6].Bagby RM, Parker JD, Taylor GJ. The twenty-item Toronto Alexithymia Scale—I. Item selection and cross-validation of the factor structure. J Psychosom Res 1994;38:23–32. [DOI] [PubMed] [Google Scholar]
- [7].Bagby RM, Taylor GJ, Parker JD, Dickens SE. The development of the Toronto Structured Interview for Alexithymia: item selection, factor structure, reliability and concurrent validity. Psychother Psychosom 2006;75:25–39. [DOI] [PubMed] [Google Scholar]
- [8].Balaban H, Semiz M, Şentürk IA, Kavakçi Ö, Cinar Z, Dikici A, Topaktas S. Migraine prevalence, alexithymia, and post-traumatic stress disorder among medical students in Turkey. J Headache Pain 2012;13:459–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Barrett LF. Are emotions natural kinds? Perspect Psychol Sci 2006;1:28–58. [DOI] [PubMed] [Google Scholar]
- [10].Bausdic S, Jayr C, Albi-Feldzer A, Fermanian J, Masselin-Dubois A, Bouhassira D, Attal N. Effect of alexithymia and emotional repression on postsurgical pain in women with breast cancer: a prospective longitudinal 12-month study. J Pain 2016;17:90–100. [DOI] [PubMed] [Google Scholar]
- [11].Bornemann B, Singer T. Taking time to feel our body: steady increases in heartbeat perception accuracy and decreases in alexithymia over 9 months of contemplative mental training. Psychophysiology 2017;54:469–82. [DOI] [PubMed] [Google Scholar]
- [12].Burger AJ, Lumley MA, Carty JN, Latsch DV, Thakur ER, Hyde-Nolan ME, Hijazi AM, Schubiner H. The effects of a novel psychological attribution and emotional awareness and expression therapy for chronic musculoskeletal pain: a preliminary, uncontrolled trial. J Psychosom Res 2016;81:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Burke AL, Mathias JL, Denson LA. Psychological functioning of people living with chronic pain: a meta-analytic review. Br J Clin Psychol 2015; 54:345–60. [DOI] [PubMed] [Google Scholar]
- [14].Cameron K, Ogrodniczuk J, Hadjipavlou G. Changes in alexithymia following psychological intervention: a review. Harv Rev Psychiatry 2014;22:162–78. [DOI] [PubMed] [Google Scholar]
- [15].Castelli L, Tesio V, Colonna F, Molinaro S, Leombruni P, Bruzzone M, Fusaro E, Sarzi-Puttini P, Torta R. Alexithymia and psychological distress in fibromyalgia: prevalence and relation with quality of life. Clin Exp Rheumatol 2012;30(6 suppl 74):70–7. [PubMed] [Google Scholar]
- [16].Castelli L, Tesio V, Di Tella M, Colonna F, Ghiggia A, Cagna M, Leombruni P, Fusaro E, Torta R. Alexithymia, pain and depression in fibromyalgia syndrome. Psychother Psychosom 2013;82:17–18. [Google Scholar]
- [17].Cerutti R, Valastro C, Tarantino S, Valeriani M, Faedda N, Spensieri V, Guidetti V. Alexithymia and psychopathological symptoms in adolescent outpatients and mothers suffering from migraines: a case control study. J Headache Pain 2016;17:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chang MC, Chen PF, Lung FW. Personality disparity in chronic regional and widespread pain. Psychiatry Res 2017;254:284–9. [DOI] [PubMed] [Google Scholar]
- [19].Cologno D, Buzzi MG, Carlesimo GA, Cicinelli P, Costa A, Fadda L, Formisano R, Marconi B, Pero S, Caltagirone C. Psychiatric disorders and pain location in unilateral migraineurs. J Headache Pain 2005;6:227–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Dere J, Tang Q, Zhu X, Cai L, Yao S, Ryder AG. The cultural shaping of alexithymia: values and externally oriented thinking in a Chinese clinical sample. Compr Psychiatry 2013;54:362–8. [DOI] [PubMed] [Google Scholar]
- [21].Di Lernia D, Serino S, Riva G. Pain in the body. Altered interoception in chronic pain conditions: a systematic review. Neurosci Biobehav Rev 2016;71:328–41. [DOI] [PubMed] [Google Scholar]
- [22].Di Tella M, Castelli L. Alexithymia in chronic pain disorders. Curr Rheumatol Rep 2016;18:41. [DOI] [PubMed] [Google Scholar]
- [23].Di Tella M, Ghiggia A, Tesio V, Romeo A, Colonna F, Fusaro E, Torta R, Castelli L. Pain experience in Fibromyalgia Syndrome: the role of alexithymia and psychological distress. J Affect Disord 2017;208:87–93. [DOI] [PubMed] [Google Scholar]
- [24].Duruk B, Sertel Berk HO, Ketenci A. Are fibromyalgia and failed back surgery syndromes actually “functional somatic syndromes” in terms of their symptomatological, familial and psychological characteristics? A comparative study with chronic medical illness and healthy controls. Agri 2015;27:123–31. [DOI] [PubMed] [Google Scholar]
- [25].Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455–63. [DOI] [PubMed] [Google Scholar]
- [26].Esin O, Gorobets E, Khairullin I, Esin R. Alexithymia as a predictor of chronic tension headaches. Bionanoccience 2017;7:272–5. [Google Scholar]
- [27].Evren B, Evren C, Guler MH. Clinical correlates of alexithymia in patients with fibromyalgia. Pain Clinic 2006;18:1–9. [Google Scholar]
- [28].Fernandez A, Sriram TG, Rajkumar S, Chandrasekar AN. Alexithymic characteristics in rheumatoid arthritis: a controlled study. Psychother Psychosom 1989;51:45–50. [DOI] [PubMed] [Google Scholar]
- [29].Galli F, Caputi M, Sances G, Vegni E, Bottiroli S, Nappi G, Tassorelli C. Alexithymia in chronic and episodic migraine: a comparative study. J Ment Health 2017;26:192–6. [DOI] [PubMed] [Google Scholar]
- [30].Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain 2012;13:715–24. [DOI] [PubMed] [Google Scholar]
- [31].Gatta M, Canetta E, Zordan M, Spoto A, Ferruzza E, Manco I, Addis A, Dal Zotto L, Toldo I, Sartori S, Battistella PA. Alexithymia in juvenile primary headache sufferers: a pilot study. J Headache Pain 2011;12:71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gatta M, Spitaleri C, Balottin U, Spoto A, Balottin L, Mangano S, Battistella PA. Alexithymic characteristics in pediatric patients with primary headache: a comparison between migraine and tension-type headache. J Headache Pain 2015;16:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ghiggia A, Romeo A, Tesio V, Tella MD, Colonna F, Geminiani GC, Fusaro E, Castelli L. Alexithymia and depression in patients with fibromyalgia: when the whole is greater than the sum of its parts. Psychiatry Res 2017;255:195–7. [DOI] [PubMed] [Google Scholar]
- [34].Glaros AG, Lumley MA. Alexithymia and pain in temporomandibular disorder. J Psychosom Res 2005;59:85–8. [DOI] [PubMed] [Google Scholar]
- [35].Gregory RJ, Manring J, Berry SL. Pain location and psychological characteristics of patients with chronic pain. Psychosomatics 2000;41: 216–20. [DOI] [PubMed] [Google Scholar]
- [36].Gregory RJ, Manring J, Wade MJ. Personality traits related to chronic pain location. Ann Clin Psychiatry 2005;17:59–64. [DOI] [PubMed] [Google Scholar]
- [37].Groenewald CB, Essner BS, Wright D, Fesinmeyer MD, Palermo TM. The economic costs of chronic pain among a cohort of treatment-seeking adolescents in the United States. J Pain 2014;15:925–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Gross JJ. Emotion regulation: current status and future prospects. Psychol Inq 2015;26:1–26. [Google Scholar]
- [39].Gross JJ. The extended process model of emotion regulation: elaborations, applications, and future directions. Psychological Inquiry 2015;26:130–7. [Google Scholar]
- [40].Gulec H Normalizing attributions may contribute to non-help-seeking behavior in people with fibromyalgia syndrome. Psychosomatics 2008;49: 212–17. [DOI] [PubMed] [Google Scholar]
- [41].Haas J, Eichhammer P, Traue HC, Hoffmann H, Behr M, Cronlein T, Pieh C, Busch V. Alexithymic and somatisation scores in patients with temporomandibular pain disorder correlate with deficits in facial emotion recognition. J Oral Rehabil 2013;40:81–90. [DOI] [PubMed] [Google Scholar]
- [42].Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. Vol. 4 Hoboken: John Wiley & Sons, 2011. [Google Scholar]
- [43].Hosoi M, Molton IR, Jensen MP, Ehde DM, Amtmann S, O’Brien S, Arimura T, Kubo C. Relationships among alexithymia and pain intensity, pain interference, and vitality in persons with neuromuscular disease: considering the effect of negative affectivity. PAIN 2010;149:273–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Huang JS, Terrones L, Simmons AN, Kaye W, Strigo I. Pilot study of functional magnetic resonance imaging responses to somatic pain stimuli in youth with functional and inflammatory gastrointestinal disease. J Pediatr Gastroenterol Nutr 2016;63:500–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Huber A, Suman AL, Biasi G, Carli G. Alexithymia in fibromyalgia syndrome: associations with ongoing pain, experimental pain sensitivity and illness behavior. J Psychosom Res 2009;66:425–33. [DOI] [PubMed] [Google Scholar]
- [46].Huguet A, Miro J. The severity of chronic pediatric pain: an epidemiological study. J Pain 2008;9:226–36. [DOI] [PubMed] [Google Scholar]
- [47].Jasinski MJ, Lumley MA, Latsch DV, Schuster E, Kinner E, Burns JW. Assessing anger expression: construct validity of three emotion expression-related measures. J Pers Assess 2016;98:640–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Jerjes W, Madland G, Feinmann C, Hopper C, Kumar M, Upile T, Kudari M, Newman S. A psychological comparison of temporomandibular disorder and chronic daily headache: are there targets for therapeutic interventions? Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007; 103:367–73. [DOI] [PubMed] [Google Scholar]
- [49].Jerlang BB. Burning mouth syndrome (BMS) and the concept of alexithymia—a preliminary study. J Oral Pathol Med 1997;26:249–53. [DOI] [PubMed] [Google Scholar]
- [50].Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH. The prevalence of chronic pain in United States adults: results of an internet-based survey. J Pain 2010;11:1230–9. [DOI] [PubMed] [Google Scholar]
- [51].Johannsen M, O’Toole MS, O’Connor M, Jensen AB, Zachariae R. Clinical and psychological moderators of the effect of mindfulness-based cognitive therapy on persistent pain in women treated for primary breast cancer—explorative analyses from a randomized controlled trial. Acta Oncol 2017;56:321–8. [DOI] [PubMed] [Google Scholar]
- [52].Kano M, Hamaguchi T, Itoh M, Yanai K, Fukudo S. Correlation between alexithymia and hypersensitivity to visceral stimulation in human. PAIN 2007;132:252–63. [DOI] [PubMed] [Google Scholar]
- [53].Karahan AY, Kucuk A, Balkarli A, Kayhan F, Ozhan N, Nas O, Gungor T, Kucuksen S. Alexithymia, depression, anxiety levels and quality of life in patients with rheumatoid arthritis. Acta Med Mediterranea 2016;32:1675–82. [Google Scholar]
- [54].Kashdan TB, Barrett LF, McKnight PE. Unpacking emotion differentiation transforming unpleasant experience by perceiving distinctions in negativity. Curr Dir Psychol Sci 2015;24:10–16. [Google Scholar]
- [55].King S, Chambers CT, Huguet A, MacNevin RC, McGrath PJ, Parker L, MacDonald AJ. The epidemiology of chronic pain in children and adolescents revisited: a systematic review. PAIN 2011;152:2729–38. [DOI] [PubMed] [Google Scholar]
- [56].Kirmayer LJ. Languages of suffering healing: alexithymia as a social and cultural process. Transcultural Psychiatric Research Review 1987;24:119–36. [Google Scholar]
- [57].Kojima M, Kojima T, Suzuki S, Takahashi N, Funahashi K, Kato D, Hanabayashi M, Hirabara S, Asai S, Ishiguro N. Alexithymia, depression, inflammation, and pain in patients with rheumatoid arthritis. Arthritis Care Res 2014;66:679–86. [DOI] [PubMed] [Google Scholar]
- [58].Kooiman C, Spinhoven P, Trijsburg R. The assessment of alexithymia: a critical review of the literature and a psychometric study of the Toronto Alexithymia Scale-20. J Psychosom Res 2002;53:1083–90. [DOI] [PubMed] [Google Scholar]
- [59].Kosturek A, Gregory RJ, Sousou AJ, Trief P. Alexithymia and somatic amplification in chronic pain. Psychosomatics 1998;39:399–404. [DOI] [PubMed] [Google Scholar]
- [60].Kugu N, Kaptanoglu E, Kavakci O, Guler E. Psychopathology, family functioning and marital relationship in female patients with fibromyalgia syndrome. Neurol Psychiatry Brain Res 2009;16:83–90. [Google Scholar]
- [61].Lane RD, Schwartz GE. Levels of emotional awareness: a cognitive-developmental theory and its application to psychopathology. Am J Psychiatry 1987;144:133–43. [DOI] [PubMed] [Google Scholar]
- [62].Leising D, Grande T, Faber R. The Toronto Alexithymia Scale (TAS-20): a measure of general psychological distress. J Res Personal 2009;43:707–10. [Google Scholar]
- [63].Leweke F, Leichsenring F, Kruse J, Hermes S. Is alexithymia associated with specific mental disorders. Psychopathology 2011;45:22–8. [DOI] [PubMed] [Google Scholar]
- [64].Li S, Zhang B, Guo Y, Zhang J. The association between alexithymia as assessed by the 20-item Toronto Alexithymia Scale and depression: a meta-analysis. Psychiatry Res 2015;227:1–9. [DOI] [PubMed] [Google Scholar]
- [65].Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, Way BM. Putting feelings into words. Psychol Sci 2007;18:421–8. [DOI] [PubMed] [Google Scholar]
- [66].Lieberman MD, Inagaki TK, Tabibnia G, Crockett MJ. Subjective responses to emotional stimuli during labeling, reappraisal, and distraction. Emotion 2011;11:468–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Lumley MA. Alexithymia and negative emotional conditions. Journal of Psychosomatic Research 2000;49:51–4. [DOI] [PubMed] [Google Scholar]
- [68].Lumley MA, Asselin LA, Norman S. Alexithymia in chronic pain patients. Compr Psychiatry 1997;38:160–5. [DOI] [PubMed] [Google Scholar]
- [69].Lumley MA, Beyer J, Radcliffe A. Alexithymia and physical health problems: a critique of potential pathways and a research agenda Emotion regulation. Berlin: Springer, 2008. pp. 43–68. [Google Scholar]
- [70].Lumley MA, Cohen JL, Borszcz GS, Cano A, Radcliffe AM, Porter LS, Schubiner H, Keefe FJ. Pain and emotion: a biopsychosocial review of recent research. J Clin Psychol 2011;67:942–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Lumley MA, Neely LC, Burger AJ. The assessment of alexithymia in medical settings: implications for understanding and treating health problems. J Pers Assess 2007;89:230–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Lumley MA, Radcliffe AM, Macklem DJ, Mosley-Williams A, Leisen JCC, Huffman JL, D’Souza PJ, Gillis ME, Meyer TM, Kraft CA, Rapport LJ. Alexithymia and pain in three chronic pain samples: comparing Caucasians and African Americans. Pain Med 2005;6:251–61. [DOI] [PubMed] [Google Scholar]
- [73].Lumley MA, Schubiner H, Lockhart NA, Kidwell KM, Harte SE, Clauw DJ, Williams DA. Emotional awareness and expression therapy, cognitive behavioral therapy, and education for fibromyalgia: a cluster-randomized controlled trial. PAIN 2017;158:2354–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Lumley MA, Smith JA, Longo DJ. The relationship of alexithymia to pain severity and impariment among patients with chronic myofascial pain: comparisons with self-efficacy, catastrophizing and depression. J Psychosom Res 2002;53:823–30. [DOI] [PubMed] [Google Scholar]
- [75].Makino S, Jensen MP, Arimura T, Obata T, Anno K, Iwaki R, Kubo C, Sudo N, Hosoi M. Alexithymia and chronic pain: the role of negative affectivity. Clin J Pain 2013;29:354–61. [DOI] [PubMed] [Google Scholar]
- [76].Margalit D, Ben Har L, Brill S, Vatine JJ. Complex regional pain syndrome, alexithymia, and psychological distress. J Psychosom Res 2014;77:273–7. [DOI] [PubMed] [Google Scholar]
- [77].Marino R, Picci RL, Ferro G, Carezana C, Gandolfo S, Pentenero M. Peculiar alexithymic traits in burning mouth syndrome: case-control study. Clin Oral Investig 2015;19:1799–805. [DOI] [PubMed] [Google Scholar]
- [78].Martinez M, Sanchez AI, Miro E, Lami MJ, Prados G, Morales A. Relationships between physical symptoms, emotional distress, and pain appraisal in fibromyalgia: the moderator effect of alexithymia. J Psychol Interdiscip Appl 2015;149:115–40. [DOI] [PubMed] [Google Scholar]
- [79].Melin EO, Thulesius HO, Persson BA. Affect School for chronic benign pain patients showed improved alexithymia assessments with TAS-20. Biopsychosoc Med 2010;4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Melis I, Agus M, Pluchino N, Di Spiezio Sardo A, Litta P, Melis GB, Angioni S. Alexithymia in women with deep endometriosis? A pilot study. J Endometriosis Pelvic Pain Disord 2014;6:26–33. [Google Scholar]
- [81].Millard RW, Kinsler BL. Evaluation of constricted affect in chronic pain: an attempt using the Toronto Alexythymia Scale. PAIN 1992;50:287–92. [DOI] [PubMed] [Google Scholar]
- [82].Mingarelli A, Casagrande M, Di Pirchio R, Nizzi S, Parisi C, Loy BC, Solano L, Rampello A, Di Paolo C. Alexithymia partly predicts pain, poor health and social difficulties in patients with temporomandibular disorders. J Oral Rehabil 2013;40:723–30. [DOI] [PubMed] [Google Scholar]
- [83].Miyaoka H, Kamijima K, Katayama Y, Ebihara T, Nagai T. A psychiatric appraisal of “glossodynia.”. Psychosomatics 1996;37:346–8. [DOI] [PubMed] [Google Scholar]
- [84].Montoro CI, Reyes del Paso GA, Duschek S. Alexithymia in fibromyalgia syndrome. Pers Individ Dif 2016;102:170–9. [Google Scholar]
- [85].Moriguchi Y, Maeda M, Igarashi T, Ishikawa T, Shoji M, Kubo C, Komaki G. Age and gender effect on alexithymia in large, Japanese community and clinical samples: a cross-validation study of the Toronto Alexithymia Scale (TAS-20). Biopsychosoc Med 2007;1:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Muftuoglu MN, Herken H, Demirci H, Virit O, Neyal A. Alexithymicfeatures in migraine patients. Eur Arch Psychiatry Clin Neurosci 2004;254:182–6. [DOI] [PubMed] [Google Scholar]
- [87].Nahin RL. Estimates of pain prevalence and severity in adults: United States, 2012. J Pain 2015;16:769–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].National Heart L, Institute B. Quality assessment tool for observational cohort and cross-sectional studies. Bethesda: National Institutes of Health, Department of Health and Human Services, 2014. [Google Scholar]
- [89].Noel M, Groenewald CB, Beals-Erickson SE, Gebert JT, Palermo TM. Chronic pain in adolescence and internalizing mental health disorders: a nationally representative study. PAIN 2016;157:1333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Nyklíček I, Vingerhoets AJ. Alexithymia is associated with low tolerance to experimental painful stimulation. PAIN 2000;85:471–5. [DOI] [PubMed] [Google Scholar]
- [91].Ogrodniczuk JS, Kealy D, Joyce AS, Abbass AA. Body talk: sex differences in the influence of alexithymia on physical complaints among psychiatric outpatients. Psychiatry Res 2018;261:168–72. [DOI] [PubMed] [Google Scholar]
- [92].Özturk AB, Özenli Y, Öztürk SB, Önel S, Söker G, Seydaoglu G. The effect of psychoeducation on anxiety and pain in patients with mastalgia. Nord J Psychiatry 2015;69:380–5. [DOI] [PubMed] [Google Scholar]
- [93].Palermo TM. Impact of recurrent and chronic pain on child and family daily functioning: a critical review of the literature. J Dev Behav Pediatr 2000;21:58–69. [DOI] [PubMed] [Google Scholar]
- [94].Panayiotou G, Leonidou C, Constantinou E, Hart J, Rinehart KL, Sy JT Björgvinsson T. Do alexithymic individuals avoid their feelings? Experiential avoidance mediates the association between alexithymia, psychosomatic, and depressive symptoms in a community and a clinical sample. Compr Psychiatry 2015;56:206–16. [DOI] [PubMed] [Google Scholar]
- [95].Parker JD, Eastabrook JM, Keefer KV, Wood LM. Can alexithymia be assessed in adolescents? Psychometric properties of the 20-item Toronto Alexithymia Scale in younger, middle, and older adolescents. Psychol Assess 2010;22:798. [DOI] [PubMed] [Google Scholar]
- [96].Pecukonis EV. Physical self-efficacy and alexithymia in women with chronic intractable back pain. Pain Manag Nurs 2009;10:116–23. [DOI] [PubMed] [Google Scholar]
- [97].Penacoba Puente C, Velasco Furlong L, Ecija Gallardo C, Cigaran Mendez M, McKenney K. Anxiety, depression and alexithymia in fibromyalgia: are there any differences according to age? J Women Aging 2013;25:305–20. [DOI] [PubMed] [Google Scholar]
- [98].Pepe L, Milani R, DiTrani M, Di Folco G, Lanna V, Solano L. Amore global approach to musculoskeletal pain: expressive writing as an effective adjunct to physiotherapy. Psychol Health Med 2014;19:687–97. [DOI] [PubMed] [Google Scholar]
- [99].Porcelli P, De Carne M, Leandro G. Alexithymia and gastrointestinal-specific anxiety in moderate to severe irritable bowel syndrome. Compr Psychiatry 2014;55:1647–53. [DOI] [PubMed] [Google Scholar]
- [100].Porcelli P, De Carne M, Leandro G. The role of alexithymia and gastrointestinal-specific anxiety as predictors of treatment outcome in irritable bowel syndrome. Compr Psychiatry 2017;73:127–35. [DOI] [PubMed] [Google Scholar]
- [101].Portincasa P, Moschetta A, Baldassare G, Altomare DF, Palasciano G. Pan-enteric dysmotility, impaired quality of life and alexithymia in a large group of patients meeting ROMEII criteria for irritable bowel syndrome. World J Gastroenterol 2003;9:2293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Rayner L, Hotopf M, Petkova H, Matcham F, Simpson A, McCracken LM. Depression in patients with chronic pain attending a specialised pain treatment centre: prevalence and impact on health care costs. PAIN 2016;157:1472–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Rieffe C, Oosterveld P, Terwogt MM. An alexithymia questionnaire for children: factorial and concurrent validation results. Pers Individ Dif 2006;40:123–33. [Google Scholar]
- [104].Rosenthal RJ. The file drawer problem and tolerance for null results. Psychological Bulletin 1979;86:638. [Google Scholar]
- [105].Saariaho AS, Saariaho TH, Mattila AK, Joukamaa MI, Karukivi M. The role of alexithymia: an 8-year follow-up study of chronic pain patients. Compr Psychiatry 2016;69:145–54. [DOI] [PubMed] [Google Scholar]
- [106].Salminen JK, Saarijarvi S, Aarela E, Toikka T, Kauhanen J. Prevalence of alexithymia and its association with sociodemographic variables in the general population of Finland. J Psychosom Res 1999;46:75–82. [DOI] [PubMed] [Google Scholar]
- [107].Sayar K, Gulec H, Topbas M. Alexithymia and anger in patients with fibromyalgia. Clin Rheumatol 2004;23:441–8. [DOI] [PubMed] [Google Scholar]
- [108].Sayin A, Derinoz O, Bodur S, Senol S, Sener S. Psychiatric symptoms and alexithymia in children and adolescents with non-organic pain: a controlled study. Gazi Med J 2007;18:170–6. [Google Scholar]
- [109].Shibata M, Ninomiya T, Jensen MP, Anno K, Yonemoto K, Makino S, Iwaki R, Yamashiro K, Yoshida T, Imada Y, Kubo C, Kiyohara Y, Sudo N, Hosoi M. Alexithymia is associated with greater risk of chronic pain and negative affect and with lower life satisfaction in a general population: the Hisayama study. PLoS One 2014;9:e90984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Sifneos PE. The prevalence of “alexithymic”characteristics in psychosomatic patients. Psychother Psychosom 1973;22:255–62. [DOI] [PubMed] [Google Scholar]
- [111].Sinikallio S, Aalto T, Airaksinen O, Herno A, Kroger H, Savolainen S, Turunen V, Viinamaki H. Depression and associated factors in patients with lumbar spinal stenosis. Disabil Rehabil 2006;28:415–22. [DOI] [PubMed] [Google Scholar]
- [112].Slavin-Spenny O, Lumley MA, Thakur ER, Nevedal DC, Hijazi AM. Effects of anger awareness and expression training versus relaxation training on headaches: a randomized trial. Ann Behav Med 2013;46:181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Steinweg DL, Dallas AP, Rea WS. Fibromyalgia: unspeakable suffering, a prevalence study of alexithymia. Psychosomatics 2011;52:255–62. [DOI] [PubMed] [Google Scholar]
- [114].Strand EB, Zautra AJ, Thoresen M, Ødegård S, Uhlig T, Finset A. Positive affect as a factor of resilience in the pain—negative affect relationship in patients with rheumatoid arthritis. J Psychosom Res 2006;60:477–84. [DOI] [PubMed] [Google Scholar]
- [115].Swart M, Kortekaas R, Aleman A. Dealing with feelings: characterization of trait alexithymia on emotion regulation strategies and cognitive-emotional processing. PLoS One 2009;4:e5751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Taylor GJ, Bagby R, Parker J. Disorders of affect regulation: alexithymia in medical and psychiatric illness. Cambridge, United Kingdom: Cambridge University Press, 1999. [Google Scholar]
- [117].Taylor GJ, Bagby RM. New trends in alexithymia research. Psychother Psychosom 2004;73:68–77. [DOI] [PubMed] [Google Scholar]
- [118].Taylor GJ. Recent developments in alexithymia theory and research. Can J Psychiatry 2000;45:134–42. [DOI] [PubMed] [Google Scholar]
- [119].Turk DC, Fillingim RB, Ohrbach R, Patel KV. Assessment of psychosocial and functional impact of chronic pain. J Pain 2016;17:T21–49. [DOI] [PubMed] [Google Scholar]
- [120].Tuzer V, Bulut SD, Bastug B, Kayalar G, Goka E, Bestepe E. Causal attributions and alexithymia in female patients with fibromyalgia or chronic low back pain. Nord J Psychiatry 2011;65:138–44. [DOI] [PubMed] [Google Scholar]
- [121].Vadacca M, Bruni R, Terminio N, Sambataro G, Margiotta D, Serino FM, Afeltra A. Alexithymia, mood states and pain experience in systemic lupus erythematosus and rheumatoid arthritis. Clin Rheumatol 2014;33:1443–50. [DOI] [PubMed] [Google Scholar]
- [122].Valkamo M, Hintikka J, Niskanen L, Viinamaki H. Psychiatric morbidity and the presence and absence of angiographic coronary disease in patients with chest pain. Acta Psychiatr Scand 2001;104:391–6. [DOI] [PubMed] [Google Scholar]
- [123].van Middendorp H, Lumley MA, Jacobs JW, van Doornen LJ, Bijlsma JW, Geenen R. Emotions and emotional approach and avoidance strategies in fibromyalgia. J Psychosom Res 2008;64:159–67. [DOI] [PubMed] [Google Scholar]
- [124].Veehof MM, Ten Klooster PM, Taal E, Westerhof GJ, Bohlmeijer ET. Psychometric properties of the Dutch five facet mindfulness questionnaire (FFMQ) in patients with fibromyalgia. Clin Rheumatol 2011. ;30:1045–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Vieira RV, Vieira DC, Gomes WB, Gauer G. Alexithymia and its impact on quality of life in a group of Brazilian women with migraine without aura. J Headache Pain 2013;14:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Villani V, Bruti G, Mostardini C, Di Stani F, Scattoni L, Dugoni D, Vanacore N, Cerbo R. Migraine in the emergency department: a psychometric study of a migraine “repeaters” sample. J Headache Pain 2005;6:301–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Villani V, Di Stani F, Vanacore N, Scattoni L, Cerbo R, Bruti G. The “repeater” phenomenon in migraine patients: a clinical and psychometric study. Headache 2010;50:348–56. [DOI] [PubMed] [Google Scholar]
- [128].Vorst HC, Bermond B. Validity and reliability of the Bermond-Vorst alexithymia questionnaire. Pers Individ Dif 2001;30:413–34. [Google Scholar]
- [129].Watson D, Pennebaker JW. Health complaints, stress, and distress: exploring the central role of negative affectivity. Psychol Rev 1989;96:234–54. [DOI] [PubMed] [Google Scholar]
- [130].White KS, McDonnell CJ, Gervino EV. Alexithymia and anxiety sensitivity in patients with non-cardiac chest pain. J Behav Ther Exp Psychiatry 2011;42:432–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Wise TN, Mann LS, Jani N, Jani S. Illness beliefs and alexithymia in headache patients. Headache 1994;34:362–5. [DOI] [PubMed] [Google Scholar]
- [132].Yalug I, Selekler M, Erdogan A, Kutlu A, Dundar G, Ankarali H, Aker T. Correlations between alexithymia and pain severity, depression, and anxiety among patients with chronic and episodic migraine. Psychiatry Clin Neurosci 2010;64:231–8. [DOI] [PubMed] [Google Scholar]
- [133].Yucel B, Kora K, Ozyalcin S, Alcalar N, Ozdemir O, Yucel A. Depression, automatic thoughts, alexithymia, and assertiveness in patients with tension-type headache. Headache 2002;42:194–9. [DOI] [PubMed] [Google Scholar]
- [134].Zeng F, Sun X, Yang B, Fu X. Life events, anxiety, social support, personality, and alexithymia in female patients with chronic pain: a path analysis. Asia Pac Psychiatry 2016;8:44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Zhu X, Yi J, Yao S, Ryder AG, Taylor GJ, Bagby RM. Cross-cultural validation of a Chinese translation of the 20-item Toronto. Alexithymia Scale 2007;48:489–96. [DOI] [PubMed] [Google Scholar]
- [136].Zimmermann G, Quartier V, Bernard M, Salamin V, Maggiori C. The 20-item Toronto Alexithymia Scale: structural validity, internal consistency and prevalence of alexithymia in a Swiss adolescent sample. L’encéphale 2007;33:941–6. [DOI] [PubMed] [Google Scholar]
- [137].Zincir SB, Sunbul M, Sunbul EA, Dalkilic B, Cengiz F, Kivrak T, Durmus E. Evaluation of alexithymia, somatosensory sensitivity, and health anxiety levels in patients with noncardiac chest pain. Biomed Res Int 2014;2014:896183. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


