Abstract
Purpose
This study investigates the biologic activity of radium-223 with vascular endothelial growth factor (VEGF)-targeted therapy in patients with advanced renal cell carcinoma (aRCC) and bone metastases.
Experimental design
Fifteen treatment-naïve patients (n=15) received pazopanib 800 mg orally once-daily and 15 previously-treated patients received sorafenib 400 mg orally twice-daily. Radium-223 55 kilobecquerel/kg was administered concurrently every four weeks for up to 6 infusions in both cohorts. The primary endpoint was decline in bone turnover markers (Procollagen I Intact N-Terminal, N-telopeptide, C-telopeptide, osteocalcin and bone-specific alkaline phosphatase) compared to baseline. Secondary endpoints included safety, rate of symptomatic-skeletal event (SSE) and time to first SSE, objective response rate, change in analgesic use and quality of life. Exploratory analysis of tumor genomic alterations was performed.
Results
Of the 30 patients enrolled, 83% had IMDC intermediate- or poor-risk disease, 33% had liver metastases and 83% had a history of SSE prior to enrolment. No dose-limiting toxicity was observed. All bone turnover markers significantly declined from baseline at week 8 and 16. Forty percent of patients experienced treatment-related grade ≥3 adverse events. Response rates were 15% and 18% per RECIST v1.1 and bone response was 50% and 30% per MD Anderson criteria, in the pazopanib and sorafenib cohort, respectively. Median SSE-free interval was 5.8 months and not reached, respectively. Analgesic use remained stable over the study time.
Conclusion
Radium-223 combined with VEGF-targeted therapy is biologically active and safe. Randomized-controlled trials are needed to define the role of radium-223 in aRCC with skeletal metastases.
Keywords: Bone Turnover Markers, Skeletal-Related Events, Kidney Cancer, Alpha-Emitter, Tyrosine Kinase Inhibitor, pazopanib, sorafenib
Translational Relevance
Bone metastases from advanced renal cell carcinoma (aRCC) are destructive and are associated with skeletal events, decreased quality of life and shorter survival. Radium-223 acts as a calcium-mimetic that targets skeletal lesions with increased bone turnover. The alpha emissions from radium-223 may impede the progression of bone metastasis by killing tumor cells and stabilizing the tumor microenvironment. Here, we assessed the biologic activity of radium-223 with standard of care VEGF-targeted therapy in 30 patients with aRCC and bone metastases. The treatment led to a significant decline in serum levels of five bone turnover markers at week 8 and 16 compared to baseline. No dose-limiting toxicity was observed. These findings suggest that radium-223 plus VEGF-targeted therapy is a safe combination with biological activity in bone metastases from aRCC. Randomized-controlled trials are needed to determine whether radium-223 may improve symptomatic-skeletal event free survival in patients with aRCC and osseous metastases.
Background
Approximately 30% of patients with advanced renal cell carcinoma (aRCC) present with bone metastases(1,2). These osseous metastases are usually destructive in nature and associated with the development of symptomatic-skeletal events (SSE) such as pathological fracture, spinal cord compression or need for surgical stabilization or radiation(3). Historically, the majority of patients (71–85%) with aRCC and bone metastases experience a skeletal-related event(3–5). These events are associated with severe disability, poor quality of life (QoL), increased opioid use and significant health care costs.(6) In addition to the morbidity of SSEs, patients with bone metastases also have poorer prognosis(7). Novel strategies are warranted to improve the QoL, SSE rates and survival of these patients.
Radium-223 is the first approved targeted alpha-emitting therapy which acts as a calcium-mimetic with natural bone-seeking proclivity. Alpha-particles induce DNA double-strand breaks leading to cellular death in areas with increased osteoblastic activity(8). Its efficacy was demonstrated in the ALSYMPCA trial, where men with castrate-resistant prostate cancer treated with radium-223 had superior overall survival (OS) and longer time to first SSE compared to placebo(9). Radium-223 is now being investigated in other tumor types with high prevalence of bone lesions, such as multiple myeloma, breast cancer and osteosarcoma(10,11).
The skeleton is a metabolically active organ that undergoes continuous remodeling, a dynamic process of bone resorption by osteoclasts and bone formation by osteoblasts(12). Though historically bone metastases secondary to aRCC have been radiographically described as osteolytic, preclinical and clinical studies of the tumor microenvironment have supported that bone metastases from aRCC result in dysregulation of both osteoblast and osteoclast function(13,14). Alpha emissions from radium-223 may interfere with this pathologic process by killing tumor cells and stabilizing the tumor microenvironment surrounding the bone lesions(15,16).
In this trial, we explored the biological activity and safety of radium-223 combined with VEGF-targeted therapy in patients with aRCC metastatic to the bone.
Patients and Methods
Study Design
The study was a two-cohort open-label trial of radium-223 (Xofigo, Bayer) combined with vascular endothelial growth factor (VEGF)-targeted therapy. The first cohort included treatment-naïve patients (n=15), while the second cohort included previously treated patients (n=15). Accrual was completed at Dana-Farber Cancer Institute and the Massachusetts General Hospital in Boston (MA, USA) between May 2015 through July 2016. The protocol was approved by the Dana Farber/Harvard Cancer Center institutional review board and all patients provided written informed consent. The trial () was conducted in full concordance with the principles of the Declaration of Helsinki and Good Clinical Practice.
Patients
Eligible patients were required to have a documented pathologic diagnosis of clear-cell or non-clear cell aRCC, radiologic evidence of one or more bone metastases, Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 1 and adequate organ and bone marrow function. Key exclusion criteria included untreated brain metastases, impending or untreated spinal cord compression, uncontrolled hypertension or cardiovascular conditions, gastrointestinal disorders at risk of perforation or fistula formation, active bleeding, recent major surgery and prior treatment with systemic radiopharmaceuticals. In the pazopanib cohort, prior systemic therapy other than cytokine was not allowed. In the sorafenib cohort, prior treatment with VEGF-targeted therapy other than sorafenib was required. Prior or concurrent bisphosphonates or denosumab was permitted.
Safety run-in
A safety run-in was conducted in the first six patients enrolled in each cohort following an observation period of eight weeks of therapy. Adverse events were recorded and graded per the Common Terminology Criteria for Adverse Events version 4.0.
Treatment
Treatment-naïve patients received pazopanib 800 mg orally once-daily, whereas previously treated patients received sorafenib 400 mg orally twice-daily with the option to dose-escalate to 600 mg orally twice-daily upon radiographic progression at the discretion of the treating investigator(17). Two dose reduction levels were allowed for pazopanib and sorafenib. All patients received radium-223 intravenously every 28 days for up to 6 doses. The initial dosing of radium-223 was 50 KBq/kg. In accordance with the National Institute of Standards and Technology (NIST) revision of January 2016, dosing of radium-223 was calibrated to the new NIST standard which corresponded to 55 KBq/kg after 16 patients were enrolled on trial. No radium-223 dose modification was allowed(18).
Study Endpoints
The primary endpoint was change in bone turnover markers (BTM) compared to baseline. Markers of bone resorption [serum c-terminal cross-linked telopeptide of type I collagen (CTX), and serum N-terminal cross-linked telopeptide of type I collagen (NTX)] and bone formation [N-terminal propeptide of procollagen type I (PINP), osteocalcin and bone-specific alkaline phosphatase (BALP)] were measured at baseline then every 8 weeks and analyzed by enzyme-linked immunosorbent assay (CTX, NTX and PINP) or electrochemiluminescence (osteocalcin and BALP) at the Mayo Medical Laboratory (Rochester, MN).
Secondary endpoints
SSE-free interval was defined as time from treatment start to the first SSE event. SSEs were defined as the use of external beam radiation to relieve bone pain, occurrence of a new symptomatic pathologic fracture, spinal cord compression, or tumor-related orthopedic surgical intervention. Progression-free survival (PFS) was defined as the time from registration to disease progression or death and overall survival (OS) was defined as the time from treatment start to death from any cause.
Imaging assessments (including computed tomography or magnetic resonance imaging and technetium-99m-MDP bone scans) occurred at baseline and every 8 weeks. Objective response rate (ORR) [partial response (PR) + complete response (CR)] and clinical benefit [ORR + stable disease (SD)] were defined per Response Evaluation Criteria In Solid Tumors (RECIST) version 1.1(19). Bone scan response was defined per MD Anderson (MDA) bone response criteria(20) (Supplement Table S1).
Analgesic use was assessed at baseline then every 4 weeks and analyzed according to step changes in the three-step World Health Organization Treatment Ladder (WHO-AL) and the eight-step Analgesic Quantification Algorithm (AQA)(21) (Supplement Table S2).
Health-related QoL questionnaires [Functional Assessment of Cancer Therapy (FACT)-Kidney Symptom Index-19 (FKSI-19) and the Brief Pain Inventory Short Form (BPI-SF)] were administered at baseline then every 8 weeks. The BPI-SF is a multi-item questionnaire with three subscales assessing: a) pain severity b) functional interference and c) pain relief by analgesic use(22). FKSI-19 questionnaire total score ranges from 0 to 76 and comprises four domains: a) Disease-Related Symptoms-Physical (DRS-P), b) Disease-Related Symptoms-Emotional (DRS-E), c) Treatment Side Effects (TSE) and d) Function Well Being (FWB). Change of 5 units in the total score was considered the minimal important difference(23,24).
Genomics
Post-hoc exploratory analysis to identify potential genomic alterations associated with clinical benefit was performed using available archival tissue from patients who provided informed consent. Deep targeted next-generation sequencing was performed using Dana-Farber Cancer Institute PROFILE test, a hybrid-capture and massively parallel sequencing assay surveying exonic DNA of 400 cancer genes(25). For the purpose of the genomic analysis only, patients were stratified as having “clinical benefit” if their best radiographic response per RECIST was CR or PR, or if they had stable disease lasting ≥4 months(26). Otherwise, they were labeled as “no clinical benefit”. Fisher exact test was used to compare the mutations between the two groups.
Statistical Methods
The study was designed to assess the change in serum level of BTM markers from baseline. With a sample size of 30 patients, there is 80% power to detect a change in BTM levels compared to baseline with an effect size of 0.465 standard deviation using a one-sample paired t-test with a two-sided alpha error of 0.10.
The analysis included all patients who received at least one study treatment. Non-parametric Wilcoxon signed rank test was used to assess the pair-wise comparisons of serum levels in BTMs with continuity corrections. Median absolute changes in BTM levels and 90% confidence internal (CI) are reported.
SSE-free interval, PFS and OS were estimated using Kaplan-Meier methods. Association between ≥50% decline in bone turnover biomarkers and objective response by RECIST v1.1 was investigated using Fisher’s exact test. Prior and on-study analgesics use was descriptively summarized per treatment arm.
Linear mixed-effects models adjusting for age and gender were used to explore changes in BPI-SF and FKSI-19 scores over time. Adjusted mean with standard error (SE) from baseline of BPI-SF domains including severity interference composite scores and pain relief were reported.
Results
Patients
Fifteen patients were enrolled in each cohort for a total of 30 patients. The median follow-up for OS was 15.8 months (95%CI, 13.4 to NA), overall, and 15.4 months (95%CI, 13.7 to NA) for pazopanib plus radium-223 arm and 25.1 months (95%CI, 13.4 to NA) for sorafenib plus radium-223, respectively. Median age was 62, 70% had clear-cell histology, 33% had liver metastases, 83% were IMDC intermediate or poor risk and 83% had a history of SSE prior to enrolment (pazopanib plus radium-223 cohort 15/15 patients; sorafenib plus radium-223 cohort 10/15 patients) Table 1. Prior SSEs consisted in radiation therapy for pain (60%), symptomatic fractures (47%), orthopedic surgical intervention (43%) and cord compression (17%).
Table 1.
Disease and patient baseline characteristics
| Radium-223 + pazopanib N=15 No. (%) |
Radium-223 + sorafenib N=15 No. (%) |
Total N=30 No. (%) |
|
|---|---|---|---|
| Male | 10(66.7) | 13(86.7) | 23(76.7) |
| Median age (IQR) | 59 (56,68) | 64 (59,68) | 62 (58,68) |
| ECOG PS | |||
| 0 | 7(47) | 6(40) | 13(43) |
| 1 | 8(53) | 9(60) | 17(57) |
| Histology | |||
| Clear-cell | 11(73) | 10(67) | 21(70) |
| Papillary | 2(13) | 5(33) | 7(23) |
| Unclassified | 2(13) | - | 2(7) |
| IDMC risk groups | |||
| Favorable | 3(20) | 2(13) | 5(17) |
| Intermediate | 9(60) | 11(73) | 20(67) |
| Poor | 3(20) | 2(13) | 5(17) |
| Lung metastasis | 9(60) | 8(53) | 17(57) |
| Liver metastasis | 3(20) | 7(47) | 10(33) |
| Prior nephrectomy | 13(87) | 12(80) | 25(83) |
| Prior line therapies | |||
| 1 | 1 (7) | 4(27) | 5(31) |
| 2 | - | 3(20) | 3(19) |
| 3 | - | 4(27) | 4(25) |
| 4 | - | 4(27) | 4(25) |
| Prior bisphosphonate or denosumab | 4(27) | 7(47) | 11(37) |
| Prior SSE | 15 (100) | 10 (67) | 25 (83) |
ECOG, Eastern Collaborative Oncologic Group, IQR, interquartile range; PS, Performance status; SSE, symptomatic skeletal events
Safety
No drug-limiting toxicity was identified during the safety run-in and the study was continued accordingly. Dose reductions occurred in one patient receiving pazopanib and four patients receiving sorafenib. Incidences of grade 3 or 4 treatment-related adverse events were 40% (6/15) and 33% (5/15) in the pazopanib plus radium-223 and sorafenib plus radium-223 cohorts, respectively (Table 2). Myelosuppression was a treatment-related adverse event of interest when combining these agents. Overall, grade 3 treatment-related anemia was observed in one patient. No grade 4 anemia, grade 3/4 thrombocytopenia or neutropenia or treatment-related deaths occurred.
Table 2.
Treatment-related adverse effects of radium-223 dichloride in combination with pazopanib or sorafenib
| Adverse Events | Pazopanib + Radium-223 | Sorafenib + Radium-223 | ||||||
|---|---|---|---|---|---|---|---|---|
| Any grade | Grade 3 or 4 | Any grade | Grade 3* | |||||
| N | % | N | % | N | % | N | % | |
| Anemia | 2 | 13 | 0 | 2 | 13 | 1 | 7 | |
| Leukopenia | 0 | 0 | 1 | 7 | 0 | 7 | ||
| Neutropenia | 2 | 13 | 0 | 0 | 0 | |||
| Thrombocytopenia | 1 | 7 | 0 | 0 | 0 | |||
| Hypothyroidism | 5 | 33 | 0 | 0 | 0 | |||
| Hypertension | 4 | 27 | 2 | 13 | 4 | 27 | 1 | 7 |
| Diarrhea | 7 | 47 | 0 | 7 | 47 | 0 | ||
| Dyspepsia | 1 | 7 | 0 | 0 | 0 | |||
| Mucositis oral | 1 | 7 | 0 | 2 | 13 | 0 | ||
| Dysgeusia | 4 | 27 | 0 | 1 | 7 | 0 | ||
| Nausea | 7 | 47 | 0 | 3 | 20 | 0 | ||
| Vomiting | 5 | 33 | 0 | 4 | 27 | 0 | ||
| Edema limbs | 0 | 0 | 1 | 7 | 0 | |||
| Fatigue | 6 | 40 | 1 | 7 | 4 | 27 | 1 | 7 |
| Rash | 0 | 0 | 7 | 47 | 3 | 20 | ||
| Palmar-plantar erythrodysesthesia | 1 | 7 | 0 | 4 | 27 | 1 | 7 | |
| Increased alanine aminotransferase | 5 | 33 | 3 | 20 | 0 | 0 | 0 | |
| Increased aspartate aminotransferase | 6 | 40 | 2 | 13 | 3 | 20 | 0 | |
| Hyperbilirubinemia | 4 | 27 | 0 | 2 | 13 | 0 | ||
| Increased creatinine | 1 | 7 | 0 | 0 | 0 | |||
| Increased lipase | 1 | 7 | 1 | 7 | 1 | 7 | 0 | |
| Increased serum amylase | 2 | 13 | 2 | 13 | 2 | 13 | 1 | 7 |
| Weight loss | 5 | 33 | 0 | 4 | 27 | 0 | ||
| Anorexia | 6 | 40 | 0 | 2 | 13 | |||
| Proteinuria | 3 | 20 | 2 | 13 | 0 | 0 | ||
| Pruritus | 0 | 0 | 2 | 13 | 0 | |||
| Adverse event per patients | ||||||||
| Any treatment-related adverse event | 14 | 93 | 6 | 40 | 14 | 93 | 5 | 33 |
No grade 4 toxicity observed in the sorafenib + radium-223 dichloride arm.
Bone turnover markers
Bone turnover markers were assessed in all thirty patients at baseline, then per protocol every 8 weeks. Seven patients came off study before the first post-baseline assessment due to toxicity (n=2), clinical progression or death (n=3) or withdrawal of consent (n=2). Markers of bone formation (BALP, PINP, osteocalcin) and resorption (CTX, NTX) decreased significantly at week 8 and 16 of treatment compared to baseline, meeting the primary endpoint of the study (Figure 1). Bone turnover markers at later time points were not analyzed due to the small number of samples available at cycle 6 to 10 at the time of data cut-off. The maximum decline in each biomarker and potential associations between ≥50% decline and clinical benefit are summarized in Table 3. Among patients with SD/PR, 83.3% (n=15/18) experienced a maximum PINP decline on study of at least 50% from baseline, compared to 40% (n=2/5) of patients with progressive disease (PD), corresponding to an odds ratio (OR) of 7.5 (90% CI, 1.21–46.6). Eleven (37%) and 8 (27%) patients received at least one dose of osteoclast-targeted therapy prior or after enrolment in the study, respectively. Post-hoc analysis revealed no association between use of osteoclast-targeted therapy and maximum decline in BTMs of ≥50%.
Figure 1.
Median absolute change from baseline in levels of bone turnover markers at baseline and every 2 cycles. * indicates significant decline compared to baseline. CTX, C-terminal cross-linked telopeptide of type I collagen; BALP, Bone-specific alkaline phosphatase; NTX, N-terminal cross-linked telopeptide of type I collagen; PINP, N-terminal propeptide of procollagen type I; OC, osteocalcin
Table 3.
Turnover markers from baseline and association between 50% or greater decline and clinical benefit
| Baseline levels (median, IQR) | Maximum decline (median, IQR) | On-study maximum decline in levels of BTM of ≥50% from baseline | ||||
|---|---|---|---|---|---|---|
| PR/SD: Prop [freq/n] (90%CI) | PD/unknown*: Prop [freq/n] (90%CI) | Odds Ratio (90%CI) | Fisher Exact P-value | |||
| CTX (ng/mL) | 0.43(0.21,0.6) | −40.7 (−59.6,−32.4) | 38.9% [7/18] (20.0%,57.8%) |
40.0% [2/5] (4.0%,76.0%) |
0.95 (0.17,5.22) | p=1.000 |
| PINP (ug/mL) | 45.5(30.25,65.75) | −59.3 (−66.7,−48.4) | 83.3% [15/18] (68.9%,97.8%) |
40.0% [2/5] (4.0%,76.0%) |
7.50 (1.21,46.60) | p=0.089* |
| BALP (ug/mL) | 11(9.1,16) | −29.2 (−42.5,−11.7) | 11.8% [2/17] (0.0%,24.6%) |
20.0% [1/5] (0.0%,49.4%) |
0.53 (0.06,4.90) | p=1.000 |
| OC(ng/mL) | 16(12.25,18.75) | −50 (−61.3,−40.6) | 61.1% [11/18] (42.2%,80.0%) |
20.0% [1/5] (0.0%,49.4%) |
6.29 (0.85,46.61) | p=0.155 |
| NTX (nM BCE) | 14.9(11.3,21.3) | −32.8 (−48,−12.3) | 27.8% [5/18] (10.4%,45.1%) |
20.0% [1/5] (0.0%,49.4%) |
1.54 (0.20,11.74) | p=1.000 |
BALP, Bone-specific alkaline phosphatase; BTM, bone turnover marker, CTX, C-terminal cross-linked telopeptide of type I collagen; IQR, Interquartile range; NTX, N-terminal cross-linked telopeptide of type I collagen; OC, osteocalcin; OR, odds ratio; PD, progressive disease; PINP, N-terminal propeptide of procollagen type I; PR, partial response; prop, proportion; SD, stable disease.
Significance assumed at p≤0.10
Efficacy outcomes
The median number of radium-223 cycles was 4.6 (IQR, 1.0, 4.6) in the pazopanib cohort and 1.9 (IQR, 0.5, 4.4) in the sorafenib cohort (Supplement Table S3). The objective response rates per RECIST v1.1 were 23% and 9% in the pazopanib plus radium-223 and sorafenib plus radium-223 cohorts, respectively, while clinical benefit rates were 85% and 64% (Supplement Figure S1 and Table S4). The objective bone response per MDA criteria was 50% (n=6/12) in the pazopanib plus radium-223 cohort and 30% (n=3/10) in the sorafenib plus radium-223 (Supplement Table S4, Figure S2).
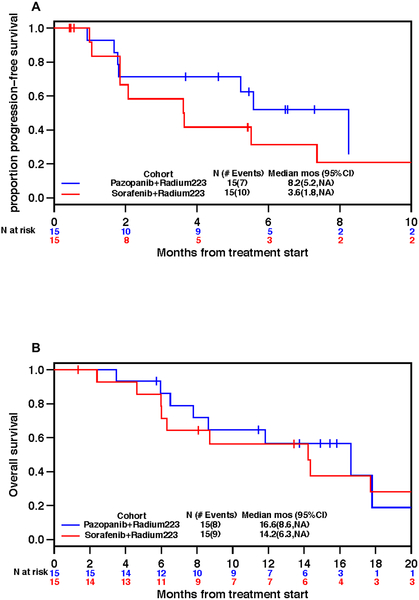
Median progression-free survival was 8.2 months (95%C I, 5.2, NA) and 3.6 months (95% CI, 1.8, NA) in the pazopanib plus radium-223 and sorafenib plus radium-223 cohort, respectively, while median OS was 16.6 months (95% CI, 8.6, NA) and 14.2 months (95% CI, 6.3, NA) (Figure 2). Nine patients (30%) experienced at least one on-study SSE; 7 in the pazopanib plus radium-223 cohort and 2 in the sorafenib plus radium-223 cohort. The median SSE-free interval was 5.8 months (95% CI, 3.4, NA) and not reached, respectively (Supplement Figure S3). Four patients had a second SSE, three in the pazopanib plus radium-223 cohort and one in the sorafenib plus radium-223 cohort. Supplement Figure S4 illustrates the temporal relationship of first on-study SSEs. Pathologic symptomatic fracture was the most common SSE (27%), followed by receipt of radiation therapy to bones (17%). There was no spinal cord compression or orthopedic surgical intervention reported on study (Supplement Table S5). Sixty two percent of the patients who experienced a fracture while on study had a fracture prior to enrollment.
Figure 2.
Kaplan-Meier plots. A- Proportion of patients with progression-free survival overtime; B- Proportion of patients surviving overtime.
Health-Related Quality of Life and Analgesic uses
QoL questionnaires were available for 29/30 patients. The adjusted FKSI-19 score slightly worsened to reach the clinically meaningful deterioration compared to baseline at cycle 3 and cycle 5 onwards. The DRS-E and FWB domain subscale scores remained unchanged over the study period, whereas the TSE and the DRS-P domain subscale scores were slightly worsened over time (Supplement Figure S5). The BPI-SF severity score stayed constant throughout the study time, whereas the interference score increased after five cycles, as the sample size became smaller. The percentage of pain relief remained constant over time (Supplement Figure S6).
Performance status and analgesic use was examined from baseline to cycle 6. Later time points were not analyzed due to small sample size. The mean performance status was 1 at baseline and remained constant for the duration of the study. Similarly, the average analgesic use as well as the proportion of patient using non-opioid analgesics or no analgesic remained unchanged during the study time (Supplement Figure S7).
Next Generation Sequencing
PROFILE gene panel was available for 16 patients; 10 who experienced clinical benefit and 6 without (Figure 3). Overall, the most frequently mutated genes were VHL (50%), SETD2 (31.3%) and PBRM1 (25%). Mutation in DNA-repair genes was overall infrequent. Tumors from three patients harbored mutations of uncertain significance in BRCA 1 and BRCA 2 genes. These variants were not associated with clinical benefit.
Figure 3.

Genomic mutations detected by targeted next generation sequencing (400 gene panel) from archival tumor specimens. Columns represent individual subjects. Rows represent selected genes of interest examined (n=7). Patients were categorized in clinical benefit (PR or SD for ≥4 months) versus no clinical benefit (PD or SD for < 4 months or not assessable). BRCA mutations were of uncertain significance. Histology: clear-cell = maroon, papillary = green. Mutation: Missense = blue, Nonsense = red, Splice site = green, frame shift insertion = orange, frame shift deletion = yellow
Discussion
Although many new targeted therapies were approved for the treatment of aRCC since 2005(27), the need to improve SSE rates and outcomes in the subset of patients with bone metastases remains a largely unmet need. We report the results of an exploratory trial investigating the biologic activity of radium-223 combined with VEGF-targeted therapy in patients with aRCC and bone metastases. This study is the first to examine the role of radium-223 in aRCC.
We found that combining radium-223 with either pazopanib or sorafenib is safe and tolerable. Adverse events were consistent with the known toxicity profile of VEGF-targeted therapies(28,29) and adding radium-223 did not result in excess hematologic toxicity or VEGF therapy dose modifications. The study met its primary endpoint as the treatment combination led to a significant decline in all BTMs early on treatment, suggesting activity in skeletal lesions. Although hypothesis-generating only, bone response rates per MDA criteria also support activity of radium-223 with VEGF-targeted therapy in skeletal lesions with ORRs up to 50% in the treatment-naïve cohort and 30% in treatment-refractory cohort.
This study is the first to measure BTMs in aRCC treated with radium-223. In absence of randomization, the fraction of BTM decrease attributable to radium-223, VEGF-targeted agents or osteoclast-targeted agents cannot be accurately quantified. While data on the effect of pazopanib and sorafenib on BTMs are sparse, radium-223 has been shown to decrease BALP, PINP as well as CTX levels at 20 weeks compared to placebo in prostate cancer (30). Similarly, in VEGF-refractory aRCC, cabozantinib, an antiangiogenic agent was also shown to decrease PINP and CTX levels at week 5 and 9 on therapy in the phase III METEOR trial(31). In contrast to our study, the BALP levels increased in cabozantinib-treated patients. Additionally, these patients had less skeletal events compared to patients receiving everolimus(31). The TUGAMO study assessed the kinetics of CTX, BALP and PINP in aRCC who received zoledronic acid with targeted therapy(13). CTX and BALP levels significantly declined at three and six months, however, as opposed to our findings, PINP levels did not decline before 12 months on zoledronic acid and standard of care targeted therapy (13). In contrast, we observed a significant and steeper decline in all BTM level after only 8 weeks of therapy despite lower incidence of osteoclast-targeting therapy use (47% of patients).
Interestingly, patients with PINP decline of ≥50% from baseline had a 7.5 higher odds of experiencing clinical benefit compared to those with no or lower decline. This finding is hypothesis-generating and further validation is needed to determine if PINP can be used as a marker of bone response to radium-223.
The overall on-study rate of SSE was 30% and the median SSE-free interval was 5.8 months in the treatment-naïve cohort and not reached in the treatment-refractory cohort. These results must be interpreted with caution bearing in mind that these patients were at very high risk of SSE since 83% of them had a prior event before enrolment. By comparison, in the METEOR trial, 16% and 34% of patients with an history of skeletal-related event at baseline experienced a subsequent skeletal-related event while on study in the cabozantinib and everolimus cohort, respectively(32). The shorter progression-free survival of patients in the previously-treated cohort may have contributed to the lower rate of SSE observed compared to the treatment-naïve cohort.
The relatively short median OS (16 months) of the treatment-naïve cohort is consistent with prior reports and highlights the poor prognosis of aRCC with osseous metastases(1). By comparison, the median OS of patients with aRCC metastatic to any site in contemporary trials of first-line VEGF-targeted therapy ranges between 25 and 30 months(27). This emphasizes the need for better therapies in this subset of patients with bone metastases.
Patients’ performance status and analgesic use stayed constant throughout the study, whereas QoL slightly trended down with time. The FKSI-19 total score worsen by five units at cycle 3 and cycle 5 onwards, which was mostly driven by changes related to treatment side effects and physical symptoms due to aRCC itself. However, similar findings were observed in the COMPARZ trial, where patients who received pazopanib experienced a decline in FKSI-19 total score from baseline hovering around 4 to 5 units at weeks 4, 10, 16 and 22(33). These changes were mostly due to worsening in TSE and DRS-P domain scores.
The BPI-SF results suggest that patients’ pain levels remained fairly constant during the study time, while no increase in analgesic use were detected on the AQA and WHO-AL scales. Although QoL or pain control remained stable on study compared to baseline, it is possible that radium-223 and VEGF-targeted therapy slowed or halted the progression of skeletal metastases and bone pain.
Since radium-223 induces double-stranded DNA damage, we evaluated whether alterations in tumor DNA-repair genes were associated with clinical benefit. We did not observe a relationship between DNA repair gene mutations and clinical benefit to therapy. The analysis may have been limited by the small sample size. The potential predictive value of DNA-repair gene mutations, such as BAP1 altered in 10% of aRCC (34,35), warrants further investigation.
The study experienced the limitations inherent to small and exploratory non-randomized trials. The absence of comparator arm limited our understanding of how the addition of radium-223 over VEGF-targeted therapy alone contributed to BTM decline, bone response per MDA criteria, SSE rates and health-related QoL scores. Measurement of BTMs is susceptible to inter-individual variability due to different burden of bone metastasis and biological factors such as gender and age(36). However, to account for this, each patient was used as his/her own control. Lastly, seven out of the thirty patients came off study before the first BTM measurement following baseline. These patients were not included in analyses of assessing BTM change/decline from baseline, potentially limiting the statistical power.
Conclusions
The combination of radium-223 and VEGF-targeted therapy in first or later lines of therapy is safe, feasible and biologically active in patients with aRCC and bone metastases. Our findings support further investigating the role of radium-223 in aRCC patients in a larger and randomized controlled trial. Given the activity of cabozantinib in aRCC patients with bone metastases, cabozantinib may be to most appropriate antiangiogenic agent to combine with radium-223 in future trials.
Supplementary Material
Disclosure of Potential Conflicts of Interest
R McKay received research funding from Bayer and Pfizer and serves on the advisory board for Novartis and Janssen; J Bellmunt is a consultant or advisor for Novartis; L Harshman is a consultant or advisor for Pfizer and received research funding from Bayer; TK Choueiri is a consultant or advisor for Bayer, Novartis and Pfizer and received research funding from Pfizer; Other co-authors have no potential conflicts of interest.
References
- 1.McKay RR, Kroeger N, Xie W, Lee J-L, Knox JJ, Bjarnason GA, et al. Impact of Bone and Liver Metastases on Patients with Renal Cell Carcinoma Treated with Targeted Therapy. European Urology 2014;65(3):577–84 doi 10.1016/j.eururo.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bianchi M, Sun M, Jeldres C, Shariat SF, Trinh QD, Briganti A, et al. Distribution of metastatic sites in renal cell carcinoma: a population-based analysis. Annals of Oncology 2012;23(4):973–80 doi 10.1093/annonc/mdr362. [DOI] [PubMed] [Google Scholar]
- 3.Woodward E, Jagdev S, McParland L, Clark K, Gregory W, Newsham A, et al. Skeletal complications and survival in renal cancer patients with bone metastases. Bone 2011;48(1):160–6 doi 10.1016/j.bone.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Santini D, Procopio G, Porta C, Ibrahim T, Barni S, Mazzara C, et al. Natural History of Malignant Bone Disease in Renal Cancer: Final Results of an Italian Bone Metastasis Survey. PLoS ONE 2013;8(12) doi 10.1371/journal.pone.0083026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coleman RE. Clinical Features of Metastatic Bone Disease and Risk of Skeletal Morbidity. Clinical Cancer Research 2006;12(20) doi 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 6.Antczak C, Trinh VQ, Sood A, Ravi P, Roghmann F, Trudeau V, et al. The Health Care Burden of Skeletal Related Events in Patients with Renal Cell Carcinoma and Bone Metastasis. The Journal of Urology 2014;191(6):1678–84 doi 10.1016/j.juro.2013.12.042. [DOI] [PubMed] [Google Scholar]
- 7.McKay RR, Lin X, Perkins JJ, Heng D, Simantov R, Choueiri TK. Prognostic Significance of Bone Metastases and Bisphosphonate Therapy in Patients with Renal Cell Carcinoma. European Urology 2014;66(3):502–9 doi 10.1016/j.eururo.2014.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henriksen G, Breistøl K, Bruland ØSS, Fodstad Ø, Larsen RH. Significant antitumor effect from bone-seeking, alpha-particle-emitting (223)Ra demonstrated in an experimental skeletal metastases model. Cancer research 2002;62(11):3120–5. [PubMed] [Google Scholar]
- 9.Parker C, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, Fosså SD, et al. Alpha Emitter Radium-223 and Survival in Metastatic Prostate Cancer. The New England Journal of Medicine 2013;369(3):213–23 doi 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 10.Coleman R, Aksnes A-K, Naume B, Garcia C, Jerusalem G, Piccart M, et al. A phase IIa, nonrandomized study of radium-223 dichloride in advanced breast cancer patients with bone-dominant disease. Breast Cancer Research and Treatment 2014;145(2):411–8 doi 10.1007/s10549-014-2939-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Subbiah V, Anderson PM, Kairemo K, Huh WW, Ravi V, Daw NC, et al. Alpha particle radium-223 dichloride (223RaCl2) in high risk osteosarcoma. Journal of Clinical Oncology 2016;34(15_suppl):11029- doi 10.1200/JCO.2016.34.15_suppl.11029. [DOI] [Google Scholar]
- 12.Raggatt LJ, Partridge NC. Cellular and Molecular Mechanisms of Bone Remodeling. Journal of Biological Chemistry 2010;285(33):25103–8 doi 10.1074/jbc.R109.041087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alcaraz A, González-López R, Morote J, de la Piedra C, Meseguer C, Esteban E, et al. Biochemical markers of bone turnover and clinical outcome in patients with renal cell and bladder carcinoma with bone metastases following treatment with zoledronic acid: The TUGAMO study. British Journal of Cancer 2013;109(1):121–30 doi 10.1038/bjc.2013.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen S-C, Kuo P-L. Bone Metastasis from Renal Cell Carcinoma. International Journal of Molecular Sciences 2016;17(6):987 doi 10.3390/ijms17060987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suominen MI, Fagerlund KM, Rissanen JP, Konkol YM, Morko JP, Peng Z, et al. Radium-223 Inhibits Osseous Prostate Cancer Growth by Dual Targeting of Cancer Cells and Bone Microenvironment in Mouse Models. Clinical Cancer Research 2017;23(15):4335–46 doi 10.1158/1078-0432.CCR-16-2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abou DS, Ulmert D, Doucet M, Hobbs RF, Riddle RC, Thorek DLJ. Whole-Body and Microenvironmental Localization of Radium-223 in Naïve and Mouse Models of Prostate Cancer Metastasis. JNCI: Journal of the National Cancer Institute 2016;108(5) doi 10.1093/jnci/djv380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Escudier B, Szczylik C, Hutson TE, Demkow T, Staehler M, Rolland F, et al. Randomized Phase II Trial of First-Line Treatment With Sorafenib Versus Interferon Alfa-2a in Patients With Metastatic Renal Cell Carcinoma. Journal of Clinical Oncology 2009;27(8):1280–9 doi 10.1200/JCO.2008.19.3342. [DOI] [PubMed] [Google Scholar]
- 18.NRC. 2016. 10–03. Notice 2016–03: Revision to the National Institute of Standards and Technology Standard for Radium-223 Impact on Dose Calibration for the Medial Use of Radium-223 Dichloride Nuclear Regulatory Commission Office of Nuclear Materials Safety and Safeguards <https://www.nrc.gov/docs/ML1526/ML15264B095.pdf>. Accessed 2017 10-03.
- 19.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). European Journal of Cancer 2009;45(2):228–47 doi 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Hamaoka T, Madewell JE, Podoloff DA, Hortobagyi GN, Ueno NT. Bone imaging in metastatic breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2004;22(14):2942–53 doi 10.1200/JCO.2004.08.181. [DOI] [PubMed] [Google Scholar]
- 21.Chung KC, Barlev A, Braun AH, Qian Y, Zagari M. Assessing analgesic use in patients with advanced cancer: development of a new scale--the Analgesic Quantification Algorithm. Pain medicine (Malden, Mass) 2014;15(2):225–32 doi 10.1111/pme.12299. [DOI] [PubMed] [Google Scholar]
- 22.Daut RL, Cleeland CS, Flanery RC. Development of the Wisconsin Brief Pain Questionnaire to assess pain in cancer and other diseases. Pain 1983;17(2):197–210. [DOI] [PubMed] [Google Scholar]
- 23.Butt Z, Peipert J, Webster K, Chen C, Cella D. General population norms for the Functional Assessment of Cancer Therapy–Kidney Symptom Index (FKSI). Cancer 2013;119(2):429–37 doi 10.1002/cncr.27688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rao D, Butt Z, Rosenbloom S, Robinson D, Roenn J, Kuzel TM, et al. A Comparison of the Renal Cell Carcinoma–Symptom Index (RCC-SI) and the Functional Assessment of Cancer Therapy–Kidney Symptom Index (FKSI). Journal of Pain and Symptom Management 2009;38(2):291–8 doi 10.1016/j.jpainsymman.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sholl LM, Do K, Shivdasani P, Cerami E, Dubuc AM, Kuo FC, et al. Institutional implementation of clinical tumor profiling on an unselected cancer population. JCI insight 2016;1(19) doi 10.1172/jci.insight.87062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Motzer RJ, Sharma P, Escudier BJ, McDermott DF, George S, Srinivas S, et al. Correlation of response with overall survival (OS) for nivolumab vs everolimus in advanced renal cell carcinoma (aRCC): Results from the phase III CheckMate 025 study. Journal of Clinical Oncology 2016;34(15_suppl):4552- doi 10.1200/JCO.2016.34.15_suppl.4552. [DOI] [Google Scholar]
- 27.Choueiri TK, Motzer RJ. Systemic Therapy for Metastatic Renal-Cell Carcinoma. N Engl J Med 2017;376(4):354–66 doi 10.1056/NEJMra1601333. [DOI] [PubMed] [Google Scholar]
- 28.Rini BI, Escudier B, Tomczak P, Kaprin A, Szczylik C, Hutson TE, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. The Lancet 2011;378(9807):1931–9 doi 10.1016/S0140-6736(11)61613-9. [DOI] [PubMed] [Google Scholar]
- 29.Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J, et al. Pazopanib versus Sunitinib in Metastatic Renal-Cell Carcinoma. The New England Journal of Medicine 2013;369(8):722–31 doi 10.1056/NEJMoa1303989. [DOI] [PubMed] [Google Scholar]
- 30.Nilsson S, Franzén L, Parker C, Tyrrell C, Blom R, Tennvall J, et al. Bone-targeted radium-223 in symptomatic, hormone-refractory prostate cancer: a randomised, multicentre, placebo-controlled phase II study. The Lancet Oncology 2007;8(7):587–94 doi 10.1016/S1470-2045(07)70147-X. [DOI] [PubMed] [Google Scholar]
- 31.Escudier B, Powles T, Motzer RJ, Olencki T, Arén Frontera O, Oudard S, et al. Cabozantinib, a New Standard of Care for Patients With Advanced Renal Cell Carcinoma and Bone Metastases? Subgroup Analysis of the METEOR Trial. Journal of Clinical Oncology 2018;36(8):765–72 doi 10.1200/JCO.2017.74.7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Escudier BJ, Powles T, Motzer RJ, Olencki T, Aren OR, Oudard S, et al. Efficacy of cabozantinib (C) vs everolimus (E) in patients (pts) with advanced renal cell carcinoma (RCC) and bone metastases (mets) from the phase III METEOR study. Journal of Clinical Oncology 2016;34(15_suppl):4558- doi 10.1200/JCO.2016.34.15_suppl.4558. [DOI] [Google Scholar]
- 33.Novartis. 2013. October/05 Pazopanib Versus Sunitinib in the Treatment of Locally Advanced and/or Metastatic Renal Cell Carcinoma (COMPARZ) NIH U.S. National Library of Medicine <https://clinicaltrials.gov/ct2/show/results/NCT00720941>. Accessed 2017 10/05. [Google Scholar]
- 34.Yu H, Pak H, Hammond-Martel I, Ghram M, Rodrigue A, Daou S, et al. Tumor suppressor and deubiquitinase BAP1 promotes DNA double-strand break repair. Proceedings of the National Academy of Sciences 2014;111(1):285–90 doi 10.1073/pnas.1309085110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brugarolas J Molecular Genetics of Clear-Cell Renal Cell Carcinoma. Journal of Clinical Oncology 2014;32(18):1968–76 doi 10.1200/JCO.2012.45.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seibel MJ. Biochemical markers of bone turnover: part I: biochemistry and variability. The Clinical biochemist Reviews 2005;26(4):97–122. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.