Abstract
Purpose.
One of the goals of identifying youth identified, based on clinical symptoms, as being at risk for developing psychosis, is to find ways to prevent or even delay the onset of the illness. Over the past 20 years, relatively few randomized control trials (RCTs), including both pharmacological and psychosocial interventions, have been conducted and often with inconsistent results. Several recent meta-analyses suggest that there are few treatments if any that might be effective and that no one treatment is seen as being more effective than any other treatment. This review aims to examine the existing RCTs and to critically review recent meta-analyses.
Recent Findings.
Individuals at clinical high risk for psychosis are a heterogenous group. Unfortunately, many interventions have not been specifically designed to address the outcome being assessed nor have participants been specifically selected for that treatment.
Summary.
The trials completed to date and the recent systematic reviews should be seen positively and used to guide the design of future trials to ensure that the right interventions are offered to the right people at the right time.
Keywords: psychosis, prodrome, clinical trials, clinical high risk, meta-analyses, treatment
Introduction
One approach to an improved understanding of the development of schizophrenia and other psychotic illnesses is the study of those who are at risk for developing psychosis. Over the past two decades, there has been an increasing amount of research focussed on early identification of those at risk of psychosis. These young people are identified based on clinical criteria, specifically, attenuated psychotic symptoms which are suggestive of being prodromal for psychosis. Since the majority of those identified will not develop a psychotic illness, these individuals are referred to as being at clinical high risk or at ultra high risk (UHR) for psychosis. There are well developed criteria for identification of risk based on structured clinical interviews such as the Scale of Psychosis-Risk Syndromes (SIPS [1]), the Comprehensive Assessment of At-Risk Mental States (CAARMS [2]) and Basic Symptoms using the Schizophrenia Proneness Interview for Adults (SPI-A)[3]. One of the hopes of this line of research was to not only identify early those at risk of psychosis, but to also intervene to prevent or delay the onset of psychosis. The overall aim of this review is to highlight the most recent treatment studies and present the current direction of this aspect of the field.
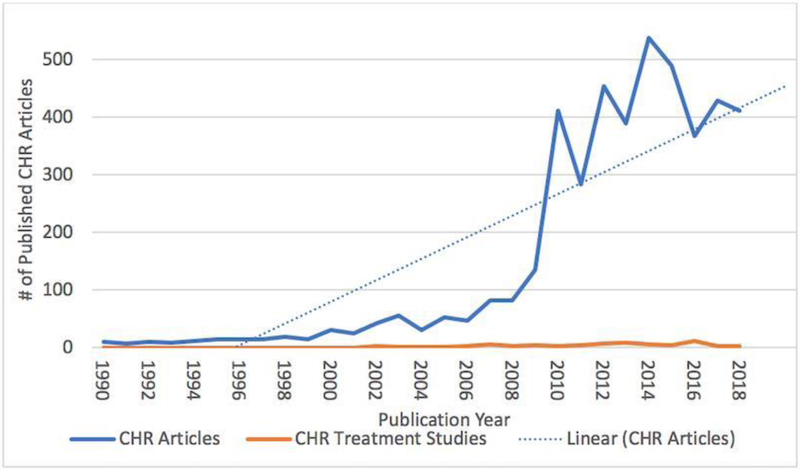
We conducted an electronic search to explore the current state of clinical high-risk research to examine the incidence of treatment publications versus other research publications stratified by time (Figure 1). Clinical high-risk non-treatment research has exponentially increased over the last decade growing from an average of 10 studies per year in 1990-1993 to an average of 400 articles per year in 2014-2018. In comparison, clinical high-risk treatment articles published in the same timeframe has remained relatively low
Figure 1.
Published CHR research studies versus published CHR treatment studies (1990-2018)
Based on our comprehensive reviews for several of the meta-analyses to be discussed below, over 50 treatment studies have been identified in the literature. However, in addition to randomized controlled trials (RCT), these include small pilot studies, open trials and trials that include mixed populations including first episode psychosis patients as well as those at clinical high-risk for psychosis. There are, to the best of our knowledge, only 20 RCTs examining the impact of treatment in samples that consisted of only individuals at clinical high-risk. These are presented in Table 1. For this review we will focus only on these 20 RCTs. We will first address the different modalities and outcomes assessed in these RCTs, followed by a brief review of the key articles for each of the different modalities. Finally, we will critically review recent meta-analyses of these treatment trials.
Table 1.
Details of RCT treatment studies in CHR (N=20)
| Author, Year |
Country | Study Design |
Intervention | Control | Treatment Duration (weeks) |
CHR Patients | ||
|---|---|---|---|---|---|---|---|---|
| N | Age M±SD |
Male N (%) |
||||||
| Cognitive Behavioral Therapy (n=5) | ||||||||
| (Addington et al., 2011) (9) | Canada | RCT | CBT | Supportive therapy |
24 | 51 | CBT: 20.8±4.5 Supportive: 21.1±3.7 |
36 (71) |
| (Morrison et al., 2004) (4) | United Kingdom | RCT | CBT | TAU | 24 | 58 | 22±4.5 | 40 (69) |
| (Morrison et al., 2012) (5) | United Kingdom | RCT | CBT + Monitoring | Monitoring | 24 | 288 | 20.7±4.3 | 180 (63) |
| (Stain et al., 2016) (10) | Australia, New Zealand | RCT | CBT + TAU | NDRL + TAU | 24 | 57 | CBT: 16.2±2.7 NDRL: 16.5±3.2 |
23 (40) |
| (Van Der Gaag et al., 2012) (8) | Netherlands | RCT | CBT + TAU | TAU | 24 | 201 | CBT: 22.9±5.6 TAU: 22.6±5.5 |
99 (49) |
| Family Therapy (n=1) | ||||||||
| (Miklowitz et al., 2014) (21) | USA + Canada | RCT | Family focused therapy | Enhanced care | 24 | 129 | 17.4±4.1 | 74 (57) |
| Cognitive Remediation (n=3) | ||||||||
| (Choi et al., 2017) (20) | USA | RCT | CR | Active control | 8 | 62 | CR: 18.2±3.8 Active Control: 18.5±3.7 |
30 (48) |
| (Loewy et al., 2016) (19) | USA | RCT | CR | Computer games | 8 | 83 | CR:17.8±3.1 Control:18.7±4.6 |
42 (51) |
| (Piskulic et al., 2015) (18) | Canada | RCT | CR | Computer games | 12 | 43 | CR:19.7±5.7 Games:17.5±3.5 |
NR |
| Integrated Psychological Therapy (n=1) | ||||||||
| (Bechdolf et al., 2012) (22) | Germany | RCT | IPT | Supportive therapy | 52 | 128 | IPT: 25.2±5.4 Supportive: 26.8±6.2 |
81 (63) |
| Risperidone plus CBT (n=2) | ||||||||
| (McGorry et al., 2002) (28) | Australia | RCT | Risperidone: 1-2 mg/day + CBT | NBI | 24 | 59 | 20±4.0 | 34 (58) |
| (McGorry et al., 2013) (32) | Australia | RCT | Risperidone: 0.5-2 mg/day + CBT or CBT + placebo | Supportive therapy + placebo or monitoring | 52 | 193 | 18.1±3.0 | 76 (39) |
| Omega-3 (n=3) | ||||||||
| (Amminger et al., 2010) (24) | Austria | RCT | Omega-3 ω-3 PUFAs: 1.2 g/day | Placebo | 12 | 81 | Omega: 16.8±2.4 Placebo: 16.0±1.7 |
27 (33) |
| (Cadenhead et al., 2017) (23) | USA + Canada | RCT | Omega-3 FAs: 740 mg EPA, 400 mg DHA/day | Placebo | 24 | 127 | NR | NR |
| (McGorry et al., 2017) (25) | Multi-national | RCT | Omega-3 ω-3 PUFAs: 1.4 g/day + CBCM | Placebo + CBCM | 24 | 304 | 19.1±4.6 | 139 (46) |
| N-methyl-D-aspartate-receptor (NMDAR) modulators (n=2) | ||||||||
| (Kantrowitz et al., 2016) (27) | USA | RCT | D-serine: 60 mg/kg/day | Placebo | 16 | 35 | D-serine: 20±4.9 placebo: 19±3.5 |
23 (65) |
| (Woods et al, 2013) (26) | USA | RCT | Glycine: 0.8 g/kg/day | Placebo | 12 | 8 | Glycine: 15.3±0.5 Placebo:16.5±2.4 |
6 (75) |
| Antipsychotics (n=3) | ||||||||
| (Ruhrmann et al., 2007) (30) | Germany | RCT | Amisulpride: mean dose 118.7 mg/day + NFI | NFI | 12 | 124 | 25.6±6.3 | 70 (57) |
| (McGlashan et al., 2006) (29) | USA + Canada | RCT | Olanzapine: 5-15 mg/day | Placebo | 52 | 60 | Olanzapine:18.2±5.5 Placebo:17.2±4.0 |
39 (65) |
| (Woods et al., 2017) (31) | USA | RCT | Ziprasidone: 20-160 mg/day | Placebo | 24 | 50 | 22.3±4.2 | 32 (64) |
Abbreviations: AD= antidepressants; AP= antipsychotics; CBT= cognitive behavioral therapy; CBCM= Cognitive-behavioral case management; CM= case management; CR= cognitive remediation; DHA= docosahexaenoic acid; EPA= eicosapentaenoic acid; FAs= fatty acids; IPT= Integrated psychological therapy; NBI= Needs based intervention; NDRL=non-directive reflective listening; NFI= Needs focused intervention; NR= not reported; PUFA= polyunsaturated fatty acid; RCT= Randomized controlled trial; TAU= treatment as usual
Treatments
Outcomes Reported by Treatment
Treatment modalities are varied. There were 10 trials using a psychosocial treatment, seven pharmacological trials, and three using both psychosocial and pharmacological treatment. In terms of psychosocial treatments, cognitive behavior therapy (CBT), cognitive remediation (CR), family interventions, and integrative psychological therapy have been assessed. Antipsychotics and Omega-3 fatty acids have been tested; however, antidepressants have only been used in naturalistic studies. Newer compounds such as d-serine and glycine have been considered in small trials and there have been a couple of trials combining risperidone and CBT. Outcomes reported were variable with the most common being rates of transition to a psychotic disorder (80%) or of attenuated psychotic symptoms (75%), and ratings of global functioning (70%).Table 2 presents the number of trials per modality with outcome targets reported in each of the studies.
Table 2.
Outcome measures reported in published treatment studies at follow-up (n=20)
| Treatment Type | APS‡ | Negative | Transition | Cognition | Disorganization | General | Total | Global Functionina |
Social Functionina |
Role Functionina |
Quality of Life | Depression | Anxiety | Mania |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cognitive Behavioral Therapy (k=5) | 3 | 2 | 5 | 0 | 0 | 0 | 2 | 5 | 1 | 0 | 3 | 4 | 4 | 0 |
| Cognitive Remediation (k=3) | 1 | 1 | 0 | 3 | 1 | 1 | 1 | 1 | 3 | 2 | 0 | 1 | 0 | 0 |
| Family Therapy (k=1) | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Integrative Psychological | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Therapy (k=1) | ||||||||||||||
| Risperidone + CBT (k=2) | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 2 | 2 | 1 | 1 |
| Antipsychotics (k=3) | 3 | 2 | 2 | 1 | 1 | 2 | 1 | 2 | 0 | 0 | 0 | 2 | 0 | 1 |
| D-serine and Glycine (k=2) | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Omega-3 (k=3) | 2 | 2 | 3 | 0 | 0 | 1 | 2 | 2 | 0 | 0 | 0 | 2 | 0 | 1 |
| TOTAL | 75% | 65% | 80% | 30% | 20% | 30% | 50% | 70% | 25% | 15% | 25% | 60% | 25% | 15% |
Antipsychotics includes Olanzapine, Amisulpride, Ziprasidone
APS=attenuated psychotic symptoms, negative= negative symptoms, general= general symptoms, total= total symptoms
Cognitive Behavioral Therapy (CBT)
The most common psychosocial intervention for clinical high-risk is CBT. Two of the key studies in this area were the EDIE and EDIE −2 trials [4, 5]. These studies compared a model of CBT specifically designed by French and Morrison [6] for youth at clinical high-risk with monitoring. In the EDIE trial, there was a 96% reduction in the odds of making a transition to psychosis for those in the CBT group compared to those in the monitoring only condition. Furthermore, the severity of attenuated psychotic symptoms and the likelihood of being prescribed antipsychotic medication was significantly reduced in the CBT group. In the multisite RCT EDIE-2 trial, there was a significant reduction in the frequency and intensity of attenuated psychotic symptoms in the CBT condition compared to the monitoring group. There was, however, no differences in transition rates between the two groups. Nevertheless, the overall transition rate for the whole study was less than 8% within 2 years; a problem that some later clinical high-risk treatment studies also faced. The Dutch EDIE trial (EDIE-NL) [7, 8] added to treatment as usual an enriched version of the French and Morrison CBT and demonstrated that transition to psychosis could be reduced by about 50% by using this CBT intervention, remaining significant at the 4-year follow-up. Two RCTs have compared an active treatment to CBT. The first did not show any differences between supportive therapy and CBT [9] in either transition or symptom improvement. In the second study, Stain and colleagues demonstrated that although both groups showed some symptom improvement, the Non-Directive Reflective Listening condition was superior to the CBT condition in decreasing the distress related to attenuated psychotic symptoms [10]. Again, in both these studies the transition rates were very low. Interestingly, three studies were powered to detect a difference [4, 5, 8], while two studies were underpowered to detect a difference in transition rates between groups [9, 10], although in design they were adequately powered expecting a transition rate that had been reported in earlier literature, but the actual transition rate was lower.
The evidence from single studies suggests that CBT may be beneficial in the reduction of attenuated psychotic symptoms and future transition to psychosis. Furthermore, in one pairwise meta-analysis [11] CBT was associated with a reduction in transition compared to monitoring at 12- and 18- month follow-ups, although the network meta-analyses [12, 11] do not support CBT as being more effective than other interventions. In other meta-analyses there was no support for CBT as being an effective treatment for negative symptoms, social functioning, depression, or anxiety, and only a trend for attenuated psychotic symptoms [13–17].
Cognitive Remediation (CR)
Three CR studies have been conducted in clinical high-risk samples [18-20]. The primary outcomes in the CR studies were cognitive outcomes. The first study compared auditory processing-based exercises, using the Brain Fitness Program (CR group) to computer games and showed no differences in cognition between groups [18]. The next CR study again using the Brain Fitness Program in comparison to computer games showed a significant improvement in verbal memory for the CR group compared to computer games [19]. Another CR study compared Processing Speed Training (PST) to computer games and found a significant improvement in processing speed and social adjustment compared to computer games [20]. In the meta-analyses, CR failed to show any significant benefit over other treatments and controls for negative symptoms, social functioning or attenuated psychotic symptoms [13-15].
Family Intervention
To date, the only RCT that used a family intervention was completed by Miklowitz and colleagues comparing three sessions of psychoeducation on stress management to Family Focused Therapy (FFT) [21]. FFT consisted of 18 sessions that focused on symptom management, communication, social and problem-solving. Findings demonstrated that over 6 months, in the FFT condition there was a decrease in attenuated psychotic symptoms, an improvement in constructive communication, and a reduction of family conflict behaviors compared to enhanced care. There were no differences in transition rates. Although both groups improved in negative symptoms and social and role functioning, the groups did not significantly differ. One of the issues with this study is that it is not clear whether any advantage for the Family Focused Therapy was a function of the actual treatment or just that this arm consisted of more sessions. Although the additional sessions required more active participation versus just receiving education.
Integrative Psychological Therapy (IPT)
Only one RCT to date has utilized an IPT design in clinical high-risk [22]. The IPT treatment consisted of CBT, skills training, cognitive remediation, and a psychoeducational multifamily group. IPT significantly reduced transition to psychosis at both 12- and 24-month follow-up compared to supportive counselling. In the meta-analyses, IPT failed to show any significant benefit over other treatments on transition, negative symptoms, and social functioning or attenuated psychotic symptoms [12-16].
Omega-3 Fatty Acids
In clinical high-risk samples, three RCTs comparing omega-3 fatty acids to placebo have been published [23-25]. The first study demonstrated very promising results with omega-3 significantly reducing both transition rates to psychosis, attenuated psychotic symptoms, negative symptoms, and general symptoms, and improving global functioning [24]. However, in two larger trials omega-3 failed to significantly reduce transition to psychosis and had no impact on symptoms or functioning compared to placebo [23, 25]. This was supported in the meta-analyses which demonstrated that omega-3 had no pooled impact on transition, attenuated psychotic symptoms, negative symptoms, and social functioning compared to placebo and other treatments [12-16].
D-serine and Glycine
Two RCTs comparing N-methyl-D-aspartate receptor modulators (D-serine or glycine) to placebo have been published. The first was a small glycine trial that did not significantly differ from placebo on any measure [26]. However, the D-serine study demonstrated a significant improvement in negative symptoms compare to placebo [27]. The meta-analyses showed that NMDAR modulators have had no significant impact on transition, attenuated psychotic symptoms, nor negative symptoms, compared to placebo and other treatments [12, 13, 15, 16].
Antipsychotics and Antipsychotics Plus CBT
The first treatment study to suggest that it might be possible to at least delay and possibly even avert progression to a full-blown psychosis for those at clinical high-risk was a low-dose risperidone plus CBT trial [28]. Since then three RCTs comparing antipsychotics to placebo have been published. The first, a comparison of olanzapine to placebo, failed to demonstrate a significant difference in transition to psychosis between groups, but did demonstrate a significant reduction in attenuated psychotic symptoms compared to placebo [29]. However, concerns were the side effects from the medications and that, although olanzapine reduced the transition rate by more than 50%, there were issues with power rendering the result non-significant. Next, a trial of amisulpride plus needs-focused intervention versus needs focused intervention alone showed a significant impact in favor of the amisulpride group on attenuated psychotic symptoms, negative symptoms, basic symptoms, depressive symptoms, and global functioning [30]. The only recent antipsychotic trial was ziprasidone versus placebo study which failed to demonstrate a difference in transition to psychosis between groups, but showed a significant reduction in attenuated psychotic symptoms compare to placebo [31]. A second study combining risperidone and CBT failed to demonstrate any impact on transition and symptoms compared to CBT alone or to supportive therapy [32]. However, in this study there was a much lower than expected transition rate in all three groups. Finally, several meta-analyses have concluded that there is no robust evidence suggesting antipsychotics are superior to placebo and other treatments for transition, attenuated psychotic symptoms, and negative symptoms [12, 13, 15, 16].
Clinical Guidelines for Treatment for clinical high-risk Individuals
Interestingly, since the International Early Psychosis Association published the first guidelines on treatment for clinical high-risk individuals in 2005 [33] here have been several published guidelines addressing those at clinical high-risk. Typically, these would consist of a few suggestions within published guidelines for Schizophrenia or for First Episode Psychosis, most likely reflecting the limited literature that would allow levels of evidence. However, in 2013 the National Institute for Health and Clinical Excellence (NICE) in the UK published detailed guidelines for the treatment of those at clinical high-risk [34] which were in 2015 by the European Psychiatry Association (EPA) guidelines [35]. The most recent and methodologically rigorous guidelines are the Canadian Treatment Guidelines for Schizophrenia published in 2017 in the Canadian Journal of Psychiatry [36], in which one chapter is devoted to nine recommendations for treatment guidelines for those at clinical high-risk for psychosis [37]. Based on the available evidence at the time only one guideline for psychological interventions received a rating of a level 1, the remainder were rated 2 or “strong” based on the NICE criteria.
Critical Review of Recent Systematic Reviews
Recently, several systematic reviews and meta-analyses have emerged to determine the efficacy of these RCTs at impacting a variety of outcomes including transition to psychosis [11, 12], attenuated psychotic symptoms [15, 16], negative symptoms [13], depression and anxiety [17] and social functioning [14]. A common methodical and meta-analytical trend that has emerged from the recent systematic reviews is the utilization of a network meta-analysis. A network meta-analysis allows for evaluations between two or more different treatments in clinical high-risk for psychosis trials that have not been compared before in a clinical trial by integrating both direct and indirect evidence. For example, a network meta-analysis in this instance allows for comparisons between the impact of glycine, ziprasidone, and risperidone plus CBT on transition to psychosis even though this comparison has not been made in an existing RCT. This comparison is possible only if all three trials use a common comparison, such as a placebo arm. In contrast, a pairwise meta-analysis pools evidence from RCTs that compare the same interventions, such as three Omega-3 trials versus control treatments. Network meta-analyses are relatively new and can impact clinical decision making and thus it is pertinent to critically review the limitations of the recent network meta-analyses in clinical high-risk samples.
First, the most recent network meta-analysis that examined transition to psychosis included 16 RCTs and concluded that there is currently no evidence to suggest that any intervention is more effective than another at preventing transition to psychosis [12]. However, this network meta-analysis had several important limitations to consider. As the authors noted a small number of RCTs (n = 16) were included in their analysis, which ultimately formed a network with many sparse connections. As a result, the network meta-analysis lacked precision and produced large confidence intervals; thus, it is possible the results were based more on noise than on the actual impact of any specific intervention on transition to psychosis. Due to this lack of precision, the results should be interpreted cautiously. Sparse connections in network meta-analyses highlight the lack of direct evidence available in the clinical high-risk literature and that a large amount of the evidence in this particular analysis was formed from indirect evidence. Thus, the results of this network meta-analysis on transition may significantly change as more evidence emerges from future RCTs. Next, the CAARMS and SIPS were the two most common clinical high-risk instruments used to determine transition to psychosis. However, these scales differ both in content and duration of symptoms required to meet criteria for transition to psychosis. Unfortunately, in this network meta-analysis, meta-regression analyses on the type of criteria used in the included RCTs could not be conducted, due to the limited number of studies.
Second, two recent network meta-analyses emerged that examined the impact of RCTs on attenuated psychotic symptoms [15, 16]. Both concluded that no specific interventions were significantly more effective at reducing attenuated psychotic symptoms compared to all other interventions. Both produced network plots with sparse connections, thus several estimates were based on little data and without direct evidence. Even though both network meta-analyses found nothing statistically noteworthy, these results should be interpreted with caution because they are based on a limited number of trials and a lack of direct comparisons, which is demonstrated by the complex geometries of the network plots. This limitation could have inflated the chances of a type 2 error, which may have led to the false-negative conclusion that a particular type of intervention had no effect. Of the RCTs included in these two network meta-analyses almost half failed to blind assessors from active versus control treatment groups. This lack of blinding of outcome assessments may have introduced important biases such as performance biases that inflated the effect of treatment on attenuated psychotic symptoms [38], thus it is possible that the impact of these interventions on attenuated psychotic symptoms is even less than reported in these reviews.
Lastly, a network meta-analysis was conducted to examine the impact of interventions on negative symptoms in clinical high-risk samples and concluded that there was neither efficacy nor effectiveness for any negative symptom treatment [13]. One limitation of this network meta-analysis was that most studies were not designed to target negative symptoms. Thus, it might not be that surprising that the psychosocial treatments included in this analysis had no impact on negative symptoms relative to other treatments because they had no specific treatment components that were specifically aimed at reducing negative symptoms. Future psychosocial interventions in clinical high-risk samples that include an active treatment to reduce negative symptoms may result in a significant reduction of negative symptoms relative to a control group. A second limitation is that this network meta-analysis pooled a variety of negative symptom scales with most studies utilizing the Scale of Prodromal Symptoms for measuring negative symptoms. Unfortunately this scale measures several negative symptoms but not restricted affect and alogia, which are symptoms that have been recommended by the negative symptom consensus group [39]. Furthermore, most studies only reported negative symptom total scores. The network meta-analysis on negative symptoms suffered from the same fundamental issues that both the transition and attenuated psychotic symptoms network meta-analyses encountered in that it lacked precision, was based on a limited number of RCTs, and produced large confidence intervals. Again, it is possible that future trials will impact the conclusions of these results.
In conclusion, the results of these recent network meta-analyses have shown little to no impact for any intervention on transition to psychosis, attenuated psychotic symptoms, and negative symptoms. However, all the network meta-analysis evidence available is based on a very limited numbers of studies and the emergence of more RCTs in the future may change the results of these network meta-analysis entirely.
Discussion
This paper reviewed the most recent RCTs assessing current treatments in youth at risk for psychosis and several systematic reviews and meta-analyses. With respect to the systematic reviews and meta-analyses, it is easy to be critical of or become pessimistic about the outcomes [40], but there are important caveats that need to be underscored when considering the results. First, there are only a small number of studies representing each type of treatment. These studies have occurred over approximately a 20-year time span with the first PACE study in Melbourne being published in 2002 [28]. Second, the majority of the trials targeted transition rates, yet the rates of transition declined over time from approximately 35% in the earlier studies to 8-11% in more recent studies, reasons for which are still unclear [41]. Third, clinical high-risk samples include individuals who achieve a complete remission within the first few months, and attenuated psychotic symptoms typically decline over time in longitudinal studies [42, 43]. Improvement was observed in these trials, and, although, it may be due to the sample improving anyway, it is possible that any kind of treatment is helpful in some way. Fourth, although many of the trials reported several outcome variables, the treatments were not necessarily chosen to target these outcomes. Most notable was that several studies reported social and role functioning outcomes, yet not one treatment was designed to improve functioning. Fifth, clinical high-risk samples were not specifically selected as needing the specific treatment being tested as may be the case in schizophrenia studies. For example, in schizophrenia studies targeting negative symptoms or cognition, inclusion criteria would include a certain level of negative symptoms or specific deficits in cognition. Sixth, in many of the trials, especially the psychosocial trials, participants may be receiving medications or other support in addition to the experimental treatment. It was recently pointed out by Nelson and colleagues [40] that in their trial in addition to the omega-3 vs placebo, both groups received cognitive-behavioral case management (CBCM) and possibly anti-depressants. Thus, there may have been enough treatment to provide a ceiling effect of improvement beyond which there was no room for Omega-3 to contribute. Finally, potential concerns with meta-analyses need to be considered. These include the possibility that since there are few trials, results could possibly change with the addition of one new trial and in network meta-analyses when there are too few studies the network has sparse connections and lacks precision. Different measures are used to determine transition to psychosis and other outcomes, plus many studies have different comparators. Moreover, when a network meta-analysis reports that one treatment is not more effective than any other, it does not necessarily mean that it is not effective, all treatments could be effective.
However, on a more positive note the results of these systematic reviews offer an excellent overview of the “state of affairs” allowing an evaluation of current treatments to ensure that future studies are designed with the past in mind. Thus, in summary, we observed that CBT has an impact on transition and attenuated psychotic symptoms, and family therapy, albeit there is only one study, may be a helpful intervention. Negative symptoms are a major concern, but are rarely addressed and social functioning, which reportedly has a role in later conversion, has never been specifically addressed. These observations lead to several recommendations. First, the heterogeneity of the clinical high-risk population needs to be considered and thus specific treatments for specific subgroups should be considered. Second, the modality of treatment studies needs to be specifically designed to address the presenting problem. For example, our research group is conducting a trial testing the effectiveness of a group intervention designed to help functioning (cognitive-behavioral social skills) compared to a psycho-education group [44]. The aim is to address the poor social functioning that is prevalent in both those who do transition to psychosis as well as those who do not [42], with entry criteria being poor social functioning. Third, treatment can be individualized and, knowing that these young clinical high-risk individuals are a heterogenous group, interventions can be designed to address the changing clinical profile and/or the treatment response of these young people [40]. These are known as adaptive interventions or sequential multiple assignment randomized trials (SMART trials) [45] in which the type and/or dose of the intervention is individualized based on clinical presentation, individual differences or treatment response. One such trial is currently underway in Melbourne, Australia [46]. In this trial, there are three steps. The first step lasts for 1.5 months and consists of support and problem solving (SPS). In the second 4.5-month step, SPS is compare to CBCM and in the third step, for 6 months CBCM +placebo is compared to CBCM + antidepressant medication. The goal of this study is to determine the most effective type and sequence of treatments for reducing the risk of developing psychosis and improving functioning.
ClinicalTrials.gov, a web-based resource maintained by the National Library of Medicine at the National Institutes of Health, provides information on clinical trials currently ongoing (see https://clinicaltrials.gov/ct2/home). In a recent survey of ClinicalTrials.gov, it appeared as if there are approximately 20 ongoing RCTs that have been registered. These studies are testing a range of interventions that include both pharmacological and psychosocial interventions. Thus, the hope for the future is that these ongoing and yet to come trials will not only focus on preventing the onset of psychosis but also on preventing any kind of disability and at the same time offer the right treatment to the right person at the right time.
Canadian Treatment Guidelines for Individuals at Clinical High Risk of Psychosis.
Recommendations*:
Anyone who appears distressed, has a decline in functioning and presents with transient or attenuated psychotic symptoms should be referred for a comprehensive assessment.
A consultant psychiatrist or trained mental health specialist in at-risk mental states should conduct the assessment.
Offer individual CBT with or without family intervention.
Offer interventions for presenting problems, (e.g. depression, anxiety, etc.)
Offer interventions to prevent development or persistence of social, educational or vocational problems.
Both psychological and pharmacological interventions can prevent a first episode of psychosis.
Above treatments should be monitored by a psychiatrist, psychologist or equivalent mental health professional.
In adults who are at clinical high-risk for psychosis a staged intervention model should be used with the least restrictive treatment approach (e.g. CBT) as first choice. If psychological treatments are ineffective and there are severe and progressive attenuated psychotic symptoms, then in adults a low-dose second-generation antipsychotic could be used.
After treatment, if symptoms, poor functioning or distress continue, monitor regularly for changes up to three years using structured and validated assessment tools.
* For more details of recommendations see reference 37.
Acknowledgements
Preparation of this article was supported by National Institute of Mental Health Grant MH105178 to Jean Addington. Olga Santesteban-Echarri is supported by a Canadian Institutes of Health Research post-doctoral scholarship and Dan Devoe by an Alberta Innovates Graduate Scholarship.
Footnotes
Conflict of Interest
Jean Addington, Daniel Devoe and Olga Santesteban-Echarri declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
References and Recommended Readings
Recently published papers of particular interest have been highlighted as:
• Of importance
- 1.McGlashan T, Walsh B, Woods S. The psychosis-risk syndrome: handbook for diagnosis and follow-up. Oxford University Press; 2010. [Google Scholar]
- 2.Yung AR, Yuen HP, McGorry PD, Phillips LJ, Kelly D, Dell'Olio M et al. Mapping the onset of psychosis: the comprehensive assessment of at-risk mental states. The Australian and New Zealand journal of psychiatry. 2005;39(11-12):964–71. doi: 10.1080/j.1440-1614.2005.01714.x. [DOI] [PubMed] [Google Scholar]
- 3.Schultze-Lutter F, Addington J, Ruhrmann S, Klosterkötter J. Schizophrenia proneness instrument, adult version (SPI-A). Rome, Italy; Giovanni Fiorito Editore: 2007. [Google Scholar]
- 4.Morrison AP, Fench P, Walford L, Lewis SW, Kilcommons A, Green J et al. Cognitive therapy for the prevention of psychosis in people at ultra-high risk. Randomised controlled trial. British Journal of Psychiatry. 2004;185:291–7. [DOI] [PubMed] [Google Scholar]
- 5.Morrison AP, French P, Stewart SLK, Birchwood M, Fowler D, Gumley AI et al. Early detection and intervention evaluation for people at risk of psychosis: Multisite randomised controlled trial. BMJ. 2012;344:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.French P, Morrison AP. Early detection and cognitive therapy for people at high risk of developing psychosis. West Sussex, England: John Wiley & Sons; 2004. [Google Scholar]
- 7.Ising HK, Kraan TC, Rietdijk J, Dragt S, Klaassen RMC, Boonstra N et al. Four-year follow-up of cognitive behavioral therapy in persons at ultra-high risk for developing psychosis: The Dutch Early Detection Intervention Evaluation (EDIE-NL) trial. Schizophrenia Bulletin. 2016;42(5):124–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Der Gaag M, Nieman DH, Rietdijk J, Dragt S, Ising HK, Klaassen RMC et al. Cognitive behavioral therapy for subjects at ultra high risk for developing psychosis: A randomized controlled clinical trial. Schizophrenia Bulletin. 2012;38(6):1180–8. doi: 10.1093/schbul/sbs105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Addington J, Epstein I, Liu L, French P, Boydell KM, Zipursky RB. A randomized controlled trial of cognitive behavioral therapy for individuals at clinical high risk of psychosis. Schizophrenia Research. 2011;125(1):54–61. doi: 10.1016/j.schres.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 10.Stain HJ, Bucci S, Baker AL, Carr V, Emsley R, Halpin S et al. A randomised controlled trial of cognitive behaviour therapy versus non-directive reflective listening for young people at ultra high risk of developing psychosis: The detection and evaluation of psychological therapy (DEPTh) trial. Schizophrenia Research journal. 2016;176(2-3):212–9. [DOI] [PubMed] [Google Scholar]
- 11.Devoe DJ, Farris MS, Townes P, Addington J. Interventions and transition in youth at risk of psychosis: a systematic review and meta-analysis. Early intervention in psychiatry. 2018;12(1):173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davies C, Cipriani A, Ioannidis JPA, Radua J, Stahl D, Provenzani U et al. Lack of evidence to favor specific preventive interventions in psychosis: a network meta-analysis. World Psychiatry. 2018;17(2): 196–209. doi: 10.1002/wps.20526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devoe DJ, Peterson A, Addington J. Negative Symptom Interventions in Youth at Risk of Psychosis: A Systematic Review and Network meta-analysis. Schizophr Bull. 2017. doi: 10.1093/schbul/sbx139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Devoe DJ, Farris MS, Townes P, Addington J. Interventions and social functioning in youth at risk of psychosis: A systematic review and meta-analysis. Early Interv Psychiatry. 2018. doi: 10.1111/eip.12689. [DOI] [PubMed] [Google Scholar]
- 15.Devoe DJ, Farris MS, Townes P, Addington J. Attenuated psychotic symptom interventions in youth at risk of psychosis: A systematic review and meta-analysis. Early Interv Psychiatry. 2018. doi: 10.1111/eip.12677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davies C, Radua J, Cipriani A, Stahl D, Provenzani U, McGuire P et al. Efficacy and Acceptability of Interventions for Attenuated Positive Psychotic Symptoms in Individuals at Clinical High Risk of Psychosis: A Network Meta-Analysis. Frontiers in Psychiatry. 2018;9:187. doi: 10.3389/fpsyt.2018.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devoe DJ, Farris MS, Addington J. Symptoms of depression and anxiety in youth at risk for psychosis: a systematic review and meta-analysis. Early intervention in psychiatry. 2018;12(1):174. [Google Scholar]
- 18.Piskulic D, Barbato M, Liu L, Addington J. Pilot study of cognitive remediation therapy on cognition in young people at clinical high risk of psychosis. Psychiatry Res. 2015;225(1-2):93–8. [DOI] [PubMed] [Google Scholar]
- 19.Loewy R, Fisher M, Schlosser DA, Biagianti B, Stuart B, Mathalon DH et al. Intensive Auditory Cognitive Training Improves Verbal Memory in Adolescents and Young Adults at Clinical High Risk for Psychosis. Schizophr Bull. 2016;42 Suppl 1:S118–26. doi: 10.1093/schbul/sbw009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi J, Corcoran CM, Fiszdon JM, Stevens M, Javitt DC, Deasy M et al. Pupillometer-based neurofeedback cognitive training to improve processing speed and social functioning in individuals at clinical high risk for psychosis. Psychiatr Rehabil J. 2017;40(1):33–42. doi: 10.1037/prj0000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miklowitz DJ, O'Brien MP, Schlosser DA, Addington J, Candan KA, Marshall C et al. Family-focused treatment for adolescents and young adults at high risk for psychosis: results of a randomized trial. Journal of the American Academy of Child & Adolescent Psychiatry. 2014;53(8):848–58. doi: 10.1016/j.jaac.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bechdolf A, Wagner M, Ruhrmann S, Harrigan S, Putzfeld V, Pukrop R et al. Preventing progression to first-episode psychosis in early initial prodromal states. Br J Psychiatry. 2012;200(1):22–9. doi: 10.1192/bjp.bp.109.066357. [DOI] [PubMed] [Google Scholar]
- 23.Cadenhead K, Addington J, Cannon T, Cornblatt B, Mathalon D, McGlashan T et al. 23. Omega-3 Fatty Acid Versus Placebo in a Clinical High-Risk Sample From the North American Prodrome Longitudinal Studies (NAPLS) Consortium. Schizophrenia Bulletin. 2017;43(suppl_1):S16–S. doi: 10.1093/schbul/sbx021.042. [DOI] [Google Scholar]
- 24.Amminger GP, Schafer MR, Papageorgiou K, Klier CM, Cotton SM, Harrigan SM et al. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch Gen Psychiatry. 2010;67(2):146–54. doi: 10.1001/archgenpsychiatry.2009.192. [DOI] [PubMed] [Google Scholar]
- 25.McGorry P, Nelson B, Markulev C, Yuen H, Schafer M, Mossaheb N et al. Effect of omega-3 polyunsaturated fatty acids in young people at ultrahigh risk for psychotic disorders: The NEURAPRO randomized clinical trial. JAMA Psychiatry. 2017;74(1):19–27. [DOI] [PubMed] [Google Scholar]
- 26.Woods S, Walsh BC, Hawkins KA, Miller TJ, Saksa JR, D'Souza DC et al. Glycine treatment of the risk syndrome for psychosis: Report of two pilot studies. European neuropsychopharmacology. 2013;23(8):931–40. doi: 10.1016/j.euroneuro.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kantrowitz JT, Woods SW, Petkova E, Cornblatt B, Corcoran CM, Chen H et al. "D-serine for the treatment of negative symptoms in individuals at clinical high risk of schizophrenia: A pilot, double-blind, placebo-controlled, randomised parallel group mechanistic proof-of-concept trial': Correction. The Lancet Psychiatry. 2016;3(7):602. [DOI] [PubMed] [Google Scholar]
- 28.McGorry PD, Yung AR, Phillips LJ, Yuen HP, Francey S, Cosgrave EM et al. Randomized controlled trial of interventions designed to reduce the risk of progression to first-episode psychosis in a clinical sample with subthreshold symptoms. Arch Gen Psychiatry. 2002;59(10):921–8. [DOI] [PubMed] [Google Scholar]
- 29.McGlashan TH, Zipursky RB, Perkins D, Addington J, Miller T, Woods SW et al. Randomized, double-blind trial of olanzapine versus placebo in patients prodromally symptomatic for psychosis. Am J Psychiatry. 2006;163(5):790–9. doi: 10.1176/ajp.2006.163.5.790. [DOI] [PubMed] [Google Scholar]
- 30.Ruhrmann S, Bechdolf A, Kuhn KU, Wagner M, Schultze-Lutter F, Janssen B et al. Acute effects of treatment for prodromal symptoms for people putatively in a late initial prodromal state of psychosis. British Journal of Psychiatry. 2007;191(SUPPL. 51):s88–s95. [DOI] [PubMed] [Google Scholar]
- 31.Woods S, Saksa J, Compton M, Daley M, Rajarethinam R, Graham K et al. Effects of ziprasidone versus placebo in patients at clinical high risk for psychosis. Schizophrenia Bulletin. 2017;Conference(States):16th International Congress on Schizophrenia Research. [Google Scholar]
- 32.McGorry PNB, Phillips LJ, Yuen HP, Francey SM, Thampi A, Berger GE, Amminger GP, Simmons MB, Kelly D, Dip G, Thompson AD, Yung AR Randomized controlled trial of interventions for young people at ultra-high risk of psychosis: twelve-month outcome. The Journal of clinical psychiatry. 2013;74(4):349–56. [DOI] [PubMed] [Google Scholar]
- 33.International, Early Psychosis Association Writing Group. International clinical practice guidelines for early psychosis. British Journal of Psychiatry. 2005;187(SUPPL. 48):0–4. [DOI] [PubMed] [Google Scholar]
- 34.National Collaborating Centre for Mental Health. Psychosis and schizophrenia in children and young people: Recognition and management2013. National Clinical Guideline Number 155. [Google Scholar]
- 35.Schmidt SJ, Schultze-Lutter F, Schimmelmann BG, Maric NP, Salokangas RKR, Riecher-Rössler A et al. EPA guidance on the early intervention in clinical high risk states of psychoses. European Psychiatry. 2015;30(3):388–404. doi: 10.1016/j.eurpsy.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 36.Pringsheim T, Addington D. Canadian schizophrenia guidelines: Introduction and guideline development process. Canadian Journal of Psychiatry. 2017;62(9):586–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Addington J, Addington D, Abidi S, Raedler T, Remington G. Canadian treatment guidelines for individuals at clinical high risk of psychosis. The Canadian Journal of Psychiatry. 2017;62(9):656–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ (Clinical research ed). 2011;343:d5928–d. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kirkpatrick B, Fenton WS, Carpenter WT Jr., Marder SR The NIMH-MATRICS consensus statement on negative symptoms. Schizophrenia Bulletin. 2006;32(2):214–9. doi: 10.1093/schbul/sbj053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nelson B, Amminger GP, McGorry PD. Recent Meta-Analyses in the Clinical High Risk for Psychosis Population: Clinical Interpretation of Findings and Suggestions for Future Research. Frontiers in Psychiatry. 2018;9(502). doi: 10.3389/fpsyt.2018.00502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yung AR, Yuen HP, Berger G, Francey S, Hung TC, Nelson B et al. Declining transition rate in ultra high risk (prodromal) services: dilution or reduction of risk? Schizophr Bull. 2007;33(3):673–81. doi: 10.1093/schbul/sbm015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Addington J, Stowkowy J, Liu L, Cadenhead KS, Cannon TD, Cornblatt BA et al. Clinical and functional characteristics of youth at clinical high-risk for psychosis who do not transition to psychosis. Psychological medicine. 2018:1–8. doi: 10.1017/s0033291718002258. [DOI] [PubMed] [Google Scholar]
- 43.Addington J, Liu L, Buchy L, Cadenhead KS, Cannon TD, Cornblatt BA et al. North American Prodrome Longitudinal Study (NAPLS 2): The Prodromal Symptoms. Journal of Nervous & Mental Disease. 2015;203(5):328–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brummitt K, Author A, Kelsven S, Devoe D, Stern L, Granholm E et al. Cognitive behavioral social skills training for youth at risk of developing psychosis. Early intervention in psychiatry. 2018;12(1):178. [Google Scholar]
- 45.Lei H, Nahum-Shani I, Lynch K, Oslin D, Murphy SA. A "SMART" design for building individualized treatment sequences. Annual review of clinical psychology. 2012;8:21–48. doi: 10.1146/annurev-clinpsy-032511-143152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nelson B, Amminger GP, Yuen HP, Wallis N, M JK, Dixon L et al. Staged Treatment in Early Psychosis: A sequential multiple assignment randomised trial of interventions for ultra high risk of psychosis patients. Early Interv Psychiatry. 2018;12(3):292–306. doi: 10.1111/eip.12459. [DOI] [PMC free article] [PubMed] [Google Scholar]