Abstract
Purpose:
This study was designed to compare survival outcomes for non-surgically managed T1-T2N0M0 small cell lung cancer (SCLC) who received either stereotactic body radiation therapy (SBRT) or conventionally fractionated radiotherapy (CFRT) using the National Cancer Data Base (NCDB).
Methods:
The was queried between 2004–2015 for patients with T1-T2N0M0 SCLC. Patients must have been treated with curative intent SBRT or CFRT (delivered daily or twice daily, 45–70 Gy) with or without chemotherapy. The primary outcome was overall survival (OS). A subset analysis of patient receiving chemotherapy was also performed. A propensity score matched (PSM) analysis was performed to compare OS among patients who received chemotherapy.
Results:
We evaluated 1378 patients in the general cohort. Multivariable Cox regression analysis(MVA) in the general cohort revealed that SBRT was significantly associated with improved survival (HR 0.68, p<0.001) along with receipt of chemotherapy (HR 0.63, p <0.001). SBRT patients were less likely to receive chemotherapy compared to CFRT patients (p<0.01). In the chemotherapy subset, of 1096 patients, on MVA, there was a trend in favor of the SBRT group (HR 0.73; p=0.06). A 3:1 PSM analysis on the chemotherapy subset found similar results on MVA with a trend in favor of SBRT (p=0.06).
Conclusion:
Patients with T1–2N0M0 SCLC treated with SBRT regimens incorporating chemotherapy had comparable outcomes to concurrent chemoradiotherapy using standard fractionation. Treatment paradigms for T1–2N0M0 SCLC incorporating SBRT warrant further exploration and should incorporate chemotherapy.
Keywords: small cell lung cancer, stereotactic body radiotherapy, early stage small cell lung cancer, national cancer database
Introduction
Small cell lung cancer (SCLC) is a poorly-differentiated, rapidly-dividing, smoking-associated neuroendocrine malignancy accounting for nearly 14% of all lung cancers with a median survival time is about 7 months.1,2 The poor prognosis of SCLC is attributable in part to the fact that two-thirds of patients present at the time of diagnosis with incurable stage IV metastatic disease.3 Standard of care approaches for definitive treatment of limited stage SCLC include chemotherapy concurrent with thoracic radiotherapy with or without prophylactic cranial irradiation.4
At the present, only a small population of SCLC patients (2–5%) present as early stage, cT1-T2N0M0 SCLC and have the highest pre-treatment probabilities of survival and cure.5–7 However, with most major medical organizations now recommending annual lung cancer screening for high risk individuals, the proportion of patients diagnosed with early stage lung cancer, including SCLC, is projected to increase.8,9 Because of the relative rarity of early stage SCLC, there are no prospective data guiding the optimal management of such patients. Currently the National Comprehensive Cancer Network (NCCN) recommends that the primary treatment of medically inoperable T1-T2 N0 patients can include Stereotactic Body Radiation Therapy (SBRT), but data is limited.10 For operable patients, lobectomy with adjuvant therapy is an option which is primarily based off a National Cancer Database (NCDB) study performed by Yang et al.11
Several institutions have reported their experience with SBRT in early stage SCLC.12–14 SBRT is an advanced method of radiotherapy delivery characterized by high dose per fraction and precise tumor targeting given over a limited number of fractions (typically 5 or less). Contemporary SBRT techniques allow for very steep radiation dose gradients outside of the targeted tumor minimizing dose to surrounding normal tissues. SBRT is the standard of care therapy for early stage node-negative NSCLC in medically inoperable patients due to its low side effect profile, short overall treatment times, and superior local control over standard fractionated radiotherapy.15 Based on these data, some practitioners have adopted the use of SBRT for early stage SCLC. A recently published NCDB study examining trends in radiotherapy delivery for stage I SCLC demonstrated an increased absolute and relative utilization of SBRT in the U.S.16 In fact, SBRT followed by adjuvant systemic therapy has recently been incorporated as a potential therapeutic strategy into the NCCN version 1.2019 guidelines17.
To better understand patient outcomes in inoperable Stage I SCLC, we undertook a NCDB analysis comparing CFRT and SBRT. The primary goal of our analysis was to evaluate overall survival (OS) differences between CFRT and SBRT. Specifically, we aimed to test the hypothesis that SBRT is an appropriate treatment modality and comparable to CFRT in early stage SCLC. A secondary goal of our study was to define the impact of adjuvant chemotherapy in non-surgically managed Stage I SCLC, either after SBRT or concurrently with CFRT.
Methods
Data Source
We performed a retrospective study using the National Cancer Database (NCDB). The NCDB is a national clinical surveillance program that abstracts approximately 70% of all new invasive malignant diagnoses in the United States each year.18 Supplied by over 1,500 hospitals, the NCDB is recognized as the largest clinical registry in the world. This study using de-identified data from the NCDB was IRB approved (#181881).
Study Population
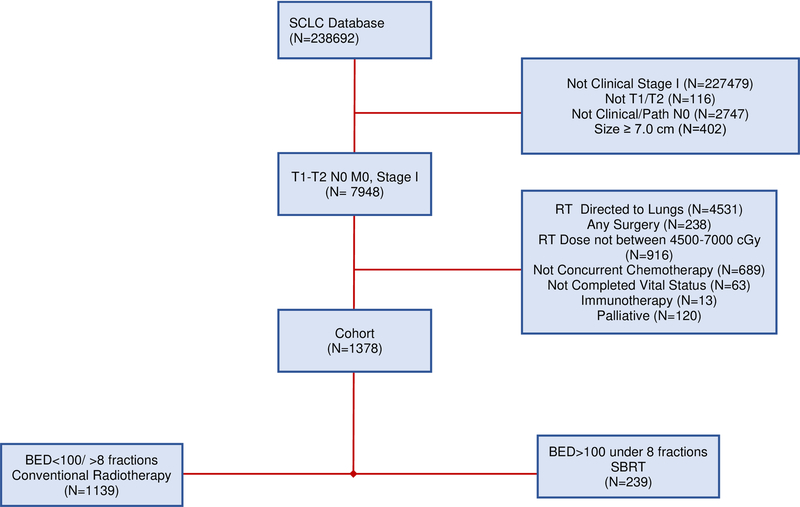
We identified patients diagnosed with cT1–2N0M0 SCLC in the NCDB between 2004 and 2015. The NCDB data dictionary references the following clinical variables described herein.19 The relative inclusion and exclusion criteria are defined below are also represented in a consort diagram depicted in Figure 1.
Figure 1:
Consort Diagram
The initial population included N = 238691 patients. For inclusion, patients needed to be Stage I, as well as T1-T2N0 with clinically negative nodes. Pathological nodes needed to be either undetermined or negative. Size was required to be less than 70 mm. Radiation anatomic target must have been administered to the lung. Patients must have received between 4500 cGy and 7000cGy of radiation. Chemotherapy administration, when delivered must have been administered no more than 3 weeks prior to radiation therapy and must have begun at minimum 2 weeks after radiation began. Patients who received any surgery, defined as the variable were excluded. Additional exclusion criteria included anyone treated with palliative intent and unknown vital status. Dose per fraction was determined by dividing the total dose by the number of treatments. Application of these criteria resulted in a total of 1378 patients.
BED was calculated in units of Gray (Gy) using the linear quadratic equation, where alpha/beta was set to 10, d was equal to the dose per fraction in Gy, and n was equal to the number of fractions:
We then defined definitive SBRT as radiation to the lung with a biologically effective dose (BED10) of ≥ 100 Gy in 8 or fewer fractions.16
Statistical Methods
The baseline characteristics are summarized in Table 1. Comparison between discrete variables groups was described as the absolute number and percentage in each category while continuous variables were described by reporting the median and interquartile range (difference between the median of the third quartile and the median of the first quartile). Continuous variables were compared with the Mann-Whitney U test. Categorical variables were compared with an uncorrected chi-square test.
Table 1:
Baseline patient characteristics. Continuous variables are expressed as the median and interquartile range(IQR) and compared via the Mann-U-Whitney test. Categorical variables are expressed as absolute number (n) and percentage.
| Covariate | SBRT N= 239 | CFRT N= 1139 | P-Value |
|---|---|---|---|
| Age median (IQR) | 75(13) | 70(13) | <0.001 |
| Female n(%) | 140(58.5) | 640(56.2) | 0.498 |
| Race n(%) | |||
| White | 216 (90.4) | 1020 (89.6) | 0.820 |
| Black | 18(7.5) | 87(7.6) | |
| Other | 5(2.1) | 32 (2.8) | |
| T2 Tumors (>30mm) n(%) | 36(15.3) | 477 (45.6) | <0.001 |
| Charlson-Deyo Score n(%) | |||
| 0 | 119(49.7) | 714(62.6) | <0.001 |
| 1 | 69(28.8) | 297(26) | |
| 2 | 36(28.8) | 104(9.13) | |
| 3 | 15(6.3) | 24(0.21) | |
| Chemotherapy n(%) | |||
| Single Agent | 3(1.25) | 22(1.9) | |
| Multi Agent | 77(32.2) | 910(79.8) | |
| Not administered | 155(64.85) | 127(11.1) | <0.001 |
| Administered unknown number of agents | 4(1.67) | 80(7) | |
| BED median(IQR) | 112.5(12.5) | 72(11.2) | <0.001 |
| Community Cancer | 7 (2.9) | 177(15.6) | <0.001 |
| Comp. Cancer center | 109 (45.6) | 608 (53.5) | |
| Academic/Research Integrated Network | 99 (41.4) | 259 (22.8) | |
| Cancer Program | 24(10.0) | 95 (8.3) | |
| Dose median(IQR) | 5000(550) | 6000(900) | <0.001 |
| Year of Diagnosis median | 2012 | 2010 | 0.01 |
| QD n(%) | NA | 996 (87.4) | NA |
| BID n(%) | 143(12.6) | ||
| Insurance n(%) | |||
| Medicare | 180 (75.3) | 770 (67.6) | |
| Private | 39 (16.3) | 256 | 0.004 |
| Medicaid | 6 (2.5) | (22.5) | |
| Uninsured | 3 (1.3) | 59 (5.2) | |
| Other Government | 10 (4.2) | 20 (1.8) | |
| Unknown | 1 (0.4) | 17 (1.5) | |
| 17 (1.5) | |||
| Pathological Nodal Evaluation | 0.704 | ||
| Not Evaluated | 8(3.3) | 44 (3.9) | |
| Path NO | 231 (96.7) | 1095 (96.1) | |
| Days From Diagnosis Until Treatment | 35(36) | 28(26) | <0.001 |
| Left | 100(41.8) | 478(41.9) | <0.001 |
| Right | 139(58.1) | 594(52.1) | |
| Unknown | 0 | 67(5.9) |
Survival analyses were performed with a Cox proportional-hazards model. We validated the assumptions of the Cox proportional hazards model by checking Schoenfeld residuals and checking the proportionality graphically. Overall survival was the primary outcome and it was measured from date of first diagnosis. We performed univariate Cox regression analysis followed by a multivariable Cox regression analysis comprised of significant variables on univariate analysis or a priori. We then generated Kaplan-Meier (KM) curves to demonstrate the respective survival analysis with a log-rank test to compare survival curves.
Since a greater proportion of patients treated with CFRT received chemotherapy in comparison to those patients treated with SBRT, we performed a subset analysis to evaluate survival among patients who received chemotherapy resulting in 1096 evaluable patients. Within this subset, we performed a similar survival analysis as previously described for the primary cohort.
We also performed a propensity score matched analysis on the chemotherapy subset. We did so by creating a multivariable logistic regression to predict the association of receiving either CFRT or SBRT. The model was comprised of age, race, sex, tumor size, Charlson-Deyo (CD) score, laterality, and days until start of therapy. We then created a 3:1 PSM of CFRT patients to SBRT patients using the nearest neighbor method. We evaluated that the standardized differences between the means was less than 0.1 for all covariates indicating an appropriate match.20
All tests were two-sided with an alpha value set to 0.05. Statistics and graphs were performed using SAS or R 3.5.0 (Vienna, Austria).
Results
General Cohort
We evaluated 239 patients treated with SBRT and 1139 patients treated with CFRT for early stage SCLC whose baseline characteristics are in Table 1. Patients treated with SBRT were more likely to be older (median 75 years) compared to those treated with CFRT (median 70 years; p < 0.001). Sex was balanced between groups with 56% comprising the CFRT group vs. 58.5% in the SBRT group (p=0.498). SBRT patients were more likely to have smaller tumors (median 19 mm) vs. CFRT patients (30 mm) (p<0.001). SBRT was also more often utilized in academic/research facilities compared to community cancer programs (p < 0.001). Patients treated with SBRT were also significantly less likely to have received chemotherapy compared to patients with CFRT (35.1% vs. 88.8%, p < 0.001).
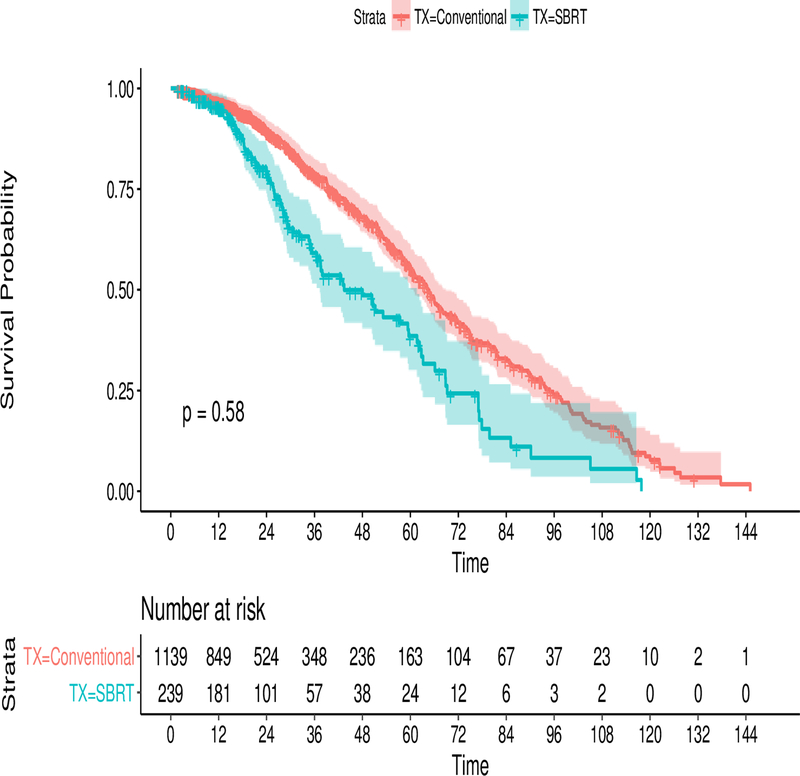
The median follow-up time for the study was 21 months. At last follow-up, 28% of CFRT patients and 38.5% of SBRT patients were alive (p < 0.001). Median survival time after treatment with CFRT and SBRT was 2.1 years (95% CI 1.9–2.3) and 2.2 years (95% CI 1.8–2.6) respectively. Comparing CFRT and SBRT, 1-year survival probability was 77% vs. 79% and 5-year survival probability was 26% vs. 27% (p = 0.58).
On univariate analysis (table 2), there was not a difference in survival between patients treated with SBRT and CFRT, HR 0.96 (95% 0.800-CI 1.143; p= 0.58). A Kaplan-Meier (KM) curve demonstrating the differences in survival is presented in Figure 2. Factors that were significantly associated with survival included age (HR 1.02, 95% CI 1.015–1.029; p < 0.001) receipt of chemotherapy (0.69; 95% CI 0.589 – 0.809; p <0.001), CD score 1.14 (95% CI 1.06–1.23, p<0.001), and female gender, HR 0.78 (0.682– 0.888; p <0.001).
Table 2:
This is a univariate Cox regression analysis for both the primary cohort and the subset analysis on the right for the patients who received chemotherapy only. It is expressed as a Hazard Ratio (HR) with 95% confidence intervals(CI)
| Covariate | Primary Cohort Hazard Ratio | 95% CI; P-value | Chemotherap y Subgroup HR | 95% CI; P-value |
|---|---|---|---|---|
| CFRT | Ref | Ref | Ref | Ref |
| SBRT | 0.96 | 0.8–1.143; 0.624 | 0.77 | 0.53–0.98; 0.038 |
| Age* | 1.02 | 1.015–1.030; <0.001 | 1.02 | 1.02–1.03; <0.01 |
| Male | Ref | Ref | Ref | Ref |
| Female | 0.78 | 0.682–0.888; <0.001 | 0.75 | 0.65–0.87; <0.01 |
| Chemotherapy: | ||||
| No | Ref | Ref | NA | NA |
| Yes | 0.69 | 0.589–0.809; <0.001 | NA | NA |
| Charlson-Deyo Score* | 1.14 | 1.06–1.23; p<0.001 | 1.13 | 1.03–1.24; <0.01 |
| Tl | Ref | Ref | Ref | Ref |
| T2 | 1.04 | 0.91–1.17;0.585 | 1.11 | 0.96–1.3; 0.13 |
| Facility: | ||||
| Community | Ref | Ref | Ref | Ref |
| Comprehensive | 0.99 | 0.81–1.21;0.957 | 0.97 | 0.82–1.3;0.78 |
| Academic | 1.087 | 0.87–1.35; 0.442 | 1.04 | 0.82–1.32;0.721 |
| Integrated | 0.96 | 0.72–1.27:0.768 | 1.11 | 0.65–1.23:0.67 |
| Laterality | ||||
| Left | Ref | Ref | Ref | Ref |
| Right | 1.12 | 0.98–1.28; 0.07 | 1.13 | 0.98–1.3; 0.09 |
| Race | ||||
| White | Ref | Ref | Ref | Ref |
| Black | 0.96 | 0.75–1.22;0.747 | 0.98 | 0.74–1.2; 0.89 |
| Other | 0.97 | 0.66–1.43; 0.899 | 1.04 | 0.67–1.6;0.83 |
| Days until treatment* | 1.00 | 0.99–1.011; 0.15 | 1.001 | 0.99–1.01;0.16 |
Values with an asterisk were analyzed as a continuous
Figure 2:
Kaplan-Meier Curve comparing survival in the SBRT arm (blue) with the CFRT arm(red) from the general cohort. At risk tables are at the bottom along with the 95% confidence intervals shaded. The P-value is resultant from the log-rank test.
On multivariate analysis in table 3, controlling for receipt of chemotherapy, female gender, age, days until treatment, CD score, and T2 tumor size, SBRT was found to be significantly associated with survival (HR 0.68, 0.55–0.84, p<0.001). Within this model, age, chemotherapy receipt, female gender, CD score, days until treatment, all retained similar hazard ratios and values.
Table 3:
This is a Multivariate Cox regression analysis comprised of the variables listed in the leftmost column forthe primary set, the chemotherapy recipient subset, and the propensity score match on the chemotherapy recipient subset . It is expressed as a hazard ratio (HR) with 95% confidence intervals (CI).
| Covariate | Hazard Ratio Primary data | 95% CI; p-value | Hazard Ratio Chemothe rapy set | 95% CI; p-value | Propensity Score HR on chemother apy subset | 95% CI; p-value |
|---|---|---|---|---|---|---|
| CFRT | Ref | Ref | Ref | Ref | Ref | Ref |
| SBRT | 0.68 | 0.55–0.84; <0.01 | 0.73 | 0.54–1.01; 0.06 | 0.72 | 0.52–1.03; 0.063 |
| Chemotherapy | ||||||
| No | Ref | Ref | NA | NA | NA | NA |
| Yes | 0.63 | 0.51–0.75; <0.01 | ||||
| Male | Ref | Ref | Ref | Ref | Ref | Ref |
| Female | 0.77 | 0.68–0.88;<0.001 | 0.77 | 0.68–0.9; <0.01 | 0.70 | 0.52–0.9; <0.01 |
| Age* | 1.02 | 1.01–1.02;<0.001 | 1.02 | 1.016–1.03; <0.001 | 1.02 | 1.008–1.04; 0.002 |
| Charlson-Deyo Score* | 1.14 | 1.05–1.22; 0.002 | 1.11 | 1.01–1.22; 0.03 | 1.05 | 0.99–1.15; 0.054 |
| Days until Treatment* | 0.99 | 0.995–1.001; 0.59 | 0.99 | 0.99–1.00; 0.49 | 1.006 | 0.99–1.01; 0.1 |
| Tl | Ref | Ref | Ref | Ref | Ref | Ref |
| T2 | 1.15 | 1.00–1.3; 0.04 | 1.15 | 0.92–1.34; 0.053 | 0.9 | 0.4–1.32; 0.4 |
| Left side | 1.06 | 0.93–1.21;0.366 | 1.08 | 0.93–1.26; 0.25 | 1.06 | 0.8–1.39;0.67 |
Values with an asterisk were analyzed as a continuous
Since chemotherapy use was largely different between the two groups, we performed a bivariate analysis with SBRT to explore whether this could be a driver for the survival advantage seen with SBRT upon multivariate but not univariable analysis. Adjusting solely for chemotherapy led to a statistically significant survival difference in favor of SBRT and chemotherapy, with a hazard ratio 0.57 (0.45–0.86; p<0.0001).
We also noted that there appeared to be an interaction term between age and SBRT with its association to survival. This is to say that while age was a significant prognostic covariate as a continuous variable, its impact as a predictor changes in a non-linear fashion. We therefore analyzed patients categorically into quartiles in order to find the cause of this interaction effect. There was no association between modality of radiation and survival for patients aged 31–64 (HR 0.91, p=0.65), 65–70 (HR 0.81, p =0.318), or 71–77 (HR 1.02, p=0.8). In contrast, we noted that in a subgroup of the oldest quartile of our patients, age 78–90, SBRT was strongly associated with a survival benefit (HR 0.63; 0.47–0.85; p=0.003).
Chemotherapy Subgroup Results
Based on the strong survival benefit we observed with receipt of chemotherapy among all subgroups, we then performed a subgroup analysis of only those patients who received chemotherapy comparing SBRT vs CFRT. In this chemotherapy treated group, there were 84 patients who received SBRT vs. 1012 patients who received CFRT. Baseline characteristics differed between groups, in a pattern similar to that observed in the primary cohort. Briefly there were 52 females (61.9%) in the SBRT group vs. 563 (55.6%) in the CFRT group (p=0.31). Median age was 72 in the SBRT group and 69 in the CF group (p=0.052). Median tumor size was 17 mm in the SBRT group and 30 mm in the CFRT group (p<0.001).
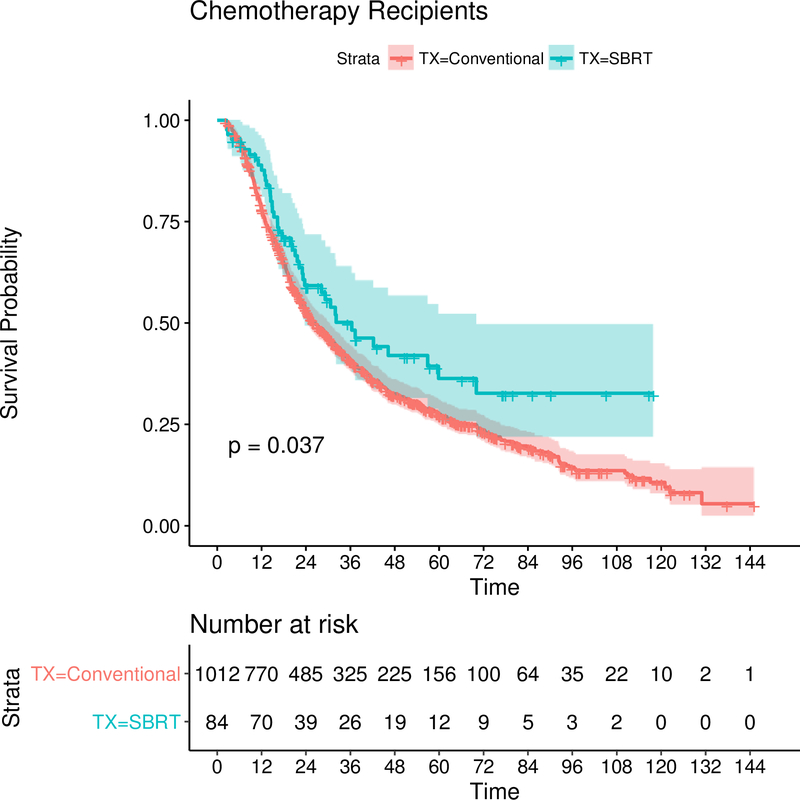
Median follow-up time in the chemotherapy subset was 23 months (range 2–147). Median survival in SBRT patients was 3.0 years (2.25–5.8) vs. 2.2 years (2.0–2.49) in the CFRT group. Survival probability in the SBRT and CFRT groups at one and five years was 89% vs. 78.0% and 36% vs. 27.5% respectively (0.038).
On univariate analysis of the chemotherapy subgroup (table 2), SBRT was significantly associated with survival, HR 0.77 (0.53–0.98, p=0.038). This is in concordance with the bivariate analyses adjusting for chemotherapy in the primary group discussed above. Female sex, age, and CD score were also significant predictors of survival by univariate analysis. Figure 3 is KM curve demonstrating the survival benefit of SBRT in the chemotherapy recipient subset.
Figure 3:
Kaplan-Meier Curve comparing survival in the SBRT arm (blue) with the CFRT arm (red) in thechemotherapy only(unmatched) dataset. At risk tables are at the bottom along with the 95% confidence intervals shaded. The P-value is resultant from the log-rank test.
On multivariable analysis adjusting for sex, age, CD score, and tumor size, the use of SBRT trended in significance towards improved survival 0.75 (0.54–1.02; p=0.06). In addition, age, female sex, and CD score were significant on multivariate analysis and retained the very similar effect sizes as they had in the primary cohort.
Propensity Matched Populations in the Chemotherapy Subset
The propensity score yielded adequate matching between groups with all standardized mean differences being less than 0.1. All matched variables were balanced between the groups. There were 252 patients in the CFRT group compared to 84 patients in the SBRT group. On univariate analysis, SBRT was associated with a favorable survival benefit with a HR of 0.72(0.53–1.005, 0.053) similar what was observed in the unmatched cohort. Age (1.02, 1.01–1.04; <0.001), female sex (0.68, 0.522–0.89; p<0.001) were strong predictors of survival. On multivariable analysis within the PS matched groups (Table 3), adjusting for age, sex, race, laterality, CD score, T2 tumor size, and days until treatment, SBRT trended with survival in a manner similar to the unmatched analysis, 0.72(0.52–1.01; p=0.063).
Discussion
Although 5% of SCLC present with T1-T2N0 SCLC, the lack of data comparing treatment modalities creates an impetus for clinicians to investigate all therapeutic strategies for patients with early stage SCLC. Herein, we report data to support that SBRT is an acceptable treatment modality for early stage inoperable SCLC. We demonstrated this via several different analytical approaches.
First, SBRT may offer patients the logistical convenience of a much shorter radiotherapy treatment course without compromising outcomes. Secondly, when delivered by experienced providers SBRT may offer lessened toxicity compared to CFRT. In NSCLC, a prospective comparison between SBRT and CFRT for stage I NSCLC demonstrated greater rates of esophagitis and pneumonitis with CFRT in comparison to SBRT.21 Finally, early stage SCLC patients treated with curative intent and aggressive local therapy (e.g. surgery or SBRT) should also receive adjuvant chemotherapy if they are fit enough to tolerate it.
Within our initial overall study population while 89% of CFRT patients received chemotherapy, only 35% of SBRT patients were treated with chemotherapy which is consistent with prior studies in this population.16 When we adjusted for all variable, our data at first suggested that patients treated with SBRT may have a survival advantage over CFRT. However, despite adjustments there were still notable imbalances in patients groups including tumor size and of course systemic chemotherapy use. This led us to more closely examine the NCCN endorsed population of patients who only receive chemotherapy. We then performed this by analyzing the difference between groups via standard multivariable cox regression analyses and propensity score matching. When we performed a subset for chemotherapy only patients, we found that SBRT trended towards a survival benefit. We confirmed this finding on PSM, which was then performed to compare a smaller subset of CFRT patients with similar characteristics as the SBRT group.
The decreased rate of chemotherapy administration across the SBRT population may be due to several factors. Since SBRT is a newer therapeutic approach in early stage SCLC and its role is still evolving, clinicians may be basing decisions on extrapolation from NSCLC data where chemotherapy is typically not administered for early stage disease. Our data suggest that this is not an acceptable practice. Alternatively, patients treated with SBRT alone (without chemotherapy) may have been deemed poor candidates for chemotherapy based on their performance status. Amongst the patients who did not receive chemotherapy, there were 35 of 155 SBRT patients with a CD comorbidity score greater than or equal to two in comparison to 19 of 127 CFRT patients. While there were relatively more patients in the SBRT group with a higher CD score, there was no significant difference between the arms on chi-squared analysis (p=0.14). Interestingly, among those in this study who were treated with SBRT, there was no significant difference in CD comorbidity score between those who received adjuvant chemotherapy and those who did not on chi-squared analysis (p=0.63).
In other case series exploring the use of SBRT for stage SCLC, use of chemotherapy has been associated with improved DFS and OS when given with SBRT compared to SBRT alone.22,23 In a case series of 6 stage I SCLC patients treated with chemotherapy and SBRT there were excellent rates of local control, (100% at one year), with only one patient experiencing distant metastases14. This is in stark contrast to a review of 43 patients in a Japanese study, where most patients (81.4%) were not treated with chemotherapy.24 It is notable that the rate of distant metastases at two years was considerably higher at 28% in this study. The results of these series, taken together with our results, underscore the importance of systemic therapy in the control of early stage SCLC and, in particular, after the use SBRT.
We acknowledge that based on retrospective data it may be premature to conclude that SBRT is an equivalent treatment modality in this group of patients. However, there is both clinical and biological rationale to believe our findings represent a true association. In early stage NSCLC, local control and OS are significantly improved when SBRT is administered in comparison to CFRT, a result that is correlated with BED delivered to tumor.25–27 As systemic therapy improves, the importance of local control in SCLC and its impact on DFS and OS will likely become more apparent. While SCLC is generally thought to be a radiosensitive tumor, local recurrences are common after concurrent chemoradiotherapy.28–29 There is data to support the rationale that higher BEDs associated with SBRT may be an important factor to reduce local recurrence in SCLC tumors. SCLC tumors contain a significant population of therapy resistant cancer stem cells (CSC), and it is hypothesized that this population is responsible for rapid recurrence of disease after therapy.30–31 Multiple lines of evidence demonstrate that CSCs are more radioresistant and the higher BED associated with SBRT may overcome this resistance in SCLC CSCs.32,33 Finally, higher dose per fraction radiotherapy such as SBRT is associated with enhancement of tumor immunogenicity and recruitment of tumor infiltrating lymphocytes in comparison to CFRT, factors which are associated with improved outcomes in both clinical and preclinical models34–36. With further improvements in systemic therapy for SCLC, the benefit of high BED SBRT in this population will likely become even more apparent.
Additionally, younger female patients with fewer comorbidities had prolonged survival. Consistent with our finding here, female sex has been previously reported as a positive prognostic factor in SCLC.37 Wheatley-Price et al. pooled SCLC clinical trial data and found univariate (HR 0.85, 95% CI 0.76–0.96, p=0.006) and multivariate (HR 0.88, 95% CI 0.79–0.99, p=0.04) survival associations with female sex in 1707 patients, although the mechanism behind this association is unknown.38 Patients with more comorbidities and as well are more likely to experience worse survival, which makes empirical sense.
There are several strengths to our study. Our data and conclusions were derived from a large, diverse population treated in community and academic centers, rather than a single-institutional report. We believe our study has several distinct differences to other published literature investigating this matter.39 We provide a transparent methodology regarding cohort selection and design. We used a carefully defined population, in which we clearly explained in our methods in order to ensure we captured all the patients who were truly T1-T2N0M0. We also used a BED-based definition for SBRT cohort selection, which enhances the plausibility of the data reported and defines a different population subset. We also performed a comprehensive, clinically relevant subset analysis to account for confounders and to explore the role of chemotherapy in this population by performing advanced methods such as matching.
There are of course limitations to this study include its retrospective nature and its associated biases including selection bias. The NCDB analysis does not provide details of chemotherapy regimens and whether there were any associated dose reductions or missed doses. Furthermore, this report does not address therapies after initial radiation and chemotherapy, and thus overall survival could be significantly skewed by choice of second-line treatments. Disease free survival and distant-disease free survival are alternative study outcomes unaffected by second-line therapies, however, such outcomes are not obtainable from this database analysis. Although death is most likely from SCLC in our study population, parameters permitting evaluation of cancer specific-survival are not available in the NCDB.
We do not have data on prophylactic cranial irradiation since the NCDB only includes the first radiation course. We suspect this would be a small confounder given the 5.4% benefit in OS benefit at 3 years in LS-SCLC.40As many SBRT patients did not receive chemotherapy, it may be reasonable to presume that that utilization of PCI paralleled that of chemotherapy, however true rate at which PCI was performed in the SBRT and CFRT study populations cannot be determined.
Finally, we emphasize that treatment with SBRT may not be suitable for all practice environments and clinical situations. SBRT requires specialized technical expertise, machinery, and physics support which may not be available at all facilities. In addition, the delivery of SBRT is not ideal when tumor directly abuts or invades central airway and vascular structures, situations where the use of SBRT can lead to high grade toxicities. The importance of clinical judgement is therapy selection cannot be overstated.
In summary, our hypothesis generating data, suggests the use of SBRT followed by adjuvant chemotherapy as an acceptable treatment option for the non-surgical management of early stage SCLC with survival outcomes comparable to CFRT with chemotherapy. This may warrant further prospective evaluation.
Acknowledgments:
The authors also wish to acknowledge the Vanderbilt Institute for Clinical and Translational Research for their funding support.
Funding: This work was supported by CTSA award No. UL1 TR002243 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Footnotes
Conflict of Interest: Author Newman Received the grant in order to support Biostatistical analysis
Ethical approval: This article does not contain any studies with human participants or animals performed by any of the authors.
References
- 1.Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: Analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006;24(28):4539–4544. doi: 10.1200/JCO.2005.04.4859 [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- 3.Demedts IK, Vermaelen KY, Van Meerbeeck JP. Treatment of extensive-stage small cell lung carcinoma: Current status and future prospects. Eur Respir J. 2010;35(1):202–215. doi: 10.1183/09031936.00105009 [DOI] [PubMed] [Google Scholar]
- 4.Turrisi AT, Kim K, Blum R, et al. Twice-Daily Compared with Once-Daily Thoracic Radiotherapy in Limited Small-Cell Lung Cancer Treated Concurrently with Cisplatin and Etoposide. N Engl J Med. 1999;340:265–271. doi: 10.1056/NEJM199901283400403 [DOI] [PubMed] [Google Scholar]
- 5.Yu JB, Decker RH, Detterbeck FC, Wilson LD. Surveillance epidemiology and end results evaluation of the role of surgery for stage I small cell lung cancer. J Thorac Oncol. 2010. doi: 10.1097/JTO.0b013e3181cd3208 [DOI] [PubMed] [Google Scholar]
- 6.Church TR, Black WC, Aberle DR, et al. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013;368:1980–1991. doi: 10.1056/NEJMoa1209120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aberle DR, Adams AM, Berg CD, et al. Reduced Lung Cancer Mortality with Low-Dose Computed Tomographic Screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oudkerk M, Devaraj A, Vliegenthart R, et al. European position statement on lung cancer screening. Lancet Oncol. 2017;18(12):e754–e766. doi: 10.1016/S1470-2045(17)30861-6 [DOI] [PubMed] [Google Scholar]
- 9.Knight SB, Crosbie PA, Balata H, Chudziak J, Hussell T, Dive C. Progress and prospects of early detection in lung cancer. Open Biol. 2017;7(9):170070. doi: 10.1098/rsob.170070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Almquist D, Mosalpuria K, Ganti AK. Multimodality Therapy for Limited-Stage Small-Cell Lung Cancer. J Oncol Pract. 2016;12(2):111–117. doi: 10.1200/JOP.2015.009068 [DOI] [PubMed] [Google Scholar]
- 11.Yang C-FJ, Chan DY, Speicher PJ, et al. Role of Adjuvant Therapy in a Population-Based Cohort of Patients With Early-Stage Small-Cell Lung Cancer. J Clin Oncol. 2016;34(10):1057–1064. doi: 10.1200/JCO.2015.63.8171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shioyama Y, Nakamura K, Sasaki T, et al. Clinical results of stereotactic body radiotherapy for Stage I small-cell lung cancer: A single institutional experience. J Radiat Res. 2013;54(1):108–112. doi: 10.1093/jrr/rrs075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ly NB, Allen PK, Lin SH. Stereotactic body radiation therapy for stage I small cell lung cancer: a single institutional case series and review of the literature. J Radiat Oncol. 2014;3(3):285–291. [Google Scholar]
- 14.Videtic GMM, Stephans KL, Woody NM, et al. Stereotactic body radiation therapy-based treatment model for stage I medically inoperable small cell lung cancer. Pract Radiat Oncol. 2013;3(4):301–306. doi: 10.1016/j.prro.2012.10.003 [DOI] [PubMed] [Google Scholar]
- 15.Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303(11):1070–1076. doi: 10.1001/jama.2010.261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stahl JM, Corso CD, Verma V, et al. Trends in stereotactic body radiation therapy for stage I small cell lung cancer. Lung Cancer. 2017;103:11–16. doi: 10.1016/j.lungcan.2016.11.009 [DOI] [PubMed] [Google Scholar]
- 17.National Comprehensive Cancer Network. (2019).Small Cell cancer (version 2.2019). Retrieved from https://www.nccn.org/professionals/physician_gls/pdf/sclc.pdf.
- 18.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: A powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15(3):683–690. doi: 10.1245/s10434-007-9747-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Cancer Database. Data Dictionary Participant User Files. http://ncdbpuf.facs.org/node/259?q=print-pdf-all. Published 2016 Accessed November 1, 2017.
- 20.Austin PC. The relative ability of different propensity score methods to balance measured covariates between treated and untreated subjects in observational studies. Med Decis Mak. 2009;29(6):661–677. doi: 10.1177/0272989X09341755 [DOI] [PubMed] [Google Scholar]
- 21.Nyman J, Hallqvist A, Lund JÅ, et al. SPACE – A randomized study of SBRT vs conventional fractionated radiotherapy in medically inoperable stage I NSCLC. Radiother Oncol. 2016;121(1):1–8. doi: 10.1016/j.radonc.2016.08.015 [DOI] [PubMed] [Google Scholar]
- 22.Verma V, Simone C, Allen PLS. Outcomes of Stereotactic Body Radiotherapy for T1–2 N0 Small Cell Carcinoma Based on Addition of Chemotherapy and Prophylactic Cranial Irradiation: A Multicenter Analysis. Clin Lung Cancer. 2017;18(6):675–681.e1. doi: 10.1016/j.cllc.2017.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmed Z, Grover P, Kennedy KF, Masood A, Davis JR, Subramanian J. Predictors for chemotherapy in early stage small cell lung carcinoma (SCLC): A National Cancer Database (NCDB) analysis. Lung Cancer. 2017;113:85–87. doi: 10.1016/j.lungcan.2017.09.004 [DOI] [PubMed] [Google Scholar]
- 24.Shioyama Y, Onishi H, Takayama K, et al. Clinical Outcomes of Stereotactic Body Radiotherapy for Patients With Stage I Small-Cell Lung Cancer: Analysis of a Subset of the Japanese Radiological Society Multi-Institutional SBRT Study Group Database. Technol Cancer Res Treat. 2018;17:1533033818783904. doi: 10.1177/1533033818783904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: Updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol. 2007;2(7 Suppl 3):S94–100. doi: 10.1097/JTO.0b013e318074de34 [DOI] [PubMed] [Google Scholar]
- 26.Jeppesen SS, Schytte T, Jensen HR, Brink C, Hansen O. Stereotactic body radiation therapy versus conventional radiation therapy in patients with early stage non-small cell lung cancer: An updated retrospective study on local failure and survival rates. Acta Oncol (Madr). 2013;52(7):1552–1558. doi: 10.3109/0284186X.2013.813635 [DOI] [PubMed] [Google Scholar]
- 27.Ball D, Mai T, Vinod S, et al. MA 13.07 A Randomized Trial of SABR vs Conventional Radiotherapy for Inoperable Stage I Non-Small Cell Lung Cancer: TROG09.02 (CHISEL). J Thorac Oncol. 2017;12(11):S1853. doi: 10.1016/j.jtho.2017.09.565 [DOI] [Google Scholar]
- 28.Winther-Larsen A, Hoffmann L, Moeller DS, Khalil AA, Knap MM. Evaluation of factors associated with loco-regional failure and survival in limited disease small cell lung cancer patients treated with chemoradiotherapy. Acta Oncol (Madr). 2015;54(9):1574–1581. doi: 10.3109/0284186X.2015.1062135 [DOI] [PubMed] [Google Scholar]
- 29.van Meerbeeck JP, Fennell DA, De Ruysscher DKM, et al. Small-cell lung cancer. Lancet. 2011;378(9804):1741–1755. doi: 10.1016/S0140-6736(11)60165-7 [DOI] [PubMed] [Google Scholar]
- 30.Sarvi S, Mackinnon AC, Avlonitis N, et al. CD133+cancer stem-like cells in small cell lung cancer are highly tumorigenic and chemoresistant but sensitive to a novel neuropeptide antagonist. Cancer Res. 2014;74(5):1554–1565. doi: 10.1158/0008-5472.CAN-13-1541 [DOI] [PubMed] [Google Scholar]
- 31.Servat J, Verlicchi A, Rosell R. Cancer stem cells in small cell lung cancer. Transl Lung Cancer Res. 2016;5(1):16–25. doi: 10.3978/j.issn.2218-6751.2016.01.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–760. doi: 10.1038/nature05236 [DOI] [PubMed] [Google Scholar]
- 33.Phillips TM, McBride WH, Pajonk F. The response of CD24-/low/CD44+breast cancer-initiating cells to radiation. J Natl Cancer Inst. 2006;98(24):1777–1785. doi: 10.1093/jnci/djj495 [DOI] [PubMed] [Google Scholar]
- 34.Moding EJ, Mowery YM, Kirsch DG. Opportunities for radiosensitization in the stereotactic body radiation therapy (SBRT) era. Cancer J (United States). 2016;22(4):267–273. doi: 10.1097/PPO.0000000000000203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brooks ED, Schoenhals JE, Tang C, et al. Stereotactic ablative radiation therapy combined with immunotherapy for solid tumors. Cancer J (United States). 2016;22(4):257–266. doi: 10.1097/PPO.0000000000000210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee Y, Auh SL, Wang Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+T cells: Changing strategies for cancer treatment. Blood. 2009;114(3):589–595. doi: 10.1182/blood-2009-02-206870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh S, Parulekar W, Murray N, et al. Influence of sex on toxicity and treatment outcome in small-cell lung cancer. J Clin Oncol. 2005;23(4):850–856. doi: 10.1200/JCO.2005.03.171 [DOI] [PubMed] [Google Scholar]
- 38.Wheatley-Price P, Ma C, Ashcroft LF, et al. The strength of female sex as a prognostic factor in small-cell lung cancer: A pooled analysis of chemotherapy trials from the Manchester Lung Group and Medical Research Council Clinical Trials Unit. Ann Oncol. 2010;21(2):232–237. doi: 10.1093/annonc/mdp300 [DOI] [PubMed] [Google Scholar]
- 39.Paximadis P, Beebe-Dimmer JL, George J, Schwartz AG, Wozniak A, Gadgeel S. Comparing Treatment Strategies for Stage I Small-cell lung Cancer. Clin Lung Cancer. 2018;19(5):e559–565. doi: 10.1016/j.cllc.2018.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Auperin A, Arriagada R, Pignon J, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylatic Cranial Irradiation Overview Collaborative Group. N Engl J Med. 1999;341(7):476–484. doi: 10.1056/NEJM199908123410703 [DOI] [PubMed] [Google Scholar]