Abstract
Angiotensin-(1–7) [Ang-(1–7)] exhibits blood pressure lowering actions, inhibits cell growth, and reduces tissue inflammation and fibrosis which may functionally antagonize an activated Ang II-AT1 receptor axis. Since the vascular actions of Ang-(1–7) and the associated receptor/signaling pathways vary in different vascular beds, the current study established the vasorelaxant properties of the heptapeptide in the renal artery of male Wistar male rats. Ang-(1–7) produced an endothelium-dependent vasodilator relaxation of isolated renal artery segments pre-contracted by a sub-maximal concentration of phenylephrine (PE) (3 × 10−7 M). Ang-(1–7) induced vasodilation of the rat renal artery with an ED50 of 3 ± 1 nM and a maximal response of 42 ± 5% (N = 10). The two antagonists (10−5 M each) for the AT7/Mas receptor (MasR) [D-Pro7]-Ang-(1–7) and [D-Ala7]-Ang-(1–7) significantly reduced the maximal response to 12 ± 1% and 18 ± 3%, respectively. Surprisingly, the AT2R receptor antagonist PD123319, the AT1R antagonist losartan and B2R antagonist HOE140 (10−6 M each) also significantly reduced Ang-(1–7)-induced relaxation to 12 ± 2%, 22 ± 3% and 14 ± 7%, respectively. Removal of the endothelium or addition of the soluble guanylate cyclase (sGC) inhibitor ODQ (10−5 M) essentially abolished the vasorelaxant response to Ang-(1–7) (10 ± 4% and 10 ± 2%, P < 0.05). Finally, the NOS inhibitor LNAME (10−4 M) reduced the response to 13 ± 2% (p < 0.05), but the cyclooxygenase inhibitor indomethacin failed to block the Ang-(1–7) response. We conclude that Ang-(1–7) exhibits potent vasorelaxant actions in the isolated renal artery that are dependent on an intact endothelium and the apparent stimulation of a NO-sGC pathway. Moreover, Ang-(1–7)-dependent vasorelaxation was sensitive to antagonists against the AT7/Mas, AT1, AT2 and B2 receptor subtypes.
Keywords: Angiotensin, Kidney, Renal artery, Endothelium, c-GMP
1. Introduction
Angiotensin-(1–7) [Ang-(1–7)] is an alternative peptide product of the renin-angiotensin-aldosterone system (RAAS) present in the circulation and various tissues including the kidney, brain and heart [8,28]. In contrast to Ang II, Ang-(1–7) exhibits blood pressure lowering actions and beneficial effects on end organ damage such as reduced fibrosis and inflammation [7,13,29]. Ang-(1–7) is formed from either Ang I by several endopeptidases (neprilysin, thimet oligopeptidase) or from Ang II by carboxypeptidases that include angiotensin-converting enzyme 2 (ACE2) and prolyl carboxypeptidase (9). Similar to bradykinin, angiotensin converting enzyme 1 (ACE) metabolizes Ang-(1–7) resulting in the formation of Ang-(1–5) and the dipeptide His-Leu [9]. Indeed, the administration of ACE inhibitors contributes to increased circulating levels of Ang-(1–7) that likely reflect both the reduced metabolism of the peptide and the processing of Ang I by neprilysin instead of ACE [7]. The cardiovascular actions of Ang-(1–7) are mediated predominantly through the G protein-coupled receptor Mas and involve the release of vasodilatory prostaglandins and formation of nitric oxide (NO) [28]. Moreover, chronic blockade of the Ang-(1–7) receptor with the selective antagonist [D-Ala7]-Ang-(1–7) (A779) partially reverses the cardiovascular effects of either ACE inhibitors or AT1 receptor antagonists [12]. Importantly, these data support the concept that at least part of the therapeutic effects of RAS blockade may be mediated through activation of the Ang-(1–7)-AT7/Mas receptor axis.
Within the kidney, the beneficial effects of Ang-(1–7) include both hemodynamic and tubular actions [12]. Acute infusion of Ang-(1–7) generally increases glomerular filtration (GFR) and augments renal sodium excretion. In contrast to Ang II, the increase in GFR by Ang-(1–7) likely reflects the vasorelaxant properties of the peptide. In higher species including dog and sheep, administration of Ang-(1–7) increases renal blood flow [15,32]. In this regard, the vascular response to Ang-(1–7) was absent in adult male sheep exposed to glucocorticoids during fetal development and the lack of a renal Ang-(1–7) response may contribute to the sustained elevation in blood pressure in this ovine model of fetal programming [12]. In pre-constricted afferent arterioles of the rabbit kidney, Ang-(1–7) induces vasodilation that is dependent on the release of NO [24]. The vasorelaxant effects of Ang-(1–7) within the kidney are consistent with the actions of the peptide on other vascular beds and the ability of the peptide to stimulate NO [6,14,26,37]. However, there is relatively little data on the vascular actions of Ang-(1–7) on isolated renal vessels. Therefore, the present study characterized the vascular responses of Ang-(1–7) in the renal artery of the normotensive Wistar rat, the major conduit vessel of the kidney, as well as the influence of various angiotensin receptor antagonists and signaling inhibitors to better define the Ang-(1–7) response in the renal vessel.
2. Materials and methods
2.1. Renal artery preparation
Male Wistar rats (age 12–15 weeks) were euthanized by decapitation under light ether anesthesia. The renal artery was isolated carefully and transferred into a Petri dish containing oxygenated Krebs’ solution. The vessel was cut into ring segments of about 5 mm. The ring segments were mounted in baths containing 50 ml Krebs-Henseleit (KH) solution at pH 7.4. The composition of KH-solution is as follows (mM): NaCl (118.3), KCl (4.7), CaCl2 (2.5), MgSO4 (1.2), NaHCO3 (25), KH2PO4 (1.2) and glucose (11.2). The tissue bath solution was maintained at 37°C and was aerated with 95% O2/5%CO2. Changes in vascular reactivity of the renal arteries were recorded by measurement of changes in the isometric tension to vasoactive agonists using computerized Automatic organ bath LSI Letica Scientific Instruments (Powerlab/8sp ADIinsturments, Panlab, Spain). A pre-tension of 0.5 g was applied and the preparations were allowed to stabilize (45 min) until a stable baseline tone was obtained. The integrity of the endothelial layer was tested before every experiment by testing the vasodilator response to carbachol (10−7 M). All studies involving animals were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication number 85–23, Revised in 1985) as approved by Kuwait University Research Administration.
2.2. Ang-(1–7) responsiveness
Following the period of equilibration, the isolated renal artery segments were pre-contracted by a sub-maximal concentration of PE (3 × 10−7 M) and a cumulative concentration response curve for Ang-(1–7) (10−12 to 3 × 10−6 M) was established. The response to any given concentration was left to stabilize before adding the next drug concentration. The relaxant responses were expressed as the percent reduction (%) of the tension induced by PE. Sigmoidal dose-response curves were established by nonlinear regression of the log dose of Ang-(1–7) and the percentage (%) of the vasorelaxation response (3 parameter) using a least squares fit for each experiment with GraphPad Prism 6.0 (GraphPad Software, San Diego, CA). Based on the constructed dose-response curves, the ED50 (concentration to reduce tone by 50%) was determined for Ang-(1–7) alone. The maximal vasorelaxation response (%) was determined for Ang-(1–7) alone or in the presence of various receptor antagonists or enzyme inhibitors.
2.3. Effect of angiotensin-receptor blockers
The effect of pre-incubation of the vessels with [sarcosine1, threonine8]-Ang II (Sarthran, 10−5 M); losartan (10−6 M); PD123319 (10−6 M); [D-Alanine7]-Ang-(1–7) (DALA, 10−5 M); [D-Proline7]-Ang-(1–7) (DPRO, 10−5 M) on the vasodilator response to Ang-(1–7) was established. The vasodilator effect to Ang-(1–7) was determined by pre-contracting the tissues with PE (3 × 10−7 M) added to the organ baths. After obtaining a steady level of contraction, the relaxant effect to Ang-(1–7) from 10−12 to 3 × 10−6 M was assessed. The tissues were then incubated for 20 min in KH solution containing the antagonists and the vasodilator effect to Ang-(1–7) was then re-assessed. The effect of the different antagonists was investigated using different tissue segments.
2.4. Effect of inhibition of nitric oxide synthase, soluble guanylate cyclase and cyclooxygenase blockade
The effect of nitro-l-arginine methyl ester (LNAME) (10−4 M), an inhibitor of nitric oxide synthase, indomethacin (10−6 M), a combination of LNAME (10−4 M) and indomethacin (10−6 M) or 1H-[1,2,4] oxadiazolo-quinoxalin (ODQ) (10−5 M), an inhibitor of soluble guanylate cyclase, on the vasodilator response induced by Ang-(1–7) was also established in the renal artery preparation. Note that the dose response range for Ang-(1–7) for these agents was 10−12 to 10−6 M. We also assessed the effect of endothelium removal on the Ang-(1–7) response (10−12 to 3 × 10−6 M). Denuded vessels were prepared by gentle rubbing of the endothelial layer which was confirmed by loss of the vasodilator response to carbachol (10−7 M). The vasodilator effect to Ang-(1–7) was determined by pre-contracting the tissues with PE (3 × 10−7 M) added to the organ baths. The contribution of the bradykinin pathway was addressed by addition of the bradykinin B2 receptor antagonist HOE140 (5 × 10−8 M) and combined HOE and LNAME (10−4 M and 3 × 10−8 M, respectively) on Ang-(1–7)-induced vasorelaxation.
2.5. Effect of potassium channel inhibitors
The effects on Ang-(1–7) dependent vasorelaxation of the potassium (K+) channel blockers glibenclamide (10−5 M) to inhibit ATP-sensitive K+ channels and iberiotoxin (5 × 10−8 M) to block calcium-activated K+ (BK) channels were also investigated in this study.
2.6. AT2 receptor binding
The assessment of the various angiotensin antagonists on AT2 receptor binding using the non-selective radioligand 125I-[Sarcosine1, Threonine8]-Ang II (Sarthran) or 125I-Ang-(1–7) was performed in isolated membranes from the rat renal artery and the pancreatic AR42J cell line (ATTC, Manassas, VA) as previously described [11]. In brief, 50 μg of the cell membrane preparation was incubated with 0.2 nM of the radioligand with or without the antagonists in 20 mM HEPES buffer pH 7.4 containing 125 mM NaCl, 5 mM MgCl2 and 2.5 mM EGTA, 0.2% BSA and a cocktail of inhibitors [22]. The binding reaction was terminated by addition of ice cold buffer and centrifugation in a high-speed microfuge at 20,000 xg. The resultant pellet was washed, centrifuged again and the pellet counted in a γ-counter. The 125I-labeled peptides were generated by chloramine T and purified by HPLC [11]. Competition curves were constructed in Prism 6 by non-linear regression using a one-site fit to determine the IC50 values [11].
2.7. Materials
Ang-(1–7), carbachol, phenylephrine, Ang II, L-NAME, HOE140, DALA, ODQ, PD123319, indomethacin, losartan and captopril were obtained from Sigma Biochemical (Chicago, USA). Sarthran was obtained from Bachem (Torrance CA) and the [D-Pro7]-Ang-(1–7) was synthesized by LifeTein, LLC (Somerset, NJ). 125I-Sarthran was prepared by the chloramine T method and purified by HPLC to a specific activity >2000 Curies/mmol as previously described [10].
2.8. Statistical analysis
The vascular data were analyzed and linear plots of the Ang-(1–7) responses were constructed using Prism 6.0. Data are presented as the mean ± SEM of ‘N’ number of experiments. The mean values for the maximal response for Ang-(1–7) alone and in the presence of the various treatments were compared using oneway analysis of variance (ANOVA) followed by Dunnett’s post hoc test. The mean responses for each dose of Ang-(1–7) among the control and treatment groups were also compared by ANOVA with Dunnett’s post hoc analysis. Statistical differences in the data were achieved at P < 0.05.
3. Results
3.1. Ang-(1–7)-induced vasorelaxation
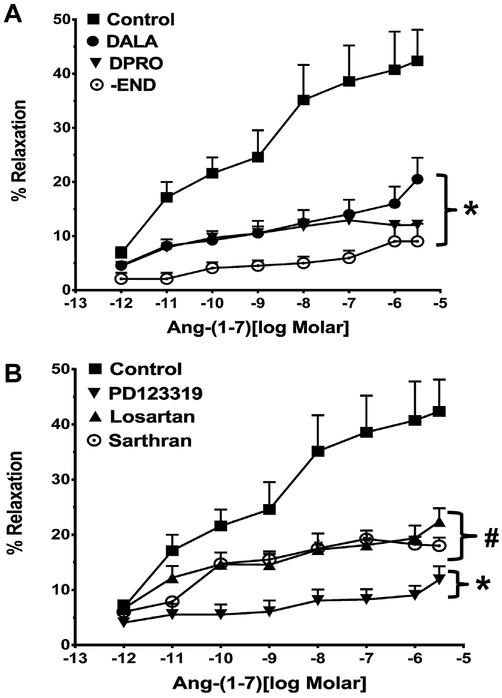
The addition of increasing concentrations of Ang-(1–7) (10−12 to 3 × 10−6 M) induced an increasingly greater degree of vasorelaxation of the rat renal artery characterized by an ED50 of 3.4 ±1.1 nM and a maximal relaxation response of 42 ± 6% (Fig. 1A, Table 1). The relaxant response of Ang-(1–7) was significantly inhibited by pre-treatment of the vessels with either the Ang-(1–7) receptor antagonists [D-Ala7]-Ang-(1–7) (DALA, A779) or [D-Pro7]-Ang-(1–7) (DPRO); DALA and DPRO reduced the maximal response to 18 ± 6% and 12 ± 6%, respectively (Table 1, Fig. 1A). Contribution of the endothelial layer to Ang-(1–7) relaxation was assessed by denuding the endothelium of the renal artery. Removal of the endothelium essentially abolished the vascular response to Ang-(1–7) (Fig. 1A, Table 2). As shown in Fig. 1B, the non-selective peptide antagonist [Sar1, Thre8]-Ang II (Sarthran, 10−5 M) also inhibited the Ang-(1–7)-dependent relaxation (Table 1, Fig. 1B). To address the contribution of Ang II receptor subtypes, we pretreated the vessels with the AT1 (losartan) or AT2 (PD123319) antagonists and assessed Ang-(1–7)-induced vasorelaxation. Both losartan and PD123319 significantly inhibited the Ang-(1–7) response and attenuated the maximal response to 22 ± 6% and 12 ± 6%, respectively (Fig. 1B; Table 1).
Fig. 1.
Influence of angiotensin receptor antagonists on the vasorelaxant response to Ang-(1–7) in the renal artery. Linear plots of percent vasorelaxation (%) and the log dose of Ang-(1–7) alone or with various antagonists. Panel A: effect of [D-Ala1]-Ang-(1–7) (DALA,10−5 M), [D-Pro1]-Ang-(1–7) (DPRO, 10−5 M) or denuding endothelium (-END) on Ang-(1–7)-induced vasodilation (10−12–3 × 10−6 M) of the isolated renalartery of the rat. Panel B: effect of [Sar1,Thre8]-Ang II (Sarthran, 10−5 M), losartan (10−6 M) and PD123319 (10−6 M) on Ang-(1–7)-induced vasodilation (10−12 to 3 × 10−6 M) of the isolated renal artery of the rat. Data are mean ± SEM (n = 4–10 per group, see Table 1) compared to Ang-(1–7) alone by ANOVA with Dunnett’s post hoc test. *P < 0.05 versus Ang-(1–7) from 10−11 to 3 × 10−6 M; #P < 0.05 versus Ang-(1–7)from 10−8 to 3 × 10−6 M.
Table 1.
Influence of Angiotensin Receptor Antagonists on Ang-(1–7)-Dependent Vasorelaxation of the Isolated Renal Artery.
| Groups | Maximal Response (%) | N |
|---|---|---|
| Ang-(1–7) | 42.4 ± 5.8 | 10 |
| D-Ala7-Ang-(1–7) | 18.0 ± 3.3* | 9 |
| D-Pro7-Ang-(1–7) | 12.3 ± 0.8* | 4 |
| Losartan | 21.9 ± 2.9* | 10 |
| PD123319 | 11.5 ± 2.4* | 10 |
| Sarthran | 18.4 ± 0.8* | 4 |
Maximal vasorelaxation response to Ang-(1–7) alone or in the presence of various angiotensin receptor antagonists.
Data are the means ± SEM (N = 4–10) analyzed by one-way ANOVA with Dunnett’s post hoc test;
P < 0.05 vs. Ang-(1–7) as control.
Table 2.
Influence of Signaling Inhibitors on Ang-(1–7)-Dependent Vasorelaxation of the Isolated Renal Artery.
| Groups | Maximal Response (%) | N |
|---|---|---|
| Control | 42.4 ± 5.8 | 10 |
| Denuded | 9.8 ± 3.5* | 4 |
| l-NAME | 13.4 ± 2.0* | 6 |
| Indomethacin | 23.7 ± 9.0 | 7 |
| l-NAME/Indomethacin | 20.5 ± 3.0* | 8 |
| ODQ | 10.0 ± 2.1* | 8 |
| HOE140 | 14.0 ± 7.0* | 9 |
| HOE/l-NAME | 10.2s ± 3.8* | 9 |
| Iberiotoxin | 22.1 ± 7.4* | 8 |
| Glibenclamide | 22.3 ± 2.3* | 8 |
Maximal vasorelaxation response to Ang-(1–7) alone or in the presence of various signaling inhibitors.
Data are the means ± SEM (N = 4–10) analyzed by one-way ANOVA with Dunnett’s post hoc test;
P < 0.05 vs. Ang-(1–7) as control.
3.2. Nitric oxide synthase and cyclooxygenase inhibitors on ang-(1–7)-induced vasodilation
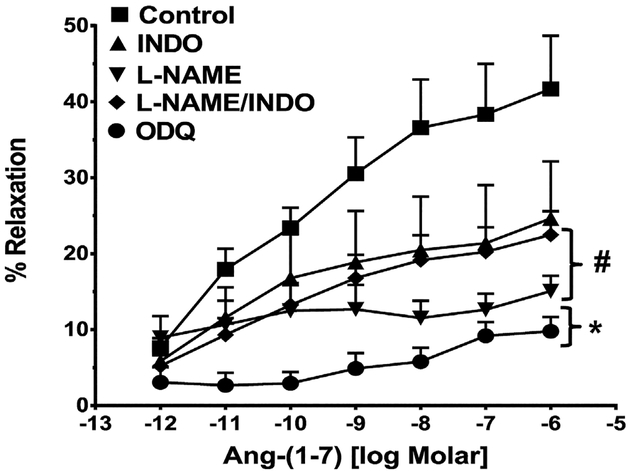
Pre-treatment of the isolated vessels with the non-selective NOS inhibitor LNAME markedly attenuated vascular relaxation to Ang-(1–7) (Table 2, Fig. 2). In contrast, the cyclooxygenase inhibitor indomethacin did not significantly reduce the Ang-(1–7) response (Table 2, Fig. 2). Combined treatment with LNAME and indomethacin produced no further inhibition of relaxation than LNAME or indomethacin alone (Table 2). Finally, pretreatment of the vessel preparation with ODQ, an inhibitor of soluble guanylate cyclase essentially abolished the Ang-(1–7)-induced vasorelaxation of the isolated renal artery (Fig. 2, Table 2).
Fig. 2.
Influence of various intracellular signaling inhibitors on the vasorelaxant response to Ang-(1–7) in the renal artery. Linear plots of percent vasorelaxation (%) and the log dose of Ang-(1–7) show the effects of indomethacin (INDO, 10−6 M), l-NAME (10−4 M), l-NAME (10−4 M) and INDO (10−6 M) or ODQ (10−5 M) on Ang-(1–7)-induced vasodilation of the isolated renal artery of the rat. Data are mean ± SEM (n = 6–10 per group; see Table 2) compared to Ang-(1–7) alone by ANOVA with Dunnett’s post hoc test. *P < 0.05 versus Ang-(1–7) from 10−11 to 10−6 M; #P < 0.05 versus Ang-(1–7) from 10−9 to 10−6 M.
3.3. Bradykinin B2 receptor antagonist on ang-(1–7)-induced vasodilation
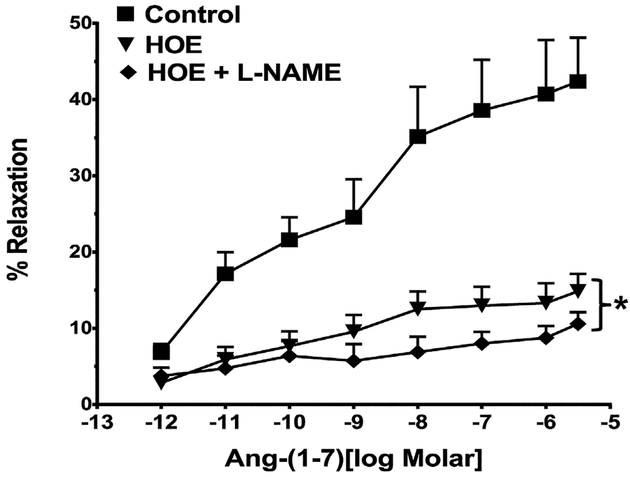
As previous studies suggested that the vascular actions of Ang-(1–7) may involve the contribution of bradykinin [6,14], we determined the effects of the bradykinin B2 receptor antagonist in the renal artery preparation. As shown in Fig. 3, the B2 receptor antagonist HOE140 markedly inhibited the vasorelaxation to Ang-(1–7). The co-treatment of the vessels with HOE140 and LNAME elicited a slightly greater degree of inhibition of the Ang-(1–7) response than the B2 antagonist alone (Fig. 3, Table 2).
Fig. 3.
Influence of the bradykinin B2 receptor antagonist HOE140 on the vasorelaxant response to Ang-(1–7) in the renal artery. Linear plots of percent vasorelaxation (%) and the log dose of Ang-(1–7) show the effects of HOE140 (HOE, 5 × 10−8M) orHOE (5 × 10−8M) and L-NAME (10−4M) on Ang-(1–7)-induced vasodilation of theisolated renal artery of rat. Data are mean ± SEM (n = 9–10 per group, see Table 2)compared to Ang-(1–7) alone by ANOVA with Dunnett’s post hoc test.*P < 0.05 versusAng-(1–7) from 10−11 to 3 × 10−6M.
3.4. K+ channel blockers on Ang-(1–7)-induced vasodilation
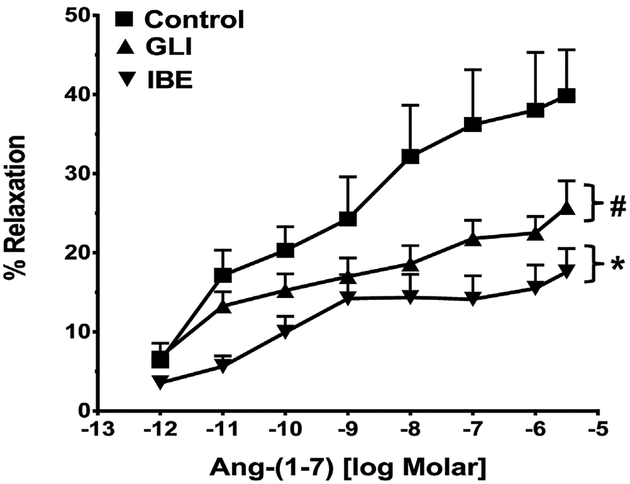
Since we previously demonstrated that the ATP-sensitive K+ channel contributes to the vasorelaxant effects of Ang-(1–7) in the corpus cavernosum [39], we determined whether K+ channels inhibitors influence the Ang-(1–7) response. The renal vessels were pretreated with either iberiotoxin to block the calcium-dependent K+ (BK) channel or glibenclamide to block the ATP-sensitive K+ channel. Iberiotoxin significantly attenuated the Ang-(1–7) response [Fig. 4, Table 2]. The addition of glibenclamide also reduced the Ang-(1–7) vasorelaxant response in the renal artery [Fig. 4, Table 2].
Fig. 4.
Influence of the K+ channel blockers on the vasorelaxant response to Ang-(1–7) in the renal artery. Linear plots of percent vasorelaxation (%) versus the log dose of Ang-(1–7) show the effects of glibenclamide (GLI, 10−5M) or iberiotoxin(IBE, 5 × 10−8M) on Ang-(1–7)-induced vasodilation of the isolated renal artery of the rat. Data are mean ± SEM (n = 8–10 per group; see Table 2) compared to Ang-(1–7) alone by ANOVA with Dunnett’s post hoc test. *P < 0.05 versus Ang-(1–7) from 10−11 to 3 × 10−6M; #P<0.05 versus Ang-(1–7) from 10−7 to 3 × 10−6M.
3.5. Competition of 125I-Sarthran binding in AR42J pancreatic cell membranes by AT1, AT2 and Ang-(1–7) receptor antagonists
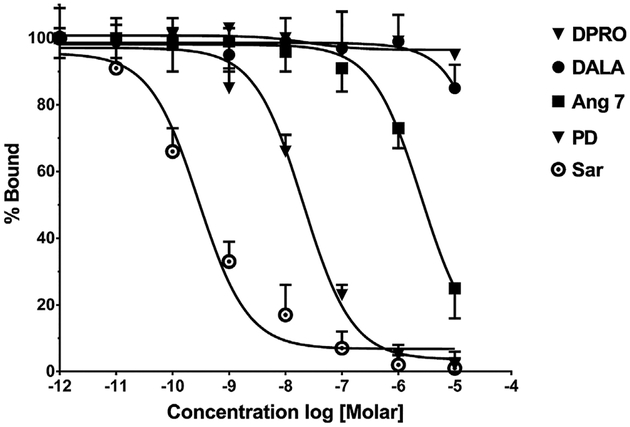
Initial attempts to characterize the influence of the various receptor antagonists on the binding of either Ang-(1–7) or Sarthran on membrane preparations of the renal artery were unsuccessful and likely reflect the low level of receptor expression and the limited amount of tissue obtained. Therefore, we utilized the rat pancreatic AR42J cells that predominantly express AT2 receptors to determine the extent of competition for Sarthran binding by Ang-(1–7) and its receptor antagonists [11]. As shown in Fig. 5, both unlabeled Sarthran and PD123319 essentially abolished binding in the AR42J cell membranes with IC50′ s of 0.3 and 20 nM, respectively. Ang-(1–7) competed for binding of 125I-Sarthan but only at a relatively high concentration (IC50 = 4.5 μM). In contrast, the Ang-(1–7) antagonists DALA and DPRO minimally competed for Sarthran binding up to a concentration of 10−5 M (Fig. 5).
Fig. 5.
Competition of 125I-[Sar1,Thr8]-Ang II binding by various angiotensin receptor antagonists in AR42J cell membranes. Competition curves for percent binding (%) of 125I-[Sar1,Thre8]-Ang II and the log dose of various competitors that include the non-selective antagonist [Sar1,Thre8]-AngII (Sar), the AT2 antagonist PD123319 (PD), Ang-(1–7) (Ang 7) the Mas receptor antagonists [D-Ala7]-Ang-(1–7) (DALA) and [D-Pro7]-Ang-(1–7) (DPRO). Data are mean ± SEM (n = 3). Competition curves were constructed in Prism 6 by non-linear regression using a one site fit.
4. Discussion
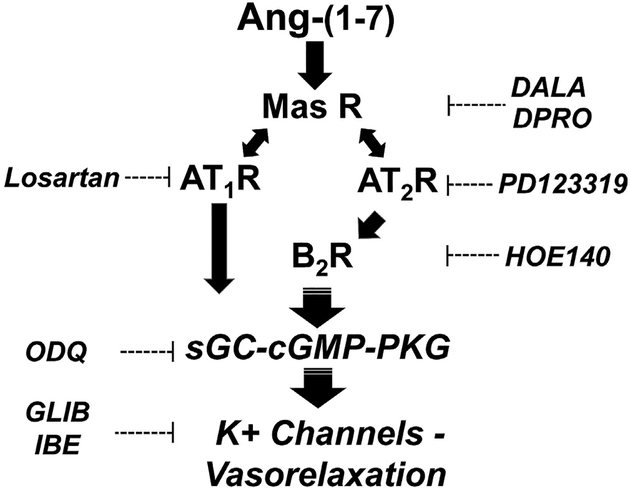
The present findings reveal that Ang-(1–7) exhibits potent vasorelaxant effects (ED50 = 3.4 nM) on phenylephrine-constricted segments of renal artery isolated from normotensive male Wistar rats. Ang-(1–7)-dependent vasorelaxation of the renal artery was essentially abolished by the AT7/Mas receptor antagonists DALA (A779) and DPRO. Surprisingly, the vascular actions of the heptapeptide were also significantly attenuated by the AT2 antagonist PD123319, the AT1 antagonist losartan and the B2 antagonist HOE140 suggesting an unusual receptor selectivity for the heptapeptide. In addition, blockade of NOS by LNAME or the soluble guanylate cyclase inhibitor ODQ reduced the vasorelaxant actions of Ang-(1–7). Thus, the downstream signaling mechanisms responsible for the vascular response to Ang-(1–7) may involve NO that ultimately stimulates soluble guanylate cyclase and the subsequent formation of cGMP. Removal of the endothelium essentially abolished the vasorelaxant effects of Ang-(1–7) in the pre-constricted vessels and suggests localization of the Ang-(1–7) receptor to this vascular compartment (Fig. 6). The vascular actions of Ang-(1–7) in the renal artery support the overall view that the Ang-(1–7)/Mas receptor pathway may antagonize the Ang II–AT1 receptor axis and other pressor systems to ameliorate an increase in blood pressure and vascular damage [12].
Fig. 6.
Potential scheme for the interaction of Ang-(1–7) with angiotensin receptors to elicit vasorelaxation of renal artery. Ang-(1–7) binds to the Mas receptor to increase cGMP and induce vasorelaxation via activation of K+ channels. The Mas receptor (Mas R) antagonists [D-Ala7]-Ang-(1–7) (DALA) and [D-Pro7]-Ang-(1–7) (DPRO) directly block the binding of Ang-(1–7) within the endothelium. Ang-(1–7) stimulation of the Mas R subsequently activates the AT1 and AT2 R through an unknown mechanism. Ang-(1–7) activation of the kinin B2 receptor (BK2 R) via the AT2R may increase cGMP levels by the stimulation of soluble guanylate cyclase (sGC) that subsequently leads to activation of protein kinase G (PKG) and K+ channels within the vascular smooth muscle. The K+ channels inhibitors glibenclamide (GLIB) and iberiotoxin (IBE) partially block the vasorelaxant effects of Ang-(1–7). The AT1R, AT2R and BK2R antagonists Losartan, PD123319 and HOE140, respectively block the vasorelaxant effects of Ang-(1–7), as well as the sGC inhibitor ODQ.
Previous studies on the vascular actions of Ang-(1–7) are in general agreement that the peptide exhibits vasorelaxant effects in various vascular beds [28]. In contrast, the identity of the receptor(s) that mediate the vascular actions of Ang-(1–7) is more equivocal. Ang-(1–7)-dependent relaxation of aortic segments was abolished by the DALA antagonist, as well as by knockdown of the receptor in the Mas null mice [28]. In this case, these investigators found no inhibitory effect of losartan or PD123319 on the vascular actions of Ang-(1–7). However, vasorelaxation of the mesenteric artery by Ang-(1–7) was blocked by DALA and the AT1 antagonists losartan and candesartan, but not the AT2 antagonist PD123319 [21]. In renal afferent arterioles, the Ang-(1–7)-induced vasodilation was abolished by DALA, but was not reversed by PD123319 or the AT1 antagonist L158809 [24]. The in vivo actions of Ang-(1–7) also support a vasodilatory role of the peptide that may contribute to a reduction in blood pressure [28]. Using a microsphere approach, Sampaio et al. reported that a low dose of Ang-(1–7) augmented blood flow in multiple vascular beds including the kidney, while a higher dose of the peptide reduced blood flow [27]. The increase in blood flow to the kidney, mesentery and skin was abolished by the Ang-(1–7) antagonist DALA; however, the influence of other angiotensin receptor antagonists was not assessed in this study [27]. Widdop and colleagues found that the depressor effects of Ang-(1–7) evident in both SHR and WKY rats following AT1 receptor blockade were abolished by PD123319 but not the DALA antagonist [36]. Consistent with the current findings, the Ang-(1–7) responses were sensitive to HOE140 and LNAME suggesting the potential interaction of AT2 and B2 receptors to stimulate NO [36]. However, these investigators recently found that both PD123319 and DALA blocked the depressor actions of Ang-(1–7) in older WKY rats (20 months versus 4 months of age) [5]. The aged WKY rats also exhibited an increase in the expression of both AT2 and the Mas receptors in the aorta, but no change in the AT1 receptor protein [5]. Lautner et al. identified an endogenous analog of Ang-(1–7) termed (Ala1)-Ang-(1–7) or almandine that interacts with the Mas-related receptor MrgD to induce relaxation of aortic rings [19]. Interestingly, both DPRO and PD123319 abolished the effects of [Ala1]-Ang-(1–7); however, DALA was ineffective suggesting that the antagonist does not apparently recognize this novel site. Thus, it is unlikely that the MrgD receptor mediates the actions of Ang-(1–7) in the renal artery or that Ang-(1–7) is readily decarboxylated to [Ala1]-Ang-(1–7) in this preparation. Although the extent that the vasorelaxant actions of Ang-(1–7) in the renal artery contribute to long-term alterations in blood pressure is equivocal in the present study, this vessel preparation may facilitate a greater elucidation of the vascular actions of Ang-(1–7) and the associated receptor-dependent pathway and downstream signaling events. Moreover, the role of Ang-(1–7) and its receptors may be particularly relevant regarding the progression of vascular damage in various pathologies including hypertension, diabetes and atherosclerosis [1–3,35,40].
We were unsuccessful in demonstrating binding of the non-selective angiotensin antagonist 125I-Sarthran or 125I-Ang-(1–7) in membrane preparations of the renal artery to attempt a further characterization of the receptor subtypes. However, given the potential role of the AT2 receptor to mediate the actions of Ang-(1–7), we established a binding assay in the rat AR42J cell line that expresses a high density of AT2 sites [11]. Both PD123319 and Sarthran essentially abolished binding, although Sarthran was a more potent competitor. Ang-(1–7) also competed for binding but exhibited a very high IC50 (>1 μM) while the [D-Ala7]-Ang-(1–7) and [D-Pro7]-Ang-(1–7) compounds did not effectively compete at the AT2 binding site; these data are consistent with previous reports on the specificity of the two antagonists evident by their lack of actions at AT2 and AT1 sites [4,30,31,35]. We interpret our results in lieu of the vascular studies to suggest a requisite role for the AT2 and B2 receptors that may partially mediate (either directly or indirectly) the vasorelaxant actions of Ang-(1–7) as opposed to non-selective actions of these antagonists to block the Mas receptor at high doses (Fig. 6). Indeed, activation of both AT2 and B2 receptors downstream from the Ang-(1–7) receptor may be critical to the stimulation of soluble guanylate cyclase and the subsequent generation of cGMP in the renal artery (Fig. 6). Carvalho and colleagues reported that Ang-(1–7)-dependent dilation of mesenteric vessels was blocked by a B2 antagonist (JE049), as well as enzyme inhibitors that prevent the generation of kinins [20]. Moreover, stimulation of NO by the Ang II-AT2 receptor was dependent on the downstream activation of the B2 receptor by bradykinin [34]. Studies in bovine endothelial cells revealed that NO stimulation by Ang-(1–7) or the non-peptide agonist AVE0991 was blocked by DALA, PD123319 and HOE140, as well as LNAME [14,37]. These studies also find that the AT1 antagonist candesartan attenuated Ang-(1–7)-dependent NO release that is consistent with the present results using losartan [14]. In our study, losartan significantly reduced the Emax for Ang-(1–7) suggesting an interaction of the antagonist with the Ang-(1–7) receptor. AT1 receptors are expressed on endothelial cells and may mediate the Ang II-dependent release of NO [33]. Transgenic mice that express a constitutively active form of the endothelial AT1 receptor exhibited lower blood pressure, enhanced eNOS activity and higher NO levels [23]. Thus, Ang-(1–7) may interact with a subset of AT1 receptors on the endothelium to stimulate NO that is sensitive to both AT1 and AT7 receptor antagonists, but may not invoke activation of either AT2 or BK2 receptors (Fig. 6). Alternatively, Walther and colleagues have demonstrated heterodimerization of the AT1 and Mas receptors which may convey novel properties of this receptor complex [18]. Additional studies are required to discern the exact role of the various angiotensin and kinin receptors on the renal vasculature that mediate the actions of Ang-(1–7).
Elucidation of the signaling cascade involved in the vasorelaxant actions of Ang-(1–7) in the renal artery apparently involves endothelial-derived NOS and cGMP. Pretreatment with the non-selective NOS inhibitor LNAME attenuated the Ang-(1–7) response. The soluble guanylate cyclase inhibitor ODQ also markedly attenuated the Ang-(1–7) induced relaxation suggesting a primary role for cGMP-dependent signaling. Additionally, removal of the endothelium abrogated the actions of Ang-(1–7) that would further implicate cGMP, although cGMP content was not assessed in Ang-(1–7)-treated vessels. The present study confirms earlier findings by Carvalho and colleagues regarding the vasodilatory actions of Ang-(1–7) and sensitivity to DALA blockade in the rat mesentery [20]. These investigators also demonstrated inhibition of the Ang-(1–7) vasorelaxant effects by both LNAME and indomethacin [20] that would support crosstalk between the NO and prostaglandin pathways [25]. Indeed, earlier studies found that intrarenal infusion of Ang-(1–7) stimulated prostaglandin release and that indomethacin attenuated the blood pressure-lowering effects of the peptide [1,16,17]. However, indomethacin treatment failed to significantly attenuate the vasorelaxant effects of Ang-(1–7), and co-treatment of indomethacin and LNAME did not appear to elicit additional inhibition compared to LNAME alone.
Finally, we note that both the K+ channel blockers glibenclamide and iberiotoxin reduced the vasorelaxant effects of Ang-(1–7). We previously reported evidence for a NO-K+ (glibenclamide-sensitive) channel pathway that mediated the Ang-(1–7)-dependent relaxation of the rabbit corpus cavernosum [39]. Moreover, Costa et al. recently reported that glibenclamide abolished the anti-nociceptive actions of Ang-(1–7) that were associated with stimulation of the NO-cGMP pathway [13]. Interestingly, Yang et al. found that Ang-(1–7) stimulation of the K+ current in a neuronal cell line was blocked by a type 1 or neuronal NOS inhibitor, although the identification of the specific K+ channel was not determined [38]. To our knowledge, the present data are the first evidence to suggest that the vasoactive effects of Ang-(1–7) in the renal artery may reflect the stimulation of K+ channels via NO/cGMP (Fig. 6).
In conclusion, the relaxation responses to Ang-(1–7) in the rat renal artery appear to involve multiple receptor systems including the AT2 and kinin B2 receptors, as well as the NO and K+ channel effector systems. The present results further emphasize the complexity of the vascular actions of Ang-(1–7) that are likely altered in hypertension and other pathologies of vascular injury [28].
Acknowledgements
This study was funded by a grant from Kuwait University Research Administration Project Number (MR04/09). Additional support was from the National Institutes of Health (HL-51952, HL-56973, and HD-08877). The authors gratefully acknowledge support in part provided by the Farley-Hudson Foundation, Jacksonville, NC.
Footnotes
Conflict of interests
The authors declare that there are no competing financial interests in the work described.
References
- [1].Benter IF, Ferraris CM, Morris M, Diz DI, Antihypertensive actions of angiotensin-(I-7) in spontaneously hypertensive rats, Am. J. Physiol 269 (1995) H313–319. [DOI] [PubMed] [Google Scholar]
- [2].Benter IF, Yousif MHM, Anim T, Cojocel C, Diz DI, Angiotensin-(1–7) prevents development of severe hypertension and end-organ damage in spontaneously hypertensive rats treated with L-NAME, Am. J. Physiol. Heart Circ. Physiol 290 (2006) H684–H691. [DOI] [PubMed] [Google Scholar]
- [3].Benter IF, Yousif MHM, Cojocel C, Al-Maghrebi M, Diz DI, Angiotensin-(1–7) prevents diabetes-induced cardiovascular dysfunction, Am. J. Physiol. Heart. Circ. Physiol 292 (2006) H666–H672. [DOI] [PubMed] [Google Scholar]
- [4].Bosnyak S, Jones ES, Christopoulos A, Aguilar MI, Thomas WG, Widdop RE, Relative affinity of angiotensin peptides and novel ligands at AT1 and AT2 receptors, Clin. Sci. (Lond.) 121 (2011) 297–303. [DOI] [PubMed] [Google Scholar]
- [5].Bosnyak S, Widdop RE, Denton KM, Jones ES, Differential mechanisms of ang (1–7)-mediated vasodepressor effect in adult and aged candesartan-treated rats, Int. J. Hypertens (2012) 1–9, 192567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Brosnihan KB, Li P, Ferrario CM, Angiotensin-(1–7) dilates canine coronary arteries through kinins and nitric oxide, Hypertension 27 (1996) 523–528. [DOI] [PubMed] [Google Scholar]
- [7].Chappell MC, Emerging evidence for a functional angiotesin-converting enzyme 2-angiotensin-(1–7) mas receptor axis; more than regulation of blood pressure? Hypertension 50 (2007) 596–599. [DOI] [PubMed] [Google Scholar]
- [8].Chappell MC, Nonclassical Renin-Angiotensin system and renal function, Compr. Physiol 2 (2012) 2733–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chappell MC, Biochemical evaluation of the renin-angiotensin system: the good, bad, and absolute? Am. J. Physiol. Heart. Circ. Physiol 310 (2016) H137–H152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chappell MC, Brosnihan KB, Diz DI, Ferrario CM, Identification of angiotensin-(1–7) in rat brain: evidence for differential processing of angiotensin peptides, J. Biol. Chem 264 (1989) 16518–16523. [PubMed] [Google Scholar]
- [11].Chappell MC, Jacobsen DW, Tallant EA, Characterization of angiotensin II receptor subtypes in pancreatic acinar AR42J cells, Peptides 16 (1995) 741–747. [DOI] [PubMed] [Google Scholar]
- [12].Chappell MC, Marshall AC, Alzayadneh EM, Shaltout HA, Diz DI, Update on the Angiotensin converting enzyme 2-Angiotensin (1–7)-MAS receptor axis: fetal programing, sex differences, and intracellular pathways, Front. Endocrinol. (Lausanne) 4 (2014) 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Costa A, Galdina G, Romero T, Solya G, Cortes S, Santos R, Diarte O, Ang-(1–7) activates the NO/cGMP and ATP-sensitive K+ channels pathway to induce peripheral anticoiception in rats, Nitric Oxide 31 (2016) 11–16. [DOI] [PubMed] [Google Scholar]
- [14].Heitsch H, Brovkovych S, Malinski T, Wiemer G, Angiotensin-(1–7)-stimulated nitric oxide and superoxide release from endothelial cells, Hypertension 37 (2001) 72–76. [DOI] [PubMed] [Google Scholar]
- [15].Heller J, Kramer HJ, Maly J, Cervenka L, Horacek V, Effect of intrarenal infusion of angiotensin-(1–7) in the dog, Kidney Blood Press. Res 23 (2000) 89–94. [DOI] [PubMed] [Google Scholar]
- [16].Hilchey SD, Bell-Quilley CP, Association between the natriuretic action of angiotensin-(1–7) and selective stimulation of renal prostaglandin I2 release, Hypertension 25 (1995) 1238–1244. [DOI] [PubMed] [Google Scholar]
- [17].Iyer SN, Yamada K, Diz DI, Ferrario CM, Chappell MC, Evidence that prostaglandins mediate the antihypertensive actions of angiotensin-(1–7) during chronic blockade of the renin-angiotensin system, J. Cardiovasc. Pharmacol 36 (2000) 109–117. [DOI] [PubMed] [Google Scholar]
- [18].Kostenis E, Milligan G, Christopoulos A, Sanchez-Ferrer CF, Heringer-Walther S, Sexton PM, Gembardt F, Kellett E, Martini L, Vanderheyden P, Schultheiss HP, Walther T, G-protein-coupled receptor Mas is a physiological antagonist of the angiotensin II type 1 receptor, Circulation 111 (2005) 1806–1813. [DOI] [PubMed] [Google Scholar]
- [19].Lautner RQ, Villela DC, Fraga-Silva RA, Silva N, Verano-Braga T, Costa-Fraga F, Jankowski J, Jankowski V, Sousa F, Alzamora A, Soares E, Barbosa C, Kjeldsen F, Oliveira A, Braga J, Savergnini S, Maia G, Peluso AB, Passos-Silva D, Ferreira A, Alves F, Martins A, Raizada M, Paula R, Motta-Santos D, Klempin F, Pimenta A, Alenina N, Sinisterra R, Bader M, Campagnole-Santos MJ, Santos RA, Discovery and characterization of alamandine: a novel component of the renin-angiotensin system, Circ. Res 112 (2013) 1104–1111. [DOI] [PubMed] [Google Scholar]
- [20].Marangoni RA, Carmona AK, Passaglia RC, Nigro D, Fortes ZB, de Carvalho MH, Role of the kallikrein-kinin system in Ang-(1–7)-induced vasodilation in mesenteric arterioles of Wistar rats studied in vivo-in situ, Peptides 27 (2006) 1770–1775. [DOI] [PubMed] [Google Scholar]
- [21].Neves LA, Averill DB, Ferrario CM, Chappell MC, Aschner JL, Walkup MP, Brosnihan KB, Characterization of angiotensin-(1–7) receptor subtype in mesenteric arteries, Peptides 24 (2003) 455–462. [DOI] [PubMed] [Google Scholar]
- [22].Pendergrass KD, Averill DB, Ferrario CM, Diz DI, Chappell MC, Differential expression of nuclear AT1 receptors and angiotensin II within the kidney of the male congenic mRen2. Lewis rat, Am. J. Physiol. Renal Physiol 290 (2006) F1497–F1506. [DOI] [PubMed] [Google Scholar]
- [23].Ramchandran R, Takezako T, Saad Y, Stull L, Fink B, Yamada H, Dikalov S, Harrison DG, Moravec C, Karnik SS, Angiotensinergic stimulation of vascular endothelium in mice causes hypotension bradycardia, and attenuated angiotensin response, Proc. Natl. Acad. Sci. U. S. A 103 (2006) 19087–19092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ren Y, Garvin JA, Carretero OA, Vasodilator action of angiotensin-(1–7) on isolated rabbit afferent arterioles, Hypertension 39 (2002) 799–802. [DOI] [PubMed] [Google Scholar]
- [25].Salvemini D, Kim SF, Mollace V, Reciprocal regulation of the nitric oxide and cyclooxygenase pathway in pathophysiology: relevance and clinical implications, Am. J. Physiol. Regul. Integr. Comp. Physiol 304 (2013) R473–R487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sampaio WO, dos Santos RA, Faria-Silva R, de Mata Machado EL, Schiffrin EL, Touyz RM, Angiotensin-(1–7) through receptor mas mediates endothelial nitric oxide synthase activation via Akt-dependent pathways, Hypertension 49 (2007) 185–192. [DOI] [PubMed] [Google Scholar]
- [27].Sampaio WO, Nasciment AA, Santos RA, Systemic and regional hemodynamic effects of angiotensin-(1–7) in rats, Am. J. Physiol. Heart Circ. Physiol 284 (2003) H1985–H1994. [DOI] [PubMed] [Google Scholar]
- [28].Santos RA, Angiotensin-(1–7), Hypertension 63 (2014) 1138–1147. [DOI] [PubMed] [Google Scholar]
- [29].Santos RA, Ferreira AJ, Simoes E, Silva AC, Recent advances in the angiotensin-converting enzyme 2-angiotensin(1–7)-Mas axis, Exp. Physiol 93 (2008) 519–527. [DOI] [PubMed] [Google Scholar]
- [30].Santos RA, Haibara AS, Campagnole-Santos MJ, Simoes E, Silva AC, Paula RD, Pinheiro SV, de Fatima LM, Lemos VS, Silva DM, Guerra MT, Khosla MC, Characterization of a new selective antagonist for angiotensin-(1–7), D-pro7-angiotensin-(1–7), Hypertension 41 (2003) 737–743. [DOI] [PubMed] [Google Scholar]
- [31].Santos RAS, Campagnole-Santos MJ, Baracho NCV, Fontes MAP, Silva LCS, Neves LAA, Oliveir DR, Caligiorne SM, Rodrigues ARV, Gropen C Jr., Carvalho WS, Silva ACSE, Khosla MC, Characterization of a new angiotensin antagonist selective for angiotensin-(1–7): evidence that the actions of angiotensin-(1–7) are mediated by specific angiotensin receptors, Brain Res. Bull 35 (1994) 293–398. [DOI] [PubMed] [Google Scholar]
- [32].Tang L, Carey LC, Bi J, Valego N, Sun X, Deibel P, Perrott J, Figueroa JP, Chappell MC, Roe JC, Gender differences in the effects of antenatal betamethasone exposure on renal function in adult sheep, Am. J. Physiol. Regul. Integr. Comp. Physiol 296 (2009) R309–R317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Toda N, Ayajiki K, Okamura T, Interaction of endothelial nitric oxide and angiotensin in the circulation, Pharmacol. Rev 59 (2007) 54–87. [DOI] [PubMed] [Google Scholar]
- [34].Tsutsumi Y, Matsubara H, Masaki H, Kurihara H, Murasawa S, Takai S, Miyazaki M, Nozawa Y, Ozono R, Nakagawa K, Miwa T, Kawada N, Mori Y, Shibasaki Y, Tanaka Y, Fujiyama S, Koyama Y, Fujiyam A, Takahashi H, Iwasaka T, Angiotensin II type 2 receptor overexpression activates the vascular kinin system and causes vasodilation, J. Clin. Invest 104 (1999) 925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Villela D, Leonhardt J, Patel N, Joseph J, Kirsch S, Hallberg A, Unger T, Bader M, Santos R, Sumners C, Stecklings UM, Angiotensin type 2 receptor and receptor Mas: a complex liason, Clin. Sci 128 (2015) 227–234. [DOI] [PubMed] [Google Scholar]
- [36].Walters PE, Gaspari TA, Widdop RE, Angiotensin-(1–7) acts as a vasodepressor agent via angiotensin II type 2 receptors in conscious rats, Hypertension 45 (2005) 960–966. [DOI] [PubMed] [Google Scholar]
- [37].Wiemer G, Dobrucki LW, Louka FR, Malinsk T, Heitsch H, AVE 0991, a nonpeptide mimic of the effects of angiotensin-(1–7) on the endothelium, Hypertension 40 (2002) 847–852. [DOI] [PubMed] [Google Scholar]
- [38].Yang RF, Yin JX, Zimmerman MC, Schultz HD, Angiotensin-(1–7) increases neruonal potassium current via a nitric oxide-dependent mechanism, Am. J. Physiol.—Cell Physiol (2011) C58–C64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].YousiF MH, Kehinde EO, Bente IF, Different responses to angionteisn-(1–7) in young, aged and diabetic corpus cavernosum, Pharmacol. Res 56 (2007) 209–2016. [DOI] [PubMed] [Google Scholar]
- [40].Yousif MH, Dhaunsi GS, Makki BM, Qabazard BA, Akhtar S, Benter IF, Characterization of Angiotensin-(1–7) effects on the cardiovascular system in an experimental model of type-1 diabetes, Pharmacol. Res 66 (2012) 269–275. [DOI] [PubMed] [Google Scholar]