Abstract
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease that typically results in death within 3–5 years after diagnosis. To date, there is no curative treatment and therefore an urgent unmet need of neuroprotective and/or neurorestorative treatments. Due to their spectrum of capacities in the central nervous system—e.g., development, plasticity, maintenance, neurogenesis—neurotrophic growth factors (NTF) have been exploited for therapeutic strategies in ALS for decades. In this review we present the initial strategy of using single NTF by different routes of administration to the use of stem cells transplantation to express a multiple NTFs-rich secretome to finally focus on a new biotherapy based on the human platelet lysates, the natural healing system containing a mix of pleitropic NTF and having immunomodulatory function. This review highlights that this latter treatment may be crucial to power the neuroprotection and/or neurorestoration therapy requested in this devastating disease.
Keywords: Amyotrophic lateral sclerosis, growth factors, therapeutic, stem cell, human platelet lysate
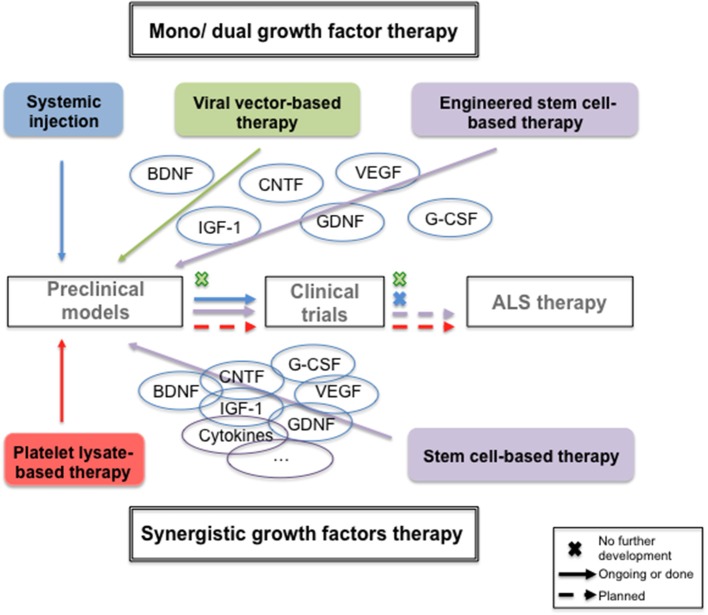
Graphical Abstract.
From single to synergistic neurotrophic growth factors therapies.
Introduction
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease affecting the upper and lower motor neurons in the cerebral cortex, brainstem and spinal cord that lead to a progressive, irreversible muscle paralysis, and swallowing and respiratory dysfunctions. Death eventually occurs 3–5 years after diagnosis (1). The majority of ALS cases (90%) are sporadic with unknown cause (2). To date, there is no curative treatment in ALS. Therefore, the development of new and effective treatment is highly urgent. Among the different approaches, the delivery of neurotrophic factors (NTFs) is explored since the 90's because NTFs are necessary to regulate several physiological processes such as neuronal differentiation and survival, axonal outgrowth and synapses maintenance (3–5), proliferation and differentiation of stem cells in the nervous system (6–9). Therefore, these trophic factors represent a promising therapeutic strategy to treat neurodegenerative diseases (10) such as ALS.
Preclinical Evidence of Neurotrophics Growth Factors Abilities to Treat Amyotrophic Lateral Sclerosis (Table 1)
Table 1.
Different routes of NTFs delivery and therapies in pre-clinical models.
| NTF | Delivery route | Model | Outcomes | References |
|---|---|---|---|---|
| RECOMBINANT NEUROTROPHIC GROWTH FACTORS | ||||
| CNTF | I.P | pmn/pmn mice (20–21 d) | MP+, S+ | (11) |
| S.C | Wobbler mice | MP+, MC+ | (12–14) | |
| BDNF | S.C | Wobbler mice | MP+ | (14) |
| GDNF | S.C | pmn/pmn mice (15–18 d) | No effect | (15) |
| VEGF | I.P | SOD1G93A mice (74 d) | MP+, DDO+, S+11 d | (16) |
| I.C.V | SOD1G93A/LSd rats (60 d) | MP+, DDO+, S+10 d | (17) | |
| I.S.P | Excitotoxic model in rats | MP+, DDO+, S+10.5 d, +5 d | (18, 19) | |
| Viral vector based gene therapy | ||||
| AAV-NTF | ||||
| IGF-1 | I.M | SOD1G93A mice (90 d) | MP+, S+22 d | (20) |
| I.S.P | SOD1G93A mice (60 d) | MP+, DDO+, S+12.3 d ♂ | (21) | |
| In D.C.N | SOD1G93A mice (88–90 d) | MP+, S+14 d | (22) | |
| I.M | SOD1G93A mice (60 and 90 d) | MP+, DDO+, S+29 d and +15 d ♂, +24 d and +14 d ♀ | (23) | |
| I.V | SOD1G93A mice (90 d) | MP+, S+10 d | (24) | |
| I.C.V | SOD1G93A mice (80–90 d) | DDO+, S+12 d | (25) | |
| VEGF | I.C.V | SOD1G93A mice (80–90 d) | DDO+, S+9 d ♂, +20 d ♀ | (25) |
| I.T | SOD1G93A mice (90 d) | DDO+, S+12 d | (26) | |
| GDNF | I.M | SOD1G93A mice (90 d) | MP+, DDO+, S+16.6 d | (27) |
| I.V | SOD1G93A rats (25 d) | MP +/–, S– | (28) | |
| G-CSF | I.S.P | SOD1G93A mice (70 d) | MP+, DDO+, S+ | (29) |
| Stem cell based therapy | ||||
| AAV-NTF | ||||
| hSC-NSC | I.S.P | SOD1G93A rats (56–62 d) | MP+, DDO+, S+11 d | (30, 31) |
| gm hNSC line (VEGF) | I.T | SOD1G93A mice (70 d) | DDO+, S+12 d | (32) |
| hSC-NPC | I.S.P | SOD1G93A mice (40 d) | MP+, S+5 d | (33) |
| gm hNPC (GDNF) | I.S.P | SOD1G93A rats (~80 d) rats (~80 d) | MP–, S– | (34, 35) |
| Cortex | SOD1G93A rats (~80 d) macaques | DDO+, S+14 d | (36) | |
| hBM-MSC | I.S.P | SOD1(G93A)dl mice (28 w) | MP+ | (37) |
| SOD1G93A mice | MP+ | (38) | ||
| mBM-MSC | I.V | SOD1G93A mice (90 d) | MP+, S+17.3 d | (39) |
| gm hBM-MSC (GDNF, VEGF, GNDF/IGF-1, BDNF) | I.M | SOD1G93A rats (80 d) | MP+, S+28 d and +18 d for GDNF, + 13 d for VEGF, +28 d for GDNF/VEGF | (40, 41) |
| mBM | I.S.P and I.M | mdf/ocd mice (6 weeks) | MP+ | (42, 43) |
| mASC | I.V | SOD1G93A mice (76–77 d) | MP+, S– | (44) |
| hASC | I.V and I.C.V | SOD1G93A mice (70 d) | MP+, DDO+, S+ | (45) |
| hUCBC | I.V | SOD1G93A mice (56 d, 66 d) | DDO+, S+21 d, +38.5 d, +23.8 d | (46–48) |
| SOD1G93A mice (60 and 90 d) | MP+, S+10 d | (49) | ||
| I.T | SOD1G93A mice | No effect | (50) | |
| I.S.P | SOD1G93A mice (40 and 90 d) | MP+, S+6 d for 40 d mice | (51) | |
| I.C.V | SOD1G93A (70 d) Wobbler mice (28 d) | MP+, S+18 d MP+ | (52) | |
| gm hUCBC (VEGF, GDNF, and/or NCAM) | I.V | SOD1(G93A)dl mice | MP+, S+ | (53, 54) |
I.P, intraperitoneal; I.M, intramuscular; I.V, intravenous; I.C.V, intracerebrovascular; I.S.P, intraspinal; I.T, intrathecal; S.C, subcutaneous; DCN, deep cerebellar nuclei; gm, genetically modified for expression of NTFs in brackets; hSC-NSC: human spinal cord-neural stem cell; m/hBM-MSC, murine/human bone marrow-mesenchymal stem cell; m/hASC, murine/human adipose derived MSC; hUCBC, human umbilical cord blood cells. Main results are summarized as follow: MP, motor performance; DDO, delay of disease onset; S, survival. The age of the model at the treatment is noted in brackets (d, days old; w, weeks). +, improvement; –, deterioration. ♂, male; ♀, female.
Recombinant NTFs Delivery by Injection
Some trophic factors have been demonstrated to promote cell survival and be protective in both in vitro and in vivo models of neuronal degeneration: Ciliary Neurotrophic Factor (CNTF), Brain-derived Neurotrophic Factor (BDNF), Glial-Derived Neurotrophic Factor (GDNF), Insulin-like Growth Factor 1 (IGF-1), Vascular Endothelial Growth Factor (VEGF), and Granulocyte-Colony Stimulating Factor (G-CSF). In vivo experiments performed in ALS models using single recombinant growth factors are described in this section.
CNTF, one of the first NTF studied in ALS models, injected intraperitonally in pmn/pmn mice, mouse model for human spinal motor neuron disease (11) or subcutaneously in wobbler mice (12) improved motor function and survival, and decreased neuronal degeneration and muscle atrophy (13). In addition, Mitsumoto et al. demonstrated a synergic effect of CNTF and BDNF, respectively, to arrest disease progression for 1 month (14).
The fusion protein BDNF with the c fragment of the tetanus toxin (BDNF-TTC) exhibited enhanced neuroprotective effect in SOD1G93A ALS mice model, but no synergic effect was observed compared to TTC alone (55). Recently, motor function improvement and less neuronal loss were observed in SOD1G93A mice treated with the flavonoid 7,8-dihydroxyflavone, a small-molecule mimicking the effect of BDNF (56). Two receptors binding the BDNF, p75NTR and TrkB.T1, were highlighted in SOD1G93A: a decreased of p75NTR expression correlated with a delay of mortality and motor impairment (57); a deletion of the TrkB.T1 increased survival and delayed motor deficit (58).
Treatment with encapsuled GDNF-secreting cells in pmn/pmn mice did not impact motor neuron degeneration and lifespan (15). The authors suggest a combined treatment for GDNF with others NTFs. Recently, astrocytic GDNF triggered by the tumor necrosis factor α (TNFα) was highlighted in the SOD1G93A mice, and found to limit motor neuron degeneration and disease progression (59).
Intraperitoneal (16) or intracerebroventricular (17) injection of VEGF at doses of 1 g/kg/d and 0.2 μg/kg/d in SOD1G93A mice and rats, respectively, increased lifespan and improved motor performance. Similar data were observed in a sporadic model of ALS rats induced by excitotoxic administration of AMPA (60, 61).
Finally, protective properties of G-CSF were observed in SOD1G93A mice when delivered continuously at dose of 30 μg/kg/d (18). Indeed, disease progression was reduced and survival increased by rescuing motoneurons. Similar results were obtained with subcutaneous injection of pegfilgrastim, a more stable analog of G-CSF (19).
As protein infusion has known drawbacks (invasive method of delivery, protein stability over time, short half-life) others strategies, such as viral vector-based gene therapy and stem cell-based therapy have been developed to express NTFs of interest and avoid chronic injection.
NTFs Delivery by Viral Vector-Based Gene Therapy
Many studies focused on IGF-1. The intramuscular injection of adeno-associated viral (AAV)-IGF-1 in SOD1G93A mice before or at the time of disease symptoms delayed disease onset and increased lifespan (20). Intraparenchymal spinal cord delivery was also tested, showing higher expression of IGF-1 but partial rescue (21), whereas a stereotaxic injection into the deep cerebellar nuclei significantly extended mice lifespan (22). Recently the injection of self-complementary adeno-associated viral vector 9 (scAAV9), a more efficient transducing agent for IGF-1, extended survival, and motor performance of SOD1G93A mice when injected either intramuscularly (23) or intravenously (24). Also, the intracerebroventricular injection of AAV4-VEGF was studied and gave similar results than AAV4-IGF-1 by slowing disease progression. No combined effect of these 2 constructions was observed in SOD1G93A mice (25). Similarly the intrathecal injection of scAAV9-VEGF showed positive impact on lifespan and motor performance in mice (26). The AAV-GDNF, injected intramuscularly in SOD1G93A allowed expression of the protein at the sites of injection, a retrograde transport in anterior horn neurons, and was associated with a delay in the onset and the progression of the disease (27). However, the systemic injection of AAV9-GDNF in SOD1G93A rats showed limited functional improvement and no survival extension (28). Finally the efficacy of intraspinal delivery was showed for AAV-G-CSF in SOD1G93A mice with minimal systemic effects (29).
NTFs Delivery by Stem Cell-Based Therapy
Different types of stem cells exist—based on their source, clonogenic capacity, differentiation potential and availability—and exert a paracrine effect, suitable for therapy in neurodegenerative disease such the ALS (62–65). We mainly focus here on stem cells with potential clinical application, engineered or used as such, e.g., a mix of NTFs.
Neuroprotection With Neural Stem Cells (NSC) and Neural Progenitor Cells (NPC)
Human NSC graft into lumbar protuberance of SOD1G93A rats was shown to delay the onset and the progression of the disease, with their integration into the spinal cord (30, 31). Similarly, the intraspinal administration of human NPC delayed the progression of the disease in SOD1G93A mice (33).
NSC were also engineered to secrete specific one. Intrathecal transplantation of human NSC overexpressing VEGF in SOD1G93A mice delayed the onset of the disease and increased survival with an integration and differentiation of NSC-VEGF into the spinal cord (32). Human neural progenitor cells NPC (hNPC) were also genetically modified to secrete GDNF. The transplantation of such engineered cells in SOD1 rats were integrated into the spinal cord, limited motoneuron degeneration but failed to improve motor function (34, 35). However, the transplantation of hPNC-GDNF into the cortex extended the survival of SOD1G93A rats and was safe for primates (36).
Mesenchymal Stromal Cells (MSC)
Bone marrow (BM) MSC (BM-MSC), when injected intraspinally (37, 38) or intravenously (39) in SOD1G93A mice, allowed decreased motoneurons degeneration, improved survival and motor function, prevented pro-inflammatory factors. Indeed, MSC display immunomodulatory properties by secreting anti-inflammatory cytokines such as TGF-β or IL-10 (66) Since neuroinflammatory markers were detected in neural tissues of ALS patients (67) promising results can be expected with MSC based therapy. Moreover, intramuscular transplantation of human BM-MSC genetically modified to secrete GDNF in SOD1G93A rats, showed a decrease in motoneuron loss and an overall increased lifespan (40). In addition they demonstrated a synergic effect of the combined intramuscular delivery of hMSC-GDNF and hMSC-VEGF with an increased survival, protection of neuromuscular junction and motoneuron degeneration, greater than either growth factor delivered individually (41). Even though human BM-MSC injections have positive effects on the disease progression, it should be noted that the whole BM intraspinally transplanted showed a greater improvement of motor functions than BM-MSC in mdf/ocd mice (42) and increased motoneurons survival when intramuscularly transplanted (43).
Others reported positive results with adipose derived MSC when administrated by systemic (44), or intracerebroventricular administration (45).
Human Umbilical Cord Blood (hUCB)
The first study performed on SOD1G93A mice irradiated and transplanted intravenously with hUBC mononuclear cells (MNC), showed a delay in the onset of symptoms and increased the survival (46, 47). Transplanted cells integrated regions of motoneuron degeneration and expressed neural markers (48). Recently, the efficiency of chronic intravenous injections of UCB MNC in symptomatic SOD1G93A mice was demonstrated, with increased lifespan and reduced inflammatory effectors (49). Similarly, the intraspinal as the intracerebroventricular injection of hUCB in pre-symptomatic SOD1G93A or wobbler mice increased survival and motor performance (51, 52). However, intrathecal administration of hUCB did not affect the lifespan of motor function of ALS mice (50).
Some authors engineered hUCB MNC to secrete some NTFs or to enhance homing at the site of degeneration (68, 69). Recently, transplanted hUCB transduced with AAV encoding VEGF, GDNF and/or neural cell adhesion molecule (NCAM), led to a high rate of SOD1G93A mice survival and improved motor function. Moreover, transplanted cells were detected 1 month after grafting into the lumbar spinal cord (53, 54).
Clinical Trials With Growth Factors: Evidence and Hypothesis for the Failure
Regarding the promising effects obtained in ALS animal models, clinical trials were conducted to examine the neuroprotective effects of these growth factors therapies in ALS patients (Table 2).
Table 2.
Clinical trials with growth factors.
| NCT number | NTF | Delivery method | Phase and status of the trial | Cohort size | Outcomes | References | Year |
|---|---|---|---|---|---|---|---|
| PROTEIN INFUSION | |||||||
| Not provided | CNTF | SC | Phase I, terminated | 57 | No adverse neurologic effects, safe, and tolerated | (70) | 1995 |
| Not provided | SC | Phase I, terminated | 570 | No beneficial effect, adverse events dose related, increased number of death at the highest dose, no beneficial effect on ALS progression | (71) | 1996 | |
| Not provided | SC | Phase II/III | 730 | Disease progression not modified, minor adverse side effects | (72) | 1996 | |
| Not provided | IT | Phase I | 4 | Pain syndromes dose-related, no systemic side effect, no improvement, or worsen of motor function | (73) | 1997 | |
| Not provided | BDNF | SC | Phase I/II, terminated | 283 | Tolerated, Trend of improved survival, less deterioration of predicted FVC | (74) | 1995 |
| Not provided | BDNF | SC | Phase III | 1 135 | Disease progression not modified, Patients with early respiratory impairment and with altered bowel function showed benefit | (75) | 1999 |
| Not provided | BDNF | IT | Phase I/II, terminated | 25 | Well tolerated, feasible | (76) | 2000 |
| Not provided | BDNF | IT | Phase III, terminated | 17 | No adverse events, no effect | (77) | 2003 |
| Not provided | BDNF | IT | Phase II/III, terminated | 13 | No effect | (78) | 2005 |
| Not provided | IGF-1 | SC | Not specify | 266 | Slowed the progression of functional impairment, slow the decline in health-related quality of life | (79) | 1997 |
| Not provided | SC | Not specify | 183 | Safe and well-tolerated, no effect | (80) | 1998 | |
| Not provided | IT | Not specify | 9 | No serious adverse effect, modest beneficial effect | (81) | 2005 | |
| NCT00035815 | SC | Phase III, completed | 330 | No benefit | (82) | 2008 | |
| Not provided | G-CSF | SC | Phase I, terminated | 13 | Safe, less decline of ALSFRS score | (83) | 2009 |
| Not provided | SC | Phase I, terminated | 39 | Safe, no significative effect on ALSFRS score | (84) | 2010 | |
| NCT00397423 | Not specify | Phase II, completed | 40 | Not available | |||
| NCT01999803 | VEGF | ICV | Phase I, terminated | 15 | Not available | ||
| NCT02269436 | ICV | Phase I, terminated | 11 | Not available | |||
| NCT01384162 | ICV | Phase I/II, terminated | 15 | Not available | |||
| STEM CELLS | |||||||
| NCT number | Type of stem cells | Delivery method | Phase and status of the trial | Cohort size | Results | References | Year |
| NCT01348451 | NSC | ISP | Phase I | 12 | No major adverse events | (85, 86) | 2012 |
| NCT01730716 | NSC | ISP | Phase II, unknown status | 18 | Not available | ||
| NCT02943850 | NPC | ISP | Phase I/IIa, active, not recruiting | 18 | Not available | ||
| NCT01640067 | NSC | ISP | Phase I, completed | 6 | Safe approach, no increase of disease progression | (87) | 2015 |
| NCT00781872 | MSC | IT, IV | Phase I/II, terminated | 19 | Safe and feasible, ALS-FRS score stable the first 6 months | (88) | 2010 |
| NCT03085706 | PBMC | ISP | Phase NA, completed | 14 | Not available | ||
| NCT01933321 | HSC | IT | Phase II/III, completed | 14 | Not available | ||
| NCT01609283 | MSC | IT | Phase I, active, not recruiting | 27 | Not available | ||
| NCT01142856 | MSC | IT | Phase I, completed | 1 | Not available | ||
| NCT00855400 | MSC | ISP | Phase I/II completed | 11 | No severe adverse event, no acceleration in the rate of decline, possible neurotrophic activity | (89) | 2012 |
| NCT02286011 | MC | IM | Phase I, active, not recruiting | 20 | Not available | ||
| NCT00855400 | MC | ISP | Phase I, completed | 11 | Safe approach, no worsening of the disease | (90) | 2016 |
| NCT03268603 | MSC | IT | Phase II, recruiting | 60 | Not available | ||
| NCT01254539 | MSC | ISP, IT | Phase I/II, completed | 63 | Infusion of MSC produces spinal changes unrelated with clinical events and disease worsening | (91) | 2013 |
| NCT01363401 | MSC | IT | Phase I/II, completed | 64 | Possible benefit lasting at least 6 months with safety | (92) | 2018 |
| NCT02917681 | MSC | IT | Phase I/II, recruiting | 28 | Not available | ||
| NCT02987413 | MSC | IT | Phase I, completed | 3 | Not available | ||
| NCT02290886 | MSC | IV | Phase I/II, active, not recruiting | 52 | Not available | ||
| NCT01051882 | MSC | IM or IT | Phase I/II, completed | 12 | Safe and tolerated, no serious adverse event, possible benefits on ALS-FRS score, and percentage of FVC | (93) | 2016 |
| NCT01777646 | MSC | IM + IT | Phase IIa, completed | 14 | |||
| NCT03280056 | MSC | IT | Phase III, Recruiting | 200 | Not available | ||
| NCT02017912 | MSC | IM, IT | Phase II, completed | 48 | Not available | ||
| NCT01759797 | MSC | IV | Phase I/II, completed | 6 | No adverse events, ALS-FRS score reduced, FVC percentage reduced | (94) | 2019 |
| NCT01771640 | MSC | IT | Phase I, completed | 8 | |||
FVC, force vital capacity; HSC, hematopoietic stem cells; I, intramuscular; ISP, intraspinal; IT, intrathecal; IV, intravenous; MC, mononuclear cell; MSC, mesenchymal stem cells; NPC, neuronal progenitor cells; NSC, neural stem cells; NTF, neurotrophic factor, PBMC, peripheral blood mononuclear cell; SC, subcutaneous.
Trials Involving NTFs Protein Systemic Injections
CNTF
In 90's the ALS CNTF Treatment study group published results obtained in phase I (70) and phase II/III (72) clinical trials where enrolled patients received subcutaneous administration of recombinant human CNTF (rHCNTF) at different doses, 15 or 30 μg/kg, three times a week for 9 months. The phase II/III randomized, placebo-controlled evaluated the safety, tolerability, and efficacy. No statistically difference between rHCNTF-treated patients and placebo-treated patients were observed and side effects were sufficiently severe to limit dosing in many patients. A second trial, same year, did not show any positive effect either (71).
One year later, Penn et al. published results of a phase I clinical trial with intrathecal pump delivery (73). The disease progression was not modified either but no systemic side effects were observed. Thus, intrathecal administration may be the preferred route of administration. To our knowledge, no further clinical study are under investigation.
BDNF
Due to a promising phase I/II clinical trial showing the safety and efficacy of subcutaneous administration of BDNF in 1995, a phase III was designed (74). Results failed to demonstrate an effect on survival but post-hoc analyses showed that those ALS patients with early respiratory impairment showed benefit (75). One year later a phase I trial showed the feasibility of intrathecal method of delivery (76) but two other trials conducted in 2003 and 2005 felt to detect any efficacy (77, 78).
IGF-1
In the late 90's, two clinical trials used IGF-1 at a dose of 0.1 mg/kg/d by subcutaneous delivery and found contradictory and opposite results (79, 80). In 2008, a phase III showed no benefit of this route of delivery in 2 years of trials (82). In a pilot study conducted in 2005, intrathecal administration had beneficial effect using high doses of IGF-1 (3 μg/kg every 2 weeks) but it was not placebo-controlled (81).
G-CSF
Ten years ago, two pilot clinical trials with subcutaneous G-CSF administration at a dose of 5 μg/kg/d reported a trend for slowing down the disease progression (84) and a delay in motor decline (83). A Phase II clinical trial is under investigation but results are not yet available.
VEGF
Three clinical trials assessed the safety, tolerability, and the possible motor function improvement as well as survival time of the intracerebroventricular administration of 4 μg/d VEGF. To our knowledge, no results are published.
6- Failure Hypothesis
Most of the clinical trials based on direct protein administration gave disappointing outcomes in view of the promising preclinical results. Different hypotheses can be raised to explain those failures (70–84):
- The route of administration: subcutaneous injection seems less efficient than the intrathecal one
- The minimal ability of these growth factors to cross the blood brain barrier
- The dose: highest safe dose in humans can be lower than those determined in animals, as the clinical trial with CNTF demonstrated
- The treatment start time: in animals, treatment start before the onset of the disease whereas in humans the diagnosis is performed at later stage
- The need of synergic association of numerous neurotrophic factors
Trials Involving Adeno-Associated Viral Gene Therapy
To our knowledge, there is no reported clinical trial using adeno-associated viral gene therapy despite promising results obtained with SOD1G93A mice. AAV2 and AAV9 are vectors having the greatest potential, one specific for neuron tissue, one passing the blood brain barrier and exhibiting neuronal tropisms, respectively. One of the drawbacks of genes therapies for ALS can be the safety. Indeed to stop delivery will not be possible if serious adverse events occur during the treatment.
Trials Involving Stem Cell Therapy
Twenty-two trials involving stem cells-based therapy are registered on ClinicalTrials.gov. Most of them use MSC from different origins and few have results available. This section is an overview of all the known clinical trials.
Neural Stem Cells
In 2012, two trials sponsored by Neuralstem used NSC by intraspinal injection. The phase I did not show any adverse events (85, 86), but the phase II has an unknown status on the ClinicalTrials.gov website.
Recently, published results of a phase I trial, proposing transplantation of human NSCs into the lumbar spinal cord, demonstrated the safety and reproducibility of this cell therapy. Moreover, because the brain tissue used was from natural miscarriages, ethical concerns may be eliminated (87). An ongoing clinical trial concern neuronal progenitor cells engineered to produce GDNF. This is a phase I/IIA trial, active but not recruiting. No results are available for now.
Blood Cells
Two clinical trials, one using autologous peripheral blood mononuclear cell for intraspinal transplantation and one in phase II/III using hematopoietic stem cells for intrathecal injection were conducted and completed but no results were reported to our knowledge. One trial using autologous bone marrow mononuclear cells (90) for intraspinal injection showed the safety of the procedure.
Mesenchymal Stromal Cells
Among 14 clinical trials using MSCs from diverse origin such as bone marrow, adipose tissue or engineered to secrete particular NTFs, through diverse types of delivery (intrathecal, intraspinal, intramuscular, intravenous, or intraventricular), 5 have no published results, 4 are ongoing, and 5 are completed with published results. All of them are listed in the Table 2 and the last 5 are detailed below and involved the use of the bone marrow derived MSCs.
In 2012, a phase I/II, using autologous bone marrow MSCs administered by intraspinal delivery, was conducted. No severe adverse event were observed, no acceleration of the disease progression noticed and an increase of the motoneurons in the treated segments compared with the untreated segments for patients who died for unrelated reasons to the procedure. Thus, this trial demonstrates the safety of intraspinal infusion of MSCs and suggests their neurotrophic activity (89). In 2013, a phase I/II confirmed the safety of BM-MSC infusion (91).
In 2016, two clinical trials in small groups of patients, phase I/II, used bone marrow MSCs engineered to secrete NTFs. Intramuscular transplantation for early ALS patients and intrathecal transplantation for progressive ALS patients were evaluated. They concluded that both route of administration are safe and provide indications of possible clinical benefits that need to be confirmed on a bigger cohort (93).
In 2018, a phase I/II trial was initiated to evaluate the safety and efficacy of these cells through intrathecal delivery. A possible benefit seems to last at least 6 months with apparent safety (92). A phase II is required to evaluate long-term efficacy and safety.
Finally, recent phase I/II trials showed safety and feasibility of intravenous and intrathecal transplantation of autologous bone marrow MSCs (94). Indeed, no adverse events were reported and the ALS-FRS score and the force vital capacity percentage were significantly reduced. Additional trials with bigger cohort are needed.
To conclude, stem cells-based therapy as a future therapy to treat ALS patients is premature due to the lack of results. As for the protein infusion, some questions need to be considered:
- The delivery method
- The timing of intervention
- The number of cells to transplant to obtain a therapeutic efficacy
- The capacity of transplanted cells to migrate to the area of interest and to mature in the hostile environment
- The evaluation of the long-term efficacy
Nevertheless, trophic factors remain essential for neuronal maintenance and survival and remain a promising candidate to treat ALS patients. Another source of those factors can be the natural healing system, namely the platelet lysate, and a continuous infusion into the brain by intracerebroventricular (ICV) injection can be a route of administration, avoiding the potential problem with the blood brain barrier crossing.
How to Improve Growth Factors Therapeutics in ALS: a New Therapeutic Approach Based on the Human Platelet Lysate
The lack of clinical efficacy of single NTF infusion, despite a good diffusion, required increasing the dose to a point where they finally induced poor tolerance (i.e., μg). A single NTF was therefore unable to induce the complex set of signaling pathways required to promote efficient neuroprotection. Platelets constitute abundant, natural sources of physiological balanced mixtures of many growth factors [e.g., Platelet Derived Growth Factor (PDGF), VEGF, IGF-1, EGF, or TGFβ) (95) and are used to enhance wound healing and tissue repair (96). In addition, they express adhesion molecules, secret chemokines (97) giving thus neuroinflammatory property to the platelate lysate that could be of an additional interest in ALS therapy. Interestingly, it was demonstrated that ICV injection of human platelet lysates significantly reduced infarct volumes in rats with permanent middle cerebral artery occlusion, improved motor function and promoted endogenous neural stem cells proliferation (98). Similar results were obtained with platelet rich plasma in ischemic rats (99). Moreover, intranasal (IN) administration of platelet lysates was demonstrated to be neuroprotective in Alzheimer and Parkinson's disease animal models (100, 101). To pursue with the neuroprotective potential of platelets lysate in neurodegenerative diseases, we developed a heated low protein human purified platelet lysate (HPPL) preparation, compatible with ICV and IN intermittent administration, to deplete fibrinogen, avoid thrombogenic, and proteolytic activities. We demonstrated its neuroprotective effect in in vitro and in vivo model of Parkinson's disease and its anti-inflammatory properties (102). To extend the concept to ALS, HPPL was tested on a motoneuron-like model and strongly protected from apoptosis and oxidative stress (103). Higher neuroprotection was obtained with HPPL compare to single growth factor or combination of 4 (PDGF, BDNF, BFGF, VEGF) and involved specific signaling pathway such as Akt and MEK (103). These results give a real hope for neuroprotective therapy and need to be confirmed in in vivo ALS model with ICV or IN administration of HPPL.
Author Contributions
FG and A-SR wrote the manuscript. J-CD, TB, and DD critically revised the manuscript. All authors read and approved the submitted version.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. The authors wish to acknowledge support from the ARSLA charity (Association pour la Recherche sur la Sclérose Latérale Amyotrophique et autres maladies du motoneurone), I-SITE-ULNE Foundation, Credit Agricole Foundation, and SATT NORD.
References
- 1.Wijesekera LC, Leigh PN. Amyotrophic lateral sclerosis. Orphanet J Rare Dis. (2009) 4:3. 10.1186/1750-1172-4-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taylor JP, Brown RH, Cleveland DW. Decoding ALS: from genes to mechanism. Nature. (2016) 539:197–206. 10.1038/nature20413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Korsching S. The neurotrophic factor concept: a reexamination. J Neurosci. (1993) 13:2739–48. 10.1523/JNEUROSCI.13-07-02739.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boonman Z, Isacson O. Apoptosis in neuronal development and transplantation: role of caspases and trophic factors. Exp Neurol. (1999) 156:1–15. 10.1006/exnr.1999.7056 [DOI] [PubMed] [Google Scholar]
- 5.Hou ST, Jiang SX, Smith RA. Permissive and repulsive cues and signalling pathways of axonal outgrowth and regeneration. Int Rev Cell Mol Biol. (2008) 267:125–81. 10.1016/S1937-6448(08)00603-5 [DOI] [PubMed] [Google Scholar]
- 6.Lamballe F, Smeyne RJ, Barbacid M. Developmental expression of trkC, the neurotrophin-3 receptor, in the mammalian nervous system. J Neurosci. (1994) 14:14–28. 10.1523/JNEUROSCI.14-01-00014.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tuttle R, O'Leary DD. Neurotrophins rapidly modulate growth cone response to the axon guidance molecule, collapsin-1. Mol Cell Neurosci. (1998) 11:1–8. 10.1006/mcne.1998.0671 [DOI] [PubMed] [Google Scholar]
- 8.Lo DC. Instructive roles of neurotrophins in synaptic plasticity. Prog Brain Res. (1998) 117:65–70. 10.1016/S0079-6123(08)64008-X [DOI] [PubMed] [Google Scholar]
- 9.Cattaneo E, McKay R. Proliferation and differentiation of neuronal stem cells regulated by nerve growth factor. Nature. (1990) 347:762–5. 10.1038/347762a0 [DOI] [PubMed] [Google Scholar]
- 10.Kotzbauer PT, Holtzman DM. Expectations and challenges in the therapeutic use of neurotrophic factors. Ann Neurol. (2006) 59:444–7. 10.1002/ana.20794 [DOI] [PubMed] [Google Scholar]
- 11.Sendtner M, Schmalbruch H, Stöckli KA, Carroll P, Kreutzberg GW, Thoenen H. Ciliary neurotrophic factor prevents degeneration of motor neurons in mouse mutant progressive motor neuronopathy. Nature. (1992) 358:502–4. 10.1038/358502a0 [DOI] [PubMed] [Google Scholar]
- 12.Mitsumoto H, Ikeda K, Holmlund T, Greene T, Cedarbaum JM, Wong V, et al. The effects of ciliary neurotrophic factor on motor dysfunction in wobbler mouse motor neuron disease. Ann Neurol. (1994) 36:142–8. 10.1002/ana.410360205 [DOI] [PubMed] [Google Scholar]
- 13.Ikeda K, Wong V, Holmlund TH, Greene T, Cedarbaum JM, Lindsay RM, et al. Histometric effects of ciliary neurotrophic factor in wobbler mouse motor neuron disease. Ann Neurol. (1995) 37:47–54. 10.1002/ana.410370110 [DOI] [PubMed] [Google Scholar]
- 14.Mitsumoto H, Ikeda K, Klinkosz B, Cedarbaum JM, Wong V, Lindsay RM. Arrest of motor neuron disease in wobbler mice cotreated with CNTF and BDNF. Science. (1994) 265:1107–1110. 10.1126/science.8066451 [DOI] [PubMed] [Google Scholar]
- 15.Sagot Y, Tan SA, Hammang JP, Aebischer P, Kato AC. GDNF slows loss of motoneurons but not axonal degeneration or premature death of pmn/pmn mice. J Neurosci. (1996) 16:2335–41. 10.1523/JNEUROSCI.16-07-02335.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng C, Nennesmo I, Fadeel B, Henter J-I. Vascular endothelial growth factor prolongs survival in a transgenic mouse model of ALS. Ann Neurol. (2004) 56:564–7. 10.1002/ana.20223 [DOI] [PubMed] [Google Scholar]
- 17.Storkebaum E, Lambrechts D, Dewerchin M, Moreno-Murciano M-P, Appelmans S, Oh H, et al. Treatment of motoneuron degeneration by intracerebroventricular delivery of VEGF in a rat model of ALS. Nat Neurosci. (2005) 8:85–92. 10.1038/nn1360 [DOI] [PubMed] [Google Scholar]
- 18.Pitzer C, Krüger C, Plaas C, Kirsch F, Dittgen T, Müller R, et al. Granulocyte-colony stimulating factor improves outcome in a mouse model of amyotrophic lateral sclerosis. Brain. (2008) 131:3335–47. 10.1093/brain/awn243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rando A, Gasco S, de la Torre M, García-Redondo A, Zaragoza P, Toivonen JM, et al. Granulocyte colony-stimulating factor ameliorates skeletal muscle dysfunction in amyotrophic lateral sclerosis mice and improves proliferation of SOD1-G93A myoblasts in vitro. Neurodegener Dis. (2017) 17:1–13. 10.1159/000446113 [DOI] [PubMed] [Google Scholar]
- 20.Kaspar BK, Lladó J, Sherkat N, Rothstein JD, Gage FH. Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science. (2003) 301:839–42. 10.1126/science.1086137 [DOI] [PubMed] [Google Scholar]
- 21.Lepore AC, Haenggeli C, Gasmi M, Bishop KM, Bartus RT, Maragakis NJ, et al. Intraparenchymal spinal cord delivery of adeno-associated virus IGF-1 is protective in the SOD1G93A model of ALS. Brain Res. (2007) 1185:256–65. 10.1016/j.brainres.2007.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dodge JC, Haidet AM, Yang W, Passini MA, Hester M, Clarke J, et al. Delivery of AAV-IGF-1 to the CNS extends survival in ALS mice through modification of aberrant glial cell activity. Mol Ther. (2008) 16:1056–64. 10.1038/mt.2008.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin H, Hu H, Duan W, Liu Y, Tan G, Li Z, et al. Intramuscular delivery of scAAV9-hIGF1 prolongs survival in the hSOD1G93A ALS mouse model via upregulation of D-amino acid oxidase. Mol Neurobiol. (2018) 55:682–95. 10.1007/s12035-016-0335-z [DOI] [PubMed] [Google Scholar]
- 24.Wang W, Wen D, Duan W, Yin J, Cui C, Wang Y, et al. Systemic administration of scAAV9-IGF1 extends survival in SOD1G93A ALS mice via inhibiting p38 MAPK and the JNK-mediated apoptosis pathway. Brain Res Bull. (2018) 139:203–10. 10.1016/j.brainresbull.2018.02.015 [DOI] [PubMed] [Google Scholar]
- 25.Dodge JC, Treleaven CM, Fidler JA, Hester M, Haidet A, Handy C, et al. AAV4-mediated expression of IGF-1 and VEGF within cellular components of the ventricular system improves survival outcome in familial ALS mice. Mol Ther. (2010) 18:2075–84. 10.1038/mt.2010.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Duan W, Wang W, Di Wen, Liu Y, Liu Y, et al. scAAV9-VEGF prolongs the survival of transgenic ALS mice by promoting activation of M2 microglia and the PI3K/Akt pathway. Brain Res. (2016) 1648:1–10. 10.1016/j.brainres.2016.06.043 [DOI] [PubMed] [Google Scholar]
- 27.Wang LJ, Lu YY, Muramatsu S, Ikeguchi K, Fujimoto K, Okada T, et al. Neuroprotective effects of glial cell line-derived neurotrophic factor mediated by an adeno-associated virus vector in a transgenic animal model of amyotrophic lateral sclerosis. J Neurosci. (2002) 22:6920–8. 10.1523/JNEUROSCI.22-16-06920.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomsen GM, Alkaslasi M, Vit JP, Lawless G, Godoy M, Gowing G, et al. Systemic injection of AAV9-GDNF provides modest functional improvements in the SOD1G93A ALS rat but has adverse side effects. Gene Ther. (2017) 24:245–52. 10.1038/gt.2017.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henriques A, Pitzer C, Dittgen T, Klugmann M, Dupuis L, Schneider A. CNS-targeted viral delivery of G-CSF in an animal model for ALS: improved efficacy and preservation of the neuromuscular unit. Mol Ther. (2011) 19:284–92. 10.1038/mt.2010.271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu L, Yan J, Chen D, Welsh AM, Hazel T, Johe K, et al. Human neural stem cell grafts ameliorate motor neuron disease in SOD-1 transgenic rats. Transplantation. (2006) 82:865–75. 10.1097/01.tp.0000235532.00920.7a [DOI] [PubMed] [Google Scholar]
- 31.Xu L, Ryugo DK, Pongstaporn T, Johe K, Koliatsos VE. Human neural stem cell grafts in the spinal cord of SOD1 transgenic rats: differentiation and structural integration into the segmental motor circuitry. J Comp Neurol. (2009) 514:297–309. 10.1002/cne.22022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hwang DH, Lee HJ, Park IH, Seok JI, Kim BG, Joo IS, et al. Intrathecal transplantation of human neural stem cells overexpressing VEGF provide behavioral improvement, disease onset delay and survival extension in transgenic ALS mice. Gene Ther. (2009) 16:1234–44. 10.1038/gt.2009.80 [DOI] [PubMed] [Google Scholar]
- 33.Knippenberg S, Rath KJ, Böselt S, Thau-Habermann N, Schwarz SC, Dengler R, et al. Intraspinal administration of human spinal cord-derived neural progenitor cells in the G93A-SOD1 mouse model of ALS delays symptom progression, prolongs survival and increases expression of endogenous neurotrophic factors. J Tissue Eng Regen Med. (2017) 11:751–64. 10.1002/term.1972 [DOI] [PubMed] [Google Scholar]
- 34.Klein SM, Behrstock S, McHugh J, Hoffmann K, Wallace K, Suzuki M, et al. GDNF delivery using human neural progenitor cells in a rat model of ALS. Hum Gene Ther. (2005) 16:509–21. 10.1089/hum.2005.16.509 [DOI] [PubMed] [Google Scholar]
- 35.Suzuki M, McHugh J, Tork C, Shelley B, Klein SM, Aebischer P, et al. GDNF secreting human neural progenitor cells protect dying motor neurons, but not their projection to muscle, in a rat model of familial ALS. PLoS ONE. (2007) 2:e689 10.1371/journal.pone.0000689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomsen GM, Avalos P, Ma AA, Alkaslasi M, Cho N, Wyss L, et al. Transplantation of neural progenitor cells expressing glial cell line-derived neurotrophic factor into the motor cortex as a strategy to treat amyotrophic lateral sclerosis. Stem Cells. (2018) 36:1122–31. 10.1002/stem.2825 [DOI] [PubMed] [Google Scholar]
- 37.Vercelli A, Mereuta OM, Garbossa D, Muraca G, Mareschi K, Rustichelli D, et al. Human mesenchymal stem cell transplantation extends survival, improves motor performance and decreases neuroinflammation in mouse model of amyotrophic lateral sclerosis. Neurobiol Dis. (2008) 31:395–405. 10.1016/j.nbd.2008.05.016 [DOI] [PubMed] [Google Scholar]
- 38.Boido M, Piras A, Valsecchi V, Spigolon G, Mareschi K, Ferrero I, et al. Human mesenchymal stromal cell transplantation modulates neuroinflammatory milieu in a mouse model of amyotrophic lateral sclerosis. Cytotherapy. (2014) 16:1059–72. 10.1016/j.jcyt.2014.02.003 [DOI] [PubMed] [Google Scholar]
- 39.Uccelli A, Milanese M, Principato MC, Morando S, Bonifacino T, Vergani L, et al. Intravenous mesenchymal stem cells improve survival and motor function in experimental amyotrophic lateral sclerosis. Mol Med. (2012) 18:794–804. 10.2119/molmed.2011.00498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suzuki M, McHugh J, Tork C, Shelley B, Hayes A, Bellantuono I, et al. Direct muscle delivery of GDNF with human mesenchymal stem cells improves motor neuron survival and function in a rat model of familial ALS. Mol Ther. (2008) 16:2002–10. 10.1038/mt.2008.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krakora D, Mulcrone P, Meyer M, Lewis C, Bernau K, Gowing G, et al. Synergistic effects of GDNF and VEGF on lifespan and disease progression in a familial ALS rat model. Mol Ther. (2013) 21:1602–10. 10.1038/mt.2013.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pastor D, Viso-Leon MC, Jones J, Jaramillo-Merchan J, Toledo-Aral JJ, Moraleda JM, et al. Comparative effects between Bone Marrow and mesenchymal stem cell transplantation in GDNF expression and motor function recovery in a motorneuron degenerative mouse model. Stem Cell Rev. (2012) 8:445–58. 10.1007/s12015-011-9295-x [DOI] [PubMed] [Google Scholar]
- 43.Pastor D, Viso-Leon MC, Botella-Lopez A, Jaramillo-Merchan J, Moraleda JM, Jones J, et al. Bone marrow transplantation in hindlimb muscles of motorneuron degenerative mice reduces neuronal death and improves motor function. Stem Cells Dev. (2013) 22:1633–44. 10.1089/scd.2012.0487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marconi S, Bonaconsa M, Scambi I, Squintani GM, Rui W, Turano E, et al. Systemic treatment with adipose-derived mesenchymal stem cells ameliorates clinical and pathological features in the amyotrophic lateral sclerosis murine model. Neuroscience. (2013) 248:333–43. 10.1016/j.neuroscience.2013.05.034 [DOI] [PubMed] [Google Scholar]
- 45.Kim KS, Lee HJ, An J, Kim YB, Ra JC, Lim I, et al. Transplantation of human adipose tissue-derived stem cells delays clinical onset and prolongs life span in ALS mouse model. Cell Transplant. (2014) 23:1585–97. 10.3727/096368913X673450 [DOI] [PubMed] [Google Scholar]
- 46.Ende N, Weinstein F, Chen R, Ende M. Human umbilical cord blood effect on sod mice (amyotrophic lateral sclerosis). Life Sci. (2000) 67:53–9. 10.1016/S0024-3205(00)00602-0 [DOI] [PubMed] [Google Scholar]
- 47.Chen R, Ende N. The potential for the use of mononuclear cells from human umbilical cord blood in the treatment of amyotrophic lateral sclerosis in SOD1 mice. J Med. (2000) 31:21–30. [PubMed] [Google Scholar]
- 48.Garbuzova-Davis S, Willing AE, Zigova T, Saporta S, Justen EB, Lane JC, et al. Intravenous administration of human umbilical cord blood cells in a mouse model of amyotrophic lateral sclerosis: distribution, migration, and differentiation. J Hematother Stem Cell Res. (2003) 12:255–70. 10.1089/152581603322022990 [DOI] [PubMed] [Google Scholar]
- 49.Garbuzova-Davis S, Rodrigues MCO, Mirtyl S, Turner S, Mitha S, Sodhi J, et al. Multiple intravenous administrations of human umbilical cord blood cells benefit in a mouse model of ALS. PLoS ONE. (2012) 7:e31254. 10.1371/journal.pone.0031254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Habisch HJ, Janowski M, Binder D, Kuzma-Kozakiewicz M, Widmann A, Habich A, et al. Intrathecal application of neuroectodermally converted stem cells into a mouse model of ALS: limited intraparenchymal migration and survival narrows therapeutic effects. J Neural Transm. (2007) 114:1395–406. 10.1007/s00702-007-0748-y [DOI] [PubMed] [Google Scholar]
- 51.Knippenberg S, Thau N, Schwabe K, Dengler R, Schambach A, Hass R, et al. Intraspinal injection of human umbilical cord blood-derived cells is neuroprotective in a transgenic mouse model of amyotrophic lateral sclerosis. Neurodegener Dis. (2012) 9:107–20. 10.1159/000331327 [DOI] [PubMed] [Google Scholar]
- 52.Bigini P, Veglianese P, Andriolo G, Cova L, Grignaschi G, Caron I, et al. Intracerebroventricular administration of human umbilical cord blood cells delays disease progression in two murine models of motor neuron degeneration. Rejuvenation Res. (2011) 14:623–39. 10.1089/rej.2011.1197 [DOI] [PubMed] [Google Scholar]
- 53.Islamov RR, Rizvanov AA, Mukhamedyarov MA, Salafutdinov II, Garanina EE, Fedotova VY, et al. Symptomatic improvement, increased life-span and sustained cell homing in amyotrophic lateral sclerosis after transplantation of human umbilical cord blood cells genetically modified with adeno-viral vectors expressing a neuro-protective factor and a neural cell adhesion molecule. Curr Gene Ther. (2015) 15:266–76. 10.2174/1566523215666150126122317 [DOI] [PubMed] [Google Scholar]
- 54.Islamov RR, Rizvanov AA, Fedotova VY, Izmailov AA, Safiullov ZZ, Garanina EE, et al. Tandem delivery of multiple therapeutic genes using umbilical cord blood cells improves symptomatic outcomes in ALS. Mol Neurobiol. (2017) 54:4756–63. 10.1007/s12035-016-0017-x [DOI] [PubMed] [Google Scholar]
- 55.Calvo AC, Moreno-Igoa M, Mancuso R, Manzano R, Oliván S, Muñoz MJ, et al. Lack of a synergistic effect of a non-viral ALS gene therapy based on BDNF and a TTC fusion molecule. Orphanet J Rare Dis. (2011) 6:10. 10.1186/1750-1172-6-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Korkmaz OT, Aytan N, Carreras I, Choi J-K, Kowall NW, Jenkins BG, et al. 7,8-Dihydroxyflavone improves motor performance and enhances lower motor neuronal survival in a mouse model of amyotrophic lateral sclerosis. Neurosci Lett. (2014) 566:286–91. 10.1016/j.neulet.2014.02.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Turner BJ, Cheah IK, Macfarlane KJ, Lopes EC, Petratos S, Langford SJ, et al. Antisense peptide nucleic acid-mediated knockdown of the p75 neurotrophin receptor delays motor neuron disease in mutant SOD1 transgenic mice. J Neurochem. (2003) 87:752–63. 10.1046/j.1471-4159.2003.02053.x [DOI] [PubMed] [Google Scholar]
- 58.Yanpallewar SU, Barrick CA, Buckley H, Becker J, Tessarollo L. Deletion of the BDNF truncated receptor TrkB.T1 delays disease onset in a mouse model of amyotrophic lateral sclerosis. PLoS ONE. (2012) 7:e39946. 10.1371/journal.pone.0039946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brambilla L, Guidotti G, Martorana F, Iyer AM, Aronica E, Valori CF, et al. Disruption of the astrocytic TNFR1-GDNF axis accelerates motor neuron degeneration and disease progression in amyotrophic lateral sclerosis. Hum Mol Genet. (2016) 25:3080–95. 10.1093/hmg/ddw161 [DOI] [PubMed] [Google Scholar]
- 60.Tovar-Y-Romo LB, Zepeda A, Tapia R. Vascular endothelial growth factor prevents paralysis and motoneuron death in a rat model of excitotoxic spinal cord neurodegeneration. J Neuropathol Exp Neurol. (2007) 66:913–22. 10.1097/nen.0b013e3181567c16 [DOI] [PubMed] [Google Scholar]
- 61.Tovar-y-Romo LB, Tapia R. Delayed administration of VEGF rescues spinal motor neurons from death with a short effective time frame in excitotoxic experimental models in vivo. ASN Neuro. (2012) 4: e0008. 10.1042/AN20110057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Czarzasta J, Habich A, Siwek T, Czaplinski A, Maksymowicz W, Wojtkiewicz J. Stem cells for ALS: an overview of possible therapeutic approaches. Int J Dev Neurosci. (2017) 57:46–55. 10.1016/j.ijdevneu.2017.01.003 [DOI] [PubMed] [Google Scholar]
- 63.Mathieu P, Battista D, Depino A, Roca V, Graciarena M, Pitossi F. The more you have, the less you get: the functional role of inflammation on neuronal differentiation of endogenous and transplanted neural stem cells in the adult brain. J Neurochem. (2010) 112:1368–85. 10.1111/j.1471-4159.2009.06548.x [DOI] [PubMed] [Google Scholar]
- 64.Reekmans K, Praet J, Daans J, Reumers V, Pauwels P, Van der Linden A, et al. Current challenges for the advancement of neural stem cell biology and transplantation research. Stem Cell Rev. (2012) 8:262–78. 10.1007/s12015-011-9266-2 [DOI] [PubMed] [Google Scholar]
- 65.Lewis CM, Suzuki M. Therapeutic applications of mesenchymal stem cells for amyotrophic lateral sclerosis. Stem Cell Res Ther. (2014) 5:32. 10.1186/scrt421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gugliandono A, Bramanti P, Mazzon E. Mesenchymal stem cells: a potential approach for Amyotrophic Lateral Sclerosis? Stem Cells Int. (2019) 2019:3675627 10.1155/2019/3675627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beers DR, Appel SH. Immune dysregulation in amyotrophic lateral sclerosis: mechanisms and emerging therapies. Lancet Neurol. (2019) 18:211–20. 10.1016/S1474-4422(18)30394-6 [DOI] [PubMed] [Google Scholar]
- 68.Rizvanov AA, Kiyasov AP, Gaziziov IM, Yilmaz TS, Kaligin MS, Andreeva DI, et al. Human umbilical cord blood cells transfected with VEGF and L(1)CAM do not differentiate into neurons but transform into vascular endothelial cells and secrete neuro-trophic factors to support neuro-genesis-a novel approach in stem cell therapy. Neurochem Int. (2008) 53:389–94. 10.1016/j.neuint.2008.09.011 [DOI] [PubMed] [Google Scholar]
- 69.Rizvanov AA, Guseva DS, Salafutdinov II, Kudryashova NV, Bashirov FV, Kiyasov AP, et al. Genetically modified human umbilical cord blood cells expressing vascular endothelial growth factor and fibroblast growth factor 2 differentiate into glial cells after transplantation into amyotrophic lateral sclerosis transgenic mice. Exp Biol Med. (2011) 236:91–8. 10.1258/ebm.2010.010172 [DOI] [PubMed] [Google Scholar]
- 70.A phase I study of recombinant human ciliary neurotrophic factor (rHCNTF) in patients with amyotrophic lateral sclerosis The ALS CNTF Treatment Study (ACTS) Phase I-II Study Group. Clin Neuropharmacol. (1995) 18:515–32. 10.1097/00002826-199512000-00004 [DOI] [PubMed] [Google Scholar]
- 71.Miller RG, Petajan JH, Bryan WW, Armon C, Barohn RJ, Goodpasture JC, et al. A placebo-controlled trial of recombinant human ciliary neurotrophic (rhCNTF) factor in amyotrophic lateral sclerosis. rhCNTF ALS Study Group. Ann Neurol. (1996) 39:256–60. 10.1002/ana.410390215 [DOI] [PubMed] [Google Scholar]
- 72.A double-blind placebo-controlled clinical trial of subcutaneous recombinant human ciliary neurotrophic factor (rHCNTF) in amyotrophic lateral sclerosis ALS CNTF Treatment Study Group. Neurology. (1996) 46:1244–9. 10.1212/WNL.46.5.1244 [DOI] [PubMed] [Google Scholar]
- 73.Penn RD, Kroin JS, York MM, Cedarbaum JM. Intrathecal ciliary neurotrophic factor delivery for treatment of amyotrophic lateral sclerosis (phase I trial). Neurosurgery. (1997) 40:94–9; discussion: 99–100. 10.1227/00006123-199701000-00021 [DOI] [PubMed] [Google Scholar]
- 74.Works in progress abstracts Ann Neurol. (1995) 38:971–3. 10.1002/ana.410380629 [DOI] [Google Scholar]
- 75.A controlled trial of recombinant methionyl human BDNF in ALS: The BDNF Study Group (Phase III) Neurology. (1999) 52:1427–33. 10.1212/WNL.52.7.1427 [DOI] [PubMed] [Google Scholar]
- 76.Ochs G, Penn RD, York M, Giess R, Beck M, Tonn J, et al. A phase I/II trial of recombinant methionyl human brain derived neurotrophic factor administered by intrathecal infusion to patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. (2000) 1:201–6. 10.1080/14660820050515197 [DOI] [PubMed] [Google Scholar]
- 77.Kalra S, Genge A, Arnold DL. A prospective, randomized, placebo-controlled evaluation of corticoneuronal response to intrathecal BDNF therapy in ALS using magnetic resonance spectroscopy: feasibility and results. Amyotroph Lateral Scler Other Motor Neuron Disord. (2003) 4:22–6. 10.1080/14660820310006689 [DOI] [PubMed] [Google Scholar]
- 78.Beck M, Flachenecker P, Magnus T, Giess R, Reiners K, Toyka KV, et al. Autonomic dysfunction in ALS: a preliminary study on the effects of intrathecal BDNF. Amyotroph Lateral Scler Other Motor Neuron Disord. (2005) 6:100–3. 10.1080/14660820510028412 [DOI] [PubMed] [Google Scholar]
- 79.Lai EC, Felice KJ, Festoff BW, Gawel MJ, Gelinas DF, Kratz R, et al. Effect of recombinant human insulin-like growth factor-I on progression of ALS. A placebo-controlled study. The North America ALS/IGF-I Study Group. Neurology. (1997) 49:1621–30. 10.1212/WNL.49.6.1621 [DOI] [PubMed] [Google Scholar]
- 80.Borasio GD, Robberecht W, Leigh PN, Emile J, Guiloff RJ, Jerusalem F, et al. A placebo-controlled trial of insulin-like growth factor-I in amyotrophic lateral sclerosis. European ALS/IGF-I Study Group. Neurology. (1998) 51:583–6. 10.1212/WNL.51.2.583 [DOI] [PubMed] [Google Scholar]
- 81.Nagano I, Shiote M, Murakami T, Kamada H, Hamakawa Y, Matsubara E, et al. Beneficial effects of intrathecal IGF-1 administration in patients with amyotrophic lateral sclerosis. Neurol Res. (2005) 27:768–2. 10.1179/016164105X39860 [DOI] [PubMed] [Google Scholar]
- 82.Sorenson EJ, Windbank AJ, Mandrekar JN, Bamlet WR, Appel SH, Armon C, et al. Subcutaneous IGF-1 is not beneficial in 2-year ALS trial. Neurology. (2008) 71:1770–5. 10.1212/01.wnl.0000335970.78664.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang Y, Wang L, Fu Y, Song H, Zhao H, Deng M, et al. Preliminary investigation of effect of granulocyte colony stimulating factor on amyotrophic lateral sclerosis. Amyotroph Lateral Scler. (2009) 10:430–1. 10.3109/17482960802588059 [DOI] [PubMed] [Google Scholar]
- 84.Nefussy B, Artamonov I, Deutsch V, Naparstek E, Nagler A, Drory VE. Recombinant human granulocyte-colony stimulating factor administration for treating amyotrophic lateral sclerosis: A pilot study. Amyotroph Lateral Scler. (2010) 11:187–93. 10.3109/17482960902933809 [DOI] [PubMed] [Google Scholar]
- 85.Riley J, Federici T, Polak M, Kelly C, Glass J, Raore B, et al. Intraspinal stem cell transplantation in amyotrophic lateral sclerosis: a phase I safety trial, technical note, and lumbar safety outcomes. Neurosurgery. (2012) 71:405–16; discussion: 416. 10.1227/NEU.0b013e31825ca05f [DOI] [PubMed] [Google Scholar]
- 86.Glass JD, Boulis NM, Johe K, Rutkove SB, Federici T, Polak M, et al. Lumbar intraspinal injection of neural stem cells in patients with amyotrophic lateral sclerosis: results of a phase I trial in 12 patients. Stem Cells. (2012) 30:1144–51. 10.1002/stem.1079 [DOI] [PubMed] [Google Scholar]
- 87.Mazzini L, Gelati M, Profico DC, Sgaravizzi G, Projetti Pensi M, Muzi G, et al. Human neural stem cell transplantation in ALS: initial results from a phase I trial. J Transl Med. (2015) 13:17. 10.1186/s12967-014-0371-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Karussis D, Karageorgiou C, Vaknin-Dembinsky A, Gowda-Kurkalli B, Gomori JM, Kassis I, et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol. (2010) 10:1187–94. 10.1001/archneurol.2010.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Blanquer M, Moraleda JM, Iniesta F, Gómez-Espuch J, Meca-Lallana J, Villaverde R, et al. Neurotrophic bone marrow cellular nests prevent spinal motoneuron degeneration in amyotrophic lateral sclerosis patients: a pilot safety study. Stem Cells. (2012) 30:1277–85. 10.1002/stem.1080 [DOI] [PubMed] [Google Scholar]
- 90.Ruiz-López FJ, Guardiola J, undIzura Verline, Gómez-Espuch J, uIniesta Fnderline, Blanquer Munderline, et al. Breathing pattern in a phase I clinical trial of intraspinal injection of autologous bone marrow mononuclear cells in patients with amyotrophic lateral sclerosis. Respir Physiol Neurobiol. (2016) 221:54–8. 10.1016/j.resp.2015.11.007 [DOI] [PubMed] [Google Scholar]
- 91.García Santos JM, Blanquer Munderline, Torres del Río S, uIniesta Fnderline, uEspuch JGnderline, Pérez-Espejo MÁ, et al. Acute and chronic MRI changes in the spine and spinal cord after surgical stem cell grafting in patients with definite amyotrophic lateral sclerosis: post-infusion injuries are unrelated with clinical impairment. Magn Reson Imaging. (2013) 8:1298–308. 10.1016/j.mri.2013.05.006 [DOI] [PubMed] [Google Scholar]
- 92.Oh KW, Noh MY, Kwon MS, Kim HY, Oh SI, Park J, et al. Repeated intrathecal mesenchymal stem cells for amyotrophic lateral sclerosis. Ann Neurol. (2018) 84:361–73. 10.1002/ana.25302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Petrou P, Gothelf Y, Argov Z, Gotkine M, Levy YS, Kassis I, et al. Safety and clinical effects of mesenchymal stem cells secreting neurotrophic factor transplantation in patients with amyotrophic lateral sclerosis: results of phase 1/2 and 2a clinical trials. JAMA Neurol. (2016) 73:337–44. 10.1001/jamaneurol.2015.4321 [DOI] [PubMed] [Google Scholar]
- 94.Nabavi SM, Arab L, Jarooghi N, Bolurieh T, Abbasi F, Mardpour S, et al. Safety, feasibility of intravenous and intrathecal injection of autologous Bone marrow derived mesenchymal stromal cells in patients with amyotrophic lateral sclerosis: an open label phase I clinical trial. Cell J. (2019) 20:592–8. 10.22074/cellj.2019.5370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nurden AT, Nurden P, Sanchez M, Andia I, Anitua E. Platelets and wound healing. Front Biosci. (2008) 13:3532–48. 10.2741/2947 [DOI] [PubMed] [Google Scholar]
- 96.Burnouf T, Goubran HA, Chen TM, Ou KL, El-Ekiaby M, Radosevic M. Blood-derived biomaterials and platelet growth factors in regenerative medicine. Blood Rev. (2013) 27:77–89. 10.1016/j.blre.2013.02.001 [DOI] [PubMed] [Google Scholar]
- 97.Nurden AT. The biology of the platelet with special reference to inflammation, wound healing and immunity. Front Biosci. (2018) 23:726–51. 10.2741/4613 [DOI] [PubMed] [Google Scholar]
- 98.Hayon Y, Dashevsky O, Shai E, Varon D, Leker RR. Platelet lysates stimulate angiogenesis, neurogenesis and neuroprotection after stroke. Thromb Haemost. (2013) 110:323–30. 10.1160/TH12-11-0875 [DOI] [PubMed] [Google Scholar]
- 99.Zhang Y, Ying G, Ren C, Jizhang Y, Brogan D, Liu Z, et al. Administration of human platelet-rich plasma reduces infarction volume and improves motor function in adult rats with focal ischemic stroke. Brain Res. (2015) 1594:267–73. 10.1016/j.brainres.2014.10.035 [DOI] [PubMed] [Google Scholar]
- 100.Anitua E, Pascual C, Antequera D, Bolos M, Padilla S, Orive G, et al. Plasma rich in growth factors (PRGF-Endoret) reduces neuropathologic hallmarks and improves cognitive functions in an Alzheimer's disease mouse model. Neurobiol Aging. (2014) 35:1582–95. 10.1016/j.neurobiolaging.2014.01.009 [DOI] [PubMed] [Google Scholar]
- 101.Anitua E, Pascual C, Perez-Gonzalez R, Orive G, Carro E. Intranasal PRGF-Endoret enhances neuronal survival and attenuates NF-kappaB-dependent inflammation process in a mouse model of Parkinson's disease. J Control Release. (2015) 203:170–80. 10.1016/j.jconrel.2015.02.030 [DOI] [PubMed] [Google Scholar]
- 102.Chou ML, Wu JW, Gouel F, Jonneaux A, Timmerman K, Renn TY, et al. Tailor-made purified human platelet lysate concentrated in neurotrophins for treatment of Parkinson's disease. Biomaterials. (2017) 142:77–89. 10.1016/j.biomaterials.2017.07.018 [DOI] [PubMed] [Google Scholar]
- 103.Gouel F, Do Van B, Chou ML, Jonneaux A, Moreau C, Bordet R, et al. The protective effect of human platelet lysate in models of neurodegenerative disease: involvement of the Akt and MEK pathways. J Tissue Eng Regen Med. (2017) 11:3236–40. 10.1002/term.2222 [DOI] [PubMed] [Google Scholar]