Abstract
Background
There is existing evidence on whether and to what degree regular exercise training improves the quality of life (QoL) among cancer survivors. However, in regards to patients with high-grade glioma (HGG; WHO grade III and IV), no conclusive study has been performed so far. The present trial aims to fill this gap by examining whether psychological well-being, sleep, QoL and physical fitness might be improved with two different types of exercise, as compared to an active control condition. Active control condition represent individuals participating at regular meetings to talk about their current life situation, though, the meetings were not intended as that of the psychotherapy group. Regular meetings are of the same frequency, duration, and intensity as the exercise interventions.
Methods
A total of 45 patients with HGG after undergoing neurosurgery and adjuvant radiotherapy, chemotherapy, or chemoradiotherapy will be consecutively and randomly assigned to (a) an endurance training, (b) a resistance training or (c) to an active control condition. The intervention will last for 6 consecutive weeks, consisting of 2 weekly sessions (30–45 min per session). Measurements would take place at three time points, namely at the beginning of the study (baseline), 3 weeks after the beginning of the study, and 6 weeks after the beginning of the study. The last measurement also represents the end of the study. Aerobic exercise performance will be assessed objectively with a 6-min walking test, and a handgrip test will be used to assess the upper body strength. Further, participants will complete a battery of questionnaires covering sociodemographic information, QoL, sleep quality and sleep patterns, coping with stress, state- and trait-anxiety, depression, and fatigue. In parallel, experts will use the Hamilton Depression Rating Scale to determine and rate participants’ symptoms of depression.
Significance
The present study will be the first to investigate and compare the impact of two different exercise modalities, namely endurance and resistance training, on physical fitness and dimensions of well-being, and sleep among patients with HGG who underwent neurosurgery followed by adjuvant radiotherapy, chemotherapy, or chemoradiotherapy. Importantly, unlike the majority of previous studies, the control condition consists of an active set-up to detect possible factual beneficial effects of exercise training, irrespective of social interactions.
Trial registration https://register.clinicaltrials.gov; identifier: NCT03775369
Keywords: Brain, Tumor, Glioma, Exercise, Training, Sleep, Control condition, Quality of life, Anxiety, Perceived stress
Background
There is increasing evidence that cancer survivors benefit from adjuvant exercise training to improve their subjective wellbeing, and improvements have been observed in regards to quality of life (QoL), coping with stress, and self-control. Here, self-control refers to the individual’s ability to control her/his thoughts, emotions, and behavior. Exercise training also has the potential to counteract series of side-effects of chemotherapy or hormone therapy such as fatigue, weight gain, muscle loss, hot flushes, nausea, or increased susceptibility to infections [1, 2]. Further, Segal et al. [3] underlined in their systematic review that exercise training is a safe intervention, which provides benefits with regard to quality of life, and muscular and aerobic performance both during and after cancer-specific treatment. However, a deeper inspection of the type of cancer reported in the systematic review of Segal et al. [3] revealed that randomized clinical trials were performed for breast cancer, colorectal cancer, prostate cancer, or just mentioned ‘cancer’ without further specifications on the location, while no randomized clinical trial has yet tested the influence of exercise interventions in patients with glioma, and more specifically with high grade glioma (HGG; WHO grade III and IV). Thus, the present randomized clinical trial was designed to address this research gap, and to create an empirical evidence base on whether exercise training can be seen as a treatment option among patients with HGG during the post-surgery state in order to improve their wellbeing, sleep and QoL.
As mentioned by Ostrom et al. [4, 5] gliomas represent 31% of all malignant brain tumors and 81% of central nervous system (CNS) tumors diagnosed in the United States. Although gliomas are rare, they can still lead to significant mortality and morbidity. Further, not all types of gliomas develop in an aggressive way, and the heterogeneity of these gliomas in terms of histopathological types and grades, clinical outcomes, and genomics makes treatment and risk management even more difficult [6].
As regards to the identification of possible risk factors, Ostrom et al. [5, 6] summarized in their systematic review that ionizing radiation and heritable genetic variants appeared to increase the risk for gliomas, whereas allergies and atopic diseases appeared to be protective. However, there is inconclusive evidence of non-ionizing radiation via cell phone use and risk of gliomas.
The usual treatment algorithm for high-grade glioma consists of neurosurgical resection of tumor tissue and postoperative combined radiochemotherapy. Depending on the genetic sub-classification of the glioma and patient-specific factors such as age and clinical condition, postoperative treatment is sometimes given in the form of only radiation therapy or chemotherapy. In the present study, however, we will investigate whether and to what degree structured exercise training may have a (beneficial) effect on the psychological wellbeing, sleep, QoL, and physical fitness during the phase of postoperative adjuvant treatment.
The underlying rationales and research findings are as follows: On a physiological level, some scholars emphasize that regular exercise training has the potential to increase the levels of serotonin, brain-derived neurotrophic factor (BDNF) and endothelial growth factor (VEGF) [7], and to increase mitochondrial activity [8]. On the psychological level, other scholars argue that exercise training has the potential to improve the patients’ mood [9, 10] and self-esteem [11], to decrease symptoms of depression [12–17], anxiety, chronic pain [18], and above all rumination [19]. Further, in a previous study on the post-treatment of patients with post-aneurysmal subarachnoid hemorrhage, we have shown that compared to a control group, a 12-week regular endurance and resistance training ameliorated the symptoms of depression, insomnia and cognitive capacities [20–22]. Next, as regards to cancer survivors, most studies focused on breast cancer survivors during or after treatment [1]. Segal et al. [23] further emphasized that physical activity and exercise interventions were safe, provided benefit in quality of life, and might have the potential to enhance social exchange. In this respect, Brand et al. [19] showed that a single session of exercise could increase interest in social contact and interactions, at least among their investigated 129 inpatients (mean age: 38.16 years; median: 36.00 years; age range: 19–63 years) with a broad variety of psychiatric disorders. However, in regards to patients with HGG, to the best of our knowledge, there has been no study to investigate the impact of exercising on the patients’ well-being and QoL, despite it is well-acknowledged that individuals with HGG who underwent radiotherapy, chemotherapy, or both radio- and chemotherapy suffer from a dramatically decreased in QoL. Accordingly, the primary aim of the present study is to examine and compare the effects of two different types of exercise training (endurance vs. resistance) on psychological wellbeing (i.e., depression, stress, anxiety), sleep, QoL and physical fitness in patients with HGG who had neurosurgery followed by radiotherapy, chemotherapy, or both, compared to an active control condition.
Methods
Trial design
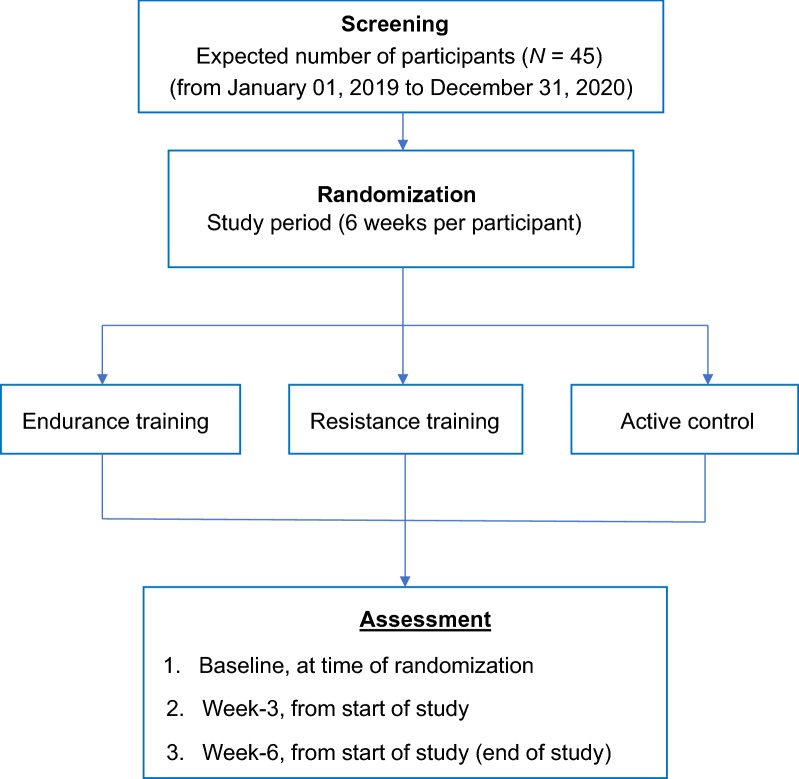
The present study is an interventional, and randomized clinical trial. Each patient will participate for 6 weeks, with pre-specified assessment time points (Table 1): Measurements would take place at three time points, namely, baseline, which is the start of the study after the randomization. The second time point is 3 weeks after the baseline/beginning of the study. The third and last time point is 6 weeks after the baseline/beginning of the study, which also represents the end of the study. After written informed consent is obtained, eligible participants from the Basel University Hospital (Department of Neurosurgery; USB, Basel, Switzerland) are to be randomly and consecutively assigned to one of the following study grouped-conditions (Fig. 1): (a) endurance training, (b) resistance training, and (c) active control.
Table 1.
Flow chart of the study: time points and the assessed dimensions at the time points
| Outcome dimensions and time points | Time points | ||||
|---|---|---|---|---|---|
| − 4 to − 1 weeksa | − 1 week | Week 0 | Week 3 | Week 6 | |
| Diagnosis and surgery | X | ||||
| Chemotherapy and/or radiotherapy at the same time | X
 X
X |
||||
| Patient’s eligibility is checked | X | ||||
| Signed written informed consent | |||||
| Randomization | X | ||||
| Assessment | |||||
| Cardiovascular performance (6MWT) and grip strength | X | X | X | ||
| Psychological functioning | X | X | X | ||
| Start study interventions | X | ||||
| Interventions | X
 X
X |
||||
| Study end | X | ||||
6MWT 6-min walking test, X this dimension is assessed; blue arrow, the time point from one period to the next
a− 4 to − 1 weeks, 4 weeks to 1 week of pre-treatment period
Fig. 1.
Study design. The enrolled participants are to be randomly assigned to one of the three study conditions: endurance training; resistance training; active control, with assessments to be taken at three pre-specified time points
Study population
Patients who meet the following inclusion and none of the exclusion criteria would be eligible for this study and consecutively enrolled in the study.
Inclusion criteria
Patients with high-grade glioma (grading criteria, WHO III and IV) after neurosurgical tumor resection or biopsy;
Had either postoperative chemotherapy, or radiotherapy, or both at the same time;
Age between 18 and 75 years;
Willing and able to follow the study intervention, and to comply with the study conditions;
Provide signed written informed consent;
Exclusion criteria
Severe psychiatric (psychosis, suicidal behavior, substance abuse disorder) issues;
Severe and somatic comorbidities (severe cardiovascular disease, severe diabetes, impairments of the musculoskeletal system); severe cardiopulmonary conditions;
Withdrawal from the study; either the patient wants to withdraw, or one of the principal investigators determines that due to unfavorable somatic or mental issues the participant is unsuitable for further participation in the study.
Recruitment
The neurosurgeon (DC; 18 years of experience) is responsible for the recruitment of eligible participants.
Participant timeline
As shown in Table 1, patients from the Department of Neurosurgery of the Basel University Hospital (Basel, Switzerland) have the following timeline: After neurosurgical treatment of the HGG, the patients would undergo either chemotherapy, or radiotherapy, or both. In parallel, they would be informed about the study content and the study aims. Once the written informed consent is signed, and the baseline assessment (cardiovascular performance and grip strengths; psychological assessments) (Table 1) is completed, the patients will be randomly assigned to one of the three study conditions. For the next 6 consecutive weeks, she/he would follow the schedule with two sessions per week. After the 3rd and 6th weeks, from the start of the study, the patients would be reassessed; similar as to the baseline assessments.
Sample size calculation
We rely on two approaches: First, for pilot studies and clinical trials, Julious [24] recommended to recruit at least 12 participants/group. Second, we performed a power analysis with G*Power [25] with the following statistical preconditions: Cohen’s f for ANOVAs with repeated measures and within and between interactions: 0.25; alpha: 0.05; Power: 0.8; number of groups: 3, number of measurements: 3; calculated total sample size: 36. To counterbalance possible drop-outs, the sample size was set at 45 participants; 15 participants per group.
Randomization
The randomizations are to be performed using an online software from the website Randomization.com (http://www.randomization.com). The online generator randomizes each subject to a single treatment using a method of randomly permuted blocks, with random block sizes for sex. Based on the generated list, a psychologist, not involved in the study, prepares 45 sealed envelopes, with no further identification, to be kept in an opaque and closed ballot box which are to be stirred before picking. Next, the psychologist would draw an envelope from the ballot and assign the study participants to one of the three study conditions picked. Once an envelope is drawn, it would be put aside. Experts rating the participants’ depression severity would be blinded to the participants’ study condition assigned.
Interventions
Endurance training
The endurance training will last for 6 consecutive weeks and consists of 2 weekly sessions (30–45 min each). Sessions would take place in small groups of 2 to 4 participants. After 5 min of warming-up and stretching, participants will exercise for 25–35 min on the treadmill at a pace of 11 to 14 points on the Borg Scale of ranging from 0 (complete rest or resting pace) to 20 (maximum pace), followed by 5 min of cooling down. In this study, cooling down represents light exercise to allow the body to gradually transition to a resting or near-resting state. Participants are allowed to adapt to their pace and to take breaks throughout the session, though they are also encouraged to keep both their speed and pace as long as possible.
Resistance training
The resistance training will also last for 6 weeks with 2 weekly training sessions (30–45 min each), in small groups of 2 to 4 participants. After 5 min of warming-up, participants will follow a structured and standardized resistance training protocol to strengthen all main bodily skeletal muscles (upper and lower arms; shoulders, upper and lower legs; abdominal muscles; core muscles). The protocol is as follows: The participants would perform each exercise in 3–5 series of 10–15 repetitions per series, at 11 to 15 points on the Borg Scale, followed by the cooling down period. Intervals between the series of 10–15 repetitions will last for about 1–3 min.
Active control condition
For 6 consecutive weeks, participants of the control condition will meet twice per week for 30–45 min but by contrast to the endurance and resistance training condition, the participants would not perform any planned physical exercise. Each session would take place in small groups of 2 to 4 participants. The control condition is not a ‘bona fide’ condition, which would be actually intended to elicit change [26, 27], that is to say, by default, group sessions in psychotherapy follow the aim to change participants’ dysfunctional cognitive-emotional concepts and information processing; By contrast, in the present control condition, topics such as successful coping strategies are not treated and not proactively proposed by the clinical psychologist responsible to monitor the content of the control conditions. Rather, the participants would be encouraged to proposing and exchanging daily life experiences.
Measures
Sociodemographic information
At baseline, the participants’ sex, age, civil status, highest educational level, and current job position will be assessed via a self-report.
Clinical- and glioma-related information
At baseline, clinical- and glioma-related information such as relevant past medical history, symptoms at initial presentation, neurosurgical procedure, histopathological diagnosis, and genetic subtype of the tumor would be retrieved from the participants’ medical records.
Psychological wellbeing
A set of self-report questionnaires will be used to assess the patients’ psychological wellbeing, including symptoms of depression, state- and trait-anxiety, and perceived stress. All questionnaires will be completed at baseline, 3 weeks into the study, and at the end of the study.
Depression
As in previous studies [28, 29], participants will complete the Beck Depression Inventory [30] to self-report symptoms of depression. The questionnaire consists of 21 items regarding typical dimensions of depression such as depressive mood, loss of appetite, sleep disorders, suicidality and similar. Answers are given on 4-point-Likert scales with the anchor points 0 (‘as always’; ‘no change’) to 3 (‘not able anymore’; ‘dramatic change’), with higher scores reflecting an increased severity of depressive symptoms. Evidence for the validity of the BDI has been previously reported [30].
State and trait anxiety
To assess state-and trait-anxiety, the State–Trait Anxiety Inventory (STAI) [31, 32] will be employed. The STAI consists of 42 items. Typical items for an anxiety state are “I feel relaxed”; “I feel nervous”, or “I feel tensed”. Typical items for anxiety traits are “I get nervous and restless when thinking at all my duties and issues”; “I can’t stop ruminating about unimportant stuff”. Answers would be given on an 8-points rating scale with the anchor points 0 (not true at all) to 7 (completely true), and with higher sum scores reflecting higher state and trait of anxiety. The STAI has been previously proved to have good psychometric properties in previous studies [31, 32].
Perceived stress
As previously described [33], we will employ the Perceived Stress Scale (PSS) [34], which has been proved to be a valid instrument in previous research [35], to assess subjectively perceived stress. The questionnaire consists of 10 items and is used to determine perceived overall stress occurring over the previous month. Answers are to be given on five-point rating scales ranging from 1 (never) to 5 (very often), with higher scores reflecting greater perceived stress.
Sleep
To assess subjective sleep complaints, participants will complete the Insomnia Severity Index [36, 37], comprising of seven items, answering on a 5-point rating scales (0, not at all, 4, very much), referring to the difficulty in falling asleep, difficulties in maintaining sleep, increased daytime sleepiness, and worriedness about sleep. The higher the overall score, the greater the respondent is assumed to suffer from insomnia. The validity of the ISI has been previously described [36, 37].
Quality of life (QoL, SF-36)
As previously described [38, 39], the short form 36 health survey (SF-36) [40] will be employed to assess the participants’ health-related QoL. This self-rating questionnaire consists of eight scales to assess impairments in physical activities (i.e., bathing, dressing, grocery shopping, using stairs, and using escalators) due to health problems (physical functioning), impairments in routine role activities at work or in other daily activities due to physical health problems (role limitations due to physical health), bodily pain, general health perceptions, energy and fatigue (vitality), impairments in social activities due to physical or emotional problems (social functioning), impairments in daily role activities at work or other everyday activities due to emotional problems (role limitations due to emotional problems), and general mental health. Answers would be given on different scales with different anchor points; interim scores would be transformed to achieve a range between 0 (worst QoL) and 100 (best QoL); thus, higher scores reflect a higher self-perceived quality of life. The validity of the SF-36 has been documented in previous research [41].
Physical fitness
To assess physical fitness, all participants will perform the 6-min walking test (6MWT) and handgrip strength test at baseline and at the end of the study.
We will use the 6MWT as a measure of cardiorespiratory fitness [42]. The 6MWT is a standardized, self-paced, submaximal exercise test, and has been designed especially for patients suffering from chronic diseases. Evidence of the validity of the 6MWT has been previously shown in various disease types [43–45] including cancer patients [46, 47]. The walking distance will be used as the main outcome measure of the 6MWT. Oxygen saturation before and after the 6MWT will be also taken into consideration.
As a measure of upper body strength, we will perform the grip strength test. As described elsewhere [48], maximum isometric grip force of the dominant hand would be assessed using a mechanic hand dynamometer (Jamar Handgrip Dynamometer; retailer: Rehaforum MEDICAL GmbH; Elmshorn, Germany). Participants will have three attempts per hand. The validity of the grip strength test has been previously documented, and researchers have shown that grip strength is associated with psychological wellbeing across different populations [49].
Statistical analyses
Irrespective of which study conditions the patients are grouped to, at baseline, we will use Pearson’s correlations and univariate ANOVAs to examine the associations between the participants’ social and demographic background and their psychological wellbeing, sleep, QoL, and physical fitness. To examine whether and to what degree the three study conditions (endurance training, resistance training, active control) would impact the outcome variables over time, a series of ANOVAs for repeated measures with the following factors and dependent variables will be performed, namely, time (baseline; week-3 and week 6 since start of study); group (endurance training; resistance training; active control), and the time-by-group interaction; dependent variables: depressive symptoms, anxiety, stress, sleep complaints, QoL, cardiorespiratory fitness, and grip strength. Given the explorative character of the present study, we follow the previously reported recommendations [50, 51] and no correction of p-values for multiple testing would be performed. Data would be analyzed both per protocol and by intent-to-treat (ITT), with the last observation carried forward (LOCF). Patients will not be further followed up as regards the study questions, though, they would undergo routine checks every 6 months to monitor the development and recovery process of the HGG. The level of significance is set at alpha ≤ 0.05. All computations would be performed with the Statistical Package for the Social Sciences (SPSS)® software (IBM Corporation, Armonk NY, USA) for Macintosh®.
Ethical aspects
The study has been approved by the Ethikkommission Nordwest- und Zentralschweiz (EKNZ) (Switzerland; ProjectID 2018-01314), and the study is to be performed in accordance with the ethical principles laid down in the seventh and current edition (2013) of the Declaration of Helsinki. All eligible participants would be fully informed about the aims of the study and the confidential data handling. Although the interventions are not potentially risky, an insurance would be provided upon eligibility for participation. The insurance covers all damages and injuries occurring during the time a person is at the hospital to participate in the study.
Protocol amendments
Important protocol modifications (i.e., change of assessment tools, inclusion, and exclusion criteria; change of interventions) would be communicated to the ethical committee (Ethikkommission Nordwest- und Zentralschweiz (EKNZ).
Confidentiality and data protection
Participants would be fully informed and assured that their data are to be handled with highest confidentiality from the very beginning of the study, throughout the study and also afterward, once the study has been completed. Accordingly, no data and information would be shared with third parties. Staff members involved in the study would strictly comply with professional confidentiality. In this line, also members of the EKNZ ethical committee would be strictly obliged to respect medical confidentiality, and to strictly avoid any kind of divulgation of participants’ participation and data.
Only staff members of the present study are allowed to have access to the data and information of the study and participants. However, by law, members of the local ethical committee and members of the Swiss ethics committees on research involving humans are allowed to organize inspections and to get access to the source data. However, again, members of both authorities would strictly comply with professional confidentiality.
Data would be kept in an electronic database. Once a patient is enrolled in the study, she/he receives a study number, and the study number would be only visible in the database, while the principal investigator (DC) not otherwise involved in patients’ assessments and randomization has the patient sheet (“key”) with both patient’s name and her/his study number. In all of the files (Case Report Forms, statistic files, etc.) only patient’s study number would be registered.
Monitoring
The source data/documents are accessible to independent monitors, who are not part of the research group, and questions are answered during monitoring. Again, audits and inspections of the authorities mentioned above may be performed to ensure proper study conduct and data handling procedures, as thoroughly described in the International Council of Harmonisation-Good Clinical Practice (ICH-GCP) guidelines and regulatory requirements.
Study harms
After every intervention/session, participants would be investigated for any pain felt or other/additional unpleasant and unexpected sensations, which can be plausibly associated with the study intervention. Adverse events would be listed and discussed if the participant is to be excluded from the study.
Ancillary and post-trial care
All patients would be treated, monitored, and routinely assessed as regards the development and recovery process of the HGG in the study center of Neurosurgery of the Basel University Hospital (Basel, Switzerland).
Dissemination policy
Upon request, participants would receive their personal profile of cardiovascular performance, grip strengths, and psychological functioning. Further, results would be presented at national and international congresses on oncology, psycho-oncology, neurosurgery, and sport sciences. The results would be published in open-access journals. Last, upon request, data might be shared with researchers, who are proven experts in this field.
Discussion
While there is a growing body of randomized clinical trials (RCTs) on the beneficial influence of regular exercise training on QoL in cancer survivors, such research refers exclusively to cancer such as breast cancer, prostate cancer or colorectal cancer [1, 3, 23]. By contrast, no such study has investigated the possible positive effects in cancer survivors with HGG. The present study expands upon previous RCTs in that: (1) two different interventions (endurance and resistance training) are compared with each other, (2) the control condition is an active control condition to ensure and to partial-out that the physical activity intervention per se, and not ‘merely’ the regular social contact with experts of the study center, is responsible for possible improvements to be observed, and (3) in that sleep quality is assessed; specifically as regards subjective sleep, there is substantive research to show that physical activity favorably impacts on sleep quality [52–54]. In our opinion, and from a methodological point of view, we believe the following reasons for the latter two aspects are of utmost importance.
First, as regards to the control condition, in a previous study [52] we successfully introduced a thoroughly assessed control condition to sort out that the effect of a regular jogging intervention in the morning on sleep and psychological functioning was related to physical activity intervention, but not to the social context. Specifically, all participants did meet at the same time in the same area of the school campus, took shower at the same time and had identical breakfast at the same time; thus, the only difference between the jogging and the control group was the physical activity intervention per se. Likewise, to assess the influence of adjuvant mindfulness-based stress reduction (MBSR) [55], participants of the control condition had the same frequency, duration, and intensity of social contacts with experts of the study center, as those participants undergoing the MBSR intervention. As mentioned, the active control intervention was not intended as being a ‘bona fide’ condition, which would have been intended to elicit dysfunctional cognitive-emotional processes. By contrast, in one of the very first studies on the influence of regular physical activity on depression among a smaller sample of female individuals with major depressive disorders [56], the intervention condition consisted of regular physical activity as group intervention, while to the control condition, no further contacts with the experts of the study center of with other patients were offered. Accordingly, Pilu et al. [56] correctly suspected that with such a methodological set-up, it was difficult to evaluate, if the improvement was due to a non-specific therapeutic effect associated with taking part in a social activity. In this view, in a former study, we showed that a single bout of physical activity could increase the interest of social interactions among individuals with psychiatric issues [19].
Second, in regards to sleep, there is a host of studies showing that regular physical activity has the potential to improve subjective and objective sleep [22, 52–54, 57–64]. However, such results are missing so far for both cancer survivors in general, and for cancer survivors with HGG. Further, there are also extant results to show that restoring sleep is associated with a broad range of cognitive, emotional, and behavioral advantages [65–70]. Accordingly, it is conceivable that also in the present study, improvements in physical activity are associated with improvements in sleep and psychological functioning.
Anticipated outcome and significance
With the present study, we intend to investigate the influence of two different types of exercising, endurance training, and resistance training, on cardiovascular fitness, handgrip strengths, and dimensions of psychological well-being and subjective sleep in patients with HGG, as compared to an active control condition. Accordingly, we expect that compared to the active control condition, improvements would be observed in the two exercising conditions. If so, this study would provide evidence for the implementation of regular exercising in the clinical treatment of HGG patients.
Acknowledgements
Not applicable.
Abbreviations
- 6MWT
6-min walking test
- ANOVA
analysis of variances
- BDNF
brain-derived neurotrophic factor
- CT
computer tomography
- DC
Dominik Cordier
- EKNZ
Ethikkommission Nordwest- und Zentralschweiz
- HGG
high-grade glioma
- ICH-GCP
International Council of Harmonisation-Good Clinical Practice
- ITT
intent-to-treat
- LOCF
last observation carried forward
- MBSR
mindfulness-based stress reduction
- MRI
magnet resonance imaging
- N
number of participants (whole sample)
- QoL
quality of life
- RCT
randomized clinical trial
- SF36
Quality of Life Questionnaire; short form (36 items)
- SPSS
Statistical Package for Social Sciences
- VEGF
vascular endothelial growth factor
- WHO
World Health Organization
Authors’ contributions
DC, MG, and SB are the principal investigators; they designed the study and are responsible for the trial design and study procedures. DC is a neurosurgeon and responsible for recruitment and patients’ information. MG and SB are responsible for the interventions and for the supervision of the study and participants. MG and SB are responsible for statistical analysis. DC, MG, and SB are responsible for the preparation of publications. All authors read and approved the final manuscript.
Funding
The study has no external funding.
Availability of data and materials
Once the study is completed, data would be available upon request from qualified researchers in the field.
Ethics approval and consent to participate
The Ethikkommission Nordwest- und Zentralschweiz (Basel, Switzerland) has approved the study (ProjectID 2018-01314), which is to be performed in accordance with the rules in the seventh and current form (2013) of the Declaration of Helsinki.
Consent of publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Dominik Cordier, Email: dominik.cordier@usb.ch.
Markus Gerber, Email: markus.gerber@unibas.ch.
Serge Brand, Email: serge.brand@upkbs.ch.
References
- 1.Courneya KS. Exercise in cancer survivors: an overview of research. Med Sci Sports Exerc. 2003;35(11):1846–1852. doi: 10.1249/01.Mss.0000093622.41587.B6. [DOI] [PubMed] [Google Scholar]
- 2.Hayes SC, Rye S, Disipio T, Yates P, Bashford J, Pyke C, et al. Exercise for health: a randomized, controlled trial evaluating the impact of a pragmatic, translational exercise intervention on the quality of life, function and treatment-related side effects following breast cancer. Breast Cancer Res Treat. 2013;137(1):175–186. doi: 10.1007/s10549-012-2331-y. [DOI] [PubMed] [Google Scholar]
- 3.Segal R, Zwaal C, Green E, Tomasone JR, Loblaw A, Petrella T. Exercise for people with cancer: a systematic review. Curr Oncol. 2017;24(4):e290–e315. doi: 10.3747/co.24.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ostrom QT, Gittleman H, Liao P, Rouse C, Chen Y, Dowling J, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro-oncology. 2014;16(Suppl 4):iv1–iv63. doi: 10.1093/neuonc/nou223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ostrom QT, Bauchet L, Davis FG, Deltour I, Fisher JL, Langer CE, et al. The epidemiology of glioma in adults: a “state of the science” review. Neuro-oncology. 2014;16(7):896–913. doi: 10.1093/neuonc/nou087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ostrom QT, Gittleman H, Stetson L, Virk S, Barnholtz-Sloan JS. Epidemiology of intracranial gliomas. Prog Neurol Surg. 2018;30:1–11. doi: 10.1159/000464374. [DOI] [PubMed] [Google Scholar]
- 7.Deslandes A, Moraes H, Ferreira C, Veiga H, Silveira H, Mouta R, et al. Exercise and mental health: many reasons to move. Neuropsychobiology. 2009;59(4):191–198. doi: 10.1159/000223730. [DOI] [PubMed] [Google Scholar]
- 8.Granata C, Jamnick NA, Bishop DJ. Principles of exercise prescription, and how they influence exercise-induced changes of transcription factors and other regulators of mitochondrial biogenesis. Sports Med. 2018;48(7):1541–1559. doi: 10.1007/s40279-018-0894-4. [DOI] [PubMed] [Google Scholar]
- 9.Knapen J, Van de Vliet P, Van Coppenolle H, David A, Peuskens J, Pieters G, et al. Comparison of changes in physical self-concept, global self-esteem, depression and anxiety following two different psychomotor therapy programs in nonpsychotic psychiatric inpatients. Psychother Psychosom. 2005;74(6):353–361. doi: 10.1159/000087782. [DOI] [PubMed] [Google Scholar]
- 10.Knapen J, Vancampfort D, Morien Y, Marchal Y. Exercise therapy improves both mental and physical health in patients with major depression. Disabil Rehabil. 2015;37(16):1490–1495. doi: 10.3109/09638288.2014.972579. [DOI] [PubMed] [Google Scholar]
- 11.Zamani Sani SH, Fathirezaie Z, Brand S, Puhse U, Holsboer-Trachsler E, Gerber M, et al. Physical activity and self-esteem: testing direct and indirect relationships associated with psychological and physical mechanisms. Neuropsychiatr Dis Treat. 2016;12:2617–2625. doi: 10.2147/ndt.s116811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stubbs B, Vancampfort D, Hallgren M, Firth J, Veronese N, Solmi M, et al. EPA guidance on physical activity as a treatment for severe mental illness: a meta-review of the evidence and Position Statement from the European Psychiatric Association (EPA), supported by the International Organization of Physical Therapists in Mental Health (IOPTMH) Eur Psychiatry. 2018;54:124–144. doi: 10.1016/j.eurpsy.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Gerber M, Holsboer-Trachsler E, Puhse U, Brand S. Exercise is medicine for patients with major depressive disorders: but only if the “pill” is taken! Neuropsychiatr Dis Treat. 2016;12:1977–1981. doi: 10.2147/NDT.S110656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schuch FB, Morres ID, Ekkekakis P, Rosenbaum S, Stubbs B. A critical review of exercise as a treatment for clinically depressed adults: time to get pragmatic. Acta Neuropsychiatr. 2016 doi: 10.1017/neu.2016.21. [DOI] [PubMed] [Google Scholar]
- 15.Stubbs B, Rosenbaum S, Vancampfort D, Ward PB, Schuch FB. Exercise improves cardiorespiratory fitness in people with depression: a meta-analysis of randomized control trials. J Affect Disord. 2016;190:249–253. doi: 10.1016/j.jad.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 16.Vancampfort D, Stubbs B, Sienaert P, Wyckaert S, De Hert M, Rosenbaum S, et al. What are the factors that influence physical activity participation in individuals with depression? A review of physical activity correlates from 59 studies. Psychiatr Danub. 2015;27(3):210–224. [PubMed] [Google Scholar]
- 17.Nebiker L, Lichtenstein E, Minghetti A, Zahner L, Gerber M, Faude O, et al. Moderating effects of exercise duration and intensity in neuromuscular vs. endurance exercise interventions for the treatment of depression: a meta-analytical review. Front Psychiatry. 2018;9:305. doi: 10.3389/fpsyt.2018.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ambrose KR, Golightly YM. Physical exercise as non-pharmacological treatment of chronic pain: why and when. Best Pract Res Clin Rheumatol. 2015;29(1):120–130. doi: 10.1016/j.berh.2015.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brand S, Colledge F, Ludyga S, Emmenegger R, Kalak N, Sadeghi Bahmani D, et al. Acute bouts of exercising improved mood, rumination and social interaction in inpatients with mental disorders. Front Psychol. 2018;9:249. doi: 10.3389/fpsyg.2018.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colledge F, Brand S, Puhse U, Holsboer-Trachsler E, Zimmerer S, Schleith R, et al. A twelve-week moderate exercise programme improved symptoms of depression, insomnia, and verbal learning in post-aneurysmal subarachnoid haemorrhage patients: a comparison with meningioma patients and healthy controls. Neuropsychobiology. 2018 doi: 10.1159/000486903. [DOI] [PubMed] [Google Scholar]
- 21.Colledge F, Brand S, Zimmerer S, Puhse U, Holsboer-Trachsler E, Gerber M. In individuals following aneurysmal subarachnoid haemorrhage, hair cortisol concentrations are higher and more strongly associated with psychological functioning and sleep complaints than in healthy controls. Neuropsychobiology. 2017;75(1):12–20. doi: 10.1159/000477966. [DOI] [PubMed] [Google Scholar]
- 22.Gerber M, Colledge F, Puhse U, Holsboer-Trachsler E, Zimmerer S, Brand S. Sleep quality, sleep EEG pattern, mental well-being and cortisol secretion in patients with ruptured aneurysm post-treatment: a comparison with post-surgery meningioma patients and controls. Neuropsychobiology. 2016;73(3):148–159. doi: 10.1159/000444492. [DOI] [PubMed] [Google Scholar]
- 23.Segal R, Zwaal C, Green E, Tomasone JR, Loblaw A, Petrella T. Exercise for people with cancer: a clinical practice guideline. Curr Oncol. 2017;24(1):40–46. doi: 10.3747/co.24.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Julious SA. Sample size of 12 per group rule of thumb for a pilot study. Pharm Stat. 2005;4(4):287–291. doi: 10.1002/pst.185. [DOI] [Google Scholar]
- 25.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–191. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- 26.Goyal M, Singh S, Sibinga EM, Gould NF, Rowland-Seymour A, Sharma R, et al. Meditation programs for psychological stress and well-being: a systematic review and meta-analysis. JAMA Intern Med. 2014;174(3):357–368. doi: 10.1001/jamainternmed.2013.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wampold BE, Mondin GW, Moody M, Stich F, Benson K, Ahn H-N. A meta-analysis of outcome studies comparing bona fide psychotherapies: empirically, “all must have prizes”. Psychol Bull. 1997;122(3):203–215. doi: 10.1037/0033-2909.122.3.203. [DOI] [Google Scholar]
- 28.Jahangard L, Sadeghi A, Ahmadpanah M, Holsboer-Trachsler E, Sadeghi Bahmani D, Haghighi M, et al. Influence of adjuvant omega-3-polyunsaturated fatty acids on depression, sleep, and emotion regulation among outpatients with major depressive disorders—results from a double-blind, randomized and placebo-controlled clinical trial. J Psychiatr Res. 2018;107:48–56. doi: 10.1016/j.jpsychires.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 29.Haghighi M, Salehi I, Erfani P, Jahangard L, Bajoghli H, Holsboer-Trachsler E, et al. Additional ECT increases BDNF-levels in patients suffering from major depressive disorders compared to patients treated with citalopram only. J Psychiatr Res. 2013;47(7):908–915. doi: 10.1016/j.jpsychires.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 30.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 31.Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the state–trait anxiety inventory. Palo Alto: Consulting Psychologists Press; 1983. [Google Scholar]
- 32.Laux L, Glanzmann P, Schaffner P, Spielberger CD. Das State-Trait-Angstinventar: Theoretische Grundlagen und Handanweisung. Weinheim: Beltz; 1981. [Google Scholar]
- 33.Brand S, Zimmerer S, Kalak N, Planta SV, Schwenzer-Zimmerer K, Muller AA, et al. Compared to controls, patients with ruptured aneurysm and surgical intervention show increase in symptoms of depression and lower cognitive performance, but their objective sleep is not affected. World J Biol Psychiatry. 2015;16(2):96–105. doi: 10.3109/15622975.2014.888093. [DOI] [PubMed] [Google Scholar]
- 34.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. doi: 10.2307/2136404. [DOI] [PubMed] [Google Scholar]
- 35.Chiu YH, Lu FJ, Lin JH, Nien CL, Hsu YW, Liu HY. Psychometric properties of the Perceived Stress Scale (PSS): measurement invariance between athletes and non-athletes and construct validity. PeerJ. 2016;4:e2790. doi: 10.7717/peerj.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. doi: 10.1016/S1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 37.Gerber M, Lang C, Lemola S, Colledge F, Kalak N, Holsboer-Trachsler E, et al. Validation of the German version of the insomnia severity index in adolescents, young adults and adult workers: results from three cross-sectional studies. BMC Psychiatry. 2016;16:174. doi: 10.1186/s12888-016-0876-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jahangard L, Fadaei V, Sajadi A, Haghighi M, Ahmadpanah M, Matinnia N, et al. Patients with OCD report lower quality of life after controlling for expert-rated symptoms of depression and anxiety. Psychiatry Res. 2018;260:318–323. doi: 10.1016/j.psychres.2017.11.080. [DOI] [PubMed] [Google Scholar]
- 39.Alirezaei P, Ahmadpanah M, Rezanejad A, Soltanian A, Sadeghi Bahmani D, Brand S. Compared to controls, individuals with Lichen Planopilaris have more depression, a lower self-esteem, and a lower quality of life. Neuropsychobiology. 2019 doi: 10.1159/000499135. [DOI] [PubMed] [Google Scholar]
- 40.Stewart AL, Hays RD, Ware JE., Jr The MOS short-form general health survey. Reliability and validity in a patient population. Med Care. 1988;26(7):724–735. doi: 10.1097/00005650-198807000-00007. [DOI] [PubMed] [Google Scholar]
- 41.Maurischat C, Ehlebracht-Konig I, Kuhn A, Bullinger M. Structural validity of the short form 36 (SF-36) in patients with rheumatic diseases. Z Rheumatol. 2005;64(4):255–264. doi: 10.1007/s00393-005-0676-x. [DOI] [PubMed] [Google Scholar]
- 42.Solway S, Brooks D, Lacasse Y, Thomas S. A qualitative systematic overview of the measurement properties of functional walk tests used in the cardiorespiratory domain. Chest. 2001;119(1):256–270. doi: 10.1378/chest.119.1.256. [DOI] [PubMed] [Google Scholar]
- 43.Bellet RN, Adams L, Morris NR. The 6-minute walk test in outpatient cardiac rehabilitation: validity, reliability and responsiveness—a systematic review. Physiotherapy. 2012;98(4):277–286. doi: 10.1016/j.physio.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 44.Buhagiar MA, Naylor JM, Harris IA, Xuan W, Adie S, Lewin A. Assessment of outcomes of inpatient or clinic-based vs home-based rehabilitation after total knee arthroplasty: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(4):e192810. doi: 10.1001/jamanetworkopen.2019.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ciani O, Piepoli M, Smart N, Uddin J, Walker S, Warren FC, et al. Validation of exercise capacity as a surrogate endpoint in exercise-based rehabilitation for heart failure: a meta-analysis of randomized controlled trials. JACC Heart Fail. 2018;6(7):596–604. doi: 10.1016/j.jchf.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 46.Schmidt K, Vogt L, Thiel C, Jager E, Banzer W. Validity of the six-minute walk test in cancer patients. Int J Sports Med. 2013;34(7):631–636. doi: 10.1055/s-0032-1323746. [DOI] [PubMed] [Google Scholar]
- 47.Granger CL, Denehy L, Parry SM, Martin J, Dimitriadis T, Sorohan M, et al. Which field walking test should be used to assess functional exercise capacity in lung cancer? An observational study. BMC Pulm Med. 2015;15:89. doi: 10.1186/s12890-015-0075-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mählmann L, Gerber M, Furlano RI, Legeret C, Kalak N, Holsboer-Trachsler E, et al. Aerobic exercise training in children and adolescents with inflammatory bowel disease: influence on psychological functioning, sleep and physical performance—an exploratory trial. Ment Health Phys Act. 2017;13:30–39. doi: 10.1016/j.mhpa.2017.09.002. [DOI] [Google Scholar]
- 49.Mahlmann L, Gerber M, Furlano RI, Legeret C, Kalak N, Holsboer-Trachsler E, et al. Psychological wellbeing and physical activity in children and adolescents with inflammatory bowel disease compared to healthy controls. BMC Gastroenterol. 2017;17(1):160. doi: 10.1186/s12876-017-0721-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harris AH, Reeder R, Hyun JK. Common statistical and research design problems in manuscripts submitted to high-impact psychiatry journals: what editors and reviewers want authors to know. J Psychiatr Res. 2009;43(15):1231–1234. doi: 10.1016/j.jpsychires.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 51.Hair JF, Black CW, Babin BJ, Anderson RE. Multivariate data analysis. 7. Essex: Pearson Education Limited; 2014. [Google Scholar]
- 52.Kalak N, Gerber M, Kirov R, Mikoteit T, Yordanova J, Puhse U, et al. Daily morning running for 3 weeks improved sleep and psychological functioning in healthy adolescents compared with controls. J Adolesc Health. 2012;51(6):615–622. doi: 10.1016/j.jadohealth.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 53.Kredlow MA, Capozzoli MC, Hearon BA, Calkins AW, Otto MW. The effects of physical activity on sleep: a meta-analytic review. J Behav Med. 2015;38(3):427–449. doi: 10.1007/s10865-015-9617-6. [DOI] [PubMed] [Google Scholar]
- 54.Chennaoui M, Arnal PJ, Sauvet F, Leger D. Sleep and exercise: a reciprocal issue? Sleep Med Rev. 2015;20:59–72. doi: 10.1016/j.smrv.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 55.Jasbi M, Sadeghi Bahmani D, Karami G, Omidbeygi M, Peyravi M, Panahi A, et al. Influence of adjuvant mindfulness-based cognitive therapy (MBCT) on symptoms of post-traumatic stress disorder (PTSD) in veterans—results from a randomized control study. Cogn Behav Ther. 2018 doi: 10.1080/16506073.2018.1445773. [DOI] [PubMed] [Google Scholar]
- 56.Pilu A, Sorba M, Hardoy MC, Floris AL, Mannu F, Seruis ML, et al. Efficacy of physical activity in the adjunctive treatment of major depressive disorders: preliminary results. Clin Pract Epidemiol Ment Health. 2007;3:8. doi: 10.1186/1745-0179-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brand S, Jossen S, Holsboer-Trachsler E, Puhse U, Gerber M. Impact of aerobic exercise on sleep and motor skills in children with autism spectrum disorders—a pilot study. Neuropsychiatr Dis Treat. 2015;11:1911–1920. doi: 10.2147/NDT.S85650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brand S, Beck J, Gerber M, Hatzinger M, Holsboer-Trachsler E. Evidence of favorable sleep-EEG patterns in adolescent male vigorous football players compared to controls. World J Biol Psychiatry. 2010;11(2 Pt 2):465–475. doi: 10.1080/15622970903079820. [DOI] [PubMed] [Google Scholar]
- 59.Brand S, Beck J, Gerber M, Hatzinger M, Holsboer-Trachsler E. ‘Football is good for your sleep’: favorable sleep patterns and psychological functioning of adolescent male intense football players compared to controls. J Health Psychol. 2009;14(8):1144–1155. doi: 10.1177/1359105309342602. [DOI] [PubMed] [Google Scholar]
- 60.Brand S, Gerber M, Beck J, Hatzinger M, Puhse U, Holsboer-Trachsler E. High exercise levels are related to favorable sleep patterns and psychological functioning in adolescents: a comparison of athletes and controls. J Adolesc Health. 2010;46(2):133–141. doi: 10.1016/j.jadohealth.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 61.Brand S, Gerber M, Kalak N, Kirov R, Lemola S, Clough PJ, et al. “Sleep well, our tough heroes!”—in adolescence, greater mental toughness is related to better sleep schedules. Behav Sleep Med. 2014;12(6):444–454. doi: 10.1080/15402002.2013.825839. [DOI] [PubMed] [Google Scholar]
- 62.Brand S, Gerber M, Kalak N, Kirov R, Lemola S, Clough PJ, et al. Adolescents with greater mental toughness show higher sleep efficiency, more deep sleep and fewer awakenings after sleep onset. J Adolesc Health. 2014;54(1):109–113. doi: 10.1016/j.jadohealth.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 63.Lang C, Kalak N, Brand S, Holsboer-Trachsler E, Puhse U, Gerber M. The relationship between physical activity and sleep from mid adolescence to early adulthood. A systematic review of methodological approaches and meta-analysis. Sleep Med Rev. 2016;28:32–45. doi: 10.1016/j.smrv.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 64.Sadeghi Bahmani D, Gerber M, Kalak N, Lemola S, Clough PJ, Calabrese P, et al. Mental toughness, sleep disturbances, and physical activity in patients with multiple sclerosis compared to healthy adolescents and young adults. Neuropsychiatr Dis Treat. 2016;12:1571–1579. doi: 10.2147/ndt.S111208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brand S, Kirov R, Kalak N, Gerber M, Puhse U, Lemola S, et al. Perfectionism related to self-reported insomnia severity, but not when controlled for stress and emotion regulation. Neuropsychiatr Dis Treat. 2015;11:263–271. doi: 10.2147/NDT.S74905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brand S, Kirov R, Kalak N, Gerber M, Schmidt NB, Lemola S, et al. Poor sleep is related to lower emotional competence among adolescents. Behav Sleep Med. 2016;14(6):602–614. doi: 10.1080/15402002.2015.1048450. [DOI] [PubMed] [Google Scholar]
- 67.Killgore WD. Effects of sleep deprivation on cognition. Prog Brain Res. 2010;185:105–129. doi: 10.1016/b978-0-444-53702-7.00007-5. [DOI] [PubMed] [Google Scholar]
- 68.Killgore WD, Kahn-Greene ET, Lipizzi EL, Newman RA, Kamimori GH, Balkin TJ. Sleep deprivation reduces perceived emotional intelligence and constructive thinking skills. Sleep Med. 2008;9(5):517–526. doi: 10.1016/j.sleep.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 69.Curcio G, Ferrara M, De Gennaro L. Sleep loss, learning capacity and academic performance. Sleep Med Rev. 2006;10(5):323–337. doi: 10.1016/j.smrv.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 70.Tempesta D, Socci V, De Gennaro L, Ferrara M. Sleep and emotional processing. Sleep Med Rev. 2018;40:183–195. doi: 10.1016/j.smrv.2017.12.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Once the study is completed, data would be available upon request from qualified researchers in the field.