Abstract
Introduction:
Human amnion membrane mesenchymal stem cells (hAMSCs) and human umbilical cord mesenchymal stem cells (hUC-MSCs) are potential, non invasive sources of stem cells used for bone tissue engineering. Phenotyping characterization is an extremely important consideration in the choice of the appropriate passage in order to maximize its osteogenic differentiation potential.
Aim:
To explore phenotype characteristics and compare osteogenic differentiation potential of hAMSCs and hUC-MSCs.
Method:
Isolation and culture were performed on hAMSCs and hUC-MSCs from a healthy woman in her 38th weeks of pregnancy. CD90, CD105 and CD73 phenotype characterization was done in passage 4-7. An osteogenic differentiation examination of hAMSCs and hUC-MSCs with Alizarin red staining and RUNX2 expression was performed in the passage that had appropriate expressions of phenotype characteristics.
Results:
The expression of CD90 hUC-MSCs was higher than that of hAMSCs in all passages. CD105 hUC-MSCs was higher in passage 4-6, while CD105 hAMSCs was equal to that of hUC-MSCs in passage 7. CD73 hUC-MSCs was higher than hAMSCs in passage 4 and 5, while in passage 6 and 7 hAMSCs was higher than hUC-MSCs. There was a decrease in the number of CD90, CD105 and CD73 on hAMSCs and hUC-MSCs in passage 5, then determined as appropriate passage. Alizarin red staining examination showed calcium deposition and revealed no significant difference, but RUNX2 expression of hUC-MSCs was significantly higher than that for hAMSCs.
Conclusion:
Both hAMSCs and hUC-MSCs had phenotype characteristics of mesenchymal stem cell and showed ostegenic differentiation potential.
Keywords: Umbilical Cord, Mesenchymal Stem Cells, Osteogenesis, Phenotype Flow Cytometry, Alizarin Red Immunohistochemistry
1. INTRODUCTION
Stem cells have the ability to renew and differentiate into various tissues such as bone as part of bone tissue engineering (1). Several sources of mesenchymal stem cells (MSCs) are human amniotic MSCs (hAMSCs) within the amniotic membrane and human umbilical cord MSCs (hUC-MSCs) derived from the umbilical cord. The advantage of hAMSC and hUC-MSCs is due to non-invasive and lack of morbidity during procurement process. In addition to ease of access, hUC-MSC is plentiful, easily reproduced and possesses high levels of immunocompatibility (2). Both hAMSCs and hUC-MSCs features a more primitive cell with the ability to differentiate into distinct, multipotent, and capable of repairing and differentiating into osteoblast (3). Recently, hUC-MSCs is being considered a conventional source. We are on our way to explore hAMSCs as an alternative for a better osteogenic potential.
Mesenchymal stem cells expressed CD90, CD105 and CD73 surface marker (4). hAMSCs have shown a larger population and more promising, 70-97% had a CD73 and 6–8% expressed CD105 (5, 6) compared to hUC-MSCs which had more than 95% of CD73, CD90 and 7.5% of CD 105.(7, 8) However, it is significantly influenced by cell passage. CD105 of hAMSCs reached its maximum 10% after passage 1, but after passage 4, fell down to 2%. CD73 remained stable in many passage (5). hUC-MSCs CD105 marker also decreased in passage 4-8 (9). hUC-MSCs showed increased activity of alkaline phosphatase and mineralization in passage 5-8 (10) attempts to isolate MSCs from umbilical cord blood (UCB). Both of amnion and umbilical cord-derived MSCs of canine model, showed poor osteogenic differentiation at early passage (11). However, the osteogenic differentiation potential of hAMSCs based on specific passage has not been widely studied.
Expansion of hAMSCs is possible until passage 5 without any morphological changes (12). Some studies kept the cells in culture for 15-20 passages before reaching senescence (13, 14). Having the same condition, the hUC-MSCs expansion could be maintained from passage 1-18 (7). Late passage should be avoid because risk of cell senescence.
Alizarin Red staining detected osteoblastic differentiation in hAMSCs and hUC-MSCs (15, 16). Runt-related transcription factor 2 (RUNX2) is an early stage osteoblastic differentiation marker which important in osteogenesis.(17). Both hAMSCs and hUC-MSCs expressed RUNX2 (18, 19), but which one is more superior, remain unclear.
2. AIM
This research aimed to explore CD90, CD105 and CD73 phenotype characteristics of hAMSCs and hUC-MSCs at various passages and determine the most appropriate passage. Osteogenic differentiation potential between hAMSCs and hUC-MSCs then compared by Alizarin red staining and RUNX2 expression examination.
3. METHODS
This is an in vitro laboratory-based experimental study using hAMSCs and hUC-MSCs of a healthy woman in her 38th weeks of pregnancy. It was granted ethical approval by The Research Ethics Committee, Dr. Soetomo General Hospital, Surabaya. The isolation procedure was performed using stem cell laboratory protocols at the Stem Cell Research and Development Centre, Airlangga University.
3.1. Isolation of hAMSCs
Human Amnion Membrane (hAM), was cut into sections and placed into a tube containing 0.25% Trypsin (Gibco BRL, Gaithersburg, MD, USA) then incubated. The solution was removed and replaced with 0.75 mg/ml Collagenase Type IV (Sigma-Aldrich, St. Louis, MO, USA) and 0.075 mg/ml DNase I solution (Takara Bio, Shiga, Japan). Pellet obtained was added to Dulbecco’s Modified Eagle’s Medium (DMEM)/Hams’s F-12 (1:1) (Gibco BRL, Gaithersburg, MD, USA). A medium containing cells was then incubated. Cell growth was observed daily, the medium being replaced every three days, on reaching confluence, passage was also performed.
3.2. Isolation of hUC-MSCs
The section of umbilical cord was cut about 1 cm and placed in a tube containing 0.25% Trypsin. Samples were immersed in Phosphate Buffered Saline (PBS) (1X, pH 7.4), containing 0.75 mg/ml of Collagenase Type IV and 0.075 mg/mL DNase I then incubated. Filtering was carried out using a cell strainer. Pellets were suspended in DMEM. A medium containing cells was then incubated. Replacement of the medium was performed every three days, with passage being carried out after confluence had occurred.
3.3. Flow cytometry Phenotypic Characterization
Characterization of hAMSCs and hUC-MSCs phenotype was performed by means of flow cytometry. In passage 4-7, MSCs were seeded in well with Alpha Minimum Essential Medium (αMEM) (Sigma-Aldrich, St. Louis, MO, USA). Afterwards, were fixed with 10% formaldehyde and incubated using the Human MSC Analysis Kit (BD Bioscience, USA) with the addition of a CD90, CD105 and CD73 and negative CD45 cocktail primary antibodies. The primary antibody was labeled using Fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse antibody (Sigma-Aldrich, St. Louis, MO, USA). The cells were then viewed and analyzed by Fluorescence Assisted Cell Sorting (FACS) Calibur flow cytometer (BD Bioscience, USA).
3.4. Osteogenic Potential Examination
3.4.1. Alizarin Red Staining.
The culture of hAMSCs and hUC-MSCs used in this study was in passage 5. Cells were cultured on a microplate containing osteogenic medium, consisting of αMEM media to which was added 50 μM of ascorbate phosphate (Sigma-Aldrich, St. Louis, MO, USA), 10 μM of glycerol phosphate (Sigma-Aldrich, St. Louis, MO, USA) and 0.1 μM dexamethasone (Sigma-Aldrich, St. Louis, MO, USA). The control group was inserted into a petri dish containing α-MEM. The hAMSCs and hUC-MSCs suspensions were implanted into microplate at a density of 2x106 cells/cm2 before an osteogenic medium was added. The medium was changed every three days. After 21 days of duration, the medium was eliminated and fixed using 10% formaldehyde. Alizarin red solution (Calcified Nodule Staining Kit, Cosmo Bio Co., Ltd., Tokyo, Japan) was added. Cell observations were performed by a 100x magnification inverted Nikon microscope (Nikon Metrology NV., Japan). Differentiated cells containing calcium mineral deposits, characteristic of osteoblast, would be colored red. The percentage of positive alizarin red-stained cells was expressed as mean ± standard deviation.
3.4.2. Immunocytochemistry
In passage 5, RUNX2 expression of hAMSCs and hUC-MSCs was examined. Cell suspensions were implanted into a microplate at a density of 2x106cells/cm2. A primary RUNX2 antibody (Abcam, Cambridge, MA, USA) and then biotinylated goat anti-polyvalent (Abcam, Cambridge, MA, USA) was added to the solution. Furthermore, streptavidin peroxidase (Abcam, Cambridge, MA, USA) was added also. One drip of 3,3’ Diaminobenzidine (DAB) (Sigma-Aldrich, St. Louis, MO, USA) Plus chromogen was added to 2 ml of 3,3’ DAB Plus Substrate, mixed and deposited into the cells and then incubated. Fluorescence microscope examination was performed and image processing was done by ImageJ (LOCI, University of Wisconsin).
3.5. Data Analysis
The data obtained was presented in the form of average value and standard deviation. The data underwent statistical analysis using R Version 3.4.0. statistics software (GNU, Auckland, New Zealand). A value of p < 0.05 were considered statistically significant.
4. RESULTS
4.1. hAMSCs and hUC-MSCs Phenotype Characteristics.
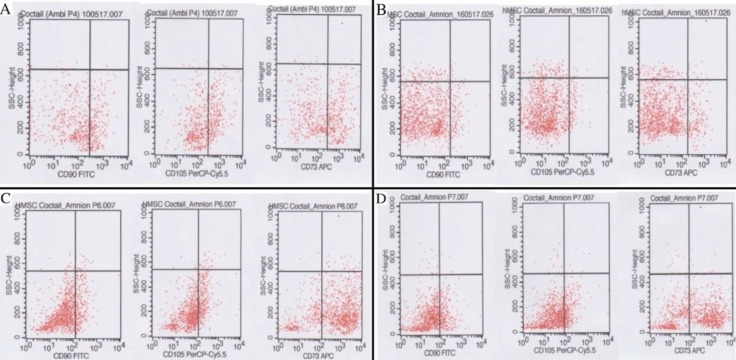
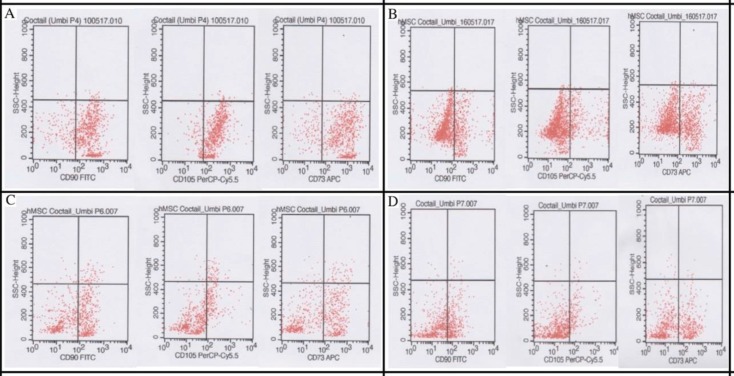
Flow cytometry result can be seen on Figure 1 and 2. Comparison of hAMSCs and hUC-MSCs phenotype characteristics on each passage shown on Figure 3.
Figure 1. CD90, CD105, CD73 of hAMSCs Flow Cytometry. (A) Passage 4; (B) Passage 5; (C) Passage 6; (D) Passage 7.
Figure 2. CD90, CD105, CD73 of hUCMSCs flow cytometry. (A) Passage 7; (B) Passage 5; (C) Passage 6; (D) Passage 7.
Based on Table 1, Mean of CD90 expression in hUC-MSCs was higher in passage 4-7 than in hAMSCs. Meanwhile, the number of CD105 hUC-MSCs was higher than hAMSCs in passage 4-6, whereas in passage 7, CD105 hAMSCs was almost the same as hUCSMCs. The number of CD73 hUCMSCs in passage 4 and 5 was higher than hAMSCs, but in passage 6 and 7 hAMSCs were more numerous than hUC-MSCs.
Table 1. Flow cytometry CD90, CD105 and CD73 comparison for hAMSCs and hUC-MSCs.
| Passage | CD | hAMSCs | hUC-MSCs |
|---|---|---|---|
| 4 | CD90 | 28.78 | 80.48 |
| CD105 | 36.95 | 86.33 | |
| CD73 | 44.41 | 84.34 | |
| 5 | CD90 | 8.79 | 23.53 |
| CD105 | 6.88 | 17.83 | |
| CD73 | 11.69 | 34.07 | |
| 6 | CD90 | 19.63 | 53.98 |
| CD105 | 27.84 | 30.79 | |
| CD73 | 74.24 | 54.67 | |
| 7 | CD90 | 21.9 | 40.08 |
| CD105 | 20.96 | 20.25 | |
| CD73 | 59.18 | 41.14 |
In passage 5, there was a decrease in the number of CD90, CD105 and CD73 in hAMSCs and hUCMSCs but in passages 6 and 7 it began to increase, although the number of each CD was not as high as in passage 4.
4.2. Osteogenic Potential of hAMSCs and hUC-MSCs
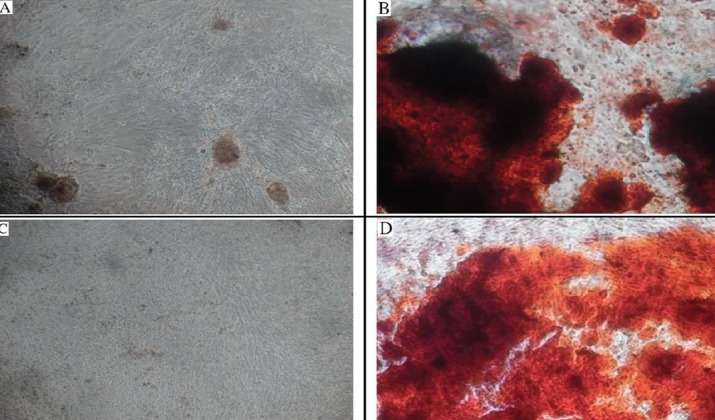
The microscopic view of the Alizarin red staining can be seen on Figure 4. Figure 5 showed percentage of alizarin red-stained cells on hAMSC was 77.3 ± 18.14 % and 75.75 ± 16.08 % on hUC-MSCs.
Figure 4. Osteogenic Differentiation as Demonstrated by Alizarin Red staining.
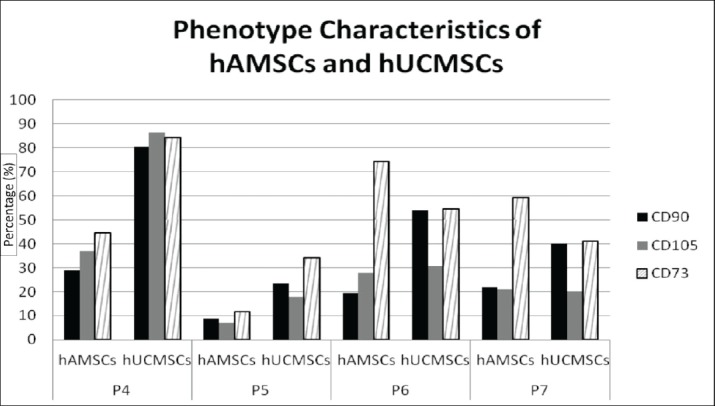
Figure 5. (A) Percentage of Positive Alizarin Red-stained Cells of hAMSCs and hUC-MSCs (%); (B) RUNX2 Expression of hAMSCs and hUC-MSCs Relative to Control. Data Presented as Mean ± SD (n=15).
Calcific Deposition by Cells of an Osteogenic Lineage was Stained Red. (A) Control hAMSCs. (B) Differentiated hAMSCs. (C) Control hUC-MSCs and (D) Differentiated hUC-MSCs.
P value of data analysis process was 0.713. No significant difference existed between the osteogenic differentiation of hAMSCs and hUC-MSCs within the Alizarin red examination as shown on Table 2.
Table 2. Osteogenic differentiation of hAMSCs and hUC-MSCs on alizarin red staining and RUNX2 expression examination.
| hAMSCs hUC-MSCs p value |
| Percentage positive alizarin 77.3 ± 18.14 % 75.75 ± 16.08 % 0.713 red-stained cells |
| RUNX2 expression 4.70 ± 0.18 6.25 ± 0.8 0.022 relative to control |
Fluorescence microscope photograph of the RUNX2 expression can be seen on Figure 6. The highest expression was found in hUC-MSCs with mean value 6.25 ± 0.82 while in hAMSCs with the mean value 4.70 ± 0.18 relative to control. The expression was counted and shown in Table 2.
Figure 6. Immunocytochemistry Photographed of RUNX2 Expression by Fluorescent Microscope. (A) Control hAMSCs, (B) Differentiated hAMSCs, (C) Control hUC-MSCs, (D) Differentiated hUC-MSCs.
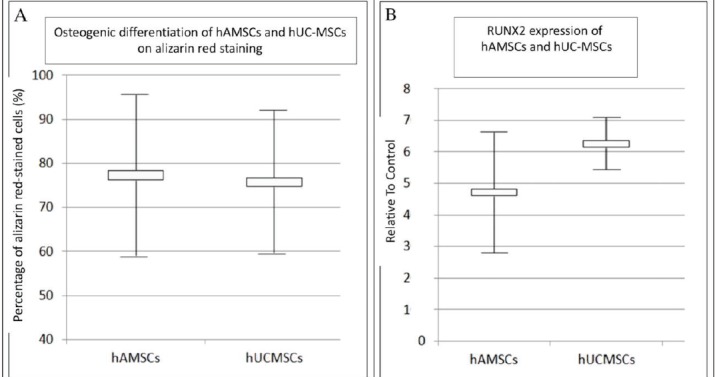
Data analysis was conducted and found that p value = 0.022, indicating significant differences in the RUNX2 expression of hAMSCs and hUC-MSCs. The RUNX2 expression of hUC-MSCs was higher than that of hAMSCs can be seen on Figure 5.
5. DISCUSSION
Based on our study, Both of hAMSCs and hUC-MSCs showed therapeutic potential because they expressed CD90, CD105 and CD73 (20) and it shows applications to numerous incurable diseases. hMSCs show several superior properties for therapeutic use compared to other types of stem cells. Different cell types are discussed in terms of their advantages and disadvantages, with focus on the characteristics of hMSCs. hMSCs can proliferate readily and produce differentiated cells that can substitute for the targeted affected tissue. To maximize the therapeutic effects of hMSCs, a substantial number of these cells are essential, requiring extensive ex vivo cell expansion. However, hMSCs have a limited lifespan in an in vitro culture condition. The senescence of hMSCs is a double-edged sword from the viewpoint of clinical applications. Although their limited cell proliferation potency protects them from malignant transformation after transplantation, senescence can alter various cell functions including proliferation, differentiation, and migration, that are essential for their therapeutic efficacy. Numerous trials to overcome the limited lifespan of mesenchymal stem cells are discussed. Level of CD90, CD105 and CD73, both of hAMSCs and hUC-MSCs, were variable in each passage. Our results are similar to previous study that cell passage affected the cell phenotype (21).
Highest CD90 expression was found in passage 4, while the lowest was in passage 5. Decreasing numbers of CD90 will result in a reduction in CD166 and reflects low pluripotency. A decreasing level of CD90 will also result in increased osteogenic differentiation which is marked by an increase in calcium mineral deposits on Alizarin red examination. A low CD90 count also plays an important role in enhancing MSC differentiation in vitro (22).
Endoglin (CD105) is a Transforming Growth Factor Beta (TGF-β) receptor III that important in TGF-β signaling during MSC chondrogenic differentiation. Low expression of CD105, as in passage 5, will increase both osteogenic differentiation in vitro and in vivo. CD105 also shows that it activates the function of TGF-β1 which serves as an inhibitor of osteogenic differentiation of MSCs (23).
The highest CD105 expression of hUCMSCs was found in passage 4 and decreased in passages 5-7. The lowest hAMSCs CD105 was revealed in passage 5, while the other passages were almost identical. This is consistent with previous study suggesting that CD105 expression decreased in passages 3-5 (24). A higher CD73 count will also increase chondrogenesis, but during the fibroblasts osteogenic differentiation process, the CD73 count will decrease (25). Moreover, absence of CD73 expression relative to control during osteogenesis was showed by Western blot analysis (26).
The highest levels of CD73, CD90 and CD105 hUC-MSCs were found in passage 4 and lowest in passage 5. This was in line with studies involving animal (canine) umbilical cords on which were performed serial passages, from passages 1-5, confirming that cell growth increased in passage 4 and then decreased in passage 5 (24). This result is contrary to study by Gong et al which hUC-MSCs exhibited similar phenotype characteristics from passage 0-15 (27).
We determined passage 5 as an appropriate passage based on positive but lowest CD73, CD105 and CD90 both of hAMSCs and hUC-MSCs. It supported by Bilic et al study, In passage 0 and 1, hAMSCs expressed CD73 >92% and CD90 > 95%, showed weak insignificant osteogenic differentiation, only less than 10% of cultured stained positive for Alkaline phosphatase (ALP) (12). In passage 5, hUC-MSCs cultured displayed osteogenesis capacity in vivo (28) and hAMSCs showed no morphological change (12).
Human amnion MSCs expressed embryonic markers such as stage specific embryonic antigen SSEA-3 and SSEA-4 by flow cytometry and octamer-binding protein Oct-3/4 by immunocytochemistry, in passage 0-1 and gradually decreased over passage 4 (12). Umbilical cord MSCs showed SSEA-4 and Oct-4 on cell culture in passages 1-3 (29). Replicative senescence was showed by hAMSCs in passages 18-22 and hUC-MSCs over passage 15 (13, 30). Therefore, appropriate passage in our study (passage 5), is less pluripotent, having lower risk of malignant transformation and provided good proliferative capacity.
Ostoegenic differentiation of MSC is observed through the presence of mineral nodules on alizarin red staining (31). This study examined hAMSCs and hUC-MSCs, proving that both were positive for alizarin red, with no significant difference statistically. Therefore, both ingredients were confirmed as having the same osteogenic potential.
The important factor in early osteogenesis is RUNX2 as major transcription factors that regulate osteoblasts and ostegenic differentiation in MSC. Experiments on rats lacking RUNX2 revealed limitations on MSC differentiation to osteoblasts (32). In our study, hAMSCs and hUC-MSCs in passage 5 showed expression of RUNX2 which indicated the differentiation of osteoblast. However, the results of statistical analysis revealed that hUC-MSCs expressed RUNX2 to a greater extent compared to hAMSCs. Osteogenic differentiation potential of our MSC was consistent with study by Shen et al that hAMSCs and hUC-MSCs showed intensive alizarin red staining and increased osteoblast protein marker (ALP, osterix, collagen I, osteocalcin and RUNX2) (33).
6. CONCLUSION
Both hAMSCs and hUC-MSCs had phenotype characteristics of MSCs. Passage 5 considered as appropriate passage because by having the lowest CD90, CD105 and CD73 expression. hAMSCs and hUC-MSCs had osteogenic differentiation potential. However, RUNX2 expression in hUC-MSCs was higher than hAMSCs.
Acknowledgments:
The author would like to thank The Stem Cell Research and Development Centre Airlangga University, Surabaya, Indonesia.
Author’s contribution:
N.H. and PH gave a substantial contribution to the conception and design of the work. NH performed data analysis. P.H. carried out the sample collection. Both authors contributed for literature search work, research report and gave final approval of the version to be published.
Conflict of interest:
There are no conflicts of interest.
Financial support:
Nil.
REFERENCES
- 1.Baraniak PR, McDevitt T. Stem Cell Paracrine Actions and Tissue Regeneration. Regen Med. 2010;5(1):121–143. doi: 10.2217/rme.09.74.Stem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang L, Ott L, Seshareddy K, Weiss ML, Michael S. Musculoskeletal Tissue Engineering with Human Umbilical Cord Mesenchymal Stromal Cells. Regen Med. 2010;6(1):95–109. doi: 10.2217/rme.10.98.Musculoskeletal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zarrabi M, Mousavi SH, Abroun S, Sadeghi B, Sc M. Potential Uses for Cord Blood Mesenchymal Stem Cells. 2010;15(4):274–281. [PMC free article] [PubMed] [Google Scholar]
- 4.Spencer ND, Gimble JM, Lopez MJ. Mesenchymal Stromal Cells: Past, Present, and Future. Vet Surg. 2010;40:129–139. doi: 10.1111/j.1532-950X.2010.00776.x. [DOI] [PubMed] [Google Scholar]
- 5.Leyva-leyva M, Barrera L, Lopez-Camarillo C, Arriaga-Pizano L, Orozco-hoyuela G, Carillo-Casas EM, Calderon-Perez J, Lopez-Diaz A, Hernandez-Aguilar F, Gonzalez-Ramirez R, Kawa S, Chimal-Monroy J, Fuentes-Mera L. Characterization of Mesenchymal Stem Cell Subpopulations from Human Amniotic Membrane with Dissimilar Osteoblastic Potential. Stem Cells Dev. 2010;00(00):1–13. doi: 10.1089/scd.2012.0359. [DOI] [PubMed] [Google Scholar]
- 6.Koike C, Zhou K, Takeda Y, Fathy M, Okabe M, Yoshida T, Nakamura Y, Kato Y, Nikaido T. Characterization of Amniotic Stem Cells. Cell Reprogram. 2010;16(4):298–305. doi: 10.1089/cell.2013.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin HJ, Bae YK, Kim M, Kwon S, Jeon HB. Comparative Analysis of Human Mesenchymal Stem Cells from Bone Marrow, Adipose Tissue, and Umbilical Cord Blood as Sources of Cell Therapy. Int J Mol Sci. 2010;14:17986–18001. doi: 10.3390/ijms140917986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin H, Kyong S, Oh W, Sun Y, Who S, Jin S. Biochemical and Biophysical Research Communications Down-regulation of CD105 is Associated with Multi-lineage Differentiation in Human Umbilical Cord Blood-derived Mesenchymal Stem Cells. Biochem Biophys Res Commun. 2010;381(4):676–681. doi: 10.1016/j.bbrc.2009.02.118. [DOI] [PubMed] [Google Scholar]
- 9.Weiss ML, Medicetty S, Bledsoe AR, Rachakatla RS, Choi M, Merchav S, Luo Y, Rao MS, Velagaliti G, Troye D. Human Umbilical Cord Matrix Stem Cells: Preliminary Characterization and Effect of Transplantation in a Rodent Model of Parkinson’s Disease. Stem Cells. 2010;24:781–792. doi: 10.1634/stemcells.2005-0330. [DOI] [PubMed] [Google Scholar]
- 10.Bieback K, Kern S, Kluter H, Eichler H. Critical Parameters for the Isolation of Mesenchymal Stem Cells from Umbilical Cord Blood. Stem Cells. 2010;22(4):625–634. doi: 10.1634/stemcells.22-4-625. [DOI] [PubMed] [Google Scholar]
- 11.Uranio MF, Dellaquila ME, Caira M, Guaricci AC, Ventura M, Catacchio CR, Martino NA, Valentini L. Characterization and In Vitro Differentiation Potency of Early- Passage Canine Amnion- and Umbilical Cord-Derived Mesenchymal Stem Cells as Related to Gestational Age. Mol Reprod Dev. 2010;81:539–551. doi: 10.1002/mrd.22322. [DOI] [PubMed] [Google Scholar]
- 12.Bilic G, Zeisberger SM, Mallik AS, Zimmermann R, Zisch AH. Comparative Characterization of Cultured Human Term Amnion Epithelial and Mesenchymal Stromal Cells for Application in Cell Therapy. Cell Transplant. 2008;17:955–968. doi: 10.3727/096368908786576507. [DOI] [PubMed] [Google Scholar]
- 13.Manochantr S, Tantrawatpan C. Isolation, Characterization and Neural Differentiation Potential of Amnion Derived Mesenchymal Stem Cells. J Med Assoc Thai. 2010;93(7):183–191. [PubMed] [Google Scholar]
- 14.Tamagawa T, Oi S, Ishiwata I, Ishikawa H, Nakamura Y. Differentiation of Mesenchymal Cells Derived from Human Amniotic Membranes into Hepatocyte-like Cells In Vitro. Hum Cell. 2010;20:77–84. doi: 10.1111/j.1749-0774.2007.00032.x. [DOI] [PubMed] [Google Scholar]
- 15.Kamadjaja MJK, Salim S, Rantam FA. Osteogenic Potential Differentiation of Human Amnion Mesenchymal Stem Cell with Chitosan-Carbonate Apatite Scaffold (In Vitro Study) Bali Med J. 2010;5(3):71–78. doi: 10.15562/bmj.v5i3.296. [DOI] [Google Scholar]
- 16.Khorshied MM, Gouda HM, Shaheen IA, Al Bolkeny T. The Osteogenic Differentiation Potentials of Umbilical Cord Blood Hematopoietic Stem Cells. Comp Clin Pathol. 2010;21:441–447. doi: 10.1007/s00580-010-1115-1. [DOI] [Google Scholar]
- 17.Franceschi RT, Xiao G, Jiang D. Transcriptional Regulation of Osteoblasts. Cells Tissues Organs. 2010;189:144–152. doi: 10.1159/000151747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Si J, Dai J, Zhang J, Liu S, Gu J, Shi J, Shen SGF, Guo L. Comparative Investigation of Human Amniotic Epithelial Cells and Mesenchymal Stem Cells for Application in Bone Tissue Engineering. Stem Cells Int. 2015:1–14. doi: 10.1155/2015/565732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L, Dormer NH, Bonewald LF, Detamore MS. Osteogenic Differentiation of Human Umbilical Cord Mesenchymal Stromal Cells in Polyglycolic Acid Scaffolds. Tissue Eng Part A. 2010;16(6):1937–1948. doi: 10.1089/ten.TEA.2009.0706. [DOI] [PubMed] [Google Scholar]
- 20.Kim HJ, Park JS. Usage of Human Mesenchymal Stem Cells in Cell-based Therapy: Advantages and Disadvantages. Dev Reprod. 2010;21(1):1–10. doi: 10.12717/DR.2017.21.1.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y, Tang SCW. Recent Progress in Stem Cell Therapy for Diabetic Nephropathy. Kidney Dis. 2010;2(1):20–27. doi: 10.1159/000441913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moraes DA, Sibov TT, Pavon LF, Alvim PQ, Bonadio RS, Da Silva JR, Pic-Taylor A, Toledo OA, Marti LC, Azevedo RB, Oliviera DM. A Reduction in CD90 (THY-1) Expression Results in Increased Differentiation of Mesenchymal Stromal Cells. Stemm Cell Res Ther. 2010;90:1–14. doi: 10.1186/s13287-016-0359-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levi B, Wan DC, Glotzbach JP, Hyun J, Januszyk M, Montoro D, Sorkin M, James AW, Nelson ER, Li S, Quarto N, Lee M, Gurtner GC, Longaker MT. CD105 Protein Depletion Enhances Human Adipose-derived Stromal Cell Osteogenesis through Reduction of Transforming Growth Factor-β1 (TGF-β1) Signaling. J Biol Chem. 2010;286(45):39497–39509. doi: 10.1074/jbc.M111.256529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee KS, Cha S, Kang HW, Song JY, Lee KW, Ko KB, Lee HT. Effects of Serial Passage on the Characteristics and Chondrogenic Differentiation of Canine Umbilical Cord Matrix Derived Mesenchymal Stem Cells. Asian-Aust J Anim Sci. 2010;26(4):588–595. doi: 10.5713/ajas.2012.12488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ode A, Schoom J, Kurtz A, Gaetjen M, Ode JE, Geissler S, Duda G, Ode A. CD73 / 5 ’-ecto-nucleotidase Acts as A Regulatory Factor in Osteo-/Chondrogenic Differentiation of Mechanically Stimulated Mesenchymal Stromal Cells. Eur Cells Mater. 2010;25:37–47. doi: 10.22203/eCM.v025a03. [DOI] [PubMed] [Google Scholar]
- 26.Ali H, Al-Yatama MK, Abu-Farha M, Behbehani K, Al Madhoun A. Multi-Lineage Differentiation of Human Umbilical Cord Wharton’s Jelly Mesenchymal Stromal Cells Mediates Changes in The Expression Profile of Stemness Markers. PLoS One. 2010;10(4):1–18. doi: 10.1371/journal.pone.0122465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gong W, Han Z, Zhao H, Wang Y, Wang J, Zhong J, Wang B, Wang S, Wang Y, Sun L, Han Z. Banking Human Umbilical Cord-derived Mesenchymal Stromal Cells for Clinical Use. Cell Transplant. 2010;21(1):207–216. doi: 10.3727/096368911X586756. [DOI] [PubMed] [Google Scholar]
- 28.Yinze D, Ma Q, Cui F, Zhong Y. Human Umbilical Cord Mesenchymal Stem Cells: Osteogenesis in vivo as seed cells for bone tissue engineering. J Biomed Mater Res–Part A. 2010;91(1):123–131. doi: 10.1002/jbm.a.32186. [DOI] [PubMed] [Google Scholar]
- 29.Li W, Pan S, Wang X, Xu W. Characterization of Stage-specific Embryonic Antigen-4 (SSEA-4)-Positive Very Small Embryonic-like Stem Cells Isolated from Human Wharton’s Jelly. Int J Clin Exp Med. 2010;10(2):4188–4199. [Google Scholar]
- 30.Scheers I, Lombard C, Paganelli M, Campard D, Najimi M, Gala J, Decottignies A, Sokal E. Human Umbilical Cord Matrix Stem Cells Maintain Multilineage Differentiation Abilities and Do Not Transform during Long-Term Culture. PLoS One. 2010;8(8):1–12. doi: 10.1371/journal.pone.0071374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ullah I, Subbarao RB, Rho GJ. Human Mesenchymal Stem Cells-Current Trends and Future Prospective. Biosci Rep. 2010;35:1–18. doi: 10.1042/BSR20150025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giuliani N, Lisignoli G, Magnani M, Racano C, Bolzoni M, Palma BD, Spolzino A, Manferdini C, Abati C, Toscani D, Facchini A, Aversa F. New Insights into Osteogenic and Chondrogenic Differentiation of Human Bone Marrow Mesenchymal Stem Cells and Their Potential Clinical Applications for Bone Regeneration in Pediatric Orthopaedics. Stem Cells Int. 2013:1–11. doi: 10.1155/2013/312501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shen C, Yang C, Xu S, Zhao H. Comparison of Osteogenic Differentiation Capacity in Mesenchymal Stem Cells Derived from Human Amniotic Membrane (AM), Umbilical Cord (UC), Chorionic Membrane (CM), and Decidua (DC) Cell Biosci. 2010;9(117) doi: 10.1186/s13578-019-0281-3. [DOI] [PMC free article] [PubMed] [Google Scholar]