Abstract
Objective
Although infant mortality because of birth defect has increased in both developed and developing countries, had not got attention like other health issues at national, regional, or local levels. Documenting the risk factors that influence the occurrence of birth defects and its seasonality will help to inform the community and to develop preventive strategies for the country.
Results
Factors associated with higher likelihood of a major structural birth defects included maternal age; neonates born from women living in urban; and in Dega; history of fever during pregnancy; intake of herbal medicine; and drinking alcohol. Counselling for pregnancy preparation and folic acid supplementation was found protective for the likelihood of birth defect.
Keywords: Birth defect, Maternal illness, Maternal medication use, Environmental exposure
Introduction
Birth defects also are known as, congenital anomalies, congenital disorder or congenital malformations [1, 2] are defined as structural or functional anomalies that occur during intrauterine development and can be identified prenatally, at birth or manifests later in life. The most common major structural birth defects include congenital heart disease, neural tube defects, orofacial clefts, limb reduction defects, and Down syndrome [2].
Every year an estimated 7.9 million children—6 percent of total births worldwide—are born with a serious birth defect of genetic or partially genetic origin. Data presented in a global report on birth defects show that at least 3.3 million children under 5 years of age die from birth defects each year and an estimated 3.2 million of those who survive may be disabled for life. This disability can have a severe human and economic toll on those affected children, their families, communities and health-care systems [3].
Although birth defects are a global problem, their impact is severe in middle- and low-income countries where more than 94% of the births with serious birth defects and 95% of the deaths of these children occur [3]. Furthermore, 15–30% of infant and child hospital admissions are due to birth defects and exact a proportionally higher health care cost than other hospitalizations [4].
Researches on birth defects and associated risk factors were not conducted adequately in Ethiopia and had not got attention like other health issues at national, regional, or local levels.
Documenting the risk factors that influence the occurrence of birth defects and its seasonality will help to inform the community and to develop preventive strategies for the country. Studying the seasonality of congenital anomalies can also help trace risk factors for birth defect.
The main objective of the study was to assess determinants and seasonality of major structural birth defects among newborns delivered at primary and referral hospital of East and West Gojjam zone, Northwest Ethiopia 2017–2018.
Main text
Subjects and methods
Study design
Institutional based unmatched case–control study design was conducted at selected primary and referral hospital of East and West Gojjam zone from September 2017 to October 2018.
Study population
All newborns in selected hospitals who was born from September 2017 to October 2018 and who fulfill the case and control definition criteria for this particular study.
Eligibility criteria
Inclusion criteria Newborns with major structural birth defect and the next three newborns (delivered after the case) without defect and whose mothers are voluntary, was included in the study.
Exclusion criteria Newborns whose mothers could not be interviewed because they were very sick, emotionally upset, and mute or die post-delivery was excluded from the study.
Sample size and sampling procedure
The sample size was calculated using the Fleiss with continuity correction factor formula with a case–control ratio of 1:3; assuming the proportion of controls exposed was 50%, the minimum odds ratio to be detected is 2.0, power of 80% and a significance level of 95%. The computed sample size was 398, (100 cases and 298 controls).
Variables of the study
Independent variables Socio demographic characteristics of mothers and fathers, obstetrical history of mother and newborn, maternal illness and medication use of the mother during pregnancy and, environmental exposures of mothers during pregnancy.
Dependent variable Patient control status of major structural birth defect.
Data collection procedure
Socio demographic characteristics of mothers and fathers; obstetrical history of mother and newborn; history of maternal illness, medication use, environmental exposures during pregnancy was assessed using structured interviewer administered questionnaire. Patient control status of the fetus/newborn was assessed by detail observation and physical examination.
Data analysis
Data was entered via Epi data and analyzed using STATA version 14. Bivariate and multivariate analyses was employed, and factors which had a p value of < 0.05 in the bivariate analysis was included in the multivariate analysis. p values of < 0.05 was considered statistically significant.
Results
A total of 398 newborns, male 219 (55%) and female 179 (45%), with gestational age (using ultrasound) of 16–43 weeks was included in this study. All 398 mothers whose newborns were included, were aged between 18 and 42 years with a mean age of 27.51 years and 388 (97.5%) were married (Table 1).
Table 1.
Socio demographic characteristics of the study sample
| Characteristics | Cases (n = 100) | Controls (n = 298) | Total |
|---|---|---|---|
| Maternal age | |||
| ≤ 20 | 10 (23.8%) | 32 (76.2%) | 42 (100.0%) |
| 20–35 | 75 (22.7%) | 256 (77.3%) | 331 (100.0%) |
| > 35 | 15 (60%) | 10 (40%) | 25 (100.0%) |
| Marital status of the mother | |||
| Married | 94 (24.2%) | 294 (75.8%) | 388 (100.0%) |
| Unmarried | 6 (60.0%) | 4 (40.0%) | 10 (100.0%) |
| Father’s age | |||
| < 35 | 51 (19.3%) | 213 (80.7%) | 264 (100.0%) |
| ≥ 35 | 49 (36.6%) | 85 (63.4%) | 134 (100.0%) |
| Residence | |||
| Urban | 51 (21.9%) | 182 (78.1%) | 233 (100.0%) |
| Rural | 49 (29.7%) | 116 (70.3%) | 165 (100.0%) |
| Primary address during 1st trimester | |||
| Dega | 25 (39.1%) | 39 (60.9%) | 64 (100.0%) |
| Woyina Dega | 34 (18.6%) | 149 (81.4%) | 183 (100.0%) |
| Kolla | 41 (27.2%) | 110 (72.8%) | 151 (100.0%) |
| Monthly income of the family | |||
| ≤ 500 | 24 (58.5%) | 17 (41.5%) | 41 (100.0%) |
| 500–10,000 | 70 (23.3%) | 230 (76.7%) | 300 (100.0%) |
| ≥ 10,000 | 6 (10.5%) | 51 (89.5%) | 57 (100.0%) |
| Father’s ethnicity | |||
| Amhara | 100 (25.3%) | 296 (74.7%) | 396 (100.0%) |
| Oromo | 0 (0.0%) | 2 (100.0%) | 2 (100.0%) |
| Mother’s ethnicity | |||
| Amhara | 96 (25.1%) | 286 (74.9%) | 382 (100.0%) |
| Oromo | 0 (0.0%) | 8 (100.0%) | 8 (100.0%) |
| Tigrie | 4 (50.0%) | 4 (50.0%) | 8 (100.0%) |
In this study, most of the major structural birth defects occur during spring and least occur during autumn. But Chi square test of seasonal patterns of birth defects shows that there is no statistically significant association between season of birth- and absence or presence of birth defects (p = 1.00).
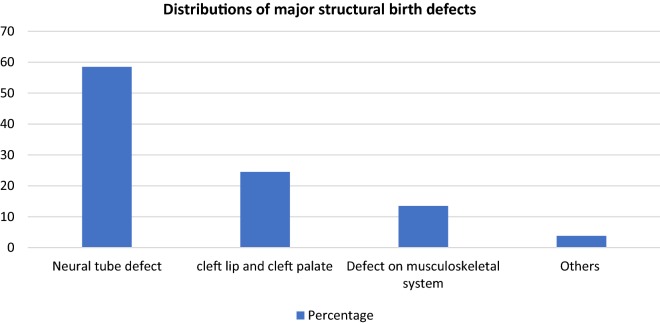
Distributions of major structural birth defects among cases
During the study period, 100 new born were found with major structural birth defects in a proportion as depicted on Fig. 1.
Fig. 1.
Distributions of major structural birth defects among cases
Determinant factors associated with major structural birth defects
Socio-demographic characteristics of mothers and fathers; the obstetrical history of the mother and newborn; the history of maternal illness, medication use, environmental exposures during pregnancy were analyzed in bivariate and multivariate logistic regression. We identified risk and protective factors for the occurrence of the major structural birth defects as depicted by Table 2.
Table 2.
Risk and protective factors associated with major structural birth defects
| Characteristics | Cases | Controls | COR with 95% CI | AOR with 95% CI |
|---|---|---|---|---|
| Count (%) | Count (%) | |||
| Maternal age | ||||
| ≤ 20 | 10 (10%) | 32 (10.7%) | 1.1 (0.5–2.2) | |
| 20–35 | 75 (75%) | 256 (85.9%) | 1 | 1 |
| > 35 | 15 (15.0%) | 10 (3.4%) | 5 (2.2–11.9) | 4.9 (1.1–23.7) |
| Residence | ||||
| Urban | 51 (21.9%) | 182 (78.1%) | 0.7 (0.4–1.2) | 6.4 (1.9–21.7) |
| Rural | 49 (29.7%) | 116 (70.3%) | 1 | 1 |
| Primary address during 1st trimester | ||||
| Dega | 25 (39.1%) | 39 (60.9%) | 2.8 (1.5–5.2) | 4.3 (1.3–14) |
| Woyina Dega | 34 (18.6%) | 149 (81.4%) | 1 | 1 |
| Kolla | 41 (27.2%) | 110 (72.8%) | 1.6 (1.0–2.7) | |
| Intake of herbal medicine during pregnancy | ||||
| Yes | 40 (40%) | 36 (12%) | 4.9 (2.9–8.2) | 10.9 (4.2–28.1) |
| No | 60 (60%) | 262 (88%) | 1 | 1 |
| Alcohol intake | ||||
| Yes | 92 (92%) | 164 (55%) | 9.4 (4.4–20) | 12.7 (3.3–48.7) |
| No | 8 (8%) | 134 (45%) | 1 | 1 |
| History of high fever during pregnancy | ||||
| Yes | 54 (54%) | 48 (16%) | 6 (3.7–10.0) | 3.4 (1.3–11.6) |
| No | 46 (46%) | 250 (84%) | 1 | 1 |
| Folic acid | ||||
| Yes | 31 (31%) | 228 (76.5%) | 1 | 1 |
| No | 69 (69%) | 70 (23.5%) | 7.2 (4.39–12.0) | 7.3 (2.9–18.8) |
| Counselling for pregnancy preparation | ||||
| Yes | 30 | 190 | 1 | 1 |
| No | 70 | 108 | 4 (2.5–6.7) | 4.8 (1.9–12.1) |
Risk factors of major structural birth defects
Factors associated with higher likelihood of a major structural birth defects included maternal age (AOR: 4.9, 95% CI 1.1–23.68); neonates born from women living in urban (AOR: 6.4, 95% CI 1.9–21.7); history of fever during pregnancy (AOR: 3.4, 95% CI 1.3–11.6); intake of herbal medicine(AOR: 10.9, 95% CI 4.2–28.1); and drinking alcohol (AOR: 12.7, 95% CI 3.3–48.7) (Table 2).
Protective factors of major structural birth defects
Counselling for pregnancy preparation (AOR: 4.8, 95% CI 1.9–12.1) and folic acid supplementation (AOR: 7.3, 95% CI 2.9–18.8) was found protective for the likelihood of birth defect (Table 2).
Discussion
Risk factors for the incidence of major structural birth defects
Many studies have been conducted to determine the association of various risk factors with the incidence of birth defects. For example, in Kenya, Tanzania and Iran the number of malformed babies appeared to increase with increasing maternal age especially from 35 years and above [5–7]. The same is true in our study; Maternal age was significantly associated with birth defect. Women above 35 years old were around five times more likely to have neonates with birth defect as compared to those women who are in the age group of 20–35 years old. However, a research done in Addis Ababa and Saudi Arabia [8, 9] showed that there is no significance difference between cases and controls regarding maternal age for occurrence of birth defects.
In rural areas of Gabon structural birth defects were rare or absent as compared to the number recorded in urban areas [10]. In this study neonates born from women living in an urban area were about six times more likely to develop birth defect as compared to those neonates born from women who live in a rural setting and the odds of women to have a newborn with the birth defect was around four times higher among women living in Dega as compared to women whose primary address was in Woyina Dega. This was supported by research on epidemiology of birth defects based on a birth defect surveillance system from 2005 to 2014 in Hunan province, China [11].
This may be due to diet diversification habits and non-fat diet in rural areas of Ethiopia as has been shown its effect in an animal model study [12] and a greater level of air pollution in urban areas [13].
Studies conducted in a variety of settings around the world have observed a significant association between maternal fever and birth defects consistent with our findings [6, 14, 15]. A population-based case control study done in Shanxi province northern China on risk factors for neural tube defects found that a history of fever during the periconceptional period was associated with almost a threefold increase in risk for neural tube defects, which persisted even after controlling for other covariates [16, 17]. This were in congruent with our study on which mothers who had history of fever during pregnancy were around three times more likely to have neonates with birth defect even after adjusting for other factors.
As reported from northern Ghana the use of herbal medicines by pregnant women poses a potential danger to the fetus and development of 70% birth defects of unknown etiology [18]. In our study intake of herbal medicine during pregnancy was significantly associated with major structural birth defects. Those women who took herbal medicine during pregnancy were around eleven times more likely to get a new born suffering from birth defect compared to women who didn’t take herbal medicine while pregnant. In Ethiopia pregnant women used herbal medicine in the first trimester of pregnancy [19] in which organogenesis and birth defect occur [20].
The 2011 Ethiopia Demographic and Health Survey found that 45% of women and 53% of men reported drinking alcohol at some point in their lives [21]. The association of alcohol drinking during and before early pregnancy and birth defect was reported as having significant association [8, 22, 23].
In our study Maternal history of alcohol intake during pregnancy was found to be significantly associated with birth defect. Women who took alcohol during their pregnancy were around thirteen times more likely to have newborns with birth defect as compared to those women who didn’t take alcohol during their pregnancy.
Both moderate and high levels of alcohol intake during early pregnancy may result in alterations in growth and morphogenesis of the fetus. Microcephaly, short palpebral fissures, epicanthal folds, maxillary hypoplasia, short nose, thin upper lip, abnormal palmar creases, joint anomalies, and congenital heart disease are also present in most infants [1].
Protective factors for the incidence of major structural birth defects
Counselling has a positive effect on a range of health outcomes like prevention of birth defects [24]. Physician counseling can reduce risk of medication-induced birth defects [25] and increase intake of folic acid before conception [26] so can reduce incidence of birth defect as explained by many researches.
Similar to above report counselling for pregnancy preparation in our study was found protective for the incidence of birth defect. Women who didn’t get counselling for pregnancy preparation were about five times more likely to have neonates with the birth defect as compared to women who got counselling for pregnancy preparation.
Nearly one-half of pregnancies are unintended so preconception care should be considered an integral part of primary care for women of reproductive age. Provide counselling regarding common issues in preconception care like family planning, screening and treatment for infectious diseases, updating appropriate immunizations, and reviewing medications for teratogenic effects can prevent poor birth outcomes including birth defect [27].
Researches in different setup showed that women who took folic acid were less likely to have babies with birth defects as compared to those who did not take folic acid during and before early pregnancy [6, 28–34].
Intake of folic acid supplementation can reduces nearly 75% of the rate of neural tube defects [35]. It is recommended to take 4 to 5 mg of folic acid daily starting 3 months before conception and continuing until 12 weeks post conception [36, 37].
In our study women who didn’t get folic acid supplementation at or before pregnancy were about seven times more likely to have neonates with birth defect as compared to those women who had been supplemented with folic acid during and before pregnancy which is consistent with other studies that have shown folic acid supplementation is protective against birth defects [37–41].
Folate acts as a cofactor for enzymes involved in DNA and RNA biosynthesis. Interruption of DNA biosynthesis or methylation reactions could prevent the proper closure of the neural tube. Such inhibition could be caused by simple deficiency of either folic acid or vitamin B12 [42].
Limitations
In this study, there is no statistically significant association between season of birth and the absence or presence of birth defects. But the data should be collected and analyzed based on the season of conception rather than the season of birth or termination. This was the main limitation of the study.
Acknowledgements
We would like to start with a heartfelt THANK YOU to each and every one who shared a kind word and gentle nudge to keep us moving forward toward completing our research. We would like to thank and express our deepest gratitude to Debre Markos University which sponsored the research, Debre Markos Referral hospital and Shegaw Motta primary hospital professionals.
Abbreviations
- AOR
adjusted odds ratio
- CI
confidence interval
Authors’ contributions
BT and DS, conceived and designed the study. AL and EA had supervised and collected data. AT, EE and BT had analyzed data. BT also wrote the initial draft of the manuscript which was critically reviewed by all authors. All authors read and approved the final manuscript.
Funding
Debre Markos University research and community service directorate supported this research for collection and interpretation of data.
Availability of data and materials
The dataset supporting the conclusion of this article is available from the authors on request.
Ethics approval and consent to participate
We took ethical approval from the institutional review board of the school of medicine, Debre Markos University. Voluntary written informed consent to take part into the study was obtained after which we continued data collection. That is parental consent has been obtained.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Binalfew Tsehay, Email: bunadmu@gmail.com.
Desalegn Shitie, Email: dessieshitie@ymail.com.
Akilog Lake, Email: akilogl@yahoo.com.
Erimiyas Abebaw, Email: fjeremy23@gmail.com.
Amisalu Taye, Email: 50amsalu@gmail.com.
Enatinesh Essa, Email: zaharaessa@gmail.com.
References
- 1.Moore KL, Persaud TVN, Torchia MG. The developing human E-book. New York: Elsevier Health Sciences; 2011. [Google Scholar]
- 2.https://www.who.int/news-room/fact-sheets/detail/congenital-anomalies. Accessed 20 July 2019.
- 3.Christianson AL, Howson CP, Modell B. Global report on birth defects: the hidden toll of dying and disabled children. New York: March of Dimes Birth Defects Foundation; 2006. [Google Scholar]
- 4.Hobbs CA, Cleves MA, Simmons CJ. Genetic epidemiology and congenital malformations: from the chromosome to the crib. Arch Pediatr Adolesc Med. 2002;156(4):315–320. doi: 10.1001/archpedi.156.4.315. [DOI] [PubMed] [Google Scholar]
- 5.Muga R, Mumah S, Juma P. Congenital malformations among newborns in Kenya. Afr J Food Agric Nutr Develop. 2009;9(3):814. [Google Scholar]
- 6.Kishimba RS, Mpembeni R, Mghamba J. Factors associated with major structural birth defects among newborns delivered at Muhimbili National Hospital and Municipal Hospitals in Dar Es Salaam, Tanzania 2011–2012. Pan Afr Med J. 2015;20(1):53. doi: 10.11604/pamj.2015.20.153.4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nili F, Jahangiri M. Risk factors for neural tube defects: a study at university affiliated hospitals in Tehran. Arch Iran Med. 2006;9(1):20–25. [PubMed] [Google Scholar]
- 8.Taye M, Afework M, Fantaye W, Diro E, Worku A. Factors associated with congenital anomalies in Addis Ababa and the Amhara Region, Ethiopia: a case–control study. BMC Pediatr. 2018;18(1):142. doi: 10.1186/s12887-018-1096-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salih MA, Murshid WR, Mohamed AG, Ignacio LC, de Jesus JE, Baabbad R, El Bushra HM. Risk factors for neural tube defects in Riyadh City, Saudi Arabia: case–control study. Sudanese J Paediatr. 2014;14(2):49. [PMC free article] [PubMed] [Google Scholar]
- 10.Mombo LE, Yangawagou-Eyeghe LM, Mickala P, Moutélé J, Bah TS, Tchelougou D, Bisseye C. Patterns and risk factors of birth defects in rural areas of south-eastern Gabon. Congenital Anomal. 2017;57(3):79–82. doi: 10.1111/cga.12201. [DOI] [PubMed] [Google Scholar]
- 11.Xie D, Yang T, Liu Z, Wang H. Epidemiology of birth defects based on a birth defect surveillance system from 2005 to 2014 in Hunan Province, China. PLoS ONE. 2016;11(1):e0147280. doi: 10.1371/journal.pone.0147280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bentham J, Michell AC, Lockstone H, Andrew D, Schneider JE, Brown NA, Bhattacharya S. Maternal high-fat diet interacts with embryonic Cited2 genotype to reduce Pitx2c expression and enhance penetrance of left–right patterning defects. Hum Mol Genet. 2010;19(17):3394–3401. doi: 10.1093/hmg/ddq251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farhi A, Boyko V, Almagor J, Benenson I, Segre E, Rudich Y, Stern E, Lerner-Geva L. The possible association between exposure to air pollution and the risk for congenital malformations. Environ Res. 2014;135:173–180. doi: 10.1016/j.envres.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 14.Fischer M, Hedberg K, Cardosi P, Plikaytis BD, Hoesly FC, Steingart KR, Bell TA, Fleming DW, Wenger JD, Perkins BA. Tobacco smoke as a risk factor for meningococcal disease. Pediatr Infect Dis J. 1997;16(10):979–983. doi: 10.1097/00006454-199710000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Dreier JW, Andersen A-MN, Berg-Beckhoff G. Systematic review and meta-analyses: fever in pregnancy and health impacts in the offspring. Pediatrics. 2014;133(3):e674–e688. doi: 10.1542/peds.2013-3205. [DOI] [PubMed] [Google Scholar]
- 16.Li Z, Ren A, Zhang L, Guo Z, Li Z. A population-based case–control study of risk factors for neural tube defects in four high-prevalence areas of Shanxi province, China. Paediat Perinat Epidemiol. 2006;20(1):43–53. doi: 10.1111/j.1365-3016.2006.00694.x. [DOI] [PubMed] [Google Scholar]
- 17.Wang W, Guan P, Xu W, Zhou B. Risk factors for oral clefts: a population-based case–control study in Shenyang, China. Paediat Perinat Epidemiol. 2009;23(4):310–320. doi: 10.1111/j.1365-3016.2009.01025.x. [DOI] [PubMed] [Google Scholar]
- 18.Meade EPKZA, Abubakar L, et al. Herbal medicine usage before and during pregnancy—a study in Northern Ghana. Int J Complement Alt Med. 2018;11(4):235–242. [Google Scholar]
- 19.Bayisa B, Tatiparthi R, Mulisa E. Use of herbal medicine among pregnant women on antenatal care at Nekemte Hospital, Western Ethiopia. Jundishapur J Nat Pharm Prod. 2014;9(4):e173682. doi: 10.17795/jjnpp-17368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sadler T. Text book of Langman’s medical embryology. In: Skeletal system 11th Ed New Delhi [South Asian edition]: Wolters kluwer 2010. p. 140.
- 21.Bālaśelṭān EYS, Macro O. Ethiopia Demographic and Health Survey, 2005: Central Statistical Authority; 2006.
- 22.Grewal J, Carmichael SL, Ma C, Lammer EJ, Shaw GM. Maternal periconceptional smoking and alcohol consumption and risk for select congenital anomalies. Birth Defects Res A. 2008;82(7):519–526. doi: 10.1002/bdra.20461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X, Li S, Wu S, Hao X, Guo S, Suzuki K, Yokomichi H, Yamagata Z. Prevalence of birth defects and risk-factor analysis from a population-based survey in Inner Mongolia, China. BMC Pediatr. 2012;12(1):125. doi: 10.1186/1471-2431-12-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Organization WH. Meeting to develop a global consensus on preconception care to reduce maternal and childhood mortality and morbidity: World Health Organization Headquarters, Geneva, 6–7 February 2012: meeting report. 2013.
- 25.Schwarz EB, Parisi SM, Handler SM, Koren G, Shevchik G, Fischer GS. Counseling about medication-induced birth defects with clinical decision support in primary care. J Women’s Health. 2013;22(10):817–824. doi: 10.1089/jwh.2013.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elsinga J, de Jong-Potjer LC, van der Pal-de KM, le Cessie S, Assendelft WJ, Buitendijk SE. The effect of preconception counselling on lifestyle and other behaviour before and during pregnancy. Women’s Health Issues. 2008;18(6):S117–S125. doi: 10.1016/j.whi.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Farahi N, Zolotor A. Recommendations for preconception counseling and care. Am Fam Phys. 2013;88(8):499–506. [PubMed] [Google Scholar]
- 28.Bortolus R, Blom F, Filippini F, van Poppel MN, Leoncini E, de Smit DJ, Benetollo PP, Cornel MC, de Walle HE, Mastroiacovo P. Prevention of congenital malformations and other adverse pregnancy outcomes with 4.0 mg of folic acid: community-based randomized clinical trial in Italy and the Netherlands. BMC Pregn Childbirth. 2014;14(1):166. doi: 10.1186/1471-2393-14-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan AC, van Essen P, Scott H, Haan EA, Sage L, Scott J, Gill TK, Nguyen A. Folate awareness and the prevalence of neural tube defects in South Australia, 1966–2007. Med J Aust. 2008;189(10):566–569. doi: 10.5694/j.1326-5377.2008.tb02183.x. [DOI] [PubMed] [Google Scholar]
- 30.De Wals P, Tairou F, Van Allen MI, Uh S-H, Lowry RB, Sibbald B, Evans JA, Van den Hof MC, Zimmer P, Crowley M. Reduction in neural-tube defects after folic acid fortification in Canada. N Engl J Med. 2007;357(2):135–142. doi: 10.1056/NEJMoa067103. [DOI] [PubMed] [Google Scholar]
- 31.Fernández N, Henao-Mejía J, Monterrey P, Pérez J, Zarante I. Association between maternal prenatal vitamin use and congenital abnormalities of the genitourinary tract in a developing country. J Pediatr Urol. 2012;8(2):121–126. doi: 10.1016/j.jpurol.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Musumeci G, Castrogiovanni P, Trovato FM, Parenti R, Szychlinska MA, Imbesi R. Pregnancy, embryo-fetal development and nutrition: physiology around fetal programming. J Histol Histopathol. 2015;2(1):1. doi: 10.7243/2055-091X-2-1. [DOI] [Google Scholar]
- 33.Shaw GM, Nelson V, Carmichael SL, Lammer EJ, Finnell RH, Rosenquist TH. Maternal periconceptional vitamins: interactions with selected factors and congenital anomalies? Epidemiology. 2002;13:625–630. doi: 10.1097/00001648-200211000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Wilson RD, Audibert F, Brock J-A, Carroll J, Cartier L, Gagnon A, Johnson J-A, Langlois S, Murphy-Kaulbeck L, Okun N. Pre-conception folic acid and multivitamin supplementation for the primary and secondary prevention of neural tube defects and other folic acid-sensitive congenital anomalies. J Obstetr Gynaecol Canada. 2015;37(6):534–549. doi: 10.1016/S1701-2163(15)30230-9. [DOI] [PubMed] [Google Scholar]
- 35.Johnson K, Posner SF, Biermann J, Cordero JF, Atrash HK, Parker CS, Boulet S, Curtis MG. Recommendations to improve preconception health and Health Care—United States: report of the CDC/ATSDR preconception care work group and the select panel on preconception care. Morbid Mortal Wkly Rep. 2006;55(6):1–4. [PubMed] [Google Scholar]
- 36.Wilson RD, Davies G, Desilets V, Reid G, Summers A, Wyatt P, Young D. The use of folic acid for the prevention of neural tube defects and other congenital anomalies. J Obstetr Gynaecol Canada. 2003;25(11):959–973. doi: 10.1016/S1701-2163(16)30248-1. [DOI] [PubMed] [Google Scholar]
- 37.Wald N, Sneddon J, Densem J, Frost C, Stone R. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet. 1991;338(8760):131–137. doi: 10.1016/0140-6736(91)90133-A. [DOI] [PubMed] [Google Scholar]
- 38.Njamnshi A, Djientcheu VDP, Lekoubou A, Guemse M, Obama M, Mbu R, Takongmo S, Kago I. Neural tube defects are rare among black Americans but not in sub-Saharan black Africans: the case of Yaounde—Cameroon. J Neurol Sci. 2008;270(1–2):13–17. doi: 10.1016/j.jns.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 39.Besser LM, Williams LJ, Cragan JD. Interpreting changes in the epidemiology of anencephaly and spina bifida following folic acid fortification of the US grain supply in the setting of long-term trends, Atlanta, Georgia, 1968–2003. Birth Defects Res A. 2007;79(11):730–736. doi: 10.1002/bdra.20401. [DOI] [PubMed] [Google Scholar]
- 40.Amarin ZO, Obeidat AZ. Effect of folic acid fortification on the incidence of neural tube defects. Paediatr Perinat Epidemiol. 2010;24(4):349–351. doi: 10.1111/j.1365-3016.2010.01123.x. [DOI] [PubMed] [Google Scholar]
- 41.Sayed AR, Bourne D, Pattinson R, Nixon J, Henderson B. Decline in the prevalence of neural tube defects following folic acid fortification and its cost-benefit in South Africa. Birth Defects Res A. 2008;82(4):211–216. doi: 10.1002/bdra.20442. [DOI] [PubMed] [Google Scholar]
- 42.Scott J. M., Weir D. G., Molloy A., Mcpartlin J., Daly L., Kirke P. Ciba Foundation Symposium 181 - Neural Tube Defects. Chichester, UK: John Wiley & Sons, Ltd.; 2007. Folic Acid Metabolism and Mechanisms of Neural Tube Defects; pp. 180–191. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset supporting the conclusion of this article is available from the authors on request.