Abstract
Working memory capacity (WMC) can predict conflict control ability. Measures of both abilities are impaired by anxiety, which is often inversely linked with mindfulness. It has been shown that a combination of high mindfulness and low anxiety is associated with better conflict control and WMC. The current study explored the electrophysiology related to such behavioral differences. Two experimental groups, one with high mindfulness and low anxiety (HMLA) and one with low mindfulness and high anxiety (LMHA), performed a color Stroop task and a change detection task, both with simultaneous electroencephalogram (EEG) recording. An advanced EEG analytical approach, Hilbert–Huang transform (HHT) analysis, was employed. This is regarded as a robust method to analyze non-linear and non-stationary signals. Lower delta activity at posterior temporal and occipital regions was seen in the HMLA group for the Stroop conflict conditions and might be generally associated with higher accuracy in this group and indicative of higher attentiveness. Higher accuracy rates and WMC were seen in the HMLA group and might be specifically associated with the higher alpha activity observed in prefrontal cortex, fronto-central and centro-parietal regions in this group. Future studies should explore how mindfulness and anxiety can independently affect these cognitive functions and their associated neurophysiology.
Keywords: conflict control, working memory, mindfulness, anxiety
Introduction
There is increasing interest in how mindfulness may alleviate levels of anxiety, which in turn, is often linked to impairments in executive functions such as conflict control and working memory (WM). Our previous study (Jaiswal et al., 2018) has shown that people with high mindfulness and low anxiety (HMLA) have better conflict control and WM capacity (WMC) than those with low mindfulness and high anxiety (LMHA). However, the electrophysiological mechanisms that might be associated with such difference in performance remained unknown. The current study aimed to explore the electrophysiological basis of this behavioral difference using an advanced electroencephalogram (EEG) analytical approach, Hilbert–Huang transform analysis (HHT) (Huang et al., 1998). HHT is regarded as a robust method to analyze non-linear and non-stationary signals such as those obtained via EEG recording (Stam, 2005). Additionally, it also provides information about instantaneous frequency and offers a high temporal resolution.
Mindfulness and anxiety are two inversely linked traits (Coffey and Hartman, 2008), which are often reported to modulate executive functions. Trait mindfulness has been defined as an innate characteristic of an individual in directing one’s attention to the present moment while adopting a non-judgmental perspective toward experiences (Kabat-Zinn, 1990; Baer et al., 2006). Trait anxiety can be regarded as affective disposition that interferes with top–down processing of cognitive control (Eysenck and Calvo, 1992; Eysenck et al., 2007). There is empirical evidence from mediational (Coffey and Hartman, 2008; Arch and Craske, 2010; Desrosiers et al., 2013), behavioral (Jaiswal et al., 2018), neurophysiological (Mocaiber et al., 2009; Brown et al., 2013) and neuroimaging (Etkin et al., 2004; Frewen et al., 2010; Way et al., 2010) studies of an inverse relationship between trait mindfulness and trait anxiety. It is suggested that both traits may be mediated by the emotion regulation system (worry, reappraisal, non-acceptance and rumination) through which they may interact in an antagonistic manner (Greeson and Brantley, 2009; Holzel et al., 2013).
According to attentional control theory (Eysenck et al., 2007), through modulating the influence of bottom-up stimuli, anxiety can impair the efficiency of conflict control (Miyake et al., 2000) required to perform a goal-directed action. Additionally, Klein and Boals (2001) suggested that responses to threatening situations in daily life may lead to lower WMC, as individuals under stress allocate part of their mental resources to curb unpleasant feelings which interfere with other goal-driven tasks. Therefore, it can be inferred that anxiety can impair conflict control, and also WMC, while mindfulness as a trait might inhibit such impairment, potentially by reducing the anxiety level. A previous study showed that, irrespective of whether an individual practiced meditation, trait mindfulness could predict the ability to monitor conflict (Moore and Malinowski, 2009). Additionally, there are two independent studies where meditators showed more efficient conflict monitoring than a control group, with one study employing an attentional network test (Jo et al., 2016) and the other a color Stroop task (CST) (Teper and Inzlicht, 2013). In a longitudinal study, Jha et al. (2010) found that with individuals with a high practice time of mindfulness training showed higher verbal WMC. Meditators are often reported to exhibit an elevated level of trait mindfulness (Kiken et al., 2015) and reduced anxiety symptoms due to meditation practice (Lau et al., 2006; Hofmann et al., 2010). Therefore, it is proposed that an elevated level of trait mindfulness and/or a reduced level of anxiety may facilitate conflict control and WMC.
Conflict control is the ability to regulate cognition and action flexibly to achieve internal goals (Miller and Cohen, 2001; Miyake and Friedman, 2012). One of the simplest ways to measure this is using a CST (Stroop, 1935), which, unlike some other conflict tasks, has the advantage of not involving any affect modulation (e.g. (Becker et al., 2001; Dresler et al., 2009)). Attentional control theory (Eysenck et al., 2007) suggests that if a non-affective stimulus can serve as an effective distractor, findings can be better generalized to develop a theory around factors that may have causal or correlational effects on behavior.
It has been suggested that conflict control in conjunction with WM (the ability to maintain target information over time) plays an important role in the operation of executive functions (Miyake et al., 2000). It has been also demonstrated that individual differences in WMC are predictive of levels of Stroop interference (Kane and Engle, 2003). To explore WMC, operation span (Unsworth et al., 2005) and change detection tasks (CDTs) (Luck and Vogel, 1997) are frequently used paradigms and measure verbal and visuospatial WM, respectively. Several studies have investigated verbal WM in relation to both mindfulness (Jha et al., 2010; Mrazek et al., 2013) and anxiety (Darke, 1988; MacLeod and Donnellan, 1993; Schmader and Johns, 2003). However, only a few studies have explored visuospatial WM in relation to anxiety (Moriya and Sugiura, 2012; Moriya, 2016; Figueira et al., 2017), and to our knowledge, no study has been carried out with respect to mindfulness.
Some studies demonstrate that conflict control and WMC are regulated by common brain regions with the prefrontal cortex principal among these (MacDonald et al., 2000; Fan et al., 2003; Bishop, 2009). Anterior cingulate cortex has also been linked with various forms of conflict monitoring (Bush et al., 2000; Fan et al., 2003; Botvinick et al., 2004), and posterior parietal lobe has also been associated with WMC (Berryhill and Olson, 2008; Tseng et al., 2012).
Electrophysiologically, conflict control has mainly been linked with theta band oscillations reported to be generated by the anterior cingulate cortex or medial frontal cortex (Womelsdorf et al., 2010; Nigbur et al., 2011; Cavanagh and Frank, 2014). However, reports also suggest that, irrespective of origin, an enhanced theta effect (Hanslmayr et al., 2008; Brittain et al., 2012; Jo et al., 2017) is distributed throughout the frontal and neighboring regions (Kahana et al., 1999; Tang et al., 2013; Zhao et al., 2015). Additionally, conflict control has also been linked with reduced beta oscillation power (Wang et al., 2014; Zhao et al., 2015). Such beta suppression has been suggested to reflect the top-down inhibition of pre-potent responses (Swann et al., 2012; Lo et al., 2013). Moreover, delta and alpha oscillations are also quite frequently observed in cognitive processes (Knyazev, 2007). Therefore, the current study evaluated the electrophysiological differences in conflict control between HMLA and LMHA groups, throughout the physiological frequency bands (delta, theta, alpha, beta, and gamma).
In WM tasks, alpha oscillations are reported to be the dominant frequency component (Jensen et al., 2002; Busch and Herrmann, 2003; Fukuda et al., 2015) that can temporally separate encoding, retention and retrieval periods (Sternberg, 1966; Luck and Vogel, 1997). Busch and Hermann (2003) suggested that object encoding and retention phases are generally influenced by object-load, while the retrieval phase is mainly regulated by feature-load. In the current study, WM was measured using the CDT (Luck and Vogel, 1997), wherein all objects were identical in shape, but varied in a single feature (color). The CDT employed here was predicted to result in modulation of alpha oscillations during the retrieval phase. Change detection is also suggested to be linked with two related yet independent processes; reactive allocation of attention, represented by correct change trials, and goal-directed allocation of attention, shown by correct no-change trials (Pessoa and Ungerleider, 2004). Therefore, to investigate change detection with a comparable attentional state, the contrast of correct change and correct no-change trials was investigated. This comparison excludes the possibility of the contamination often associated with the comparison of ‘detected or correct’ and ‘undetected or incorrect’ trials (Ress et al., 2000; Pessoa et al., 2002). To estimate the neural correlates of differences in WMC between HMLA and LMHA groups, modulation of alpha oscillations was explored during the retrieval phase. Additionally, several WM studies using different paradigms and populations have addressed a role of gamma oscillations in this process (Howard et al., 2003; Jokisch and Jensen, 2007; Roux et al., 2012; Lundqvist et al., 2016). Consequently, the current study also explored how gamma oscillations might reflect the differences between the groups and set sizes effects in the CDT.
Brain oscillations can indicate distinct cognitive processes (Fellinger et al., 2011) which are often altered by various affective states or traits. The current study explored the time-frequency characteristics of the oscillations recorded during the cognitive tasks to investigate the cognitive effects of trait mindfulness and trait anxiety. To analyze the time-frequency spectrum of these cognitive process-related oscillations, HHT analysis (Huang et al., 1998) was employed. Based on the previously mentioned studies, we expected to observe characteristic high theta and low beta power in the CST and dynamic alpha power in both groups. More importantly, we explored the entire spectrum from delta to gamma for the CST to investigate any group differences that might account for higher accuracy rates observed in a HMLA group compared to a LMHA group. In the CDT, principally alpha frequency with additional beta-gamma frequencies were explored to also investigate if they showed any change as a function of set sizes or differences between groups.
Methods
Participants
Participants were recruited based on their mindfulness and anxiety scores. People with any prior experience of mindfulness through Zen, Tai Chi, Qi Gong or Yoga were excluded. Prospective participants undergoing psychiatric treatment, pharmacological treatment or with any neurological disease were not recruited. Two groups of participants were recruited; HMLA and LMHA (for details see Supplementary Text, ST1), initially with 30 individuals in each group. Four participants were excluded, one due to having a WMC more than 2 SD higher than the group mean, and three due to technical issues during their EEG recordings (for demographic information, see Table 1).
Table 1.
Demographic and trait information of participants
| MAAS scores | STAI-T scores | Sample size | Mean age (years) | |
|---|---|---|---|---|
| High mindfulness-low anxiety (HMLA) | 74.7 ± 3.3 | 33.7 ± 3.9 | N = 27 (14 Females) | 21.2 ± 1.4 |
| Low mindfulness-high anxiety (LMHA) | 44.5 ± 7.5 | 60.0 ± 4.8 | N = 29 (15 Females) | 20.8 ± 1.9 |
MAAS: Mindful Attention Awareness Scale (MAAS) (Brown & Ryan, 2003)
STAI-Trait: State Trait Anxiety Inventory (STAI-T) (Spielberger et al., 1970)
All values are means ± standard deviations.
All the participants had normal vision or corrected-to-normal vision. Each participant gave written informed consent in accordance with the Declaration of Helsinki before participating in the experiment. All the experimental procedures were approved by the Institutional Review Board of National Taiwan University, Taipei, Taiwan.
Task procedure
The two cognitive tasks, CST and CDT, were performed by all the participants with simultaneous EEG recording. Tasks were administered in a counterbalanced order across participants in both groups with a break of at least 2–5 min between the presentations of each. Participants were either monetarily compensated or assigned course credits after completion of the tasks.
For the CST, participants had to identify the color of the target and disregard the meaning of the stimulus. There were 72 practice trials and a total of 432 trials in the formal experiment, with a 2:1 ratio of congruent to incongruent trials. For the CDT, participants were instructed to detect if the memory and test arrays were different or identical. There were 36 trials and 288 trials during practice and formal sessions, respectively. There were an equal number of change and no-change trials, and the change occurred with equal frequency in the left and right visual field. A total of 96 trials were presented for each set size in the task (for details see Figure 1).
Fig. 1.
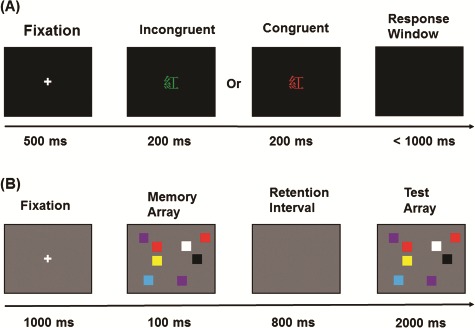
Schematic time frame of cognitive tasks. (A) CST: a fixation cross was presented for 500 ms at the center of the screen 1. This was followed by display of the target stimulus for 200 ms. The targets presented were Chinese characters for different colors [red ( ), green (
), green ( ), blue (
), blue ( ) or yellow (
) or yellow ( )] either with same ink color as its meaning (congruent) or with ink different from the meaning (incongruent). The maximum response period was 1000 ms, which terminated at the time limit or when a key was pressed. This was followed by a 1000 ms inter-trial interval. Responses were collected through keys (‘d’, ‘f’, ‘k’ and ‘l’) labeled with the colors. (B) CDT: a central fixation was displayed for 1000 ms, followed by the memory array. This was one of three set sizes, with 2, 4 or 8 randomly arranged colored squares presented for 100 ms using a pool of highly discriminable colors for the squares (red, blue, violet, green, yellow, black and white). There followed a retention interval of 800 ms and subsequent presentation of a test array for 2000 ms. The participants had to respond by pressing the corresponding keys assigned ‘1’ if the test array differed or ‘2’ if it was the same as the initial stimulus.
)] either with same ink color as its meaning (congruent) or with ink different from the meaning (incongruent). The maximum response period was 1000 ms, which terminated at the time limit or when a key was pressed. This was followed by a 1000 ms inter-trial interval. Responses were collected through keys (‘d’, ‘f’, ‘k’ and ‘l’) labeled with the colors. (B) CDT: a central fixation was displayed for 1000 ms, followed by the memory array. This was one of three set sizes, with 2, 4 or 8 randomly arranged colored squares presented for 100 ms using a pool of highly discriminable colors for the squares (red, blue, violet, green, yellow, black and white). There followed a retention interval of 800 ms and subsequent presentation of a test array for 2000 ms. The participants had to respond by pressing the corresponding keys assigned ‘1’ if the test array differed or ‘2’ if it was the same as the initial stimulus.
Analysis
Behavioral analyses
All the statistical analyses for behavioral data were identical to Jaiswal et al. (2018). Detailed explanation and statistical parameters can be found in the supplementary information (ST2).
EEG acquisition and analysis
EEG recording parameters
EEG data were acquired using 36-channel Ag/AgCl electrodes mounted in an elastic cap (Electrocap International) according to the International 10–20 System (FP1, FP2, F7, F3, Fz, F4, F8, FT7, FC3, FCz, FC4, FT8, T3, C3, Cz, C4, T4, TP7, CP3, CPZ, CP4, TP8, T5, P3, Pz, P4, T6, O1, Oz, O2, HEOL, HEOR, VEOU, VEOL, A1, A2), offline referenced to the left and right mastoid. The impedances of all EEG electrodes were kept below 5 kΩ, and data were recorded with a Neuroscan amplifier (Nuamps) and Neuroscan 4.5 Software with a sampling rate of 1000 Hz and a bandwidth of DC-260 Hz.
After epoching of each trial, vertical eye movements were removed by independent component analysis (Jung et al., 2000) using the script in EEGLAB (Delorme and Makeig, 2004). HHT analysis was conducted with in-house MATLAB (MathWorks) scripts comprising ensemble empirical mode decomposition (EEMD) package algorithms proposed by Wu and Huang (2009) (these scripts can be obtained from the corresponding author upon request). A conceptual description of HHT and EEMD has been provided in supplementary item ST3.
First, the EEG signal was decomposed into a fixed number of intrinsic mode functions (IMFs) by EEMD algorithms. Secondly, the Hilbert transform was calculated for each IMF to acquire the instantaneous frequencies. The resultant frequency bands obtained were delta (0.9–3.5 Hz), theta (3.5–7 Hz), alpha (7–14 Hz), beta (14–28 Hz) and gamma (28–56 Hz). The methodological and theoretical basis for determining the range of these frequency bands have been provided in supplementary item ST4. SPM12 for MEG/EEG (Wellcome Department of Cognitive Neurology, London, UK1) was used to perform further data processing and data analysis.
For statistical analyses, a two-tailed cluster-based non-parametric permutation (CBnPP) test was conducted (Maris and Oostenveld, 2007; Groppe et al., 2011; Liang et al., 2014) on the multichannel HHT spectra (channels × frequency × time points). The neighboring distance between two EEG sensors was defined as 60 mm, and 2000 permutations were performed for each test. CBnPP is a powerful approach to detect significant effects, and particularly clustered effects, in EEG data. This approach is less conservative in comparison with Bonferroni or false discovery rate correction yet can protect against multiple comparison errors.
EEG analysis: CST
The HHT time-frequency spectrum was plotted for the CST from 100 ms before and 800 ms following stimulus onset. A range of frequencies from 0.9 to 56 Hz was explored to analyze differences in conflict processes within and between groups. Five comparisons across the scalp for time–frequency distributions were made for this task. Two paired t-tests were performed to test for differences in EEG activity between incongruent trials and congruent trials for both groups. Two independent t-tests were conducted to investigate differences in EEG activity between the two groups on incongruent and congruent trials. Finally, another independent t-test was performed on differences in the power of incongruent and congruent trials between the two groups. This set of comparisons tested whether one or both groups differed under different congruency conditions.
EEG analysis: CDT
An overall analysis was performed using the sum of change and no-change trials in both groups to see the effect of set sizes and groups on the alpha to gamma frequencies (7–56 Hz). The HHT time–frequency spectrum was plotted for the CDT from 100 ms before and 500 ms following the onset of the test array. Primarily, alpha frequencies (7–14 Hz) were explored to assess within- and between-group differences as a consequence of set sizes. The power of change and no-change trials were attributed as the primary electrophysiological index of change detection (Pessoa and Ungerleider, 2004), across all comparisons.
A total of three categories of comparisons across the scalp for time–frequency distributions were made. In categories 1 and 2, two paired t-tests were performed to demonstrate the contrast in EEG activity between change and no-change trials for both groups on each set size separately. To assess the contrast between the two groups, two independent t-tests were performed on the difference of power between change and no-change trials for set sizes 4 and 8, respectively. In category 3, employing the difference in power of change and no-change trials, two paired t-tests were performed to test differences in EEG activity for set size 8 compared to set size 4 for both groups. To test the between-group differences, an independent t-test was performed on the difference of power between set sizes 8 and 4 in addition to differences in power of change and no-change trials. This set of comparisons provided an overview of whether or not one or both groups differed under different set size conditions.
Results
Behavioral results
On the CST, the HMLA group showed a significantly higher accuracy rate (p < 0.05) than the LMHA group for both the congruent and incongruent conditions. A descriptive summary of conflict task performance is shown in Table 2.
Table 2.
Color Stroop task behavioral data summary
| Color Stroop Task | Median Accuracy (%) | Reaction Times ± SEM (ms) | ||
|---|---|---|---|---|
| Congruent | Incongruent | Congruent | Incongruent | |
| HMLA group | 97.57 | 94.44 | 341.39 ± 11.47 | 442.44 ± 18.86 |
| LMHA group | 94.1 | 90.28 | 365.86 ± 17.95 | 455.52 ± 22.74 |
| Statistical estimate | -2.728$ | -3.021$ | -1.13@ | -0.439@ |
| p-value* | 0.006 | 0.006 | 0.264 | 0.662 |
$Non-parametric estimate of Mann-Whitney U test z-value.
@Parametric estimate of twosample test t-value.
*p-values reported here are after Holm–Bonferroni corrections.
On the CDT, the HMLA group showed a higher accuracy rate and WMC than the LMHA group, significantly so for set size 4 (P < 0.05) and marginally for set size 8 (P < 0.15). A descriptive summary of CDT performance is given in Table 3.
Table 3.
Results of validation and control analyses
| Change Detection Task | Mean Accuracy ± SEM (%) | Pashler's K (Kp) ± SEM | ||
|---|---|---|---|---|
| Set 4 | Set 8 | Set 4 | Set 8 | |
| HMLA group | 88.81 ± 1.06 | 69.83 ± 1.16 | 3.435 ± 0.071 | 3.664 ± 0.206 |
| LMHA group | 82.26 ± 1.54 | 66.24 ± 1.35 | 2.962 ± 0.092 | 3.186 ± 0.249 |
| t-value | 3.461 | 2.002 | 4.012 | 1.467 |
| p-value* | 0.003 | 0.1 | < 0.001 | 0.148 |
*p-values reported here are after Holm–Bonferroni corrections.
Time–frequency HHT spectrum results
Color Stroop task
Data from all trials were time-locked to the target onset (the target was always presented for a fixed interval of 200 ms, represented by the target offset point) and contrast between incongruent and congruent trials was performed for both groups. Significantly higher theta power was seen for the incongruent than for the congruent condition in both groups from 200 ms after target offset until ~500 ms across all electrodes (P < 0.05, CBnPP). Transient (~100 ms) higher alpha activity was seen in the fronto-central areas in both groups (P < 0.05, CBnPP). This was followed by later suppression of alpha activity 300 ms after target offset in both groups across all electrodes. There was also significantly lower activity in beta oscillations for all electrodes for both groups 300 ms after target offset (P < 0.05, CBnPP). There was also unexpected significantly lower delta activity throughout the trials in the HMLA group only (P < 0.05, CBnPP) (Figure 2A and B).
Fig. 2.
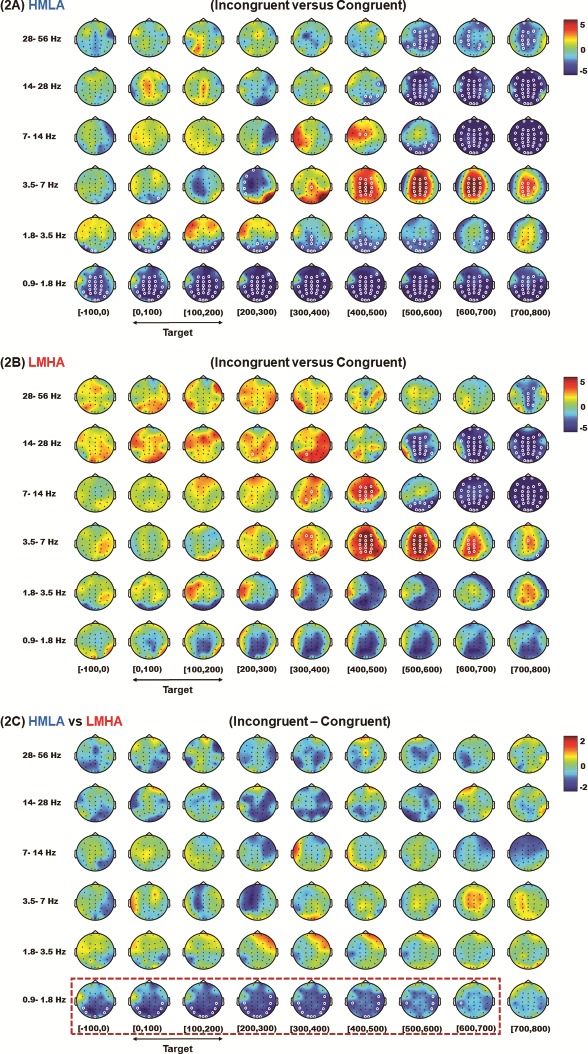
HHT time–frequency spectrum of the CST EEG. Data for analysis from all trials were time-locked to the target onset (the target was always presented for a fixed interval of 200 ms). A contrast between incongruent and congruent trials was carried out for the HMLA (A) and LMHA (2B) groups and a contrast between the two groups were also performed for the difference in power between incongruent trials and congruent trials (2C). (The white circles indicate the regions of significance, P < 0.05 CBnPP).
When the differences of power for the incongruent and congruent trials were compared, a significant difference between the two groups was seen for delta oscillations. The HMLA group showed lower delta activity than the LMHA group initially from 100 ms prior to the target onset in posterior temporal and occipital areas that was sustained and later extended to temporo-parietal areas until 700 ms after the target onset (P < 0.05, CBnPP) (Figure 2C). There were no group differences observed for either oscillation bands or periods when compared for congruent and incongruent conditions separately (P > 0.05, CBnPP) (see Supplementary Figure S2A and B).
Change detection task
The overall analysis showed the predominance of alpha activity in the LMHA group only in the retrieval phase. However, there was no effect of gamma oscillations observed across set sizes and groups (see Supplementary Figure S3). Primarily, the analysis was carried out with data for all trials time-locked to the onset of the test array. For category 1 comparisons, on set size 4 of the task, the contrast between change and no-change trials did not show any significant difference in alpha activity for either group (P > 0.05, CBnPP) (Figure 3A and 3B). There was also no difference between the two groups when the difference in power between change, and no-change trials was compared (P > 0.05, CBnPP) (Figure 3C).
Fig. 3.
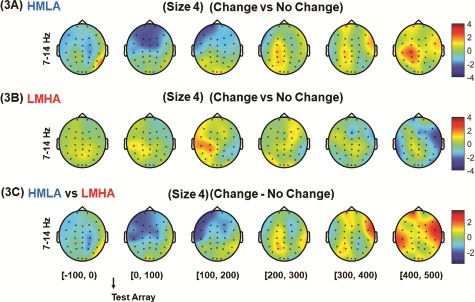
HHT time–frequency spectrum of CDT EEG data for set size 4. Analysis was carried out with data for all trials time-locked to the onset of the test array. For category 1 comparisons, on set size 4 of the task, the contrast between change and no change trials was performed for HMLA (A) and LMHA (B) groups and a contrast between the two groups were also performed on difference in power between change trials and no change trials (C).
For category 2 comparisons, on set size 8, the contrast between change and no-change trials did not show any significant difference in alpha activity for either group (P > 0.05, CBnPP) (Figure 4A and B). When the difference in power from change to no-change trials was compared, the HMLA group showed higher alpha activity beginning at the onset of the test array for the right fronto-central area that gradually expanded to right centro-parietal, fronto-central midline and right parietal areas as well as left fronto-central areas. This increase in alpha activity disappeared 400 ms after the onset of the test array (P < 0.05, CBnPP) (Figure 4C).
Fig. 4.
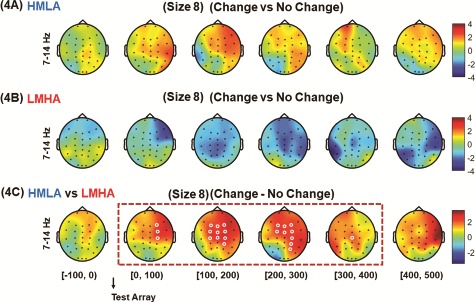
HHT time–frequency spectrum of CDT EEG data for set size 8. Analysis was carried out with data for all trials time-locked to the onset of the test array. For category 2 comparisons, on set size 8 of the task, the contrast between change and no change trials was performed for HMLA (A) and LMHA (B) groups and a contrast between the two groups were also performed on difference in power between change trials and no change trials (C). (The white circles indicate the regions of significance, P < 0.05 CBnPP).
For category 3 comparisons, the contrast between set size 8 and set size 4 on differences in power between change and no-change trials showed a significant difference in alpha activity in the HMLA group (P < 0.05, CBnPP), but not in the LMHA group (P > 0.05, CBnPP) (Figure 5A and B). In the HMLA group, alpha activity for set size 8 was significantly higher than for set size 4 beginning 100 ms before onset of the test array at the right fronto-central area and subsequently spreading to the fronto-central midline to the left frontal region (P < 0.05 CBnPP). This increase in alpha activity was no longer significant 200 ms after the onset of the test array. Furthermore, the contrast between groups for the difference in power for set sizes 8 and 4 using subtracted power of change trials and no-change trials, showed that the HMLA group had higher alpha activity than the LMHA group beginning 100 ms prior to onset of the test array at the right fronto-central area. This gradually expanded to bilateral fronto-central, centro-parietal and fronto-central midline areas (P < 0.05, CBnPP) and was no longer significant 300 ms after onset of the test array (P > 0.05, CBnPP) (Figure 5C).
Fig. 5.
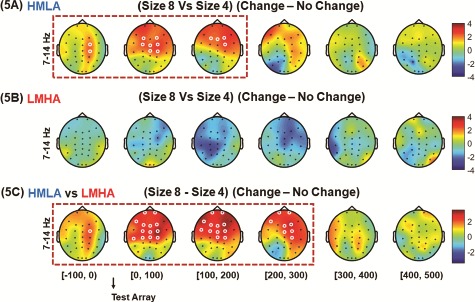
HHT time–frequency spectrum of CDT on difference of power from set size 8 to set size 4. Analysis was carried out with data for all trials time-locked to the onset of the test array. For category 3 comparisons, the contrast between set size 8 and set size 4 for differences in power between change and no change trials was performed for HMLA (A) and LMHA (B) groups. Furthermore, a contrast between the two groups was also performed on difference in power between change trials and no change trials as well as difference in set size 8 and set size 4 (C). (The white circles indicate the regions of significance, P < 0.05 CBnPP).
Discussion
As reported previously differences in both the CST and the CDT were seen for the LMHA and HMLA groups (Jaiswal et al., 2018). The differences seen here in the neurophysiology of the groups during task performance may explain the mechanisms underlying these differences. Higher accuracy on the CST observed in the HMLA group might have been due to lower delta (0.9–1.8 Hz) activity. Additionally, higher accuracy and higher WMC on the CDT in this group might be associated with higher alpha power (7–14 Hz). However, no role of gamma oscillations (28–56 Hz) was observed in the current WM task (for more details see Supplementary information, ST5). The neural dynamics of the observed brain oscillations on the two cognitive tasks are discussed below.
Neural dynamics of conflict control
HHT analysis of the EEG data showed characteristic higher theta (3.5–7 Hz) and lower beta (14–28 Hz) oscillations for the contrast between incongruent and congruent conditions in both groups. This is consistent with several previous studies (Hanslmayr et al., 2008; Wang et al., 2014; Zhao et al., 2015; Jo et al., 2017), adding more evidence for the emergence of theta and beta frequencies as a consequence of conflict monitoring. The appearance of a transient elevation of alpha (7–14 Hz) and theta activity and subsequent suppression of alpha activity is in line with initial high alpha power being associated with inhibition of the motor response and/or increasing the signal-to-noise ratio (Klimesch, 1999; Hummel et al., 2002). Simultaneously elevated theta may indicate a conflict that might be resolved (Hanslmayr et al., 2008; Cavanagh et al., 2009). Subsequent alpha suppression may relate to the release of inhibition processes (Knyazev, 2007) and facilitation of correct responses. The observation of topographically widespread alpha desynchronization is in line with this being seen in almost any kind of cognitive task that requires maintenance of general attentional resources (Pfurtscheller and Lopes da Silva, 1999).
There was, surprisingly, significant lower delta (0.9–1.8 Hz) activity in the HMLA group than the LMHA group for the CST, mainly over posterior temporal, temporo-parietal and occipital areas. This lower delta activity did not seem to be cognitive task specific, as it was evident even before the target onset. This might reflect a more alert attentive state (Steriade et al., 1993; Constantinople and Bruno, 2011) and might have facilitated better behavioral performance on the CST. An alternative explanation might be related to salience detection and impulsivity (Knyazev, 2007). A recent study showed that individuals who had weaker coupling between salience and visual networks showed successful resistance of tempting distractors (Steimke et al., 2017). It has also been suggested that the salience network plays an important role in the maintenance of attentional control in mindful people (Malinowski, 2013). It might be that the lower delta activity observed in the HMLA group during performance of the task might not be specific to the Stroop task. Rather it may be a general effect in this group individuals related to suppression of distractibility and the tendency to automatically read the color words.
Neural dynamics related to WMC
The contrasts between change and no-change conditions showed the time window where most the prevalent difference in alpha activity (7–14 Hz) was observed. This was immediately after onset of the test array and remained for up to 400 ms, for within and between-group comparisons. There were no within-group differences in alpha activity for the contrast of change and no-change conditions for both set sizes. However, the HMLA group showed higher alpha activity than LMHA group for the difference in power of the two trial types. Furthermore, subtracted power of the lower set size from that of the higher size showed that only the HMLA group had within as well as between group differences in alpha activity. Thus, the higher accuracy and WMC for both set sizes in the HMLA group may potentially be associated with such higher alpha power in this group. This suggests that under higher WM load, the HMLA group may allocate higher mental resources, while the LMHA group might be performing close to the ceiling at a lower memory load. Therefore, in the harder condition (higher memory load), the LMHA group might not have resources available to allocate and so show poorer performance than the HMLA group. The current observations are in agreement with the report by Dong et al. (2015) that higher alpha activity might be associated with higher WM for the HMLA group. However, it should be noted that Dong et al. (2015) employed a different paradigm (an n-back task) and observed the low WM group showed much stronger alpha desynchronization than the high WM group.
According to the traditional alpha-inhibition hypothesis (Klimesch, 1996; Pfurtscheller, 2003) alpha suppression in a given topographical area follows in response to relevant sensory stimuli (Palva and Palva, 2007). In accordance with this hypothesis, a previous WM study (Gevins et al., 1997) showed a decrease in alpha activity with an increase in WM load. This is quite contrary to both the current study and some previous studies (Krause et al., 1996; Jensen et al., 2002; Tuladhar et al., 2007) where high alpha activity was observed to be associated with an increase in memory load. This difference might be explained by the fact that Gevins et al. (1997) employed an n-back task that might encompass temporally overlapping operations required in encoding, scanning of memorized items, responding and ‘deleting’ items previously held in memory. This is quite distinct from a paradigm such as the CDT where encoding, retention and retrieval phases are temporally separated (Jensen et al., 2002). The current observation of higher alpha (7–14 Hz) activity in the HMLA group may be reconciled with the traditional alpha-inhibition hypothesis by an alternative explanation given by Klimesch (1999). It was proposed that alpha activity denotes inhibited brain activity in the region where it is seen in a similar pattern to when brain areas are not active in a relaxed state. Thus, during such an inhibited state the brain resources may be available to be allocated for retention of memory items by preventing the flow of non-essential information for the task into these areas (Klimesch, 1999; Jensen et al., 2002; Knyazev, 2007). In the current study, the HMLA group showed higher alpha activity than the LMHA group during performance of the CDT, indicating that the HMLA group might have been in a more relaxed or mindful state even when under a high WM load, and this might have led to better performance in this group.
Although the primary source of alpha activity in humans is centered on parietal regions, prefrontal alpha activity has been suggested to play an important role in the regulation of executive functions including WM (Sauseng et al., 2005; Hsu et al., 2014). The higher alpha activity in prefrontal areas observed in the HMLA group, and possibly indicating a relationship with higher WM, is in line with previous reports (Goldman-Rakic, 1996; Sauseng et al., 2005), suggesting better top-down processing through enhanced alpha activity during the WM task. The topographical distribution of alpha activity in the current research partially echoes findings from an fMRI study (Pessoa and Ungerleider, 2004) that showed activation of frontal gyrus, anterior cingulate cortex, and anterior parietal sulcus during change detection. In the current study, more alpha activity in the HMLA group than the LMHA group was observed in fronto-central, central and centro-parietal areas in addition to the PFC region. Thus, considering the alternative explanation of the alpha-inhibition hypothesis, it can be inferred that the HMLA group, showing higher alpha activity in the above brain areas, might have been in a more relaxed or mindful state than the LMHA group. This might have led to the allocation of these brain areas for successful maintenance and retrieval of memory items (Jensen et al., 2002) in addition to the simultaneous enhancement of the neural signal to noise ratio (Klimesch, 1999; Hummel et al., 2002).
Limitations and future directions
One important limitation of the current study is the way the two experimental groups were created by combining mindfulness and anxiety scores such that the principal contributions of each to behavioral or neurophysiological differences could not be teased apart. Additionally, while the current study encompassed mindfulness and anxiety, it did not involve manipulation of either. Therefore, future studies should explore how interventions involving mindfulness or manipulation of anxiety state can modulate behavioral performance and the associated neural correlates of such performance.
Conclusions
The previously shown behavioral differences between two groups with HMLA and LMHA in accuracy rate on a CST, and different accuracy rates and WMC on a CDT, could be accounted for by the variation in delta and alpha electrophysiological activities. The higher accuracy rate on the CST in the HMLA group might be generally linked to lower delta activity in posterior temporal and occipital areas, indicating a more attentive state in this group. The higher accuracy rate and WMC in the HMLA group might be specifically linked to enhanced alpha activity in PFC, fronto-central and centro-parietal regions during the retrieval phase and indicating active allocation of brain resources in this group. Thus, the findings indicate that high mindful, low anxious people may possess better conflict ability, which may be generally due to lower delta activity, whereas they may have higher WMC specifically associated with higher alpha activity. Future studies should explore the specific predictions regarding the topography and directionality of changes in power pertaining to the groups and tasks.
Supplementary Material
Footnotes
Funding
This work was sponsored by the Ministry of Science and Technology, Taiwan, (107-2420-H-008-009, 107-2628-H-008-002-MY3, 106-2410-H-008-038-MY3, 106-2628-H-008-002-MY4 and 107-2410-H-008-040-MY3) and sponsored by Taiwan Ministry of Education’s ‘Academic Strategic Alliance: Taiwan and Oxford University’ project grant (MOE Oxford-NCU-BRC collaborative project).
Conflict of interest
None declared.
References:
- Arch J.J., Craske M.G. (2010). Laboratory stressors in clinically anxious and non-anxious individuals: the moderating role of mindfulness. Behaviour Research and Therapy, 48(6), 495–505. [DOI] [PubMed] [Google Scholar]
- Baer R.A., Smith G.T., Hopkins J., Krietemeyer J., Toney L. (2006). Using self-report assessment methods to explore facets of mindfulness. Assessment, 13(1), 27–45. [DOI] [PubMed] [Google Scholar]
- Becker E.S., Rinck M., Margraf J., Roth W.T. (2001). The emotional Stroop effect in anxiety disorders general emotional or disorder specificity? Journal of Anxiety Disorders, 15(3), 147–59. [DOI] [PubMed] [Google Scholar]
- Berryhill M.E., Olson I.R. (2008). Is the posterior parietal lobe involved in working memory retrieval? Evidence from patients with bilateral parietal lobe damage. Neuropsychologia, 46(7), 1767–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop S.J. (2009). Trait anxiety and impoverished prefrontal control of attention. Nature Neuroscience, 12(1), 92–8. [DOI] [PubMed] [Google Scholar]
- Botvinick M.M., Cohen J.D., Carter C.S. (2004). Conflict monitoring and anterior cingulate cortex: an update. Trends in Cognitive Sciences, 8(12), 539–46. [DOI] [PubMed] [Google Scholar]
- Brittain J.S., Watkins K.E., Joundi R.A., et al. (2012). A role for the subthalamic nucleus in response inhibition during conflict. The Journal of Neuroscience, 32(39), 13396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K.W., Goodman R.J., Inzlicht M. (2013). Dispositional mindfulness and the attenuation of neural responses to emotional stimuli. Social Cognitive and Affective Neuroscience, 8(1), 93–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch N.A., Hermann C.S. (2003). Object-load and feature-load modulate EEG in a short-term memory task. Neuroreport, 14(13), 1721–4. [DOI] [PubMed] [Google Scholar]
- Bush G., Luu P., Posner M.I. (2000). Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences, 4(6), 215–22. [DOI] [PubMed] [Google Scholar]
- Cavanagh J.F., Cohen M.X., Allen J.J. (2009). Prelude to and resolution of an error: EEG phase synchrony reveals cognitive control dynamics during action monitoring. The Journal of Neuroscience, 29(1), 98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh J.F., Frank M.J. (2014). Frontal theta as a mechanism for cognitive control. Trends in Cognitive Sciences, 18(8), 414–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey K.A., Hartman M. (2008). Mechanisms of action in the inverse relationship between mindfulness and psychological distress. Complementary Health Practice Review, 13(2), 79–91. [Google Scholar]
- Constantinople C.M., Bruno R.M. (2011). Effects and mechanisms of wakefulness on local cortical networks. Neuron, 69(6), 1061–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darke S. (1988). Anxiety and working memory capacity. Cognition and Emotion, 2(2), 145–54. [Google Scholar]
- Delorme A., Makeig S. (2004). EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134(1), 9–21. [DOI] [PubMed] [Google Scholar]
- Desrosiers A., Vine V., Klemanski D.H., Nolen-Hoeksema S. (2013). Mindfulness and emotion regulation in depression and anxiety: common and distinct mechanisms of action. Depression and Anxiety, 30(7), 654–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresler T., Meriau K., Heekeren H.R., van der Meer E. (2009). Emotional Stroop task: effect of word arousal and subject anxiety on emotional interference. Psychological Research, 73(3), 364–71. [DOI] [PubMed] [Google Scholar]
- Dong S., Reder L.M., Yao Y., Liu Y., Chen F. (2015). Individual differences in working memory capacity are reflected in different ERP and EEG patterns to task difficulty. Brain Research, 1616, 146–56. [DOI] [PubMed] [Google Scholar]
- Etkin A., Klemenhagen K.C., Dudman J.T., et al. (2004). Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron, 44(6), 1043–55. [DOI] [PubMed] [Google Scholar]
- Eysenck M.W., Calvo M.G. (1992). Anxiety and performance: the processing efficiency theory. Cognition and Emotion, 6(6), 409–34. [Google Scholar]
- Eysenck M.W., Derakshan N., Santos R., Calvo M.G. (2007). Anxiety and cognitive performance: attentional control theory. Emotion, 7(2), 336–53. [DOI] [PubMed] [Google Scholar]
- Fan J., Flombaum J.I., McCandliss B.D., Thomas K.M., Posner M.I. (2003). Cognitive and brain consequences of conflict. NeuroImage, 18(1), 42–57. [DOI] [PubMed] [Google Scholar]
- Fellinger R., Klimesch W., Schnakers C., et al. (2011). Cognitive processes in disorders of consciousness as revealed by EEG time-frequency analyses. Clinical Neurophysiology, 122(11), 2177–84. [DOI] [PubMed] [Google Scholar]
- Figueira J.S.B., Oliveira L., Pereira M.G., et al. (2017). An unpleasant emotional state reduces working memory capacity: electrophysiological evidence. Social Cognitive and Affective Neuroscience, 12(6), 984–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frewen P.A., Dozois D.J., Neufeld R.W., et al. (2010). Individual differences in trait mindfulness predict dorsomedial prefrontal and amygdala response during emotional imagery: an fMRI study. Personality and Individual Differences, 49(5), 479–84. [Google Scholar]
- Fukuda K., Mance I., Vogel E.K. (2015). α Power modulation and event-related slow wave provide dissociable correlates of visual working memory. Journal of Neuroscience, 35(41), 14009–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevins A., Smith M.E., McEvoy L., Yu D. (1997). High-resolution EEG mapping of cortical activation related to working memory: effects of task difficulty, type of processing, and practice. Cerebral Cortex, 7(4), 374–85. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic P.S. (1996). Regional and cellular fractionation of working memory. Proceedings of the National Academy of Sciences of the United States of America, 93(24), 13473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greeson J., Brantley J. (2009). Mindfulness and anxiety disorders: developing a wise relationship with the inner experience of fear In: Clinical Handbook of Mindfulness. New York: Springer, pp. 171–88. [Google Scholar]
- Groppe D.M., Urbach T.P., Kutas M. (2011). Mass univariate analysis of event-related brain potentials/fields II: simulation studies. Psychophysiology, 48(12), 1726–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanslmayr S., Pastotter B., Bauml K.H., Gruber S., Wimber M., Klimesch W. (2008). The electrophysiological dynamics of interference during the Stroop task. Journal of Cognitive Neuroscience, 20(2), 215–25. [DOI] [PubMed] [Google Scholar]
- Hofmann S.G., Sawyer A.T., Witt A.A., Oh D. (2010). The effect of mindfulness-based therapy on anxiety and depression: a meta-analytic review. Journal of Consulting and Clinical Psychology, 78(2), 169–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzel B.K., Hoge E.A., Greve D.N., et al. (2013). Neural mechanisms of symptom improvements in generalized anxiety disorder following mindfulness training. NeuroImage Clinical, 2, 448–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard M.W., Rizzuto D.S., Caplan J.B., et al. (2003). Gamma oscillations correlate with working memory load in humans. Cerebral Cortex, 13(12), 1369–74. [DOI] [PubMed] [Google Scholar]
- Hsu T.Y., Tseng P., Liang W.K., Cheng S.K., Juan C.H. (2014). Transcranial direct current stimulation over right posterior parietal cortex changes prestimulus alpha oscillation in visual short-term memory task. NeuroImage, 98, 306–13. [DOI] [PubMed] [Google Scholar]
- Huang N.E., Shen Z., Long S.R., et al. (1998). The empirical mode decomposition and the Hilbert spectrum for nonlinear and non-stationary time series analysis. Proceedings of the Royal Society of London Series A, 454(1971), 903–95. [Google Scholar]
- Hummel F., Andres F., Altenmuller E., Dichgans J., Gerloff C. (2002). Inhibitory control of acquired motor programmes in the human brain. Brain, 125(Pt 2), 404–20. [DOI] [PubMed] [Google Scholar]
- Jaiswal S., Tsai S.-Y., Juan C.-H., Liang W.-K., Muggleton N.G. (2018). Better cognitive performance is associated with the combination of high trait mindfulness and low trait anxiety. Frontiers in Psychology, 9(627). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O., Gelfand J., Kounios J., Lisman J.E. (2002). Oscillations in the alpha band (9–12 Hz) increase with memory load during retention in a short-term memory task. Cerebral Cortex, 12(8), 877–82. [DOI] [PubMed] [Google Scholar]
- Jha A.P., Stanley E.A., Kiyonaga A., Wong L., Gelfand L. (2010). Examining the protective effects of mindfulness training on working memory capacity and affective experience. Emotion, 10(1), 54–64. [DOI] [PubMed] [Google Scholar]
- Jo H.-G., Malinowski P., Schmidt S. (2017). Frontal theta dynamics during response conflict in Long-term mindfulness meditators. Frontiers in Human Neuroscience, 11(299). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo H.G., Schmidt S., Inacker E., Markowiak M., Hinterberger T. (2016). Meditation and attention: a controlled study on long-term meditators in behavioral performance and event-related potentials of attentional control. International Journal of Psychophysiology, 99, 33–9. [DOI] [PubMed] [Google Scholar]
- Jokisch D., Jensen O. (2007). Modulation of gamma and alpha activity during a working memory task engaging the dorsal or ventral stream. The Journal of Neuroscience, 27(12), 3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung T.P., Makeig S., Humphries C., et al. (2000). Removing electroencephalographic artifacts by blind source separation. Psychophysiology, 37(2), 163–78. [PubMed] [Google Scholar]
- Kabat-Zinn J. (1990). Full Catastrophe Living: Using the Wisdom of your Body and Mind to Face Stress, Pain, and Illness. New York: Delacorte Press. [Google Scholar]
- Kahana M.J., Sekuler R., Caplan J.B., Kirschen M., Madsen J.R. (1999). Human theta oscillations exhibit task dependence during virtual maze navigation. Nature, 399(6738), 781. [DOI] [PubMed] [Google Scholar]
- Kane M.J., Engle R.W. (2003). Working-memory capacity and the control of attention: the contributions of goal neglect, response competition, and task set to Stroop interference. Journal of Experimental Psychology: General, 132(1), 47. [DOI] [PubMed] [Google Scholar]
- Kiken L.G., Garlan E.L., Bluth K., Palsson O.S., Gaylord S.A. (2015). From a state to a trait: trajectories of state mindfulness in meditation during intervention predict changes in trait mindfulness. Personality and Individual Differences, 81, 41–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein K., Boals A. (2001). The relationship of life event stress and working memory capacity. Applied Cognitive Psychology, 15(5), 565–79. [Google Scholar]
- Klimesch W. (1996). Memory processes, brain oscillations and EEG synchronization. International Journal of Psychophysiology, 24(1–2), 61–100. [DOI] [PubMed] [Google Scholar]
- Klimesch W. (1999). EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Research. Brain Research Reviews, 29(2–3), 169–95. [DOI] [PubMed] [Google Scholar]
- Knyazev G.G. (2007). Motivation, emotion, and their inhibitory control mirrored in brain oscillations. Neuroscience and Biobehavioral Reviews, 31(3), 377–95. [DOI] [PubMed] [Google Scholar]
- Krause C.M., Lang A.H., Laine M., Kuusisto M., Porn B. (1996). Event-related EEG desynchronization and synchronization during an auditory memory task. Electroencephalography and Clinical Neurophysiology, 98(4), 319–26. [DOI] [PubMed] [Google Scholar]
- Lau M.A., Bishop S.R., Segal Z.V., et al. (2006). The Toronto mindfulness scale: development and validation. Journal of Clinical Psychology, 62(12), 1445–67. [DOI] [PubMed] [Google Scholar]
- Liang W.K., Lo M.T., Yang A.C., et al. (2014). Revealing the brain's adaptability and the transcranial direct current stimulation facilitating effect in inhibitory control by multiscale entropy. NeuroImage, 90, 218–34. [DOI] [PubMed] [Google Scholar]
- Lo Y.H., Liang W.K., Lee H.W., et al. (2013). The neural development of response inhibition in 5- and 6-year-old preschoolers: an ERP and EEG study. Developmental Neuropsychology, 38(5), 301–16. [DOI] [PubMed] [Google Scholar]
- Luck S.J., Vogel E.K. (1997). The capacity of visual working memory for features and conjunctions. Nature, 390(6657), 279–81. [DOI] [PubMed] [Google Scholar]
- Lundqvist M., Rose J., Herman P., Brincat S.L., Buschman T.J., Miller E.K. (2016). Gamma and Beta bursts underlie working memory. Neuron, 90(1), 152–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald A.W., Cohen J.D., Stenger V.A., Carter C.S. (2000). Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science, 288(5472), 1835–8. [DOI] [PubMed] [Google Scholar]
- MacLeod C., Donnellan A.M. (1993). Individual differences in anxiety and the restriction of working memory capacity. Personality and Individual Differences, 15(2), 163–73. [Google Scholar]
- Malinowski P. (2013). Neural mechanisms of attentional control in mindfulness meditation. Frontiers in Neuroscience, 7, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris E., Oostenveld R. (2007). Nonparametric statistical testing of EEG- and MEG-data. Journal of Neuroscience Methods, 164(1), 177–90. [DOI] [PubMed] [Google Scholar]
- Miller E.K., Cohen J.D. (2001). An integrative theory of prefrontal cortex function. Annual Review of Neuroscience, 24(1), 167–202. [DOI] [PubMed] [Google Scholar]
- Miyake A., Friedman N.P., Emerson M.J., Witzki A.H., Howerter A., Wager T.D. (2000). The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: a latent variable analysis. Cognitive Psychology, 41(1), 49–100. [DOI] [PubMed] [Google Scholar]
- Miyake A., Friedman N.P. (2012). The nature and Organization of Individual Differences in executive functions: four general conclusions. Current Directions in Psychological Science, 21(1), 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocaiber I., Pereira M.G., Erthal F.S., et al. (2009). Regulation of negative emotions in high trait anxious individuals: an ERP study. Psychology and Neuroscience, 2(2), 211. [Google Scholar]
- Moore A., Malinowski P. (2009). Meditation, mindfulness and cognitive flexibility. Consciousness and Cognition, 18(1), 176–86. [DOI] [PubMed] [Google Scholar]
- Moriya J. (2016). Attentional networks and visuospatial working memory capacity in social anxiety. Cognition and Emotion, 1–9. [DOI] [PubMed] [Google Scholar]
- Moriya J., Sugiura Y. (2012). High visual working memory capacity in trait social anxiety. PLoS One, 7(4), e34244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrazek M.D., Franklin M.S., Phillips D.T., Baird B., Schooler J.W. (2013). Mindfulness training improves working memory capacity and GRE performance while reducing mind wandering. Psychological Science, 24(5), 776–81. [DOI] [PubMed] [Google Scholar]
- Nigbur R., Ivanova G., Sturmer B. (2011). Theta power as a marker for cognitive interference. Clinical Neurophysiology, 122(11), 2185–94. [DOI] [PubMed] [Google Scholar]
- Palva S., Palva J.M. (2007). New vistas for alpha-frequency band oscillations. Trends in Neurosciences, 30(4), 150–8. [DOI] [PubMed] [Google Scholar]
- Pessoa L., Gutierrez E., Bandettini P.A., Ungerleider L.G. (2002). Neural correlates of visual working memory: fMRI amplitude predicts task performance. Neuron, 35(5), 975–87. [DOI] [PubMed] [Google Scholar]
- Pessoa L., Ungerleider L.G. (2004). Neural correlates of change detection and change blindness in a working memory task. Cerebral Cortex, 14(5), 511–20. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G. (2003). Induced oscillations in the alpha band: functional meaning. Epilepsia, 44(Suppl 12), 2–8. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G., Lopes da Silva F.H. (1999). Event-related EEG/MEG synchronization and desynchronization: basic principles. Clinical Neurophysiology, 110(11), 1842–57. [DOI] [PubMed] [Google Scholar]
- Ress D., Backus B.T., Heeger D.J. (2000). Activity in primary visual cortex predicts performance in a visual detection task. Nature Neuroscience, 3(9), 940. [DOI] [PubMed] [Google Scholar]
- Roux F., Wibral M., Mohr H.M., Singer W., Uhlhaas P.J. (2012). Gamma-band activity in human prefrontal cortex codes for the number of relevant items maintained in working memory. The Journal of Neuroscience, 32(36), 12411–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauseng P., Klimesch W., Doppelmayr M., Pecherstorfer T., Freunberger R., Hanslmayr S. (2005). EEG alpha synchronization and functional coupling during top-down processing in a working memory task. Human Brain Mapping, 26(2), 148–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmader T., Johns M. (2003). Converging evidence that stereotype threat reduces working memory capacity. Journal of Personality and Social Psychology, 85(3), 440–52. [DOI] [PubMed] [Google Scholar]
- Stam C.J. (2005). Nonlinear dynamical analysis of EEG and MEG: review of an emerging field. Clinical Neurophysiology, 116(10), 2266–301. [DOI] [PubMed] [Google Scholar]
- Steimke R., Nomi J.S., Calhoun V.D., et al. (2017). Salience network dynamics underlying successful resistance of temptation. Social Cognitive and Affective Neuroscience, 12(12), 1928–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M., McCormick D.A., Sejnowski T.J. (1993). Thalamocortical oscillations in the sleeping and aroused brain. Science, 262(5134), 679–85. [DOI] [PubMed] [Google Scholar]
- Sternberg S. (1966). High-speed scanning in human memory. Science, 153(3736), 652–4. [DOI] [PubMed] [Google Scholar]
- Stroop J.R. (1935). Studies of interference in serial verbal reactions. Journal of Experimental Psychology, 18(6), 643. [Google Scholar]
- Swann N.C., Cai W., Conner C.R., et al. (2012). Roles for the pre-supplementary motor area and the right inferior frontal gyrus in stopping action: electrophysiological responses and functional and structural connectivity. NeuroImage, 59(3), 2860–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D., Hu L., Chen A. (2013). The neural oscillations of conflict adaptation in the human frontal region. Biological Psychology, 93(3), 364–72. [DOI] [PubMed] [Google Scholar]
- Teper R., Inzlicht M. (2013). Meditation, mindfulness and executive control: the importance of emotional acceptance and brain-based performance monitoring. Social Cognitive and Affective Neuroscience, 8(1), 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng P., Hsu T.Y., Chang C.F., et al. (2012). Unleashing potential: transcranial direct current stimulation over the right posterior parietal cortex improves change detection in low-performing individuals. The Journal of Neuroscience, 32(31), 10554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuladhar A.M., Huurne N.t., Schoffelen J.M., Maris E., Oostenveld R., Jensen O. (2007). Parieto-occipital sources account for the increase in alpha activity with working memory load. Human Brain Mapping, 28(8), 785–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsworth N., Heitz R.P., Schrock J.C., Engle R.W. (2005). An automated version of the operation span task. Behavior Research Methods, 37(3), 498–505. [DOI] [PubMed] [Google Scholar]
- Wang K., Li Q., Zheng Y., Wang H., Liu X. (2014). Temporal and spectral profiles of stimulus–stimulus and stimulus–response conflict processing. NeuroImage, 89, 280–8. [DOI] [PubMed] [Google Scholar]
- Way B.M., Creswell J.D., Eisenberger N.I., Lieberman M.D. (2010). Dispositional mindfulness and depressive symptomatology: correlations with limbic and self-referential neural activity during rest. Emotion (Washington, D.C.), 10(1), 12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womelsdorf T., Johnston K., Vinck M., Everling S. (2010). Theta-activity in anterior cingulate cortex predicts task rules and their adjustments following errors. Proceedings of the National Academy of Sciences, 107(11), 5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., Huang N.E. (2009). Ensemble empirical mode decomposition: a noise-assisted data analysis method. Advances in Adaptive Data Analysis, 1(01), 1–41. [Google Scholar]
- Zhao J., Liang W.-K., Juan C.-H., Wang L., Wang S., Zhu Z. (2015). Dissociated stimulus and response conflict effect in the Stroop task: evidence from evoked brain potentials and brain oscillations. Biological Psychology, 104, 130–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


