Abstract
Few studies have used matched affective paradigms to compare humans and non-human primates. In monkeys with amygdala lesions and youth with anxiety disorders, we examined cross-species pupillary responses during a saccade-based, affective attentional capture task. Given evidence of enhanced amygdala function in anxiety, we hypothesized that opposite patterns would emerge in lesioned monkeys and anxious participants. A total of 53 unmedicated youths (27 anxious, 26 healthy) and 8 adult male rhesus monkeys (Macaca mulatta) completed matched behavioral paradigms. Four monkeys received bilateral excitotoxic amygdala lesions and four served as unoperated controls. Compared to healthy youth, anxious youth exhibited increased pupillary constriction in response to emotional and non-emotional distractors (F(1,48) = 6.28, P = 0.02, η2p = 0.12). Pupillary response was associated significantly with anxiety symptoms severity (F(1,48) = 5.59, P = 0.02, η2p = 0.10). As hypothesized, lesioned monkeys exhibited the opposite pattern i.e. decreased pupillary constriction in response to distractors, compared to unoperated control monkeys (F(1,32) = 24.22, P < 0.001, η2 = 0.33). Amygdala lesioned monkeys and youth with anxiety disorders show opposite patterns of pupil constriction in the context of an affective distractor task. Such findings suggest the presence of altered amygdala circuitry functioning in anxiety. Future lesion and human neuroimaging work might examine the way in which specific amygdala sub-nuclei and downstream circuits mediate these effects.
Keywords: anxiety, amygdala, pupillometry, attention, emotion, rhesus
Introduction
Studies directly comparing data in non-human primates and humans provide a critical foundation for translational research. Despite increasing emphasis on such work (Cole et al., 2009; Wallis, 2011; Callaghan et al., 2014; Callaghan and Tottenham, 2016), only a few studies have collected data using matched behavioral paradigms in humans and monkeys (Margulies et al., 2009; Kagan et al., 2010). While this type of cross-species work can be difficult given the complexities of working with human clinical samples and monkey populations, it can yield convergent and complementary evidence in understanding psychopathology. The current study collected data in healthy youth and youth with anxiety disorders on an adapted version of a task sensitive to amygdala lesions in primates (Dal Monte et al., 2015). The study presents data on the task in these youths as well as new data in primates with and without amygdala lesions, in a search for complementary cross-species findings.
The current study examines the impact of emotional stimuli on attention in pediatric anxiety. A focus on youth is important, given that most anxiety disorders first arise during child/adolescent development (Kessler et al., 2005). Correlates of anxiety observed in adults may reflect sequelae of chronic anxiety and/or confounding features such as treatment. Prior research, using various tasks, has consistently demonstrated biases in the capture of attention among children and adolescents with anxiety disorders (Bar-Haim et al., 2007). While imaging studies suggest that the amygdala and related circuitry mediates such biases (Shechner et al., 2012; Weber et al., 2016; White et al., 2016), strong conclusions regarding a causal role for amygdala circuitry are not possible due to the correlational nature of brain imaging methods. Thus, the combination of human and animal models can be critically informative in mapping relations among amygdala function, attention bias and anxiety, given long-standing evidence that amygdala lesions disrupt fear and anxiety behavior in monkeys, e.g. Weiskrantz (1956). To implement such work, the current study utilized a saccade-based attentional capture task similar to the gap/overlap task used in prior studies but incorporating affective stimuli. Examining saccade latencies on this current task, Dal Monte et al. (2015) found that threatening distractors strongly disrupt attention in monkeys, but that this disruption was eliminated in monkeys with amygdala lesions. The monkeys also completed an additional paradigm where full monkey faces were freely viewed in the absence of a cognitive task. During free viewing, compared to unoperated controls, monkeys with amygdala lesions showed reduced exploration of the eyes and less pupil constriction to facial stimuli (Dal Monte et al., 2015). However, Dal Monte et al. did not examine pupil response to monkey faces in the context of the saccade-based attentional capture task on which the monkeys manifested perturbed saccade patterns. As task demands may influence pupillary responses, here we combine analysis of these previously unexamined pupillometry data from the attentional capture task in monkeys from Dal Monte et al. (2015) with a parallel study in humans in order to examine cross-species convergence.
While considerable research uses response latency to index attention, studies suggest that pupillary response may more precisely quantify aspects of attention during cognitive tasks. Nevertheless, both attention and affect are likely to influence both response latency and pupillary response. Direct comparisons in humans and monkeys might guide future attempts to understand any commonalities and differences across species, outcome measures and task demands. Pupil size is shaped by parasympathetic and sympathetic pathways (Sirois and Brisson, 2014). As in humans, work in rhesus monkeys implicates sympathetic innervation of the iris in modulating pupil diameter and responsivity (Clarke et al., 2003). Cognitive load appears to increase pupil diameter and reduce the pupillary light reflex (Steinhauer et al., 2000) and anticipation of an aversive event also reduces the pupillary light reflex (Bitsios et al., 1996). Clinical studies in humans extend such findings by linking altered pupillary responses to clinical and trait-level anxiety (Bakes et al., 1990; Nagai et al., 2002) and to family history of affective disorders (Burkhouse et al., 2014). Again, comparing data in humans and monkeys may provide an important guide to focus future work.
The current study examined saccade latency and pupillometry in both humans and non-human primates using parallel behavioral paradigms that required saccades away from a central face stimulus distractor and toward a peripheral target. This provides an important first step to guide future, more definitive studies. Overall, we broadly hypothesized that amygdala lesions in rhesus monkeys, compared to anxiety in human participants, would elicit opposite effects on attentional capture, providing one piece of complementary evidence for amygdala circuit disruption in clinical anxiety. The broad nature of these hypotheses reflects the limited previous work of this nature. First, we examined saccade latency in unmedicated youth with or without anxiety disorders with the hypothesis that anxiety would exaggerate attention capture by negative face stimuli distractors, opposite to the effect of amygdala lesions shown previously in monkeys (Dal Monte et al., 2015). Second, we sought to extend this work to compare pupillary responses on this task in monkeys with amygdala lesions to pupillary responses in anxious youth. Again, given prior neuroimaging evidence of amygdala circuitry ‘hyper-responsivity’ in anxious youth, we hypothesized that amygdala lesions would cause pupillary responses ‘opposite’ to those seen in anxious youth. Observing such patterns would be a first step toward implicating the amygdala and related circuitry in the etiology of perturbed pupillary function in human anxiety disorders.
Materials and methods
Study 1: anxiety-related differences in humans
Participants
A total of 53 children and adolescents (8–18 years old), including 27 unmedicated anxious patients and 26 healthy volunteers, completed the current task. Participants enrolled in an institutional review board-approved protocol at the National Institute of Mental Health in Bethesda, MD. Youth and their guardians provided written informed assent and consent, respectively. Children’s psychiatric diagnoses were assessed using parent and child report on the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime version (Kaufman et al., 1997). Participants either met criteria for an anxiety disorder [generalized anxiety disorder (GAD), social anxiety disorder and/or separation anxiety disorder] or did not meet criteria for any Axis I diagnosis (healthy volunteers). Participants were excluded for pervasive developmental disorders, intelligence quotient (IQ) lower than 70 on the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999), substance abuse within the past 2 months, current diagnoses of major depressive disorder, obsessive–compulsive disorder, or post-traumatic stress disorder, or lifetime history of psychosis, bipolar disorder or major trauma. Additionally, no participants met criteria for attention deficit hyperactivity disorder, conduct disorder, oppositional defiant disorder or disruptive mood dysregulation disorder.
Participants and their guardians also completed questionnaires assessing clinical symptoms. Parent and child report was acquired on the Screen for Anxiety Related Disorders (SCARED; Birmaher et al., 1997) to assess recent anxiety symptoms (average of parent and child report) as well as on the Affective Reactivity Index (ARI; Stringaris et al., 2012) to assess irritability symptoms in the past 6 months (average of parent and child report). Child report was acquired on the Children’s Depression Inventory (CDI; Kovacs, 1985) to assess recent depressive symptoms as well as on the State-Trait Anxiety Inventory (STAI-S/T; Spielberger et al., 1970) to assess state anxiety at the time of the task and trait-level anxiety.
Saccade-based attentional capture task
Participants completed a saccade-based behavioral task (Figure 1A) to assess attentional capture by emotional face distractors. The task was based on prior work examining attentional capture in rhesus monkeys (Dal Monte et al., 2015). The current task was presented to participants using Eprime v2.0 (Psychology Software Tools, 2012), and eye movements were recorded at 300 Hz using a Tobii TX300 eye tracking camera via Tobii Studio 3.2.1. (Tobii Technology AB, Danderyd, Sweden).
Fig. 1.
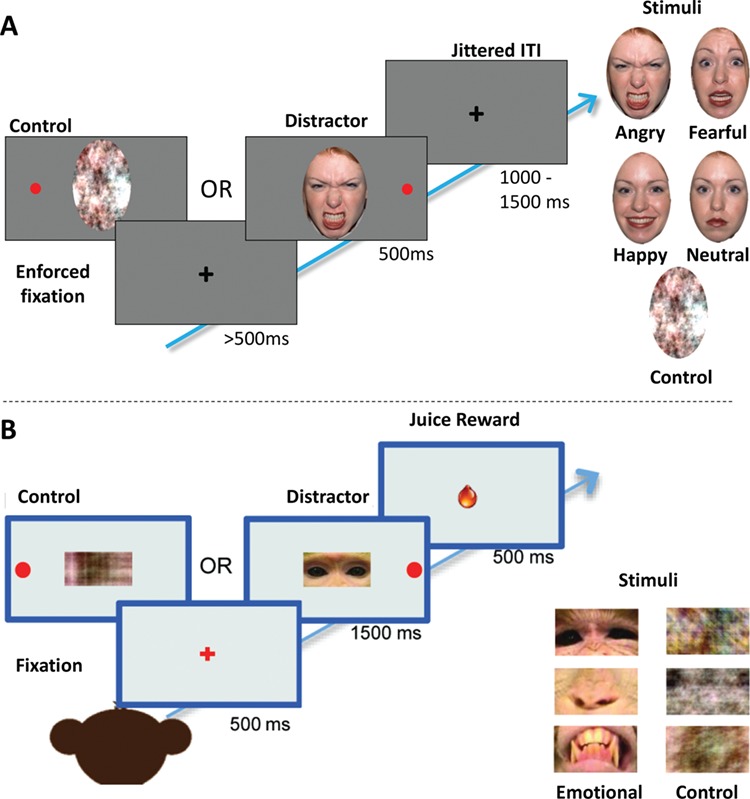
Saccade-based attentional capture tasks. The saccade-based attentional capture task used with human participants is displayed in Panel A. Participants were instructed to fixate on a black central fixation cross and to make a saccade to a peripheral red dot target that would appear on either the left or right side of the screen simultaneously with a centrally presented face distractor for 500 ms. The fixation cross remained on the screen between trials for a jittered period of at least 1000–1500 ms but would remain on the screen indefinitely until participants fixated here for 500 ms before each trial. The central distractors were oval cut-outs of angry, fearful, happy or neutral faces or non-face control distractors (examples are shown). Distractors were either presented with the eyes or the mouth at the central fixation point. All stimuli appeared on a gray background. The task used with monkeys is shown in panel B. Animals faced a computer monitor on stimuli were shown. After holding fixation for 500 ms, a central distractor was simultaneously presented with a peripheral saccade target either to the left or right of center. Monkeys were juice rewarded for making correct saccades and holding fixation on the target for 500 ms. Distractors (examples shown) were either social (portion of a monkey face) or control (scrambled part of a face) images of a specific face part (eyes, nose or mouth) showing a particular facial expression (neutral, submissive, threatening, affiliative).
At the start of the task, participants completed a calibration phase (standard 9-point Tobii calibration), during which the geometric characteristics of the eyes were estimated to acquire accurate gaze point calculations. If large offsets were observed between the calibration points and the participant’s gaze, they were asked to re-calibrate before beginning the task. Then, participants were instructed to fixate on a black central fixation cross and to make a saccade to a peripheral red dot target that would appear on either the left or right side of the screen simultaneously with a centrally presented face stimulus for 500 ms. The fixation cross remained on the screen between trials for a jittered period of at least 1000–1500 ms but would remain on the screen indefinitely until participants fixated on it for the 500 ms preceding each trial. The central distractors were oval cut-outs of angry, fearful, happy or neutral faces from the NimStim database (Tottenham et al., 2009). The pixels in the face stimuli were scrambled to create a non-face control condition matched on color and luminance. Stimuli were either presented with the eyes or the mouth at the central fixation point. All stimuli appeared on a gray background.
Participants completed 20 practice trials with scrambled face control stimuli to acclimate to making responses via saccade. The task then consisted of four runs of 100 trials, with a break between each run. These 400 trials included 40 instances of each distractor condition (angry, fearful, happy, neutral, scrambled face control) at each presentation location (eyes, mouth). Participants received feedback on their performance after every 10 trials indicating whether they were performing well or not (seeing the feedback ‘Keep it up!’ if their saccades were accurate and within the response window on 8 of the prior 10 trials vs ‘Too slow!’). Accurate responses were logged in Eprime as trials on which the gaze position reached the target circle within the response time window.
Saccade latency analysis
Gaze position data were analyzed using R v3.3.2 (R Core Team, 2015). The main variable of interest was latency to saccade to the target on each trial. This was calculated as the time from the onset of the distractor and target to the time that the gaze position first left a rectangular region enclosing the face stimulus. Trials with a saccade time of <100 ms or during which the eye tracker lost the gaze for more than 25% of acquisition frames were excluded. Additionally, any trials during which participants made an anticipatory saccade to the location of the prior target were excluded. Only accurate trials were examined, i.e. those during which the participant successfully made a saccade to the target location during the response window.
Repeated measures analysis of covariance (ANCOVA) were used to examine group and symptom-related differences in saccade latency. Greenhouse–Geisser correction was used to adjust for non-sphericity. Partial eta-squared values (η2p) are presented to indicate effect size. A within-subject factors for distractor condition (fearful, angry, happy, neutral faces or scrambled control) was the main task condition of interest. Location (eyes or mouth) were examined; as there was a main effect but not interaction with distractor emotion (see Results; Supplementary Figure S1), location was collapsed across eyes and mouth in subsequent analyses to increase power. Age was included as a covariate in all analyses to control for potential developmental effects in this age range. In a categorical analysis, participant group (anxious patient or healthy volunteer) was included as a between-subjects fixed effect. In continuous analyses, SCARED, ARI and STAI-S scores were examined. CDI and STAI-T scores were not examined as independent predictors of interest as they correlated very highly with SCARED scores (r > 0.8).
Pupillometry analysis
Pupil size during the task was examined using TimeStudio v3.17 (Nyström et al., 2016). Data were excluded if >75% of acquisitions were missing from a given trial. Missing or excluded data were interpolated (up to five adjacent missing data points). A sliding window average (five time point window) applied at each time point. Individual trials were detrended and z-scored. The 200 ms period before stimulus onset was used as a baseline for each trial. The baseline period was chosen to correspond to a period when participants maintained central fixation. Based on visualization of the average response profile, collapsed across groups, we extracted changes in pupil diameter on each trial from 500 to 1000 ms following trial onset. This window covered a relatively wide time frame and followed the typical period of saccade performance (~300 ms), likely alleviating any potential effects of saccades on pupil estimation. As described in the saccade latency section, repeated measures ANCOVAs were used to examine effects of distractor condition, group, SCARED, ARI and STAI-S scores covarying for age. One anxious participant consistently showed outlier values (>3SD from the mean) for pupil size across emotional conditions and was excluded from this analysis.
Study 2: effects of amygdala lesions in macaques
Participants
Eight adult male rhesus monkeys (Macaca mulatta; 5–9 years old) were tested as part of the current study. Four monkeys received bilateral excitotoxic lesions of the amygdala and four were retained as unoperated controls. Procedures have been presented in full previously (Dal Monte et al., 2015), but will be summarized briefly here and additional information regarding rearing and experimental history are provided in the supplement. Animals were housed individually, on a 12 h light/dark cycle with 24 h/day access to food, but controlled access to water during testing. All procedures were reviewed and approved by the National Institute of Mental Health Animal Care and Use Committee.
Amygdala lesions were made by ibotenic acid injection, as previously described (Baxter et al., 2000). The injections were carried out in two stages (balanced for hemisphere of first surgery) separated by at least 2 weeks. After the bilateral amygdala lesion was complete, monkeys recovered for 10–14 days. Lesions were intended to include the entire amygdala, i.e. the basolateral nuclear group and the central, medial and cortical nuclei. The extent of amygdala damage was evaluated from post-operative T2-weighted MRI scans obtained within 10 days of surgery (see Figure 1 in Dal Monte et al., 2015). After at least 60 days from the lesion surgery, monkeys received a surgically implanted head post to fix the head for accurate video tracking of eye movements. Monkeys received an additional 30–40 days of recovery before task training. Prior testing of two of the monkeys examined here shows these lesions to alter fear-related behavior (Chudasama et al., 2009).
Saccade-based attentional capture task
Monkeys were trained and tested on a saccade-based attentional capture task (Figure 1B). On each trial, monkeys fixated a cross presented at the center of the display for 500 ms. The fixation cross was then replaced with a distractor image, while a peripheral saccade target was presented randomly to the left or right of the distractor. The monkeys were trained to saccade to the peripheral target, as quickly as possible, and maintain fixation for 500 ms to earn a juice reward. Of note, Dal Monte et al. (2015) reported data on saccade latency and accuracy from this task as well as eye tracking and pupillometry data from a free-viewing paradigm. Critically, data on pupillary responses within the context of this saccade-based attentional capture task have not been previously examined or reported. Such data facilitate comparisons with data in humans collected on the adapted saccade-based task, as cognitive demands and other subtle task features can influence pupillary responding.
Distractor images were either social distractors (eyes, nose or mouth of monkey faces displaying a neutral, affiliative, submissive or threatening expression) or control distractors (scrambled portions of monkey faces). An eye-tracking system (Arrington ViewPoint) recorded eye movements and pupil size while monkeys performed the task. Five sessions (generally <20 min) were collected per animal with up to 480 correct trials per session. Each daily session used a different stimulus set (i.e. novel face identities), balanced across expression and comprised of 480 unique images (80 eyes, 80 noses, 80 mouths, 80 eyes scrambled, 80 noses scrambled, 80 mouths scrambled). Images were selected from a set of images of 50–60 individual adult male monkeys displaying various facial expressions.
Pupillometry analysis
For each session, pupil size traces were extracted for each trial and screened for outliers or artifacts. Outliers related to lid closure or loss of the eye-tracking signal (<1% of all samples) during the free-viewing period were detected and linearly interpolated over using the nearest valid, adjacent samples. Pupil diameter was then baseline normalized and expressed as a percent change from baseline for each trial by subtracting and then dividing by the mean pupil diameter for the 250 ms prior to stimulus onset. The baseline period was chosen to correspond to a period when the monkeys maintained central fixation. Based on visualization of the average response profile, collapsed across the lesion and control groups, we extracted mean percent changes in pupil diameter on each trial from 400 to 700 ms following trial onset. Effects on change in pupil size were tested via mixed-effects analysis of variance (ANOVA) that specified group (lesion and control), distractor condition, face part and social vs control distractor as fixed factors and nested monkey in group and session under monkey and group as random effects. Partial eta-squared values (η2p) are presented to indicate effect size.
Results
Study 1: anxiety-related differences in humans
Demographics
Demographic and clinical characteristics of the two participant groups are presented in Table 1. Healthy and anxious participants were matched on age, sex and IQ. Anxious participants exhibited significantly higher levels of anxiety, irritability and depressive symptoms. Of the 27 anxious patients, 17 were diagnosed with both GAD and social anxiety, 7 were diagnosed with GAD but not social anxiety and 3 were diagnosed with social anxiety but not GAD.
Table 1.
The demographic and clinical characteristics of the sample are presented here by diagnostic group. Mean and standard deviation values are presented for age, IQ and clinical questionnaire scores. Group differences in these values were assessed by independent samples t-test. The number and percent within group of female participants is presented and group differences were tested by chi-squared test
| Healthy (N = 26) | Anxious (N = 27) | |
|---|---|---|
| Age | 14.20 (2.50) | 13.72 (2.90) |
| Sex (n/% female) | 18 (69.23%) | 16 (61.53%) |
| IQ a | 106.29 (12.3) | 111.27 (12.78) |
| SCARED total *** | 7.59 (4.88) | 29.01 (9.33) |
| STAI-T *** | 27.32 (6.16) | 37.26 (8.98) |
| STAI-S * | 27.89 (3.90) | 30.78 (4.61) |
| ARI b,** | 0.81 (1.35) | 3.135 (2.98) |
| CDI c,*** | 3.53 (4.34) | 11.33 (7.86) |
* P < 0.05, **P < 0.01, ***P < 0.001.
a N = 50 participants completed the Wechsler Abbreviated Scale of Intelligence.
b N = 44 participants completed the ARI.
c N = 43 participants completed the CDI.
Saccade latency
Examining general task effects (Supplementary Figure S1), we identified a main effect of distractor condition (F(2.89,150.22) = 22.82, P < 0.001, η2p = 0.31) and of location (F(1,52) = 19.51, P < 0.001, η2p = 0.27). Specifically, saccade latencies were longer for angry and fearful distractors than happy and neutral distractors, with all faces eliciting longer saccade latencies than scrambled distractors. Distractors located with the eyes vs the mouth at fixation elicited longer latencies. As noted in the methods, given that there was no significant interaction between distractor condition and location (F(3.43,178.34) = 1.29, P = 0.28, η2p = 0.02), trials were collapsed across location to increase power to examine effects of distractor condition in subsequent analyses.
There were no significant effects of group (F(1,50) = 0.99, P = 0.32, η2p = 0.02) or interactions between group and emotion (F(2.84,141.86) = 0.70, P = 0.55, η2p = 0.01) predicting saccade latency. Similarly, there were no main effects or interactions with SCARED, STAI-S or ARI scores (all Ps > 0.18, η2p < 0.04). There was a significant main effect of age (F(1,50) = 17.09, P < 0.001, η2p = 0.25), where age negatively correlated with latencies across all distractor conditions (Supplementary Figure S2).
Pupillometry
In a repeated measures ANCOVA, distractor condition (F(3.61,173.40) = 9.47, P < 0.001, η2p = 0.16) and group (F(1,48) = 6.28, P = 0.02, η2p = 0.12; Figure 2B) were significant predictors of pupil size, with no significant interaction between condition and group. The main effect of distractor condition was observed in the healthy (F(3.29,75.62) = 11.31, P < 0.001, η2p = 0.33) and anxious groups (F(3.32,79.69) = 10.21, P < 0.001, η2p = 0.30) separately. Across both groups, greater constriction was observed for scrambled distracted compared to face distractors. The anxious patients showed greater constriction to all distractor conditions as compared to the healthy volunteers. Age was not a significant predictor and did not interact with emotion (Ps > 0.43, η2p < 0.02).
Fig. 2.
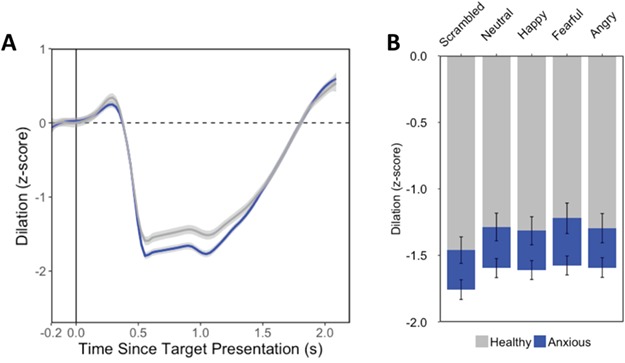
Results of the pupilometry analyses from Study 1 examining pupil response in humans are displayed here. (A) Time courses of change in pupil size are shown for the anxious (blue) and healthy (blue) groups [baseline normalized to the first 200 ms before trial onset (at 0 ms)]. The shaded regions indicate ±1 standard error of the mean (SEM). (B) Mean percent change in pupil size from 500 to 1000 ms following trial onset is presented for each group split by distractor category. Error bars indicate ±1 SEM.
Similarly, there was a significant main effect when examining SCARED scores as a continuous measure of anxiety symptoms (F(1,48) = 5.59, P = 0.02, η2p = 0.10; Supplementary Figure S3), where again higher anxiety was related to more constriction across emotions. There were no significant main effects or interactions with ARI, STAI-S and age (Ps > 0.13, η2p < 0.04).
Study 2: effects of amygdala lesions in macaques
As noted above, the reaction time effects during this task have been published previously (Dal Monte et al., 2015). The pupillary effects in this task have not been examined previously and are presented in Figure 3. Similar to the results of Study 1, all monkeys showed pupillary constriction following stimulus onset. Moreover, for social stimuli, the facial expression of the distractor significantly modulated pupil size (F(3,96) = 23.85, P < 0.001, η2p = 0.43) in both the unoperated controls (F(3,48) = 14.29, P < 0.001, η2p = 0.47) and lesion group (F(3,48) = 10.15, P < 0.001, η2p = 0.38). This effect also varied based on the displayed facial feature (Distractor Condition × Face Part, F(6,192) = 46.25, P < 0.001, η2p = 0.35). Specifically, this interaction was driven by the display of mouth distractors for threat faces, which led to less pupillary constriction compared to displays of mouth distractors for other facial expressions. Critically, the lesion and control groups differed in the overall magnitude of pupil size changes (F(1,32) = 24.22, P < 0.001, η2p = 0.33) with lesioned monkeys exhibiting less pupillary constriction compared to unoperated controls in response to both scrambled (F(1,36) = 18.97, P < 0.001, η2p = 0.33) and social (F(1,32) = 23.63, P < 0.001, η2p = 0.34) distractors. Thus, the effect of the amygdala lesions on pupil size were opposite of what was observed in the patients with anxiety. Also consistent with our earlier findings in patients and healthy comparisons, we did not observe a significant interaction between group and distractor condition (F(3,96) = 2.21, P = 0.092, η2p = 0.02).
Fig. 3.
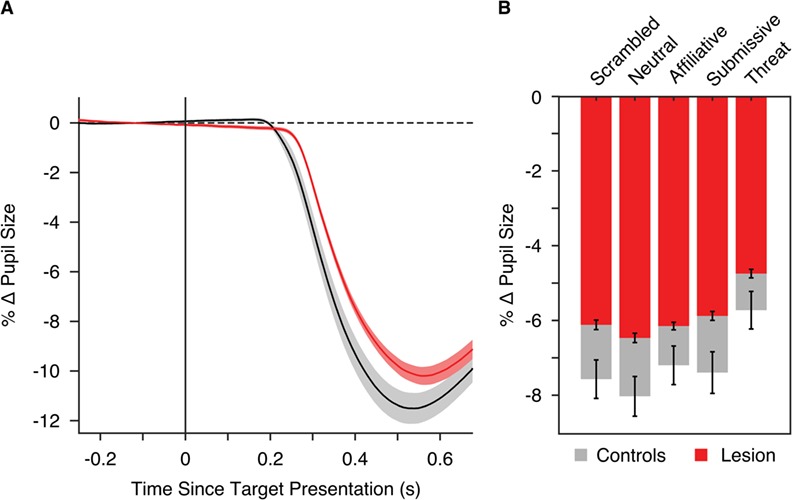
Results of the pupilometry analyses from Study 2 examining pupil response in monkeys are displayed here. (A) Time courses of change in pupil size are shown for the lesion (red) and control (gray) groups [baseline normalized to the 250 ms before trial onset (at 0 ms)] averaged across face part. The shaded regions indicate ±1 SEM. (B) Mean percent change in pupil size from 400 to 700 ms following trial onset is presented for each group split by distractor category. Error bars indicate ±1 SEM.
Discussion
The current study presents a translational, cross-species examination of pupillary responses in the context of an attention capture task. This combines a lesion-based study in non-human primates with examination of clinically affected patients, using closely matched tasks. While difficult, use of similar tasks is critical to maximize the probability that similar neural systems will be engaged across species (Averbeck and Chafee, 2016). Our human study found that, relative to psychiatrically healthy youth, youth with anxiety exhibit more pupillary constriction to all distractor conditions. Similarly, the non-human primate data indicated that amygdala lesions in monkeys impacted on pupillary constriction in response to all distractor conditions. However, the finding in monkeys was in an opposite direction to that in patients, with ‘less’ pupillary constriction to all distractors in lesioned relative to control monkeys.
At a relatively broad level, these results implicate the amygdala and related circuitry in pupillary responses during an affective attention capture task. Thus, taken together, the two studies broadly and indirectly link amygdala circuitry function to perturbed pupillary responses in anxiety patients. Nevertheless, such findings represent only a first step in comparing findings across the two species. This is because such alterations in pupil dilation could reflect many mechanisms, only some of which are shared across the two experiments. Pupil dilation may increase visual information flow in contexts where this information is relevant to the well-being of the individual. This includes situations where threat avoidance or reward consumption is present. Moreover, dilation also appears to track potential overall changes in internal arousal that might contribute to anxiety, at least in humans, though the underlying mechanisms of this need to be elucidated further. Further, amygdala lesions could reduce attention to stimuli by removing amygdala effects on the locus coeruleus or by causing an overall change in sympathetic or parasympathetic tone thus impacting pupil dilation. Future studies that pharmacologically manipulate arousal states in combination with amygdala lesions or inactivation could help arbitrate between specific mechanistic explanations.
In monkeys, threatening stimuli during saccade-based attentional capture task elicited reduced pupillary constriction compared to other stimuli, and this effect was particularly driven by the mouth portion of the face. However, while reduced pupillary constriction to threatening stimuli was seen in all monkeys, the amygdala lesion showed a robust effect on constriction across stimulus type. Explicit task demands are known to alter pupillary responses patterns, and such an effect emerged in these new data. Hence, these new results extend previously reported findings of monkey pupillary response during free viewing of full face stimuli, i.e. without a required response or task demands (Dal Monte et al., 2015). Specifically, previously reported free-viewing data exhibited a three-way interaction where lesion effects on pupillary response differed as a function of stimulus properties, i.e. facial expression and which facial feature (eyes or mouth) were presented at central fixation (Dal Monte et al., 2015). No such effects manifested in the new data from the attentional capture task and data from a matched free-viewing paradigm were not available from the human participants. Thus, the current data from monkeys build on the prior results of the free-viewing paradigm in demonstrating that the explicit demands of the new task for saccadic response impact on pupillary responses. Moreover, pupillary response data from the saccade task, as opposed to the free-viewing task, more closely mirror data from the human participants, who were also studied with the saccade-based attentional capture task. As such, data collected with the same task yielded greater cross-species convergence. This informs future research in monkeys to elucidate neural factors related to anxiety disorders and reveal mechanisms accounting for distinct pupillary responses to threats across free-viewing and task-based paradigms. Additionally, observations of cross-species parallels in pupillary data but discrepancies in saccade-related data suggest further need to develop novel perspectives for evaluating cross-species data in ways that link these data to models of individual differences in behavior related to neural circuitry function.
Previously reported data from the monkeys also showed a strong effect of amygdala lesion on saccade reaction time during the attentional capture task, where lesions reduced the effect of threat-related distractors on saccade latency (Dal Monte et al., 2015). In humans, negative emotional compared to neutral, distractors similarly delayed saccades to targets. However, this slowing did not differ as a function of anxiety. Cross-species effects on pupillary responses but not saccade latencies may reflect several factors. First, the monkeys were head posted, highly trained and juice rewarded for performance on the task. This facilitates consistent responses and data acquisition for saccades, relative to the more variable and unconstrained human task performance. Further, we noted an association between age and saccade data but not pupillary data. These age effects may increase inter-participant variability and limit power to test associations between anxiety and saccade performance.
Second, full amygdala lesions in monkeys may have produced a larger effect on saccade performance than would be expected from subtler anxiety-related differences in human amygdala circuit function. To that end, while a strength of the study was that the youth in this sample were all unmedicated and currently anxious, more robust effects may be observed in a more chronically or severely affected sample. Also, we cannot rule out potential differences due to the adult age of the monkeys. This should be examined in future work performing amygdala lesions in pediatric monkeys, though this may lead to additional challenges in terms of neural development. That said, relatively subtle amygdala alterations in human anxiety may manifest with milder alteration in saccade function or may be more selective to pupillary response. Indeed, such findings in humans mirror primate research documenting heterogeneity in the contributions of amygdala sub-nuclei to threat-response behavior (Kalin et al., 2004; Fox et al., 2015).
Prior data in primates suggest that heterogeneity of amygdala sub-nuclei function manifests in both behavioral effects and anatomical connections (Stefanacci and Amaral, 2002; Kalin et al., 2004; Fox et al., 2015). Increasing activity in some sub-nuclei facilitates threat responses, whereas increasing activity in other sub-nuclei reduces threat responses. Moreover, the lateral nucleus receives extensive cortical input and influences stimulus-reinforcement learning, while the basal nucleus influences saccades through a multi-synaptic pathway. The central nucleus, in contrast, influences autonomic and other physiologic responses to threat through direct outputs to the brainstem (Janak and Tye, 2015). While full amygdala lesions lead to alterations in both saccades and pupillary responses, the selective effect on pupillary response observed in humans could reflect associations between anxiety and dysfunction within those amygdala sub-nuclei most heavily involved in autonomic responses. For example, in monkeys, bilateral amygdala lesions of both central and basolateral nuclei cause elevations in baseline heart rate and reduced heart rate variability, indicating disrupted parasympathetic tone (Mitz et al., 2017). Thus, future basic science studies can probe the degree to which the associations observed in the current study result from effects within specific amygdala sub-nuclei. Additionally, while post-surgical recovery time is necessary before performing this study in the monkeys, neural compensation may have occurred and thus the results may reflect alterations in amygdala-related circuitry that arose due to the lesion rather than the lesion specifically. Future work could probe this issue further in human and non-human primates using neuroimaging to examine amygdala-related circuit effects.
Our findings in children were specific, linking pupil response or saccade performance to trait anxiety symptoms only, not to irritability or state-level anxiety. This contrasts with results from other attention capture tasks that utilize reaction time, where comparable findings manifest across symptom scales (Bar-Haim et al., 2007; Salum et al., 2017). Importantly, clinically anxious youth show both elevated trait- and state-level anxiety, yet the pupil effect we observed was driven by trait-level differences as opposed to in-the-moment anxiety levels. Thus, this may implicate these pupillary response effects as a potential marker of illness, i.e. stable trait-level anxiety, rather than state anxiety in the moment. While we found specific associations between attentional measures and anxiety symptoms, it is unclear whether these associations depend on particular features of our task. For example, a prior study found ‘increased’ pupillary dilation after aural and visual presentation of negative words associated with increased anxiety severity (Shechner et al., 2015). This suggests that the pupillary constriction we found may be specific to the affective face stimuli or other demands of the current task and thus effects may differ as a function of the paradigm examined, e.g. Bakes et al. (1990) and Bitsios et al. (2002). Furthermore, increased luminance or salience upon stimulus onset relative to the fixation period may have contributed to the overall constriction observed here. Critically, luminance was matched across stimuli, and group differences were observed in the context of this constriction. This may be controlled further in future work.
In sum, the current study used parallel methods across species to identify complementary findings from human patients with clinical anxiety and non-human primate models with amygdala lesions. The saccade-based attentional capture paradigm elicited affective face-related differences in pupillary response across species. Across affective facial and control stimuli, human anxiety vs amygdala lesions in monkeys exhibited opposite effects on overall pupillary responses. This work implicated elevated amygdala responsivity in anxiety underlying enhanced pupillary constriction to stimuli during an attentional capture paradigm.
Funding
This study was supported by the National Institute of Mental Health Intramural Research Program (ZIAMH002781).
Conflict of interest.
None declared.
Supplementary Material
Acknowledgements
We would like to thank the patients and their families for participating. We would also like to thank the staff of the Veterinary Medicine and Resources Branch at National Institute of Mental Health, National Institute of Health for offering their facilities and support during data collection.
References
- Averbeck B.B., Chafee M.V. (2016). Using model systems to understand errant plasticity mechanisms in psychiatric disorders. Nature Neuroscience, 19(11), 1418–25 10.1038/nn.4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakes A., Bradshaw C.M., Szabadi E. (1990). Attenuation of the pupillary light reflex in anxious patients. British Journal of Clinical Pharmacology, 30(3), 377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y., Lamy D., Pergamin L., Bakermans-Kranenburg M.J., van IJzendoorn M.H. (2007). Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychological Bulletin, 133(1), 1–24 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Baxter M.G., Parker A., Lindner C.C., Izquierdo A.D., Murray E.A. (2000). Control of response selection by reinforcer value requires interaction of amygdala and orbital prefrontal cortex. The Journal of Neuroscience, 20(11), 4311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B., Khetarpal S., Brent D., et al. (1997). The screen for child anxiety related emotional disorders (SCARED): scale construction and psychometric characteristics. Journal of the American Academy of Child and Adolescent Psychiatry, 36(4), 545–53 10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- Bitsios P., Szabadi E., Bradshaw C.M. (1996). The inhibition of the pupillary light reflex by the threat of an electric shock: a potential laboratory model of human anxiety. Journal of Psychopharmacology, 10(4), 279–87 10.1177/026988119601000404. [DOI] [PubMed] [Google Scholar]
- Bitsios P., Szabadi E., Bradshaw C.M. (2002). Relationship of the ‘fear-inhibited light reflex’ to the level of state/trait anxiety in healthy subjects. International Journal of Psychophysiology, 43(2), 177–84doi: Pii S0167-8760(01)00173-8. [DOI] [PubMed] [Google Scholar]
- Burkhouse K.L., Siegle G.J., Gibb B.E. (2014). Pupillary reactivity to emotional stimuli in children of depressed and anxious mothers. Journal of Child Psychology and Psychiatry, 55(9), 1009–16 10.1111/jcpp.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan B.L., Sullivan R.M., Howell B., Tottenham N. (2014). The international society for developmental psychobiology Sackler symposium: early adversity and the maturation of emotion circuits—a cross-species analysis. Developmental Psychobiology, 56(8), 1635–50 10.1002/dev.21260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan B.L., Tottenham N. (2016). The neuro-environmental loop of plasticity: a cross-species analysis of parental effects on emotion circuitry development following typical and adverse caregiving. Neuropsychopharmacology, 41(1), 163–76 10.1038/npp.2015.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y., Izquierdo A., Murray E.A. (2009). Distinct contributions of the amygdala and hippocampus to fear expression. The European Journal of Neuroscience, 30(12), 2327–37 10.1111/j.1460-9568.2009.07012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke R.J., Zhang H., Gamlin P.D. (2003). Characteristics of the pupillary light reflex in the alert rhesus monkey. Journal of Neurophysiology, 89(6), 3179–89 10.1152/jn.01131.2002. [DOI] [PubMed] [Google Scholar]
- Cole M.W., Yeung N., Freiwald W.A., Botvinick M. (2009). Cingulate cortex: diverging data from humans and monkeys. Trends in Neurosciences, 32(11), 566–74 10.1016/j.tins.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Monte O., Costa V.D., Noble P.L., Murray E.A., Averbeck B.B. (2015). Amygdala lesions in rhesus macaques decrease attention to threat. Nature Communications, 6, 10161. 10.1038/ncomms10161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A.S., Oler J.A., Tromp D.P.M., Fudge J.L., Kalin N.H. (2015). Extending the amygdala in theories of threat processing. Trends in Neurosciences, 38(5), 319–29 10.1016/j.tins.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janak P.H., Tye K.M. (2015). From circuits to behaviour in the amygdala. Nature, 517(7534), 284–92 10.1038/nature14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan I., Iyer A., Lindner A., Andersen R.A. (2010). Space representation for eye movements is more contralateral in monkeys than in humans. Proceedings of the National Academy of Sciences of the United States of America, 107(17), 7933–8 10.1073/pnas.1002825107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin N.H., Shelton S.E., Davidson R.J. (2004). The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. The Journal of Neuroscience, 24(24), 5506–15 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J., Birmaher B., Brent D., et al. (1997). Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry, 36(7), 980–8 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Berglund P., Demler O., Jin R., Merikangas K.R., Walters E.E. (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry, 62(6), 593–602 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kovacs M. (1985). The Children’s Depression Inventory (CDI). Psychopharmacology Bulletin, 21(4), 995–8. [PubMed] [Google Scholar]
- Margulies D.S., Vincent J.L., Kelly C., et al. (2009). Precuneus shares intrinsic functional architecture in humans and monkeys. Proceedings of the National Academy of Sciences of the United States of America, 106(47), 20069–74 10.1073/pnas.0905314106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitz A.R., Chacko R.V., Putnam P.T., Rudebeck P.H., Murray E.A. (2017). Using pupil size and heart rate to infer affective states during behavioral neurophysiology and neuropsychology experiments. Journal of Neuroscience Methods, 279, 1–12 10.1016/j.jneumeth.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai M., Wada M., Sunaga N. (2002). Trait anxiety affects the pupillary light reflex in college students. Neuroscience Letters, 328(1), 68–70. [DOI] [PubMed] [Google Scholar]
- Nyström P., Falck-Ytter T., Gredebäck G. (2016). The TimeStudio Project: an open source scientific workflow system for the behavioral and brain sciences. Behavior Research Methods, 48(2), 542–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psychology Software Tools (2012). E-Prime 2.0. Available: http://www.pstnet.com.
- R Core Team (2015). R: A Language and Environment for Statistical Computing, Vienna, Austria: R Foundation for Statistical Computing, Available: http://www.R-project.org. [Google Scholar]
- Salum G.A., Mogg K., Bradley B.P., et al. (2017). Association between irritability and bias in attention orienting to threat in children and adolescents. Journal of Child Psychology and Psychiatry, 58(5), 595–602 10.1111/jcpp.12659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechner T., Britton J.C., Perez-Edgar K., et al. (2012). Attention biases, anxiety, and development: toward or away from threats or rewards? Depression and Anxiety, 29(4), 282–94 10.1002/da.20914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechner T., Jarcho J.M., Wong S., Leibenluft E., Pine D.S., Nelson E.E. (2015). Threats, rewards, and attention deployment in anxious youth and adults: an eye tracking study. Biological Psychology, 122, 121–9. 10.1016/j.biopsycho.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirois S., Brisson J. (2014). Pupillometry. Wiley Interdisciplinary Reviews: Cognitive Science, 5(6), 679–92 10.1002/wcs.1323. [DOI] [PubMed] [Google Scholar]
- Spielberger C.D., Gorsuch R.L., Lushene R.E. (1970). Manual for the State-Trait Anxiety Inventory, Consulting Psychologists Press, Palo Alto. [Google Scholar]
- Stefanacci L., Amaral D.G. (2002). Some observations on cortical inputs to the macaque monkey amygdala: an anterograde tracing study. The Journal of Comparative Neurology, 451(4), 301–23 10.1002/cne.10339. [DOI] [PubMed] [Google Scholar]
- Steinhauer S.R., Condray R., Kasparek A. (2000). Cognitive modulation of midbrain function: task-induced reduction of the pupillary light reflex. International Journal of Psychophysiology, 39(1), 21–30. [DOI] [PubMed] [Google Scholar]
- Stringaris A., Goodman R., Ferdinando S., et al. (2012). The Affective Reactivity Index: a concise irritability scale for clinical and research settings. Journal of Child Psychology and Psychiatry, 53(11), 1109–17 10.1111/j.1469-7610.2012.02561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N., Tanaka J.W., Leon A.C., et al. (2009). The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Research, 168(3), 242–9 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis J.D. (2011). Cross-species studies of orbitofrontal cortex and value-based decision-making. Nature Neuroscience, 15(1), 13–9 10.1038/nn.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M.A., Morrow K.A., Rizer W.S., Kangas K.J., Carlson J.M., Walla P. (2016). Sustained, not habituated, activity in the human amygdala: a pilot fMRI dot-probe study of attentional bias to fearful faces. Cogent Psychology, 3(1), 10.1080/23311908.2016.1259881. [DOI] [Google Scholar]
- Wechsler D. (1999). Wechsler Abbreviated Scale of Intelligence, San Antonio: The Psychological Corporation. [Google Scholar]
- Weiskrantz L. (1956). Behavioral changes associated with ablation of the amygdaloid complex in monkeys. Journal of Comparative and Physiological Psychology, 49(4), 381–91. [DOI] [PubMed] [Google Scholar]
- White L.K., Britton J.C., Sequeira S., et al. (2016). Behavioral and neural stability of attention bias to threat in healthy adolescents. NeuroImage, 136, 84–93 10.1016/j.neuroimage.2016.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


