Abstract
Context
Patients with lipodystrophy have high prevalence of proteinuria.
Objective
To assess kidney disease in patients with generalized (GLD) vs partial lipodystrophy (PLD), and the effects of metreleptin on proteinuria in patients with lipodystrophy.
Design, Setting, Patients, Intervention
Prospective, open-label studies of metreleptin treatment in patients with GLD and PLD at the National Institutes of Health.
Outcome Measures
The 24-hour urinary albumin and protein excretion rates, estimated glomerular filtration rate (eGFR), and creatinine clearance (CrCl) were measured at baseline and during up to 24 months of metreleptin treatment. Patients with increases in medications affecting outcome measures were excluded.
Results
At baseline, patients with GLD had significantly greater albuminuria, proteinuria, eGFR, and CrCl compared with patients with PLD. CrCl was above the normal range in 69% of patients with GLD and 39% with PLD (P = 0.02). With up to 24 months of metreleptin treatment, there were significant reductions in albuminuria and proteinuria in patients with GLD, but not in those with PLD. No changes in eGFR or CrCl were observed in patients with GLD or PLD during metreleptin treatment.
Conclusions
Patients with GLD had significantly greater proteinuria than those with PLD, which improved with metreleptin treatment. The mechanisms leading to proteinuria in lipodystrophy and improvements in proteinuria with metreleptin are not clear. Hyperfiltration was also more common in GLD vs PLD but did not change with metreleptin.
Patients with generalized lipodystrophy (GLD) had greater proteinuria and more hyperfiltration vs those with partial lipodystrophy (PLD). Metreleptin improved proteinuria in GLD, but not PLD.
Lipodystrophy syndromes comprise a group of genetic or acquired rare diseases characterized by lack of adipose tissue. The deficiency of body fat results in deficiency of the adipocyte-derived hormone leptin, as well as increased ectopic fat storage, and metabolic abnormalities including extreme insulin resistance, diabetes, and dyslipidemia (1). Lipodystrophy syndromes can be broadly categorized as generalized lipodystrophy (GLD), in which there is near-complete loss of adipose tissue, or partial lipodystrophy (PLD), in which adipose tissue is lost in selected depots (most often the buttocks and lower extremities) but preserved in other depots such as the head, neck, and trunk. Although both GLD and PLD are associated with metabolic disease, complications of lipodystrophy are often more severe and present at younger ages in patients with GLD (1, 2).
Proteinuria is a frequent finding in patients with lipodystrophy. Prevalence estimates for macroalbuminuria (≥300 mg albumin/24 h urine) were 35% to 60% in GLD and 21% in PLD, whereas prevalence of nephrotic range proteinuria (>3.5 g/d) was 14% to 20% in GLD and 5% in PLD (3, 4). In addition, there have been case reports suggesting a link between proteinuric nephropathy and PLD caused by p.R349W LMNA mutation (5, 6). Hyperfiltration, as assessed by estimated glomerular filtration rate (eGFR) or creatinine clearance (CrCl), has also been reported in patients with GLD. A variety of renal pathologic changes have been reported in lipodystrophy syndromes, including glomerular hypertrophy, mesangial expansion, podocyte injury, diabetic nephropathy, focal segmental glomerulosclerosis, and membranoproliferative glomerulonephritis (3, 7). Surprisingly, only a small minority of patients with lipodystrophy who underwent kidney biopsy had pathology consistent with diabetic nephropathy. Based on the diversity of renal pathologic changes observed in lipodystrophy, the pathophysiologic mechanisms leading to the propensity to glomerular hyperfiltration and proteinuric nephropathy in lipodystrophy are likely to be equally complex.
A prior small study demonstrated that leptin replacement therapy with recombinant human methionyl leptin (metreleptin) for 4 to 36 months reduced urinary protein excretion and creatinine clearance in 11 of 15 (73%) patients with GLD (4). However, this study did not control for use of angiotensin antagonists, which may have confounded the results. The effects of metreleptin on urine protein excretion and filtration rates in patients with PLD have not yet been established.
In the current study, we examined the effects of 2 years of metreleptin treatment on proteinuria and glomerular filtration rate in patients with both GLD and PLD who did not increase doses of angiotensin antagonists.
Methods
Patients
Patients with lipodystrophy participated in prospective, open-label interventional studies of metreleptin at the National Institutes of Health (NIH) Clinical Center, Bethesda, MD. Studies were approved by the institutional review board of the National Institute of Diabetes and Digestive and Kidney Diseases. All subjects or their legal guardians provided written informed consent before participation. Minors provided assent.
Eligibility criteria included a clinical diagnosis of non-HIV-associated lipodystrophy, age 6 month or older, leptin level <12 ng/mL in females and <8 ng/mL in males, and at least one metabolic abnormality, including diabetes per 2007 American Diabetes Association criteria, fasting hyperinsulinemia (>30 μU/mL), or hypertriglyceridemia (>200 mg/dL).
Study design
Data collection
Patients were evaluated every 6 months for the first year of metreleptin treatment, and annually thereafter. During each visit, patients underwent a physical examination along with blood and urine analysis. Systolic and diastolic blood pressures (SBP and DBP, respectively) from each visit were used to determine whether a patient was hypertensive. For adult patients, hypertension was defined as either SBP ≥140 mm Hg or DBP ≥90 mm Hg. For patients younger than 18 years of age, hypertension was defined as either SBP or DBP ≥95th percentile based on age, sex, and height (8).
At each visit, 24-hour urine collection was performed for albumin excretion rate, protein excretion rate, and glucose excretion rate. Urinary protein excretion ≥150 mg/d was considered abnormal. Microalbuminuria was defined as urinary albumin excretion rate between 30 and 300 mg/24 h. Macroalbuminuria was defined as albumin excretion ≥300 mg/24 h. Nephrotic range proteinuria was defined as protein excretion ≥3.5 g/24 h. Blood samples were obtained after an 8- to 12-hour fast for measurement of triglycerides, low-density lipoprotein cholesterol (LDL-c), glucose, hemoglobin A1c, and creatinine using the standard techniques of the NIH Clinical Center laboratory. Leptin concentration in fasting serum samples was measured before metreleptin initiation by radioimmunoassay (EMD-Millipore, Billerica, MA). CrCl was calculated from serum and 24-hour urine data. eGFR was calculated from serum creatinine using age-appropriate equations: Bedside Schwartz equation for patients ≤18 years of age and Chronic Kidney Disease Epidemiology Collaboration creatinine equation for patients >18 years. CrCl or eGFR >130 mL/min/1.73 m2 was considered elevated.
We report data at baseline and 6 (range, 3 to <9), 12 (range, 9 to <18), and 24 (range 18 to <33) months after initiation of metreleptin treatment. Patients began metreleptin therapy following their baseline visit. If more than one visit occurred within the specified time window, the visit closest to the designated time point was selected for analyses.
Metreleptin dosing
Full metreleptin dosing information has been published previously (9). Metreleptin was dosed once or twice daily, with dosing according to weight (sex- and age-dependent). Nine patients were enrolled in the pilot study of the use of metreleptin in lipodystrophy, during which dose titrations were scheduled. Dose titrations in patients in the subsequent long-term study of metreleptin in lipodystrophy were based on clinical response and clinician judgment.
Medication changes
Doses of diabetes and lipid-lowering medications were not increased during the first year of metreleptin treatment but were lowered or discontinued if clinically indicated (e.g., for hypoglycemia). After the first year of metreleptin, diabetes and lipid-lowering medications were increased or decreased as clinically indicated.
Changes in other medications (e.g., renin-angiotensin system antagonists) were not restricted per study protocol. In the current analysis, to account for confounding effects of angiotensin-converting enzyme inhibitors (ACEis) and/or angiotensin receptor blockers (ARBs) on kidney function and proteinuria, we reviewed patients’ medication changes throughout the treatment period. Data from visits in which patients had either initiated or increased their dose of ACEi and/or ARB relative to their baseline (premetreleptin) medication use were excluded from the analyses. If patients resumed their baseline dose of renin-angiotensin system antagonists at a subsequent visit, that visit was included in the analysis.
Statistical analyses
Continuous outcomes are reported as mean ± SD or median [25th,75th percentile], based on distribution of the data. Nonnormally distributed variables were log-transformed before analysis. To compare baseline differences between GLD and PLD, unpaired t test or Mann-Whitney test was used for continuous variables, and χ2 test for categorical variables. Baseline differences between GLD and PLD for proteinuria, albuminuria, CrCl, and eGFR were also performed with adjustment for baseline use of renin-angiotensin system antagonists using mixed models. To evaluate intrasubject changes of each of outcome throughout the treatment period, a linear mixed model was used for each outcome with visit (baseline and 6, 12, and 24 months) as a main factor to account for correlation among multiple observations within each subject, with Bonferroni-corrected post hoc comparisons among individual visits.
We investigated the potential utility of using clinical and laboratory parameters to predict subsequent changes in renal outcomes. The set of preliminary predictors was selected by examining each predictor separately using a linear mixed model with a significance level of 10%. Potential predictors of changes in renal outcomes with metreleptin included: lipodystrophy type, affected gene (if known), duration of diabetes, age at visit, use of diuretics, use of renin-angiotensin system antagonists, hemoglobin A1c, triglycerides, LDL-c, insulin total daily dose, 24-hour urinary glucose excretion rate, SBP, DBP, presence of hypertension at visit, smoking status at visit, smoking history, and endogenous leptin concentration. Next, we obtained a final model that included visit number and the predictors selected by performing a variable selection out of the selected set of preliminary predictors. If a baseline value of each renal outcome was included in the final model, then renal outcome values without baseline were used.
All analyses except for selection of the initial set of potential predictors were two-tailed tests based on a significance level of 0.05 and conducted using SAS, version 9.4 (SAS Institute, Cary, NC).
Results
Baseline characteristics
Baseline characteristics of patients are presented in Table 1. There were 115 patients; 73 had GLD and 42 had PLD. Most (83%) had genetic forms of lipodystrophy; no patient had acquired PLD associated with complement disorders or membranoproliferative glomerulonephritis. Compared with patients with PLD, patients with GLD were younger, had lower proportion of females, lower endogenous leptin levels, shorter duration of diabetes, and a lower proportion of ACEi/ARB usage. Patients with GLD had more severe insulin resistance as evidenced by higher fasting insulin and homeostasis model assessment of insulin resistance, but other measures of metabolic disease severity including hemoglobin A1c and triglycerides were comparable in GLD and PLD.
Table 1.
Baseline Patient Characteristics
| Parameter | All (N = 115) | GLD (N = 73) | PLD (N = 42) | P Value (GLD vs PLD) |
|---|---|---|---|---|
| Age, y | 18 [14,35] | 16 [12,21] | 35 [19,46] | <0.0001 |
| Sex, % female | 83 | 77 | 95 | 0.01 |
| Lipodystrophy subtype, N | 48 CGL | 48 CGL | 42 FPL | — |
| 19 AGL | 19 AGL | |||
| 42 FPL | 6 Atypical progeria | |||
| 6 Atypical progeria | ||||
| Affected gene, N | — | 29 AGPAT2 | 23 LMNA | — |
| 17 BSCL2 | 9 PPAR-gamma | |||
| 7 LMNA | 1 PCYT1a | |||
| 19 None | 9 Unknown | |||
| 1 Unknown | ||||
| Diabetes, % | 80 | 78 | 83 | 0.63 |
| Diabetes duration, y | 6.8 [3.3,12] | 4.5 [2.8,9.4] | 11 [4.7,18] | 0.001 |
| Fasting insulin, mcU/mL | 42 [21,90] | 53 [26,104] | 26 [13,52] | 0.0005 |
| HOMA-IR | 17.0 [7.5,36.0] | 25.5 [12.0,44.9] | 8.9 [5.3,17.5] | <0.0001 |
| Hypertension, % | 29a | 31a | 26 | 0.62 |
| ACEi/ARB usage, % | 43 | 36 | 55 | 0.05 |
| Leptin, ng/mL | 1.7 [0.97,4.6] | 1.1 [0.80,1.8] | 6.2 [3.9,9.4] | <0.0001 |
| Hemoglobin A1c, % | 8.4 ± 2.3 | 8.6 ± 2.4 | 8.1 ± 2.1 | 0.23 |
| Triglycerides, mg/dL | 432 [231,1140] | 441 [229,1187] | 422 [233,1007] | 0.92 |
| LDL-c | 90 [66,117] | 90 [69,115] | 92 [65,134] | 0.98 |
| Urinary glucose excretion, g/24 h | 4.9 [0.39,23] | 5.1 [0.77,26] | 4.0 [0.18,15] | 0.08 |
| Urinary albumin excretion, mg/24 h | 83 [15,554] | 244 [31,978] | 18 [7.9,79] | <0.0001 |
| Normal, <30 mg/24 h, % | 41.6 | 25.5 | 67.7 | |
| Microalbuminuria, 30-300 mg/24 h, % | 26.9 | 29.1 | 23.5 | 0.001 |
| Macroalbuminuria, >300 mg/24 h, % | 31.5 | 45.4 | 8.8 | |
| Urinary protein excretion, mg/24 h | 293 [148,1244] | 605 [198,2250] | 165 [119,249] | <0.0001 |
| Elevated, >150 mg/24 h, % | 75.0 | 82.8 | 61.8 | 0.04 |
| Nephrotic range, >3.5 g/24 h, % | 8.7 | 13.8 | 0 | 0.02 |
| eGFR, mL/min/1.73 m2 | 129 [99,155] | 143 [112,169] | 105 [85,129] | <0.0001 |
| Elevated, >130 mL/min/1.73 m2, % | 47.8 | 63.9 | 19.5 | <0.0001 |
| CrCl, mL/min | 141 [106,209] | 174 [115,233] | 111 [95,163] | 0.005 |
| Elevated, >130 mL/min, % | 57.9 | 66.7 | 42.9 | 0.04 |
Results are presented as median [25th,75th percentile] or mean ± SD unless otherwise noted.
Abbreviations: AGL, acquired general lipidystrophy; CGL, congenital generalized lipidystrophy; FPL, familial partial lipodystrophy; HOMA-IR, homeostasis model assessment of insulin resistance [glucose (mg/dL) × insulin (mcU/mL)/405].
One patient with GLD younger than 2 years of age was excluded from evaluation of hypertension.
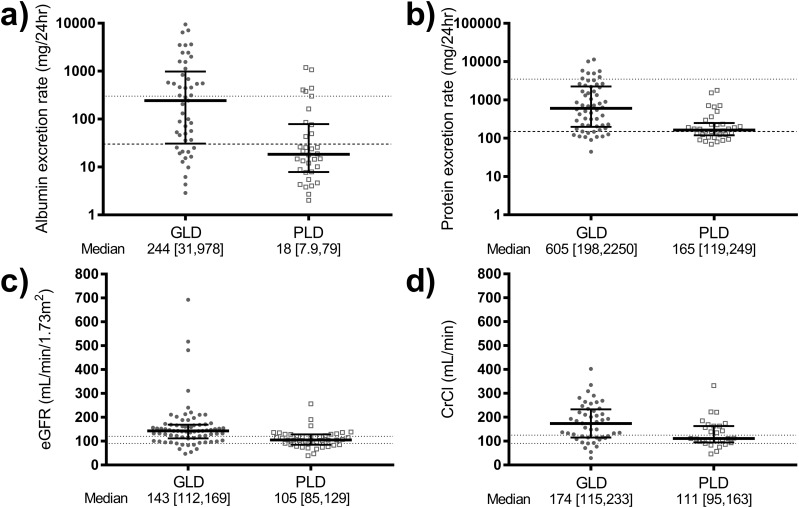
Patients with GLD had more severe proteinuric nephropathy at baseline compared with PLD. This was evidenced by higher median urinary protein and albumin excretion rates, as well as higher prevalence of macroalbuminuria (>300 mg albumin excretion/24 h) and nephrotic range proteinuria (>3.5 g/24 h), in GLD compared with PLD (Table 1, Fig. 1). In four of the eight patients with GLD and nephrotic range proteinuria, urine protein electrophoresis was performed. In all cases, this demonstrated a predominance of albuminuria (79% to 85%) with no paraproteinuria. Patients with GLD also had higher median CrCl and eGFR compared with those with PLD, and were more likely to have hyperfiltration as evidenced by CrCl >130 mL/min or eGFR >130 mL/min/1.73 m2 (Table 1, Fig. 1). Differences between patients with GLD and PLD for urinary protein and albumin excretion rates, CrCl, and eGFR remained statistically significant after adjustment for baseline use of renin-angiotensin system antagonists.
Figure 1.
Baseline kidney parameters in patients with GLD (circles) and PLD (squares). (a) Twenty-four-hour urinary albumin excretion (dashed line, cut-point for microalbuminuria; dotted line, cut-point for macroalbuminuria). (b) Twenty-four-hour urinary protein excretion (dashed line, upper limit of normal urinary protein excretion; dotted line, cut-point for nephrotic range proteinuria). (c) eGFR (dotted lines, normal range). (d) CrCl (dotted lines, normal range) were all greater in GLD vs PLD (P ≤ 0.005 for all). The 24-h urinary albumin and protein excretion rates were log-transformed before analysis.
Changes with metreleptin treatment
The median duration of follow-up data during metreleptin treatment included in this analysis was 23.6 months [17.3,24.9]. Because of initiation of renin-angiotensin system antagonists or an increase in the medication dose, data from 12/63 patients with GLD and 2/34 with PLD were excluded from the 6-month visit, data from 16/55 patients with GLD and 7/39 with PLD were excluded from the 12-month visit, and data from 17/55 patients with GLD and 9/32 with PLD were excluded from the 24-month visit.
Changes in metabolic outcomes with metreleptin are shown in Table 2. In patients with GLD, metreleptin treatment reduced triglycerides from a median of 441 [230,1164] mg/dL at baseline to 190 [130,289] by 6 months, with improvements maintained thereafter (P < 0.0001). Metreleptin also improved glycemia control in patients with GLD, as demonstrated by reductions after 6 months in hemoglobin A1c by 2.1% and fasting glucose by 53 mg/dL; these improvements were maintained at all follow-up visits (P < 0.0001 for all). Insulin sensitivity also improved, as assessed by insulin dose reduction among the 43 insulin users. The baseline median insulin dose was 225 U/d, which decreased by a median of 75 U/d after 6 months, and 200 U/d after 12 and 24 months (P = 0.002). Seventeen (40%) insulin users with GLD discontinued insulin after metreleptin initiation, from a baseline median dose of 160 U/d. Metabolic improvements with metreleptin were of smaller magnitude in patients with PLD, including a nonsignificant decrease in triglycerides from 422 [234,910] mg/dL to 318 [219,501] at 6 months, with similar levels maintained thereafter (P = 0.065). Patients with PLD did experience significant improvements in glycemia control with metreleptin, as demonstrated by reductions after 6 months in hemoglobin A1c by 0.8% and fasting glucose by 44 mg/dL; these improvements were maintained at all follow-up visits (P = 0.0044 and P = 0.0004, respectively). Metreleptin did not significantly lower insulin dose in patients with PLD, and only two patients with PLD discontinued insulin (from baseline doses of 39 and 172 U/d).
Table 2.
Changes in Metabolic Parameters With Metreleptin
| GLD | PLD | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline (N = 73) | 6 Mo (N = 51) | 12 Mo (N = 39) | 24 Mo (N = 38) | P a | Baseline (N = 42) | 6 Mo (N = 32) | 12 Mo (N = 33) | 24 Mo (N = 24) | P a | |
| Insulin dose (insulin users only), U/d | 225 [100,420] | 58 [0,150] | 0 [0,50] | 0 [0,30] | 0.002b | 181 [98,280] | 120 [50,220] | 100 [50,200] | 150 [70,232] | 0.13 |
| Triglycerides, mg/dL | 441 [230,1164] | 190 [130,289] | 176 [106,332] | 171 [87,337] | <0.0001c | 422 [234,910] | 318 [219,501] | 311 [179,498] | 324 [200,481] | 0.065 |
| Glucose, mg/dL | 188 ± 83 | 130 ± 52 | 110 ± 44 | 116 ± 43 | <0.0001c | 173 ± 82 | 124 ± 32 | 135 ± 59 | 137 ± 63 | 0.001c |
| Hemoglobin A1c, % | 8.6 ± 2.4 | 6.4 ± 1.7 | 6.0 ± 1.3 | 6.2 ± 1.6 | <0.0001c | 8.1 ± 2.1 | 7.1 ± 1.3 | 7.5 ± 1.9 | 7.4 ± 1.7 | 0.002c |
| Urinary glucose excretion, g/24 h | 5.1 [0.8,26] | 0.3 [0.1,4.9] | 0.3 [0.1,2.9] | 0.8 [0.2,4.9] | <0.0001c | 4.0 [0.2,14.1] | 0.5 [0.1,4.4] | 2.4 [0.3,15.7] | 2.3 [0.3,9.6] | 0.005d |
Results are presented as median [25th,75th percentile] or mean ± SD.
P value for mixed model across all time points.
P < 0.05 for month 12 vs baseline with Bonferroni correction for multiple comparisons.
P < 0.05 for all follow-up time points vs baseline with Bonferroni correction for multiple comparisons.
P < 0.05 for month 6 vs baseline with Bonferroni correction for multiple comparison.
Compared with baseline, urinary albumin and protein excretion decreased significantly after up to 24 months of metreleptin in patients with GLD (Fig. 2). An 83% reduction in urinary albumin excretion and 56% reduction in urinary protein excretion were seen by 6 months of metreleptin, with no further improvement thereafter. There were no significant changes in CrCl or eGFR in patients with GLD after up to 24 months of metreleptin (Fig. 2). By contrast, metreleptin therapy did not lead to significant changes in albuminuria, proteinuria, or glomerular filtration rate in patients with PLD (Fig. 2).
Figure 2.
Kidney outcomes during up to 24 months of metreleptin treatment in GLD (left) and PLD (right). The 24-h urinary albumin excretion (dashed line, cut-point for microalbuminuria; dotted line, cut-point for macroalbuminuria) significantly decreased with metreleptin. (a) GLD (P < 0.001 for overall time effect, with significant changes between baseline and all subsequent time points on post hoc analysis with Bonferroni correction). (b) There was no change in 24-h urinary albumin excretion in PLD. (c) The 24-h urinary protein excretion (dashed line, upper limit of normal urinary protein excretion; dotted line, cut-point for nephrotic range proteinuria) significantly decreased with metreleptin in GLD (P < 0.001 for overall time effect, with significant changes between baseline and all subsequent time points on post hoc analysis with Bonferroni correction). (d) There was no change in 24-h urinary protein excretion in PLD. There were no changes after metreleptin treatment in eGFR (dotted lines, normal range) in either (e) GLD or (f) PLD. (g) There were no changes after metreleptin treatment in CrCl (normal range: dotted lines) in GLD or (h) PLD. The 24-h urinary albumin and protein excretion rates were log-transformed before analysis. Closed circles, GLD; open squares, PLD.
Multivariate analyses of predictors of metreleptin response showed that, in patients with GLD after up to 24 months of metreleptin, baseline urinary albumin excretion rate (P < 0.0001) and triglycerides (P = 0.02) were independent predictors of change in albuminuria. In patients with GLD, only baseline urinary protein excretion rate (P < 0.0001) was an independent predictor of change in proteinuria.
Development of end-stage kidney disease in metreleptin-treated patients
Five patients developed end-stage kidney disease during or after metreleptin treatment. Patient NIH2 [congenital generalized lipidystrophy (CGL) resulting from AGPAT2 mutation] had biopsy-proven focal segmental glomerulosclerosis before metreleptin treatment. She was withdrawn from metreleptin treatment after 3 years because of noncompliance and died of end-stage kidney disease 9 years after metreleptin discontinuation. Patient NIH6 (CGL resulting from AGPAT2 mutation) withdrew from metreleptin treatment after 1 year for personal reasons. She subsequently restarted metreleptin 7 years later and continued for an additional 1.5 years until her death from end-stage kidney disease; biopsy was not performed. Patient NIH7 (familial partial lipodystrophy resulting from LMNA mutation) reportedly had a diagnosis of membranoproliferative glomerulonephritis before metreleptin treatment (biopsy results not available); she progressed to dialysis after 6 years of metreleptin treatment and underwent kidney transplant 2.5 years later. She has had stable function of the transplanted kidney for 9 years. Patient NIH9 (acquired GLD) was withdrawn from metreleptin treatment after 14 months because of progression of kidney disease; biopsy demonstrated membranoproliferative glomerulonephritis. She progressed to dialysis and died secondary to hepatorenal failure 9 months after metreleptin withdrawal. Patient NIH14 (acquired GLD) was withdrawn from metreleptin after 9 months because of progressive kidney disease; biopsy demonstrated membranoproliferative glomerulonephritis. Metreleptin was restarted 7 months later and continued thereafter. He progressed to end-stage kidney disease and underwent kidney transplant 7 years after metreleptin initiation. He died 15 years after metreleptin initiation secondary to end-stage kidney disease.
Discussion
In the current study, we assessed baseline kidney disease in metreleptin-naïve patients with GLD and PLD and examined the effects of metreleptin treatment on kidney disease in the largest cohort of patients with lipodystrophy reported. We confirmed that patients with lipodystrophy have proteinuric nephropathy at baseline before metreleptin, accompanied by hyperfiltration. These abnormalities were more frequent in patients with GLD compared with those with PLD. Following up to 24 months of metreleptin treatment, we observed significant reductions in albuminuria and proteinuria in patients with GLD, but not PLD, despite stable or decreasing doses of renin-angiotensin system antagonists. A recent meta-analysis demonstrated that, in patients with diabetic nephropathy, a sustained 30% reduction in albuminuria predicted lower progression to end-stage kidney disease (10). Although the median improvement in albuminuria with metreleptin treatment in patients with GLD was well above this threshold, it is not clear whether this improvement will lead to reduced risk of end-stage kidney disease. In contrast to a prior small study by our group (4), we did not observe a reduction in hyperfiltration in this large cohort of patients with GLD after metreleptin treatment; the previously reported reduction in eGFR and CrCl may have been secondary to changes in renin-angiotensin system antagonists.
Although patients with lipodystrophy predominantly have causes of kidney disease other than diabetic nephropathy, they have features in common with diabetic nephropathy, including a predominance of albuminuria consistent with tubular (rather than glomerular) injury. We therefore hypothesized that, as in diabetic nephropathy, improvements in proteinuria with metreleptin might be secondary to improved glycemia control. The Diabetes Control and Complications Trial demonstrated that long-term intensive glycemic control in patients with type 1 diabetes reduces prevalence of albuminuria and declines in eGFR (11), and similar findings have been demonstrated in patients with type 2 diabetes (12). Although metreleptin treatment significantly reduced A1c in patients with GLD, A1c was not an independent predictor of changes in proteinuria. Similarly, although metreleptin decreased urinary glucose excretion in GLD, urinary glucose excretion was not an independent predictor of changes in proteinuria. Moreover, metreleptin also decreased A1c and urinary glucose excretion in patients with PLD (albeit the magnitude of reduction was smaller), yet there was no change in proteinuria in PLD, suggesting that changes in proteinuria were not mediated by changes in glycemia.
Metreleptin might also improve proteinuria via improvements in insulin resistance. There are strong epidemiologic data linking insulin resistance with albuminuria in healthy, nondiabetic subjects (13), as well as patients with both type 1 (14, 15) and type 2 diabetes (16). Moreover, a meta-analysis of randomized, controlled trials of the insulin-sensitizing drug class of thiazolidinediones in patients with type 2 diabetes demonstrated decreased albuminuria and proteinuria. It is not possible to know if this beneficial effect of thiazolidinediones was mediated through improvements in insulin resistance, glycemia, or other factors. Although we did not find an association between changes in insulin or C-peptide and proteinuria or albuminuria, these are poor markers of insulin resistance, particularly among patients with diabetes and impaired beta-cell function. Although the current study did not have a robust measure of insulin resistance, such as the hyperinsulinemic, euglycemic clamp, we and others have previously demonstrated improved insulin sensitivity with metreleptin in patients with both generalized and PLD (17–20).
In patients with GLD, we identified baseline urinary albumin excretion rate and triglycerides as independent predictors of change in albuminuria with metreleptin treatment. Baseline urinary protein excretion rate was the only independent predictor of change in proteinuria with metreleptin treatment in GLD. We previously demonstrated that improvements in diabetes and hypertriglyceridemia with metreleptin are greater in patients with lower endogenous leptin and those with more severe metabolic derangements before treatment (2). Thus, the reduction in albuminuria and proteinuria in GLD, but not PLD, observed in this study may simply reflect the more severe baseline albuminuria and proteinuria in GLD vs PLD. Triglycerides have been shown to be an independent predictor of albuminuria incidence in patients with type 1 diabetes (21) and have been positively associated with increases in albuminuria in patients with type 2 diabetes (22). In the current study, the inclusion of triglycerides as a predictor of change in albuminuria may reflect overall metabolic improvements with metreleptin.
We cannot determine from this study whether improvements in urinary protein excretion with metreleptin were caused by direct effects of metreleptin on the kidney, systemic actions of metreleptin, and/or improvements in metabolic disease. There are rodent data supporting some direct actions of leptin within the kidney. Although most of leptin’s systemic effects are mediated via the long-form Ob-Rb receptor, expression of the short-form Ob-Ra receptor has been observed in mouse renal cortex and isolated glomeruli (23, 24) and in rat proximal straight tubules, loops of Henle, distal tubules, and collecting ducts. Rodent models have demonstrated that leptin may have direct effects in the kidneys via its receptor (24).
Systemic treatment with leptin in mice has demonstrated improvements in kidney morphology and function. In two rodent models of leptin deficiency (leptin gene deletion and lipodystrophy), leptin replacement reversed a diabetic nephropathy phenotype. In BTBR ob/ob leptin-deficient obese mice with diabetic nephropathy, leptin treatment resulted in restoration of podocyte density and reduction in glomerular volume, suggestive of reduction in glomerular hypertrophy (23). The authors also observed upregulation of phosphorylated-STAT3 within glomerular and tubular epithelial cells, suggesting that leptin led to signal transduction within the kidney (23). Overexpression of leptin in a mouse model of GLD (A-ZIP) resulted in improvement in hyperglycemia and attenuation of development of diabetic nephropathy. Although A-ZIP mice have glomerular hypertrophy and mesangial expansion, double transgenic mice with lipodystrophy and leptin overexpression have complete inhibition of glomerular hypertrophy and mesangial expansion as well as significantly reduced albuminuria, compared with A-ZIP mice. These results demonstrate the effects of leptin replacement on albuminuria and renal histological changes in a mouse model of lipoatrophic diabetes (25).
In rodent models, but not in humans, hyperleptinemia has been linked to fibrotic effects in both the liver (26, 27) and the kidney (15, 28). It is thus of at least theoretical concern that supraphysiologic doses of metreleptin given to patients with lipodystrophy could contribute to the development of kidney injury such as focal segmental glomerulosclerosis. Although we do not have kidney biopsies before and after metreleptin treatment to verify effects of leptin on fibrosis, our data are reassuring in that metreleptin treatment actually improved proteinuria rather than exacerbate it. Five patients treated with metreleptin in this study progressed to end-stage kidney disease; however, the data are insufficient to determine whether this represented the natural history of their disease, vs possible exacerbation by metreleptin therapy.
Our study is the largest to report baseline kidney disease in patients with GLD and PLD. We have also evaluated changes in renal outcomes with metreleptin treatment in the largest sample of patients with GLD and PLD. Although the study was not designed specifically to assess kidney function, and thus changes in ACEi/ARB medications were permitted, we restricted the current analyses to patients without increases in these drugs. A limitation of this study is that kidney biopsies were not routinely collected of before and after metreleptin treatment, thus we cannot determine whether improvements in proteinuria with metreleptin correlated with structural changes in the kidney. The study was also limited by the evaluation of renal filtration using equations for eGFR. Because creatinine correlates with lean body mass, which is elevated in both GLD and PLD (29, 30), our eGFR data may be falsely elevated. However, CrCl is not affected by lean body mass, and was also elevated in our patient population.
In conclusion, our study found that patients with GLD have more severe proteinuria than patients with PLD, and metreleptin significantly lowered urinary protein excretion in GLD. In contrast to a prior smaller study in GLD from our group that did not account for changes in ACEi/ARB medications (4), we did not observe significant reductions in eGFR or CrCl with metreleptin. Further mechanistic studies are needed to address whether the reduction in proteinuria with metreleptin is due to direct effects of leptin in the kidneys or an overall improved metabolic state with metreleptin.
Acknowledgments
Financial Support: This work was supported by the intramural research program of the National Institute of Diabetes and Digestive and Kidney Diseases.
Clinical Trial Information: ClinicalTrials.gov nos. NCT00005905 (registered 12 June 2000), NCT00025883 (registered 29 October 2001), and NCT01778556 (registered 29 January 2013).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- ACEi
angiotensin-converting enzyme inhibitor
- ARB
angiotensin receptor blocker
- CGL
congenital generalized lipidystrophy
- CrCl
creatinine clearance
- DBP
diastolic blood pressure
- eGFR
estimated glomerular filtration rate
- GLD
generalized lipodystrophy
- LDL-c
low-density lipoprotein cholesterol
- NIH
National Institutes of Health
- PLD
partial lipodystrophy
- SBP
systolic blood pressure
References
- 1. Garg A. Clinical review#: lipodystrophies: genetic and acquired body fat disorders. J Clin Endocrinol Metab. 2011;96(11):3313–3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Diker-Cohen T, Cochran E, Gorden P, Brown RJ. Partial and generalized lipodystrophy: comparison of baseline characteristics and response to metreleptin. J Clin Endocrinol Metab. 2015;100(5):1802–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Akinci B, Unlu SM, Celik A, Simsir IY, Sen S, Nur B, Keskin FE, Ozgen Saydam B, Kutbay Ozdemir N, Sarer Yurekli B, Ergur BU, Sonmez M, Atik T, Arslan A, Demir T, Altay C, Tunc UA, Arkan T, Gen R, Eren E, Akinci G, Yilmaz AA, Bilen H, Ozen S, Celtik A, Savas Erdeve S, Cetinkaya S, Onay H, Sarioglu S, Oral EA. Renal complications of lipodystrophy: a closer look at the natural history of kidney disease. Clin Endocrinol (Oxf). 2018;89(1):65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Javor ED, Moran SA, Young JR, Cochran EK, DePaoli AM, Oral EA, Turman MA, Blackett PR, Savage DB, O’Rahilly S, Balow JE, Gorden P. Proteinuric nephropathy in acquired and congenital generalized lipodystrophy: baseline characteristics and course during recombinant leptin therapy. J Clin Endocrinol Metab. 2004;89(7):3199–3207. [DOI] [PubMed] [Google Scholar]
- 5. Thong KM, Xu Y, Cook J, Takou A, Wagner B, Kawar B, Ong AC. Cosegregation of focal segmental glomerulosclerosis in a family with familial partial lipodystrophy due to a mutation in LMNA. Nephron Clin Pract. 2013;124(1-2):31–37. [DOI] [PubMed] [Google Scholar]
- 6. Fountas A, Giotaki Z, Dounousi E, Liapis G, Bargiota A, Tsatsoulis A, Tigas S.. Familial partial lipodystrophy and proteinuric renal disease due to a missense c.1045C > T LMNA mutation. Endocrinol Diabetes Metab Case Rep. 2017;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Musso C, Javor E, Cochran E, Balow JE, Gorden P. Spectrum of renal diseases associated with extreme forms of insulin resistance. Clin J Am Soc Nephrol. 2006;1(4):616–622. [DOI] [PubMed] [Google Scholar]
- 8. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2):555–576. [PubMed] [Google Scholar]
- 9. Brown RJ, Oral EA, Cochran E, Araújo-Vilar D, Savage DB, Long A, Fine G, Salinardi T, Gorden P. Long-term effectiveness and safety of metreleptin in the treatment of patients with generalized lipodystrophy. Endocrine. 2018;60(3):479–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coresh J, Heerspink HJL, Sang Y, Matsushita K, Arnlov J, Astor BC, Black C, Brunskill NJ, Carrero JJ, Feldman HI, Fox CS, Inker LA, Ishani A, Ito S, Jassal S, Konta T, Polkinghorne K, Romundstad S, Solbu MD, Stempniewicz N, Stengel B, Tonelli M, Umesawa M, Waikar SS, Wen CP, Wetzels JFM, Woodward M, Grams ME, Kovesdy CP, Levey AS, Gansevoort RT; Chronic Kidney Disease Prognosis Consortium and Chronic Kidney Disease Epidemiology Collaboration. Change in albuminuria and subsequent risk of end-stage kidney disease: an individual participant-level consortium meta-analysis of observational studies. Lancet Diabetes Endocrinol. 2019;7(2):115–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. DCCT/EDIC research group. Effect of intensive diabetes treatment on albuminuria in type 1 diabetes: long-term follow-up of the Diabetes Control and Complications Trial and Epidemiology of Diabetes Interventions and Complications study. Lancet Diabetes Endocrinol. 2014;2(10):793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 13. Mykkänen L, Zaccaro DJ, Wagenknecht LE, Robbins DC, Gabriel M, Haffner SM. Microalbuminuria is associated with insulin resistance in nondiabetic subjects: the insulin resistance atherosclerosis study. Diabetes. 1998;47(5):793–800. [DOI] [PubMed] [Google Scholar]
- 14. Ekstrand AV, Groop PH, Grönhagen-Riska C. Insulin resistance precedes microalbuminuria in patients with insulin-dependent diabetes mellitus. Nephrol Dial Transplant. 1998;13(12):3079–3083. [DOI] [PubMed] [Google Scholar]
- 15. Orchard TJ, Chang YF, Ferrell RE, Petro N, Ellis DE. Nephropathy in type 1 diabetes: a manifestation of insulin resistance and multiple genetic susceptibilities? Further evidence from the Pittsburgh Epidemiology of Diabetes Complication Study. Kidney Int. 2002;62(3):963–970. [DOI] [PubMed] [Google Scholar]
- 16. Groop L, Ekstrand A, Forsblom C, Widén E, Groop PH, Teppo AM, Eriksson J. Insulin resistance, hypertension and microalbuminuria in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1993;36(7):642–647. [DOI] [PubMed] [Google Scholar]
- 17. Brown RJ, Valencia A, Startzell M, Cochran E, Walter PJ, Martin Garraffo HM, Cai H, Gharib AM, Ouwerkerk R, Courville AB, Bernstein S, Brychta RJ, Chen KY, Walter M, Auh S, Gorden P. Metreleptin improves insulin sensitivity independent of food intake in humans with lipodystrophy. J Clin Invest. 2018;128(8):3504–3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Petersen KF, Oral EA, Dufour S, Befroy D, Ariyan C, Yu C, Cline GW, DePaoli AM, Taylor SI, Gorden P, Shulman GI. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J Clin Invest. 2002;109(10):1345–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vatier C, Fetita S, Boudou P, Tchankou C, Deville L, Riveline J, Young J, Mathivon L, Travert F, Morin D, Cahen J, Lascols O, Andreelli F, Reznik Y, Mongeois E, Madelaine I, Vantyghem M, Gautier J, Vigouroux C. One-year metreleptin improves insulin secretion in patients with diabetes linked to genetic lipodystrophic syndromes. Diabetes Obes Metab. 2016;18(7):693–697. [DOI] [PubMed] [Google Scholar]
- 20. Ebihara K, Kusakabe T, Hirata M, Masuzaki H, Miyanaga F, Kobayashi N, Tanaka T, Chusho H, Miyazawa T, Hayashi T, Hosoda K, Ogawa Y, DePaoli AM, Fukushima M, Nakao K. Efficacy and safety of leptin-replacement therapy and possible mechanisms of leptin actions in patients with generalized lipodystrophy. J Clin Endocrinol Metab. 2007;92(2):532–541. [DOI] [PubMed] [Google Scholar]
- 21. Bjornstad P, Maahs DM, Wadwa RP, Pyle L, Rewers M, Eckel RH, Snell-Bergeon JK. Plasma triglycerides predict incident albuminuria and progression of coronary artery calcification in adults with type 1 diabetes: the Coronary Artery Calcification in Type 1 Diabetes Study. J Clin Lipidol. 2014;8(6):576–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tien KJ, Tu ST, Chen HC, Hsiao JY, Hsieh MC. Triglycerides are independently associated with albuminuria in Taiwanese Type 2 diabetic patients. J Endocrinol Invest. 2012;35(9):800–803. [DOI] [PubMed] [Google Scholar]
- 23. Pichaiwong W, Hudkins KL, Wietecha T, Nguyen TQ, Tachaudomdach C, Li W, Askari B, Kobayashi T, O’Brien KD, Pippin JW, Shankland SJ, Alpers CE. Reversibility of structural and functional damage in a model of advanced diabetic nephropathy. J Am Soc Nephrol. 2013;24(7):1088–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wolf G, Hamann A, Han DC, Helmchen U, Thaiss F, Ziyadeh FN, Stahl RA. Leptin stimulates proliferation and TGF-beta expression in renal glomerular endothelial cells: potential role in glomerulosclerosis [seecomments]. Kidney Int. 1999;56(3):860–872. [DOI] [PubMed] [Google Scholar]
- 25. Suganami T, Mukoyama M, Mori K, Yokoi H, Koshikawa M, Sawai K, Hidaka S, Ebihara K, Tanaka T, Sugawara A, Kawachi H, Vinson C, Ogawa Y, Nakao K. Prevention and reversal of renal injury by leptin in a new mouse model of diabetic nephropathy. FASEB J. 2005;19(1):127–129. [DOI] [PubMed] [Google Scholar]
- 26. Imajo K, Fujita K, Yoneda M, Nozaki Y, Ogawa Y, Shinohara Y, Kato S, Mawatari H, Shibata W, Kitani H, Ikejima K, Kirikoshi H, Nakajima N, Saito S, Maeyama S, Watanabe S, Wada K, Nakajima A. Hyperresponsivity to low-dose endotoxin during progression to nonalcoholic steatohepatitis is regulated by leptin-mediated signaling. Cell Metab. 2012;16(1):44–54. [DOI] [PubMed] [Google Scholar]
- 27. Reitman ML. Leptin in the liver: a toxic or beneficial mix? Cell Metab. 2012;16(1):1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gunduz Z, Dursun N, Akgun H, Ozturk F, Okur H, Koc N. Renal effects of long-term leptin infusion and preventive role of losartan treatment in rats. Regul Pept. 2005;132(1-3):59–66. [DOI] [PubMed] [Google Scholar]
- 29. Savage DB, Murgatroyd PR, Chatterjee VK, O’Rahilly S. Energy expenditure and adaptive responses to an acute hypercaloric fat load in humans with lipodystrophy. J Clin Endocrinol Metab. 2005;90(3):1446–1452. [DOI] [PubMed] [Google Scholar]
- 30. Christensen JD, Lungu AO, Cochran E, Collins MT, Gafni RI, Reynolds JC, Rother KI, Gorden P, Brown RJ. Bone mineral content in patients with congenital generalized lipodystrophy is unaffected by metreleptin replacement therapy. J Clin Endocrinol Metab. 2014;99(8):E1493–E1500. [DOI] [PMC free article] [PubMed] [Google Scholar]