Abstract
Objective
Maternal obesity and gestational diabetes mellitus (GDM) are associated with adverse outcomes, particularly with a male fetus. The composition and amount of substrate supplied to the placenta are altered in these conditions. We hypothesized that there are sexually dimorphic differences in utilization of glucose, fatty acids, and glutamine between trophoblast of lean women, women with obesity, and women with GDM.
Design
Trophoblasts were isolated from term male or female placentas from lean women, women with obesity, or women with GDM (n = 4 to 6 per group), and syncytiotrophoblast formed during 72 hours before measuring mitochondrial respiration by a fuel flex assay (Seahorse XF96 analyzer). Dependency, capacity, and flexibility for use of glucose, glutamine, and fatty acids were measured with western blot of glucose transporter GLUT1, glutaminase, and carnitine palmitoyltransferase 1A.
Results
Sexual dimorphism in syncytiotrophoblast fuel utilization was seen in women with GDM vs lean women with a significant increase in glucose dependency in males and glucose capacity in females, whereas for glutamine, capacity was significantly decreased in males and females but dependency significantly decreased only in females. Fatty acid dependency and capacity significantly increased in male trophoblast and capacity in female trophoblast of women with GDM vs either lean women or women with obesity. In male but not female trophoblast, flexibility to use all three fuels significantly decreased from lean women to women with obesity and women with GDM. In male trophoblast there were significant associations between GLUT1 and glucose dependency (positive) and flexibility (negative).
Conclusions
Human syncytiotrophoblast utilizes glutamine for mitochondrial respiration. Utilization of glucose, fatty acids, and glutamine changes in a sexually dimorphic manner with obesity and GDM, predominantly with a male placenta.
Measuring use of glucose, glutamine, and fatty acids for syncytiotrophoblast mitochondrial respiration revealed sexual dimorphism, particularly in males, of metabolic reprogramming with obesity and GDM.
The incidence of maternal obesity continues to increase, with up to 25% of the pregnant population in the United States being obese [body mass index (BMI) >30] (1, 2). Maternal obesity is associated with adverse outcomes for mother and fetus, including hypertensive disorders, fetal macrosomia, and perinatal death (3, 4) but it also is a risk factor for development of gestational diabetes mellitus (GDM), a common metabolic disorder of pregnancy, which affects ∼7% of pregnancies in the United States (5). Elevated blood glucose levels in the mother result in elevated glucose in the fetus and increased fetal insulin secretion, causing macrosomia, an increased risk of cesarean delivery, and birth trauma, including vaginal tears, shoulder dystocia, and asphyxia (6). Importantly, both maternal obesity and GDM program the offspring for disease in later life, including cardiovascular disease, metabolic syndrome, obesity, and diabetes (7–11). Current treatments of GDM can reduce but not prevent these adverse outcomes. Importantly, although the size of the delivered infant can be reduced to normal birth weight centiles, the infant may still be at risk for programming for obesity and diabetes in later life.
The placenta regulates maternal metabolism during pregnancy to increase the availability of substrates for transfer to the fetus, which then determines fetal growth and development (12). Appropriate development of the placenta is then crucial to normal fetal development (13). In normal pregnancy maternal insulin resistance increases circulating glucose and fatty acids for transfer, and this is further exacerbated with obesity and GDM (12). However, the placenta does not simply serve as a conduit for transfer of nutrients to the fetus; indeed, the placenta consumes substantial quantities of these substrates to provide energy for its considerable anabolic activity (14) and also to store the substrates to perhaps buffer the transfer to the fetus. Studies in pregnant sheep showed that only 55% of oxygen and 28% of glucose taken up by the pregnant uterus were used by the fetus (15), with the remainder being used by the considerable metabolic activity of the placenta. This was subsequently confirmed by studies of placental metabolism in different species (16), showing the placenta’s oxygen and glucose consumption to be several times higher per unit weight than that of the fetus. Of the placenta’s oxygen consumption for energy generation, one third is used for de novo synthesis of proteins (e.g., peptide hormones), and one third is used to maintain the cation gradient across the cell membrane, supporting transport mechanisms (17). Hence, by virtue of its prodigious consumption of substrates, the placenta provides a large and fundamentally important contribution to determining both the quality and quantity of nutrients available to the fetus. Therefore, as the placenta regulates nutrient composition and supply from mother to fetus and is the source of hormonal signals that affect maternal and fetal metabolism, consideration of placental metabolism per se is essential to complete our understanding of how the placenta regulates nutrient transfer, energy balance, fetal growth, and hence fetal programming (18).
Surprisingly, however, placental metabolism, particularly human placental metabolism, has not been examined in depth, and even less so in contemporary clinical scenarios. Although it is often stated that glucose is the major substrate used by the placenta (19), only 2% to 4% of fatty acids taken up by the placenta are transferred to the fetus (20, 21), with the remainder being either stored in the placenta or used in fatty acid oxidation to generate energy. It has been claimed that in the setting of GDM, placental glucose consumption increases and fatty acid oxidation is decreased by 40% to 50% (22, 23), with this adaptation serving as a regulatory step toward fetal nutrition (24).
Currently, the changes in placental mitochondrial function that generate energy with various pregnancy complications (e.g., diabetes and obesity) remain understudied. We have previously shown that trophoblast mitochondrial respiration is significantly reduced with maternal obesity (25) and is further significantly reduced with type A2 GDM (A2GDM; i.e., requiring medication to control) (26). In the setting of obesity, this reduced mitochondrial respiration is also associated with metabolic inflexibility (25), that is, the inability to use alternative substrates when glucose availability is blocked. In addition to glucose and fatty acids, amino acids, particularly glutamine, can be used, via the tricarboxylic acid (TCA) cycle, to generate acetyl coenzyme A for oxidative phosphorylation. Glutamine is the most abundant amino acid in the circulation (27), with concentrations in the pregnant uterine circulation reaching almost 900 µM (28), and it is used avidly by rapidly dividing cells (29), typified by tumors and also potentially trophoblast. Furthermore, the human placenta possesses glutaminase activity (30–32) responsible for glutamine decomposition to glutamate but is also capable of converting glutamate to glutamine via glutamine synthetase (33). Glutamate can be converted by glutamate dehydrogenase into α-ketoglutarate (2-oxoglutarate) (33) as an anaplerotic reaction feeding the TCA cycle and then providing the reducing equivalents for oxidative phosphorylation. In vivo human placenta takes up glutamate from the fetal circulation (34) and secretes large amounts of glutamine in to both the fetal and maternal circulations (28, 35). Glutamine is regarded as a conditionally essential amino acid, in particular for fetal development (36).
The Seahorse fuel flex kit allows the use of glucose, fatty acids, and glutamine to be determined in cultured cells. We have used the fuel flex kit to test the hypothesis that there are differences in the utilization of glucose, fatty acids, and glutamine between trophoblast isolated from lean women, women with obesity, and women with GDM. It is well known that the male fetus is at greater risk for an adverse outcome than is the female fetus (37), and we and others have previously shown sexual dimorphism in placental function, including antioxidant defenses (38), expression of miR-210, which regulates mitochondrial respiration (39), and of PGC-1α (40), the master regulator of mitochondrial biogenesis. Hence, we have investigated whether there is sexual dimorphism in trophoblast fuel flexibility with obesity and GDM.
Patients and Methods
Placentas were collected from three groups of women with either a male or a female fetus: lean (prepregnancy BMI, 18.5 to 24.9; n = 6 male, 7 female), obese (prepregnancy BMI, 30 to 45; n = 5 male, 5 female), and A2GDM (requiring insulin, matched for BMI with women with obesity; n = 4 male, 5 female). In all cases the weight of the neonate was appropriate for their respective gestational age. Women with any other pregnancy complication, for example, preeclampsia, preterm birth or with a small-for-gestational-age or large-for-gestational-age fetus, were excluded. Placentae were collected from the Labor and Delivery Unit at the University Hospital into a repository under a protocol approved by the Institutional Review Board of the Oregon Health and Science University with informed consent from the patients. All tissues and clinical data were deidentified before being made available to the investigative team.
Tissue collection and trophoblast isolation
Placental tissue was collected following elective cesarean section at term in the absence of labor. Villous tissue was sampled from five random sites of the placenta and flash-frozen in liquid nitrogen and stored at −80°C. Additionally, ∼35 g of tissue was taken from five random sites, and primary cytotrophoblasts were isolated using a protocol adapted from Eis et al. (41) using trypsin DNase digestion and Percoll gradient purification. Isolated cytotrophoblast cells were cultured for 72 hours in Iscove’s modified Dulbecco’s medium (with 25 mM glucose, 4 mM glutamine, and 1 mM pyruvate) containing 10% FBS and 1% penicillin/streptomycin to allow syncytialization prior to the Mito fuel flex assay (Agilent Technologies) using the Seahorse XFe96 analyzer that we have previously used for assay of trophoblast mitochondrial respiration (25).
Mitochondrial respiration
The Mito fuel flex test determines the rate of oxidation of each substrate (glucose, fatty acid, or glutamine) by measuring mitochondrial respiration of the cell [oxygen consumption rate (OCR) normalized to nuclear DNA] in the presence or absence of specific fuel pathway inhibitors using the Seahorse XFe96 analyzer. The fuel pathway inhibitors used were (i) UK5099, an inhibitor of the glucose oxidation pathway, which noncompetitively blocks the action of mitochondrial pyruvate carrier, which transports the pyruvate produced from glucose by glycolysis into the TCA cycle; (ii) bis-2-(5-phenylacetamido-1,3,4-thiadiazol-2-yl)ethyl sulfide (BPTES), a noncompetitve allosteric inhibitor of glutamine oxidation, which blocks glutaminase (GSL1) responsible for conversion of glutamine to glutamate, which can be converted to α-ketoglutarate and oxidized in the TCA cycle; and (iii) etomoxir, an irreversible inhibitor of long-chain fatty acid oxidation, which blocks the enzyme carnitine palmitoyltransferase 1A, which translocates long-chain fatty acids into the mitochondria for β oxidation.
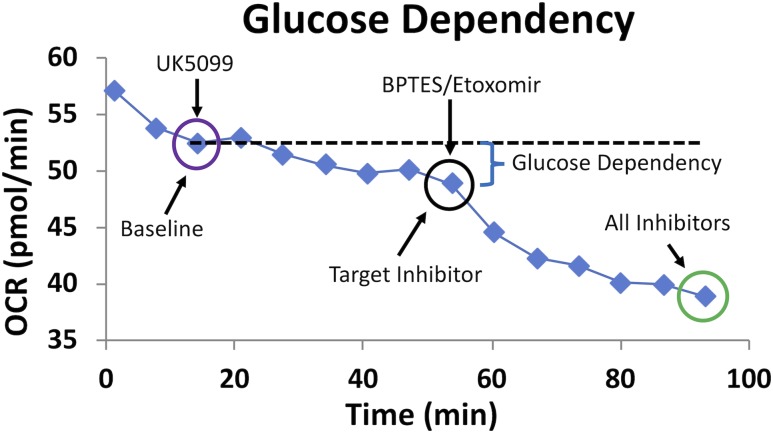
Following 72 hours of culture to allow syncytialization, media were changed to Seahorse base media supplemented with additional glucose (25 mM), glutamine (4 mM), and pyruvate (1mM) 1 hour before fuel flex assay. In the fuel flex assay, baseline OCR of trophoblast in the presence of all three fuel sources is first measured and then subsequently measured after inhibition of one fuel pathway followed by OCR measurement when the other two pathways are inhibited (Fig. 1). The final concentrations of the inhibitors in the assay were BPTES (3 µM), UK5099 (2 µM), and etomoxir (4 µM). From the fuel flex data the following parameters were the calculated for each fuel: fuel dependency, the relative amount of basal mitochondrial oxidation from a single fuel that cannot be compensated through oxidation of the other two fuels by the cell; fuel capacity, the relative ability of a cell to oxidize a specific fuel in the basal state when oxidation of the other two fuels are blocked; and fuel flexibility (percent capacity minus percent dependency), the relative ability of a cell in the basal state to switch or compensate mitochondrial oxidation from one fuel to another.
Figure 1.
Representative fuel flex analysis showing assessment of glucose dependency. Dependency for glucose is tested by first injecting the inhibitor of glucose oxidation pathway, UK5099, followed by inhibition of the two alternative pathways with BPTES and etomoxir. Dependency is calculated using the equation: Dependency (%) = [(Baseline OCR − Target inhibitor OCR)/(Baseline OCR − All inhibitors OCR)] × 100%.
The following equations were used in the calculations of these parameters:
Dependency (%) = [(Baseline OCR − Target inhibitor OCR)/(Baseline OCR − All inhibitors of OCR)] × 100%
Capacity (%) = 1 − [(Baseline OCR − Other two inhibitors of OCR)/(Baseline OCR − All inhibitors of OCR)] × 100% Flexibility (%) = Capacity (%) − Dependency (%).
Western blotting
For western blotting analysis, cytotrophoblast cells were plated in six-well tissue culture plates plate (4 million cells per well. Media were changed at 48 hours, and after 96 hours media were removed and cells rinsed in ice-cold PBS, and protein was harvested in 80 µL of RIPA buffer with added protease/phosphatase inhibitors. Each well was then scraped using a cell scraper and lysate was centrifuged at 1000 × g for 10 minutes at 4°C to remove cellular debris. Total proteins (10 μg) were separated on 4% to 20% precast linear gradient gels (Bio-Rad Laboratories), transferred to nitrocellulose membranes, and blocked with 5% (w/v) nonfat milk in Tris-buffered saline with Tween 20 for 1 hour. Membranes were incubated overnight at 4°C with primary antibody diluted in 5% BSA (w/v) in Tris-buffered saline with Tween 20 and detected using an appropriate peroxidase-conjugated secondary antibody diluted in the same manner. Products were visualized by ECL chemiluminescence (Millipore). Band intensities were measured using the G-box system (Syngene) and normalized to β-actin used as a loading control. Although all gels for the same target were run on the same day to control for variability, a calibration standard was made from a pool of three male and three female trophoblast samples from lean women and run on each gel. Band intensities were adjusted for change in the signal of the calibrator. Primary antibodies used were GLUT1 and carnitine palmitoyltransferase 1A (CPT1A; rabbit, Cell Signaling Technology, catalog nos. 12939 and 12252, respectively), glutaminase (mouse, Novus Biologicals, catalog no. NBP2-43762), all used at 1:1000 dilution, and β-actin (mouse, Sigma-Aldrich).
Statistical analysis
Data are reported as box-and-whisker plots (median, interquartile range, upper and lower values) or as individual values with mean ± SEM. Comparisons between two groups were performed using nonparametric statistics, the Mann–Whitney test or Kruskal–Wallis with a Dunn post hoc test for multiple comparisons in data sets with more than two groups. P < 0.05 was considered significant. The OCR parameters were analyzed by regression and correlation analysis against maternal BMI using Excel and GraphPad Prism 5.0.
Results
Clinical characteristics of study patients
There were no significant differences in maternal age, gestational age at delivery, fetal or placental weight, or the fetal/placental weight ratio between the women of differing BMI or with GDM. All of the women in the study were white. By experimental design, the women with A2GDM were matched for BMI with the women with obesity, and both had a significantly greater BMI than that of the lean group (Table 1).
Table 1.
Patient Characteristics
| Patient Group | Fetal Sex | Maternal Age (y) | Gestational Age (wk) | BMI (kg/m2) | Fetal Weight (g) | Placental Weight (g) | Fetal/Placental Ratio |
|---|---|---|---|---|---|---|---|
| Lean | Male | 34.3 ± 3.9 | 38.7 ± 1.4 | 22.6 ± 1.9 | 3345 ± 407 | 547 ± 76 | 6.2 ± 1.3 |
| n = 6 | |||||||
| Female | 33.4 ± 4.0 | 39.1 ± 0.8 | 23.8 ± 1.8 | 3416 ± 355 | 525 ± 94 | 6.6 ± 0.7 | |
| n = 7 | |||||||
| Obese | Male | 31.6 ± 4.7 | 38.7 ± 1.9 | 37.9 ± 6.2a | 3611 ± 559 | 523 ± 105 | 7.1 ± 1.4 |
| n = 5 | |||||||
| Female | 29.8 ± 5.0 | 39.3 ± 0.2b | 36.4 ± 4.4a | 3537 ± 301 | 494 ± 91 | 7.3 ± 1.1 | |
| n = 5 | |||||||
| A2GDM | Male | 33.0 ± 1.6 | 38.5 ± 1.0 | 40.1 ± 8.4a | 3839 ± 447 | 662 ± 81 | 5.6 ± 0.7 |
| n = 4 | |||||||
| Female | 31.2 ± 3.7 | 37.3 ± 1.0a | 37.4 ± 1.5a | 3161 ± 712 | 531 ± 193 | 5.7 ± 1.2 | |
| n = 5 |
All data are expressed as mean ± SD.
P < 0.05 vs lean of corresponding sex.
P < 0.05 vs A2GDM of corresponding sex.
Fuel flex assays
A typical curve obtained from the Seahorse analyzer during a fuel flex assay is illustrated in Fig. 1. OCR (µmol/min) is measured with and without the combinations of inhibitors as well as dependency, capacity, and flexibility calculated for each of the three fuels, that is, glucose, glutamine, and fatty acid. In this example dependency for glucose in syncytiotrophoblast is first tested by injection of UK5099 to inhibit glucose oxidation followed by inhibition of the two alternative pathways (glutamine and fatty acids by BPTES and etomoxir, respectively). Whereas glucose and glutamine are added exogenously in the fuel flex assay media, fatty acids were not, and hence we are examining the metabolism of endogenous fatty acids.
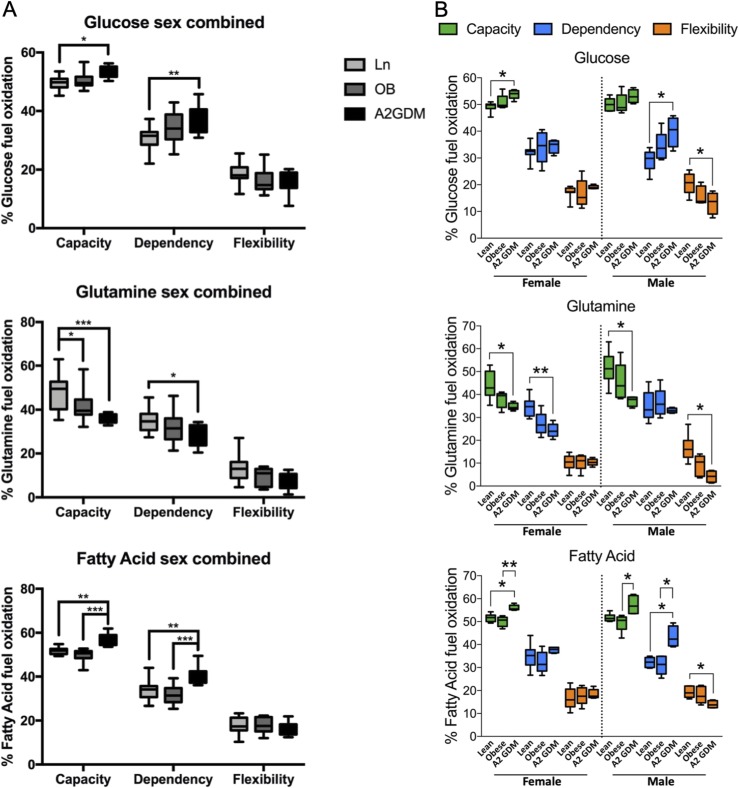
Comparison of these parameters for each fuel was made between trophoblast from lean women, women with obesity, and women with GDM with both sexes combined (Fig. 2A) or separately for either a male or female fetus (Fig. 2B). When data for both sexes were combined, we see increasing dependency and capacity for glucose in trophoblast from women with obesity and women with A2GDM compared with lean women, with significant differences seen between women with A2GDM vs lean women (P < 0.01 and P < 0.05 for dependency and capacity, respectively). Similarly, dependency and capacity for fatty acids were both significantly greater in trophoblast of women with A2GDM vs lean women (P < 0.01) or women with obesity (P < 0.001). However, flexibility was not significantly different using either glucose or fatty acid as fuels between the various conditions. In contrast, both dependency (P < 0.05) and capacity (P < 0.001) for glutamine were significantly lower in trophoblast of women with A2GDM vs lean women and in capacity (P < 0.05) for women with obesity vs lean women. Although flexibility for glutamine was reduced in trophoblast of women with obesity and women with A2GDM vs lean women, the effect was not significant. We subsequently examined the parameters by fetal (placental) sex (Fig. 2B). With glucose utilization, we saw a significant increase (∼30%, P < 0.05) in dependency and a decrease in flexibility (also ∼30%) for male trophoblast from women with A2GDM vs lean women (P < 0.05). Whereas no difference in glucose capacity was seen in trophoblast of the male fetus between conditions, there was a small but significant increase in capacity for glucose in the female trophoblast from women with A2GDM vs lean women (P < 0.05) and no change in dependency or flexibility. With glutamine as the fuel (Fig. 2B), there was a significant decrease in both capacity (∼30%, P < 0.05) and flexibility (∼75%, P < 0.05) in male trophoblast from women with A2GDM vs lean women, whereas in female trophoblast there were significant decreases in capacity (∼30%, P < 0.05) and dependency (∼30%, P < 0.01) with women with A2GDM vs lean women. When fatty acids were the fuel (Fig. 2B), there was significantly increased dependency of ∼30% in trophoblast of males from women with A2GDM vs either lean women or women with obesity (both P < 0.05) and capacity of ∼10% in trophoblast of A2GDM vs lean and A2GDM vs obese (P < 0.05) and a decrease in flexibility in trophoblast of A2GDM vs lean (P < 0.05). In female trophoblasts with fatty acids as fuel the only significant change was an increase in capacity (∼10%) between trophoblast of women with A2GDM vs lean women (P < 0.05) and women with A2GDM vs women with obesity (P < 0.01).
Figure 2.
Fuel flex analysis of syncytiotrophoblast of lean women, women with obesity, and women with A2GDM with either a male or female fetus. (A and B) Dependency, capacity, and flexibility for glucose, glutamine, and fatty acids with (A) both sexes combined (n = 13 lean, 10 obese, and 9 A2GDM) or (B) in male and female trophoblast of lean women, women with obesity, and women with A2GDM (n = 6, 5, and 4 males for lean, obese, and GDM, respectively, and n =7, 5, and 5 females for lean, obese, and GDM, respectively). Median, minimum, and maximum values and interquartile range are shown. *P < 0.05, **P < 0.01, *** P < 0.001, as measured by a Kruskal–Wallis test with a Dunn post hoc test.
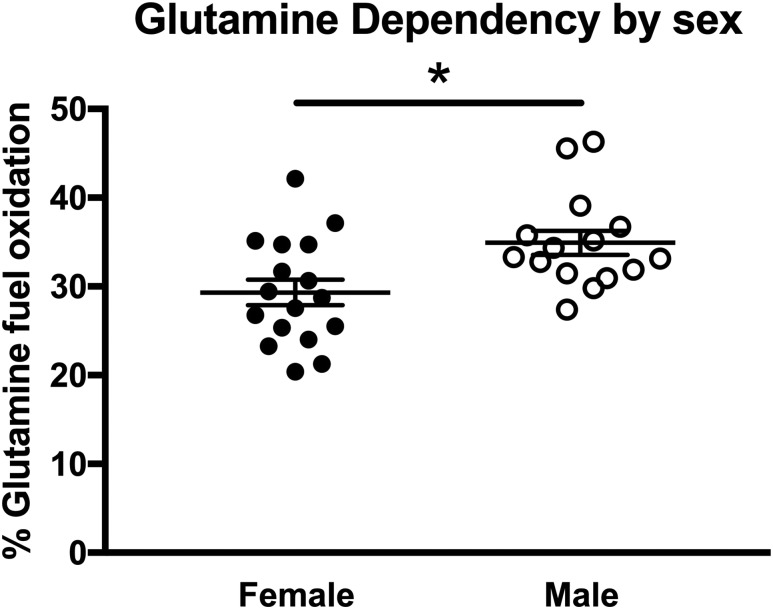
When only sex of trophoblast was considered with all maternal conditions combined, only glutamine dependency was found to be significantly different, being greater in male vs female trophoblast (P < 0.05, Fig. 3A). Hence, trophoblast fuel oxidation is responsive to the maternal metabolic milieu. Glucose and fatty acid dependency and capacity increase modestly but significantly with obesity and A2GDM, whereas glutamine dependency and capacity decrease. However, there is a sexual dimorphism in these responses, with male trophoblast accounting for the increase in glucose and fatty acid dependency and decreased glutamine capacity, but female trophoblast showing a decrease in both glutamine dependency and capacity but an increase in glucose and fatty acid capacity. There is a significant decrease in flexibility for glucose, glutamine, and fatty acids in male trophoblast from women with A2GDM vs lean women that is not seen in female trophoblast.
Figure 3.
Glutamine dependency in relationship to placental sex. Glutamine dependency was determined for all conditions combined in male (n = 15) and female (n = 17) trophoblast (mean ± SEM). *P < 0.05, Mann–Whitney test.
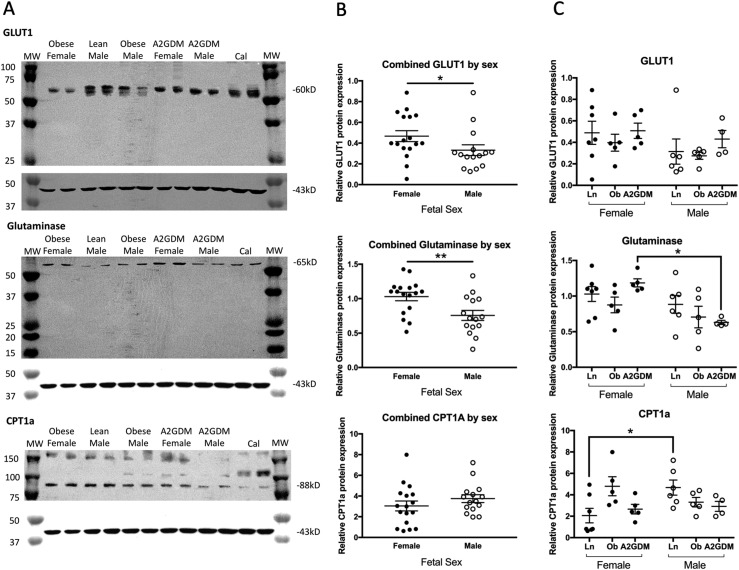
Western blot analysis of syncytiotrophoblast homogenates for GLUT1, glutaminase, and CPT1A was performed to assess the contribution of this transporter and enzymes to the activity of the three fuel pathways. Protein bands were found at the expected sizes for each protein, that is, GLUT1 at 54 kDa, glutaminase at 64 kDa, and CPT1A at 88 kDa (Fig. 4A). Quantitation of these bands revealed that overall expression of GLUT1 and glutaminase was significantly lower in trophoblast of male vs female placentas (P < 0.05 and P < 0.01 respectively), but with no difference in CPT1A (Fig. 4B). When both sexes were combined, no differences in protein expression for GLUT1, glutaminase, or CPT1A were seen across the three conditions (lean, obese, or A2GDM, data not shown). However, when stratifying by placental sex, glutaminase expression decreased with worsening metabolic milieu such that expression in male trophoblast from women with A2GDM was significantly less than in female trophoblast from women with A2GDM (P < 0.05, Fig. 4C). For CPT1A, expression in male trophoblast of lean women was significantly higher than that of female trophoblast of lean women (P < 0.05, Fig. 4C) but decreased with poor metabolic milieu such that it was then not significantly different.
Figure 4.
Western blot analysis of GLUT1, glutaminase, and CPT1A protein expression in human syncytiotrophoblast of male and female placentas of lean women, women with obesity, and women with A2GDM. (A) Representative western blots. β-Actin (43 kDa, each lower panel) was used as a loading control, and each gel contained a calibrator sample (Cal) to control for variability between gels. (B) Gels were imaged and bands were quantitated with normalization to β-actin. GLUT1 and glutaminase expression were significantly lower (*P < 0.05 and **P < 0.01, respectively, Mann–Whitney test) in trophoblast of male (n = 15) vs female (n = 17) placentas when all conditions were combined. (C) Individual values of band intensity for GLUT1, glutaminase, and CPT1A were plotted for each condition, and trophoblast sex and comparisons were made by a Kruskal–Wallis test with a Dunn post hoc test and a Mann–Whitney test, respectively. *P < 0.05 (n = 6, 5, and 4 males and n =7, 5, and 5 females from lean women, women with obesity, and women with A2GDM, respectively). Bars show mean ± SEM.
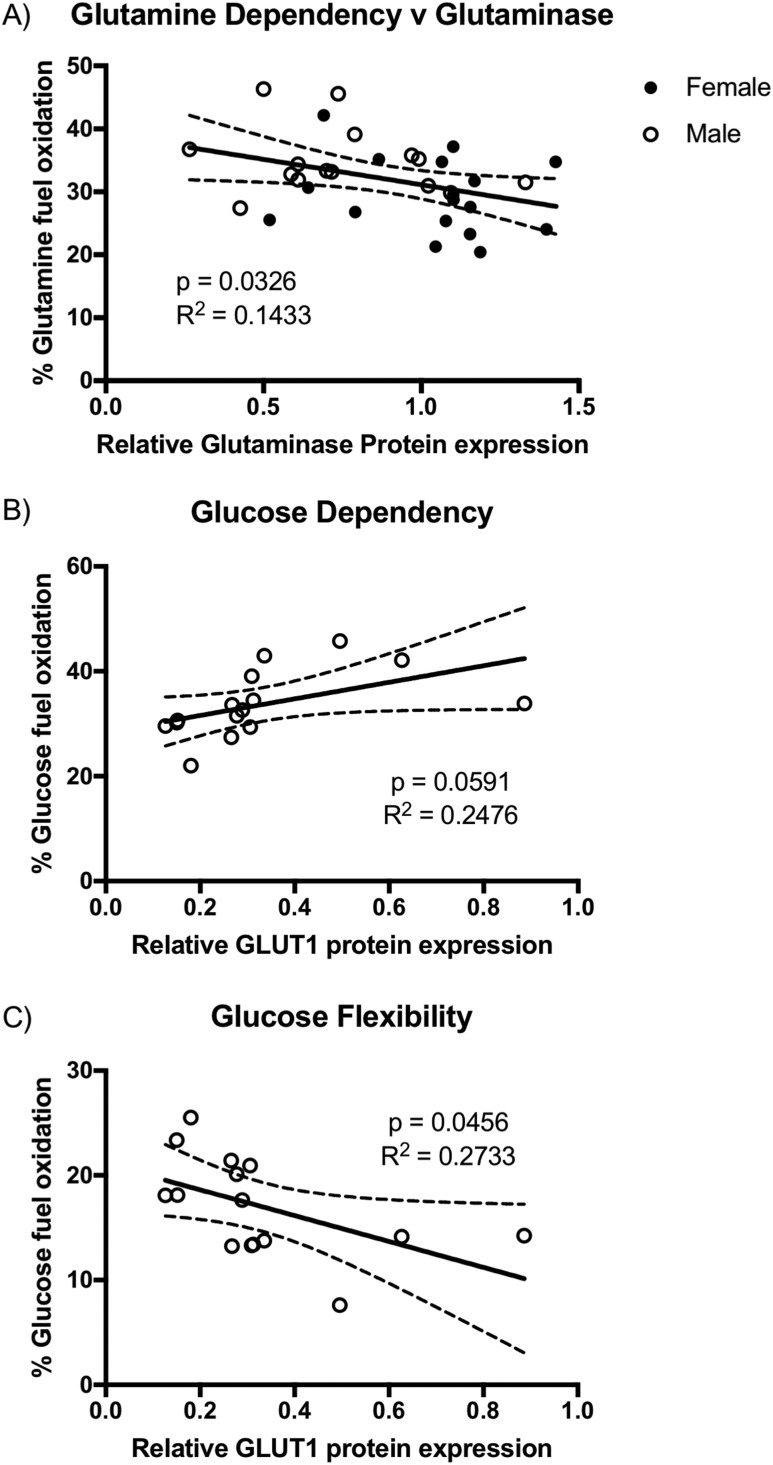
We correlated GLUT1, glutaminase, and CPT1A expression to the individual fuel flex parameter. Having noted that glutamine dependency was significantly greater in male trophoblast vs female trophoblast and that glutaminase expression was significantly decreased in male vs female trophoblast, we found that glutamine dependency was significantly inversely related to glutaminase expression when data from male and female trophoblasts across all three conditions were combined (P < 0.05, Fig. 5A). However, neither glutamine flexibility nor capacity was related to glutaminase expression. Similar regression analysis for GLUT1 expression and glucose fuel parameters revealed a positive association of glucose dependency and GLUT1 expression in male trophoblast, but that just failed to reach significance (P = 0.059, Fig. 5B), and a significant inverse relationship (P < 0.05) between glucose flexibility and GLUT1 expression, but again only in male trophoblast (Fig. 5C). There was no relationship between glucose capacity and GLUT1 or for any of the fatty acid fuel parameters and CPT1A expression (data not shown).
Figure 5.
(A–C) Relationship of (A) trophoblast glutaminase expression and glutamine dependency with male (n = 15) and female (n = 17) trophoblast combined, (B) glucose dependency and GLUT1 expression, and (C) glucose flexibility and GLUT1 expression, both in male trophoblast only (n = 15). A significant negative correlation of glutamine dependency with glutaminase expression (P < 0.05), a positive correlation of glucose dependency with GLUT1 (P = 0.059), and a negative correlation of glucose flexibility with GLUT1 expression (P < 0.05) were found.
Discussion
The increased maternal concentrations of glucose and fatty acids in the setting of obesity and GDM (42, 43) affects both placental uptake and consumption of these substrates but also the type and amount of substrate then available to be supplied to the fetus, thus impacting fetal growth and development (24). We have previously shown decreased mitochondrial respiration in trophoblast from women with obesity and a lack of metabolic flexibility (25), which is further exacerbated with type A2GDM (26). In this study, we have undertaken a more detailed examination of fuel flexibility in trophoblast from lean women, women with obesity, and women with GDM. As we have previously shown sexual dimorphism in trophoblast mitochondrial respiration (39, 44) and in placental antioxidant defenses (38), we stratified our study by placental sex. Strengths of the study are the comparison of three patient groups, the investigation of sexual dimorphism, and the comprehensive analysis of fuel usage in relationship to oxygen consumption for energy generation.
Our data clearly demonstrate, to our knowledge for the first time, the dependency of trophoblast on glutamine for baseline respiration. Glutamine is converted to glutamate by glutaminase, which is abundant in placenta, and then glutamate dehydrogenase yields α-ketoglutarate, an intermediate in the TCA cycle that generates the reducing equivalents for the electron transport chain, producing ATP. Interestingly this dependency for glutamine is of a similar magnitude (∼35% in trophoblast of lean women) to that for fatty acids and slightly larger than that for glucose (30%), which is commonly assumed to be the major substrate for trophoblast energy generation (19). Given the often made comparison of placental tissue to tumor tissue, utilization of glutamine, which is a hallmark of tumors (29), by trophoblast is perhaps not surprising. In lean women there was no difference in dependency for these three fuels between male and female trophoblast. However, with the changing maternal metabolic milieu of obesity and A2GDM, that is, hyperglycemia and hyperlipidemia, we find an increased dependency on glucose and fatty acids for baseline respiration but only with a male placenta. This suggests an increased reliance of the male trophoblast on these substrates for basal respiration. However, this is accompanied by significantly decreased flexibility for use of both glucose and fatty acids, and additionally significantly decreased flexibility for glutamine; that is, male trophoblast cannot adapt by increasing oxidation of other fuels when one is removed. This decreased flexibility seen in male and not female trophoblast may contribute to the increased risk of the male fetus for adverse outcomes (37). The changes we see in dependency and flexibility for glucose and fatty acids show incremental changes from lean women to women with obesity and are further exacerbated with BMI-matched A2GDM, suggesting that the effect is not due to obesity alone but may reflect the continuum of worsening hyperglycemia and hyperlipidemia from obesity to A2GDM. Indeed, we had previously shown a worsening of trophoblast mitochondrial respiration from obesity to A2GDM (26).
The capacity (overall ability to use a fuel when other pathways are inhibited) for glucose and fatty acids was increased slightly (∼10%) but significantly in both male and female trophoblast of women with A2GDM compared with lean women. This observation is distinct from that of Visiedo et al. (22, 23) who found a reduction of fatty acid oxidation of up to 50% together with an increase in glucose uptake in placental explants of women with GDM compared with controls. These investigators attributed the reduced fatty acid oxidation and a corresponding reduction in CPT1A to high glucose in GDM (23). These differences may be the result of combining male and female placentas together and examining oxidation of exogenous fatty acid using whole placental tissue explants vs our measurement of endogenous fatty acid metabolism in mitochondrial respiration in isolated trophoblast from either male or female placentas. We do, however, see evidence for sexual dimorphism in CPT1A expression, and that expression decreases in male trophoblast from lean women to women with A2GDM. Interestingly, we see the capacity for glutamine decreased significantly by approximately one third in male and female trophoblast with women with A2GDM compared with lean women, suggesting that a component of the obese or A2GDM metabolic milieu limits the ability to use glutamine when glucose and fatty acids are inhibited. There is also sexual dimorphism in the effect of obesity or A2GDM on glutamine utilization, as flexibility decreases significantly with increasing adiposity and A2GDM in the male trophoblast but dependency decreases significantly in the same setting with female trophoblast. In this study where the women with A2GDM were treated with insulin, we see no increase in GLUT1 expression between lean women, women with obesity, and women with A2GDM, unlike our previous report of increased GLUT1 in women with GDM treated with glyburide vs treated with diet (26), a finding since confirmed by others (45). GLUT1 is predominantly expressed in the microvillus membrane of trophoblast; however, expression in basement membrane is thought to be rate limiting for glucose transfer to the fetus (46). We find that glucose dependency is positively correlated but glucose flexibility is negatively correlated with total GLUT1 expression measured in trophoblast, but both only in a male trophoblast. In human placenta, microvillus membrane GLUT1 expression has been found to negatively correlate with uteroplacental glucose uptake, which itself positively correlates with placental glucose consumption (47). Hence, there may be a relationship between dependency on glucose for baseline metabolism and the ability to increase glucose oxidation when other pathways are inhibited and the supply of glucose to the placenta, which is increased with obesity and gestational diabetes (42, 43).
In addition to our previous description of altered fuel flexibility with maternal obesity (25), another example of placental metabolic reprogramming is seen in pregnancies at high altitude where the hypoxia has been inferred to lead to increased placental anaerobic glycolysis at the expense of mitochondrial respiration to spare oxygen to support the fetus, this resulting in increased glucose utilization in the placenta with consequent decreased glucose delivery to the fetus leading to growth restriction (48). Recent findings using a four-vessel sampling technique in humans has shown that the placenta consumes 30% of glucose taken up from maternal blood and that placental consumption modulates maternal-to-fetal glucose transfer and fetal glucose consumption such that high placental use of glucose limits fetal glucose delivery and consumption (47). Studies in the isolated perfused placental cotyledon coupled with computational modeling showed that placental metabolism also influences fatty acid transfer to the fetus, with the vast majority of fatty acids taken up being incorporated into placental lipid pools (49). Hence, the placenta is not simply the passive conduit for substrate transport to the fetus, but its metabolism influences substrate supply to the fetus.
It is clear from our data that trophoblast respiration in lean women appears to use glucose, fatty acids, and glutamine for roughly equivalent proportions of energy production; however, the changing metabolic milieu (obesity and A2GDM) alters this usage, but in a sexually dimorphic manner. The increased availability of glucose and fatty acids in obesity and A2GDM may lead to their increased usage by the placenta for oxidation. Indeed, in the recent paper by Michelsen et al. (47) uteroplacental glucose uptake was positively correlated with placental glucose consumption. This is particularly evident in males who adopt a growth strategy vs the preservation strategy of females in utero, with the downside being the lack of flexibility in fuel usage in male trophoblast placing the placenta in a high-risk position when faced with further insults, with the ultimate outcome being stillbirth. The impact of altered placental consumption of fuels in the setting of obesity and A2GDM where there are greater amounts of maternal fuel, on amounts transferred to the fetus and ultimately fetal growth, remains to be explored. Furthermore, the altered placental metabolism of fuels may serve to buffer the fetus from the altered maternal levels in obesity and A2GDM.
In summary, our findings show the significant contribution of the conditionally essential amino acid glutamine to oxidative phosphorylation and energy production in the placenta and that placental utilization of glucose, fatty acids, and glutamine for energy generation changes in the setting of obesity and gestational diabetes in a sexually dimorphic manner, predominantly when the placenta is that of a male who is at greater risk for adverse pregnancy outcomes. Our findings add additional weight to the concept of a “selfish placenta,” that is, that placental metabolism and consumption of substrates affects delivery to the fetus, again in sexually dimorphic manner. Current therapeutic approaches for diabetic pregnancies focus on normalizing maternal glucose levels, which may reduce fetal macrosomia but not completely ameliorate the effects of a diabetic pregnancy. Our findings suggest other metabolic approaches might be useful and that sex-specific strategies should be considered.
Acknowledgments
Financial Support: This work was supported by National Institutes of Health/National Institute of Child Health and Human Development Grant HD095610 (to L.M.).
Disclosure Summary: The authors have nothing to disclose
Glossary
Abbreviations:
- A2GDM
type A2 GDM
- BMI
body mass index
- BPTES
bis-2-(5-phenylacetamido-1,3,4-thiadiazol-2-yl)ethyl sulfide
- CPT1A
carnitine palmitoyltransferase 1A
- GDM
gestational diabetes mellitus
- OCR
oxygen consumption rate
- TCA
tricarboxylic acid
References and Notes
- 1. Guelinckx I, Devlieger R, Beckers K, Vansant G. Maternal obesity: pregnancy complications, gestational weight gain and nutrition. Obes Rev. 2008;9(2):140–150. [DOI] [PubMed] [Google Scholar]
- 2. Galtier-Dereure F, Boegner C, Bringer J. Obesity and pregnancy: complications and cost. Am J Clin Nutr. 2000;71(5Suppl):1242S–1248S. [DOI] [PubMed] [Google Scholar]
- 3. Kristensen J, Vestergaard M, Wisborg K, Kesmodel U, Secher NJ. Pre-pregnancy weight and the risk of stillbirth and neonatal death. BJOG. 2005;112(4):403–408. [DOI] [PubMed] [Google Scholar]
- 4. Ehrenberg HM, Mercer BM, Catalano PM. The influence of obesity and diabetes on the prevalence of macrosomia. Am J Obstet Gynecol. 2004;191(3):964–968. [DOI] [PubMed] [Google Scholar]
- 5. Finer S, Mathews C, Lowe R, Smart M, Hillman S, Foo L, Sinha A, Williams D, Rakyan VK, Hitman GA. Maternal gestational diabetes is associated with genome-wide DNA methylation variation in placenta and cord blood of exposed offspring. Hum Mol Genet. 2015;24(11):3021–3029. [DOI] [PubMed] [Google Scholar]
- 6. Kjos SL, Buchanan TA. Gestational diabetes mellitus. N Engl J Med. 1999;341(23):1749–1756. [DOI] [PubMed] [Google Scholar]
- 7. Barker DJ. The fetal and infant origins of adult disease. BMJ. 1990;301(6761):1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Catalano PM. Obesity and pregnancy—the propagation of a viscous cycle? J Clin Endocrinol Metab. 2003;88(8):3505–3506. [DOI] [PubMed] [Google Scholar]
- 9. Forsén T, Eriksson JG, Tuomilehto J, Teramo K, Osmond C, Barker DJ. Mother’s weight in pregnancy and coronary heart disease in a cohort of Finnish men: follow up study. BMJ. 1997;315(7112):837–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lawlor DA, Ronalds G, Clark H, Smith GD, Leon DA. Birth weight is inversely associated with incident coronary heart disease and stroke among individuals born in the 1950s: findings from the Aberdeen Children of the 1950s prospective cohort study. Circulation. 2005;112(10):1414–1418. [DOI] [PubMed] [Google Scholar]
- 11. Pettitt DJ, Aleck KA, Baird HR, Carraher MJ, Bennett PH, Knowler WC. Congenital susceptibility to NIDDM. Role of intrauterine environment. Diabetes. 1988;37(5):622–628. [DOI] [PubMed] [Google Scholar]
- 12. Lain KY, Catalano PM. Metabolic changes in pregnancy. Clin Obstet Gynecol. 2007;50(4):938–948. [DOI] [PubMed] [Google Scholar]
- 13. Myatt L. Placental adaptive responses and fetal programming. J Physiol. 2006;572(Pt 1):25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bax BE, Bloxam DL. Energy metabolism and glycolysis in human placental trophoblast cells during differentiation. Biochim Biophys Acta. 1997;1319(2–3):283–292. [DOI] [PubMed] [Google Scholar]
- 15. Meschia G, Battaglia FC, Hay WW, Sparks JW. Utilization of substrates by the ovine placenta in vivo. Fed Proc. 1980;39(2):245–249. [PubMed] [Google Scholar]
- 16. Bloxam DL. Human placental energy metabolism: its relevance to in vitro perfusion. Contrib Gynecol Obstet. 1985;13:59–69. [PubMed] [Google Scholar]
- 17. Carter AM. Placental oxygen consumption. Part I: in vivo studies—a review. Placenta. 2000;21(Suppl A):S31–S37. [DOI] [PubMed] [Google Scholar]
- 18. Hay WW., Jr The placenta. Not just a conduit for maternal fuels. Diabetes. 1991;40(Suppl 2):44–50. [DOI] [PubMed] [Google Scholar]
- 19. Martinez F, Milan R, Flores-Herrera O, Olvera-Sanchez S, Gomez-Chang E, Espinosa-Garcia M. The role of mitochondria in syncytiotrophoblast cells: bioenergetics and steroidogenesis. In: Zheng J, ed. Recent Advances in Research on the Human Placenta. Shanghai, China: InTech; 2012:397–428. [Google Scholar]
- 20. Dancis J, Jansen V, Kayden HJ, Schneider H, Levitz M. Transfer across perfused human placenta. II. Free fatty acids. Pediatr Res. 1973;7(4):192–197. [DOI] [PubMed] [Google Scholar]
- 21. Lewis RM, Desoye G. Placental lipid and fatty acid transfer in maternal overnutrition. Ann Nutr Metab. 2017;70(3):228–231. [DOI] [PubMed] [Google Scholar]
- 22. Visiedo F, Bugatto F, Quintero-Prado R, Cózar-Castellano I, Bartha JL, Perdomo G. Glucose and fatty acid metabolism in placental explants from pregnancies complicated with gestational diabetes mellitus. Reprod Sci. 2015;22(7):798–801. [DOI] [PubMed] [Google Scholar]
- 23. Visiedo F, Bugatto F, Sánchez V, Cózar-Castellano I, Bartha JL, Perdomo G. High glucose levels reduce fatty acid oxidation and increase triglyceride accumulation in human placenta. Am J Physiol Endocrinol Metab. 2013;305(2):E205–E212. [DOI] [PubMed] [Google Scholar]
- 24. Catalano PM, Hauguel-De Mouzon S. Is it time to revisit the Pedersen hypothesis in the face of the obesity epidemic? Am J Obstet Gynecol. 2011;204(6):479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mele J, Muralimanoharan S, Maloyan A, Myatt L. Impaired mitochondrial function in human placenta with increased maternal adiposity. Am J Physiol Endocrinol Metab. 2014;307(5):E419–E425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Muralimanoharan S, Maloyan A, Myatt L. Mitochondrial function and glucose metabolism in the placenta with gestational diabetes mellitus: role of miR-143. Clin Sci (Lond). 2016;130(11):931–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bergström J, Fürst P, Norée LO, Vinnars E. Intracellular free amino acid concentration in human muscle tissue. J Appl Physiol. 1974;36(6):693–697. [DOI] [PubMed] [Google Scholar]
- 28. Holm MB, Bastani NE, Holme AM, Zucknick M, Jansson T, Refsum H, Mørkrid L, Blomhoff R, Henriksen T, Michelsen TM. Uptake and release of amino acids in the fetal-placental unit in human pregnancies. PLoS One. 2017;12(10):e0185760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reitzer LJ, Wice BM, Kennell D. Evidence that glutamine, not sugar, is the major energy source for cultured HeLa cells. J Biol Chem. 1979;254(8):2669–2676. [PubMed] [Google Scholar]
- 30. Klimek J, Makarewicz W, Swierczynski J, Bossy-Bukato G, Zelewski L. Mitochondrial glutamine and glutamate metabolism in human placenta and its possible link with progesterone biosynthesis. Placenta. 1993;14(Suppl 1):77–86. [Google Scholar]
- 31. Luschinsky HL. The activity of glutaminase in the human placenta. Arch Biochem Biophys. 1951;31(1):132–140. [DOI] [PubMed] [Google Scholar]
- 32. Makarewicz W, Swierczynski J. Phosphate-dependent glutaminase in the human term placental mitochondria. Biochem Med Metab Biol. 1988;39(3):273–278. [DOI] [PubMed] [Google Scholar]
- 33. Jozwik M, Pietrzycki B, Jozwik M, Anthony RV. Expression of enzymes regulating placental ammonia homeostasis in human fetal growth restricted pregnancies. Placenta. 2009;30(7):607–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Battaglia FC. Glutamine and glutamate exchange between the fetal liver and the placenta. J Nutr. 2000;130(4S Suppl):974S–977S. [DOI] [PubMed]
- 35. Day PE, Cleal JK, Lofthouse EM, Goss V, Koster G, Postle A, Jackson JM, Hanson MA, Jackson AA, Lewis RM. Partitioning of glutamine synthesised by the isolated perfused human placenta between the maternal and fetal circulations. Placenta. 2013;34(12):1223–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Parimi PS, Kalhan SC. Glutamine supplementation in the newborn infant. Semin Fetal Neonatal Med. 2007;12(1):19–25. [DOI] [PubMed] [Google Scholar]
- 37. Eriksson JG, Kajantie E, Osmond C, Thornburg K, Barker DJP. Boys live dangerously in the womb. Am J Hum Biol. 2010;22(3):330–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Evans L, Myatt L. Sexual dimorphism in the effect of maternal obesity on antioxidant defense mechanisms in the human placenta. Placenta. 2017;51:64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Muralimanoharan S, Guo C, Myatt L, Maloyan A. Sexual dimorphism in miR-210 expression and mitochondrial dysfunction in the placenta with maternal obesity. Int J Obes. 2015;39(8):1274–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jiang S, Teague AM, Tryggestad JB, Aston CE, Lyons T, Chernausek SD. Effects of maternal diabetes and fetal sex on human placenta mitochondrial biogenesis. Placenta. 2017;57:26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Eis AL, Brockman DE, Pollock JS, Myatt L. Immunohistochemical localization of endothelial nitric oxide synthase in human villous and extravillous trophoblast populations and expression during syncytiotrophoblast formation in vitro. Placenta. 1995;16(2):113–126. [DOI] [PubMed] [Google Scholar]
- 42. Catalano PM, Huston L, Amini SB, Kalhan SC. Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus. Am J Obstet Gynecol. 1999;180(4):903–916. [DOI] [PubMed] [Google Scholar]
- 43. Okereke NC, Huston-Presley L, Amini SB, Kalhan S, Catalano PM. Longitudinal changes in energy expenditure and body composition in obese women with normal and impaired glucose tolerance. Am J Physiol Endocrinol Metab. 2004;287(3):E472–E479. [DOI] [PubMed] [Google Scholar]
- 44. Muralimanoharan S, Gao X, Weintraub S, Myatt L, Maloyan A. Sexual dimorphism in activation of placental autophagy in obese women with evidence for fetal programming from a placenta-specific mouse model. Autophagy. 2016;12(5):752–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Díaz P, Dimasuay KG, Koele-Schmidt L, Jang B, Barbour LA, Jansson T, Powell TL. Glyburide treatment in gestational diabetes is associated with increased placental glucose transporter 1 expression and higher birth weight. Placenta. 2017;57:52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jansson T, Wennergren M, Illsley NP. Glucose transporter protein expression in human placenta throughout gestation and in intrauterine growth retardation. J Clin Endocrinol Metab. 1993;77(6):1554–1562. [DOI] [PubMed] [Google Scholar]
- 47. Michelsen TM, Holme AM, Holm MB, Roland MC, Haugen G, Powell TL, Jansson T, Henriksen T. Uteroplacental glucose uptake and fetal glucose consumption: a quantitative study in human pregnancies. J Clin Endocrinol Metab. 2019;104(3):873–882. [DOI] [PubMed] [Google Scholar]
- 48. Illsley NP, Caniggia I, Zamudio S. Placental metabolic reprogramming: do changes in the mix of energy-generating substrates modulate fetal growth? Int J Dev Biol. 2010;54(2–3):409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Perazzolo S, Hirschmugl B, Wadsack C, Desoye G, Lewis RM, Sengers BG. The influence of placental metabolism on fatty acid transfer to the fetus. J Lipid Res. 2017;58(2):443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]