Abstract
Context
Placental transport capacity influences fetal glucose supply. The syncytiotrophoblast is the transporting epithelium in the human placenta, expressing glucose transporters (GLUTs) and insulin receptors (IRs) in its maternal-facing microvillous plasma membrane (MVM) and fetal-facing basal plasma membrane (BM).
Objective
The objectives of this study were to (i) determine the expression of the insulin-sensitive GLUT4 glucose transporter and IR in the syncytiotrophoblast plasma membranes across gestation in normal pregnancy and in pregnancies complicated by maternal obesity, and (ii) assess the effect of insulin on GLUT4 plasma membrane trafficking in human placental explants.
Design, Setting, and Participants
Placental tissue was collected across gestation from women with normal body mass index (BMI) and mothers with obesity with appropriate for gestational age and macrosomic infants. MVM and BM were isolated.
Main Outcome Measures
Protein expression of GLUT4, GLUT1, and IR were determined by western blot.
Results
GLUT4 was exclusively expressed in the BM, and IR was predominantly expressed in the MVM, with increasing expression across gestation. BM GLUT1 expression was increased and BM GLUT4 expression was decreased in women with obesity delivering macrosomic babies. In placental villous explants, incubation with insulin stimulated Akt (S473) phosphorylation (+76%, P = 0.0003, n = 13) independent of maternal BMI and increased BM GLUT4 protein expression (+77%, P = 0.0013, n = 7) in placentas from lean women but not women with obesity.
Conclusion
We propose that maternal insulin stimulates placental glucose transport by promoting GLUT4 trafficking to the BM, which may enhance glucose transfer to the fetus in response to postprandial hyperinsulinemia in women with normal BMI.
Our data suggest that maternal insulin stimulates human placental glucose transport by promoting trafficking of GLUT4 to the fetal-facing syncytiotrophoblast basal plasma membrane.
Fetal growth is critically dependent on glucose availability, and increased fetal glucose supply is thought to accelerate growth by stimulating the secretion of insulin, IGF-I, and IGF-II (1, 2). Fetal glucose supply is determined by multiple factors, including maternal glucose levels (3) and placental glucose transport capacity.
Almost two-thirds of American women now enter pregnancy either overweight or obese (4, 5), which can be associated with hyperglycemia and increased glucose transfer across the placenta. Maternal obesity is associated with a multitude of medical and obstetric short- and long-term complications (6), including an increased risk to deliver an infant that is macrosomic or large for gestational age (LGA) (7, 8), has increased adiposity (9), and/or is insulin resistant at birth (10). Additionally, babies born to obese mothers, particularly when they are large at birth, are at risk for obesity, diabetes, and cardiovascular disease later in life (11–13). Maternal obesity is associated with fetal hyperglycemia despite normal maternal fasting blood glucose levels implicating increased placental glucose transport capacity (14). However, placental glucose transport in women with obesity has not been studied in detail.
Transplacental glucose transport is facilitated by a family of glucose transporters (GLUTs), which mediate stereo-specific, sodium-independent diffusion of glucose down a concentration gradient. The syncytiotrophoblast, the transporting epithelium of the human placenta, acts as a barrier between the maternal and fetal circulations, and it mediates the transport of nutrients, such as glucose, across the placenta. GLUTs are expressed in both the maternal-facing microvillous plasma membrane (MVM) and the fetal-facing basal plasma membrane (BM) of the polarized syncytiotrophoblast. Multiple isoforms of GLUTs have been identified in the human placenta, including GLUT1, GLUT3, GLUT4, GLUT8, GLUT9, GLUT10, and GLUT12 (15–21). GLUT1 expression is particularly high in the syncytiotrophoblast plasma membranes, with threefold higher expression in the MVM compared with the BM, reflecting the higher glucose transport activity in the MVM (15). Given the sixfold to sevenfold larger surface area at term of the syncytiotrophoblast MVM compared with the BM, it has been estimated that the total glucose transport capacity of the MVM may be 20-fold higher than that of the BM. Based on these findings, it has been proposed that transport across the BM constitutes the rate-limiting step of transplacental glucose transfer (15), a model that is supported by experimental data (22). GLUT3 is highly expressed in the MVM in early pregnancy, and its expression decreases across gestation (21). GLUT4 is an insulin-regulated transporter predominantly expressed in traditional insulin-sensitive tissues, such as adipose tissue, as well as skeletal and cardiac muscle. Although GLUT4 has been reported to be expressed in intravillous stromal cells of the human placenta (23), previous studies suggested that GLUT4 was not localized to the syncytiotrophoblast at term (24–26). In contrast, GLUT4 protein was found to be expressed in the cytoplasm and MVM of first trimester syncytiotrophoblast (19).
The insulin receptor (IR) is a heterotetramer with each homodimer comprised of a ligand-binding α-subunit and a catalytic β-subunit. The IR exists in two splice variant isoforms, IRA and IRB, dependent on the absence or presence of a 12–amino acid segment at the C-terminal of the extracellular α-subunit. Although the two IR isoforms have similar binding affinity for insulin, the isoforms have different affinity for IGF-II and proinsulin, which are bound by IRA but not IRB. Insulin-sensitive tissues predominantly express the IRB isoform, whereas IRA is mainly thought to be expressed by fetal tissue and the placenta (27, 28). However, the precise subcellular localization of IRs in the human placenta remains to be fully established (23, 29–31). Using immunohistochemistry, Desoye et al. (29, 30) demonstrated that placental IRs were predominantly expressed in the syncytiotrophoblast MVM in early pregnancy, whereas IR protein expression at term was primarily found in the fetoplacental vessels. In contrast, other investigators have reported that IRs are abundantly expressed in the syncytiotrophoblast MVM at term (14, 31–33), and there is significant evidence in the literature supporting that the human placenta is insulin responsive. For example, insulin increases glucose transport in the term placenta as shown by in vitro and placental perfusion studies (34, 35). Ericsson et al. (19, 36) reported that insulin increases glucose uptake in first trimester placental villous explants, suggesting that glucose transport in the placenta can be regulated by insulin in early pregnancy. Additionally, it is well established that insulin stimulates amino acid transport in term villous explants (37) and cultured primary human trophoblasts (38, 39). Information on how maternal insulin impacts the expression and signaling through IR and GLUT4 transporters in the placenta is important because it will help us better understand how postprandial insulin modifies placental glucose transport to the fetus.
The objective of this study was to determine the expression of the insulin-sensitive GLUT4 glucose transporter and IR in the syncytiotrophoblast plasma membranes across gestation in normal pregnancy and in pregnancies complicated by maternal obesity. Additionally, we assessed the effect of insulin on GLUT4 plasma membrane trafficking in human placental explants in early pregnancy and at term. We hypothesized that GLUT4 is expressed in both MVM and BM, that insulin promotes placental GLUT4 plasma membrane trafficking, and that placental GLUT4 and IR expression increases across gestation and in maternal obesity.
Materials and Methods
Tissue collection
Informed written consent was obtained from all participants for collection of placental tissue and for the use of their protected health information, under protocols approved by the Institutional Review Board at the University of Colorado, Anschutz Medical Campus (COMIRB 14-1073 and 06-1098). Placental tissue was collected from 29 healthy women who were undergoing elective surgical termination of pregnancy at 8 to 22 weeks of gestation [18 ± 0.57 weeks (mean ± SEM)]. Placental tissue was processed on average ∼1 to 2 hours after collection, during which time the placenta was stored on ice. Trophoblast/placental tissue was identified and rinsed and all trophoblast tissue/the entire placenta were then homogenized with a Polytron (15,000 rpm, 2 minutes) in ice-cold buffer D [250 mM sucrose, 10 mM HEPES (pH 7.4), with protease and phosphatase inhibitors].
Placentas were also obtained at term immediately after vaginal or cesarean delivery from healthy women with normal body mass index (BMI; early pregnancy BMI, 18 to 24.9 kg/m2), women with obesity (early pregnancy BMI >30 kg/m2) with appropriate for gestation age (AGA) babies, and women with obesity with macrosomic babies (>4000 g). The amniotic sac, chorionic plate, and decidua were removed and ∼100 g of villous tissue was collected from all areas of the placenta. Villous tissue was rinsed in saline and homogenized as above. All processes were carried out on ice, and homogenized tissue was snap-frozen in liquid nitrogen and stored at −80°C until analyses or further processing.
Isolation of syncytiotrophoblast plasma membranes
Syncytiotrophoblast MVM and BM were isolated according to previously described protocols (40, 41). Briefly, placental tissue was thawed, rehomogenized, and centrifuged for 15 minutes at 10,000 × g at 4°C. The supernatant was collected, and the pellet was suspended in buffer D, homogenized, and centrifuged for 10 minutes at 10,000 × g at 4°C. The supernatant from both spins was centrifuged for 30 minutes at 125,000 × g at 4°C. The pellet was resuspended in buffer D with 12 mM MgCl2, which was gently stirred on ice for 20 minutes and centrifuged for 15 minutes at 2500 × g at 4°C. The supernatant, containing the MVM fraction, was centrifuged for 30 minutes at 125,000 × g at 4°C. The pellet, containing the BM fraction, was resuspended in buffer D, homogenized, and layered onto a sucrose gradient and centrifuged for 60 minutes at 144,000 × g at 4°C. The supernatants containing MVM and BM fractions were centrifuged for 30 minutes at 125,000 × g at 4°C. The pellets were resuspended in buffer D containing protease and phosphatase inhibitors, snap-frozen in liquid nitrogen, and stored at −80°C until analysis.
As previously described (19, 42), MVM enrichment was determined by the MVM/homogenate ratio of alkaline phosphatase activity. Mean MVM enrichment was 12.3 ± 1.5 and 12.6 ± 1.0 (mean ± SEM) in early gestation and at term, respectively. Enrichment of the BM was determined by the BM/homogenate ratio of the protein expression of voltage-dependent anion-selective channel 1 (VDAC1), which is exclusively expressed in the BM of human placenta (43), using western blot. Mean BM enrichment was 38.1 ± 7.8 and 40.0 ± 4.0 (mean ± SEM) in early gestation and at term, respectively.
Western blot
Briefly, protein was loaded onto precast polyacrylamide gels (Bio-Rad Laboratories, Hercules, CA), samples were randomly loaded onto gels, all groups were represented on each gel, and an equalizer sample was used to correct for variation between gels. After electrophoresis the proteins were transferred onto polyvinylidene difluoride membranes overnight at 4°C. Following transfer, membranes were blocked in either 5% (w/v) milk in Tris-buffered saline with 0.1% (v/v) Tween 80 (TBST) or 5% (w/v) BSA in TBST. Membranes were incubated with primary antibodies: Merck Millipore (Burlington, MA): GLUT1 (07-1401), pIRS-1 T612 (09-432), tIRS-1 (06-248); Cell Signaling Technology (Danvers, MA): GLUT4 (2213), IRβ (3025), pAkt T308 (5106), pAkt S473 (9271), and tAkt (9272); Abcam (Cambridge, UK): GLUT4 (ab654) and VDAC1 (ab15895); Santa Cruz Biotechnology (Santa Cruz, CA): IR β-subunit (sc711), pIRS-1 T612 (09-432), and tIRS-1 (06-248). Membranes were incubated with appropriate peroxidase-labeled secondary antibody (Cell Signaling Technology). Bands were visualized using enhanced chemiluminescence detection reagents (Thermo Fisher Scientific, Waltham, MA). Densitometry analysis was performed using GeneTools version 4.3.8 (Syngene, Cambridge, UK). Amido black total protein staining was used to control for any variations in protein loading and transfer efficiency. Full western blot membranes are shown in an online repository (44).
Immunohistochemistry
Term placental tissue samples were rinsed in physiological saline and placed in zinc fixative [0.1 M Tris buffer (pH 7.4), 2.8 mM calcium acetate, 37 mM zinc chloride, and 23 mM zinc acetate) for 12 hours. After fixation, samples were rinsed in PBS, embedded in OCT compound (Thermo Fisher Scientific) and cut into sections (16 µm), which were mounted onto slides. The slides were incubated in methanol with 1% (v/v) H2O2 for 30 minutes at room temperature and washed in PBS. A Vectastain ABC horseradish peroxidase kit (Vector Laboratories, Peterborough, UK) was used, and the manufacturer’s instructions were followed. In brief, slides were blocked for 30 minutes at room temperature. Sections were incubated overnight at 4°C in primary antibodies: GLUT4 (1:1500, Abcam), GLUT1 (1:1500, Merck Millipore), and IRβ (1:100, Cell Signaling Technology). The sections were incubated in Vectastain ABC kit secondary biotinylated antibody for 60 minutes at room temperature, washed in PBS, and incubated in ABC Vectastain for 30 minutes at room temperature. To visualize the antigen stain, a diaminobenzidine substrate kit (Vector Laboratories) was used. Slides were washed in PBS before a hematoxylin QS nuclear counterstain (Vector Laboratories) was used to visualize the nuclei. Slides were then rinsed in PBS and mounted. Four term placentas were sectioned, and eight sections per placenta were used for each antibody.
Insulin stimulation in fresh villous fragments
Villous tissue was collected from 10 terminations of pregnancy from 17.2 to 21.6 weeks gestation with a mean BMI of 24 kg/m2 (19.6 to 32.2 kg/m2) and from placentas delivered at term, eight from women with normal BMI (18.5 to 24.9 kg/m2) and from six women with obesity (>30 kg/m2). Villous tissue was isolated, washed in 0.9% (w/v) saline and dissected, as previously described (45). Villous tissue from each placenta was incubated in medium made up of a 1:1 mix of high-glucose (25 mM) DMEM (Sigma-Aldrich, St. Louis, MO) and Ham’s F12 (Thermo Fisher Science), containing antibiotics (gentamicin, penicillin, and streptomycin), with or without 1 nM insulin (Sigma-Aldrich) for 4 hours at 37°C with gentle movement (68 rpm) on an incubator shaker. Following incubation, villous tissue was washed in ice-cold Dulbecco’s PBS and centrifuged at 500 × g for 1 minute. The pellets were resuspended in buffer D with protease and phosphatase inhibitors, homogenized, snap-frozen in liquid nitrogen, and stored at −80°C. Syncytiotrophoblast MVM and BM were isolated as above. In villous tissue homogenates total and phosphorylated Akt (S473, T308) were determined, and in isolated syncytiotrophoblast membranes GLUT1, GLUT4, and IR protein expression was examined using western blot.
Data presentation and statistical analyses
Data are presented as mean ± SEM. All statistical tests were performed using GraphPad Prism version 6.03 and R version 3.5.1. Two-sample t tests, paired t tests, and ANOVA were used to determine significant differences. All t tests and ANOVA used a Welch correction to allow for potential heteroscedasticity (unequal group variances). For a given ANOVA, pairwise comparisons of multiple group means used Bonferroni adjusted significance level (i.e., α* = 0.05/no. of comparisons). All other tests were evaluated at a significance level of α = 0.05.
Lastly, a sensitivity analysis was conducted (results not shown) to assess the robustness of any significant findings. Because t tests and ANOVA may be invalid given nonnormal residuals in small samples, a sensitivity analysis was conducted that allowed for potential nonnormality (nonparametric bootstrap/permutation-based tests for comparing group means). When a test result differed between the main and sensitivity analyses, we did not draw any conclusions from that discrepant test. However, when a test result agreed between the main and sensitivity analyses, this gives further evidence that the test result is valid. All significant findings in the manuscript were confirmed by the sensitivity analysis (results not shown).
Results
Clinical characteristics of study subjects
Placental tissue was collected from 19 women (mean BMI, 26.2 ± 1.12 kg/m2) undergoing elective terminations of pregnancy (mean gestational age, 17.5 ± 0.79 weeks) and from 28 women at term for protein expression studies in isolated syncytiotrophoblast plasma membranes. The clinical characteristics of term pregnancies, which included women with normal BMI (control), women with obesity who delivered AGA babies, and mothers with obesity who gave birth to macrosomic babes, are provided in Table 1. By design, early pregnancy BMI was different in both obese and obese/macrosomia groups compared with the control BMI group (P < 0.0001). Birth weight was also different in the obese/macrosomia group compared with the control and obese BMI group (P = 0.011). There were no other significant differences in clinical characteristics, including gestational age at collection.
Table 1.
Clinical Characteristics of Study Subjects: Syncytiotrophoblast Plasma Membrane Isolation at Term
| Control, Mean (SD) (n =11) |
Obese, Mean (SD) (n = 12) |
Obese/Macrosomic, Mean (SD) (n = 5) |
P Value | |
|---|---|---|---|---|
| Gestational age, wk | 39.27 (1.21) | 39.30 (0.61) | 39.54 (1.15) | 0.9075 |
| Early pregnancy BMI, kg/m2 | 22.36 (1.81) | 35.58 (3.39) | 35.58 (2.38) | <0.0001 |
| Birth weight, g | 3396.09 (259.47) | 3279.75 (314.92) | 4613.80 (715.42) | 0.0113 |
| Placental weight, g | 647.77 (123.76) | 614.08 (97.98) | 918.94 (251.44) | 0.0850 |
| Delivery mode | ||||
| Cesarean | 7 (63.6%) | 8 (66.7%) | 5 (100.0%) | 0.4073 |
| Vaginal | 4 (36.4%) | 4 (33.3%) | 0 (0.0%) | |
| Fetal sex | ||||
| Female | 8 (72.7%) | 6 (50.0%) | 2 (40.0%) | 0.4129 |
| Male | 3 (27.3%) | 6 (50.0%) | 3 (60.0%) |
Boldface signifies statistical significance.
Placental villi were collected from 10 women undergoing elective terminations of pregnancy; the mean maternal BMI was 24.0 ± 0.56 kg/m2, and the mean gestational age was 19.1 ± 1.14 weeks. Placental villous tissue was also collected from term pregnancies from eight women with normal BMI and six women with obesity. The clinical characteristics of these pregnancies are provided in Table 2. By design, early pregnancy BMI was statistically different between the normal and obese BMI groups (P = 0.005). There were no significant differences in subject characteristics, including gestational age at delivery, birth weight, and placental weight in this subset of samples.
Table 2.
Clinical Characteristics of Study Subjects: Term Villous Explant Experiments
| Normal, Mean (SD) (n = 8) |
Obese, Mean (SD) (n = 6) |
P Value | |
|---|---|---|---|
| Gestational age, wk | 38.98 (0.76) | 39.22 (0.77) | 0.5709 |
| Early pregnancy BMI, kg/m2 | 22.25 (1.81) | 34.18 (6.40) | 0.0052 |
| Birth weight, g | 3403.75 (466.64) | 3529.67 (611.61) | 0.6837 |
| Placental weight, g | 649.27 (135.31) | 701.83 (102.32) | 0.4241 |
| Delivery mode | |||
| Cesarean | 5 (62.5%) | 3 (50.0%) | 1.0000 |
| Vaginal | 3 (37.5%) | 3 (50.0%) | |
| Fetal sex | |||
| Female | 2 (25.0%) | 3 (50.0%) | 0.5804 |
| Male | 6 (75.0%) | 3 (50.0%) |
Boldface signifies statistical significance.
Localization of GLUT4, GLUT1, and IR in term human placenta
Immunohistochemistry was performed to determine the cellular localization of GLUT4, GLUT1, and IR in sections from term healthy placenta. GLUT4 was predominantly expressed in the BM of the syncytiotrophoblast (Fig. 1A). In contrast, GLUT1 (Fig. 1B) and IR (Fig. 1C) were highly polarized to the MVM, with less expression in the BM.
Figure 1.
Cellular localization of GLUT4, GLUT1, and IRβ. (A) GLUT4 (brown) is highly expressed in the BM of the syncytiotrophoblast and not in the MVM. (B) GLUT1 (brown) and (C) IRβ (brown) are predominantly expressed in the MVM, with less expression in the BM. (D) Negative control without primary antibody added.
GLUT4 and IR expression in the syncytiotrophoblast plasma membranes across gestation
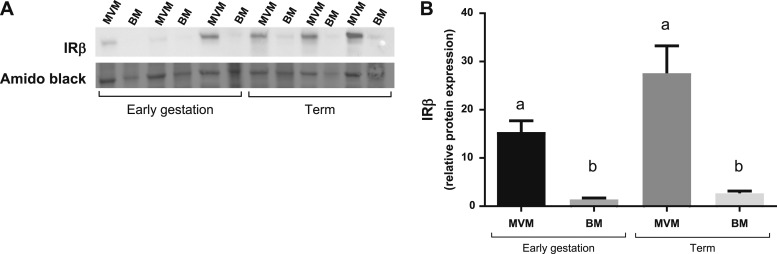
GLUT4 protein was exclusively expressed in the fetal-facing BM of term syncytiotrophoblast (Fig. 2A). We confirmed these results using a second GLUT4 antibody (Fig. 2B). In the BM, protein expression of GLUT4 was 84% higher at term (n = 16) compared with early gestation (n = 19, P = 0.04; Fig. 2C and 2D). Although IRβ protein was expressed in MVM and BM (Fig. 3A), IRβ expression was ∼10-fold higher in the MVM than in the BM in early pregnancy (n = 6, P < 0.0001) and at term (n = 6, P = 0.0005; Fig. 3B). IRβ protein expression in the MVM (P = 0.08) and in the BM (P = 0.047) increased across gestation (Fig. 3B).
Figure 2.
GLUT4 protein is exclusively expressed in the syncytiotrophoblast BM. Representative western blots of GLUT4 protein expression in the MVM and BM of term syncytiotrophoblast are shown. (A and B) Antibodies from (A) Santa Cruz Biotechnology and (B) Cell Signaling Technology. (C) Representative western blot of BM GLUT4 protein expression across gestation. (D) BM GLUT4 protein expression increases significantly (P = 0.04) from early gestation (11 to 24 wk, n = 19) to term (n = 16) and was not expressed in the MVM at any gestational age (not shown). Mean ± SEM. *P < 0.05, two-sample t test.
Figure 3.
IR protein expression in syncytiotrophoblast plasma membranes. IR protein expression in the maternal-facing syncytiotrophoblast MVM and fetal-facing BM across gestation is shown. (A) Representative western blot of IRβ expression across gestation in MVM and BM. (B) Mean IRβ protein expression was compared across four groups (n = 6 per group) for a total of six pairwise comparisons. Data are shown as mean + SEM. ANOVA with pairwise t tests using Bonferroni adjusted significance level α* = 0.05/6 = 0.0083. Groups with different superscript letters are significantly different at α* = 0.0083. Mean IRβ expression was significantly higher in the MVM compared with the BM in early gestation (11 to 24 wk, P < 0.0001) and at term (P < 0.0001). Mean IRβ expression tended to increase across gestation in the MVM (P = 0.085) and the BM (P = 0.047), but these differences did not reach statistical significance.
Effect of maternal obesity on GLUT1, GLUT4, and IR protein expression at term
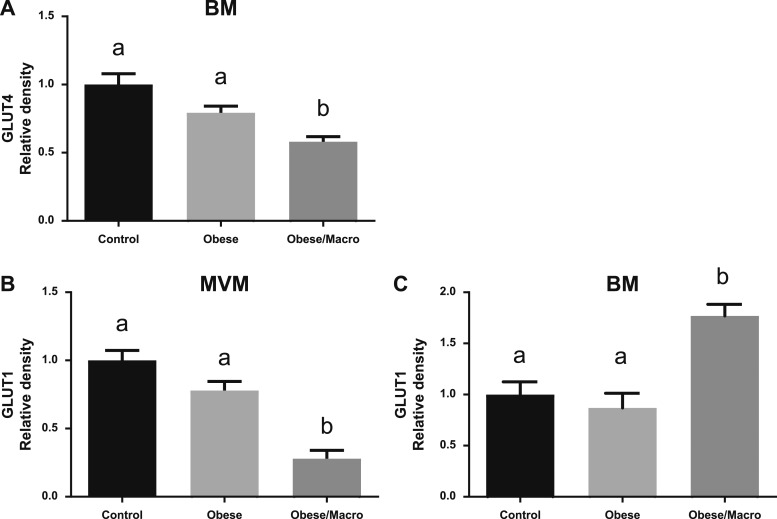
We determined glucose transporter and IRβ protein expression in the BM and MVM of term placentas from women with normal BMI (control, n = 11), women with obesity who delivered AGA babies (n = 12), and women with obesity who delivered macrosomic babies (n = 5). Contrary to our hypothesis, GLUT4 expression was decreased in the BM of placentas from women with obesity who delivered macrosomic babies compared with controls (−42%, P = 0.0073) and compared with women with obesity who delivered AGA babies (−27%, P = 0.0098; Fig. 4A). There was no difference in BM GLUT4 expression in placentas from control women compared with women with obesity (Fig. 4A).
Figure 4.
Glucose transporter protein expression in the MVM and BM in maternal obesity. GLUT4 protein expression in the BM and GLUT1 protein expression in the MVM and BM of placentas from women with normal BMI (Control, n = 11), women with obesity delivering AGA infants (n=12) or a macrosomic infant (Obese/Macro, n = 5), measured by Western blot, are shown. Data are shown as mean ± SEM. Each ANOVA contains three pairwise t tests and uses Bonferroni adjusted significance level α* = 0.05/3 = 0.0167. Groups with different superscript letters are significantly different at α* = 0.0167. (A) BM GLUT4 protein expression in Obese/Macro was significantly lower as compared with Obese (P = 0.007) and Control (P = 0.001) placentas; there was no significant difference in protein expression between Control and Obese placentas. (B) In the MVM GLUT1 protein expression was significantly lower in Obese/Macro as compared with Obese (P = 0.0001) and Control placentas (P < 0.0001). (C) In the BM GLUT1, protein expression was significantly higher in Obese/Macro placentas compared with Control (P = 0.0006) and Obese (P = 0.0002) placentas.
MVM GLUT1 protein expression was lower in placentas from mothers with obesity delivering macrosomic babies compared with controls (−72%, P < 0.0001) and mothers with obesity delivering AGA babies (−64%, P = 0.0001; Fig. 4B). There was no difference in MVM GLUT1 expression in placentas from control women compared with women with obesity (Fig. 4B). In the BM, GLUT1 protein expression was higher in obese/macrosomic placentas compared with placentas from control women (+77%, P = 0.0006) and women with obesity (+104%, P = 0.0002; Fig. 4C).
Effect of insulin treatment on GLUT and IR protein expression and Akt phosphorylation in villous explants
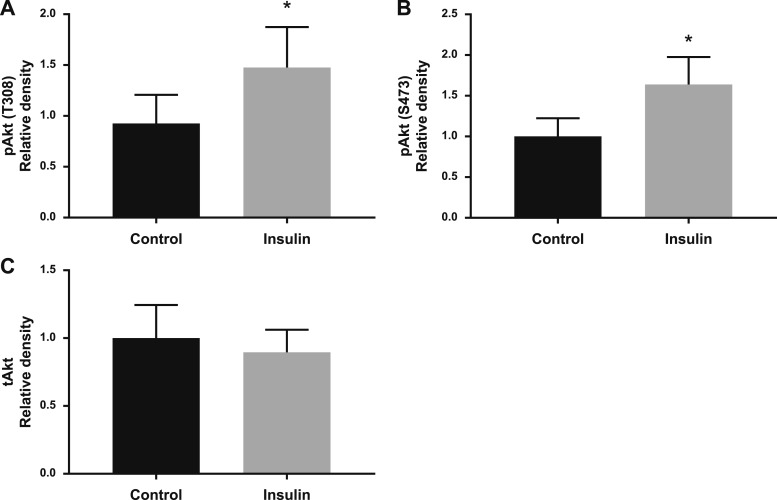
Placental villous explants from early gestation (n = 10) and term (n = 14) placentas were treated with insulin for 4 hours, and the protein expression of glucose transporters, IRβ, and key components of the insulin signaling pathway were examined. In villous explants from early pregnancy there were no differences in the protein expression of GLUT1 in the MVM (Fig. 5A) or BM (Fig. 5B) or IRβ in the MVM (Fig. 5C) or BM (Fig. 5D) after insulin stimulation. There was a 49% increase in GLUT4 protein expression in the BM (P = 0.07) when villous explants from early gestation placentas were treated with insulin (Fig. 5E). In response to insulin, phosphorylation of Akt at T308 (+59%, P = 0.011; Fig. 6A) and S473 (+64%, P = 0.024; Fig. 6B) increased in villous explants isolated from early gestation placentas; however, there was no difference in total Akt expression (Fig. 6C).
Figure 5.
Effect of insulin on GLUT and IR protein expression in villous explants from early gestation placentas. Villous explants from early pregnancy placentas were treated with and without insulin and (A) MVM GLUT1, (B) BM GLUT1, (C) MVM IRβ, (D) BM IRβ, and (E) BM GLUT4 protein expression was determined by western blot (n = 6 to 10 per group). Data are shown as mean ± SEM. Significance was evaluated with a paired t test.
Figure 6.
Insulin stimulates the phosphorylation of Akt in villous explants from early gestation. Villous explants from early pregnancy placentas were treated with and without insulin and phosphorylation: (A) Akt (T308), P = 0.01; (B) Akt (S473), P = 0.02; and (C) total expression of Akt was measured by western blot (n = 4 to 7 per group). Data are shown as mean ± SEM. *P < 0.05, paired t test.
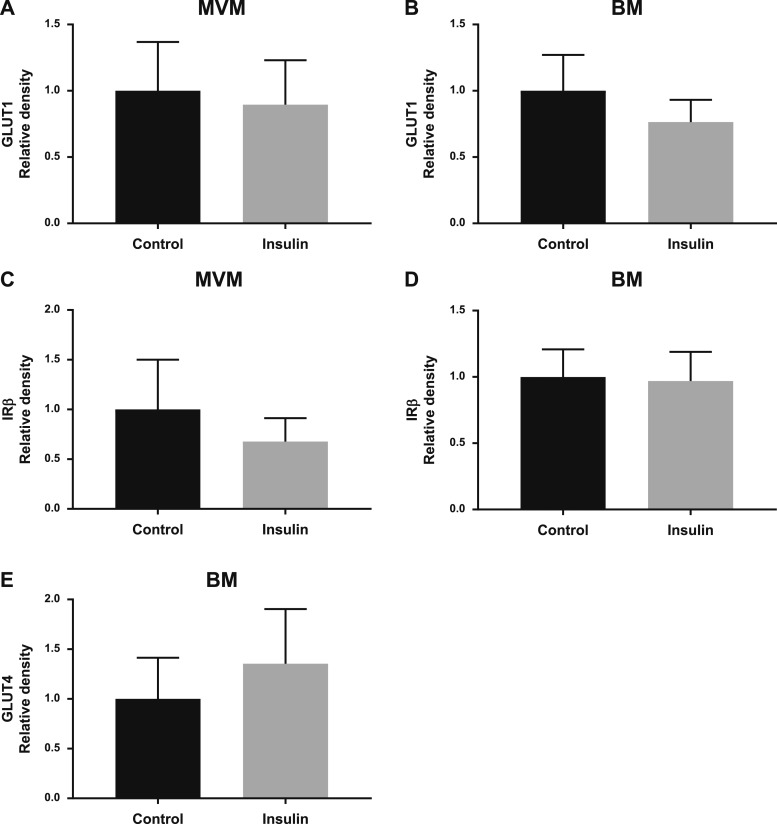
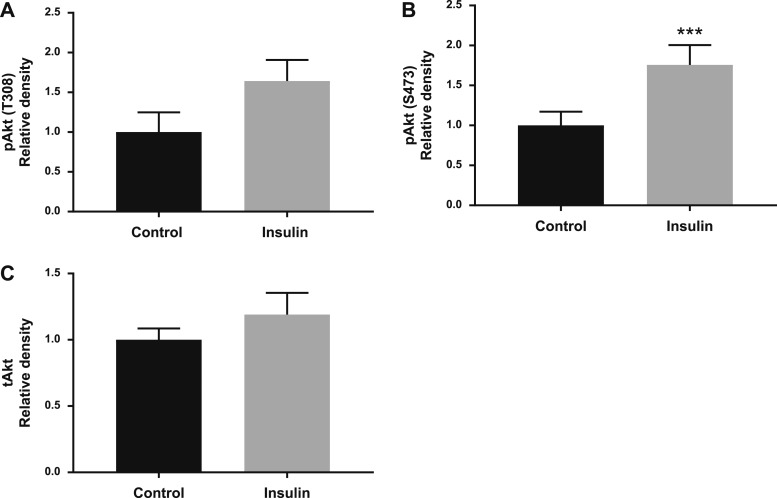
Insulin did not change the protein expression of GLUT1 in the MVM (Fig. 7A) or BM (Fig. 7B) or IRβ in the MVM (Fig. 7C) or BM (Fig. 7D) in villous explants from term placentas from women with a normal BMI. In contrast, insulin increased BM GLUT4 protein expression by 77% (P = 0.001; Fig. 7E) in villous explants isolated from normal BMI mothers. In term placentas from women with obesity, insulin did not alter the protein expression of GLUT1 in the MVM (Fig. 8A) or BM (Fig. 8B), IRβ in the MVM (Fig. 8C) or BM (Fig. 8D), or GLUT4 in the BM (Fig. 8E). Akt phosphorylation in term placental villous explants in response to insulin was similar in women with normal BMI and women with obesity, and the results from these two groups are therefore presented together. Phosphorylation of Akt at T308 (+64%, P = 0.10; Fig. 9A) and at S473 (+76%, P = 0.0003; Fig. 9B) were increased when term placental villous explants were treated with insulin. There was no difference in total Akt expression (Fig. 9C).
Figure 7.
Insulin stimulates BM GLUT4 protein expression in villous explants from term placentas of women with normal BMI. Villous explants from term placentas were treated with and without insulin, and (A) MVM GLUT1, (B) BM GLUT1, (C) MVM IRβ, (D) BM IRβ, and (E) BM GLUT4 protein expression (P = 0.0013) was measured by western blot (n = 7 to 8 per group). Data are shown as mean ± SEM. **P < 0.01, paired t test.
Figure 8.
Insulin does not stimulate GLUT or IR protein expression in villous explants from term placentas of women with obesity. Villous explants from term placentas were treated with and without insulin, and (A) MVM GLUT1, (B) BM GLUT1 (C) MVM IRβ, (D) BM IRβ, and (E) BM GLUT4 protein expression was measured by western blot (n = 6 per group). Data are shown as mean ± SEM. Significance was evaluated with a paired t test.
Figure 9.
Insulin stimulates Akt phosphorylation in term villous explants isolated from normal BMI women and women with obesity. Villous explants from term placentas were treated with and without insulin, and phosphorylation of (A) Akt (T308) and (B) Akt (S473) (P = 0.0003) and (C) total expression of Akt were measured by western blot (n = 12 to 14 per group). Data are shown as mean ± SEM. ***P < 0.001, paired t test.
Discussion
To our knowledge, this is the first study to show that the insulin-sensitive glucose transporter GLUT4 is exclusively expressed on the fetal-facing BM of the syncytiotrophoblast and that expression increases across gestation. In accordance with our hypothesis, we found that the IR is expressed predominantly in the maternal-facing MVM, with increasing expression across gestation. Our explant culture data suggest that maternal insulin promotes the transport of glucose across the placenta to the fetus by upregulating GLUT4 expression in the BM. These findings are consistent with the model that the human placenta contributes to postprandial insulin-mediated glucose disposal in the mother by promoting transplacental transport of glucose to the fetus. This represents a previously unknown mechanism by which maternal glucose is allocated for fetal growth, which may contribute to the positive correlation between maternal glucose levels and birth weight in women with normal glucose tolerance (3).
The GLUT4 transporter is primarily expressed in skeletal muscle and adipose tissue where insulin-stimulated glucose uptake is critical for rapid postprandial glucose disposal. In the absence of stimulation, GLUT4 is almost completely excluded from the plasma membrane in these tissues; however, insulin stimulation initiates rapid GLUT4 translocation from its intracellular location to the plasma membrane (46). Although previous studies indicate that insulin stimulates glucose transport in the term (34, 35) and first trimester placenta (19), numerous investigators have failed to detect GLUT4 in the human placenta (25, 26, 47). However, using immunohistochemistry, Xing et al. (23) reported that GLUT4 is expressed in intravillous stromal cells of human term placenta. Additionally, we have previously demonstrated that GLUT4 is present in perinuclear membranes in the cytosol of the syncytiotrophoblast in early pregnancy (19). In the current study we isolated the maternal-facing microvillous and fetal-facing basal syncytiotrophoblast plasma membranes across gestation in the human placenta and using two separate antibodies demonstrated that GLUT4 is expressed in the BM but not in the MVM. Additionally, BM GLUT4 protein expression was higher at term compared with early gestation, demonstrating that the expression of this transporter increases across gestation in parallel to the increased fetal nutrient demand to support the rapid growth of the fetus. BM GLUT4 expression may not have been identified by previous studies (25, 26, 47) because of a focus on expression in either whole placental homogenate or only in the MVM.
GLUT4 translocation to the plasma membrane is stimulated following insulin binding to the IR, which is a heterotetramer consisting of two α-subunits and two β-subunits, in the plasma membrane of target cells. These subunits have distinct functions: the extracellular α-subunit binds insulin, whereas the β-subunit possesses intrinsic tyrosine kinase activity. It has previously been reported that the placenta expresses the IR across gestation predominantly in the MVM (31–33, 48), suggesting that the IR interacts with maternal insulin. Functional IR activity has long been established in the MVM of human term placenta due to its role in activating amino acid transporters (37, 49). The results from the current study confirm these findings, with the IR found to be expressed predominantly in the MVM across gestation, with little expression in the BM. Using immunohistochemistry, Desoye et al. (29) reported that although expressed in the MVM in early pregnancy, in late gestation IR is mostly expressed in the fetal capillary endothelium with little expression in the MVM, leading to the proposal that fetal insulin, rather than maternal insulin, controls placental function in late gestation. Although our study did not investigate the expression of the IR in fetal vessels of the human placenta, our findings of 10-fold higher IR expression in the MVM as compared with the BM at term is at odds with the previous study and supports the concept that maternal insulin is likely to be more important than fetal insulin in regulating syncytiotrophoblast function across gestation, including at term. The discrepancy between the previous study and our results may be related to differences in the specificity of the antibodies used in the two studies and the fact that we determined IR expression in purified MVM isolated by a well-established and highly validated protocol.
Women with obesity are more likely to deliver an LGA or macrosomic infant, which is associated with increased risk of perinatal complications and linked to short- and long-term health problems in the offspring. Fetal growth is determined, in part, by transplacental nutrient transport, as reflected by increased expression and activity of placental nutrient transporters for glucose, amino acids, and fatty acids in women with obesity who give birth to LGA/macrosomic babies (14, 37, 50). These findings are in agreement with in vivo studies in a mouse model of diet-induced maternal obesity associated with fetal overgrowth, in which placental transport of glucose and amino acids is increased (51, 52). Our findings in the current study indicate that BM GLUT1 expression is increased in women with obesity delivering macrosomic infants, an observation in line with our previous report that BM GLUT1 expression is positively correlated with birth weight in nondiabetic mothers with obesity (14). These findings are in general agreement with the concept that BM GLUT1 expression and activity are the rate-limiting steps in transplacental glucose transfer (15, 22) and that an increased abundance of GLUT1 in the fetal-facing BM of the syncytiotrophoblast may contribute to increased glucose delivery to the fetus and contribute to fetal overgrowth. Conversely, our results demonstrate reduced expression of the GLUT1 transporter in the MVM and GLUT4 in the BM in women with obesity delivering large babies.
In muscle and adipose tissue insulin binds to the α-subunit of the IR, leading to autophosphorylation of IRβ-subunit tyrosine residues. The IR substrate family-1 (IRS-1) interacts with the phosphorylated IR, which facilitates IRS-1 phosphorylation, leading to the recruitment of effector molecules, such as PI3K. Activation of P13K leads to engagement of both PDK-1 and mTORC2 to phosphorylate both serine and threonine Akt and phosphorylation of the Akt substrate of 160 kDa (AS160/TBC1D4). It is known that PI3K signaling bifurcates, leading to activation of both Akt and actin remodeling, which involves activation of the Rho family G proteins. Insulin-induced actin remodeling implicated in GLUT4 translocation differs between muscle and adipose cells; in muscle cells Rac1 is activated, whereas in adipocytes TC10α activation is required (53). It is unknown whether the placenta utilizes insulin-stimulated actin remodeling in GLUT4 translocation and which Rho family G proteins are activated in this pathway.
As expected, insulin stimulation of placental villi resulted in phosphorylation of serine/threonine Akt in women with normal BMI. Similarly, using Akt phosphorylation as the functional readout, villous explants from pregnancies complicated by obesity responded to insulin, consistent with the possibility that the placenta retains normal insulin responsiveness in maternal obesity. However, insulin did not increase BM GLUT4 abundance in placentas from pregnancies complicated by maternal obesity, which may indicate defects in the insulin signaling pathway downstream of Akt or in phosphatidylinositol 3-kinase–mediated actin remodeling. The signaling events linking IR activation to GLUT4 trafficking to the syncytiotrophoblast fetal-facing BM are currently unknown and warrant further investigation.
In summary, we propose that maternal insulin stimulates placental glucose transport by promoting GLUT4 trafficking to the fetal-facing syncytiotrophoblast BM. These mechanisms may enhance glucose transfer to the fetus in response to postprandial hyperinsulinemia in women with normal BMI. Further studies are needed to investigate the insulin signaling pathways involved in GLUT4 translocation in both normal pregnancies and in pregnancy complications such as maternal obesity and/or GDM.
Acknowledgments
The authors thank Anna Vavra for her assistance.
Financial Support: This work was supported by National Institutes of Health Grant HD068370 and National Institutes of Health/National Center for Advancing Translational Sciences Colorado CTSA Grant UL1 TR002535. Contents are the authors’ sole responsibility and do not necessarily represent official National Institutes of Health views.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- AGA
appropriate for gestational age
- BM
basal plasma membrane
- BMI
body mass index
- GLUT
glucose transporter
- IR
insulin receptor
- LGA
large for gestational age
- MVM
microvillous plasma membrane
References and Notes
- 1. Gicquel C, Le Bouc Y.. Hormonal regulation of fetal growth. Horm Res. 2006;65(Suppl 3):28–33. [DOI] [PubMed] [Google Scholar]
- 2. Hay WW., Jr Recent observations on the regulation of fetal metabolism by glucose. J Physiol. 2006;572(Pt 1):17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, Hadden DR, McCance DR, Hod M, McIntyre HD, Oats JJ, Persson B, Rogers MS, Sacks DA; HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358(19):1991–2002. [DOI] [PubMed] [Google Scholar]
- 4. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009–2010. NCHS Data Brief. 2012;(82):1–8. [PubMed] [Google Scholar]
- 5. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Catalano PM, Ehrenberg HM. The short- and long-term implications of maternal obesity on the mother and her offspring. BJOG. 2006;113(10):1126–1133. [DOI] [PubMed] [Google Scholar]
- 7. Baeten JM, Bukusi EA, Lambe M. Pregnancy complications and outcomes among overweight and obese nulliparous women. Am J Public Health. 2001;91(3):436–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ehrenberg HM, Mercer BM, Catalano PM. The influence of obesity and diabetes on the prevalence of macrosomia. Am J Obstet Gynecol. 2004;191(3):964–968. [DOI] [PubMed] [Google Scholar]
- 9. Sewell MF, Huston-Presley L, Super DM, Catalano P. Increased neonatal fat mass, not lean body mass, is associated with maternal obesity. Am J Obstet Gynecol. 2006;195(4):1100–1103. [DOI] [PubMed] [Google Scholar]
- 10. Catalano PM, Presley L, Minium J, Hauguel-de Mouzon S. Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care. 2009;32(6):1076–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115(3):e290–e296. [DOI] [PubMed] [Google Scholar]
- 12. Cnattingius S, Villamor E, Lagerros YT, Wikstrom AK, Granath F. High birth weight and obesity—a vicious circle across generations. Int J Obes (Lond). 2012;36(10):1320–1324. [DOI] [PubMed] [Google Scholar]
- 13. Lawlor DA, Smith GD, O’Callaghan M, Alati R, Mamun AA, Williams GM, Najman JM. Epidemiologic evidence for the fetal overnutrition hypothesis: findings from the mater-university study of pregnancy and its outcomes. Am J Epidemiol. 2007;165(4):418–424. [DOI] [PubMed] [Google Scholar]
- 14. Acosta O, Ramirez VI, Lager S, Gaccioli F, Dudley DJ, Powell TL, Jansson T. Increased glucose and placental GLUT-1 in large infants of obese nondiabetic mothers. Am J Obstet Gynecol. 2015;212(2):227.e1–7. [DOI] [PubMed] [Google Scholar]
- 15. Jansson T, Wennergren M, Illsley NP. Glucose transporter protein expression in human placenta throughout gestation and in intrauterine growth retardation. J Clin Endocrinol Metab. 1993;77(6):1554–1562. [DOI] [PubMed] [Google Scholar]
- 16. Doege H, Schürmann A, Bahrenberg G, Brauers A, Joost HG. GLUT8, a novel member of the sugar transport facilitator family with glucose transport activity. J Biol Chem. 2000;275(21):16275–16280. [DOI] [PubMed] [Google Scholar]
- 17. Dawson PA, Mychaleckyj JC, Fossey SC, Mihic SJ, Craddock AL, Bowden DW. Sequence and functional analysis of GLUT10: a glucose transporter in the type 2 diabetes-linked region of chromosome 20q12-13.1. Mol Genet Metab. 2001;74(1–2):186–199. [DOI] [PubMed] [Google Scholar]
- 18. Gude NM, Stevenson JL, Rogers S, Best JD, Kalionis B, Huisman MA, Erwich JJ, Timmer A, King RG. GLUT12 expression in human placenta in first trimester and term. Placenta. 2003;24(5):566–570. [DOI] [PubMed] [Google Scholar]
- 19. Ericsson A, Hamark B, Powell TL, Jansson T. Glucose transporter isoform 4 is expressed in the syncytiotrophoblast of first trimester human placenta. Hum Reprod. 2005;20(2):521–530. [DOI] [PubMed] [Google Scholar]
- 20. Bibee KP, Illsley NP, Moley KH. Asymmetric syncytial expression of GLUT9 splice variants in human term placenta and alterations in diabetic pregnancies. Reprod Sci. 2011;18(1):20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brown K, Heller DS, Zamudio S, Illsley NP. Glucose transporter 3 (GLUT3) protein expression in human placenta across gestation. Placenta. 2011;32(12):1041–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vardhana PA, Illsley NP. Transepithelial glucose transport and metabolism in BeWo choriocarcinoma cells. Placenta. 2002;23(8–9):653–660. [DOI] [PubMed] [Google Scholar]
- 23. Xing AY, Challier JC, Lepercq J, Caüzac M, Charron MJ, Girard J, Hauguel-de Mouzon S. Unexpected expression of glucose transporter 4 in villous stromal cells of human placenta. J Clin Endocrinol Metab. 1998;83(11):4097–4101. [DOI] [PubMed] [Google Scholar]
- 24. Zhou J, Bondy CA. Placental glucose transporter gene expression and metabolism in the rat. J Clin Invest. 1993;91(3):845–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Barros LF, Yudilevich DL, Jarvis SM, Beaumont N, Baldwin SA. Quantitation and immunolocalization of glucose transporters in the human placenta. Placenta. 1995;16(7):623–633. [DOI] [PubMed] [Google Scholar]
- 26. Kainulainen H, Järvinen T, Heinonen PK. Placental glucose transporters in fetal intrauterine growth retardation and macrosomia. Gynecol Obstet Invest. 1997;44(2):89–92. [DOI] [PubMed] [Google Scholar]
- 27. Westermeier F, Sáez T, Arroyo P, Toledo F, Gutiérrez J, Sanhueza C, Pardo F, Leiva A, Sobrevia L. Insulin receptor isoforms: an integrated view focused on gestational diabetes mellitus. Diabetes Metab Res Rev. 2016;32(4):350–365. [DOI] [PubMed] [Google Scholar]
- 28. Mosthaf L, Grako K, Dull TJ, Coussens L, Ullrich A, McClain DA. Functionally distinct insulin receptors generated by tissue-specific alternative splicing. EMBO J. 1990;9(8):2409–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Desoye G, Hartmann M, Blaschitz A, Dohr G, Hahn T, Kohnen G, Kaufmann P. Insulin receptors in syncytiotrophoblast and fetal endothelium of human placenta. Immunohistochemical evidence for developmental changes in distribution pattern. Histochemistry. 1994;101(4):277–285. [DOI] [PubMed] [Google Scholar]
- 30. Desoye G, Hartmann M, Jones CJ, Wolf HJ, Kohnen G, Kosanke G, Kaufmann P. Location of insulin receptors in the placenta and its progenitor tissues. Microsc Res Tech. 1997;38(1–2):63–75. [DOI] [PubMed] [Google Scholar]
- 31. Tavaré JM, Holmes CH. Differential expression of the receptors for epidermal growth factor and insulin in the developing human placenta. Cell Signal. 1989;1(1):55–64. [DOI] [PubMed] [Google Scholar]
- 32. Whitsett JA, Lessard JL. Characteristics of the microvillus brush border of human placenta: insulin receptor localization in brush border membranes. Endocrinology. 1978;103(4):1458–1468. [DOI] [PubMed] [Google Scholar]
- 33. Nelson DM, Smith RM, Jarett L. Nonuniform distribution and grouping of insulin receptors on the surface of human placental syncytial trophoblast. Diabetes. 1978;27(5):530–538. [PubMed] [Google Scholar]
- 34. Kaur Anand R, Kanwar U, Nath Sanyal S. Characteristics of glucose transport across the microvillous membranes of human term placenta. Nutr Hosp. 2006;21(1):38–46. [PubMed] [Google Scholar]
- 35. Acevedo CG, Márquez JL, Rojas S, Bravo I. Insulin and nitric oxide stimulates glucose transport in human placenta. Life Sci. 2005;76(23):2643–2653. [DOI] [PubMed] [Google Scholar]
- 36. Ericsson A, Hamark B, Jansson N, Johansson BR, Powell TL, Jansson T. Hormonal regulation of glucose and system A amino acid transport in first trimester placental villous fragments. Am J Physiol Regul Integr Comp Physiol. 2005;288(3):R656–R662. [DOI] [PubMed] [Google Scholar]
- 37. Jansson N, Rosario FJ, Gaccioli F, Lager S, Jones HN, Roos S, Jansson T, Powell TL. Activation of placental mTOR signaling and amino acid transporters in obese women giving birth to large babies. J Clin Endocrinol Metab. 2013;98(1):105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jones HN, Jansson T, Powell TL. Full-length adiponectin attenuates insulin signaling and inhibits insulin-stimulated amino acid transport in human primary trophoblast cells. Diabetes. 2010;59(5):1161–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Karl PI, Alpy KL, Fisher SE. Amino acid transport by the cultured human placental trophoblast: effect of insulin on AIB transport. Am J Physiol. 1992;262(4 Pt 1):C834–C839. [DOI] [PubMed] [Google Scholar]
- 40. Illsley NP, Wang ZQ, Gray A, Sellers MC, Jacobs MM. Simultaneous preparation of paired, syncytial, microvillous and basal membranes from human placenta. Biochim Biophys Acta. 1990;1029(2):218–226. [DOI] [PubMed] [Google Scholar]
- 41. Jansson T, Ekstrand Y, Björn C, Wennergren M, Powell TL. Alterations in the activity of placental amino acid transporters in pregnancies complicated by diabetes. Diabetes. 2002;51(7):2214–2219. [DOI] [PubMed] [Google Scholar]
- 42. Bowers GN Jr, McComb RB. A continuous spectrophotometric method for measuring the activity of serum alkaline phosphatase. Clin Chem. 1966;12(2):70–89. [PubMed] [Google Scholar]
- 43. Oh SY, Hwang JR, Lee Y, Choi SJ, Kim JS, Kim JH, Sadovsky Y, Roh CR. Isolation of basal membrane proteins from BeWo cells and their expression in placentas from fetal growth-restricted pregnancies. Placenta. 2016;39:24–32. [DOI] [PubMed] [Google Scholar]
- 44. James-Allan LB, Arbet J, Teal SB, Powell TL, Jansson T. Data from: Insulin stimulates GLUT4 trafficking to the syncytiotrophoblast basal plasma membrane in the human placenta. figshare 2019. Deposited 26 April 2019. 10.6084/m9.figshare.8036117. [DOI] [PMC free article] [PubMed]
- 45. Jansson N, Greenwood SL, Johansson BR, Powell TL, Jansson T. Leptin stimulates the activity of the system A amino acid transporter in human placental villous fragments. J Clin Endocrinol Metab. 2003;88(3):1205–1211. [DOI] [PubMed] [Google Scholar]
- 46. Bryant NJ, Govers R, James DE. Regulated transport of the glucose transporter GLUT4. Nat Rev Mol Cell Biol. 2002;3(4):267–277. [DOI] [PubMed] [Google Scholar]
- 47. Hauguel-de Mouzon S, Leturque A, Alsat E, Loizeau M, Evain-Brion D, Girard J. Developmental expression of Glut1 glucose transporter and c-fos genes in human placental cells. Placenta. 1994;15(1):35–46. [DOI] [PubMed] [Google Scholar]
- 48. Whitsett JA, Johnson CL, Hawkins K. Differences in localization of insulin receptors and adenylate cyclase in the human placenta. Am J Obstet Gynecol. 1979;133(2):204–207. [DOI] [PubMed] [Google Scholar]
- 49. Roos S, Kanai Y, Prasad PD, Powell TL, Jansson T. Regulation of placental amino acid transporter activity by mammalian target of rapamycin. Am J Physiol Cell Physiol. 2009;296(1):C142–C150. [DOI] [PubMed] [Google Scholar]
- 50. Lager S, Ramirez VI, Gaccioli F, Jang B, Jansson T, Powell TL. Protein expression of fatty acid transporter 2 is polarized to the trophoblast basal plasma membrane and increased in placentas from overweight/obese women. Placenta. 2016;40:60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rosario FJ, Kanai Y, Powell TL, Jansson T. Increased placental nutrient transport in a novel mouse model of maternal obesity with fetal overgrowth. Obesity (Silver Spring). 2015;23(8):1663–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Aye IL, Rosario FJ, Powell TL, Jansson T. Adiponectin supplementation in pregnant mice prevents the adverse effects of maternal obesity on placental function and fetal growth. Proc Natl Acad Sci USA. 2015;112(41):12858–12863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. JeBailey L, Rudich A, Huang X, Di Ciano-Oliveira C, Kapus A, Klip A. Skeletal muscle cells and adipocytes differ in their reliance on TC10 and Rac for insulin-induced actin remodeling. Mol Endocrinol. 2004;18(2):359–372. [DOI] [PubMed] [Google Scholar]