Abstract
Objectives:
Published cost estimates for cystic fibrosis (CF) are based on older data and do not reflect increased use of specialty drugs in recent years. We assessed recent trends in healthcare expenditures for CF patients in the United States (US) with employer-sponsored health insurance.
Methods:
The study is a retrospective analysis of claims data for privately insured individuals aged 0–64 years who were continuously enrolled in non-capitated plans for at least 1 calendar year during 2010–2016. Mean annual expenditures during a calendar year were calculated for individuals who met a claims-based CF case definition. Average annual growth rates were calculated through linear regression of the natural logarithm of annual expenditures.
Results:
The annual CF prevalence was 1.1–1.4 per 10 000 adults and 2.9–3.0 per 10 000 children. Average spending adjusted for inflation nearly doubled from roughly $67 000 per patient in 2010 and 2011 to approximately $131 000 per patient in 2016. Inflation-adjusted spending on outpatient and inpatient care increased by 0.5% and 2.5% per year, respectively, whereas pharmaceutical spending increased by 20.2% per year. Virtually all of the growth in pharmaceutical spending was accounted for by spending on specialty drugs; inflation-adjusted spending on other medications increased by 1.3% per year. The annual growth rate in pharmaceutical spending rose by 33.1% during 2014–2016, the years during which lumacaftor/ivacaftor was introduced.
Conclusions:
Per-patient expenditures for privately-insured patients with CF almost doubled during 2010–2016; specialty drugs were largely responsible for this increase, with a major contribution from new, genotype-targeted CFTR modulator medications.
Keywords: cystic fibrosis, drugs, health expenditures, precision medicine, rare diseases
1 |. INTRODUCTION
Up-to-date estimates of healthcare expenditures for US patients with cystic fibrosis (CF) are lacking. Expenditures per patient with CF with enrolled in fee-for-service employer-sponsored insurance (ESI) plans during 2006 averaged $48 098, with a median of $30 508.1 Rising healthcare expenditures in the United States have been paced by spending on prescription medications, which in 2015 accounted for 17% of aggregate expenditures.2 Cost estimates used in economic evaluations of CF therapies should reflect up-to-date clinical practices.
Much pharmaceutical spending is on specialty drugs, defined in the US context as medications costing at least $1000 per prescription or per month.3 Specialty drugs include orphan drugs that target rare diseases such as CF, which affects about 35 000 people in the United States.4 Specialty drugs for CF include pulmonary medications that treat chronic infection, airway mucus obstruction, and inflammation, and pancreatic enzyme products to treat malabsorption. Until recently, pancreatic enzymes as nutritional supplements were unregulated in the United States. After the U.S. Food and Drug Administration (FDA) began to require approval of marketing of pancreatic enzyme products on the basis of evidence of clinical efficacy and safety, FDA approved six pancreatic enzyme products for CF during 2009–2012.5
CF has become a model system for precision medicine, which includes medications targeted to specific protein defects associated with individual mutations or genotypes.6 In particular, new genome-specific biologic medications, namely cystic fibrosis transmembrane conductance regulator (CFTR) modulator therapies that target defects in the CFTR protein caused by specific mutations in the CFTR gene, have been introduced. Ivacaftor (Kalydeco™) was first approved by FDA in January 2012 for patients aged ≥6 years with a G551D CFTR mutation, about 4–5% of CF patients.7,8 FDA subsequently expanded its approved use to include nine additional mutations (February 2014), patients ≥2 years old (March 2015) and, most recently, an additional 23 mutations (May 2017).9 Uptake of ivacaftor use following FDA approval was rapid, with roughly 80% of eligible patients initiating use in the first year.10 A combination drug, lumacaftor/ivacaftor (Orkambi™) was approved in July 2015 for patients aged ≥12 years with the most common CFTR genotype (delF508 homozygous),11 and the indication was expanded in 2016 to children aged 6–11 years. These two approved CFTR medications are an order of magnitude more expensive than other CF specialty drugs.11 No studies have reported the contribution of spending on these products to overall expenditures for insured patients with CF, although one modeling study was published in 2016.12 In this study, we describe current trends in health care expenditures for commercially insured patients with CF in the US.
2 |. METHODS
2.1 |. Data source
The IBM Watson Truven Health MarketScan® Commercial Database is a nationwide convenience sample of claims data from ESI plans. Numerous peer-reviewed articles have used those data, including two CF cost studies published in this journal.1,13 Participating plans, both capitated and non-capitated, are of two types; large, self-insured employers that contract with plans to administer payments, and health plans that sell insurance to smaller, fully-insured employers.
We accessed the MarketScan data via Treatment Pathways 4.0, an online analytic platform using a dynamic version of the data that is stored on IBM Watson servers and is regularly updated. Specifically, on December 31, 2017, we accessed the 100% Treatment Pathways sample of data from January 1, 2010 through July 31, 2017. Treatment Pathways is restricted to plans with prescription drug coverage. Those plans include roughly 75% of all MarketScan enrollees. Treatment Pathways combines data from the MarketScan Commercial and Medicare Supplemental databases. MarketScan data are deidentified, and their analysis is not classified by the Centers for Disease Control and Prevention as human subjects research. Truven Health Analytics, which is an IBM Company, maintains data validity and integrity.
2.2 |. Data analysis
We analyzed data on patients with CF who were continuously enrolled in non-capitated plans for at least 1 calendar year during 2010–2016 and were aged 0–64 years at the beginning of a calendar year. We identified patients with CF using an algorithm that required either one inpatient claim with an International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis code of 277.0× or International Classification of Disease, Tenth Revision, Clinical Modification (ICD-10-CM) code of E84x (Supplemental Table S1) or two outpatient claims with the same set of codes at least 30 days apart between January 1, 2010 and July 31, 2017.
Expenditures were calculated by calendar year from 2010 through 2016 and restricted to patients with continuous enrollment during the calendar year. Patients were classified as pediatric if they were reported to be aged ≤17 years at the beginning of the calendar year and adults were those aged 18–64 years.
Annual mean and median expenditures were calculated for all health care services, with or without a CF code, and mean expenditures were calculated separately for inpatient services, outpatient services, and outpatient pharmaceuticals. Prescription expenditures were further stratified into four groups: ivacaftor, lumacaftor/ivacaftor, other CF-specific specialty drugs (pulmonary medications and pancreatic enzyme products), and all other pharmaceuticals (Supplemental Table S2). Outpatient expenditures were classified in this analysis into outpatient encounters, emergency room (with discharge home), laboratory tests, and durable medical equipment for respiratory support.
Per-person mean current calendar-year expenditures were calculated and graphed. Average annual growth rates were calculated through linear regression of the natural logarithm of annual mean or median expenditures on calendar year for specified time periods; the regression coefficient expresses the average exponential increase for that period.14 Growth rates were calculated for the entire 2010–016 period and for sub-periods determined with regard to inflection points ascertained through visual inspection of the data.
In addition to current-year expenditures, mean and median expenditures were calculated in constant-year terms (expressed in 2016 US dollars) through two different methods of inflation adjustment. First, the total personal consumption expenditures (PCE) index of the US Bureau of Economic Analysis was used to adjust expenditures from different years to overall purchasing power. Second, the PCE healthcare index by function was used to adjust expenditures for medical inflation. The latter measure is an unbiased measure of medical inflation, whereas the commonly used medical care component of the Consumer Price Index has consistently overstated the rate of overall medical inflation.15
3 |. RESULTS
3.1 |. Population characteristics
Numbers of unique MarketScan enrollees ≤64 years with continuous enrollment in non-capitated plans with prescription drug coverage during a calendar year peaked at 25.8 million in 2012 and declined to 15.6 million in 2016. In each year, 24–26% of the sample were aged ≤17 years. Most of the decline after 2012 was in the category of fully-insured health plans sponsored by smaller employers. Numbers enrolled in large, self-insured employer plans during a calendar year was 14.6 million (57% of total enrollment) in 2012 and 12.3 million (79% of total enrollment) in 2016.
The number of unique individuals in the database with continuous enrollment in non-capitated plans by calendar year varied from 18 million to 28 million. The number of patients within each year’s defined cohort who had ≥2 outpatient claims or ≥1 inpatient claim associated with CF within the calendar year varied from 2745 to 4291. The ratio of the two numbers is the administrative prevalence of CF in the subsample of persons continuously-enrolled in non-capitated plans. The annual administrative prevalence was 1.1–1.4 per 10 000 working-age adults and 2.8–3.0 per 10 000 children each year from 2010 through 2016 (Table 1). The administrative prevalence in children was similar to the birth prevalence of CF in the United States, estimated at 2.9 per 10 000 births.4 The lower frequency of CF in adults in this sample is a product of both excess mortality and sample selection for relatively healthy adults with ESI.
TABLE 1.
Cystic fibrosis prevalence per 10 000 enrollees ages 0–17 and 18–64 years per calendar year, 2010–2016
| Age group | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 |
|---|---|---|---|---|---|---|---|
| Children ages 0–17 | 2.9 | 3.0 | 2.9 | 2.9 | 2.9 | 2.9 | 2.8 |
| Adults ages 18–64 | 1.1 | 1.2 | 1.3 | 1.4 | 1.3 | 1.3 | 1.4 |
3.2 |. Overall expenditures
Spending per patient at current prices grew rapidly between 2010 and 2016. Median expenditures increased from $32 586 in 2010 to $67 760 in 2016, more than doubling in a period of 5 years (Table 2). Similarly mean expenditures more than doubled at current prices, from $61 591 to $130 879. Expenditures were lower for pediatric patients, for example, median $62 014 and mean $116 171 in 2016, than for adult patients, median $72 846 and mean $140 564.
TABLE 2.
Mean expenditures (current US dollars) per person with cystic fibrosis (CF) per year, 2010–2016
| All CF patients <65 years | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 |
|---|---|---|---|---|---|---|---|
| Median total payment | 32 586 | 35 977 | 38 660 | 41 897 | 46 596 | 56 190 | 67 760 |
| Mean total payment | 61 591 | 62 424 | 71 010 | 73 166 | 86 662 | 100 875 | 130 879 |
| Inpatient | 24 626 | 23 140 | 24 716 | 21 776 | 28 824 | 25 617 | 29 482 |
| Outpatient | 14 898 | 15 237 | 15 615 | 15 077 | 16 316 | 16 449 | 17 557 |
| Pharmacy | 22 067 | 24 047 | 30 679 | 36 313 | 41 522 | 58 760 | 83 839 |
| Ivacaftor | - | - | 4586 | 6237 | 7585 | 11 197 | 13 921 |
| Lumacaftor/Ivacaftor | - | - | - | - | - | 10 919 | 28 331 |
| Specialty drugs | 15 774 | 18 845 | 20 892 | 24 669 | 27 484 | 29 962 | 35 043 |
| Pancreatic enzymes | 3510 | 5219 | 6004 | 7027 | 8392 | 9738 | 11 836 |
| Pulmonary | 12 264 | 13 626 | 14 889 | 17 641 | 19 354 | 20 211 | 23 206 |
| Other medications | 6293 | 5202 | 5201 | 5407 | 6453 | 6681 | 6545 |
| Pediatric CF patients | |||||||
| Median total payment | 33 267 | 35 020 | 37 756 | 40 915 | 44 099 | 52 109 | 62 014 |
| Total mean payment | 58 212 | 56 079 | 62 614 | 68 088 | 75 150 | 87 336 | 116 171 |
| Inpatient | 22 686 | 19 280 | 18 669 | 18 697 | 22 916 | 19 231 | 22 704 |
| Outpatient | 12 649 | 12 509 | 12 735 | 12 920 | 12 627 | 13 092 | 14 006 |
| Pharmacy | 22 877 | 24 290 | 31 210 | 36 472 | 39 607 | 55 013 | 79 461 |
| Ivacaftor | - | - | 5170 | 6138 | 6789 | 10 889 | 14 108 |
| Lumacaftor/Ivacaftor | - | - | - | - | - | 9656 | 27 570 |
| Specialty drugs | 18 141 | 20 749 | 22 569 | 26 801 | 28 932 | 30 404 | 33 948 |
| Pancreatic enzymes | 4168 | 5701 | 6368 | 7403 | 8503 | 9903 | 10 883 |
| Pulmonary | 13 973 | 15 049 | 16 201 | 19 398 | 20 429 | 20 468 | 23 065 |
| Other medications | 4737 | 3540 | 3472 | 3533 | 3886 | 4064 | 3835 |
| Adult CF patients | |||||||
| Median total payment | 32 080 | 36 718 | 39 484 | 42 643 | 48 326 | 62 449 | 72 846 |
| Total mean payment | 64 577 | 67 536 | 77 455 | 76 624 | 94 544 | 110 160 | 140 564 |
| Inpatient | 26 341 | 26 250 | 29 358 | 23 873 | 32 868 | 29 996 | 33 946 |
| Outpatient | 16 886 | 17 434 | 17 825 | 16 546 | 18 842 | 18 835 | 19 895 |
| Pharmacy | 21 350 | 23 852 | 30 272 | 36 205 | 42 834 | 61 329 | 86 722 |
| Ivacaftor | - | - | 4138 | 6305 | 8130 | 11 409 | 13 798 |
| Lumacaftor/Ivacaftor | - | - | - | - | - | 11 785 | 28 832 |
| Specialty drugs | 13 682 | 17 311 | 19 606 | 23 216 | 26 493 | 29 660 | 35 763 |
| Pancreatic enzymes | 2929 | 4832 | 5724 | 6772 | 8316 | 9625 | 12 464 |
| Pulmonary | 10 753 | 12 479 | 13 882 | 16 445 | 18 617 | 20 035 | 23 300 |
| Other medications | 7668 | 6541 | 6528 | 6684 | 8211 | 8475 | 8329 |
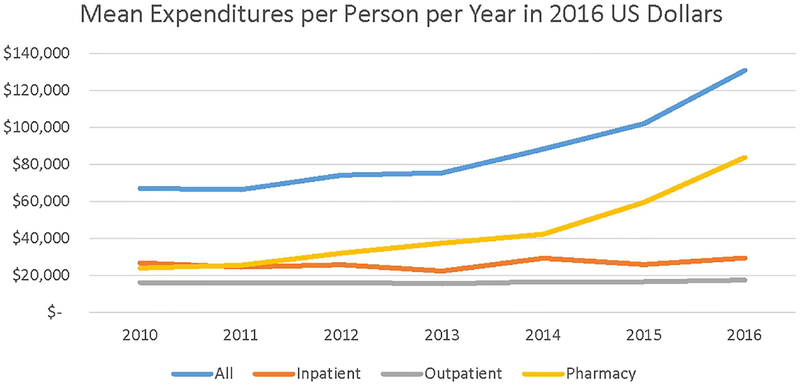
Mean expenditures began to increase substantially in 2012 and even more rapidly beginning in 2015 (Figure 1). The annual exponential growth in mean expenditures during 2010–2016 was 12.2% in current dollars, 10.8% relative to general prices, and 10.6% relative to medical prices. The exponential growth rates in median expenditures were slightly lower, 11.7%, 10.3%, and 10.2%, respectively.
FIGURE 1.
Mean Expenditures in 2016 US Dollars per Person with Cystic Fibrosis in MarketScan Commercial Data, 2010–2016. Expenditures are adjusted for inflation using the Personal Consumption Expenditures index, Bureau of Economic Analysis
Changes over time in per-patient expenditures were similar for self-insured and fully-insured plans. The ratio of mean expenditures between fully-insured and self-insured plans was 1.00 in 2010 and 0.96 in 2016 and fluctuated in the range 0.86–1.05. Greater variability occurred in the ratios of mean expenditures by age group across years, 0.89–1.15 for the pediatric sample and 0.79–1.10 for the adult sample.
Per-person spending on inpatient care and non-pharmaceutical outpatient care grew relatively modestly. From 2010 to 2016, inpatient spending increased in current dollars by 3.2% per year and outpatient spending by 2.5% per year. The average changes in constant-dollar per-person spending were 1.8% per year for inpatient services and 1.1% per year for outpatient non-pharmaceutical services relative to general consumer prices (Table 3) and by 1.7% and 1.0% per year relative to medical prices.
TABLE 3.
The natural logarithm of mean expenditures in 2016 US dollars per person per year, 2010–2016, and the average annual growth rates (ie, exponentiated coefficients from regressions of the logarithm of mean expenditures on calendar year), for total payments and by type of payment
| All CF patients | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | Increase per year 2010–2016a |
|---|---|---|---|---|---|---|---|---|
| Total payment | 11.11 | 11.10 | 11.21 | 11.23 | 11.39 | 11.53 | 11.78 | 10.8% |
| Inpatient | 10.20 | 10.11 | 10.16 | 10.02 | 10.29 | 10.16 | 10.29 | 1.8% |
| Outpatient | 9.70 | 9.69 | 9.69 | 9.65 | 9.72 | 9.72 | 9.79 | 1.1% |
| Pharmacy | 10.09 | 10.15 | 10.37 | 10.53 | 10.65 | 10.99 | 11.34 | 20.4% |
| Ivacaftor | 8.47 | 8.77 | 8.95 | 9.34 | 9.54 | |||
| Lumacaftor/Ivacaftor | 9.31 | 10.25 | ||||||
| Pancreatic enzymes | 8.25 | 8.62 | 8.74 | 8.89 | 9.05 | 9.20 | 9.38 | 17.3% |
| Pulmonary | 9.50 | 9.58 | 9.65 | 9.81 | 9.89 | 9.93 | 10.05 | 9.2% |
| Other medications | 8.83 | 8.62 | 8.60 | 8.63 | 8.79 | 8.82 | 8.79 | 1.6% |
Adjusted for inflation using the Personal Consumption Expenditures index, Bureau of Economic Analysis.
3.3 |. Pharmaceutical expenditures
Most of the growth in per-person expenditures was in the category of outpatient pharmaceuticals, which grew by 21.8% per year in current dollars and by 20.2–20.4% per year in constant dollars. The share of pharmaceuticals in total spending increased from 35.8% in 2010 to 64.1% in 2016. The pharmaceutical share of spending was highest for children and adolescents, 39.3% in 2010 and 68.4% in 2016. The increase in pharmaceutical spending was not steady over time. During 2011–2014 the annual growth rate in inflation-adjusted prescription payments was 16.5–16.7%; that rate more than doubled to 33.7–34.2% during 2014–2016.
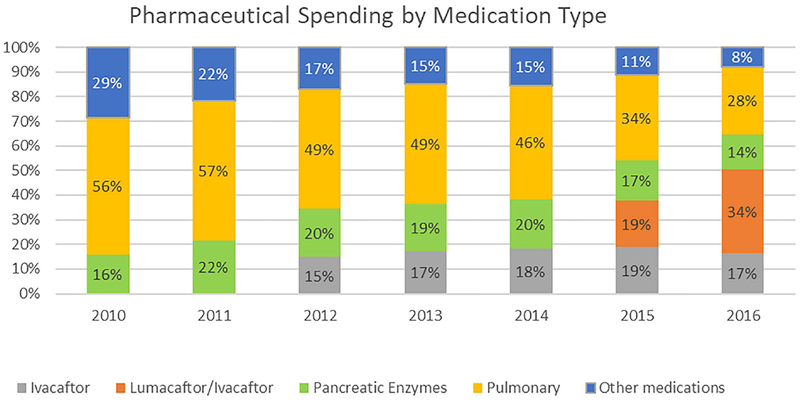
Changes in spending by type of medication for the whole sample are shown in Figure 2. Virtually all of the growth in pharmaceutical spending was accounted for by growth in spending on specialty drugs, including the new CFTR modulators. Spending on all other medications grew by just 1.6% per year in real terms, with the share of drug spending accounted for by non-specialty medications falling from 29% in 2010 to 8% in 2016.
FIGURE 2.
Distribution of Pharmacy Prescription Expenditures in 2016 US Dollars by Medication Type per Person with Cystic Fibrosis in MarketScan Commercial data, 2010–2016. Pharmaceutical spending by medication type in marketscan commercial data, 2010–2016
Ivacaftor accounted for 15–19% of drug spending each year from 2012 through 2016. Among the 5.8% of CF patients who took ivacaftor in 2016, the drug accounted for 85% of pharmaceutical spending. Lumacaftor/ivacaftor accounted for 19% of all pharmaceutical spending for the CF population in 2015 and 34% in 2016. In 2016, lumacaftor/ivacaftor was taken by 17.6% of CF patients in this sample, and it accounted for 74% of their total pharmaceutical spending. Excluding spending on CFTR modulators, the average annual growth in inflation-adjusted spending per privately insured patient with CF was 4.8%, compared with 10.8% annual growth in overall inflation-adjusted spending.
Growth in spending on other specialty drugs, both pulmonary and pancreatic, was also rapid. Inflation-adjusted spending on pancreatic enzyme and pulmonary therapies grew by 17.1–17.3% and 9.1–9.2% per year, respectively. Because growth in spending on CFTR modulators was even more rapid, the share of other specialty drugs fell from 72% of pharmaceutical spending in 2010 (56% for pulmonary drugs and 16% for pancreatic enzymes) to 42% in 2016 (28% for pulmonary and 14% for pancreatic enzymes).
The increases in per-patient spending on pancreatic enzyme and pulmonary products were largely accounted for by increased spending per filled prescription, which grew in inflation-adjusted terms by 12.8% and 7.4% per year. There were modest increases in the proportions of patients using pancreatic enzymes or pulmonary medications, from 54% and 55% in 2010 to 62% and 60% in 2016, respectively and also modest increases in the number of filled prescriptions per user. For pancreatic enzymes, there was a one-time jump from 4.0 fills per user in 2010 to 5.1 in 2011.
4 |. DISCUSSION
Reported per-patient mean expenditures for privately-insured patients with CF enrolled in non-capitated employer-sponsored plans in the United States almost doubled in inflation-adjusted dollars during the study years, from $67 127 in 2010 to $130 879 in 2016. The comparable figure for 2006 per-person mean expenditures 1 in 2016 dollars adjusted for general price inflation was $56 252. Growth in median expenditures was similarly rapid, from $35 515 in 2010 to $67 760 in 2016. The mean expenditure for the patient population is appropriate for assessing aggregate expenditures, whereas the median is useful for characterizing costs for typical patients.1
The remarkably rapid growth in per-person expenditures in recent years for the privately insured US population living with CF calls into question the continued use of published cost estimates derived from time periods when new therapies were either unavailable or were less commonly used. Simply adjusting estimates from previous years for inflation is not sufficient; current treatment costs for this population requires up-to-date real-world data on expenditures. The present study only applies to the privately insured population with ESI enrolled in non-capitated plans, who comprise about 85% of the MarketScan Commercial sample. The key limitation of the study is that it would not be valid to extrapolate from trends in expenditures for privately insured patients with CF to healthcare costs for the overall population with CF. Since public payer reimbursements are substantially lower,16 a similar analysis of healthcare expenditures for publicly insured patients with CF would also provide important information.
Most of the increase in per-person expenditures for privately insured patients with CF since 2010 was accounted for by increased spending on CF-specific specialty drugs (CFTR modulators, pulmonary medications, and pancreatic enzyme products). Excluding such products, inpatient and outpatient spending rose by 1–2% per year, respectively, relative to inflation. Pharmaceutical spending rose from one-third of all spending on CF care during 20061 and 36% in 2010 to 64% in 2016. In particular, the CFTR modulators, although taken by a subset of patients, dominated overall growth in spending after 2014. During 2016, CFTR modulators were taken by almost one-fourth of privately insured patients with CF and accounted for over one-half of all reported drug spending for the CF patient population with ESI. More than one-half of all growth between 2010 and 2016 in inflation-adjusted spending for privately insured patients with CF was accounted for by spending on CFTR modulators.
The role of specialty drugs as cost drivers in overall healthcare spending has attracted growing attention from stakeholders including insurers, self-insured employers, pharmacy benefit managers, and public policymakers.3,17 In 2015, specialty drugs accounted for almost 40% of drug spending in the United States, and is expected to reach 50% by 2018.17 The increase in spending has ramifications for insurance benefit design, drug formularies, and coverage criteria. The high cost of treatments could also increase out-of-pocket spending for patients, which have implications for adherence to therapies and treatment decision-making.
Despite the notable increase in specialty drug spending for people with CF, it is important to consider the improvements in health outcomes that occurred during this period. Life expectancy continues to rise with the introduction of new therapies and advances in care delivery. Over the past decade, the CF Foundation has issued peer-reviewed, evidence-based guidelines for chronic respiratory medications that support early use of CF-specific specialty medications.18 Long-term use of specialty drugs, such as inhaled tobramycin and dornase alfa, has been linked to decreased mortality.19 In addition, CF care centers adopting more aggressive monitoring in their patient populations have been shown to have better overall health outcomes.20 Over this period, therefore, treatment complexity in CF has increased in both pediatric and adult patient populations.21 Notably, a recent registry-based comparison of CF health outcomes found that children and young adults in the United States had better lung function than those in the United Kingdom, and that specialty drugs, such as dornase alfa, were much more commonly prescribed in the United States.22 All of these factors that have improved health likely also contributed to the rise in pharmaceutical expenditures observed in this analysis.
Breakthrough drugs, such as ivacaftor, represent both the greatest promise for dramatic improvement in CF health outcomes and the largest impact on healthcare spending. Both ivacaftor and lumacaftor/ivacaftor have proven efficacy in clinical trials, although the magnitude of clinical benefit in eligible populations in terms of improved lung function is greater for ivacaftor monotherapy than for lumacaftor/ivacaftor.8,23 Effectiveness studies using real-world patient registry data have shown sustained benefits of ivacaftor in terms of slowing disease progression and improving nutritional status.24 An analysis of MarketScan claims data found a two-thirds reduction in inpatient admissions with a CF diagnosis among patients who initiated ivacaftor therapy during 2012.25
Currently up to one-half of the CF population is eligible for ivacaftor or lumacaftor/ivacaftor. Additional modulators are in clinical development and, if they are found to be efficacious, will be made available to patients with a broader array of genotypes. The long-term impact of the therapies—physiological, healthcare use, survival, and health-related quality of life—is still unknown. Ongoing evaluation of these therapies is needed to ensure that increased spending on them is associated with optimal outcomes (i.e., the value proposition).
Researchers are expected to provide information on costs and outcomes to payers and other stakeholders so that decision makers can determine which clinical services provide good value.26 The UK National Health Service (NHS) in 2012 commissioned a health technology assessment of ivacaftor.27 The incremental cost-effectiveness ratio (ICER) was calculated to be 10–15 times higher than the threshold range used, but within the range of other “ultra-orphan” medicines covered by the NHS.28 On that basis, ivacaftor was approved for coverage.
Like any study using administrative healthcare data, this study has limitations.29 One is the restriction to a convenience sample of private insurance plans sponsored by employers. The MarketScan Commercial data have been found to be comparable in demographics to the US population with ESI,30 which in turn comprises >90% of the US population with private insurance.31 It has been reported that 56% of a representative US sample of children with CF had private insurance.32 MarketScan claims data have previously been analyzed for information on expenditures for privately-insured patients with CF.1,13 The study findings, however, cannot be extrapolated to people with private plans that do not include prescription drugs.
A second limitation is the use of ICD diagnosis codes to identify persons with CF, since ICD codes are subject to multiple types of errors.33 Consequently, some people without CF might have been incorrectly coded as having CF. An algorithm of ≥2 outpatient claims on separate dates or ≥1 inpatient claim is commonly used in health services research to minimize coding errors.34,35 Conversely, some insured persons with CF who were mildly affected and had few CF-specific care episodes might not have met the case algorithm. On the other hand, the administrative prevalence of CF in the pediatric population in this study is consistent with the estimated birth prevalence of CF in the United States.4 A medical record review of children with billing codes for conditions at high risk of influenza (eg, asthma) found a sensitivity of 72% and specificity of 95%; the one case of CF was correctly coded.36
Third, and most seriously, information on health care use and expenditures is incomplete. For example, if patients obtain drugs through coupons from manufacturers and do not file a claim for reimbursement we have no record of those prescriptions. Most critically, rebates that health plans and pharmacy benefit managers receive from drug manufacturers at the end of the year can result in overstatement of net expenditures on drugs reported in payments recorded to pharmacies.29,37 Overall, net revenues of manufacturers after rebates and discounts in 2016 were 28.2% less than gross revenues.37,38 Not only do private plan expenditures on prescription medications appear overstated using administrative claims data, but the overstatement may be growing over time. From 2015 to 2016, aggregate national spending on medications calculated using invoice prices rose by 5.8% but after taking into account manufacturer rebates and other discounts it rose by just 4.8%.38
We did not censor patients who received lung transplants. In 2016, 2% of continuously enrolled patients (n = 58) had received a lung transplant at some point during 2010–2016. Their mean and median expenditures during 2016 were $667 012 and $515 750, respectively. Excluding those patients reduced mean expenditures for the remaining patients by 5%.
5 |. CONCLUSIONS
Rapid growth in private expenditures for CF in recent years appears to be largely due to increases in specialty drug spending, particularly as new high-priced precision medicine therapies are being introduced. Expenditures on the treatment of CF in the United States, as assessed through administrative private insurance claims, is increasingly dominated by specialty drugs. Ongoing evaluation of the benefits of existing and new therapies, as well as how they affect overall healthcare use, is needed to ensure patients receive high-quality, high-value care. A challenge for researchers, though, is a lack of accurate information as to how much payers are actually spending on CF medications after taking into account discounts and rebates.
Supplementary Material
Footnotes
Publisher's Disclaimer: DISCLAIMER
Publisher's Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. The authors have no financial support to report for the preparation of this manuscript.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Ouyang L, Grosse SD, Amendah DD, Schechter MS. Healthcare expenditures for privately insured people with cystic fibrosis. Pediatr Pulmonol. 2009;44:989–996. [DOI] [PubMed] [Google Scholar]
- 2.Observations on trends in prescription drug spending In: health and human services. Office of The Assistant Secretary for Planning and Evaluation, editor, ASPE Issue Brief 2016. [Google Scholar]
- 3.Johnson SG, Sims KA. Optimizing specialty drug use. Pharmacotherapy 2017. [DOI] [PubMed] [Google Scholar]
- 4.Knapp EA, Fink AK, Goss CH, et al. The Cystic Fibrosis Foundation Patient Registry. Design and methods of a national observational disease registry. Ann Am Thorac Soc. 2016;13:1173–1179. [DOI] [PubMed] [Google Scholar]
- 5.U.S. Food and Drug Administration. Updated Questions and Answers for Healthcare Professionals and the Public: use an Approved Pancreatic Enzyme Product (PEP). Volume 2017.
- 6.Martiniano SL, Sagel SD, Zemanick ET. Cystic fibrosis: a model system for precision medicine. Curr Opin Pediatr. 2016;28:312–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis PB, Yasothan U, Kirkpatrick P. Ivacaftor. Nat Rev Drug Discov. 2012;11:349. [DOI] [PubMed] [Google Scholar]
- 8.Ramsey BW, Davies J, McElvaney NG, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365:1663–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.U.S. Food and Drug Administration. FDA expands approved use of Kalydeco to treat additional mutations of cystic fibrosis Volume 2017.
- 10.Sawicki GS, Dasenbrook E, Fink AK, Schechter MS. Rate of uptake of ivacaftor use after U.S. Food and Drug Administration approval among patients enrolled in the U.S. Cystic Fibrosis Foundation Patient Registry. Ann Am Thorac Soc. 2015;12:1146–1152. [DOI] [PubMed] [Google Scholar]
- 11.Mayer M. Lumacaftor-ivacaftor (Orkambi) for cystic fibrosis: behind the ‘breakthrough’. Evid Based Med. 2016;21:83–86. [DOI] [PubMed] [Google Scholar]
- 12.Dilokthornsakul P, Hansen RN, Campbell JD. Forecasting US ivacaftor outcomes and cost in cystic fibrosis patients with the G551D mutation. Eur Respir J. 2016;47:1697–1705. [DOI] [PubMed] [Google Scholar]
- 13.Briesacher BA, Quittner AL, Fouayzi H, Zhang J, Swensen A. Nationwide trends in the medical care costs of privately insured patients with cystic fibrosis (CF), 2001–2007. Pediatr Pulmonol. 2011;46:770–776. [DOI] [PubMed] [Google Scholar]
- 14.Feng LB, Pines JM, Yusuf HR, Grosse SD. U.S. trends in computed tomography use and diagnoses in emergency department visits by patients with symptoms suggestive of pulmonary embolism, 2001–2009. Acad Emerg Med. 2013;20:1033–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunn A, Grosse SD, Zuvekas S. Adjusting health expenditures for inflation: a review of measures for health services research in the United States. Health Serv Res. 2018;53:175–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selden TM, Karaca Z, Keenan P, White C, Kronick R. The growing difference between public and private payment rates for inpatient hospital care. Health affairs (Project Hope). 2015;34:2147–2150. [DOI] [PubMed] [Google Scholar]
- 17.National Business Group on Health. Policy Recommendations to Promote Sustainable, Affordable Pricing for Specialty Pharmaceuticals 2017.
- 18.Mogayzel PJ Jr., Naureckas ET, Robinson KA, et al. Cystic fibrosis pulmonary guidelines. Chronic medications for maintenance of lung health. Am J Respir Crit Care Med. 2013;187:680–689. [DOI] [PubMed] [Google Scholar]
- 19.Sawicki GS, Signorovitch JE, Zhang J, et al. Reduced mortality in cystic fibrosis patients treated with tobramycin inhalation solution. Pediatr Pulmonol. 2012;47:44–52. [DOI] [PubMed] [Google Scholar]
- 20.Johnson C, Butler SM, Konstan MW, Morgan W, Wohl ME. Factors influencing outcomes in cystic fibrosis: a center-based analysis. Chest. 2003;123: 20–27. [DOI] [PubMed] [Google Scholar]
- 21.Sawicki GS, Ren CL, Konstan MW, et al. Treatment complexity in cystic fibrosis: trends over time and associations with site-specific outcomes. J Cyst Fibros. 2013;12:461–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goss CH, MacNeill SJ, Quinton HB, et al. Children and young adults with CF in the USA have better lung function compared with the UK. Thorax. 2015;70:229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wainwright CE, Elborn JS, Ramsey BW, et al. Lumacaftor-ivacaftor in patients with cystic fbrosis homozygous for Phe508del CFTR. N Engl J Med. 2015;373:220–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sawicki GS, McKone EF, Pasta DJ, et al. Sustained benefit from ivacaftor demonstrated by combining clinical trial and cystic fibrosis patient registry data. Am J Respir Crit Care Med. 2015; 192:836–842. [DOI] [PubMed] [Google Scholar]
- 25.Suthoff ED, Bonafede M, Limone B, O’Callaghan L, Sawicki GS, Wagener JS. Healthcare resource utilization associated with ivacaftor use in patients with cystic fibrosis. J Med Econ. 2016;19:845–851. [DOI] [PubMed] [Google Scholar]
- 26.Neumann PJ, Cohen JT. ICER’s revised value assessment framework for 2017–2019: a critique. Pharmacoeconomics 2017. [DOI] [PubMed] [Google Scholar]
- 27.Whiting P, Al M, Burgers L, et al. Ivacaftor for the treatment of patients with cystic fibrosis and the G551D mutation: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2014;18:1–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.NHS Commissioning Board. Clinical commissioning policy: ivacaftor for cystic fibrosis. 2013.
- 29.Motheral B, Brooks J, Clark MA, et al. A checklist for retrospective database studies-report of the ISPOR Task Force on Retrospective Databases. Value Health. 2003;6:90–97. [DOI] [PubMed] [Google Scholar]
- 30.Aizcorbe A, Liebman E, Pack S, Cutler DM, Chernew ME, Rosen AB. Measuring health care costs of individuals with employer-sponsored health insurance in the U.S.: a comparison of survey and claims data. Stat J IAOS. 2012;28:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Center for Health Statistics; Centers for Disease Control and Prevention. Health, United States, 2015. Hyattsville, MD: 2016. [Google Scholar]
- 32.Schechter MS, McColley SA, Silva S, et al. Association of socioeconomic status with the use of chronic therapies and healthcare utilization in children with cystic fibrosis. J Pediatr. 2009;155:634–639 e631–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Malley KJ, Cook KF, Price MD, Wildes KR, Hurdle JF, Ashton CM. Measuring diagnoses: ICD code accuracy. Health Serv Res 2005;40: 1620–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCullough PA, Philbin EF, Spertus JA, Kaatz S, Sandberg KR, Weaver WD. Confirmation of a heart failure epidemic: findings from the Resource Utilization Among Congestive Heart Failure (REACH) study. J Am Coll Cardiol. 2002;39:60–69. [DOI] [PubMed] [Google Scholar]
- 35.Grosse SD, Boulet SL, Amendah DD, Oyeku SO. Administrative data sets and health services research on hemoglobinopathies: a review of the literature. Am J Prev Med. 2010;38:S557–S567. [DOI] [PubMed] [Google Scholar]
- 36.Daley MF, Barrow J, Pearson K, et al. Identification and recall of children with chronic medical conditions for influenza vaccination. Pediatrics. 2004;113:e26–33. [DOI] [PubMed] [Google Scholar]
- 37.Augustine NR, Madhavan G, Nass SJ. Making Medicines Affordable: A National Imperative: National Academies Press; 2017. [PubMed] [Google Scholar]
- 38.IQVIA Institute. Medicines Use and Spending in the U.S. A Review of 2016 and Outlook to 2021. 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.