Abstract
Preservation of glomerular structure and function is pivotal in the prevention of glomerulonephritis, a category of kidney disease characterized by proteinuria which can eventually lead to chronic and end-stage renal disease. The glomerulus is a complex apparatus responsible for the filtration of plasma from the body. In disease, structural integrity is lost and allows for the abnormal leakage of plasma contents into the urine. A method to isolate and examine glomeruli in culture is critical for the study of these diseases. In this protocol, an efficient method of retrieving intact glomeruli from adult rat kidneys while conserving structural and morphological characteristics is described. This process is capable of generating high yields of glomeruli per kidney with minimal contamination from other nephron segments. With these glomeruli, injury conditions can be mimicked by incubating them with a variety of chemical toxins, including protamine sulfate, which causes foot process effacement and proteinuria in animal models. Degree of injury can be assessed using transmission electron microscopy, immunofluorescence staining, and western blotting. Nephrin and Wilms Tumor 1 (WT1) levels can also be assessed from these cultures. Due to the ease and flexibility of this protocol, the isolated glomeruli can be utilized as described or in a way that best suits the needs of the researcher to help better study glomerular health and structure in diseased states.
Keywords: Medicine, Issue 141, Kidney, Foot Process Effacement, Glomerular Injury, Proteinuria, Glomerular Permeability, Protamine Sulfate, Renal Pathology, Glomerular Disease
Introduction
The glomerulus is a highly specialized tuft of capillaries responsible for the filtration of circulating plasma. It forms the beginning of the nephron, which is the basic functional unit of the kidney. Glomerular function is defined by a uniquely fenestrated capillary endothelium, the slit diaphragm of podocytes, and an intervening basement membrane. These layers form a semipermeable barrier to allow for the selective excretion of substances into the filtrate. Water, ions, and other small molecules generally pass through, while larger molecules are retained in the plasma. Podocytes are specialized epithelial cells that spread over the basement membrane, surrounding the capillaries with cytoplasmic projections known as foot processes. The foot processes of adjacent podocytes interdigitate and are crossed by slit diaphragms comprised of proteins such as nephrin, podocin, P-cadherin, and ZO-11. In cross section, these foot processes are evenly arranged over the basement membrane. In diseased glomeruli, the foot processes become grossly abnormal or “effaced,” leading to abnormal leak of plasma contents into the filtrate. As such, glomerular damage is generally characterized by the presence of abnormally large amounts of protein (e.g., proteinuria) and/or red blood cells (e.g., hematuria) in the urine. In addition, injured podocytes lose expression of nephrin as well as its regulator Wilms Tumor 1 (WT1), a key protein responsible for maintenance of differentiation2,3. The glomeruli are a primary target of damage in diabetic nephropathy and other glomerulonephritides such as minimal change disease, membranous nephropathy, and focal segmental glomerulosclerosis. These diseases are major causes of progressive kidney failure and the development of end-stage renal disease, a condition in which survival relies upon dialysis or renal transplantation. Therefore, it is important to study glomeruli to better understand chronic kidney disease (CKD) pathology.
A cell culture system is critical to studying glomerular biology. Due to its central role in generating the slit diaphragm, as well as the existence of specific proteinuric diseases due to slit diaphragm protein mutations, much research has understandably utilized the podocyte in isolation. This has led to the generation of primary podocyte cell lines to utilize in vitro. These cells can be cultured under a variety of conditions and can even be grown on permeable supports to assess permeability4. However, the isolation of proliferating cells often selects dedifferentiated cells that have lost some of their podocyte markers. This has led to the generation of conditionally immortalized podocytes derived from a transgenic mouse strain carrying a temperature-sensitive mutant of the SV40 large T gene (e.g. immortomouse), which could be grown in culture but also be differentiated to express a full array of podocyte markers5. These methods of primary culture have been pivotal in understanding podocyte biology4,6,7.
Nevertheless, cultures containing single cell types lack the intercellular relationships that occur in vivo as well as the support structure and matrices, and monolayers of these cells do not necessarily recapitulate the three-dimensional architecture of glomeruli. The immortalized podocytes can also be cumbersome and challenging to culture8, and require possession of either the immortomouse or a starting aliquot of cells from established investigators to get started. Further, the glomerulus is comprised of not only podocytes, but also capillary endothelial cells and the basement membrane, as well as mesangial cells which provide support for the structure. It is therefore useful to develop an ex vivo approach available to all investigators for the study of intact glomeruli that retain their native architecture as well as all the cells constituting the normal glomerulus.
In 1958, Cook and Pickering described the first isolation of glomeruli from the rabbit kidney. After observations that fat emboli became lodged in glomeruli, they postulated that particles of the same size could be used to specifically isolate these structures. Indeed, the infusion of iron oxide particles into the kidney led to the trapping of these particles in glomeruli. After mechanical dissociation and sieving of the kidney, the glomeruli could be isolated intact and with purity through the use of magnetic separation9. In 1971, Misra showed that the iron oxide infusions could be omitted, and glomerular isolation achieved with sieving of minced human, dog, rabbit or rat kidney tissue10. This technique has been modified since then depending on the goal of the investigators but has essentially resulted in purified preparations that could be further studied or from which primary cell cultures could be established11,12,13,14,15,16,17.
Here we describe a protocol for the isolation of intact viable glomeruli from the rat kidney. The entire protocol takes just a few hours. Although they do not proliferate, experimental plans of any size can be supported by simply increasing the number of kidneys as starting material. While there are published protocols for the magnetic bead separation of glomeruli, they require an intravenous injection of beads, are more expensive, and may alter biology since the beads are either retained by the glomeruli in culture or require glomerular “lysing” and removal by centrifugation19. Compared to mouse glomeruli, the larger size of rat glomeruli (nearly 100 μm in two month old rats18) makes it much easier to separate them from other kidney structures using a simple sieving technique.
As evidence of their usefulness, we have characterized the glomeruli to demonstrate the different cell types. They can also be exposed to agents known to injure glomeruli in vivo, and we demonstrate the adverse effects of protamine sulfate (PS) on these cultures. PS is a polycation that neutralizes the anionic sites along the glomerular capillary wall20. This neutralization has a dramatic effect on the glomerular filtration barrier and therefore increases proteinuria and foot process effacement. These glomeruli can be assessed with immunoblots for key proteins such as nephrin and WT1 to assess overall health. Furthermore, their structure can be evaluated with light, immunofluorescence, and electron microscopy.
Overall, this protocol is accessible to most investigators (one only needs access to the animals and some simple equipment). With morphological features left undamaged, the researcher is able to analyze the glomeruli and see how other important cell types and matrix preservation in the glomeruli affect function and disease progression, a shortcoming of podocyte cultures.
Protocol
All methods described here have been approved by the Institutional Animal Care and Use Committee (IACUC) of University of Pittsburgh.
1. Isolation of Rat Glomeruli
- To prepare a sterile 1 % isolation buffer, add 5 g of bovine serum albumin (BSA) to a 600 mL beaker.
- Add 500 mL of 1× phosphate buffered saline (PBS) to the beaker and stir with a magnetic stirring rod until all BSA is dissolved.
-
Sterile filter the 1 % BSA/PBS buffer in a cell culture hood.NOTE: The sterile 1 % BSA/PBS buffer can be kept at 4 °C for up to a week.
- Obtain 2 to 4 Sprague-Dawley rats weighing 150 to 200 g each.
- Euthanize rats via carbon dioxide inhalation using a chamber in which 100% carbon dioxide is introduced at a rate of 10–30% of the chamber volume per minute. Observe animals for cessation of respiration and faded eye color before removal from carbon dioxide.
- Prepare the skin over the anterior abdomen with 70% ethanol and utilize hair clippers to remove hair, if desired. Make a midline incision in the skin using surgical scissors or scalpel. Make another midline incision through the muscle layer to expose internal organs. Extend the incision superiorly through the diaphragm and sternum or rib cage as a secondary method of euthanasia.
-
Isolate and remove both kidneys and place into a sterile 50 mL plastic conical tube with 30 mL of Hanks’ buffered salt solution (HBSS) on ice.NOTE: Several rats can be euthanized in the same chamber and all of the kidneys can be placed in the same conical tube.
-
Keep the kidneys on ice and transport to a sterile cell culture hood. Transfer the kidneys to a sterile Petri dish containing 5 mL of HBSS, also over ice, and remove and discard perirenal fat with scissors and sharp forceps. If still present, also remove and discard the capsule surrounding the kidney by making a small superficial incision and then use sharp forceps to gently pull it away from the organ.
NOTE: Always keep kidney isolates on ice and practice sterile technique throughout. A piece of sterile gauze can be placed in the Petri dish as a textured surface to hold the kidney in place during manipulation.- Cut kidneys in half lengthwise (midsagittal section) and remove and discard the medulla (which is darker in color) with a scalpel in a second Petri dish with 5 mL of HBSS.
- Transfer the remaining pieces, which are predominantly kidney cortex, into a third Petri dish with 5 mL of HBSS and mince with a sterile razor blade until the pieces are less than 1 mm in size, or until a paste is formed.
Wet the top and bottom of a 180 μm sieve with 1 % BSA/PBS over a 500 mL waste beaker. This step is critical as it coats the sieve with protein and reduces adherence of glomeruli, which will improve yield.
-
Place the minced cortex on a small edge of the sieve and use the textured plunger flange (the side opposite the rubber seal) of a 10 mL syringe to mush the tissue through the sieve into a bottom pan sitting on ice. Rinse periodically with HBSS but use as little as possible to avoid sample dilution.
NOTE: Do not exceed 30 mL of buffer total, so only 25 mL more can be used here. The reason for placing the minced tissue on only one edge of the sieve is to reduce adherence of glomeruli by limiting exposure to part of the sieve rather than the entire sieve surface area.- To facilitate this, reuse the fluid collecting in the bottom pan to rinse the sieve. Once sieving is complete, carefully wash the bottom of the sieve once more with 1 % BSA/PBS buffer from the bottom of the pan to capture any glomeruli that may be loosely adhered.
- Collect all of the fluid in the bottom pan into several 10 mL syringes equipped with 20 G needles and pass it through the needle at least 2 additional times. On the last collection, keep the glomeruli-containing fluid in the syringe until ready to pass it through the 90 μm sieve.
- While the fluid is stored in the syringes, wash the bottom pan by flushing with 1% BSA/PBS into waste beaker and wet the top and bottom of a 90 μm sieve with 1% BSA/PBS. Place the sieve on top of the bottom pan on ice.
- Apply the sample in the syringes to one edge of the sieve and mush through the sieve with another syringe flange as describe above. Wash with solution from the bottom pan to collect everything on one edge of the sieve.
- Once sieving is complete, carefully wash the bottom of the sieve as in step 1.5.1. Collect all the fluid in the bottom pan in a 50 mL plastic conical tube.
- Wash the bottom pan with 1 % BSA/PBS into a waste beaker and wet the top and bottom of a 75 μm sieve with 1 % BSA/PBS. Place the sieve on top of the bottom pan on ice.
- Apply the sample to one edge of the sieve. Liquid should flow through easily. Rinse through the top with at least 20 mL of 1% BSA/PBS into waste beaker. Carefully wash the bottom of the sieve as well. The glomeruli will remain on the top of this 75 μm sieve.
- Use HBSS to collect glomeruli in a Petri dish by rinsing through the sieve upside down. Use as much HBSS as needed here. Collect into one or more 50 mL plastic conical tube(s) on ice.
Centrifuge at 1800 × g for 5 min at 4 °C, remove supernatant carefully with a pipette, and resuspend pellet in 10 mL of cold HBSS. Combine the samples if multiple conical tubes were collected in 1.7.2. Repeat the centrifugation step.
Resuspend the glomeruli in 5 mL of HBSS until ready to proceed to the next step.
-
If desired, take a count of the total glomeruli. For this, take a 10 μL sample with a pipette and place the drop on a glass slide. View under a microscope, count the glomeruli in the field and multiply by 500 to get total yield.
NOTE: Purity may be determined by counting the number of tubules seen in the field and dividing by total number of glomeruli and tubule structures. There should be > 95% glomeruli in the visual field.- If there is significant tubular contamination, repeat steps 1.6.1 onward, and make sure to wash thoroughly when completing step 1.7.1. This is the step in which tubular contamination is most likely to occur.
2. Injury of Glomeruli
-
Collect the glomeruli in a 15 mL plastic conical tube and spin down at 200 × g. Resuspend in an appropriate amount of HBSS based on the total yield so that there are approximately 9,000 glomeruli per well. Pipette 450 μL of sample into each well of a 24-well plate, or any plate format that fits the downstream assays required.
NOTE: Pipetting up and down to mix glomeruli in buffer ensures a homogeneous solution. The glomeruli are very sticky and heavy and will stick to each other as well as settle at the bottom of a solution quickly and onto the sides of the tube.
-
To make 6 mg/mL protamine sulfate (PS) solution, first dissolve 1 g of PS into 10 mL of dH2O heated to 40 °C in a 15 mL plastic conical tube and vortex thoroughly. Take 30 μL of the solution and add to 470 μL of dH2O.
NOTE: This will produce a 6 mg/mL solution, and when 50 μL are added to the well as described below, the final concentration is 0.6 mg/mL.- In duplicate, add 50 μL of protamine sulfate (this is a 1:10 dilution) to each well of one group, while supplying another group 50 μL of HBSS (negative control).
Incubate the plate for 4 h at 37 °C.
Spin down contents of each well in a microcentrifuge tube (4000 × g, 4 °C, 10 – 15 s) and wash twice with 1 mL of PBS each.
Aspirate off the final wash and resuspend in 300 μL of PBS. Split into three aliquots to be prepared for protein isolation, transmission electron microscope (TEM) visualization, and immunofluorescence staining (IF).
3. Preparing Glomeruli for Analysis
Spin down the contents of the three aliquots (4000 × g, 4 °C, 10 – 15 s) and aspirate off remaining buffer and proceed to sample analysis by TEM, IF, or protein isolation.
- Processing for TEM
- Fix glomerular pellets in 150 μL of cold 2.5% glutaraldehyde in 0.01 M PBS. Remove the fixative carefully from the pellet using a Pasteur pipette, making sure not to disrupt the pellet, then rinsed it with PBS and post-fix it in 1% osmium tetroxide with 1% potassium ferricyanide.
- Dehydrate the sample through a graded series of ethanol (30, 50, 70, 90, 100, 100, 100%) and propylene oxide then embed in epoxy embedding material.
- Cut semi-thin (300 nm) sections on an ultramicrotome. Stain with 0.5% Toluidine Blue in 1 % sodium borate and examine them under the light microscope.
- Cut ultrathin sections (65 nm), stain them with uranyl acetate and Reynold’s lead citrate, and examine on a transmission electron microscope.
- Processing for IF
- Add 150 μL of a 12% gelatin in PBS solution and immediately place on dry ice to form a pellet. Soak the pellet in 500 μL of 2% paraformaldehyde/1x PBS in a microcentrifuge tube overnight at 4 °C.
- Place the pellet in a base mold and embed by adding optimal cutting temperature (OCT) medium over dry ice. Cut 5–10 μm sections on a cryotome and proceed with immunofluorescence/immunohistochemistry or standard histological stains.
- For Protein Isolation
- Add 150 μL of RIPA buffer to centrifuged glomerular pellet with 1.5 μL of protease inhibitor and dounce with tissue homogenizers.
- Proceed to protein quantification and immunoblotting or other protein analysis.
Representative Results
The protocol from the time of euthanasia to isolation of the glomeruli can be completed in as little as 2 h and has a high throughput and efficiency. With proper utilization of the technique, the yield of glomeruli per rat kidney ranges from 6,000 – 10,000 glomeruli when starting with 8 kidneys. The final suspension is densely packed with glomeruli and has an overall purity > 95%, showing minimal contamination from tubular segments or other cell types (Figure 1A, B). In addition, the OCT-embedded glomeruli can be stained with Hematoxylin and Eosin (H & E) stains to see the morphology (Figure 1C). These glomeruli maintain their structure throughout the whole protocol, even after processing. We demonstrate that isolated glomeruli possess intact and viable podocytes (Figure 2A), mesangial cells (Figure 2B), and endothelial cells (Figure 2C).
Figure 1. Typical appearance of rat glomerular cultures after isolation.
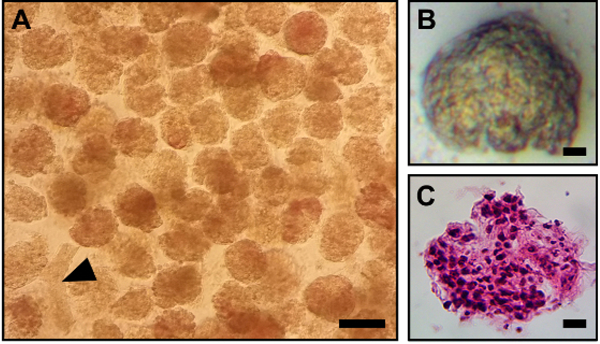
(A) Brightfield image of glomerular culture. Although we chose a field in which there is a single renal tubule in this micrograph (arrowhead), the cultures obtained are generally > 95% pure. Scale bar equals 100 μm. (B) Enlarged image of a single glomerulus. (C) Hematoxylin and eosin stain of a single glomerulus. Scale bars in B and C equal 10 μm.
Figure 2. Constituent cell types are retained in isolated glomeruli.
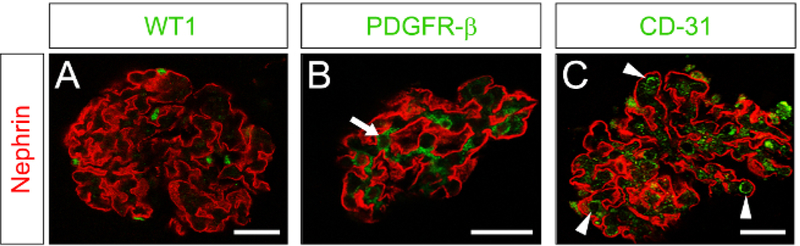
Confocal immunofluorescence microscopy was performed for nephrin (red, podocytes) and cell-specific markers to identify podocytes, mesangial cells, and the endothelium. (A) Costain for nephrin and WT1 (green, podocytes). (B) Costain for nephrin and PDGFR-β (green, mesangial cells, arrow). (C) Costain for nephrin and CD31 (green, endothelial cells, vessels in cross section marked by arrowheads). Scale bar = 25 μm on all panels.
Once isolated, the glomeruli can be exposed to well-known chemical injuries to simulate in vivo pathology. In this case, protamine sulfate (PS) was chosen for its ability to disrupt the charge of the glomerular filtration barrier, which eventually leads to foot process effacement. PS-treated glomeruli have a striking reduction in nephrin (red) and a number of nuclei positive for WT1 (green) via immunofluorescence (Figure 3A, B). The glomeruli can also be prepared for transmission electron microscopy (Figure 3C). Control samples have normal podocyte morphology and foot processes whereas the PS-treated glomeruli have foot process effacement (Figure 3D), which is a sign of podocyte dysfunction and is seen in in vivo models using PS21. This also corresponded to a decrease in nephrin detected by immunoblotting (Figure 3E, F).
Figure 3. Podocyte injury can be induced in vitro using isolated rat glomeruli.
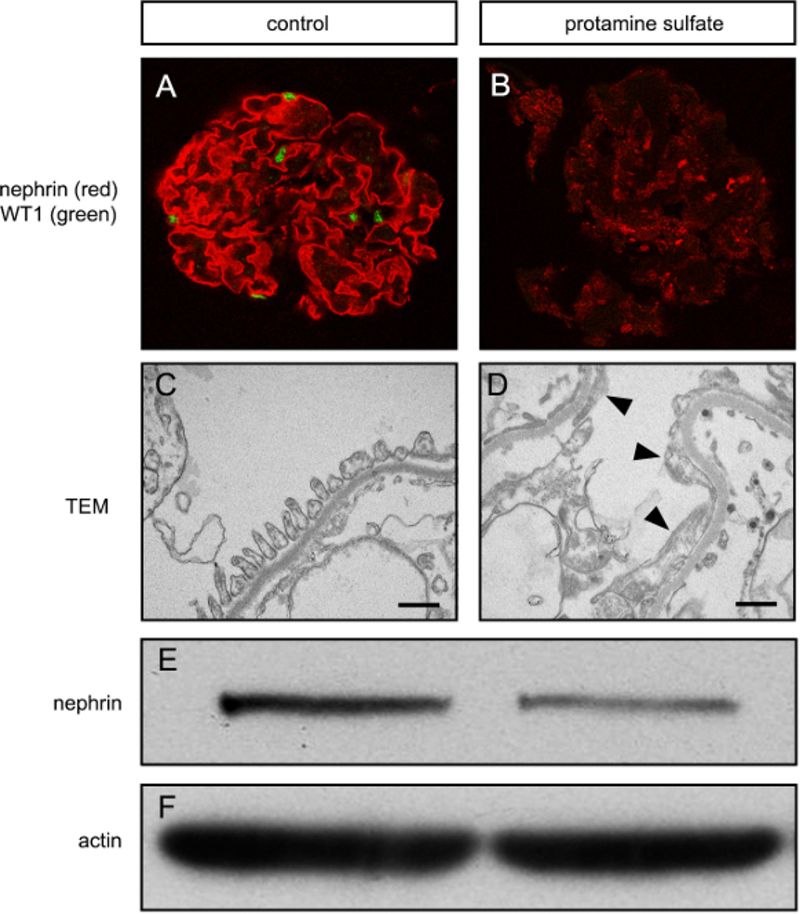
(A) Confocal immunofluorescence microscopy for nephrin and WT1 in an untreated glomerular culture. Note the linear nephrin staining (red) and presence of WTI-positive nuclei (green) which are typically seen when there are healthy podocytes. (B) After protamine sulfate treatment, nephrin expression is decreased and non-linear, and WT1 is absent, indicating podocyte injury. (C) Transmission electron microscpy (TEM) image showing foot processes, basement membrane, and a fenestrated endothelium typical of normal glomerular structure. Bar equals 500 nm. (D) After protamine sulfate, foot processes are elongated or effaced (arrowheads), which indicates podocyte injury. (E) Western blot for nephrin showing decreased nephrin after protamine sulfate treatment. (F) Actin loading control for western blot is shown.
To determine viability, cleaved caspase-3 was assessed as a marker of apoptosis. Using immunofluorescence, cleaved caspase-3 first appears in a few cells starting 2 h after isolation (Figure 4). The number of cells expressing this protease became more abundant over time, with the highest levels seen at 24 and 48 h. This suggests that apoptosis does occur relatively early in culture and that downstream applications should be performed soon after isolation for best results.
Figure 4. Assessment of cell viability in isolated glomeruli.
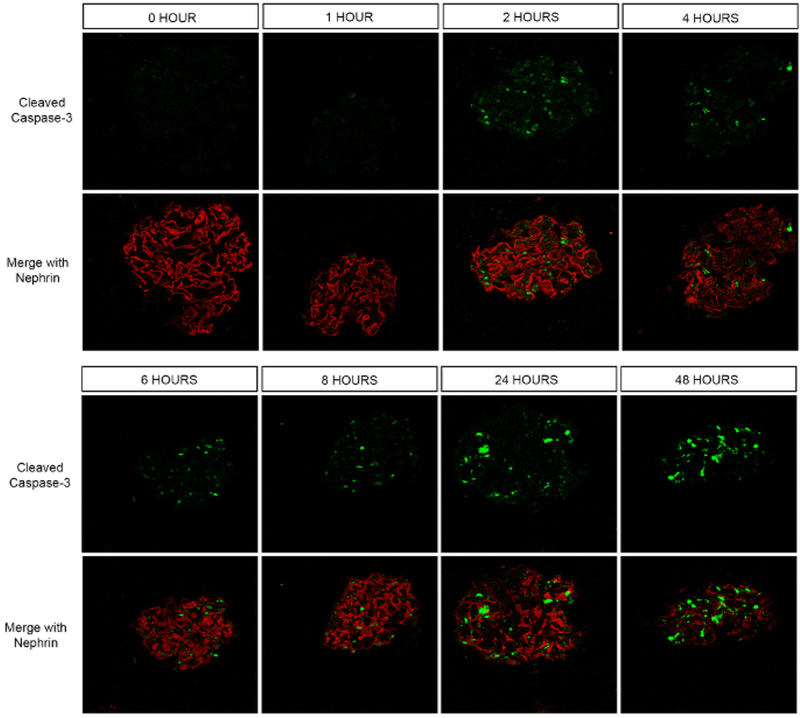
Confocal immunofluorescence microscopy for cleaved caspase-3 (green) was performed. A nephrin co-stain was performed to easily locate the glomeruli. While there was no cleaved caspase-3 positivity at 0 and 1 h after isolation of glomeruli, fluorescence signal in a few cells was noted at 2 h. Progressively more cells turned positive the longer after isolation they were examined. The greatest number of caspase-3 positive cells were noted at 24 and 48 h.
Discussion
This is an efficient method for recovering glomeruli from rat kidney using inexpensive, reusable equipment with a simple protocol. As with all procedures, there are limitations to its usefulness. First, although we obtain > 95% purity, because of the sieving nature of the protocol and the starting material it is impossible to exclude all contaminants, and a few red blood cells and the occasional tubular segment will be present in the culture. We do not anticipate these small contaminants to be a problem for the vast majority of applications. Second, the sieving protocol relies on the use of rat kidneys, in which glomeruli are much larger than in mice. If mouse glomeruli are needed, a technique using commercial magnetic beads (e.g., Dynabeads) has been published22. Third, it has been shown that specific mRNAs (plasminogen activator inhibitor-1 and collagen I) in isolated glomeruli derive almost entirely from Bowman’s capsule rather than intraglomerular cells23. This could lead to inappropriate conclusions if mRNA isolation is attempted from these glomeruli. It should be noted that in our hands the majority of glomeruli are decapsulated and should therefore lack significant contributions from Bowman’s capsules. Fourth, cell death begins to occur relatively quickly under culture conditions.
We found that the apoptotic program, as evidenced by appearance of cleaved caspase-3, was activated starting at 2 h of culture. This is in general agreement with previous reports showing that apoptosis, as assessed by DNA fragmentation, TUNEL, and histologic analysis, begins to occur within 1–2 h after isolation24. However, it should also be noted that early observations showed that metabolic activity could be detected from isolated sieved glomeruli when cultured for at least 3 h, suggesting considerable cellular viability at this timepoint16. Nevertheless, based on the results, we believe that it would be prudent to utilize isolated glomeruli immediately in intended experiments for the best results. We recommend that all experiments be performed before 72 h as we have observed that the entire glomerular structure begins to deteriorate beyond that timepoint.
It is much easier to obtain higher yields when starting with 4–8 kidneys as opposed to only 2 because a certain number of glomeruli are lost due to adherence to the various sieves, but once the sieves are maximally coated there is no additional loss. For the same reason, it is important to soak the sieves in the BSA/PBS buffer prior to use as glomeruli are more likely to adhere to a dry sieve, and to limit tissue exposure to one edge of the sieve. We suggest starting with no fewer than 6 kidneys (3 rats) to obtain a reasonable yield.
Some investigators have isolated primary podocyte cultures from isolated glomeruli which tend to grow out of the glomeruli during culture17. We have noted that some isolated glomeruli will adhere to the culture dish plastic and that cells will begin to migrate out of the glomerular structure at late timepoints (72 h after isolation). The study of these cells is beyond the scope of this isolation protocol, and there is some controversy as to whether these cells are podocytes, parietal epithelial cells, or both15,25. Notably, Mundel et al., have reported that cobblestone cells harvested from sieved glomeruli may be induced to differentiate into mature podocytes under specific culturing conditions26. Some of the confusion regarding cell identity may depend on whether the isolated glomeruli are encapsulated (including Bowman’s capsule which is populated by parietal epithelial cells) or decapsulated in the sieving procedure. In our hands, using sequential sieve sizes of 180, 90, and 75 μm led to the majority of glomeruli being decapsulated.
Most forms of proteinuric chronic kidney disease are due to increases in glomerular permeability. Several authors have utilized isolated glomeruli in ex vivo permeability experiments. In one method, the change in glomerular volume after altering the osmotic content of the surrounding media was used to estimate permeability27. Recently, it was established that leakage of a fluorescent probe from isolated glomeruli could be quantified to measure permeability and could be performed in rodents exposed to glomerular disease experimental models28.
We have noted that in the TEM preparation process, we sometimes see the podocyte foot processes lift off the basement membrane. We do not feel this is happening in the culture and is more likely an artifact during TEM sample preparation. Notably, even when this occurs, it is clear whether the foot processes look “normal” or are effaced.
Overall, this protocol provides a method by which one can evaluate morphologic and cellular changes in response to injury. We anticipate that it may be used as a companion technique to isolated podocyte cultures, especially when interactions with extracellular matrix or other native cell types are being considered. It holds promise for increasing understanding of proteinuric CKD, which would improve the ability to develop future therapeutics for this debilitating disease.
Acknowledgements
This work was supported by American Heart Association grant FTF 16990086, NIH P30 DK079307, American Society of Nephrology Gottschalk Award, a University of Pittsburgh Medical Center Competitive Medical Research Fund Award, and a University of Pittsburgh Physicians Academic Foundation Award. We thank Gerard Apodaca for his technical suggestions for glomerular preservation for histologic analysis, Mara Sullivan and Ming Sun for assistance with electron microscopy, and Yingjian Li and Youhua Liu for their technical input in this protocol. We also thank Cynthia St. Hilaire for the CD31 antibody.
Footnotes
Disclosures
The authors have nothing to disclose.
Video Link
The video component of this article can be found at https://www.jove.com/video/58162/
References
- 1.Grahammer F, Schell C, & Huber T. Molecular understanding of the slit diaphragm. Pediatric Nephrology. 28 (10), 1957–1962, (2013). [DOI] [PubMed] [Google Scholar]
- 2.Tan RJ, Zhou L, Zhou D, Lin L, & Liu Y Endothelin receptor a blockade is an ineffective treatment for adriamycin nephropathy. PLoS One. 8 (11), e79963, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wagner N, Wagner KD, Xing Y, Scholz H, & Schedl A The major podocyte protein nephrin is transcriptionally activated by the Wilms’ tumor suppressor WT1. Journal of the American Society of Nephrology. 15 (12), 3044–3051, (2004). [DOI] [PubMed] [Google Scholar]
- 4.Rogacka D et al. Insulin increases filtration barrier permeability via. TRPC6-dependent activation of PKGIalpha signaling pathways. Biochimica et Biophysica Acta. 1863 (6), 1312–1325, (2017). [DOI] [PubMed] [Google Scholar]
- 5.Mundel P et al. Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Experimental Cell Research. 236 (1), 248–258, (1997). [DOI] [PubMed] [Google Scholar]
- 6.Moller CC et al. Induction of TRPC6 channel in acquired forms of proteinuric kidney disease. Journal of the American Society of Nephrology. 18 (1), 29–36, (2007). [DOI] [PubMed] [Google Scholar]
- 7.Tian D et al. Antagonistic regulation of actin dynamics and cell motility by TRPC5 and TRPC6 channels. Science Signaling. 3 (145), ra77, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shankland SJ, Pippin JW, Reiser J, & Mundel P Podocytes in culture: past, present, and future. Kidney Internationalernational. 72 (1), 26–36, (2007). [DOI] [PubMed] [Google Scholar]
- 9.Cook WF, & Pickering GW A rapid method for separating glomeruli from rabbit kidney. Nature. 182 (4642), 1103–1104, (1958). [DOI] [PubMed] [Google Scholar]
- 10.Misra RP Isolation of glomeruli from mammalian kidneys by graded sieving. American Journal of Clinical Pathology. 58 (2), 135–139, (1972). [DOI] [PubMed] [Google Scholar]
- 11.Burlington H, & Cronkite EP Characteristics of cell cultures derived from renal glomeruli. Proceedings of the Society for Experimental Biology and Medicine. 142 (1), 143–149, (1973). [DOI] [PubMed] [Google Scholar]
- 12.Harper PA, Robinson JM, Hoover RL, Wright T., & Karnovsky MJ. Improved methods for culturing rat glomerular cells. Kidney International. 26 (6), 875–880, (1984). [DOI] [PubMed] [Google Scholar]
- 13.Kreisberg JI, Hoover RL, & Karnovsky MJ Isolation and characterization of rat glomerular epithelial cells in vitro. Kidney International. 14 (1), 21–30, (1978). [DOI] [PubMed] [Google Scholar]
- 14.Weinstein T, Cameron R, Katz A, & Silverman M Rat glomerular epithelial cells in culture express characteristics of parietal, not visceral, epithelium. Journal of the American Society of Nephrology. 3 (6), 1279–1287, (1992). [DOI] [PubMed] [Google Scholar]
- 15.Yaoita E, Kurihara H, Sakai T, Ohshiro K, & Yamamoto T Phenotypic modulation of parietal epithelial cells of Bowman’s capsule in culture. Cell and Tissue Research. 304 (3), 339–349, (2001). [DOI] [PubMed] [Google Scholar]
- 16.Meezan E, Brendel K, Ulreich J, & Carlson EC Properties of a pure metabolically active glomerular preparation from rat kidneys. I. Isolation. Journal of Pharmacology and Experimental Therapeutics. 187 (2), 332–341, (1973). [PubMed] [Google Scholar]
- 17.Piwkowska A et al. Insulin increases glomerular filtration barrier permeability through PKGIalpha-dependent mobilization of BKCa channels in cultured rat podocytes. Biochimica et Biophysica Acta. 1852 (8), 1599–1609, (2015). [DOI] [PubMed] [Google Scholar]
- 18.Kotyk T et al. Measurement of glomerulus diameter and Bowman’s space width of renal albino rats. Computer Methods and Programs in Biomedicine. 126 143–153, (2016). [DOI] [PubMed] [Google Scholar]
- 19.Liu X et al. Isolating glomeruli from mice: A practical approach for beginners. Exp Ther Med. 5 (5), 1322–1326, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leeuwis JW, Nguyen TQ., Dendooven A., Kok RJ., & Goldschmeding R. Targeting podocyte-associated diseases. Advanced Drug Delivery Reviews. 62 (14), 1325–1336, (2010). [DOI] [PubMed] [Google Scholar]
- 21.Subramanian B et al. Mice with mutant Inf2 show impaired podocyte and slit diaphragm integrity in response to protamine-induced kidney injury. Kidney International. 90 (2), 363–372, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takemoto M et al. A new method for large scale isolation of kidney glomeruli from mice. American Journal of Pathology. 161 (3), 799–805, (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinmetz OM et al. A pitfall of glomerular sieving: profibrotic and matrix proteins derive from the Bowman’s capsule and not the glomerular tuft in rats with renovascular hypertension. Nephrology Dialysis Transplantation. 22 (10), 3055–3060, (2007). [DOI] [PubMed] [Google Scholar]
- 24.Ishikawa Y, & Kitamura M Spontaneous apoptosis of podocytes in explanted glomeruli. Technical note. Kidney International. 54 (6), 2008–2013, (1998). [DOI] [PubMed] [Google Scholar]
- 25.Krtil J, Platenik J, Kazderova M, Tesar V, & Zima T Culture methods of glomerular podocytes. Kidney and Blood Pressure Research. 30 (3), 162–174, (2007). [DOI] [PubMed] [Google Scholar]
- 26.Mundel P, Reiser J, & Kriz W Induction of differentiation in cultured rat and human podocytes. Journal of the American Society of Nephrology. 8 (5), 697–705, (1997). [DOI] [PubMed] [Google Scholar]
- 27.McCarthy ET et al. TNF-alpha increases albumin permeability of isolated rat glomeruli through the generation of superoxide. Journal of the American Society of Nephrology. 9 (3), 433–438, (1998). [DOI] [PubMed] [Google Scholar]
- 28.Desideri S et al. A novel assay provides sensitive measurement of physiologically relevant changes in albumin permeability in isolated human and rodent glomeruli. Kidney International. 93 (5), 1086–1097, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]


