Abstract
Cyclic imines, generated in situ from their corresponding N-lithiated amines and a ketone hydride acceptor, undergo reactions with a range of organometallic nucleophiles to generate α-functionalized amines in a single operation. Activation of the transient imines by Lewis acids that are compatible with the presence of lithium alkoxides was found to be crucial to accommodate a broad range of nucleophiles including lithium acetylides, Grignard reagents, and aryllithiums with attenuated reactivities.
Graphical Abstract

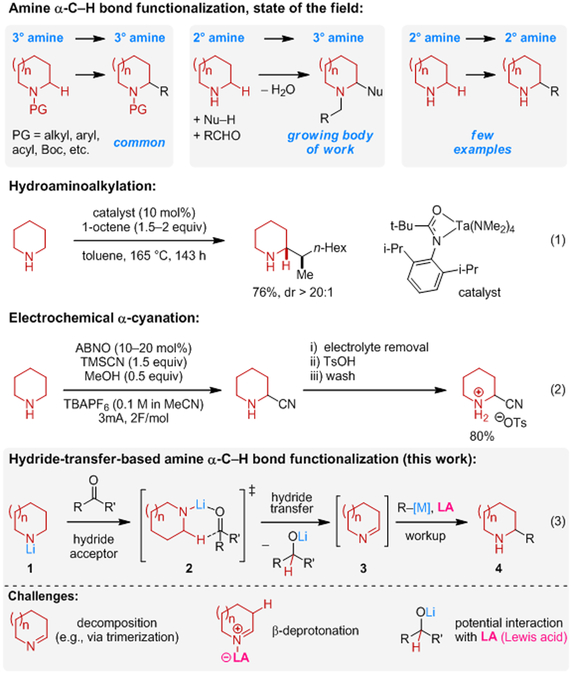
Saturated nitrogen heterocycles remain one of the most important classes of compounds in drug discovery.1 C─H bond functionalization of the parent heterocyclic frameworks represents an attractive strategy for accessing more complex saturated amines from simple ones, and remains a topic of widespread interest.2 Particularly well-studied are α-C─H bond functionalizations of cyclic amines (Scheme 1).3 However, despite considerable advances, the vast majority of the various activation modes developed to date are incompatible with the presence of an N─H bond, limiting their utility to 3° (often N-aryl) or protected 2° amines. Directing groups are frequently required and can be difficult to remove, hampering the use of the underlying methods. As an alternative, condensation-based methods involving α-C─H bond functionalization of 2° cyclic amines have recently emerged.2m,2o While attractive for a number of reasons, the products of these transformations are necessarily 3° amines. Methods for the direct synthesis of α-functionalized 2° cyclic amines from their corresponding parent amines remain limited. Hydroaminoalkylation enables the introduction of α-branched aliphatic substituents and is relatively well-developed for acyclic amines.4 However, applications to the functionalization of 2° cyclic amines remain underdeveloped and typically require elevated temperatures and prolonged reaction times (Scheme 1, eq 1).5 An interesting recent advance is the electrochemical α-cyanation of NH-piperidines (Scheme 1, eq 2).6 Here we report a generalizable method for the α-C─H bond functionalization of 2° cyclic amines, utilizing a broad range of easily accessible organometallic nucleophiles (Scheme 1, eq 3).
Scheme 1.
Overview and Strategy
Inspired by seminal studies on the hydride donor ability of lithium amides by Wittig et al.,7 we recently developed a new strategy for amine α-C─H bond functionalization (Scheme 1, eq 3).8 In the process, an N-lithiated amine 1 reduces a ketone to form a lithium alkoxide and cyclic imine 3, presumably via a transition state related to 2.9 Imine 3 subsequently engages an organolithium reagent, providing an α-functionalized amine 4.10 While applicable to a relatively broad range of amines, our original procedure is limited to the use of highly reactive organolithium nucleophiles. Organolithiums with attenuated reactivities (e.g., lithium acetylides) and, importantly, Grignard reagents, failed to effectively engage the transient imine species. We hypothesized that a Lewis acid may serve to activate cyclic imines towards nucleophilic addition. While there is precedent showing that such a strategy can be successful with acyclic imines and nonenolizable cyclic imines,11–13 it was not obvious whether previously developed methods would be applicable to enolizable cyclic imines such as 1-pyrroline and 1-piperideine. These species are known to be unstable and prone to trimerization,14 a process that could potentially be accelerated by Lewis acids. Imine trimers tend to be stable under basic conditions and don’t react with organolithium compounds even at elevated temperatures. Conversion of the imine trimers to the corresponding monomers is possible but requires protic conditions and/or heating, conditions that are incompatible with strong nucleophiles.15 Further, while Lewis acid activation of an enolizable cyclic imine should serve to increase its electrophilicity, it also increases the acidity of the β-proton and may thus further enhance the known propensity of these imines to undergo deprotonation.7 Finally, it was unclear whether any Lewis acid with sufficient activity would be compatible with the lithium alkoxide produced in the imine-generating step. Needless to say, having to first remove said alkoxide from the reaction mixture would diminish the attractiveness of the method.
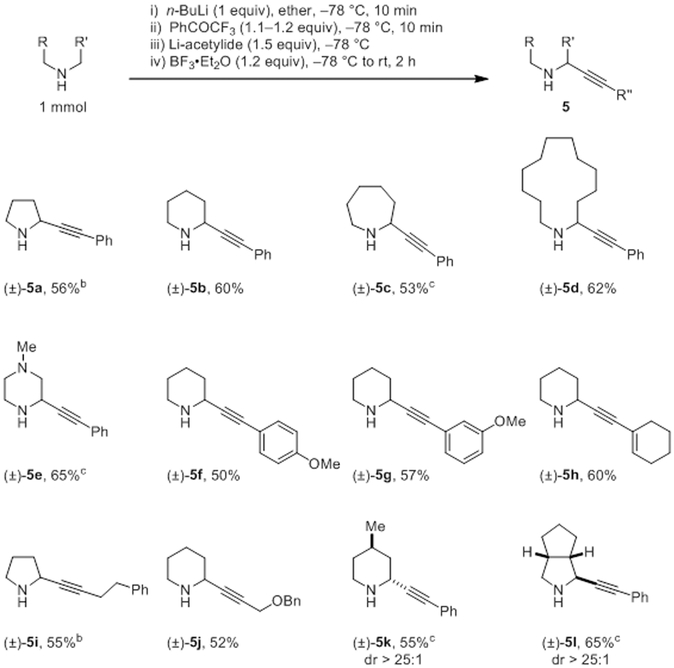
Upon extensive evaluation of a range of reaction parameters, conditions were identified that allowed for the one-pot α-alkynylation of various amines with lithium acetylides (Scheme 2).16 Briefly, following the generation of the cyclic imine, Li-acetylide was added, immediately followed by addition of BF3 etherate. The order of addition and the timing was found to be crucial.16 Regardless of ring size, 2° cyclic amines readily underwent substitutions with Li-phenylacetylide. A range of alkynes participated in this reaction. 4-Methyl piperidine and a bicyclic pyrrolidine derivative underwent alkynylation to provide products in highly diastereoselective fashion. Little or no product formation was observed in the absence of BF3 etherate.
Scheme 2. α-Alkynylation of Aminesa.
a All yields correspond to isolated yields of purified products. The Li-acetylide was prepared in a 4:1 mixture of PhMe and THF. b Benzophenone was used as the hydride acceptor c The Li-acetylide was prepared in THF.
Similar reaction conditions were successfully applied to substitution reactions of amines with aryllithium compounds possessing attenuated nucleophilicities, enabling the introduction of furan, benzofuran, and various substituted indole moieties (Scheme 3). Several of the nucleophiles had previously been evaluated in the absence of a Lewis acid. For instance, 2-furyllithium failed to provide any product without added BF3 etherate. Others, such as 2-lithiated N-methylindole provided only trace amounts of products in the absence of a Lewis acid.
Scheme 3. α-Arylation of Amines With Heteroaryllithiumsa.
a All yields correspond to isolated yields of purified products. Yields in parenthesis correspond to results obtained in the absence of BF3 etherate. b Trifluoroacetophenone was used as the hydride acceptor.
Grignard reagents, an important class of easily accessible organometallic nucleophiles, failed to provide notable yields in the addition to cyclic imines in the absence of Lewis acids. While BF3 etherate also enabled the use of these nucleophiles, trimethylsilyl trifluoromethanesulfonate (TMSOTf) provided the best results in the α-substitution of amines with Grignard reagents.16 The scope of this transformation is summarized in Scheme 4. Phenyl- and benzyl substituents were readily introduced into amines with different ring sizes. To obtain easily isolable nonvolatile products in the introduction of lower molecular weight substituents, these reactions were evaluated with azacyclotridecane. Linear, α-branched, and β-branched alkyl groups, as well as vinyl, allyl, and ethynyl groups were readily introduced. Notably, while methyllithium was previously found to be a poor nucleophile for cyclic imines, methylmagnesium chloride in combination with TMSOTf readily provided product 7g. High levels of diastereoselectivity were achieved in the α-phenylation of a bicyclic amine (product 7o), the introduction of an α-homoallyl substituent in 4-benzylpiperidine (product 7p), and the α-n-butylation of an intermediate used in the synthesis of the commercial drug risperidone (product 7q).17 Notably, the preparation of 7q failed with n-butyllithium as the nucleophile, presumably due to the incompatibility of this reagent with the benzisoxazole moiety. Further expansion of scope was achieved with active nucleophiles obtained via the turbo Grignard method (Scheme 5).18
 |
(4) |
 |
(5) |
Scheme 4. α-Functionalization of Amines With Grignard Reagentsa.
a All yields correspond to isolated yields of purified products.
Scheme 5. α-Functionalization of Amines With Activated Grignard Reagentsa.
a All yields correspond to isolated yields of purified products.
Products derived from the Lewis acid promoted α-C─H bond functionalization of 2° cyclic amines could be further functionalized regioselectively on the α′-position. For instance, piperidine 6f underwent α′-phenylation to provide product 9 in 60% yield (eq 4), whereas 10 was obtained in 64% yield via the α′-n-butylation of 7d (eq 5).
In summary, we have achieved the α-C─H bond functionalization of 2° cyclic amines with a broad range of organometallic nucleophiles. This method significantly improves the availability of valuable building blocks for synthesis.
Supplementary Material
ACKNOWLEDGMENT
Financial support from the NIH–NIGMS (Grant R01GM101389) is gratefully acknowledged.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
Experimental procedures and characterization data (PDF)
The authors declare no competing financial interests.
REFERENCES
- (1) a).Rings in Drugs. Taylor RD; MacCoss M; Lawson ADG J. Med. Chem. 2014, 57, 5845–5859; [DOI] [PubMed] [Google Scholar]; b) Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. Vitaku E; Smith DT; Njardarson JT J. Med. Chem. 2014, 57, 10257–10274. [DOI] [PubMed] [Google Scholar]
- (2).a) Selected recent reviews on amine C─H functionalization: Direct sp3 C-H bond activation adjacent to nitrogen in heterocycles. Campos KR Chem. Soc. Rev. 2007, 36, 1069–1084; [DOI] [PubMed] [Google Scholar]; b) Functionalization of Organic Molecules by Transition-Metal-Catalyzed C(sp3)-H Activation. Jazzar R; Hitce J; Renaudat A; Sofack-Kreutzer J; Baudoin O Chem. Eur. J. 2010, 16, 2654–2672; [DOI] [PubMed] [Google Scholar]; c) Catalytic Dehydrogenative Cross-Coupling: Forming Carbon-Carbon Bonds by Oxidizing Two Carbon-Hydrogen Bonds. Yeung CS; Dong VM Chem. Rev. 2011, 111, 1215–1292; [DOI] [PubMed] [Google Scholar]; d) Direct alpha-Functionalization of Saturated Cyclic Amines. Mitchell EA; Peschiulli A; Lefevre N; Meerpoel L; Maes BUW Chem. Eur. J. 2012, 18, 10092–10142; [DOI] [PubMed] [Google Scholar]; e) Oxidative Coupling of Tertiary Amines: Scope, Mechanism and Challenges. Jones KM; Klussmann M Synlett 2012, 23, 159–162; [Google Scholar]; f) The Redox-Neutral Approach to C-H Functionalization. Peng B; Maulide N Chem. Eur. J. 2013, 19, 13274–13287; [DOI] [PubMed] [Google Scholar]; g) The Cross-Dehydrogenative Coupling of C sp3-H Bonds: A Versatile Strategy for C-C Bond Formations. Girard SA; Knauber T; Li C-J Angew. Chem. Int. Ed. 2014, 53, 74–100; [DOI] [PubMed] [Google Scholar]; h) C-H Bond Functionalization through Intramolecular Hydride Transfer. Haibach MC; Seidel D Angew. Chem. Int. Ed. 2014, 53, 5010–5036; [DOI] [PubMed] [Google Scholar]; i) Advancement in Cascade [1,n]-Hydrogen Transfer/Cyclization: A Method for Direct Functionalization of Inactive C(sp3)-H Bonds. Wang L; Xiao J Adv. Synth. Catal. 2014, 356, 1137–1171; [Google Scholar]; j) Synthesis of Saturated N-Heterocycles. Vo C-VT; Bode JW J. Org. Chem 2014, 79, 2809–2815; [DOI] [PubMed] [Google Scholar]; k) The redox-A3 reaction. Seidel D Org. Chem. Front. 2014, 1, 426–429; [DOI] [PMC free article] [PubMed] [Google Scholar]; l) Catalytic asymmetric α-C(sp3)─H functionalization of amines. Qin Y; Lv J; Luo S Tetrahedron Lett. 2014, 55, 551–558; [Google Scholar]; m) The Azomethine Ylide Route to Amine C─H Functionalization: Redox-Versions of Classic Reactions and a Pathway to New Transformations. Seidel D Acc. Chem. Res. 2015, 48, 317–328; [DOI] [PMC free article] [PubMed] [Google Scholar]; n) Amine Functionalization via Oxidative Photoredox Catalysis: Methodology Development and Complex Molecule Synthesis. Beatty JW; Stephenson CRJ Acc. Chem. Res. 2015, 48, 1474–1484; [DOI] [PMC free article] [PubMed] [Google Scholar]; o) Classical-Reaction-Driven Stereo- and Regioselective C(sp3)─H Functionalization of Aliphatic Amines. Mahato S; Jana CK Chem. Rec. 2016, 16, 1477–1488; [DOI] [PubMed] [Google Scholar]; p) Organocatalysis in Inert C─H Bond Functionalization. Qin Y; Zhu L; Luo S Chem. Rev. 2017, 117, 9433–9520; [DOI] [PubMed] [Google Scholar]; q) Recent Advances in the Enantioselective Oxidative α-C─H Functionalization of Amines. Cheng M-X; Yang S-D Synlett 2017, 28, 159–174; [Google Scholar]; r) Transition metal-catalyzed α-alkylation of amines by C(sp3)‒H bond activation. Gonnard L; Guérinot A; Cossy J Tetrahedron 2019, 75, 145–163; [Google Scholar]; s) Construction of N-Heterocycles through Cyclization of Tertiary Amines. Liu S; Zhao Z; Wang Y Chem. Eur. J. 2019, 25, 2423–2441. [DOI] [PubMed] [Google Scholar]
- (3).a) Selected recent reports on amine C─H functionalization: Palladium-catalysed transannular C─H functionalization of alicyclic amines. Topczewski JJ; Cabrera PJ; Saper NI; Sanford MS Nature 2016, 531, 220–224; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Native functionality in triple catalytic cross-coupling: sp3 C─H bonds as latent nucleophiles. Shaw MH; Shurtleff VW; Terrett JA; Cuthbertson JD; MacMillan DWC Science 2016, 352, 1304–1308; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Enantioselective amine α-functionalization via palladium-catalysed C─H arylation of thioamides. Jain P; Verma P; Xia G; Yu J-Q Nat. Chem. 2017, 9, 140–144; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Palladium-Catalyzed Enantioselective C─H Activation of Aliphatic Amines Using Chiral Anionic BINOL-Phosphoric Acid Ligands. Smalley AP; Cuthbertson JD; Gaunt MJ J. Am. Chem. Soc. 2017, 139, 1412–1415; [DOI] [PubMed] [Google Scholar]; e) Synthesis of Ring-Fused 1-Benzazepines via [1,5]-Hydride Shift/7-Endo Cyclization Sequences. Suh CW; Kwon SJ; Kim DY Org. Lett. 2017, 19, 1334–1337; [DOI] [PubMed] [Google Scholar]; f) Direct Intermolecular C─H Functionalization Triggered by 1,5-Hydride Shift: Access to N-Arylprolinamides via Ugi-Type Reaction. Zhen L; Wang J; Xu Q-L; Sun H; Wen X; Wang G Org. Lett. 2017, 19, 1566–1569; [DOI] [PubMed] [Google Scholar]; g) Synthesis of Spirooxindoles via the tert-Amino Effect. Ramakumar K; Maji T; Partridge JJ; Tunge JA Org. Lett. 2017, 19, 4014–4017; [DOI] [PubMed] [Google Scholar]; h) Construction of the tetrahydroquinoline spiro skeleton via cascade [1,5]-hydride transfer-involved C(sp3)-H functionalization “on water”. Zhu S; Chen C; Xiao M; Yu L; Wang L; Xiao J Green Chem. 2017, 19, 5653–5658; [Google Scholar]; i) Organocatalytic C(sp3)─H Functionalization via Carbocation-Initiated Cascade [1,5]-Hydride Transfer/Cyclization: Synthesis of Dihydrodibenzo[b,e]azepines. Li S-S; Zhou L; Wang L; Zhao H; Yu L; Xiao J Org. Lett. 2018, 20, 138–141; [DOI] [PubMed] [Google Scholar]; j) Intramolecular hydride transfer onto arynes: redox-neutral and transition metal-free C(sp3)-H functionalization of amines. Idiris FIM; Majeste CE; Craven GB; Jones CR Chem. Sci. 2018, 9, 2873–2878; [DOI] [PMC free article] [PubMed] [Google Scholar]; k) Redox-triggered cascade dearomative cyclizations enabled by hexafluoroisopropanol. Li S-S; Lv X; Ren D; Shao C-L; Liu Q; Xiao J Chem. Sci. 2018, 9, 8253–8259; [DOI] [PMC free article] [PubMed] [Google Scholar]; l) Second-Generation Palladium Catalyst System for Transannular C─H Functionalization of Azabicycloalkanes. Cabrera PJ; Lee M; Sanford MS J. Am. Chem. Soc. 2018, 140, 5599–5606; [DOI] [PMC free article] [PubMed] [Google Scholar]; m) Chiral Magnesium Bisphosphate-Catalyzed Asymmetric Double C(sp3)─H Bond Functionalization Based on Sequential Hydride Shift/Cyclization Process. Mori K; Isogai R; Kamei Y; Yamanaka M; Akiyama T J. Am. Chem. Soc. 2018, 140, 6203–6207; [DOI] [PubMed] [Google Scholar]; n) C─H Functionalization of Amines via Alkene-Derived Nucleophiles through Cooperative Action of Chiral and Achiral Lewis Acid Catalysts: Applications in Enantioselective Synthesis. Shang M; Chan JZ; Cao M; Chang Y; Wang Q; Cook B; Torker S; Wasa M J. Am. Chem. Soc. 2018, 140, 10593–10601; [DOI] [PMC free article] [PubMed] [Google Scholar]; o) Borane-Catalyzed Synthesis of Quinolines Bearing Tetrasubstituted Stereocenters by Hydride Abstraction-Induced Electrocyclization. Maier AFG; Tussing S; Zhu H; Wicker G; Tzvetkova P; Flörke U; Daniliuc CG; Grimme S; Paradies J Chem. Eur. J. 2018, 24, 16287–16291. [DOI] [PubMed] [Google Scholar]
- (4).Early transition metal-catalyzed C─H alkylation: hydroaminoalkylation for Csp3–Csp3 bond formation in the synthesis of selectively substituted amines. Edwards PM; Schafer LL Chem. Commun. 2018, 54, 12543–12560. [DOI] [PubMed] [Google Scholar]
- (5).Tantalum Catalyzed Hydroaminoalkylation for the Synthesis of α- and β-Substituted N-Heterocycles. Payne PR; Garcia P; Eisenberger P; Yim JCH; Schafer LL Org. Lett. 2013, 15, 2182–2185. [DOI] [PubMed] [Google Scholar]
- (6).Electrochemical Aminoxyl-Mediated α-Cyanation of Secondary Piperidines for Pharmaceutical Building Block Diversification. Lennox AJJ; Goes SL; Webster MP; Koolman HF; Djuric SW; Stahl SS J. Am. Chem. Soc. 2018, 140, 11227–11231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7) a).Über Lithium diäthylamid als Hydrid Donator. Wittig G; Schmidt HJ; Renner H Chem. Ber. 1962, 95, 2377–2383; [Google Scholar]; b) Zur Reaktionsweise N-metallierter acyclischer und cyclischer sekundärer Amine. Wittig G; Hesse A Liebigs Ann. Chem. 1971, 746, 149–173; [Google Scholar]; c) Hydrid-Übertragung von Lithium-pyrrolidid auf Azomethine. Wittig G; Hesse A Liebigs Ann. Chem. 1971, 746, 174–184; [Google Scholar]; d) Über die Reaktivität von metallierten Aminen als Hydrid-Donatoren. Wittig G; Häusler G Liebigs Ann. Chem. 1971, 746, 185–199. [Google Scholar]
- (8).Direct α-C─H bond functionalization of unprotected cyclic amines. Chen W; Ma L; Paul A; Seidel D Nat. Chem. 2018, 10, 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).For a review on the hydricity of lithium amides, see: Reduction with lithium dialkylamides. Majewski M; Gleave DM J. Organomet. Chem. 1994, 470, 1–16. [Google Scholar]
- (10) Precedent for these types of reactions is limited: a).Regioselective 2-alkylation and 2-arylation of piperidine and pyrrolidine via organolithiation of cyclic imines. Scully FE J. Org. Chem. 1980, 45, 1515–1517; [Google Scholar]; b) A Convenient Preparation of 3-Aza-2-phenyl Bicyclo[3.2.2]nonane and Related 2-Substituted Cyclic Amines. Healy MAM; Smith SA; Stemp G Synth. Commun. 1995, 25, 3789–3797. [Google Scholar]
- (11) Examples of imine activation with BF3 etherate or TMS triflate: a).1. Boron trifluoride activated 3-thiazolines. An efficient preparation of functionalized thiazolidines. Meltz CN; Volkmann RA Tetrahedron Lett. 1983, 24, 4503–4506; [Google Scholar]; b) Addition of alkynyl anions to aldimines containing α-hydrogens: a novel synthesis of β-aminoacetylenes. Wada M; Sakurai Y; Akiba K.-y. Tetrahedron Lett. 1984, 25, 1083–1084; [Google Scholar]; c) The Activation of Imines to Nucleophilic Attack by Grignard Reagents. Brook MA; Jahangir Synth. Commun. 1988, 18, 893–898; [Google Scholar]; d) Alkylation of 3,4-Dihydro-beta-carboline. Kawate T; Nakagawa M; Yamazaki H; Hirayama M; Hino T Chem. Pharm. Bull. 1993, 41, 287–291; [Google Scholar]; e) BF3-Mediated Addition of Lithium Phenylacetylide to an Imine: Correlations of Structures and Reactivities. BF3·R3N Derivatives as Substitutes for BF3·Et2O. Aubrecht KB; Winemiller MD; Collum DB J. Am. Chem. Soc. 2000, 122, 11084–11089; [Google Scholar]; f) BF3-Mediated Additions of Organolithiums to Ketimines: X-ray Crystal Structures of BF3–Ketimine Complexes. Ma Y; Lobkovsky E; Collum DB J. Org. Chem. 2005, 70, 2335–2337. [DOI] [PubMed] [Google Scholar]
- (12).For the activation of imines with TMS benzotriazole see: 1-(Trimethylsilyl)benzotriazole-Assisted Addition of Grignard Reagents to Imines: A Versatile Approach to Aliphatic Secondary Amines. Katritzky AR; Hong Q; Yang Z J. Org. Chem. 1994, 59, 7947–7948. [Google Scholar]
- (13) For the addition of organometallics to BF3-activated pyridines, see: a).Transition-Metal-Free BF3-Mediated Regioselective Direct Alkylation and Arylation of Functionalized Pyridines Using Grignard or Organozinc Reagents. Chen Q; du Jourdin XM; Knochel P J. Am. Chem. Soc. 2013, 135, 4958–4961; [DOI] [PubMed] [Google Scholar]; b) Transition-Metal-Free BF3-Mediated Oxidative and Non-Oxidative Cross-Coupling of Pyridines. Chen Q; León T; Knochel P Angew. Chem. Int. Ed. 2014, 53, 8746–8750. [DOI] [PubMed] [Google Scholar]
- (14).For instance, 1-piperideine has a strong propensity to trimerize: Copper-Catalyzed Asymmetric Propargylation of Cyclic Aldimines. Fandrick DR; Hart CA; Okafor IS; Mercadante MA; Sanyal S; Masters JT; Sarvestani M; Fandrick KR; Stockdill JL; Grinberg N; Gonnella N; Lee H; Senanayake CH Org. Lett. 2016, 18, 6192–6195. [DOI] [PubMed] [Google Scholar]
- (15) Selected applications of imine trimers: a).Facile synthesis of N-acyl-2-pyrrolines. Kraus GA; Neuenschwander K J. Org. Chem. 1981, 46, 4791–4792; [Google Scholar]; b) Introduction of carbon unit at the alpha-position of alicyclic amines utilizing a decarboxylative reaction with malonic acid derivatives. Fukawa H; Terao Y; Achiwa K; Sekiya M Chem. Lett. 1982, 11, 231–232; [Google Scholar]; c) A New Method for the Introduction of Arylthio Groups at the alpha-Position of Alicyclic Amines. Terao Y; Yasumoto Y; Ikeda K; Sekiya M Chem. Pharm. Bull. 1986, 34, 105–108; [Google Scholar]; d) A new route to ene carbamates, precursors to benzoindolizinones through sequential asymmetric hydrogenation and cyclization. Couture A; Deniau E; Lebrun S; Grandclaudon P; Carpentier J-F J. Chem. Soc., Perkin Trans 1 1998, 1403–1408; [Google Scholar]; e) Design and Photochemical Characterization of a Biomimetic Light-Driven Z/E Switcher. Sampedro D; Migani A; Pepi A; Busi E; Basosi R; Latterini L; Elisei F; Fusi S; Ponticelli F; Zanirato V; Olivucci M J. Am. Chem. Soc. 2004, 126, 9349–9359; [DOI] [PubMed] [Google Scholar]; f) The reaction of cyclic imines with the Ruppert–Prakash reagent. Facile approach to α-trifluoromethylated nornicotine, anabazine, and homoanabazine. Shevchenko NE; Vlasov K; Nenajdenko VG; Röschenthaler G-V Tetrahedron 2011, 67, 69–74. [Google Scholar]
- (16).See the Supporting Information for details.
- (17).Discovering risperidone: the LSD model of psychopathology. Colpaert FC Nat. Rev. Drug Discov. 2003, 2, 315–320. [DOI] [PubMed] [Google Scholar]
- (18) a).A LiCl-Mediated Br/Mg Exchange Reaction for the Preparation of Functionalized Aryl- and Heteroarylmagnesium Compounds from Organic Bromides. Krasovskiy A; Knochel P Angew. Chem. Int. Ed. 2004, 43, 3333–3336; [DOI] [PubMed] [Google Scholar]; b) Progress and developments in the turbo Grignard reagent i-PrMgCl·LiCl: a ten-year journey. Li-Yuan Bao R; Zhao R; Shi L Chem. Commun. 2015, 51, 6884–6900; [DOI] [PubMed] [Google Scholar]; c) Improving the Halogen–Magnesium Exchange by using New Turbo-Grignard Reagents. Ziegler DS; Wei B; Knochel P Chem. Eur. J. 2019, 25, 2695–2703. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.