Abstract
It is becoming increasingly apparent that the tumor microenvironment plays a critical role in human breast cancer onset and progression. Therefore, we isolated cancer-associated fibroblasts (CAFs) from human breast cancer lesions and studied their properties, as compared with normal mammary fibroblasts (NFs) isolated from the same patient. Here, we demonstrate that 8 out of 11 CAFs show dramatic downregulation of caveolin-1 (Cav-1) protein expression; Cav-1 is a well-established marker that is normally decreased during the oncogenic transformation of fibroblasts. Next, we performed gene expression profiling studies (DNA microarray) and established a CAF gene expression signature. Interestingly, the expression signature associated with CAFs encompasses a large number of genes that are regulated via the RB-pathway. The CAF gene signature is also predictive of poor clinical outcome in breast cancer patients that were treated with tamoxifen mono-therapy, indicating that CAFs may be useful for predicting the response to hormonal therapy. Finally, we show that replacement of Cav-1 expression in CAFs (using a cell-permeable peptide approach) is sufficient to revert their hyper-proliferative phenotype and prevent RB hyper-phosphorylation. Taken together, these studies highlight the critical role of Cav-1 downregulation in maintaining the abnormal phenotype of human breast cancer-associated fibroblasts.
Keywords: caveolin-1, invasive breast cancer, mammary fibroblasts
Introduction
Until recently, the study of breast cancer onset and progression has been focused on the epithelial components of the tumor, with little attention given to the surrounding tumor stroma. New evidence has now emerged suggesting a key interaction between mammary epithelia and the adjacent tumor stroma, which is changing the way breast cancer is perceived.
During breast cancer onset, a developmental switch occurs, changing the normal stroma composed of fat, basement membrane and fibroblasts, to a desmoplastic or reactive stroma. This new reactive stroma shows increased collagen and extracellular matrix deposition produced by hyper-proliferative activated fibroblasts, with myofibroblast characteristics.1 This tumor microenvironment (or cancer stroma) also contains more angiogenic components and increased inflammatory cell recruitment.2,3 Interestingly, tumor-associated fibroblasts behave similarly to wound repair fibroblasts, which have increased contractility, and induce angiogenesis, as well as increase epithelial growth through the secretion of cytokines, ECM and growth factors.4,5 However, unlike wound-healing fibroblasts, CAFs remain activated and do not undergo spontaneous quiescence or apoptosis, as seen during wound closure.6
Also, there is increasing evidence suggesting that CAFs could be involved in the degradation of the matrix surrounding the tumor, causing stromal invasion. Indeed, CAFs have been shown to secrete important proteolytic enzymes such as matrix-degrading metalloproteinases (MMPs), transforming growth factor (TGF)β, platelet-derived growth factors (PDGF), hepatocyte growth factor (HGF) and other growth factors, suggestive an active role in tumor invasion.7,8
Caveolins (Cav-1, −2 and −3) are the principal structural proteins coating caveolae, small omega-shaped invaginations of the plasma membrane, measuring 50–100-nm in diameter. In cell culture, the transformation of NIH-3T3 fibroblasts with various activated oncogenes, such as H-Ras (G12V), Bcr-Abl or v-Abl causes dramatic reductions in caveolin-1 (Cav-1) protein expression.9,10 Interestingly, the levels of Cav-1 protein expression inversely correlate with the ability of these fibroblasts to undergo anchorage-independent growth in soft agar; that is lower Cav-1 levels result in larger colony size.10 In these cells, Cav-1 behaves as a transformation suppressor protein as anchorage-independent growth can be reversed by the re-expression of Cav-1 via an inducible system.9 Furthermore, the knockdown of endogenous Cav-1 in NIH-3T3 fibroblasts, using an antisense approach, promotes anchorage-independent growth in soft agar and tumor formation in nude mice, which again could be reversed by Cav-1 re-expression, demonstrating a direct role for Cav-1 in regulating fibroblast cell growth.11 Finally, genetic evidence has been presented that Cav-1 functions as a negative regulator of cell cycle progression, as Cav-1 (−/−) fibroblasts are hyper-proliferative and Cav-1 re-expression drives their arrest in the G0/G1 phase of the cell cycle.12 The ability of Cav-1 to drive cell cycle arrest has been previously mapped to the caveolin-scaffolding domain (residues 82–101), which also functions as a broad-spectrum kinase inhibitor.13 Thus, loss of Cav-1 is a marker of oncogenic transformation in fibroblasts, where it normally behaves as a transformation suppressor that prevents cell cycle progression. These findings may have important implications for understanding the growth-promoting properties of the tumor micro-enviroment. However, the status of Cav-1 expression and function in human tumor-associated fibroblasts has never been assessed.
Although CAFs have been shown to demonstrate enhanced proliferation capacity, increased migration, and the ability to enhance tumor growth in co-culture experiments, very little is known about the molecular mechanisms regulating their hyper-proliferative phenotype.14,15 Here, we show that Cav-1 levels are decreased in CAFs, when compared to matching normal fibroblasts (NFs) from the same patient. Additionally, we functionally demonstrate that loss of Cav-1 plays a key role in maintaining the hyper-proliferative phenotype of CAFs. Thus, understanding the role of Cav-1 in the proliferation of CAFs could be an important new step in the development of novel therapeutic strategies targeting the tumor micro-environment.
Results
Breast tissue and tumor morphology following surgical resection
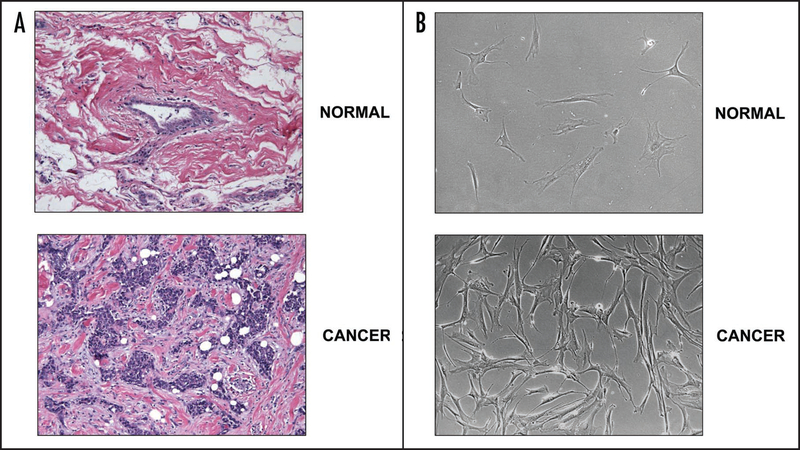
In order to visualize the reactive stroma in breast tumors removed from breast cancer patients, hematoxylin and eosin (H&E) staining was performed on each tumor, and compared with matching normal adjacent tissue from the same patient. A representative example is shown in Figure 1A. Briefly, these H&E stained sections show (i) a normal mammary duct surrounded by normal stroma (upper) and (ii) an invasive tumor surrounded by reactive stroma, with an increased population of fibroblasts (lower).
Figure 1.
Morphology of breast tissue and mammary stromal fibroblasts. (A) Representative images of H&E stained sections from the tissues used to generate the primary cultures of breast stromal fibroblasts are shown. Upper and lower panels correspond to normal breast tissue and invasive ductal carcinoma (IDC), respectively. The stroma surrounding the invasive ductal carcinoma is highly reactive, containing many fibroblasts, as demonstrated by small and numerous hematoxylin-stained nuclei. All tumors were matched with normal tissue from the same patient. Images were taken with a 20x objective using an Olympus BX51 microscope, a Qimaging Micropublisher 5.0 camera and iVision software. (B) Phase images of primary cultures of fibroblasts isolated from invasive ductal carcinomas (lower) and matching fibroblasts from adjacent normal breast tissue (upper) of the same patient. Fibroblasts isolated from the breast tumor appear more elongated and spindle-shaped. Images were taken at 10x.
Morphology of primary cultures of normal fibroblasts (NFs) and cancer-associated fibroblasts (CAFs)
Primary fibroblast cell cultures were generated from the tissues obtained after surgical resection of the breast tumor masses from 11 female patients with invasive ductal carcinoma (IDC). Figure 1B shows phase images of normal mammary fibroblasts (NFs) isolated from unaffected adjacent tissue (upper) and cancer-associated fibroblasts (CAFs) isolated from the mammary tumor mass (lower). Interestingly, CAFs are more numerous and appear elongated as compared to NFs, suggestive of a transformed phenotype.
Caveolin-1 protein levels are decreased in CAFs, consistent with a transformed phenotype
Loss of Cav-1 protein expression has been shown to be a marker of oncogenic transformation in fibroblasts.9,10 Thus, we next subjected CAFs and matched NFs from the same patient to Western blot analysis with anti-Cav-1 IgG. The results are shown in Figure 2.
Figure 2.
Downregulation of Cav-1 protein levels in CAFs versus NFs. (A) Fold changes in Cav-1 protein expression in CAFs vs. NFs generated from 11 breast cancer patients. Patient results were divided into three groups: (A), loss of Cav-1 expression; (B), no change in Cav-1 expression; and (C), increased Cav-1 expression. Note that most of the tumor-associated fibroblasts (from 8 of 11 patients) had a reduction in Cav-1 expression (n = 8), while one had no change, and two showed an increase. Thus, we focused our efforts on the patients showing a loss of Cav-1 expression. G corresponds to group and N to number of patients. (B) Immunoblot analysis of Cav-1 expression shows a decrease in Cav-1 levels in cancer-associated fibroblasts (C) when compared to adjacent normal fibroblasts (N). All the tumors analyzed had a matched normal tissue control from the same patient. β-actin was used as a loading control to assure equal loading. 30 μg of total protein lysate was loaded in each lane.
Interestingly, when densitometry analysis was performed, Cav-1 levels were significantly decreased in the majority of cases analyzed (8 out of 11 patients examined; Fig. 2A). The immunoblots for Cav-1 are shown in Figure 2B, where the levels of Cav-1 are decreased in CAFs, as compared to matching NFs. The results were normalized against β-actin. Subsequent analyses were focused on those CAFs with reduced Cav-1 expression, as this appeared to be the predominant phenotype.
Breast CAFs are hyperproliferative
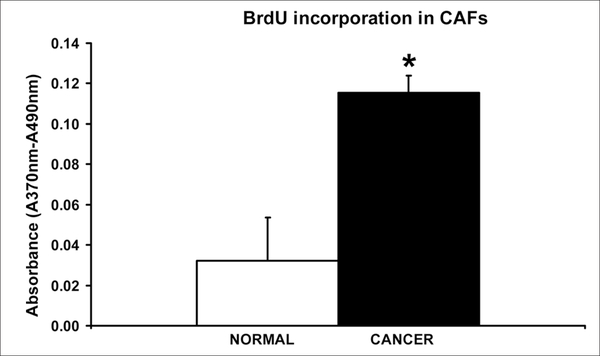
To determine if the CAFs isolated from invasive breast tumor specimens are more proliferative than the normal fibroblasts (NFs) isolated from the same patient, we performed BrdU incorporation assays. BrdU is a pyrimidine analogue that is incorporated into DNA when cells undergo DNA replication following a G1-S transition. When both cell types (CAFs versus NFs) were plated at identical densities and left to grow for 72 hrs, the CAFs incorporated ~3.6 fold more BrdU than their normal counterparts (Fig. 3; p < 0.05). The absorbance (A370 nm–A490 nm) is proportional to the BrdU incorporated by the cells after two hours of incubation with the analogue. It is important to note that similar results were obtained when BrdU incorporation was measured in CAFs and NFs isolated from several different patients who showed decreased Cav-1 levels in their CAFs.
Figure 3.
CAFs exhibit a hyper-proliferative phenotype. Equal numbers of normal and cancer fibroblasts were plated for 72 hrs and given a two hour pulse of BrdU. Using an ELISA kit, the absorbance was then measured at 370 nm with a reference of 490 nm. The absorbance is reflective of the amount of BrdU incorporated by the cells. CAFs show a ~3.6 fold increase in BrdU incorporation. *p < 0.05. Quantitatively similar results were obtained when BrdU incorporation was measured in CAFs and NFs isolated from several different patients.
Using gene expression profiling to mechanistically dissect the hyper-proliferative phenotype of CAFs
To understand the mechanisms responsible for the increased proliferation in CAFs, we randomly selected three matched pairs of NFs and CAFs (that showed loss of Cav-1 expression), and subjected them to gene expression microarray analyses. Each matched pair of NFs and CAFs demonstrated significant changes in gene expression, with ~1,500 to 3,000 altered transcripts per pair. Thus, we defined gene sets that were commonly upregulated or downregulated in CAFs vs. NFs (See Suppl. Data for a detailed list).
By comparing all three pairs of NFs and CAFs, we generated a gene signature that consists of 118 upregulated known genes (Table 1) and 66 downregulated known genes (Table 2) (See also Fig. 4A). All of these genes were changed more than 2-fold.
Table 1.
Breast cancer-associated fibroblast (CAF) gene signature—upregulated transcripts
| ADAM12 | ADAM metallopeptidase domain 12 (meltrin alpha) |
| ALDH1A3 | aldehyde dehydrogenase 1 family, member A3 |
| ANLN | anillin, actin binding protein (scraps homolog, Drosophila) |
| APOBEC3B | apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like 3B |
| ARSI | arylsulfatase family, member I |
| AURKA | aurora kinase A |
| BHLHB2 | basic helix-loop-helix domain containing, class B, 2 |
| BIRC5 | baculoviral IAP repeat-containing 5 (survivin) |
| BLM | Bloom syndrome |
| BRCA1 | breast cancer 1, early onset |
| BUB1 | BUB1 budding uninhibited by benzimidazoles 1 homolog (yeast) |
| BUB1B | BUB1 budding uninhibited by benzimidazoles 1 homolog beta (yeast) |
| C13orf3 | chromosome 13 open reading frame 3 |
| C14orf145 | chromosome 14 open reading frame 145 |
| C20orf42 | chromosome 20 open reading frame 42 |
| C5orf13 | chromosome 5 open reading frame 13 |
| C9orf140 | chromosome 9 open reading frame 140 |
| C9orf46 | chromosome 9 open reading frame 46 |
| CALB2 | calbindin 2, 29 kDa (calretinin) |
| CASC5 | cancer susceptibility candidate 5 |
| CBR3 | carbonyl reductase 3 |
| CCDC34 | coiled-coil domain containing 34 |
| CCNB2 | cyclin B2 |
| CCNF | cyclin F |
| CD44 | CD44 molecule (Indian blood group) |
| CDC2 | cell division cycle 2, G1 to S and G2 to M |
| CDC20 | CDC20 cell division cycle 20 homolog (S. cerevisiae) |
| CDC45L | CDC45 cell division cycle 45-like (S. cerevisiae) |
| CDCA1 | cell division cycle associated 1 |
| CDCA3 | cell division cycle associated 3 |
| CDCA5 | cell division cycle associated 5 |
| CDCA8 | cell division cycle associated 8 |
| CENPA | centromere protein A |
| CENPF | centromere protein F, 350/100ka (mitosin) |
| CENPK | centromere protein K |
| CENPM | centromere protein M |
| CEP55 | centrosomal protein 55 kDa |
| CKAP2L | cytoskeleton associated protein 2-like |
| COTL1 | coactosin-like 1 (Dictyostelium) |
| DCBLD2 | discoidin, CUB and LCCL domain containing 2 |
| DEPDC1 | DEP domain containing 1 |
| DKFZP761M1511 | NA |
| DKFZp762E1312 | NA |
| DLG7 | discs, large homolog 7 (Drosophila) |
| DTL | denticleless homolog (Drosophila) |
| E2F7 | E2F transcription factor 7 |
| E2F8 | E2F transcription factor 8 |
| EPHA4 | EPH receptor A4 |
| FABP5 | fatty acid binding protein 5 (psoriasis-associated) |
| FAM83D | family with sequence similarity 83, member D |
| FANCD2 | Fanconi anemia, complementation group D2 |
| FKBP5 | FK506 binding protein 5 |
| FOXM1 | forkhead box M1 |
| GINS2 | GINS complex subunit 2 (Psf2 homolog) |
| GTSE1 | G-2 and S-phase expressed 1 |
| HCAP-G | non-SMC condensin I complex, subunit G |
| HMMR | hyaluronan-mediated motility receptor (RHAMM) |
| IL27RA | interleukin 27 receptor, alpha |
| KCNMA1 | potassium large conductance calcium-activated channel, subfamily M, alpha member 1 |
| KIAA0101 | KIAA0101 |
| KIAA1794 | KIAA1794 |
| KIF11 | kinesin family member 11 |
| KIF14 | kinesin family member 14 |
| KIF15 | kinesin family member 15 |
| KIF20A | kinesin family member 20A |
| KIF23 | kinesin family member 23 |
| KIF2C | kinesin family member 2C |
| KIF4A | kinesin family member 4A |
| KNTC2 | kinetochore associated 2 |
| LOC146909 | NA |
| LOC150084 | NA |
| LOC283824 | NA |
| LOC653594 | NA |
| LPXN | leupaxin |
| MAD2L1 | MAD2 mitotic arrest deficient-like 1 (yeast) |
| MCM10 | MCM10 minichromosome maintenance deficient 10 (S. cerevisiae) |
| MCM2 | MCM2 minichromosome maintenance deficient 2, mitotin (S. cerevisiae) |
| MCM5 | MCM5 minichromosome maintenance deficient 5, cell division cycle 46 (S. cerevisiae) |
| MELK | maternal embryonic leucine zipper kinase |
| MET | met proto-oncogene (hepatocyte growth factor receptor) |
| MKI67 | antigen identified by monoclonal antibody Ki-67 |
| MLF1IP | MLF1 interacting protein |
| MMP1 | matrix metallopeptidase 1 (interstitial collagenase) |
| MND1 | meiotic nuclear divisions 1 homolog (S. cerevisiae) |
| MT1M | metallothionein 1 M |
| NEBL | nebulette |
| NEFL | neurofilament, light polypeptide 68 kDa |
| NEK2 | NIMA (never in mitosis gene a)-related kinase 2 |
| NET1 | neuroepithelial cell transforming gene 1 |
| NPHP1 | nephronophthisis 1 (juvenile) |
| NRG1 | neuregulin 1 |
| NUSAP1 | nucleolar and spindle associated protein 1 |
| ODZ2 | odz, odd Oz/ten-m homolog 2 (Drosophila) |
| OIP5 | Opa interacting protein 5 |
| PBK | PDZ binding kinase |
| PLAUR | plasminogen activator, urokinase receptor |
| PLK1 | polo-like kinase 1 (Drosophila) |
| PLP2 | proteolipid protein 2 (colonic epithelium-enriched) |
| POLE2 | polymerase (DNA directed), epsilon 2 (p59 subunit) |
| PPAPDC1A | phosphatidic acid phosphatase type 2 domain containing 1A |
| PRC1 | protein regulator of cytokinesis 1 |
| PRIM1 | primase, polypeptide 1, 49 kDa |
| PTHLH | parathyroid hormone-like hormone |
| PTTG1 | pituitary tumor-transforming 1 |
| RACGAP1 | Rac GTPase activating protein 1 |
| RAD51AP1 | RAD51 associated protein 1 |
| RAD54L | RAD54-like (S. cerevisiae) |
| RNASEH2A | ribonuclease H2, subunit A |
| RRM2 | ribonucleotide reductase M2 polypeptide |
| SHCBP1 | SHC SH2-domain binding protein 1 |
| SLC16A3 | solute carrier family 16, member 3 (monocarboxylic acid transporter 4) |
| SMS | spermine synthase |
| SPAG5 | sperm associated antigen 5 |
| SPBC24 | spindle pole body component 24 homolog (S. cerevisiae) |
| SPBC25 | spindle pole body component 25 homolog (S. cerevisiae) |
| STMN1 | stathmin 1/oncoprotein 18 |
| STMN3 | stathmin-like 3 |
| TCF19 | transcription factor 19 (SC1) |
| TK1 | thymidine kinase 1, soluble |
| TOP2A | topoisomerase (DNA) II alpha 170 kDa |
| TPX2 | TPX2, microtubule-associated, homolog (Xenopus laevis) |
| TRIP13 | thyroid hormone receptor interactor 13 |
| TSPAN13 | tetraspanin 13 |
| TTK | TTK protein kinase |
| TYMS | thymidylate synthetase |
| XYLT1 | xylosyltransferase I |
Genes shown in BOLD are part of an RB/E2F gene signature. Table represents 118 known genes and 8 known transcripts.
Table 2.
Breast cancer-associated fibroblast (CAF) gene signature—downregulated transcripts
| ADAMTS5 | ADAM metallopeptidase with thrombospondin type 1 motif, 5 (aggrecanase-2) |
| ADD3 | adducin 3 (gamma) |
| ADH1B | alcohol dehydrogenase IB (class I), beta polypeptide |
| AKR1C3 | aldo-keto reductase family 1, member C3 (3-alpha hydroxysteroid dehydrogenase, type III) |
| ALS2CR19 | amyotrophic lateral sclerosis 2 (juvenile) chromosome region, candidate 1 |
| BANK1 | B-cell scaffold protein with ankyrin repeats 1 |
| BEX1 | brain expressed, X-linked 1 |
| CASP1 | caspase 1, apoptosis-related cysteine peptidase (interleukin 1, beta, convertase) |
| CD36 | CD36 molecule (thrombospondin receptor) |
| COL16A1 | collagen, type XVI, alpha 1 |
| COP1 | constitutive photomorphogenic protein |
| CYP2U1 | cytochrome P450, family 2, subfamily U, polypeptide |
| DBC1 | deleted in bladder cancer 1 |
| DSCR1L1 | Down syndrome critical region gene 1-like 1 |
| EEA1 | early endosome antigen 1, 162 kD |
| EMX2 | empty spiracles homolog 2 (Drosophila) |
| EMX2OS | empty spiracles homolog 2 (Drosophila) opposite strand |
| FABP3 | fatty acid binding protein 3, muscle and heart (mammary-derived growth inhibitor) |
| FHL1 | four and a half LIM domains 1 |
| GALNTL1 | UDP-N-acetyl-alpha-D-galactosamine:polypeptide, N-acetylgalactosaminyltransferase-like 1 |
| HNMT | histamine N-methyltransferase |
| INMT | indolethylamine N-methyltransferase |
| ITGA1 | integrin, alpha 1 |
| ITPR1 | inositol 1,4,5-triphosphate receptor, type 1 |
| KCTD12 | potassium channel tetramerisation domain containing 12 |
| LEPR | leptin receptor |
| LMCD1 | LIM and cysteine-rich domains 1 |
| LOC221091 | NA |
| LOC387763 | NA |
| LRRC32 | leucine rich repeat containing 32 |
| MACF1 | microtubule-actin crosslinking factor 1 |
| MALAT1 | metastasis associated lung adenocarcinoma transcript 1 (non-coding RNA) |
| MAN1C1 | mannosidase, alpha, class 1C, member 1 |
| MID1 | midline 1 (Opitz/BBB syndrome) |
| MRVI1 | murine retrovirus integration site 1 homolog |
| MTBP | Mdm2, transformed 3T3 cell double minute 2, p53 binding protein (mouse) binding protein, 104 kDa |
| MYH10 | myosin, heavy polypeptide 10, non-muscle |
| NANOS1 | nanos homolog 1 (Drosophila) |
| NID2 | nidogen 2 (osteonidogen) |
| PAPPA | pregnancy-associated plasma protein A, pappalysin 1 |
| PCSK5 | proprotein convertase subtilisin/kexin type 5 |
| PDGFD | platelet derived growth factor D |
| PDGFRB | platelet-derived growth factor receptor, beta polypeptide |
| PELO | pelota homolog (Drosophila) |
| PKIB | protein kinase (cAMP-dependent, catalytic) inhibitor beta |
| PLGLA1 | plasminogen-like A1 |
| PLXND1 | plexin D1 |
| PPARG | peroxisome proliferative activated receptor, gamma |
| PPL | periplakin |
| PRKG1 | protein kinase, cGMP-dependent, type I |
| RBMS3 | RNA binding motif, single stranded interacting protein |
| RSPO3 | R-spondin 3 homolog (Xenopus laevis) |
| SLC40A1 | solute carrier family 40 (iron-regulated transporter), member 1 |
| SLC5A3 | solute carrier family 5 (inositol transporters), member 3 |
| SNTB1 | syntrophin, beta 1 (dystrophin-associated protein A1, 59 kDa, basic component 1) |
| STAT1 | signal transducer and activator of transcription 1, 91 kDa |
| STMN2 | stathmin-like 2 |
| STAU2 | staufen, RNA binding protein, homolog 2 (Drosophila) |
| SUSD2 | sushi domain containing 2 |
| SVEP1 | sushi, von Willebrand factor type A, EGF and pentraxin domain containing 1 |
| SYNPO2 | synaptopodin 2 |
| THRB | thyroid hormone receptor, beta (erythroblastic leukemia viral (v-erb-a) oncogene homolog 2, avian) |
| TMEM16D | transmembrane protein 16D |
| TNFRSF21 | tumor necrosis factor receptor superfamily, member 21 |
| TNXB | tenascin XB |
| UBE2E2 | ubiquitin-conjugating enzyme E2E 2 (UBC4/5 homolog, yeast) |
| ZCCHC7 | zinc finger, CCHC domain containing 7 |
| ZFP36L2 | zinc finger protein 36, C3H type-like 2 |
Table represents 66 known genes and 2 known transcripts.
Figure 4.
High expression of the breast CAF gene signature is associated with poor clinical outcome in breast cancer patients treated with tamoxifen monotherapy. (A) Venn diagrams summarizing how the two gene signatures were derived by comparing and intersecting the gene sets from matched NFs and CAFs from three different patients. (B) Gene expression data from 60 ER-positive human breast tumors that were both micro- and macrodissected were analyzed for the expression pattern of 118 genes upregulated in CAFs. A core of proliferation associated genes that are regulated by the RB/E2F pathway (marked in red) strongly co-segregated in this analyses. (C) A Kaplan-Meyer survival analysis was conducted, wherein the recurrence of those tumors in the highest quartile of overall expression was compared against the remainder of the cohort (p < 0.001). Patients in the High CAF gene expression group had a poor prognosis on Tamoxifen mono-therapy, with greater than a 3.8-fold reduction in recurrence-free survival.
Gene ontology analysis revealed that the 118 upregulated transcripts exhibit a strong enrichment for genes involved in cell cycle control (Table 3). Correspondingly, 44 genes within those upregulated in CAFs (Table 4) are part of an RB/E2F gene signature, associated with RB functional inactivation.23 Similarly, we found that MET and its co-receptor CD44 are both upregulated and are part of the 118 gene signature, suggesting that the HGF/MET signaling axis is also activated in CAFs. In contrast, the downregulated genes exhibited only a weak enrichment for genes involved in extracellular matrix biology and adhesion.
Table 3.
Gene ontology analysis of human breast CAF gene sets
| Gene ontology terms/biological process | p-value |
|---|---|
| CAFs—upregulated genes | |
| Mitotic cell cycle | 3.40E-30 |
| M phase | 2.40E-26 |
| Regulation of cell cycle | 8.60E-14 |
| Organelle organization and biogenesis | 8.10E-09 |
| Sister chromatid segregation | 9.60E-06 |
| Establishment of organelle localization | 1.90E-05 |
| DNA repair | 1.10E-04 |
| Chromosome localization | 3.80E-04 |
| Interphase | 6.00E-04 |
| Biopolymer metabolism | 2.30E-03 |
| Establishment of cellular localization | 5.10E-03 |
| Cellular localization | 5.40E-03 |
| Cytokinesis | 8.20E-03 |
| CAFs—downregulated genes | |
| Cell-matrix adhesion | 1.50E-02 |
Table 4.
Breast cancer-associated fibroblast (CAF) RB/E2F gene signature—44 upregulated genes
| ANLN | anillin, actin binding protein (scraps homolog, Drosophila) |
| AURKA | aurora kinase A |
| BIRC5 | baculoviral IAP repeat-containing 5 (survivin) |
| BLM | Bloom syndrome |
| BRCA1 | breast cancer 1, early onset |
| BUB1 | BUB1 budding uninhibited by benzimidazoles 1 homolog (yeast) |
| CCNB2 | cyclin B2 |
| CCNF | cyclin F |
| CDC2 | cell division cycle 2, G1 to S and G2 to M |
| CDC20 | CDC20 cell division cycle 20 homolog (S. cerevisiae) |
| CDC45L | CDC45 cell division cycle 45-like (S. cerevisiae) |
| CDCA3 | cell division cycle associated 3 |
| CDCA5 | cell division cycle associated 5 |
| CDCA8 | cell division cycle associated 8 |
| CENPA | centromere protein A |
| FANCD2 | Fanconi anemia, complementation group D2 |
| FOXM1 | forkhead box M1 |
| GTSE1 | G-2 and S-phase expressed 1 |
| KIF11 | kinesin family member 11 |
| KIF20A | kinesin family member 20A |
| KIF23 | kinesin family member 23 |
| KIF2C | kinesin family member 2C |
| KIF4A | kinesin family member 4A |
| MAD2L1 | MAD2 mitotic arrest deficient-like 1 (yeast) |
| MCM10 | MCM10 minichromosome maintenance deficient 10 (S. cerevisiae) |
| MCM2 | MCM2 minichromosome maintenance deficient 2, mitotin (S. cerevisiae) |
| MCM5 | MCM5 minichromosome maintenance deficient 5, cell division cycle 46 (S. cerevisiae) |
| MKI67 | antigen identified by monoclonal antibody Ki-67 |
| NEK2 | NIMA (never in mitosis gene a)-related kinase 2 |
| NUSAP1 | nucleolar and spindle associated protein 1 |
| PLK1 | polo-like kinase 1 (Drosophila) |
| PRC1 | protein regulator of cytokinesis 1 |
| PRIM1 | primase, polypeptide 1, 49 kDa |
| PTTG1 | pituitary tumor-transforming 1 |
| RAD51AP1 | RAD51 associated protein 1 |
| RAD54L | RAD54-like (S. cerevisiae) |
| RRM2 | ribonucleotide reductase M2 polypeptide |
| STMN1 | stathmin 1/oncoprotein 18 |
| TCF19 | transcription factor 19 (SC1) |
| TK1 | thymidine kinase 1, soluble |
| TOP2A | topoisomerase (DNA) II alpha 170 kDa |
| TRIP13 | thyroid hormone receptor interactor 13 |
| TTK | TTK protein kinase |
| TYMS | thymidylate synthetase |
Those genes that were consistently upregulated in CAFs were utilized to cluster a breast cancer data set to determine their impact on disease outcome. Specifically, we observed that the 118 upregulated gene expression signature correlated with an increased risk of recurrence after tamoxifen mono-therapy (Fig. 4B and C). Thus, high expression of the breast CAF gene signature was associated with a 3.8-fold decrease in recurrence-free survival, in patients treated with tamoxifen mono-therapy.
Interestingly, Cav-1 transcript levels in CAFs were either increased ~2.3–2.4-fold or not changed, suggesting that the loss of Cav-1 protein expression we observed occurs at a post-transcriptional or post-translational level (data not shown).
Phospho-RB, PCNA and MCM-7 are increased in CAFs, consistent with cell cycle progression
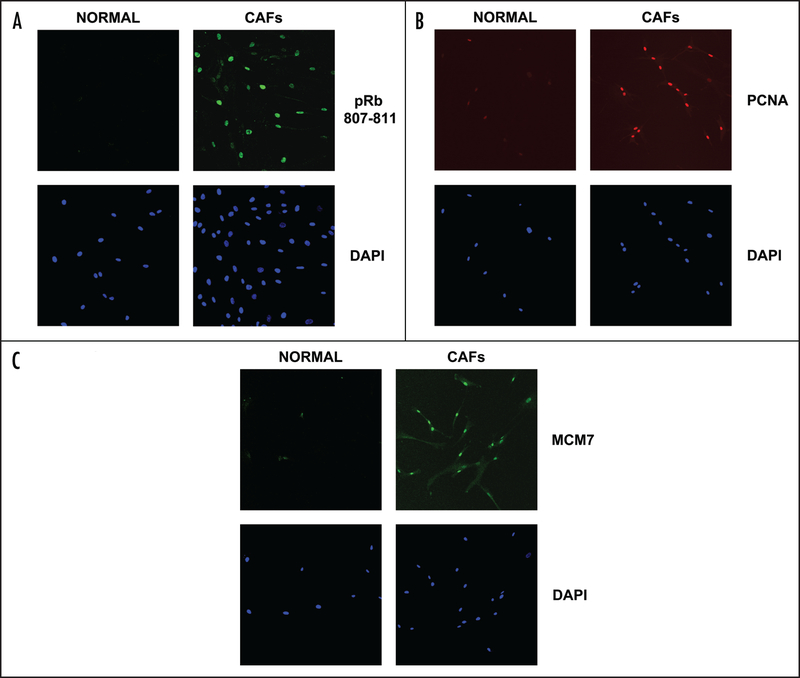
To validate the mechanisms responsible for the increased proliferation in CAFs, we analyzed the levels of well established cell cycle regulators such as phospho-RB (Ser 807/811), PCNA, and MCM7 by immunofluorescence. While the normal fibroblasts expressed these cell cycle proteins very faintly, the CAFs showed an increase in all three nuclear markers, as assessed by their co-localization with DAPI (Fig. 5). The images shown are representative of CAFs isolated from several patients, as compared with matching control NFs from the same patient.
Figure 5.
RB phosphorylation and RB-regulated gene products are increased in CAFs. (A) CAFs have increased phosphorylated RB as compared with normal adjacent fibroblasts, as shown by immunufluorescence (Upper panels), using a phopho-specific antibody that recognizes endogeneous RB only when phosphorylated at serine 807/811. (B) CAFs show an increase in the levels of PCNA when compared with normal adjacent fibroblasts, as shown by confocal microscopy (Upper). (C) CAFs show increases in MCM7 expression, as seen by confocal microscopy (Upper). In (A–C), DAPI staining shows the nuclei of the cells imaged (Lower). Images were taken at 20x. Virtually identical results were obtained using CAFs and NFs isolated from several different patients. Representative images are shown.
Treatment of CAFs with a Cav-1 mimetic peptide rescues their hyper-proliferative phenotype
In order to functionally assess the possible causative role of Cav-1 downregulation in driving the hyperproliferative phenotype of CAFs, we next replaced Cav-1 in CAFs using a cell-permeable peptide approach.
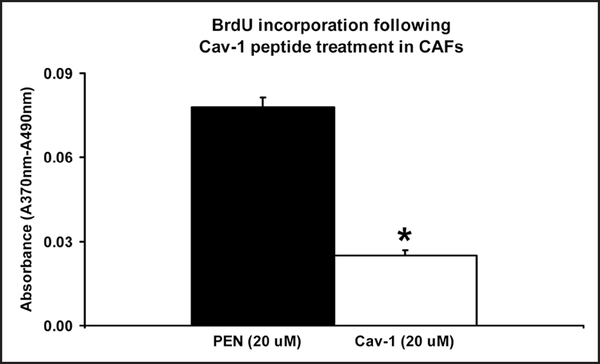
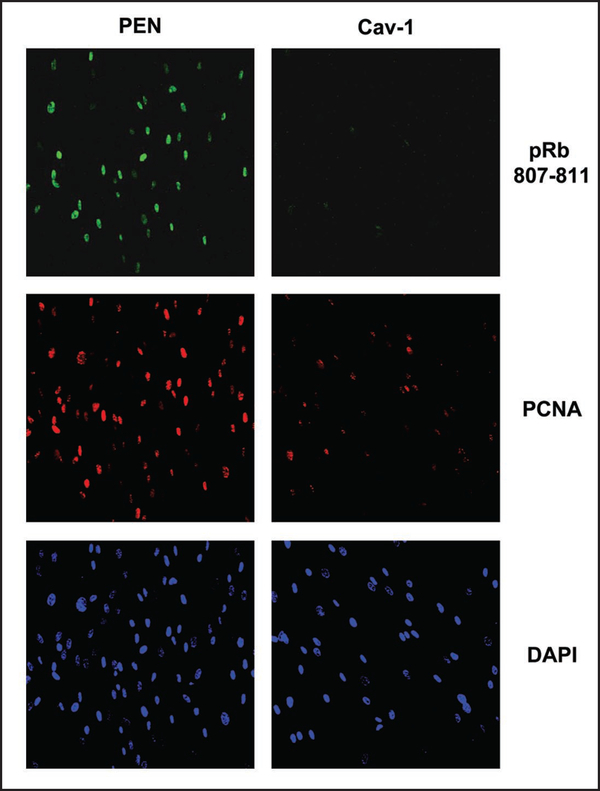
As predicted, treatment with a cell-permeable Cav-1 mimetic peptide was sufficient to reverse their hyper-proliferative phenotype, resulting in a 3-fold reduction in cell proliferation, as measured by BrdU incorporation (Fig. 6). In accordance with these results, phosphorylation of RB on serine 807/811 was completely reversed and PCNA levels were drastically decreased (Fig. 7).
Figure 6.
BrdU incorporation is inhibited by a Cav-1 mimetic peptide in CAFs. Equal numbers of cancer-associated fibroblasts were plated for 24 hrs and given a 20 μM dose of a cell-permeable Cav-1 mimetic peptide attached to penetratin. Following 48 hrs of treatment, the cells were given a two hour pulse of BrdU. Using an ELISA kit, the absorbance was then measured at 370 nm with a reference of 490 nm. CAFs show a 3-fold decrease in BrdU incorporation following Cav-1 treatment. *p < 0.05. Equivalent results were obtained using CAFs isolated from several different patients.
Figure 7.
RB-phosphorylation is inhibited by a Cav-1 mimetic peptide in CAFs. Treatment of CAFs with a Cav-1 mimetic peptide inhibited the phosphorylation of RB as shown by immunufluorescence (Upper), using a phospho-specific antibody that recognizes endogeneous RB only when phosphorylated at serine 807/811. PCNA levels were also decreased by the Cav-1 peptide (Middle). Penetratin (Pen) alone did not affect the levels of phospho-RB or PCNA. DAPI staining shows the nuclei of the cells imaged (Lower). Images were taken at 20x. Virtually identical results were obtained with 5, 10 and 20 μM dosages of the Cav-1 mimetic peptide.
Discussion
Numerous studies now suggest an important and dynamic role for the tumor micro-environment (cancer-associated stromal tissue) in regulating the growth and metastasis of primary tumors. For example, in co-culture experiments, carcinoma-associated fibroblasts (CAFs) extracted from human breast carcinomas were shown to promote the growth of breast carcinoma cells significantly more than normal mammary fibroblasts (NFs) derived from the same patients.24 In vivo, CAFs promote angiogenesis by recruiting endothelial progenitor cells (EPCs), an effect mediated in part by SDF-1.24 Another study demonstrated that prostatic epithelial cells grown in the presence of CAFs become permanently transformed. Importantly, this effect is not observed when normal prostatic fibroblasts are used.25 Similarly, when exposed to the conditioned media of pancreatic CAFs, pancreatic epithelial cells showed an increase in proliferation, migration, invasion and colony formation.26
Since CAFs are thought to play a key role in tumor growth, understanding the mechanisms governing their hyper-proliferation becomes an important focus for targeted tumor therapies. Very little attention has been given to the mechanisms driving their hyperproliferation. To address this issue, we isolated mammary fibroblasts from invasive breast tumors and adjacent normal tissue from the same patient. Then, we subjected these CAFs to a detailed phenotypic analysis. Our results demonstrate for the first time that the Cav-1 protein is downregulated in human breast CAFs. Importantly, Cav-1 is a well-known marker of oncogenic transformation in fibroblasts.10 Loss of Cav-1 expression in CAFs did not seem to be related to the patient’s tumor grade, or breast cancer marker status (ER, PR or Her2), indicating that loss of Cav-1 expression may be a generalized or common event involved in breast cancer initiation.
Next, we used transcriptome analysis (gene expression profiling) to gain mechanistic insight into the hyper-proliferative phenotype of CAFs. Interestingly, our results directly show the dramatic upregulation of a gene expression profile that is known to be part of an RB/E2F gene signature. More specifically, these genes are known to be upregulated when RB is deleted and E2F activity is increased. It is important to note that breast cancer patients that exhibited the high expression CAF signature had a poor prognosis on Tamoxifen monotherapy, with greater than a 3.8-fold reduction in recurrence-free survival. Importantly, this same proliferative gene expression signature has been associated with poor disease outcome in multiple settings, suggesting that gene expression within stromal compartments may be similarly relevant for predicting disease outcome.27–30
Normally, when RB is hypo-phosphorylated in quiescent or differentiated cells, it interacts with and sequesters E2F family transcription factors, repressing the transcription of genes essential for cell cycle progression.31 Replication factors such as PCNA (Proliferating Cell Nuclear Antigen) and MCM7 (Minichromosome Maintenance Protein) are well-characterized RB target gene products. PCNA is a transcription factor which helps the DNA polymerase delta bind to DNA.32 When MCM7 is bound to RB, it inhibits DNA replication and when RB is phosphorylated it releases MCM7 and promotes its assembly in the pre-replicative complex.33 Interestingly, MCM proteins have been shown to be regulated by oncogenes.34
To validate our DNA microarray results, we examined the state of RB-phosphorylation in NFs and CAFs. As predicted, RB was hypo-phosphorylated in NFs and hyper-phosphorylated in CAFs, as demonstrated using an antibody specific for the phospho-serine 807/811 site within the RB protein product. Also, the levels of PCNA and MCM7 were increased in CAFs, consistent with RB inactivation by hyper-phosphorylation. This increase in PCNA and MCM7 in response to RB inactivation was previously demonstrated in other cell systems. For example, a complete knockdown of RB in MCF7 cells with a shRNA causes an increase in PCNA and MCM7 protein levels, when compared to vector alone transfected cells.23 In RB loxP/loxP mouse adult fibroblasts (MAFs), in which the RB gene was excised/inactivated with CRE recombinase, PCNA and MCM7 were also greatly increased as compared with RB (+/+) positive cells.35
Thus far, we have demonstrated that CAFs show Cav-1 downregulation, RB hyper-phosphorylation, and the upregulation of a gene signature associated with RB inactivation. To establish a cause-effect relationship between Cav-1 protein expression and RB activation status, we functionally replaced Cav-1 in CAFs using a cell-permeable Cav-1 mimetic peptide. This peptide was previously shown to block tumor progression in mice as shown by a reduction in Evans blue extravasation within the tumor.36 In a different study, the administration of this Cav-1 peptide has also been shown to decrease inflammation in carrageenan-injected mice.37 We have also previously demonstrated that this Cav-1 mimetic peptide can be used to prevent the development of right ventricular hypertrophy, pulmonary hypertension and pulmonary artery medial hypertrophy in a monocrotaline rat model.38
Interestingly, functional replacement of Cav-1 in CAFs reverted their hyper-proliferative phenotype almost to the level of normal fibroblasts, as shown by decreased BrdU incorporation and a loss of RB-phosphorylation. This negative regulatory effect of Cav-1 on the proliferation of CAFs is in agreement with its known tumor-suppressor function in both mammary epithelia (MCF-7 cells) and oncogene-transformed NIH-3T3 fibroblasts.9,39
Cav-1 has also previously been shown to negatively regulate RB-phosphorylation and PCNA expression in whole animal models, such as Cav-1 (−/−) mice. Following carotid artery ligation, Cav-1 (−/−) mice develop severe neointimal hyperplasia accompanied by an increase in PCNA expression levels and RB-phosphorylation.40 In the intestinal epithelium of Cav-1 (−/−) mice, PCNA levels are increased compared to the wild type mice.41 Similarly, genetic ablation of Cav-1 expression in MMTV-PyMT mice (an established mouse model of mammary tumorigenesis) results in dramatic increases in primary tumor formation and lung metastasis.42 Analysis of primary mammary tumors from these PyMT/Cav-1 (−/−) mice reveals dramatic increases in RB-phosphorylation.42 Interestingly, implantation of PyMT/Cav-1 (+/+) mammary tumor tissue in the mammary fat pads of Cav-1 (−/−) mice results in up to a ~2-fold increase in tumor growth, indicating that the mammary stroma of Cav-1 (−/−) mice has tumor promoting properties.43
In conclusion, this study demonstrates for the first time that Cav-1 is downregulated in breast cancer-associated fibroblasts found within invasive human tumors. Loss of Cav-1 expression can account for their hyper-proliferative phenotype, as replacement of Cav-1 function with a cell-permeable Cav-1 mimetic peptide reverts this hyper-proliferative behavior. We also show that Cav-1 mediates its effects in CAFs via the inhibition of RB phosphorylation and decreases in downstream RB targets such as MCM7 and PCNA. Importantly, these proliferative markers are known to contribute to poor outcome in breast cancer patients. Understanding the role of Cav-1 in regulating the proliferation of CAFs within the cancer stroma will undoubtedly open new possibilities for stromal-targeted therapies to inhibit tumor progression.
Methods
Materials
The Cav-1 scafolding domain was attached to a biotinylated cell permeable Drosophila antennapedia peptide (AP or Penetratin) [(biotin)-RQPKIWFPNRRKPWKK-(OH)] to generate the following sequence [(biotin)-RQPKIWFPNRRKPWKK-DGIWKASFTTFTVTKYWFYR-(OH)]. Both peptides (AP and AP-Cav-1) were custom synthesized (at the Tufts University Core Facility). Anti-caveolin-1 rabbit polyclonal antibody (N-20) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). A mouse monoclonal antibody to β-actin was purchased from Sigma (St. Louis, MO). Horseradish peroxidase-conjugated secondary antibodies [anti-mouse (1:20,000 dilution) (Pierce, Rockford, IL) or anti-rabbit (1:20,000 dilution) (BD Biosciences, San Jose, CA)] were used to visualize bound primary antibodies with the super-signal chemiluminescence substrate (Pierce). Mouse monoclonal antibodies to MCM7 and PCNA were purchased from Santa Cruz Biotechnology and the rabbit polyclonal phospho-Rb (Ser 807/811) was purchased from Cell Signaling Technology, Danvers, MA). Fluorescein (FITC) and rhodamine (TRITC)-conjugated secondary antibodies were purchased from Jackson Immuno Research (West Grove, PA).
Breast tissue samples
After informed consent was obtained, breast tissues from cancer patients and normal adjacent tissue from the same patient were collected. The tissues were stained with hematoxylin and eosin (H&E) and the TNM staging system (AAJCC Cancer Staging Manual, 6th edition, 2002) along with the Nottingham system, were used to determine the tumor stage for every patient.16 Tumor grade, estrogen receptor (ER), progesterone receptor (PR) and Her2 status were also recorded. All tissues obtained were processed within 1-hour of surgical resection to isolate the primary fibroblasts. Breast cancer tissues were obtained from 11 female patients affected by invasive ductal carcinoma, median age of 50.7 years (range 32–66), undergoing surgical resection at the Thomas Jefferson University. This study was approved by the Institutional Review Board (IRB) at Thomas Jefferson University.
Culture of cancer-associated fibroblasts
Mammary fibroblasts were isolated, essentially as previously described.17,18 Briefly, 1-hour or less following surgical resection, the tissue samples were washed and kept in PBS containing antibiotics (100 U/ml penicillin, 100 μg/ml streptomycin, 1.5 g/ml Fungizone) (Invitrogen Corporation, Carlsbad, CA) at 4°C and minced into 1–2 mm fragments. Then, the tissues were digested overnight at 37°C with 0.1% collagenase III (Worthington Biochemical Corp., Lakewood, NJ) in DMEM containing penicillin and streptomycin and 10% fetal bovine serum (FBS, Invitrogen Corporation). The following day, the epithelial cells were separated from the stromal cells by differential centrifugation, as previously described.19 The stromal cells were washed in PBS before being plated in 60 mm dishes (Corning Incorporated, Corning, NY) and incubated in a humidified 5% CO2 at 37°C. The cells were grown in 10% DMEM media and changed every other day. All experiments were performed between passages 3–10. In addition, another protocol for fibroblasts isolation was performed, as previously described.18 In parallel, when the tissues were resected, a 1 mm piece of normal and tumor tissue were deposited in a 60 mm dish and left to grow in DMEM 20% FBS at 37°C in a 5% CO2 incubator. The dishes were trypsinized when a population of fibroblasts appeared around each piece of tissue (2–3 weeks). Cells were separated by centrifugation and resuspended in DMEM 10% FBS. Monolayer cultures were grown until 60–70% confluence and passaged for a maximum of 10 passages.
Western blotting
Fibroblasts were grown to subconfluency (50–60%) in DMEM 10% FBS containing antibiotics and harvested in appropriate volume of lysis buffer (10 mM Tris, pH 7.5, 150 mM NaCl, 1% Triton X-100, 60 mM octylglucoside), containing protease and phosphatase inhibitors (Roche Applied Science, Indianapolis, IN). Cell lysates were sonicated with a Branson Sonifier 250 (VWR International, West Chester, PA) and centrifuged at 12,000 × g for 10 min to remove insoluble debris. Protein concentrations were analyzed using the BCA reagent (Pierce) and 30 μg of protein was loaded and separated by SDS-PAGE (12% acrylamide) and transferred to a 0.2 μm nitrocellulose transfer membrane (Fisher Scientific, Pittsburgh, PA). Membranes were blocked for 30 min at room temperature (10 mM Tris, pH 8.0, 150 mM NaCl, 0.05% Tween-20, 5% nonfat dry milk). Membranes were incubated with the primary antibody for 1 hr followed by washes and 30 min incubation with secondary antibody at room temperature in blocking solution. The immublots were washed and treated with a chemiluminescence substrate and visualized by exposing the film for 30–60 sec.
BrdU assay
Cell proliferation was determined using a standard BrdU assay (Roche). The incorporation of a pyrimidine analogue (BrdU) was measured in fibroblasts, as suggested by the manufacturer. Briefly, fibroblasts were trypsinized and plated in a 96-well plate (Corning) at a density of 2,000 cells/well. The cancer associated fibroblasts were treated the next day with 5, 10 or 20 μM vehicle plus peptide (either AP-alone versus AP-Cav-1) for 48 hrs or left untreated. Equal numbers of normal fibroblasts were plated at the same time and left untreated to measure their proliferation. All cells were given a BrdU pulse of 2 hrs at 37°C.
Confocal microscopy
Fibroblasts were trypsinized and plated on glass coverslips (Fisher Scientific) and left to grow overnight in DMEM 10% FBS. The next day, cells were fixed with cold (−20°C) methanol (Fisher Scientific) for 20 min, washed with PBS (1X) and incubated with primary antibodies at 37°C in IF buffer (PBS (1X), 5% BSA, 0.5% NP-40) for 45 min. The cells were then washed three times with PBS (1X) and incubated with secondary antibodies in IF buffer for 30 minutes at 37°C. Finally, the cells were washed twice and incubated with DAPI (Invitrogen Corporation) for five minutes, washed and mounted. The cells were imaged with a confocal microscope (Zeis LSM 510). All images were acquired with a 20X objective.
Gene profiling
Gene profiling (DNA microarray) was performed on primary cultures from three different patients with invasive breast carcinomas and compared to their matching NFs. These studies were carried out essentially as we have previously described for other cell types.20 Briefly, RNA was extracted from fibroblasts by TRIzol method (Invitrogen Corporation) according to the manufacturer’s instructions. The RNA was further purified using RNeasy Micro Kit (Qiagen, Valancia, CA) and reverse transcribed using Superscript III First-Strand Synthesis System (Invitrogen Corporation) and T7-dT24 primer (Sigma Genosys). The single stranded cDNA was converted to double stranded cDNA and purified. The double stranded cDNA was used as a template to generate biotinylated cRNA using RNA Transcription Labeling Kit (Enzo, New-York, NY) and the labeled cRNA was purified. The cRNA (15 μg) was fractionated to produce fragments of between 35–200 bp and hybridized to the human 133A Plus 2.0 array (Affymetrix, Santa Clara, CA). The hybridization was carried out in accordance with Affymetrix protocols. The arrays were scanned at 570 nm with a confocal scanner from Affymetrix.
Array data analysis
Analysis of the arrays was performed as previously described using the statistical package R and the limma library of the Bioconductor software package.20,21 Normalization of the array was performed using a robust multiarray analysis (RMA). A fold change of greater than two was used as a criterion for differential gene expression. Gene ontology analyses was performed using the DAVID 2007 bioinformatics resource. Gene lists were uploaded with the Homo Sapien genome as the background. Microarray data (series GSE1378 and GSE1379) from X.J. Ma et al.22 were obtained from the National Center for Biotechnology Information Gene Expression Omnibusweb site (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GPL1223) and manipulated using GeneSpring GX software (version 7.2) (Agilent Technologies). For each series, the raw data was obtained from GEO as log2 of normalized Cy5/Cy3 ratio, where tumor sample RNA and human universal reference RNA were labeled with Cy5 and Cy3, respectively. The raw data were transformed from log2 to linear values followed by per-gene median normalization in GeneSpring. The expression levels of CAF associated genes were clustered based on standard correlation as the similarity measurement. Subsequently, a condition tree based on distance correlation was created to order the tumor specimens. The quartile exhibiting the highest expression level of the CAF gene signature was utilized to define the impact of the CAF signature on disease outcome. For Kaplan-Meier analysis, statistical calculations were performed using GraphPad Prism 4.0 software.
Statistical analysis
Abundance of Cav-1 expression in fibroblasts (CAFs and NFs) and BrdU incorporation were analyzed using a two-tailed paired Student t-test. Differences were considered statistically significant when p < 0.05.
Acknowledgements
M.P.L. and his laboratory were supported by grants from the NIH/NCI (R01-CA-80250; R01-CA-098779; R01-CA-120876), the American Association for Cancer Research (AACR), and the Department of Defense-Breast Cancer Research Program (Synergistic Idea Award). I.M. was supported by a Post-doctoral Fellowship from the Susan G. Komen Breast Cancer Foundation. F.S. was supported by grants from the Elsa U. Pardee Foundation, the W.W. Smith Charitable Trust, and a Research Scholar Grant from the American Cancer Society (ACS). J.F.J. was supported by a Career Catalyst Award from the Susan G. Komen Breast Cancer Foundation.
This project is funded, in part, under a grant with the Pennsylvania Department of Health (to M.P.L.). The Department specifically disclaims responsibility for any analyses, interpretations or conclusions.
Footnotes
Supplementary materials can be found at: www.landesbioscience.com/supplement/MercierCBT7-8-Sup.xls
References
- 1.Sappino AP, Skalli O, Jackson B, Schurch W, Gabbiani G. Smooth-muscle differentiation in stromal cells of malignant and non-malignant breast tissues. Int J Cancer 1988; 41:707–12. [DOI] [PubMed] [Google Scholar]
- 2.Ronnov-Jessen L, Petersen OW, Bissell MJ. Cellular changes involved in conversion of normal to malignant breast: importance of the stromal reaction. Physiol Rev 1996; 76:69–125. [DOI] [PubMed] [Google Scholar]
- 3.Tlsty TD, Hein PW. Know thy neighbor: stromal cells can contribute oncogenic signals. Curr Opin Genet Dev 2001; 11:54–9. [DOI] [PubMed] [Google Scholar]
- 4.Mueller MM, Fusenig NE. Friends or foes—bipolar effects of the tumour stroma in cancer. Nat Rev Cancer 2004; 4:839–49. [DOI] [PubMed] [Google Scholar]
- 5.Serini G, Gabbiani G. Mechanisms of myofibroblast activity and phenotypic modulation. Exp Cell Res 1999; 250:273–83. [DOI] [PubMed] [Google Scholar]
- 6.Desmouliere A, Redard M, Darby I, Gabbiani G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol 1995; 146:56–66. [PMC free article] [PubMed] [Google Scholar]
- 7.Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts I. Paracrine cells important in health and disease. Am J Physiol 1999; 277:1–9. [DOI] [PubMed] [Google Scholar]
- 8.Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts II. Intestinal subepithelial myofibroblasts. Am J Physiol 1999; 277:183–201. [DOI] [PubMed] [Google Scholar]
- 9.Engelman JA, Wykoff CC, Yasuhara S, Song KS, Okamoto T, Lisanti MP. Recombinant expression of caveolin-1 in oncogenically transformed cells abrogates anchorage-independent growth. J Biol Chem 1997; 272:16374–81. [DOI] [PubMed] [Google Scholar]
- 10.Koleske AJ, Baltimore D, Lisanti MP. Reduction of caveolin and caveolae in oncogenically transformed cells. Proc Natl Acad Sci USA 1995; 92:1381–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galbiati F, Volonte D, Engelman JA, Watanabe G, Burk R, Pestell RG, Lisanti MP. Targeted downregulation of caveolin-1 is sufficient to drive cell transformation and hyperactivate the p42/44 MAP kinase cascade. Embo J 1998; 17:6633–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, Macaluso F, Russell RG, Li M, Pestell RG, Di Vizio D, Hou H Jr., Kneitz B, Lagaud G, Christ GJ, Edelmann W, Lisanti MP. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem 2001; 276:38121–38. [DOI] [PubMed] [Google Scholar]
- 13.Galbiati F, Volonte D, Liu J, Capozza F, Frank PG, Zhu L, Pestell RG, Lisanti MP. Caveolin-1 expression negatively regulates cell cycle progression by inducing G(0)/G(1) arrest via a p53/p21(WAF1/Cip1)-dependent mechanism. Mol Biol Cell 2001; 12:2229–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schor SL, Schor AM, Rushton G. Fibroblasts from cancer patients display a mixture of both foetal and adult-like phenotypic characteristics. J Cell Sci 1988; 90:401–7. [DOI] [PubMed] [Google Scholar]
- 15.Schor SL, Schor AM, Rushton G, Smith L. Adult, foetal and transformed fibroblasts display different migratory phenotypes on collagen gels: evidence for an isoformic transition during foetal development. J Cell Sci 1985; 73:221–34. [DOI] [PubMed] [Google Scholar]
- 16.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. C.W. Elston & I.O. Ellis. Histopathology 1991; 19:403–410. Histopathology 2002; 41:151–2. [DOI] [PubMed] [Google Scholar]
- 17.Casey TM, Eneman J, Crocker A, White J, Tessitore J, Stanley M, Harlow S, Bunn JY, Weaver D, Muss H, Plaut K. Cancer associated fibroblasts stimulated by transforming growth factor beta1 (TGFbeta1) increase invasion rate of tumor cells: a population study. Breast Cancer Res Treat 2007; 110:39–49. [DOI] [PubMed] [Google Scholar]
- 18.Leroy EC. Connective tissue synthesis by scleroderma skin fibroblasts in cell culture. J Exp Med 1972; 135:1351–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Speirs V, White MC, Green AR. Collagenase III: a superior enzyme for complete disaggregation and improved viability of normal and malignant human breast tissue. In Vitro Cell Dev Biol Anim 1996; 32:72–4. [DOI] [PubMed] [Google Scholar]
- 20.Li Z, Wang C, Jiao X, Katiyar S, Casimiro MC, Prendergast GC, Powell MJ, Pestell RG. Alternate cyclin D1 mRNA splicing modulates p27KIP1 Binding and cell migration. J Biol Chem 2008; 283:7007–15. [DOI] [PubMed] [Google Scholar]
- 21.Wang C, Li Z, Lu Y, Du R, Katiyar S, Yang J, Fu M, Leader JE, Quong A, Novikoff PM, Pestell RG. Cyclin D1 repression of nuclear respiratory factor 1 integrates nuclear DNA synthesis and mitochondrial function. Proc Natl Acad Sci USA 2006; 103:11567–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma XJ, Wang Z, Ryan PD, Isakoff SJ, Barmettler A, Fuller A, Muir B, Mohapatra G, Salunga R, Tuggle JT, Tran Y, Tran D, Tassin A, Amon P, Wang W, Wang W, Enright E, Stecker K, Estepa-Sabal E, Smith B, Younger J, Balis U, Michaelson J, Bhan A, Habin K, Baer TM, Brugge J, Haber DA, Erlander MG, Sgroi DC. A two-gene expression ratio predicts clinical outcome in breast cancer patients treated with tamoxifen. Cancer Cell 2004; 5:607–16. [DOI] [PubMed] [Google Scholar]
- 23.Bosco EE, Wang Y, Xu H, Zilfou JT, Knudsen KE, Aronow BJ, Lowe SW, Knudsen ES. The retinoblastoma tumor suppressor modifies the therapeutic response of breast cancer. J Clin Invest 2007; 117:218–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 2005; 121:335–48. [DOI] [PubMed] [Google Scholar]
- 25.Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res 1999; 59:5002–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hwang RF, Moore T, Arumugam T, Ramachandran V, Amos KD, Rivera A, Ji B, Evans DB, Logsdon CD. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res 2008; 68:918–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Desmedt C, Sotiriou C. Proliferation: the most prominent predictor of clinical outcome in breast cancer. Cell Cycle 2006; 5:2198–202. [DOI] [PubMed] [Google Scholar]
- 28.Bosco EE, Knudsen ES. RB in breast cancer: at the crossroads of tumorigenesis and treatment. Cell Cycle 2007; 6:667–71. [DOI] [PubMed] [Google Scholar]
- 29.Carter SL, Eklund AC, Kohane IS, Harris LN, Szallasi Z. A signature of chromosomal instability inferred from gene expression profiles predicts clinical outcome in multiple human cancers. Nat Genet 2006; 38:1043–8. [DOI] [PubMed] [Google Scholar]
- 30.Deeb KK, Michalowska AM, Yoon CY, Krummey SM, Hoenerhoff MJ, Kavanaugh C, Li MC, Demayo FJ, Linnoila I, Deng CX, Lee EY, Medina D, Shih JH, Green JE. Identification of an integrated SV40 T/t-antigen cancer signature in aggressive human breast, prostate and lung carcinomas with poor prognosis. Cancer Res 2007; 67:8065–80. [DOI] [PubMed] [Google Scholar]
- 31.Bartek J, Bartkova J, Lukas J. The retinoblastoma protein pathway and the restriction point. Curr Opin Cell Biol 1996; 8:805–14. [DOI] [PubMed] [Google Scholar]
- 32.Moldovan GL, Pfander B, Jentsch S. PCNA, the maestro of the replication fork. Cell 2007; 129:665–79. [DOI] [PubMed] [Google Scholar]
- 33.Gladden AB, Diehl JA. The cyclin D1-dependent kinase associates with the pre-replication complex and modulates RB. MCM7 binding. J Biol Chem 2003; 278:9754–60. [DOI] [PubMed] [Google Scholar]
- 34.Shohet JM, Hicks MJ, Plon SE, Burlingame SM, Stuart S, Chen SY, Brenner MK, Nuchtern JG. Minichromosome maintenance protein MCM7 is a direct target of the MYCN transcription factor in neuroblastoma. Cancer Res 2002; 62:1123–8. [PubMed] [Google Scholar]
- 35.Srinivasan SV, Mayhew CN, Schwemberger S, Zagorski W, Knudsen ES. RB loss promotes aberrant ploidy by deregulating levels and activity of DNA replication factors. J Biol Chem 2007; 282:23867–77. [DOI] [PubMed] [Google Scholar]
- 36.Gratton JP, Lin MI, Yu J, Weiss ED, Jiang ZL, Fairchild TA, Iwakiri Y, Groszmann R, Claffey KP, Cheng YC, Sessa WC. Selective inhibition of tumor microvascular permeability by cavtratin blocks tumor progression in mice. Cancer Cell 2003; 4:31–9. [DOI] [PubMed] [Google Scholar]
- 37.Bucci M, Gratton JP, Rudic RD, Acevedo L, Roviezzo F, Cirino G, Sessa WC. In vivo delivery of the caveolin-1 scaffolding domain inhibits nitric oxide synthesis and reduces inflammation. Nat Med 2000; 6:1362–7. [DOI] [PubMed] [Google Scholar]
- 38.Jasmin JF, Mercier I, Dupuis J, Tanowitz HB, Lisanti MP. Short-term administration of a cell-permeable caveolin-1 peptide prevents the development of monocrotaline-induced pulmonary hypertension and right ventricular hypertrophy. Circulation 2006; 114:912–20. [DOI] [PubMed] [Google Scholar]
- 39.Fiucci G, Ravid D, Reich R, Liscovitch M. Caveolin-1 inhibits anchorage-independent growth, anoikis and invasiveness in MCF-7 human breast cancer cells. Oncogene 2002; 21:2365–75. [DOI] [PubMed] [Google Scholar]
- 40.Hassan GS, Jasmin JF, Schubert W, Frank PG, Lisanti MP. Caveolin-1 deficiency stimulates neointima formation during vascular injury. Biochemistry 2004; 43:8312–21. [DOI] [PubMed] [Google Scholar]
- 41.Li J, Hassan GS, Williams TM, Minetti C, Pestell RG, Tanowitz HB, Frank PG, Sotgia F, Lisanti MP. Loss of caveolin-1 causes the hyper-proliferation of intestinal crypt stem cells, with increased sensitivity to whole body gamma-radiation. Cell Cycle 2005; 4:1817–25. [DOI] [PubMed] [Google Scholar]
- 42.Williams TM, Medina F, Badano I, Hazan RB, Hutchinson J, Muller WJ, Chopra NG, Scherer PE, Pestell RG, Lisanti MP. Caveolin-1 gene disruption promotes mammary tumorigenesis and dramatically enhances lung metastasis in vivo. Role of Cav-1 in cell invasiveness and matrix metalloproteinase (MMP-2/9) secretion. J Biol Chem 2004; 279:51630–46. [DOI] [PubMed] [Google Scholar]
- 43.Williams TM, Sotgia F, Lee H, Hassan G, Di Vizio D, Bonuccelli G, Capozza F, Mercier I, Rui H, Pestell RG, Lisanti MP. Stromal and epithelial caveolin-1 both confer a protective effect against mammary hyperplasia and tumorigenesis: Caveolin-1 antagonizes cyclin D1 function in mammary epithelial cells. Am J Pathol 2006; 169:1784–801. [DOI] [PMC free article] [PubMed] [Google Scholar]