Abstract
Aim:
Metastatic melanoma patients were treated with patient-specific vaccines consisting of autologous dendritic cells loaded with antigens from irradiated cells from short-term autologous tumor cell lines.
Patients & methods:
A total of 72 patients were enrolled in a single-arm Phase I/II (NCT00948480) trial or a randomized Phase II (NCT00436930).
Results:
Toxicity was minimal. Median overall survival (OS) was 49.4 months; 5-year OS 46%. A 5-year OS was 72% for 18 recurrent stage 3 without measurable disease when treated and 53% for 30 stage 4 without measurable disease when treated. A total of 24 patients with measurable stage 4 when treated (median of four prior therapies) had an 18.5 months median OS and 46% 2-year OS.
Conclusion:
This dendritic cell vaccine was associated with encouraging survival in all three clinical subsets. Clinicaltrial.gov NCT00436930 and NCT00948480.
Keywords: : autologous tumor antigens, dendritic cells, melanoma, patient-specific therapy, therapeutic vaccine
Based on the responsiveness of metastatic melanoma to immunotherapies [1,2], immuno-oncology investigators have been pursuing therapeutic vaccines to treat advanced melanoma for more than 25 years. Unfortunately, various approaches have met with limited success [3]. Most notable disappointments were large-randomized trials of an allogeneic cell line vaccine [4], a gp100 peptide antigen vaccine [5], and a combination of HLA-restricted peptides injected with or without GM–CSF [6]. The first putative therapeutic vaccine to receive regulatory approval for cancer treatment was sipuleucel-T, a mixture of dendritic cells (DC) and lymphocytes exposed to prostatic acid phosphatase and GM–CSF and infused intravenously for castrate-resistant prostate cancer [7]. Approval was based on a 4-month (18%) improvement in overall survival (OS). In 2015 intralesional injection of talimogene laherparepvec, a cytolytic Herpes virus modified to secrete GM–CSF, was approved based on durable responses in about 25% of patients with primarily regionally advanced or soft-tissue distant metastatic melanoma [8]. That approach is based on autologous tumor antigens (ATA), but the systemic immune benefit may be limited by injecting into the immunosuppressive tumor microenvironment. In fact, most responses were in the injected lesions with limited responses in more distant lesions, suggesting that systemic immunization effects were limited.
In recent years, evidence has accumulated suggesting that the best source of antigens for vaccines is autologous tumor because of unique neoantigens that result from nonsynonymous mutations [9,10]. Immunogenomics have made it possible to identify nonsynonymous mutations, determine messenger sequences that can be transcribed and translated, and predict the neoantigenicity and HLA-binding potential of specific molecules [11,12]. The best way to present such ATA may be on autologous DC rather than directly injecting antigens [13–15]. Three different preclinical animal models demonstrated that injections of DC loaded with specific neoantigens induced effective CD4-mediated recognition of the same neoantigens and was associated with therapeutic benefit [16]. Similarly, in melanoma patients, neoantigens derived from nonsynonymous mutations and loaded on DC were associated with new or increased immunoreactivity to the specific neoantigens [17]. A less complex approach is the use of autologous tumor, especially short-term autologous cell lines as a source of ATA in as much as they include the entire repertoire of neoantigens unique to that patient, including antigens that may be unique to the patient’s tumor initiating cells [18–20].
The role of adjuvants in cancer vaccines is not clear, although historically adjuvants have been added to induce inflammation at the site of cutaneous vaccine injections. There is a good rationale for using GM–CSF as an adjuvant with vaccines [21,22], and it is a component of the two therapeutic cancer vaccines that have been approved for marketing [7,8]. The GM–CSF has been used as a treatment in melanoma for many years [23], but never received regulatory approval for that purpose. Repeated injections of subcutaneous GM–CSF monotherapy (daily for 2 weeks, off for 2 weeks) showed promise in single arm studies [24,25] but was no better than placebo in patients with stage 3 or stage 4 metastatic melanoma that had been surgically resected [6], and was inferior to intralesional cytolytic virus vaccine in patients with metastases that were accessible for injection [8].
For several years, we conducted clinical trials with autologous DC loaded with ATA (DC–ATA) derived from short-term cell cultures and then admixed with GM–CSF at the time of injection [11,26–31]. The mechanism of action for this DC vaccine (DCV) is believed to be the induction of new immune responses to ATA or enhancement of weak existing immune responses. Two trials were conducted with DC–ATA in patients with metastatic melanoma. A single-arm Phase I–II trial established safety and suggested an improvement in OS [26,27]. A randomized Phase II trial confirmed safety and longer survival compared with an autologous tumor cell vaccine (TCV) consisting of irradiated autologous tumor cells from short-term cell lines that were admixed with GM–CSF at the time of subcutaneous injection [28,29]. In this report, we present 5-year survival data for all 72 metastatic melanoma patients who were treated with patient-specific DCV. They were treated during 2001–2011 prior to adoption of anti-BRAF/MEK treatment for patients whose tumors expressed BRAF mutations and prior to adoption of monoclonal antibody checkpoint inhibitors including anti-CTLA-4 ipilimumab, and antiprogrammed death molecule-1 (PD-1) products nivolumab and pembrolizumab. The purposes of this article are to: provide additional information regarding manufacturing of the patient-specific DCV, present adverse-event data for all 72 patients, report survival for specific cohorts of patients defined by stage and tumor burden at the time of treatment and compare survival in those cohorts with published results for similar patients.
Patients & methods
Patients
All human subjects were treated in clinical trials that followed the principles outlined in the Declaration of Helsinki and were approved by an appropriate institutional review board. Written informed consent was obtained from all participants involved. Patients received their DC–ATA product in two sequential trials: an open-label Phase II (clinicaltrials.gov NCT00436930, registered 15 February 2007) [26,27], and a randomized Phase II (NCT00948480, registered 28 July 2009) [28,29]. Both trials were investigator-initiated and conducted under BB-IND 8554. The single-arm trial enrolled 54 patients from 27 December 2000 to 17 April 2007. The 1:1 randomized Phase II trial enrolled patients from 9 October 2007 to 13 December 2010. Eligibility criteria were identical in both protocols. These included a diagnosis of either stage 4 distant metastatic or recurrent stage 3 melanoma, establishment of a short-term autologous tumor cell line from a resected metastatic lesion, a Karnofsky Performance Status of 70–100, typical Phase II criteria for organ function, and referral for enrollment by the patient’s managing physician. Patients with treated brain metastases were eligible unless they had uncontrolled lesions. Patients with a diagnosis of an active autoimmune disease, or an immunosuppressive disease, or receiving immunosuppressive medications were ineligible as were patients with chronic infections including hepatitis B, hepatitis C and HIV. Concurrent anticancer therapy was not permitted. All patients had undergone excision of a metastatic lesion (locoregional or distant) from which a cell line had been established; thus, all had stage 4 or recurrent stage 3 melanoma at the time of tumor harvest, but differed in terms of stage and measurability of disease at the time of treatment. Patients were referred from all over the USA; however, per request of the US FDA, all injections were administered at one geographical location.
Intermediate & final products
Short-term tumor cell lines
Typically patients underwent surgery as part of standard of care, usually to render them disease free, or as a palliative or diagnostic procedure. However, in some instances patients with more widespread disease underwent resection of a lesion that was easily accessible to surgery, specifically for an effort to establish a tumor cell line that might be used in making an investigational agent for a clinical trial. At the time of surgical resection, tumor samples were collected locally in Newport Beach, California or at various geographic locations throughout the USA. Tumor was transported in tissue culture media containing antibiotics provided in a transport kit for overnight delivery to a cell biology laboratory located in Hoag Cancer Center in Newport, CA, USA.
The methods used to establish cell lines were the same in both eras in which these trials were conducted as published previously [32–34]. Briefly, tissue was mechanically minced and/or enzymatically digested to create a single cell suspension that was placed in tissue culture media and incubated. Alternatively, processed tumor samples were cryopreserved and placed into cell culture at a later date. A successful cell culture was defined by morphology typical of melanoma cells, continuous proliferation and initial growth to 50 million cells. Cultures were then expanded to 120 million cells in the single arm trial and to 220 million cells in the randomized trial. Immunophenotyping was conducted with a panel of antibodies to various melanoma-associated antigens. For the single arm trial, tumor cell cultures included coincubation with IFN-γ for 72 h just prior to irradiation, but IFN-γ was not added at the end of manufacturing of melanoma cell lines in the randomized trial. For both trials, tumor cells were irradiated at a dose of 100 Gy and cryopreserved before incubation with autologous DC. Irradiated tumor cells were confirmed to be nonproliferative in a nonradioactive proliferation assay then stored in in AIM 5 serum-free and antibiotic-free medium with 8% DMSO until they were thawed and washed prior to incubation with autologous DC.
Tumor cells for delayed type hypersensitivity skin testing
Individual aliquots containing 1 million irradiated tumor cells were stored in AIM 5 serum-free medium with 8% DMSO. At the time of intradermal injection, these were thawed, washed and resuspended in 1 ml normal saline. Total 1 week before starting vaccine (week 0) patients were injected intradermally on the volar surface of the forearm. Within 48–72 h, the test was interpreted by nursing staff as negative (no induration), weakly positive (5–9 mm induration) or positive (≥1 cm induration). The test and interpretation was repeated 1 week after 3 weekly injections had been administered (week 4), and at weeks 12 and 24 in patients who remained on study.
Dendritic cells
The procedures for producing DC from peripheral blood monocytes were previously described [26–29,35]. Peripheral blood mononuclear cells (PBMC) were obtained by leukapheresis procedures carried out in the Hoag Cancer Center. For the single arm trial, PBMC were isolated by Ficoll–Paque™ (Amersham Biosciences, NJ, USA) density gradient separation of the leukapheresis product and subsequent plastic adherence/wash steps. During the randomized trial, the Elutra® Cell Separation System (Terumo BCT, CO, USA) closed cell selection system was used to enrich for monocytes. After isolation of monocytes, immature DC was generated in a 6-day culture in AIM5 serum-free media to which the cytokines IL-4 (250 IU/ml) and GM–CSF (1000 IU/ml) were added. During this time, CD14-positive monocytes were converted into DC. For characterization purposes, monoclonal antibodies and flow cytometry were used to determine the percentage of cells expressing CD80, CD86 and CD11c before and after this culture process. Cell number and viability were recorded.
Autologous DC vaccines
All treatment doses for each patient were manufactured a single batch in which the DC and cryopreserved irradiated tumor cells were coincubated for 12–18 h at a ratio of about 3:1 in T25 flasks. For each treatment dose, cells were divided into ten fractions containing 1–33 million DC that had been coincubated with about 10 million irradiated tumor cells. These were cryopreserved in individual vials containing AIM 5 serum-free medium and 8% DMSO. One vial was used for safety testing including exclusion of bacterial or mycoplasma contamination and confirmation of an endotoxin level <350 IU/ml.
Administration of DC vaccines
Preparation for injection
Just prior to administration, DCV was rapidly thawed in a 37°C water bath. The thawed cell suspension was then rinsed in 10 ml of sterile AIM-V (antibiotic-free) medium in a 50 ml conical tube. A cell count was performed prior to centrifugation and decantation of the wash phase. The cell pellet was then suspended in 1 ml saline and 500 μg of GM–CSF in a 1.5 ml vial, and finally drawn into a 3.0 ml syringe for injection with a 25-gauge needle within 5 h of thawing.
Treatment schedule
In both trials, each vaccine dose was injected subcutaneously in an extremity once a week for 3 weeks, and then once a month for 5 months at weeks 8, 12, 16, 20 and 24. The same extremity was not used for consecutive injections. Patients were monitored for toxicity, objective tumor response and progression free survival (PFS) and OS. Concurrent anticancer therapy was not allowed, but patients could interrupt vaccine therapy for specific interventions, then resume vaccine treatment.
Statistical analysis
Survival curves were generated using the method of Kaplan–Meier. Comparisons of proportions were made using Pearson’s χ2 test or Fisher’s exact test. Averages were compared using the student’s t-test. Median survival was estimated from the survival curve calculations.
Survival analyses were performed for the entire study population and for cohorts defined as: recurrent stage 3 with no measurable disease following surgical resection, previously confirmed distant stage 4 disease but no measurable disease at the time of treatment after surgical resection(s) and/or systemic therapy and measurable distant stage 4 disease. Survival was calculated from the date of enrollment for intent to treat; each patient was treated with his/her vaccine product.
The OS was defined by the date of death regardless of cause. The PFS was defined by date of disease progression or date of death, whichever came first. The PFS was estimated by the investigator based on medical and radiology reports, and patient reporting. The protocol requested that scans can be performed at any time that there were symptoms or signs of disease progression, and otherwise until disease progression at 3 months intervals in the first year, every 6 months during the next 2 years, and then every 6 months out to 5 years.
Results
Cell products
Anatomic sites from which tumors were resected
Table 1 shows the anatomic origins of tumors from which the cell lines were derived that served as sources of patient-specific ATA. Most tumors were obtained from readily accessible lymph nodes or cutaneous lesions, but 28% were obtained from viscera. At the time of tumor harvest, 29 patients had recurrent stage 3 disease, but only 18 were still stage 3 at the time of treatment.
Table 1. . Anatomic sources of tissue from which tumor cell lines were derived.
| Tissue source | Patients (n) |
|---|---|
| Lymph nodes | (37) |
| Axilla and/or supraclavicular | 15 |
| Inguinal/femoral/iliac | 10 |
| Neck | 5 |
| Mediastinum | 3 |
| Retroperitoneal | 2 |
| Parotid | 1 |
| Popliteal | 1 |
| Viscera | (20) |
| Lung | 7 |
| Breast | 3 |
| Liver | 2 |
| Spleen | 2 |
| Small bowel | 2 |
| Adrenal | 2 |
| Bone | 2 |
| Soft tissue | (15) |
| Leg | 6 |
| Chest wall | 4 |
| Arm | 2 |
| Back | 1 |
| Orbit | 1 |
| Paraspinal | 1 |
At time of tissue harvest, 29 were recurrent stage 3 and 43 were stage 4. At time of treatment, highest stage was 18 recurrent stage 3, 54 stage 4.
Production of tumor cells
The overall success rate for tumor cell lines was 188/419 (44.9%) for all samples submitted, regardless of the quality of the sample in terms of size, viability and contamination. Some patients had more than one sample submitted. During the period in which the open-label trial was conducted cell lines were established for possible use for 110 patients from 227 metastatic melanoma samples (48.5%) [27]. During the period in which the randomized Phase II trial was conducted, 78 cell lines were established from 192 metastatic melanoma samples (40.6%) [29]. As shown in Table 2, it took an average of little over 3 months to establish tumor cell lines, and there were no differences in time to successful establishment of a melanoma cell line by anatomic origin of the resected tumor specimen, geographic location from which tumor was submitted, or whether patients had stage 4 or recurrent stage 3 melanoma at the time of resection.
Table 2. . Days to a successful culture and expansion of cancer cells.
| Variable | Patients (n) | Mean number of days to successful culture ± standard deviation | p-value |
|---|---|---|---|
| Tissue source: | |||
| – Lymph nodes | 37 | 116.0 ± 69.2 | |
| – Viscera | 20 | 88.6 ± 61.0 | 0.34 |
| – Cutaneous | 15 | 87.4 ± 68.0 | 0.20 |
| Stage at time of tumor collection: | |||
| – Recurrent stage 3 | 29 | 108.7 ± 69.1 | |
| – Stage 4 | 43 | 98.2 ± 66.8 | 0.50 |
| Geographic source of tumor: | |||
| – California | 48 | 105.9 ± 75.5 | 0.56 |
| – Non-California | 24 | 95.4 ± 47.5 | |
| Specific trial and era: | |||
| – Single arm (2000–2007) | 54 | 101.9 ± 66.1 | |
| – Randomized (2007–2010) | 18 | 103.8 ± 72.7 | 0.92 |
Production of dendritic cells
Table 3 summarizes data related to the generation of DC and the final DCV product. The DC production was successful for all patients and an additional 13 patients during 2001–2006 for whom a DCV-treatment product was manufactured, but never used because the patient was not referred for treatment (n = 12) or was ineligible (n = 1). One patient underwent two leukapheresis procedures because of contamination of the first DC product; thus, DC was successfully produced for clinical use for 85/86 leukapheresis products. From a DC production perspective, the major difference between trials was the reliance on plastic adherence as opposed to the Elutra for monocyte isolation for the randomized trial. Because of the increased efficiency for monocyte isolation by elutriation, fewer total PBMC were obtained during the shorter leukapheresis procedure used during the randomized trial. However, more monocytes were placed into culture in the randomized trial because of the improved efficiency of monocyte collection.
Table 3. . Summary data related to the generation of dendritic cells and the final dendritic cell vaccine products.
| Cell characteristics | Single arm | Randomized | Combined |
|---|---|---|---|
| Mean number of cells obtained by apheresis (n) | 10.0 × 109 (46) |
6.7 × 109 (18) |
9.1 × 109 (64) |
| Monocytes (CD14+), % (n) | 17.3%
(54) |
17.5%
(18) |
17.4% (72) |
| Mean number of monocytes placed in culture to produce DC (n) | 2.0 × 108 (44) |
4.2 × 108 (18) |
2.6 × 108 (62) |
| CD80 positive pre co-culture with irradiated tumor cells, % (n) | 39.1%
(42) |
15.3%
(17) |
32.2%
(59) |
| CD86 positive pre co-culture with irradiated tumor cells, % (n) | 75.5%
(42) |
67.7%
(17) |
73.3%
(59) |
| CD11c positive pre co-culture with irradiated tumor cells, % (n) | 99.3%
(42) |
86.4%
(17) |
95.6%
(59) |
| Mean number irradiated tumor cells in co-culture (n) | 80 × 106 (54) |
115 × 106 (18) |
88.8 × 106 (72) |
| Mean total number of cells in co-culture (n) | 213 × 106 (54) |
165 × 106 (18) |
201 × 106 (72) |
| CD80 positive post co-culture with ITC, % (n) | 37.5%
(52) |
100%
(17) |
52.9%
(69) |
| CD86 positive post co-culture with ITC, % (n) | 71.9%
(52) |
40.3%
(17) |
64.1%
(69) |
| CD11c positive post co-culture with ITC, % (n) | 87.7%
(52) |
51.8%
(16) |
79.2%
(68) |
| Number of DC (CD11c positive) per treatment dose (n) | 17.7 × 106 (52) |
8.7 × 106 (16) |
15.6 × 106 (68) |
| Number of TC (CD11c negative) per treatment dose (n) | 2.1 × 106 (52) |
4.2 × 106 (16) |
2.6 × 106 (68) |
| Total number of cells per treatment dose (n) | 19.8 × 106 (52) |
12.9 × 106 (16) |
18.2 × 106 (68) |
DC: Dendritic cell; ITC: Irradiated tumor cell; TC: Tumor cell.
Production of DC vaccine
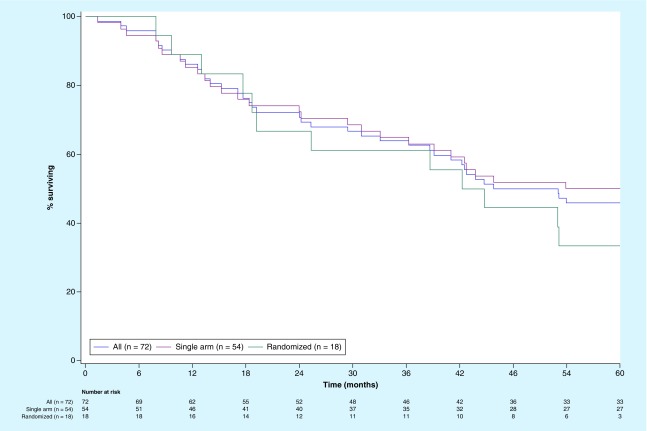
In Table 3, the apparent differences in various phenotypic parameters on the final product likely reflect slight differences in the way in which DC was manufactured for each trial, and the effect of exposure of TC to IFN-γ, which was associated with a more rapid phagocytosis during incubation with DC. As a result, there were more residual CD11c-negative cells in vaccine preparations for the randomized trial in which IFN-γ was not added at the end of the cell culture, which reflects residual irradiated tumor cells that were not phagocytosed by DC. The DCV products used in the randomized trial contained an average of 33% cells that were CD11c-negative, while in the open-label trial they represented only about 10%. Less than 2% of CD11c-negative cells in these samples were lymphocytes; the majority of CD11c-negative cells were irradiated tumor cells that were not phagocytosed by DC. This raised the issue of whether such a difference might have resulted in a different potency than could have affected survival. However, as shown in Figure 1, OS curves for the two trials were quite similar. It is not clear what the contribution of intact irradiated tumor cells was to the vaccine, but the DCV was associated with much longer survival compared with TCV as a historical control [27], and as a contemporary control in the randomized trial of DCV versus TCV [28,29].
Figure 1. . Overall survival for all 72 melanoma patients treated with autologous dendritic cell vaccines loaded with autologous tumor antigens and survival in a single arm Phase II trial (n = 54) and in a randomized Phase II trial (n = 18).
Pooled clinical trial results
Patient characteristics
Median age was 52 years (range: 17–83 years), and was also 52 years in each of the Phase II trials. Table 4 shows the proportions of different characteristics for all 72 patients and for the cohorts whose most advanced stage of disease at the time of treatment were recurrent stage 3 (n = 18) without measurable disease, stage 4 without measurable disease (n = 30) or stage 4 with measurable disease per response evaluation criteria in solid tumors (RECIST; n = 24). A patient’s most advanced stage of disease was considered to be recurrent stage 3 (n = 18) even if they had detectable or equivocal radiographic findings of distant metastatic disease if they had not been confirmed histologically (n = 3). The median time from tumor collection to enrollment for treatment was 7.1 months, and the median time from tumor collection to efforts to initiate a cell culture was 4.0 months. A total of 29 patients had recurrent stage 3 at the time of tumor collection, but 11 (38%) had experienced distant progression by the time of enrollment for treatment, and nine had measurable disease at that time. Of 33 patients with stage 4 disease at the time of tumor collection, 15 (45%) had recurred and had measurable disease at the time of treatment, while 18 (55%) had no evidence of disease at that time, often because of additional treatment procedures – including surgical resection of new metastases and stereotactic radiation for brain metastases. It is noteworthy that of the 54 patients, whose most advanced stage was stage 4, 14 (26%) had experienced brain metastases, and ten (19%) had experienced hepatic metastases.
Table 4. . Baseline characteristics by stage and measurable disease at time of treatment.
| Clinical feature | Recurrent stage 3 (n = 18) | Nonmeasurable stage 4 (n = 30) | Measurable stage 4 (n = 24) | All (n = 72) |
|---|---|---|---|---|
| Age >60 years (median 52 years) | 4 (22%) | 6 (20%) | 9 (38%) | 19 (26%) |
| Proportion male | 9 (50%) | 21 (70%) | 15 (62%) | 45 (62%) |
| Unknown primary | 1 (6%) | 7 (23%) | 7 (29%) | 15 (21%) |
| Acrolentiginous primary | 1 (6%) | 2 (7%) | 1 (4%) | 4 (6%) |
| Ocular primary | 0 | 1 (3%) | 1 (4%) | 2 (3%) |
| Mucosal primary | 0 | 0 | 0 | 0 |
| Stage 3 at time of tumor collection | 17 (100%) | 2 (7%) | 9 (42%) | 29 (40%) |
| Stage 4 at time of tumor collection | 0 | 28 (93%) | 15 (62%) | 43 (60%) |
| Lymph node source of tissue | 14 (78%) | 7 (23%) | 11 (46%) | 32 (44%) |
| Soft tissue source of tissue | 4 (22%) | 9 (30%) | 8 (33%) | 17 (24%) |
| Lung source of tissue | – | 5 (17%) | 2 (8%) | 7 (10%) |
| Visceral met (not lung) | – | 9 (30%) | 3 (12%) | 14 (19%) |
| Ave number prior therapies | 3.0 | 3.3 | 3.9 | 3.4 |
| Prior lymph node and/or soft tissue metastases | 18 (100%) | 16 (53%) | 21 (88%) | 55 (76%) |
| Prior lung metastases | – | 8 (27%) | 7 (29%) | 20 (28%) |
| Prior breast metastases | – | 2 (7%) | 2 (8%) | 4 (6%) |
| Prior bone metastases | – | 2 (7%) | 2 (7%) | 4 (6%) |
| Prior gallbladder metastases | – | 0 | 1 (4%) | 1 (1%) |
| Prior adrenal metastases | – | 1 (3%) | 1 (4%) | 2 (3%) |
| Prior bowel metastases | – | 3 (10%) | 4 (17%) | 7 (10%) |
| Prior splenic metastases | – | 4 (13%) | 1 (4%) | 5 (7%) |
| Prior liver metastases | – | 7 (23%) | 3 (12%) | 10 (14%) |
| Prior brain metastases | – | 6 (20%) | 8 (33%) | 14 (19%) |
| ECOG 0 or KPS 100 | 17 (94%) | 23 (77%) | 7 (29%) | 47 (65%) |
| Elevated LDH at treatment | 3 (17%) | 7 (23%) | 13 (54%) | 23 (32%) |
| Anergic to common skin tests | 3 (17%) | 11 (37%) | 3 (17%) | 17 (24%) |
| Tumor DTH positive at baseline | 0 | 2 (7%) | 1 (4%) | 3 (4%) |
| Tumor DTH positive after vaccine | 3 (17%) | 8 (27%) | 2 (8%) | 13 (18%) |
DTH: Delayed type hypersensitivity test; ECOG: Eastern Cooperative Oncology Group; Mets: Metastasis; KPS: Karnofsky Performance Status; NED: No evidence of disease; RT: Radiation therapy.
Staging and substaging for these patients was assigned per the seventh edition of AJCC staging [36]. Among the patients with recurrent stage 3 melanoma, 14 had recurred one-, two-, three- and four-times. The highest substages for these were N1b (n = 5), N2b (n = 1), N2c (n = 3) and N3 (n = 9). Among the 30 patients who had experienced distant metastatic disease (stage 4) but did not have measurable disease per RECIST at the time of treatment, their highest substages were M1a (n = 10), M1b (n = 4) and M1c (n = 16). Previous sites of metastases that were not measurable at the time of treatment included liver (n = 6), brain (n = 5), bowel (n = 3) and bone (n = 2). Among these 30 patients, the previous number of recurrences during the course of their disease were one (n = 5), two (n = 16), three (n = 7), four (n = 1) and six (n = 1). Among the 24 patients with measurable disease at the time of treatment, stages at that time were M1a (n = 5, 21%), M1b (n = 5, 21%) and M1c (n = 14, 58%). Among these 24 patients, the number of previous recurrences during the course of their disease were one (n = 1), two (n = 11), three (n = 5), four (n = 3), five (n = 2) and six (n = 2).
As shown in Table 5, these patients were very heavily treated prior to receiving their patient-specific vaccines. As shown in Table 4, the average number of prior therapies was 3.0 (range: 1–5) for the stage 3 cohort, 3.3 (range: 1–7) for the stage 4 nonmeasurable disease cohort and 3.9 (range: 1–7) for those with stage 4 measurable disease. Table 5 also shows the categories of treatments received after initiating vaccine therapy. Among patients with stage 3 disease, there were six who received no additional therapy and remained disease free and alive at 5 years of follow-up. Two other patients underwent resection of one regional thigh metastasis after completion of DCV and then remained disease free and alive at 5 years of follow-up without other therapy. Another patient continued on GM–CSF, then underwent resection and radiation to a distant bone metastasis, then remained disease free and alive at 5 years of follow-up.
Table 5. . Antimelanoma therapy pre- and post-dendritic cell vaccine.
| Other therapies received | Stage 3 NMD (n = 18) | Stage 4 NMD (n = 30) | Stage 4 MD (n = 24) | |||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| Metastasectomy (ies) locoregional | 18 | 3 | 2 | 1 | 9 | 2 |
| Metastasectomy (ies) distant | 0 | 6 | 23 | 17 | 11 | 7 |
| RT (not brain) | 7 | 4 | 6 | 6 | 7 | 6 |
| Brain RT | 0 | 2 | 6 | 5 | 7 | 8 |
| Chemotherapy | 8 | 4 | 14 | 7 | 19 | 16 |
| IL-2 | 6 | 2 | 15 | 7 | 13 | 2 |
| IFN-α | 9 | 1 | 10 | 1 | 11 | 1 |
| GM-CSF | 5 | 3 | 11 | 4 | 8 | 1 |
| Anti-VEGF | 0 | 1 | 1 | 1 | 4 | 2 |
| Anti-VEGF-TKI | 0 | 0 | 0 | 0 | 1 | 4 |
| Vaccine | 2 | 2 | 7 | 3 | 0 | 1 |
| Anti-BRAF | 0 | 0 | 0 | 2 | 0 | 1 |
| Anti-BRAF + Anti-MEK | 0 | 0 | 0 | 0 | 0 | 1 |
| Anti-CTLA4 | 0 | 0 | 0 | 4 | 1 | 5 |
| Anti-PD1 | 0 | 0 | 0 | 1 | 0 | 0 |
| None and no PD | 0 | 6 | 0 | 3 | 0 | 2 |
CSF: Cerebrospinal fluid; LR: Locoregional; MD: Measurable disease; NMD: No measurable disease; PD: Progressive disease; PD1: Programmed death molecule 1; RT: Radiation therapy; TKI: Tyrosine kinase inhibitor.
Among the 30 patients who had experienced distant metastases but did not have measurable disease at the time of DCV treatment, 11 were alive and disease free after 5 years of follow-up. Of these 11, three received no additional therapy, two were disease free but received some additional GM–CSF (both) and subcutaneous IL-2 (one), four underwent resection of a single site of distant metastatic disease and then remained disease free (metastases to soft tissue back, small bowel, celiac node and rectosigmoid), one underwent resection of a small bowel mass and received whole brain irradiation followed by temozolomide and one underwent cyberknife treatment of a brain metastasis followed by temozolomide. Among the 16 who survived 5 years, one subsequently received ipilimumab and later IL-2 with tumor infiltrating lymphocytes, and one received nivolumab and later ipilimumab.
Among the 24 patients with measurable disease, three remained disease free and were alive 5 years after starting DCV. One received no additional therapy, one received radiation therapy to a residual adrenal cell metastasis and one had resection of a residual lymph node. Among the four patients who survived 5 years, none received nivolumab, pembrolizumab or ipilimumab, but one did receive dabrafinib plus trametinib in addition to chemotherapy, pazoponib, surgical resection and radiation for brain metastases, and resection of a small bowel metastasis.
Efficacy
Survival curves are shown in Figures 1 and 2. All survival curves are actual to 5 years rather than actuarial in as much as all patients were either deceased or followed to 5 years with none lost to follow-up. Two patients died of unrelated cerebrovascular events, one stage 4 without measurable disease and one stage 4 with measurable disease.
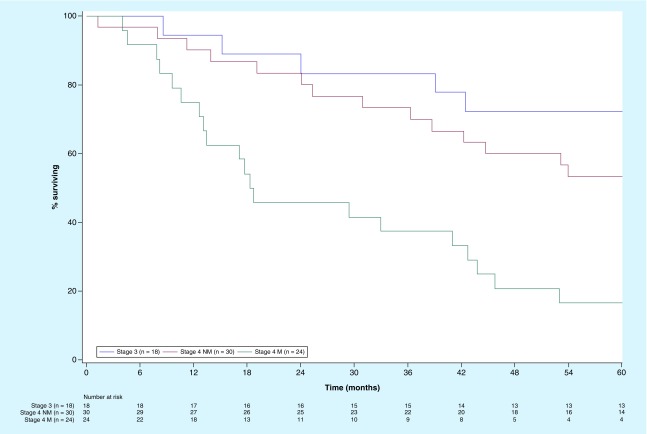
Figure 2. . Overall survival for melanoma patients treated with autologous dendritic cell vaccines loaded with autologous tumor antigens whose stages of disease at the time of treatment were stage 3 (n = 18), stage 4 with nonmeasurable disease per RECIST (n = 30), or measurable (M) disease per RECIST (n = 24).
M: Measurable; NM: Not measurable.
OS for all & by trial
Figure 1 displays the OS for all 72 patients treated with DCV and for those treated in the single arm (n = 54) and randomized trials (n = 18). All three curves were similar. The median survival for all 72 patients was 49.4 months (95% CI: 38.6–60 months). The 3-year survival rate was around 60% overall and in each trial. In the single arm open-label trial, median survival was greater than 60.0 months (95% CI lower limit 36.3 months) and the 5-year survival 50% [27], while the 18 patients treated with DCV in the randomized trial had a median survival of 43.4 months (95% CI: 18.6–60 months) and 5-year survival was 33% [29].
OS for all & by most advanced stage
The 5-year OS for the 18 patients whose most advanced stage was recurrent stage 3 was 72%. For the 54 patients whose most advanced stage was stage 4, the median OS was 42.5 months with 1-, 2-, 3-, 4- and 5-year survival rates of 83, 67, 57, 43 and 37%, respectively. However, this included patients who had measurable or nonmeasurable disease at the time of treatment. Figure 2 shows OS curves for patients based on the extent of disease at the time of treatment. Median OS was greater than 60 months for recurrent stage 3 (95% CI lower limit: 38.7 months; n = 18) and for nonmeasurable stage 4 (n = 30), compared with 18.5 months (95% CI: 12.6–42.7 months) for patients with measurable stage 4 disease (n = 24). The 3-year survival rates were 83, 73 and 38%, respectively.
Progression-free survival by most advanced stage
For all 72 patients, the median PFS was 5.4 months. Median PFS was 32 months for recurrent stage 3, 7.4 months for nonmeasurable stage 4, and 3.4 months for measurable stage 4. The 3-year PFS rates were 44, 23 and 4%, respectively.
Survival comparisons with published results
Because of the unique circumstances associated with harvesting tumor, establishing a cell line and creating a patient-specific autologous TCV, there are no perfectly comparable patient populations for outcomes comparisons. In terms of resected stage 3 disease, a large randomized trial was conducted in such patients, but most had N1a and N2a disease [4]. In that trial, patients randomized to bacillus Calmette–Guérin (BCG) plus an allogeneic cell line vaccine had a 5-year survival of 59%, and those randomized to BCG plus placebo had a 5-year survival of 68% [4]. The 5-year survival in our trial for the 18 patients with recurrent stage 3 disease was 72% even though all had N1b, N2b, N2c or N3 stage 3 disease, which are associated with a worse prognosis.
In a similar trial, patients with surgically resected stage 4 disease who were still free of disease were randomized to BCG plus an allogeneic vaccine or BCG plus placebo [4]. Patients randomized to the placebo + BCG had a median survival of 39 months and a 5-year OS of 45%, while those randomized to BCG + allogeneic vaccine had a median survival of 32 months and 5-year OS of 40%. Another trial enrolled patients with resected stage 4 disease, who had no disease at the time of treatment with an antimelanoma MART-1 peptide vaccine [37].
Their median survival was 46 months and 5-year OS 43%. In our DCV trial, patients surgically resected stage 4 disease who did not have measurable disease at the time of DCV therapy, the median survival was not reached at 5 years and the 5-year OS was 53%.
Another trial was limited to patients who were still free of disease after resection of either stage 3 or stage 4 melanoma, and were randomized to a multipeptide vaccine or placebo and GM–CSF or placebo if they had the appropriate HLA type, or to GM–CSF or placebo if they did not have the appropriate HLA type [6]. The 5-year OS rates were 54% for those who received the multipeptide vaccine, 51% for those who received placebo and 52% for those who received GM–CSF. In our DCV trial, the 48 patients who had nonmeasurable stage 3 or stage 4 melanoma had a 5-year OS of 60%.
There are several reports of immunotherapy in patients with measurable metastatic melanoma. A compilation of trials with the anti-CTLA4 antibody ipilimumab in patients with measurable metastatic disease revealed a median survival of 11.4 months and 2-year OS of 22% [38]. A clinical trial of the anti-PD-1 monoclonal antibody nivolumab in previously treated melanoma patients was associated with a median OS of 16.8 months and a 5-year OS of 43% [39]. A randomized trial in patients healthy enough to receive high-dose IL-2 resulted in a median survival of 17.8 months and 5-year OS of 30% in those who received IL-2 + gp100 peptide vaccine, and 11.1 months and 18% OS in those who received IL-2 alone [40]. In our trial, the patients with measurable melanoma at the time of DCV therapy had a median OS of 18.5 months and a 2-year OS of 46%. Thus, the survival for DCV-treated patients compared favorably with other immunotherapies in all cohorts.
Toxicity
Treatment with these patient-specific vaccines was well tolerated and consistent with the adverse event profile for a single injection of 500 µg of GM–CSF. Table 6 displays the adverse events reported for the 72 patients. There were no grade 4 adverse events (AE), and only five patients experienced a grade 3 AE; three reported a grade 3 headache and two reported a grade 3 skin rash. In contrast to what has been reported for monoclonal antibody checkpoint inhibitors, there were no immune-related adverse events resulting from autoimmune organ inflammation [41–43].
Table 6. . Adverse events and laboratory abnormalities considered possibly attributable to autologous dendritic cell vaccine (n = 72).
| Event & grade (%) | 0 | 1 | 2 | 3 | 4 | 5 | Any |
|---|---|---|---|---|---|---|---|
| Injection site reaction | 24 | 43 | 33 | 0 | 0 | 0 | 76 |
| Anemia | 58 | 35 | 7 | 0 | 0 | 0 | 42 |
| WBC (high or low) | 67 | 29 | 4 | 0 | 0 | 0 | 33 |
| Bone/joint pain | 72 | 28 | 0 | 0 | 0 | 0 | 28 |
| Transaminasemia | 76 | 22 | 1 | 0 | 0 | 0 | 24 |
| Chills/rigors | 78 | 22 | 0 | 0 | 0 | 0 | 22 |
| Nausea/vomiting | 83 | 12 | 7 | 0 | 0 | 0 | 19 |
| Headache | 82 | 12 | 3 | 4 | 0 | 0 | 18 |
| Rash/itch | 85 | 11 | 1 | 3 | 0 | 0 | 15 |
| Fatigue/malaise | 89 | 11 | 0 | 0 | 0 | 0 | 11 |
| Fever | 94 | 4 | 1 | 0 | 0 | 0 | 6 |
WBC: White blood count.
Delayed type hypersensitivity skin test reactivity
All 72 patients had a baseline DTH test at week 0 and a repeat test at week 4. Only three had a positive tumor DTH test at baseline; they survived 45, 53 and 60+ months. It is generally believed that patients who have a positive DTH at baseline may already have an active immune response that could contribute to greater survival, regardless of any biologic activity of a vaccine. Of the 69 with a negative DTH at baseline, eight (11.6%) converted to positive at week 4, and five others had a positive tumor DTH at a later time point. One patient whose test converted to positive died at 1.3 months of a cerebrovascular event that was not related to his melanoma or to treatment. Median survival was >60 months for the ten who had a positive DTH at week 0 or 4 compared with 43.8 months for those who tested negative on both occasions. The proportions surviving more than 44 months were 8/10 versus 30/62 (p = 0.090)
Discussion
This report summarizes manufacturing and clinical results for the 72 metastatic melanoma patients who were treated with DCV between 2001 and 2011. It provides important information regarding the manufacturing and reproducibility of comparable products in biologically heterogeneous patients. It also demonstrates the differing benefits among patients stratified by stage and disease measurability suggesting survival benefit for all patient groups in an era prior to general availability of BRAF/MEK and immune checkpoint inhibitors. All datasets on which the conclusions of the report rely are available on request.
Feasibility
The ability to establish autologous tumor cell lines was similar regardless of the anatomic site of resection, regardless of the stage of the patient at the time of resection and regardless of the geographic location where the tumor specimen was resected. The success rate for establishing tumors was similar in both trials as was the time it took to establish a tumor cell line. From a clinical and commercial perspective, it would be highly desirable if the cancer cell lines could be established more reliably and more rapidly.
The need to perform a surgical excision limits the pool of patients who could be treated with this approach. While biopsies or needle aspirates are less invasive means of obtaining tumor tissue, the nonhomogeneous location of cancer initiating cells within a tumor mass is likely to be associated with sampling error which would decrease the rate of cell-line success. Our limited experience with needle biopsies of resected tumor lesions supports this concern. It would also be highly desirable if such lines could be reliably established and expanded from blood samples containing circulating tumor cells rather than having to rely on resected specimens.
The DC was reliably produced for all patients in both trials, but there were some differences between the trials in terms of DC production and ATA loading. From a DC production perspective, the major difference was the reliance on plastic adherence as opposed to the Elutra for monocyte isolation. The most striking difference in the final product was the number of residual CD11c-negative cells in the treatment product in the randomized trial, which reflects residual irradiated tumor cells that were not phagocytosed. For those 18 patients, on average 33% of each dose were residual irradiated tumor cells, while in the open-label trial they represented only about 10%. These differences did not translate into a difference in clinical outcome following vaccine therapy. The immune response to DCV is quite different than that observed in patients treated with an irradiated tumor cell TCV [29]. Of particular interest was the change in IL-17 production, which is associated with a Th17 long-term memory helper T cell response [44,45]. There was no evidence that differences in DC number, TC number or their relative proportions in a given vaccine, was associated with any difference in outcome nor was OS dissimilar between the two trials. Antigen loading might be better accomplished by lysing the tumor cells, which would also result in a more consistent percentage of DC in the final product.
Toxicity
Repeated injections of these patient-specific vaccines were associated with minimal toxicity, and those adverse events that were observed are typical of injections of GM–CSF. There were no severe or life-threatening immune related adverse events. The only grade 3 toxicities were headaches that occurred in three patients, and grade 3 skin rashes reported for two patients. This is in sharp contrast to the rates of grade 3–5 toxicities associated with IL-2 (near 100%), anti-CTLA-4 (30%), anti-PD-1 (15%), anti-CTLA4 plus anti-PD-1 (60%) and anti-BRAF/MEK (60%) [41–43].
Clinical efficacy & comparison with other immunotherapies
These 72 patients were treated during 2001–2011. The major strength of the clinical data presented here is the maturity of the follow-up data such that 5-year survival data was available for every patient with no patients lost to follow-up, and the sample size of 72 patients which permitted some subset analyses based on stage and tumor measurability. This is important because stage and presence of measurable disease were strongly associated with both PFS and OS. The median survival of 49 months and the 5-year OS of 46% for all 72 patients is impressive for this era, but there is no comparable historical control group for comparison because of the varying stages and extent of disease at the time of treatment. However, as discussed in the results section, survival for DCV-treated patients compared favorably with other treatments in every cohort including patients who had distant measurable disease at the time of treatment. The DCV data becomes even more impressive when one considers the differences in the patient populations in these trials. For instance, in the multipeptide trial 61% of the patients had resected stage 3 disease including primary stage 3 [6], while in the DCV trials only 25% had stage 3 disease and all were recurrent. The allogeneic vaccine trial included mostly primary stage 3 patients in one trial, and excluded patients who had had brain metastases in the stage 4 trial [4]. The only data included for nivolumab was from the initial trial that included heavily pretreated patients. No data for pembrolizumab was included because none of the published studies provided survival data for patients with measurable disease who had received more than one systemic therapy previously.
The PFS associated with DCV was much less impressive than the OS. Similarly, among the 24 patients who had measurable disease per RECIST, no objective tumor remissions were reported during or immediately following the period of treatment. We now know that absence of high objective response rates or prolonged PFS does not preclude a survival benefit for some immunotherapies. Such discordance was anticipated based on experience in earlier trials with TCV [46]. Such discordance was also observed in trials of the anti-CTLA-4 antibody ipilimumab [5], and the anti-prostate cancer cell immunotherapy sipuleucel-T [7].
The standard treatment of metastatic melanoma has changed since these vaccine trials were conducted and response rates and long-term survival in advanced melanoma patients have steadily improved since 2010 as newer agents have moved into first-line therapy. These include the anticytotoxic T lymphocyte antigen-4 (CTLA-4) monoclonal antibody ipilimumab [5,37], targeted signal transduction inhibitors in patients with B600E/K BRAF mutations [47–49] and monoclonal antibody inhibitors of the immune checkpoint PD-1 [40,50–54]. Treatment continues to evolve as anti-BRAF/anti-MEK and anti-PD-1 therapies have become standard treatments in the adjuvant setting for patients with regionally advanced melanoma or oligometastatic disease but are at high risk for recurrence after surgical resection [55–58]. Initial treatment with combined anti-CTLA4 and anti-PD-1 has resulted in higher response rates and long-term survival, but at the expense of grade 3–4 adverse events in more than 50% of patients [59–61].
Anti-BRAF/MEK combinations are now approved for patients with BRAF mutations as first-line therapy for unresectable and distant metastatic melanoma, and as adjuvant treatment for patients with high-risk stage 3 melanoma or resected stage 4 melanoma. The checkpoint inhibitors anti-CTLA-4 (ipilimumab) and anti-PD-1 antibodies nivolumab and pembrolizumab as well as the combination of nivolumab plus ipilimumab have also been approved as first-line therapies for unresectable and distant metastatic melanoma, and as adjuvant therapies for patients with high risk stage 3 melanoma or resected stage 4 melanoma. However, there appears to still be an unmet need for vaccine strategies that can induce or enhance recognition of patient-specific ATA in addition to using checkpoint inhibitors to block factors that are suppressing existing immune responses [19,20].
The vast majority of DCV-treated patients were injected before the wide-spread availability of anti-BRAF and effective checkpoint inhibitors such as ipilimumab, nivolumab, or pembrolizumab, and few patients ever received these other immune therapies; so their survival was not impacted by those agents. DCV should be additive or synergistic with such therapies because of its mechanism of action to induce or enhance ATA recognition, rather than blocking the suppression of existing anti-ATA immune responses. Treatment-related toxicity associated with DCV was minimal; so it may easy to use in conjunction with other therapies. Because of its limited toxicity, and unique mechanism of action resulting in targeting of patient-specific ATA including those on cancer initiating cells, DCV may add additional benefit to these therapies, especially if ATA unique to cancer stem cells and early progenitors are being targeted in this approach [14,18,62–64].
Conclusion
Patient-specific DCV therapeutic vaccines were well tolerated and associated with encouraging survival results in patients with metastatic melanoma, including those with resected recurrent stage 3 disease, stage 4 patients both with and without measurable disease at the time of treatment. For wider testing of this approach, the source of autologous tumor antigen must be available more reliably and in a shorter period of time.
Future perspective
During the past 5 years, BRAF-MEK inhibitors and immune checkpoint inhibitors have displaced IL-2, IL-α and cytotoxic chemotherapy as the treatments of choice for patients with advanced melanoma. During the next 5–10 years, the benefits and limitations of BRAF-MEK inhibitors and immune checkpoint inhibitors will be further clarified. It is already recognized that less than half of all metastatic melanoma patients derive long-term benefit from these therapies; so, there is still an unmet need either for more effective and/or less toxic agents, or nontoxic agents that are additive to or synergistic with, the anti-PD-1 checkpoint inhibitors. A high proportion of patients with metastatic melanoma lacks a BRAF mutation and therefore do not benefit from anti-BRAF/MEK therapy. A high proportion of patients also lack an underlying immune response, and therefore do not benefit from anti-PD-1 therapy. During the next 5–10 years, there were will be continued testing of vaccines in an effort to induce new immune responses or enhance weak existing responses to patient-specific neoantigens. With further improvement in manufacturing, DC vaccines loaded with autologous tumor cell antigens, including those expressed on tumor initiating cells, will be poised to become an important adjunct to existing immunotherapies.
Summary points.
A total of 45 men and 27 women with metastatic melanoma were treated with patient-specific therapeutic vaccines during 2001–2011.
The vaccines consisted of autologous dendritic cells loaded with antigens from irradiated autologous tumor cells from a short-term cell culture derived from one or more surgically resected metastases.
Tumor sources were lymph nodes (n = 37), viscera (n = 20) and soft tissue (n = 15).
The antigen-loaded dendritic cells were admixed with GM–CSF (500 µg) and injected subcutaneously weekly for 3 weeks and monthly for 5 months at weeks 8, 12, 16, 20 and 24.
The average number of doses injected was 7.3 of a possible 8.
Treatment was well tolerated, highest grade adverse event was 3 in five of 72 patients.
Median survival was 49.4 months and 5-year survival rate was 46%.
A total of 5-year survival rates based on cohorts defined at the time of vaccine treatment were, 72% for 18 patients who were stage 3 without measurable disease and 53% for 30 patients who were stage 4 without measurable disease.
For the 24 patients who were stage 4 with measurable disease at the time of treatment, median survival was 18.5 months and 2-year survival rate was 46%.
Acknowledgments
The authors would like to acknowledge the contributions of S Minassian who prepared the survival curves. S Selvan and P Schiltz supervised the manufacturing of tumor cell lines, dendritic cells and dendritic cell vaccine product in the Hoag Cancer Center Cell Biology during 2000–2008. D Carbonell participated in the manufacturing of intermediate and final products during 2002–2011. L Beutel provided quality assurance and regulatory oversight during 2000–2011. Medical oncologist NM Barth referred seven patients during 2003–2007. Medical oncologist PF Sheehy referred five patients during 2003–2010. Research associates R Ellis (2001–2014), CD Leon (2000–2011), C Mayorga (2001–2011) and A Bodega (2014–2016) interacted with patients and collected data. K Austin supervised leukapheresis procedures during 2001–2011 and C Hendrix supervised the Cancer Outpatient Treatment Clinic where patients received the vaccine injections during 2001–2011.
Footnotes
Author contributions
RO Dillman submitted the INDs and designed the clinical trials for this investigator initiated research while we was Medical Director of Hoag Cancer Center where all patient-specific products were made and where all patients were treated, participated in data analysis and wrote the manuscript. AN Cornforth was in charge of manufacturing of the Patient-specific products, participated in the writing of the methods section and reviewed the manuscript. EF McClay contributed 14 of the 72 patients for the study during 2000–2010, managed their care and provided their medical information for data collection and reviewed the manuscript. C Depriest was the Senior Study Coordinator for these trials, supervised the research coordinators and data managers in the collection of data and completion of case report forms, compiled the data and reviewed the manuscript.
Financial & competing interests disclosure
Manufacturing of vaccines and the conduct and monitoring of the clinical trials was financed by the Hoag Hospital Foundation and Hoag Hospital. This included financial support of the Hoag Cell Biology Laboratory, the clinical trials staff and RO Dillman. The recent analysis and preparation of this manuscript was supported by AIVITA Biomedical, Inc. RO Dillman is now the Chief Medical Officer for AIVITA Biomedical, Inc., and is an employee and has equity in that company. AN Cornforth is an employee and has equity in TCRR Therapeutics, Inc. RO Dillman was previously the holder of the INDs, and author and principle investigator for both clinical trials, which were conducted while he was medical director of the Hoag Cancer Center in Newport Beach, CA, USA. AN Cornforth was a scientist in the Hoag Cell Biology Laboratory until the fall of 2011. EF McClay and C Depriest have nothing to disclose. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
All human subjects were treated in clinical trials that followed the principles outlined in the Declaration of Helsinki and were approved by an appropriate institutional review board. Written informed consent was obtained from all participants involved.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Atkins MB, Lotze MT, Dutcher JP. et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J. Clin. Oncol. 17(7), 2105–2116 (1999). [DOI] [PubMed] [Google Scholar]
- 2.Kirkwood JM, Manola J, Ibrahim JG. et al. A pooled analysis of Eastern Cooperative Oncology Group and intergroup trials of adjuvant high-dose interferon for melanoma. Clin. Cancer Res. 10(5), 1670–1677 (2004). [DOI] [PubMed] [Google Scholar]
- 3.Dillman RO. Melanoma vaccines: trials and tribulations. Vaccine Dev. Ther. 3, 57–78 (2013). [Google Scholar]
- 4.Morton DL, Mozzillo N, Thompson JF. et al. An international, randomized, Phase III trial of bacillus–Calmette-Guerin (BCG) plus allogeneic melanoma vaccine (MCV) or placebo after complete resection of melanoma metastatic to regional or distant sites. J. Clin. Oncol. 25(Suppl. 18), 8508 (2007). [Google Scholar]
- 5.Hodi FS, O’Day SJ, McDermott DF. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363(8), 711–723 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lawson DH, Lee S, Zhao F. et al. Randomized, placebo-controlled, Phase III trial of yeast-derived granulocyte–macrophage colony–stimulating factor (GM–CSF) versus peptide vaccination versus GM–CSF plus peptide vaccination versus placebo in patients with no evidence of disease after complete surgical resection of locally advanced and/or stage IV melanoma: a trial of the Eastern Cooperative Oncology Group – American College of Radiology Imaging Network Cancer Research Group (E4697). J. Clin. Oncol. 33(34), 4066–4076 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kantoff PW, Higano CS, Shore ND. et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 363(5), 411–422 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Andtbacka RH, Kaufman HL, Collichio F. et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J. Clin. Oncol. 33(25), 2780–2788 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science 438(6230), 69–74 (2015). [DOI] [PubMed] [Google Scholar]; •• Excellent review summarizing establishing existence of patient-specific neoantigens and importance as targets for immunotherapy.
- 10.Tran E, Robbins PF, Rosenberg SA. ‘Final common pathway’ of human cancer immunotherapy: targeting random somatic mutations. Nat. Immunol. 18(3), 255–262 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hundal J, Miller CA, Griffith M. et al. Cancer immmunogenomics: computational neoantigens identification and vaccine design. Cold Spring Harb. Symp. Quant. Biol. 81, 105–111 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Türeci Ö, Vormehr M, Diken M. et al. Targeting the heterogeneity of cancer with individualized neoepitope vaccines. Clin. Cancer Res. 22(8), 1885–1896 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Dillman RO, Selvan SR, Schiltz PM. Patient-specific dendritic-cell vaccines for metastatic melanoma. N. Engl. J. Med. 355(11), 1179–1181 (2006). [DOI] [PubMed] [Google Scholar]
- 14.Dillman RO, Cornforth AN, Nistor GI. Dendritic cell vaccines for melanoma: past, present, and future. Melanoma Manag. 3(4), 267–283 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Review of dendritic cell vaccines in treatment of melanoma and emphasis on the importance of antigen source.
- 15.Balan S, Finnigan J, Bhardwaj N. Dendritic cell strategies for eliciting mutation-derived tumor antigen responses in patients. Cancer J. 23(2), 131–137 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kreiter S, Vormehr M, van de Roemer N. et al. MHC class II epitopes drive therapeutic immune responses to cancer. Nature 520(7549), 692–696 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carreno BM, Magrini V, Becker-Hapak M. et al. Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science 348(6236), 803–808 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dillman RO, Cornforth AN, Nistor G. Cancer stem cell antigen-based vaccines: the preferred strategy for active specific immunotherapy of metastatic melanoma? Expert Opin. Biol. Ther. 13(5), 643–656 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Dillman RO. Is there a role for therapeutic cancer vaccines in the age of checkpoint inhibitors? Hum. Vaccin. Immunother. 13(3), 528–532 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dillman RO. An update on the relevance of vaccine research for the treatment of metastatic melanoma. Melanoma Manage. 4(4), 203–215 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Summary of rational and evidence for role of vaccines as an adjunct to checkpoint inhibition in patients with metastatic melanoma.
- 21.Dranoff G, Jaffee E, Lazenby A. et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte–macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc. Natl Acad. Soc. USA 90(8), 3539–3543 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dillman RO, Wiemann M, Nayak SK. et al. Interferon-gamma or granulocyte–macrophage colony-stimulating factor administered as adjuvants with a vaccine of irradiated autologous tumor cells from short-term cell line cultures: a randomized Phase II trial of the Cancer Biotherapy Research Group. J. Immunother. 26(4), 367–373 (2003). [DOI] [PubMed] [Google Scholar]
- 23.Kaufman HL, Ruby CE, Hughes T, Slingluff CL Jr. Current status of granulocyte–macrophage colony-stimulating factor in the immunotherapy of melanoma. J. Immunother. Cancer. 2(1), 11 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spitler LE, Grossbard ML, Ernstoff MS. et al. Adjuvant therapy of stage III and IV malignant melanoma using granulocyte–macrophage colony-stimulating factor. J. Clin. Oncol. 18(8), 1614–1621 (2000). [DOI] [PubMed] [Google Scholar]
- 25.Spitler LE, Weber RW, Allen RE. et al. Recombinant human granulocyte–macrophage colony-stimulating factor (GM–CSF, sargramostim) administered for 3 years as adjuvant therapy of stages II (T4), III, and IV melanoma. J. Immunother. 32(6), 632–637 (2009). [DOI] [PubMed] [Google Scholar]
- 26.Dillman R, Selvan S, Schiltz P. et al. Phase I/II trial of melanoma patient-specific vaccine of proliferating autologous tumor cells, dendritic cells, and GM–CSF: planned interim analysis. Cancer Biother. Radiopharm. 19(5), 658–665 (2004). [DOI] [PubMed] [Google Scholar]
- 27.Dillman RO, Selvan SR, Schiltz PM. et al. Phase II trial of dendritic cells loaded with antigens from self-renewing, proliferating autologous tumor cells as patient-specific anti-tumor vaccines in patients with metastatic melanoma: final report. Cancer Biother. Radiopharm. 24(3), 311–319 (2009). [DOI] [PubMed] [Google Scholar]; • Results of single arm trial of dendritic cell vaccine in patients with metastatic melanoma, and doubling of median survival compared with historical control of similar patients treated with autologous irradiated tumor cell vaccine.
- 28.Dillman RO, Cornforth AN, Depriest C. et al. Tumor stem cell antigens as consolidative active specific immunotherapy: a randomized Phase II trial of dendritic cells versus tumor cells in patients with metastatic melanoma. J. Immunother. 35(8), 641–649 (2012). [DOI] [PubMed] [Google Scholar]
- 29.Dillman RO, Cornforth AN, McClay EF. et al. Randomized Phase II trial of autologous dendritic cell vaccines versus autologous tumor cell vaccines in patients with metastatic melanoma: 5-year follow up and additional analyses. J. Immunother. Cancer. 6(1), 19 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Results of randomized trial in patients with metastatic melanoma in which autologous dendritic cell vaccine was associated with doubling of median survival compared with patients treated with autologous irradiated tumor cell vaccine.
- 30.Dillman RO, Depriest C. Dendritic cell vaccines presenting autologous tumor antigens from self-renewing cancer cells in metastatic renal cell cancer. J. Exploratory Res. Pharmacol. 3(4), 93–101 (2018). [Google Scholar]
- 31.Wang X, Bayer ME, Chen X. et al. Phase I trial of active specific immunotherapy with autologous dendritic cells pulsed with autologous irradiated tumor stem cells in hepatitis B-positive patients with hepatocellular carcinoma. J. Surg. Oncol. 111(7), 862–867 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dillman RO, Nayak SK, Beutel L. Establishing in vitro cultures of autologous tumor cells for use in active specific immunotherapy. J. Immunother. Emphasis Tumor Immunol. 14(1), 65–69 (1993). [DOI] [PubMed] [Google Scholar]
- 33.Selvan SR, Carbonell DJ, Fowler AW. et al. Establishment of stable cell lines for personalized melanoma cell vaccine. Melanoma Res. 20(4), 280–292 (2010). [DOI] [PubMed] [Google Scholar]
- 34.Cornforth AN, Fowler AW, Carbonell DJ. et al. Characterization of interferon-γ-treated melanoma tumor cells for use in dendritic cell-based immunotherapy. Cancer Biother. Radiopharm. 26, 345–351 (2011). [DOI] [PubMed] [Google Scholar]
- 35.Cornforth AN, Lee G, Dillman RO. Autologous peripheral blood mononuclear cell recognition of autologous proliferating tumor cells in the context of a patient-specific vaccine trial. J. Biomed. Biotechnol. 2011, 635850 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balch CM, Gershenwald JE, Soong SJ. et al. Final version of 2009 AJCC melanoma staging and classification. J. Clin. Oncol. 27(36), 6199–6206 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tagawa ST, Cheung E, Banta W. et al. Survival analysis after resection of metastatic disease followed by peptide vaccines in patients with stage IV melanoma. Cancer 106(6), 1353–1357 (2006). [DOI] [PubMed] [Google Scholar]
- 38.Schadendorf D, Hodi S, Robert C. et al. Pooled analysis of long-term survival data from Phase II and Phase III trials of ipilimumab in unresectable or metastatic melanoma. J. Clin. Oncol. 33(17), 1889–1894 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Topalian SL, Sznol M, McDermott DF. et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J. Clin. Oncol. 32(10), 1020–1030 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwartzentruber DJ, Lawson DH, Richards JM. et al. Gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N. Engl. J. Med. 364(22), 2119–2127 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Naidoo J, Page DB, Li BT. et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann. Oncol. 26(12), 2375–2391 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang PF, Chen Y, Song SY. et al. Immune-related adverse events associated with anti-PD1/PD-L1 treatment for malignancies: a meta-analysis. Front. Pharmacol. 18(8), 730 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baxi S, Yang A, Gennarelli RL. Immune-related adverse events for anti-PD-1 and anti-PD-L-1 drugs: systematic review and meta-analysis. BMJ 360, k793 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muranski P, Boni A, Antony PA. et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood 112(2), 362–373 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kryczek I, Banerjee M, Cheng P. et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood 114(6), 1141–1149 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dillman RO, DePriest C, DeLeon C. et al. Patient-specific vaccines derived from autologous tumor cell lines as active specific immunotherapy: results of exploratory Phase I/II trials in patients with metastatic melanoma. Cancer Biother. Radiopharm. 22(3), 309–321 (2007). [DOI] [PubMed] [Google Scholar]
- 47.Robert C, Karaszewska B, Schachter J. et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N. Engl. J. Med. 372(1), 30–39 (2015). [DOI] [PubMed] [Google Scholar]
- 48.Long GV, Weber JS, Infante JR. et al. Overall survival and durable responses in patients with BRAF V600-mutant metastatic melanoma receiving dabrafenib combined with trametinib. J. Clin. Oncol. 34(8), 871–878 (2016). [DOI] [PubMed] [Google Scholar]
- 49.Long GV, Flaherty KT, Stroyakovskiy D. et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: long-term survival and safety analysis of a Phase III study. Ann. Oncol. 28(7), 1631–1639 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robert C, Long GV, Brady B. et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 372(4), 320–330 (2015). [DOI] [PubMed] [Google Scholar]
- 51.Robert C, Schachter J, Long GV. et al. Pembrolizumab versus ipilimumab in advanced melanoma. N. Engl. J. Med. 372(26), 2532–2532 (2015). [DOI] [PubMed] [Google Scholar]
- 52.Weber JS, Hodi FS, Wolchok JD. et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J. Clin. Oncol. 35(7), 7885–792 (2017). [DOI] [PubMed] [Google Scholar]
- 53.Ribas A, Puzanov I, Dummer R. et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-0002): a randomised, controlled, Phase II trial. Lancet Oncol. 16(8), 908–918 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ribas A, Hamid O, Daud A. et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA 315(15), 1600–1609 (2016). [DOI] [PubMed] [Google Scholar]
- 55.Eggermont AM, Chiarion-Sileni V, Grob JJ. et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N. Engl. J. Med. 375(19), 1845–1855 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Long GV, Hauschild A, Santinami M. et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N. Engl. J. Med. 377(19), 1813–1823 (2017). [DOI] [PubMed] [Google Scholar]
- 57.Weber J, Mandala M, Del Vecchio M. et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N. Engl. J. Med. 377(19), 1824–1835 (2017). [DOI] [PubMed] [Google Scholar]
- 58.Eggermont AM, Blank CU, Mandala M. et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N. Engl. J. Med. 378(19), 1789–1801 (2018). [DOI] [PubMed] [Google Scholar]
- 59.Postow MA, Chesney J, Pavlick AC. et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N. Engl. J. Med. 372(21), 2006–2017 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Larkin J, Chiarion-Sileni V, Gonzalez R. et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 373(1), 23–34 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wolchok JD, Chiarion-Sileni V, Gonzalez R. et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 377(14), 1345–1356 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zabierowski SE, Herlyn M. Melanoma stem cells: the dark seed of melanoma. J. Clin. Oncol. 26(17), 2890–2894 (2008). [DOI] [PubMed] [Google Scholar]
- 63.Schmidt P, Kopecky C, Hombach A. et al. Eradication of melanomas by targeted elimination of a minor subset of tumor cells. Proc. Natl Acad. Sci. USA 108(6), 2474–2479 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ning N, Pan Q, Zheng F. et al. Cancer stem cell vaccination confers significant antitumor immunity. Cancer Res. 72(7), 1853–1864 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]