Abstract
Pollen collection is necessary for bee survival and important for flowering plant reproduction, yet if and how pollen extraction motor routines are modified with experience is largely unknown. Here, we used an automated reward and monitoring system to evaluate modification in a common pollen-extraction routine, floral sonication. Through a series of laboratory experiments with the bumblebee, Bombus impatiens, we examined whether variation in sonication frequency and acceleration is due to instrumental learning based on rewards, a fixed behavioral response to rewards, and/or a mechanical constraint. We first investigated whether bees could learn to adjust their sonication frequency in response to pollen rewards given only for specified frequency ranges and found no evidence of instrumental learning. However, we found that absence versus receipt of a pollen reward did lead to a predictable behavioral response, which depended on bee size. Finally, we found some evidence of mechanical constraints, in that flower mass affected sonication acceleration (but not frequency) through an interaction with bee size. In general, larger bees showed more flexibility in sonication frequency and acceleration, potentially reflecting a size-based constraint on the range over which smaller bees can modify frequency and acceleration. Overall, our results show that although bees did not display instrumental learning of sonication frequency, their sonication motor routine is nevertheless flexible.
Keywords: Bombus impatiens, buzz pollination, foraging, innate behavior, learned behavior, Solanum
The interdependency of pollinating insects and plants creates an ideal arena to study the flexibility of complex behavior. Pollinator behavior includes both how a pollinator moves between flowers and how a pollinator physically interacts with flowers. In many cases, pollinators must perform specific behaviors to extract nectar and pollen rewards from a plant and to successfully fertilize a flower. Floral sonication (also called floral vibration or buzz pollination, see Buchmann 1983; Vallejo-Marín 2018) is a widespread behavior in which bees use vibrations to extract pollen from anthers (Cardinal et al. 2018). Bees show extensive variation (both within and among individuals) in how they sonicate flowers but little is known about why this variation in behavior exists (Morgan et al. 2016; Russell et al. 2016, 2017; Switzer and Combes 2017).
Much of the previous research on pollination behavior focuses on how and why insects forage for nectar, leaving pollen-foraging behavior poorly understood (but see Morgan et al. 2016; Muth et al. 2016b; Nicholls and Hempel de Ibarra 2014; Raine and Chittka 2007; Russell et al. 2016, 2017). Bees collect and consume pollen as an essential source of proteins, lipids, vitamins, and minerals (Nicolson 2011). Many flowers conceal pollen in anthers or corolla tubes with small pores through which the pollen can be released (Buchmann 1983; Russell et al. 2017). These poricidal flowers require vibration (or floral sonication) to shake the pollen out of the pores. Many bees, including bumblebees, utilize sonication while foraging for pollen (Cardinal et al. 2018; De Luca and Vallejo-Marín 2013). Different bee species approach and grasp the flowers in different ways while sonicating (Switzer et al. 2016), and different species tend to sonicate at different frequencies and amplitudes (Burkart et al. 2011; Rosi-Denadai et al. 2018). There is also extensive variation among individual bees within a species in the mechanical characteristics of sonication (Switzer and Combes 2017). Finally, individual bumblebees sonicate flowers from different species of plants at different frequencies (Switzer and Combes 2017).
To begin exploring the underlying causes of the wide variation in sonication behavior, we address potential sources of within- and among-individual variation in the common eastern bumblebee, Bombus impatiens. First, individuals may learn specific routines over time by increasing behaviors that provide them with a reward (i.e., instrumental learning) (Dukas and Real 1991; Laverty 1994; Raine and Chittka 2007; Srinivasan 2012; Loukola et al. 2017). For example, a variety of pollinators, including honey bees and bumblebees, demonstrate learning of complex behaviors that are relatively far removed from natural foraging activities, in response to nectar rewards, such as string-pulling and ball-rolling (Alem et al. 2016; Loukola et al. 2017). While bees also modify their pollen foraging behavior on diverse flowers (Laverty and Plowright 1988; Raine and Chittka 2007; Morgan et al. 2016; Whitehorn et al. 2017), no previous studies have examined whether bees modify sonication frequency and amplitude as a result of instrumental learning. Therefore, we hypothesize that variation in sonication behavior may reflect bees learning to perform behaviors that result in greater pollen rewards.
Second, within-individual variation in complex behavioral routines may be due to innately specified responses to variable foraging conditions. If a particular set of behavioral rules or routines work well on most flowers, or when the time and energy required to learn, a complex behavior is high, then insects may rely on innately-specified foraging strategies. In this case, variation in sonication behavior may be explained in part by an innate, predictable response to variation in reward.
Finally, mechanical constraints may result in variation in bee behavior within and/or among individuals, due to the physical properties (e.g., mass) of both bees and flowers. Among-individual variation could arise from differences in bee body size (Burkart et al. 2011) or possibly level of satiation. Though satiation is not addressed in this study, prior work has shown satiation to affect learning (Menzel and Müller 1996) and foraging behavior (Dyer 2002; Mayack and Naug 2015). During floral sonication, bees use their flight muscles to vibrate flowers to release pollen (King and Buchmann 1996). Bumblebees within a single colony can exhibit an 8-fold variation in mass (Goulson et al. 2002; Heinrich 2004) and thoracic muscle averages 26.1% of body mass (Buchwald and Dudley 2010); thus, larger bees typically have larger flight muscles. Because larger flight muscles in insects can produce greater forces (Marden 1987), larger bees may be able to produce a greater range of sonication frequencies and/or accelerations, whereas smaller bees may not be able to vibrate certain flowers with enough energy to release pollen.
In addition, within-bee variation in sonication could arise from physical differences in the flowers the bee visits (Switzer and Combes 2017; Arroyo-Correa et al. 2019). For a given force produced by a sonicating bee, the acceleration a flower experience should be inversely proportional to its mass (force = mass × acceleration). Generalist bumblebees visit flowers varying greatly in mass (Galen and Newport 1987; Switzer and Combes 2017). In addition, a flower attached to a stem behaves as a mechanically oscillating system (analogous to cantilevered beam) when disturbed from rest, vibrating at a natural (resonant) frequency (Meirovitch 1975) that depends on the mass and material properties of the flower and stem. It is possible that bees could optimize sonication by matching their sonication frequency to the natural vibration frequency of the flower, increasing flower displacement and/or minimizing the effort required to move the flower. Altogether, we hypothesize that differences in pollinator size may explain some among-individual variation in sonication properties, and differences in flower mass may explain some within-individual variation.
In this study, we address these 3, non-mutually exclusive hypotheses by investigating how pollen rewards and flower properties influence bee sonication behavior. In 3 separate experiments, we used bumblebees, B. impatiens, sonicating on Solanum dulcamara flowers to examine how individuals varied their sonication frequency and acceleration, using a computer-controlled behavioral classification and reward delivery system (i.e., operant conditioning chamber).
We tested the first hypothesis that bees use instrumental learning to alter their sonication behavior by examining individual responses to treatments in which pollen rewards were delivered only when bees sonicated within pre-defined vibrational frequency ranges. We focused on frequency because past work has shown that sonication frequency differs when bees sonicate different plant species (Switzer and Combes 2017), though there is little evidence that different flowers require sonication at different frequencies to release pollen (Rosi-Denadai et al. 2018). If bees use instrumental learning to shape sonication behavior in functional ways, we expected that over time, bees would sonicate more often within the range of frequencies that produced a pollen reward, explaining some within-individual variation in bee behavior; however, in nature, learning may also play a role in explaining among-individual variation. We tested the second hypothesis that bees display a predictable and innate response to variation in reward by observing how bees changed their sonication frequency and acceleration when they received a reward versus when they did not. A predictable, innate response could explain within-individual variation in behavior; however, it may also explain variation among individuals, if different bees have different innate responses. Finally, we tested the third hypothesis that flower mass and bee size affect sonication acceleration and/or frequency by allowing bees of various sizes to visit flowers with experimentally altered mass, providing potential explanations for both within- and among individual variation in sonication.
Materials and Methods
Experimental setup
We built cubic plywood flight chambers (60-cm sides) with painted, flat grey interiors (San Antonio grey latex paint; Supplementary Figure S1). The front of each flight chamber had a clear vinyl panel that could be closed with Velcro strips (60 cm × 30 cm). Each chamber was illuminated on a 14/10 light/dark cycle, using ∼900 cool white Light Emitting Diodes (LEDs) (5000 K, HitLights, Baton Rouge, LA). Cages were kept in the lab at room temperature (∼21°C). Temperature and ambient humidity (unmonitored) were not modified beyond lab conditions.
We purchased 5 B.impatiens colonies from Koppert Biological Systems (Howell, MI), and used flower-naive bees for all experiments. Supplementary Table S1 shows colony identity and sample sizes for each experiment. We attached each flight chamber to a single colony and maintained nectar (∼40% table sugar by weight) and pollen feeders (as described in Russell and Papaj 2016) inside the flight chambers. We also built an experimental chamber, identical in size to the flight chambers that allowed us to quantify and reward sonication behavior (Supplementary Figure S1). The experimental chamber did not contain nectar or pollen feeders.
Inside the experimental chamber, we built an automated experimental setup to measure bees’ sonication behavior and to deliver pollen rewards. The experimental chamber contained an array of 8 artificial flowers (each consisting of 5 circular petals, total maximum diameter of ∼2.4 cm), punched from 2-mm thick, blue foam sheets (Fibre Craft, Skokie, IL) and placed on a 3-by-3 grid, with side length of ∼10 cm (the top, center position in the grid contained the pollen dispenser, rather than an artificial flower). Artificial flowers were used to visually attract the bee to this area of the experimental chamber (see Supplementary Figure S2). We attached a single, fresh S.dulcamara flower to a needle protruding from the center flower in the array by sliding the needle through the receptacle and into the anther cone, but not protruding through the anther cone. The flower was attached so that the anther cone was pointing out at a horizontal angle (see Supplementary Figure S2). Prior to placing the flower on the needle, we removed the style and placed a drop of clear glue (Elmer’s school glue, High Point, North Carolina) on the tip of the anther cone, to prevent pollen from being released from the poricidal anthers (poricidal anthers have small pores that can be blocked to prevent the release of pollen).
We recorded bee vibrations on the central flower using a ceramic shear accelerometer (352A24, PCB Piezotronics, Depew, NY) that was attached to the needle behind the flower (see Supplementary Figure S2). Accelerometer data traveled through a signal conditioner (482C05, PCB Piezotronics, Depew, NY) to a data acquisition board (USB-6229, National Instruments, Austin, TX), and a computer, where we processed it using custom-written code in Python (Python Software Foundation 2018). We collected accelerometer data at 200,000 samples sec−1 and we converted the differential voltage reading from the accelerometer to acceleration (in m s−2), based on the factory calibration (10.17 mV/m/s2).
Sonication is often recorded with laser vibrometers, microphones, or accelerometers. Past studies have defined and reported sonication “amplitude” in many different ways—as peak velocity of motion (King and Buchmann 2003), peak decibels of the sound produced (Morgan et al. 2016), or peak acceleration of motion (De Luca and Vallejo-Marín 2013; Arroyo-Correa et al. 2019). Displacement (distance moved) is rarely reported (but see King and Buchmann 2003). In this paper, we report the acceleration resulting from bee sonication, and we use the word “acceleration” to refer to a measurement of the maximum magnitude of the acceleration signal relative to equilibrium (in m s−2). Note that acceleration was not measured directly at the bee’s mandibles or on the flower—we were recording accelerations from slightly behind the flower (Supplementary Figure S2). The acceleration at the bees’ mandibles was likely larger than at the middle of the needle, where the accelerometer was attached; thus, our measures were likely a conservative underestimate of accelerations produced at the bee’s mandibles.
For the accelerometer data, we calculated frequency in 0.1-s sampling windows by using Fast Fourier Transform (FFT) and selecting the dominant peak from the frequency spectrum (Supplementary Figure S3). We characterized acceleration by the amplitude [(maximum – minimum)/2] of the acceleration wave, which we refer to simply as “acceleration.” We used this calculation because many of the recordings were not centered at zero, and the peak amplitude of the acceleration was not constant (Supplementary Figure S3).
We positioned a custom-built, automated, pollen dispensing system above the experimental flower. The system consisted of a microcontroller (Arduino, Scarmagno, Italy) that turned a stepper motor attached to a screw; this screw worked like an auger to push pollen out of a tube above the bee (see Supplementary Figure S2). We rewarded a bee by dropping pollen out of the tube onto it while it was sonicating the flower. A bee often rotated around the flower, and as a result groomed pollen from its venter and dorsum. We did not notice any bees that did not groom and pack pollen into their corbiculae, indicating that bees likely treated pollen dispensed from artificial flowers as they do pollen dispensed from live flowers. Heterantherous flowers, for example, can deposit pollen onto a bee’s dorsum (Papaj et al. 2017). We covered the tube with insect netting to prevent bees from collecting pollen directly from the tube. Bees occasionally landed on the netting, but we did not note any that spent time collecting pollen from the netting. Each rewarding event released 1.8 ± 0.13 (mean ± SD) mg of apple pollen (Firman Pollen Company, Yakima, WA) above the bee. We removed fresh flowers S. dulcamara from plants less than 4 h before using them in the experiments and stored them at room temperature, floating them in water until use. Flowers were placed onto the needle in the central position of the array less than 5 min before a bee was placed in the experimental chamber.
Experimental procedures
Experiment 1: instrumental learning hypothesis
We first investigated whether bees could learn to adjust their sonication frequency in response to pollen rewards given only for sonications within specified frequency ranges. We focused this experiment on frequency because past work has shown that sonication frequency differs with the flower species being sonicated (Switzer and Combes 2017). For each bee, the first trial (∼50 sonications) was a within-individual control treatment (rewarded over the full frequency range of 220–450 Hz). This provided a baseline measurement for each bee’s behavior, allowing us to identify changes during subsequent, experimental trials. After the first trial, bees were randomly assigned to 1 of 3 treatments. In the full-frequency treatment, bees were rewarded any time they sonicated within the range of 220–450 Hz. We chose the lower bound to exclude wingbeats (∼ 175 Hz for commercial colonies; Buchwald and Dudley 2010; Mountcastle and Combes 2013), and we chose the upper bound because bumblebee sonications have rarely been reported above 450 Hz (De Luca et al. 2014; Switzer and Combes 2017). In the high-frequency treatment, we rewarded bees for sonicating between 340 and 390 Hz. We chose the lower limit of the high-frequency treatment, because it was approximately the mean sonication frequency during a pilot study. We chose the upper limit of 390 Hz to exclude harmonics of bees sonicating at lower frequencies. In the low-frequency treatment, we rewarded bees for sonicating only between 220 and 330 Hz. For this and all further experiments, we set a lower threshold for accelerations of 0.2 m s−2 (max − min voltage = 0.004 V), which was above the threshold of electronic noise. This reduced erroneous pollen dispensing, and it was well below any accelerations caused by bees.
To choose a bee for each trial of the experiment, we put a S. dulcamara flower (with anthers glued shut) into the flight chamber connected to the colony, and waited until a bee visited the flower and began sonicating. While the bee was on the flower, we picked up the flower with a gloved hand and moved it from the flight chamber to the experimental chamber. We did not touch the bee during the move. In the experimental chamber, we gently brushed the bee from the flower and removed that flower, whereupon the bee found and sonicated the experimental flower (a different, S. dulcamara flower mounted on the accelerometer array), already within the experimental chamber. We tested a single bee in the experimental chamber at a time. If a bee did not visit the experimental flower for more than 5 min after being moved to the experimental chamber, we stopped the trial.
We used this same procedure to move bees from the flight chamber to the experimental chamber for every trial. For the first trial with each bee, we aimed to record at least 50 sonications to provide a baseline measurement. We stopped the first trial only when a bee completed at least 50 sonications and then flew away from the flower or if the bee did not visit the flower for more than 5 min. On subsequent trials, we allowed each bee in the experimental chamber until it stopped visiting the flower for more than 5 min. Though this experiment was designed to test changes in sonication frequency due to instrumental learning, we also analyzed acceleration amplitude, because these variables may change together, and we aimed to be consistent with the analyses of the other experiments.
After the first (baseline) trial, we applied individual markings to bees with paint (oil-based paint pens, Sharpie, Oak Brook, Illinois), randomly assigned them to a treatment group, let them return to the colony box, and then conducted additional treatment trials after they had deposited pollen loads and resumed foraging in the flight cage. At the end of the remaining trials, we captured the bee in a clear container and returned it directly back into the colony box. We interspersed trials on different individual bees throughout this experiment (i.e., we did not conduct all trials with a single bee before collecting data from another bee).
At the end of the experiment, we removed the bees that had been used from the colonies, froze them, and recorded the distance between the inner margins of the tegula across the thoracic dorsum (intertegular or “IT” span) with digital calipers (Cane 1987). IT span is a convenient proxy for bee size that has been used in previous studies (Cane 1987; Greenleaf et al. 2007). We measured and included IT span in the statistical analysis because it has been shown to explain some of the variation in sonication frequency both within and among bee species (Burkart et al. 2011; Switzer and Combes 2017). We were unable to attain IT span measurements for 2 bees because their marks wore off. To fill in the missing data for these 2 individuals, we used regression imputation based on sonication frequency for the first trial (∼50 sonications per bee). To evaluate how much these imputations affected the results, we conducted our analyses with and without these 2 bees and found no major changes in regression coefficients or statistical significance.
Experiment 2: predictable, innate response hypothesis
We next investigated whether receipt versus absence of a pollen reward led to a predictable response in sonication behavior. Each bee received 2 treatments within a single foraging bout (trial) in the experimental chamber—in 1 treatment the bee was rewarded for all sonications, and in another treatment, the bee was not rewarded for any sonications. The flower and all other experimental conditions remained the same throughout each trial, and only the delivery or absence of a pollen reward was changed. We systematically alternated the order in which bees received the treatments—approximately half of the bees were rewarded for the first 50 sonications and then not rewarded for the next 50 sonications. The other half received treatments in the opposite order. If a bee did not visit the flower for more than 5 min, we stopped the trial, froze the bee, and then measured its IT span. Each bee completed only 1 trial and was never returned to the colony box.
Experiment 3: physical constraint hypothesis
Last, we investigated how bees’ sonication frequency and acceleration varied, depending on the mass of the flower and the size of the bee. We implemented an “increased-mass” treatment, in which we modified experimental flowers by placing a small piece of wire (1 mm × 3 mm, ∼15 mg of metal added to the mass of the flower) inside the anther cone before the anthers were glued shut. For comparison, the body mass of adult worker bumblebees B. impatiens has been reported as 109–372 mg (Buchwald and Dudley 2010). To account for any potential effects of damage caused by the wire, we performed a control treatment (“sham flower”) by placing a piece of wire inside the anther cone, and then removing the wire before the anthers were glued. We systematically alternated the treatment order; half of the bees got the sham flower first, and half of the bees got the increased-mass flower first. Halfway through each trial, whereas the bee was still in the experimental chamber, we switched the flower treatment by quickly removing and replacing the S. dulcamara flower on the needle in the experimental cage. During this entire experiment, bees were rewarded with pollen for any sonications between 220 and 450 Hz. We switched the treatment once the bee stopped contacting (flew away from) the flower, after first having performed at least 25 sonications. Thus, the treatment switch did not happen at exactly 25 sonications for each bee, because we wanted to let the bee leave the flower naturally before switching out the flower (to avoid disrupting the bee). We recorded frequency and acceleration of bees’ sonications on both types of flowers. If the bee did not visit the experimental flower for 5 min, we stopped the trial. After each trial, we removed the bee, froze it, and measured its IT span. Each bee completed only 1 trial and was never returned to the colony box.
Statistical analysis
We made figures with the R (R Core Team 2018 ver 3.5.1) package, ggplot2 (Wickham 2016) and Python (Python Software Foundation 2018) package matplotlib (Hunter 2007). We used linear mixed effects models (LMMs) with the R package, lme4 (Bates et al. 2015), to model frequency and acceleration. We log-transformed (base e) acceleration so that the data would fit the assumptions of linear regression (Ramsey and Schafer 2012). This transformation was necessary because plots of residuals versus fitted values showed heteroskedasticity when data were not log-transformed. All error indicators (shaded regions or error bars) on the plots show 95% CIs that were calculated from 10,000 bootstrap samples that were based on fixed effects only.
For Experiment 2 (predictable, innate response hypothesis), in addition to analyzing sonication frequency and acceleration, we recorded how many times bees sonicated before they left the flower for 5 min, and we analyzed these data with Cox proportional hazards regression (R package survival; Therneau and Grambsch 2000) to determine if there was a significant difference in the probability of leaving the flower in the 2 treatment groups. We plotted the resulting survival curves with the R package, survminer (Kassambara et al. 2017).
Model selection
Our general approach to model selection was as follows: we started with a large model that included all predictors and interactions that we had a priori reasons to expect may affect the response variable, and then we performed a backward stepwise procedure to remove terms that did not improve the model, according to Bayesian Information Criterion (BIC). We chose to use BIC because we had many data points, we prioritized model interpretability and simplicity, and we wanted to minimize overfitting (Aho et al. 2014). Though BIC often agrees with Akaike Information Criterion (AIC) or likelihood ratio tests (LRTs) in selecting models, BIC penalizes the inclusion of more predictors in the model more than either of these 2 methods (Aho et al. 2014). Because we used BIC, we do not report P-values for predictors that were removed during the model-selection process. In addition, since experimental treatment was of interest in all models, we kept it in all models as a predictor (see Colegrave and Ruxton 2017).
Within each experiment, we report reduced and full models for acceleration and frequency that have the same predictors. In the reduced models, we included all predictors that resulted in a decrease in BIC in either the frequency of acceleration model. For example, in the second experiment, the use of BIC suggested that we drop IT span in the model for frequency but not the model for acceleration. However, we included IT span in both models reported in the Supplementary Tables. For Experiment 1, we conducted post-hoc pairwise comparisons of the treatment groups, using the R package, multcomp (Hothorn et al. 2008) with a conservative Bonferroni correction of P-values (Abdi 2007). We centered IT span in all models.
For Experiment 1 (instrumental learning hypothesis), we analyzed sonication frequency and acceleration with LMMs that included the following fixed effects: treatment (rewarded at high, low, or full range of frequencies), IT span, colony, trial number, and the interaction of treatment and IT span (Supplementary Tables S2 and S3). We did not investigate nonlinear terms because we had a relatively low number of individuals in this experiment. We included a random intercept of bee ID and added a random slope of trial number within bee ID. We allowed for correlation between bee ID and trial number.
For Experiment 2 (predictable, innate response hypothesis), we analyzed sonication frequency and acceleration using LMMs that included the following predictors: treatment order (rewarded first or unrewarded first), treatment (rewarded or unrewarded), IT span, colony, visit number within each treatment, the interaction of treatment and IT span, the interaction of treatment and visit number, the interaction of treatment order and visit number, the interaction of treatment order and treatment, and the 3-way interaction of treatment order: treatment: visit number (Supplementary Tables S4 and S5). Note that we included colony as a fixed effect, because even though it was not a variable of interest, we did not have data from enough colonies to justify including colony as a random effect (Crawley 2002). We included bee ID as a random effect. We investigated the nonlinear terms of visit number in this experiment by using a Generalized Additive Mixed Model (R package, gamm4, Wood and Scheipl 2017), but found no significant nonlinearities. Thus, we report the results of the LMMs. For the Cox proportional hazards model, we used the same selection procedure (BIC), with the following predictors: treatment order (rewarded first or unrewarded first), treatment (rewarded or unrewarded), IT span, colony, and the interaction of treatment order and IT span. We report P-values for the predictors in the reduced model, based on LRTs.
For Experiment 3 (physical constraint hypothesis), we used LMMs that included the following fixed effects: treatment order (increased mass first or sham first), treatment (increased mass or sham), IT span, colony, visit number within each treatment, the interaction of treatment and IT span, the interaction of treatment and visit number, the interaction of treatment order and visit number, the interaction of treatment order and treatment, and the 3-way interaction of treatment order: treatment: visit number (Supplementary Tables S6 and S7). As with Experiment 2, we included colony as a fixed effect, because we did not have data from enough colonies to justify including colony as a random effect (Crawley 2002). We included bee ID as a random effect. As in Experiment 2, we used a Generalized Additive Mixed Model to investigate nonlinear effects of visit number but found no significant nonlinearities—thus we report the results of the LMMs.
Results
Experiment 1: no evidence for instrumental learning
We measured sonication frequency (cycles per second) and acceleration [(maximum accel. – minimum accel.)/2] for 42 individual bees in this experiment; however, only 24 individuals completed more than the first trial (baseline measurement of ∼50 sonications) and moved onto the treatment trials. The 18 bees that completed only the first trial sonicated 32.5–51.5 times during the first trial, whereas the 24 bees that completed more than 1 trial sonicated 50–55 times during the first trial (95% bootstrap CI for median). In all, most bees performed more than 30 sonications, with only 6/42 bees sonicating fewer than 30 times during the first trial. We recorded 6,505 sonications by 6 bees in the high frequency group, 12,703 sonications by 8 bees in the low frequency group, and 2,997 sonications from 10 bees in the full frequency group that completed more than the first trial (see Supplementary Figures S4 and S5 for plots of raw data and learning curves, respectively). We also recorded 1,920 sonications on the first trial for all 42 bees. During the experimental trials (after the baseline), bees in the full frequency group were rewarded for 100% of their sonications, whereas bees that were rewarded for high-frequency sonications were rewarded for 32.5–49.4% of their sonications, and bees that were rewarded for low-frequency sonications were rewarded for 15.8–45.4% of their sonications (95% bootstrap CI for mean).
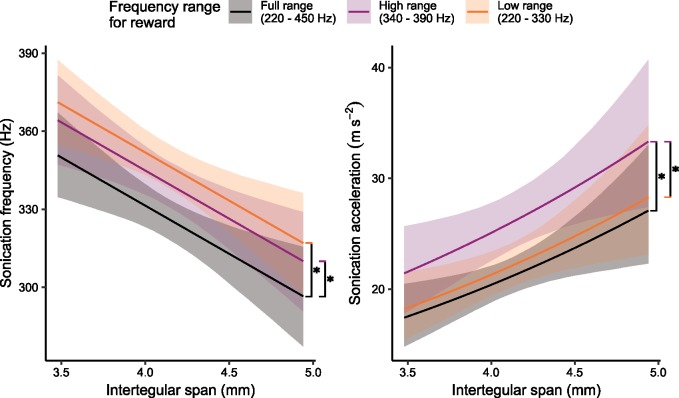
In our statistical analysis, the final model showed that frequency was affected by treatment, trial number, and IT span (Table 1, Supplementary Table S2). We found that bees sonicated significantly higher by about 14 Hz, when they were rewarded for only high sonication frequencies, relative to when they were rewarded for the full range of frequencies (Post-hoc pairwise test, P < 0.001, Table 1, Supplementary Table S2, Figure 1). Notably however, when bees were rewarded for sonicating only within the lower frequency range, they also tended to increase their sonication frequency by about 20 Hz (Table 1, Figure 1, Supplementary Table S2, Post-hoc pairwise test, P < 0.001). The increase in sonication frequency when bees were rewarded for only low-frequency sonications disagrees with our prediction that bees would match the frequency range that was reinforced (see Supplementary Figure S5 for learning curves). Furthermore, we found no significant difference in sonication frequency between the groups that were rewarded for high versus low frequency sonications (Figure 1, Supplementary Table S2, Post-hoc pairwise test, P = 0.26).
Table 1.
Regression coefficients and abbreviations for all 3 experiments
| Predictor | Coefficient abbreviation | Freq. Estimate (Hz) | Accel. Estimate [log (base e) m/s/s] | exp (Accel. Estimate) | |
|---|---|---|---|---|---|
| Experiment 1 (instrumental learning) | (Intercept) | β0 | 326.2 | 3.024 | 20.57 |
| Treatment – high | β(high) | 13.5 | 0.207 | 1.23 | |
| Treatment – low | β(low) | 20.4 | 0.044 | 1.04 | |
| Trial Number | β(Tr. Num.) | 0.9 | 0.010 | 1.01 | |
| IT span, centered (mm) | β(IT) | −37.1 | 0.302 | 1.35 | |
| Experiment 2 (predictable, innate response | (Intercept) | β0 | 339.5 | 3.281 | 26.60 |
| Treatment order – Unrewarded -> Rewarded | β(Trt. Ord.) | −14.7 | −0.100 | 0.90 | |
| Treatment – Rewarded | β(reward) | −11.0 | −0.127 | 0.88 | |
| IT span, centered (mm) | β(IT) | −12.0 | 0.407 | 1.50 | |
| Colony – 5 | β(Col5) | 22.8 | −0.239 | 0.79 | |
| Treatment: IT span | β(Trt: IT) | −14.8 | −0.171 | 0.84 | |
| Treatment order: Treatment | β(Trt. Ord.: Trt) | 10.8 | 0.007 | 1.01 | |
| Experiment 3 (physical constraint hypothesis | (Intercept) | β0 | 346.6 | 2.855 | 17.37 |
| IT span, centered (mm) | β(IT) | −9.5 | 0.057 | 1.06 | |
| Treatment – Increased-mass | β(mass) | 2.4 | −0.284 | 0.75 | |
| IT span: Treatment | β(IT: Trt) | 12.8 | 0.333 | 1.40 |
For full statistical models, see Supplementary tables.
Figure 1.
Estimated sonication frequency and acceleration for different reward ranges and IT spans. (Left) Larger bees sonicated at lower frequencies than smaller bees in all treatment groups. When rewarded for only high or low frequency sonications, bees sonicated at significantly higher frequencies than when they were rewarded for the full range of frequencies. (Right) Larger bees tended to produce higher accelerations in all treatment groups, though adding IT span to the acceleration model did not result in a decrease in Bayesian Information Criteria (BIC). When rewarded for only high-frequency sonications, bees produced significantly higher accelerations than when they were rewarded over the full range or for only low frequency sonications. Regression lines indicate estimated means (holding trial number constant) and shaded regions indicate 95% bootstrap confidence intervals. Vertical black lines with asterisks indicate significant differences (post-hoc pairwise comparisons, P < 0.01).
In addition to the results concerning the experimental treatment, we found a negative relationship between IT span and sonication frequency such that an increase of 1 mm in IT span was associated with a decrease in sonication frequency of 37 Hz (Table 1, Figure 1, Supplementary Table S2). Supplementary Table S6 shows the distribution of IT spans for bees in this experiment. For both models (frequency and acceleration), we found that trial number did not contribute greatly to the model as a fixed effect, but we included it as a fixed effect, to allow for a correlation of bee ID and trial number as random effects.
For acceleration, we report a model with the same predictors as the frequency model, for consistency. According to BIC, the model for acceleration should have all of the same predictors as the frequency model, except IT span. However, we report the effect of IT span on acceleration, so that this model has the same predictors as the model for frequency (Table 1, Supplementary Table S2). Since acceleration was log-transformed, the regression lines for acceleration for Figure 1 are curved, and the exponentiated regression coefficients can be interpreted as ratios of geometric means (see Table 1). For example, the exponentiated coefficient for IT span (centered) is the ratio of the expected geometric mean for bees of 1 mm larger than average IT span over the expected geometric mean for bees of average IT span, when all other variables are held constant. As other variables change, the geometric means change, but the ratio remains constant. To interpret the regression coefficient for IT span (centered), an increase in IT span of 1 mm is associated with a 35% increase in sonication acceleration, holding other variables constant (Table 1, Supplementary Table S3, Figure 1).
The frequency range in which bees received a pollen reward was also associated with changes in sonication acceleration. When bees were rewarded only for high-frequency sonications, they sonicated 23% higher in acceleration than when they were rewarded for the full range of frequencies (Post-hoc pairwise test, P < 0.001, Table 1, Supplementary Table S3, Figure 1). When bees were rewarded for low-frequency sonications, they showed only a small, non-significant, increase of about 4% in sonication acceleration, compared with when they were rewarded for the full range of frequencies (Table 1, Supplementary Table S3, Figure 1, Post-hoc pairwise test, P = 0.47) and decrease of 13% compared with bees that were rewarded for only high frequencies (Post-hoc pairwise test, P = 0.002, Supplementary Table S3, Figure 1). As with the frequency model, trial number did not contribute greatly to predict acceleration, though we left the predictor in the model to allow for a correlation between trial number and bee ID (Supplementary Table S3).
Experiment 2: evidence for predictable, innate response to reward variation
In our second experiment, we observed 96 bees, recording 3,660 sonications from 50 bees that received the reward treatment before the no-reward treatment and 2,191 sonications from 46 bees that received then no-reward treatment before the reward treatment. We found that the treatment variable, the receipt or absence of a pollen reward, affected both the frequency and acceleration of bee sonications (Table 1, Figure 2, Supplementary Tables S4 and S5).
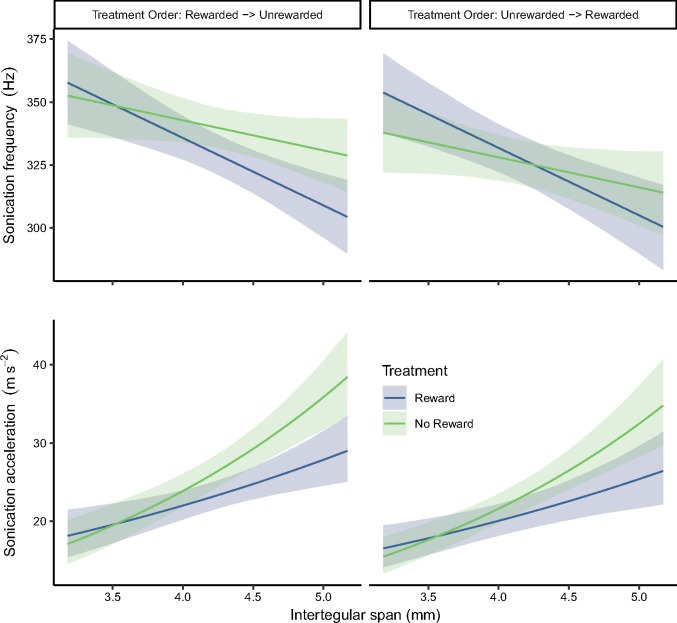
Figure 2.
Estimated sonication frequency (Top) and acceleration (Bottom) for bees that were rewarded versus unrewarded with pollen. Data are shown for frequency (top row) and acceleration (bottom row) and by treatment order and treatment order (columns). We found that both the receipt versus absence of a pollen reward and bee size (IT span) affected sonication frequency and acceleration. Treatment order affected sonication frequency but did not have a strong effect on acceleration. In this figure, we show estimates for only 1 colony (colony 4), but the plots for both colonies can be found in Supplementary Figure S8 and Supplementary Figure S9. Plotted lines indicate estimated means and shaded regions indicate 95% bootstrap confidence intervals. The curve of the acceleration line is due to the log-transformation for data used in statistical models.
Sonication frequency was predicted by a model including an interaction of treatment (reward versus no reward) with treatment order and IT span (Table 1, Supplementary Table S4). For example, bees with an average IT span (IT span = 4.27 mm) that were rewarded first were estimated to increase sonication frequency by about 11 Hz, during the second, unrewarded trial (Table 1, Figure 2, Supplementary Table S4). However, bees of average IT span that were rewarded second showed little change in frequency between the first (unrewarded) and second (rewarded) treatments, with an estimated reduction in sonication frequency of only about 0.2 Hz, during the second, rewarded treatment [β(reward) + β(Trt. Ord: Trt), Table 1, Figure 2, Supplementary Table S4]. The response of bees to lack of a reward changed for bees of different sizes. For a very small bee (IT span = 3.27 mm) that was rewarded first, the pollen reward was estimated to result in a decrease in sonication frequency of 4 Hz during the second (unrewarded) treatment [−1 × β(Trt: IT) + β(reward), Table 1, Figure 2, Supplementary Table S4]. If we estimate the same change for a very large bee (IT span = 5.27 mm), we find an increase of 26 Hz during the second, unrewarded treatment [1 × β(Trt: IT) + β(reward), Table 1, Figure 2, Supplementary Table S4]. Increasing IT span was generally associated with a decrease in sonication frequency, but the relationship strength depended on whether bees were rewarded with pollen (Figure 2). Supplementary Figure S7 shows the distribution of IT spans for bees in this experiment.
We also found differences between colonies—colony 5 tended to sonicate 23 Hz higher than colony 4, while holding other variables constant (Table 1, Supplementary Figure S4). Since colony differences were not of particular interest in this study, we have shown results for only colony 4 in Figure 2. Data for both colonies can be found in Supplementary Figure S8.
For the acceleration model, we did not find strong evidence of an interaction between treatment and treatment order. As with Experiment 1, the exponentiated regression coefficients for acceleration are interpreted as ratios of geometric means. For example, bees of average size (centered IT span = 4.27 mm) that were rewarded first sonicated about 12% lower in acceleration during the first, rewarded trial, compared with the second, unrewarded trail (Table 1, Figure 2, Supplementary Figure S5). Similarly, bees of average IT span that were rewarded second produced lower accelerations by 11% during the second, unrewarded treatment {exp[β(reward) + β(Trt. Ord.: Trt)], Table 1, Figure 2, Supplementary Figure S5}.
However, acceleration was affected by an interaction between treatment and bee size (Supplementary Figure S5), with the largest bees showing the greatest changes in acceleration between rewarded and unrewarded treatments (Figure 2). Increasing IT span by 1 mm, when was associated with a 50% increase in sonication acceleration when bees were not rewarded (Table 1, Figure 2, Supplementary Figure S5) and a 27% increase in sonication acceleration when they were rewarded {exp[β(IT) + β(Trt: IT)], Table 1, Figure 2, Supplementary Figure S5}. Bees with IT span 1-mm larger than the mean (IT span = 5.27 mm) that were rewarded first, produced accelerations that were about 26% lower during the first, rewarded, trial, compared with the second, unrewarded trial {exp[β(reward) + 1 × β(Trt: IT)], Table 1, Figure 2, Supplementary Figure S5}. A smaller bee (IT span 1-mm smaller than average), on the contrary, produced accelerations that were 5% larger during the first, rewarded trial than the second, unrewarded trial {exp[β(reward) + −1 × β(Trt: IT)], Table 1, Figure 2, Supplementary Figure S5}.
We also found differences between colonies—we estimate that colony 5 sonicated at 21% lower accelerations than colony 4, whereas holding other variables constant (Table 1, Supplementary Figure S5). Since colony differences were not of particular interest in this study, we have shown results for only colony 4 in Figure 2. Data for both colonies can be found in Supplementary Figure S9. For a plot of raw data that shows performance over time, see Supplementary Figure S10.
Finally, receiving no pollen reward increased the probability of bees abandoning their sonication attempts; unrewarded bees were more likely to leave the flower and not return within 5 min (resulting in the end of the trial). From the Cox proportional hazards regression, we estimate that for a fixed point in time, individuals that were rewarded were ∼0.4 times as likely to leave the flower than when they were not rewarded {exp[β(Treatment, rewarded)] = 0.41; β(Treatment, rewarded) = −0.892, LRT for pollen reward: χ21 = 10.0, P = 0.002, Supplementary Figure S11}. We also found significant colony-level differences—bees in colony 5 were ∼2.4 times as likely to leave the flower as bees in colony 4 {exp[β(Colony, 5)] = 2.4; β(Colony, 5) = 0.873, LRT for colony: χ21 = 10.75, P = 0.001, Supplementary Figure S11}.
Experiment 3: evidence for some physical constraints on sonication variation
All 36 bees in this experiment experienced both increased-mass and sham flower treatments and produced a total of 2,360 sonications. The effect of flower mass on sonication frequency was relatively small, but flower mass did affect acceleration resulting from sonication (Figure 3, Supplementary Tables S6 and S7).
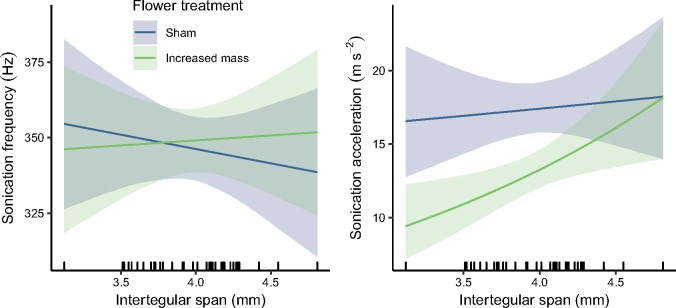
Figure 3.
Sonication frequency (Left) and acceleration (Right) for bumblebees on sham versus increased-mass flowers. Lines represent estimated means of sonication frequency and acceleration. Flower mass did not have a large effect on sonication frequency, but did affect accelerations produced by sonication, though an interaction with IT span. Shaded regions indicate 95% bootstrap confidence intervals. The black, vertical lines (“rug”) at the bottom of the plot show the distribution of bees’ IT spans in this experiment. The curve of the acceleration lines is due to the log-transformation for data used in statistical models.
None of the variables in this Experiment 3 improved the model for the sonication frequency (Figure 3, Supplementary Table S6); however, we present the coefficient interpretations here to allow for comparison to the acceleration model. For bees of average IT span (IT span = 3.97 mm), the increased mass treatment was associated with an increase in sonication frequency of 2 Hz (Table 1, Figure 3, Supplementary Table S6). An increase in IT span by 1 mm was associated with a decrease in sonication frequency of 10 Hz (Table 1, Figure 3, Supplementary Table S6) when bees were on the sham flower and about 3 Hz when bees were on the increased-mass flower [β(IT) + β(IT: Trt), Table 1, Figure 3, Supplementary Table S6].
In contrast to sonication frequency, flower mass, bee size and the interaction of the 2 improved the model for acceleration. For bees of average IT span, the increased mass treatment was associated with a 25% reduction in acceleration produced by sonication (Table 1, Figure 3, Supplementary Table S7). For bees with an IT span 1-mm smaller than the average (IT span = 2.9 mm), the increased mass treatment had a large effect, reducing acceleration by 46% {exp[β(mass) + −1 × β(IT: Trt)], Table 1, Supplementary Table S7}. Bees with an IT span 1-mm larger than average (IT span = 4.97 mm) displayed acceleration that were only 5% lower on the increased-mass flower {exp[β(mass) + 1 × β(IT: Trt)], Table 1, Figure 3, Supplementary Table S7}.
For a plot of raw data that shows performance over time, see Supplementary Table S12. Because some of the frequency data on Supplementary Table S12 appeared to fall into distinct clusters, we have conducted a separate analysis after removing the low-frequency data for each bee, which may have been due to high-frequency wingbeats (rather than low-frequency sonications). Clusters and outliers were identified with unsupervised clustering methods, Density-based spatial clustering of applications with noise (DBSCAN) (Ester et al. 1996) and Gaussian mixture model clustering (Benaglia et al. 2009), and the re-analyzed data are presented in Supplementary Tables S13 and S14, and Supplementary Tables S8–S11.
Discussion
Extracting resources efficiently from various kinds of flowers may require behavioral modification on the part of foragers. In some cases, insect foragers learn to perform a specific motor routine to obtain a food reward (Dukas and Real 1991; Dukas 1995; Giurfa 2012; Srinivasan 2012; Wolf and Chittka 2016). Though many experiments have tested the learning ability of bees in a nectar foraging context (Laverty 1994; Wolf and Chittka 2016; Loukola et al. 2017), there is comparatively little research on how bees modify their pollen-collecting behavior (Raine and Chittka 2007; Morgan et al. 2016; Muth et al. 2016a, 2016b; Papaj et al. 2017; Russell et al. 2017). In this paper, we investigated proximate causes of variation in bumblebee sonication behavior when foraging for pollen.
We tested 3 hypotheses about why sonication behavior varies within and among individuals of the same species. First, we hypothesized that bees display instrumental learning of sonication behavior and can learn to tune the frequency of their sonications to acquire a pollen reward (leading to within-individual variation). We found no evidence to support this hypothesis. As expected, bees show extensive among- and within-individual variation in sonication frequency, but the relationship between frequency and reward was unexpected. We predicted that bumblebees would sonicate at higher frequencies when only high-frequency buzzes were rewarded and sonicate at low frequencies when only low frequency buzzes were rewarded. In fact, we found that if bees were not rewarded, they sometimes increased their sonication frequency (Figures 1 and 2). Notably, in Experiment 1, when bees were rewarded for only low-frequency sonications, they increased their sonication frequency by about 20 Hz, relative to bees in the full-frequency group (Table 1, Figure 1). We did not find evidence of instrumental learning for frequency, which is consistent with previous studies showing that floral sonication has a strong innate component (Morgan et al. 2016; Russell et al. 2016, 2017). While we did not investigate whether additional components of floral sonications are learned, other studies have shown that pollen-foraging bees can learn floral cues, reward quality, how to groom themselves, and potentially how to adjust their body position during pollen collection (Raine and Chittka 2007; Nicholls and Hempel de Ibarra 2014; Morgan et al. 2016; Muth et al. 2016b; Russell et al. 2016, 2017; Nicholls et al. 2017).
The drop-off in return visits may be due to several possible reasons. First, individual foragers may make infrequent foraging trips (Russell et al. 2017 shows many foragers make 0–5 foraging trips per day). Second, the drop-off may also be due to satiation; that is, several bees may not have treated pollen as a reward. For instance, of the 6 bees that did not perform more than 30 sonications during the first trial, 5 of them completed only 1 trial. Last, although we observed the bees in our experiment foraging and grooming as they do in nature, our artificial pollen delivery system may not simulate floral conditions accurately enough to allow for expression of instrumental learning. Nevertheless, our results do provide a simple explanation for why other studies have shown that experience has only a modest influence on the floral sonication motor routine (Morgan et al. 2016; Russell et al. 2016, 2017; Nicholls et al. 2017).
Our finding that bees often increase sonication acceleration and sometimes frequency when they are unrewarded (Figure 2), however, is consistent with our second hypothesis, though it does not rule out the possibility of instrumental learning. We predicted that bees may exhibit a pre-specified, innate response to reward variation. Our second experiment explicitly tested whether bees changed their behavior depending on whether they were or were not rewarded with pollen. We found that the largest bees increased their sonication acceleration when they did not receive a reward, but the smallest bees showed negligible changes (Figure 2). The relationship of sonication frequency and reward was less clear. Our statistical model estimated that the largest bees would show an large increase in sonication frequency when not rewarded compared with when they were rewarded; average sized bees would show no change in sonication frequency, and the smallest bees may even show a decrease in sonication frequency when not rewarded (shown by the interaction of Treatment and IT span in Supplementary Table S4). The relationship was also affected by an interaction with treatment order (Figure 2, Supplementary Table S4). Consistent with previous experiments (Buchmann and Cane 1989), we also found that bees were more likely to stop visiting flowers when they did not receive a reward (Supplementary Figure S11).
Our first 2 experiments taken together showed no evidence that bees tune sonications to the particular frequency range to obtain pollen from flowers, but instead may have a pre-specified (unlearned) response to unrewarding flowers. Several previous studies (De Luca et al. 2013; Rosi-Denadai et al. 2018) have found that plants (S.lycopersicum and S.rostratum) do not exhibit an optimal sonication frequency for pollen release. Instead, increasing sonication acceleration and/or frequency results in increased pollen release (De Luca et al. 2013; Rosi-Denadai et al. 2018). While increasing acceleration and frequency likely increases energetic cost of foraging for the bee, this relationship between sonication and pollen release may be broadly predictable and stimulate an innate response to lack of pollen reward. The relationship between sonication frequency and pollen release shown previously in S. lycopersicum also depended on other factors—if velocity or displacement was held constant, an increase in frequency resulted in an increase in pollen release, but if acceleration was held constant, then an increase in frequency corresponded to a slight decrease in pollen release (Rosi-Denadai et al. 2018). This suggests that bees can increase pollen release by simply increasing sonication frequency and/or acceleration, without tuning their sonication to particular plants. In addition, this flexible foraging strategy may be especially effective for extracting the last remaining amount of pollen from flowers.
Throughout our trials, we measured both sonication frequency and acceleration produced by sonication. Many studies on floral sonication measure only frequency, because, unlike acceleration, frequency is easily quantified with a microphone (De Luca et al. 2018). Acceleration is an important measure in sonication studies, because increasing sonication acceleration has been shown to increase pollen release in some flowers (De Luca et al. 2013; Rosi-Denadai et al. 2018). In our first 2 experiments, our results suggested that variation in acceleration were generally driven by similar factors as variation in frequency. Larger bees produced greater acceleration when they were not rewarded, and the magnitude of this effect was highly dependent on the bee size. Larger bees showed more behavioral plasticity, which may reflect a physiological limitation on smaller bees (which possess smaller flight muscles that limit their maximum force production). One interesting difference between the frequency and acceleration results in our first 2 experiments was that the bees that were rewarded only for low-frequency sonications in the first experiment did not show a significant increase in sonication acceleration (they did increase frequency), even though they were rewarded infrequently (Figure 1). The bees in the low-frequency treatment happened to be smaller on average (see Supplementary Figure S6) and were rewarded less often than the bees in the high-frequency group, which may have influenced their response in terms of sonication frequency and acceleration. Together, these results indicate that when bees (particularly larger bees) do not receive a pollen reward, they vibrate flowers harder and/or faster (consistent with Russell et al. 2016)—these higher-energy vibrations may be more successful for releasing the last bits of pollen out of a flower that is almost depleted of pollen.
We also found support for our third hypothesis—that differences in physical characteristics (i.e., mass) of bees and flowers explain some within and among-bee variation in sonication. First, we found that some among-individual variation in sonication behavior can be explained by differences in bee mass. The largest bees increased sonication frequency when they did not receive a pollen reward, but the estimated change in behavior was negligible (or even opposite) for the smallest bees (Figure 2)—this is indicated by the large estimated interaction of IT span and Treatment [β(Trt: IT) = −14.8, Supplementary Table S4 and Table 1] and is visualized as the crossing lines for frequency on Figure 2. This may be because smaller bees are already performing near their physical maximum, while larger bees, with larger muscles, are able to increase their sonication frequency and acceleration to a greater extent.
In addition to bee mass affecting sonication, we found that flower mass affects the accelerations produced by sonication, whereas flower mass does not affect sonication frequency (Figure 3). Because bees did not change their sonication frequency on increased-mass versus sham flowers, our results corroborate past research, suggesting that bees do not necessarily match their vibrations to the resonant frequency of the flower (King and Buchmann 2003). However, the lower acceleration produced by sonication on increased-mass versus control flowers is consistent with the view of the flower and bee as a mechanical system, in which the acceleration of the flower results from the force applied by the bee divided by the flower’s mass (rearranged from force = mass x acceleration). Our results suggest that the mass of the flower affects the maximum accelerations that bees can produce. Flowers with greater mass caused a decrease in sonication acceleration, for smaller bees in particular, which would be expected if they were not changing their sonication behavior (Figure 3). This agrees with previous work showing that smaller bees tend to sonicate on smaller flowers (Corbet and Huang 2014). Larger bees may be less affected by increasing the mass of flowers, since their larger flight muscles may provide them with a greater operating range, allowing them to increase maximum force output to maintain higher flower acceleration.
In addition, for bees (e.g., smaller bees) that are already producing the largest force that they can, sonication frequency and acceleration are likely linked; when the mass of the flower increases, acceleration will necessarily decline if the bee holds its sonication frequency constant. In this way, acceleration may be constrained by sonication frequency or vice versa. A plot of sonication frequency versus acceleration from our study provides further support for the idea that these 2 properties may not be independent (Supplementary Figure S15).
Our findings provide a foundation for future work. First, we did not measure the acceleration of the bee’s mandibles or muscles—our measurement was on an object that the bee was moving that was part of a coupled, mechanical system. To obtain the most complete understanding of sonication behavior (independent from interactions with the mechanical system of the flower/stem), it would be helpful to perform direct measurements of acceleration on the bee’s body. In addition, future studies aimed at investigating the source of sonication differences between colonies could have important implications. Though there was not an obvious difference in the size of bees used from different colonies (see Supplementary Figure S7), there may be other differences between colonies (e.g., colony stores, larvae, and other colony-level variables) that could explain differences in both sonication behavior and pollen-foraging motivation.
In conclusion, our results suggest that bumblebees may rely more heavily on an innate foraging strategy, rather than learning, to improve pollen release during sonication. If sonicating at a certain frequency and acceleration produces no pollen reward, a bee may either give up or it may increase its sonication frequency and/or acceleration, which likely entails a greater energetic expense for the bee.
Supplementary Material
Acknowledgments
We thank the Arnold Arboretum staff, particularly, Kea Woodruff, Plant Growth Facilities Manager, who maintained the plants used in the study. C.M.S. was supported by the following: Air Force Office of Scientific Research Grant FA9550-14-1-0398, Komen Endowed Chair, National Defense Science and Engineering Graduate Fellowship, University of Washington Data Science Grant from the Alfred P. Sloan Foundation, Gordon and Betty Moore Foundation, and Washington Research Foundation. The work was also supported in part by the National Science Foundation grant (CAREER IOS-1253677) to SAC and the Graduate and Professional Student Council of the University of Arizona to ALR. Associated data and custom scripts are deposited at Zenodo (doi: 10.5281/zenodo.2530941, Switzer et al. 2019).
Authors’ Contributions
C.M.S. curated data, performed formal analysis, wrote software, produced visualizations and prepared the original draft. C.M.S., R.H., and A.L.R. conceptualized the study and designed the experiments. C.M.S. and A.L.R. conducted pilot experiments. C.M.S. conducted the experiments presented in the paper. All authors performed review and editing and acquired funding.
References
- Abdi H, 2007. Bonferroni and Šidák corrections for multiple comparisons In: Salkind NJ, editor. Encyclopedia of Measurement and Statistics. Thousand Oaks (CA: ): Sage; 103–107. [Google Scholar]
- Aho K, Derryberry D, Peterson T, 2014. Model selection for ecologists: the worldviews of AIC and BIC. Ecology 95:631–636. [DOI] [PubMed] [Google Scholar]
- Alem S, Perry CJ, Zhu X, Loukola OJ, Ingraham T et al. , 2016. Associative mechanisms allow for social learning and cultural transmission of string pulling in an insect. PLoS Biol 14:e1002564. doi: 10.1371/journal.pbio.1002564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo-Correa B, Beattie CE, Vallejo-Marín M, 2019. Bee and floral traits affect the characteristics of the vibrations experienced by flowers during buzz–pollination. J Exp Biol 222:jeb198176. doi: 10.1242/jeb.198176 [DOI] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, Walker S, 2015. lme4: linear mixed–effects models using Eigen and S4. J Stat Softw 67:1–48. [Google Scholar]
- Benaglia T, Chauveau D, Hunter D, Young D, 2009. mixtools: an R package for analyzing finite mixture models. J Stat Softw 32:1–29. [Google Scholar]
- Buchmann SL, 1983. Buzz pollination in angiosperms In: Jones CE, Little RJ, editors. Handbook of Experimental Pollination Biology. New York (NY: ): Van Nostrand Reinhold Company; 73–113. [Google Scholar]
- Buchmann SL, Cane JH, 1989. Bees assess pollen returns while sonicating Solanum flowers. Oecologia 81:289–294. [DOI] [PubMed] [Google Scholar]
- Buchwald R, Dudley R, 2010. Limits to vertical force and power production in bumblebees (Hymenoptera: bombus impatiens). J Exp Biol 213:426–432. [DOI] [PubMed] [Google Scholar]
- Burkart A, Lunau K, Schlindwein C, 2011. Comparative bioacoustical studies on flight and buzzing of neotropical bees. J Pollinat Ecol 6:118–124. [Google Scholar]
- Cane JH, 1987. Estimation of bee size using intertegular span (Apoidea.). J Kans Entomol Soc 60:145–147. [Google Scholar]
- Cardinal S, Buchmann SL, Russell AL, 2018. The evolution of floral sonication, a pollen foraging behavior used by bees (Anthophila). Evolution 72:590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colegrave N, Ruxton GD, 2017. Statistical model specification and power: recommendations on the use of test-qualified pooling in analysis of experimental data. Proc R Soc Lond, B, Biol Sci 284:20161850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbet SA, Huang SQ, 2014. Buzz pollination in eight bumblebee–pollinated Pedicularis species: does it involve vibration–induced triboelectric charging of pollen grains? Ann Bot 114:1665–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley MJ, 2002. Statistical Computing: An Introduction to Data Analysis Using S–plus. Chichester, England: Wiley. [Google Scholar]
- De Luca PA, Bussière LF, Souto–Vilaros D, Goulson D, Mason AC et al. , 2013. Variability in bumblebee pollination buzzes affects the quantity of pollen released from flowers. Oecologia 172:805–816. [DOI] [PubMed] [Google Scholar]
- De Luca PA, Cox D, Vallejo-Marín M, 2014. Comparison of pollination and defensive buzzes in bumblebees indicates species–specific and context–dependent vibrations. Naturwissenschaften 101:331–338. [DOI] [PubMed] [Google Scholar]
- De Luca PA, Giebink N, Mason AC, Papaj DR, Buchmann SL, 2018. How well do acoustic recordings characterize properties of bee (Anthophila) floral sonication vibrations? Bioacoustics doi: 10.1080/09524622.2018.1511474. [Google Scholar]
- De Luca PA, Vallejo-Marín M, 2013. What’s the ‘buzz’ about? The ecology and evolutionary significance of buzz–pollination. Curr Opin Plant Biol 16:429–435. [DOI] [PubMed] [Google Scholar]
- Dukas R, 1995. Transfer and interference in bumblebee learning. Animal Behav 49:1481–1490. [Google Scholar]
- Dukas R, Real LA, 1991. Learning foraging tasks by bees: a comparison between social and solitary species. Animal Behav 42:269–276. [Google Scholar]
- Dyer LA, 2002. A quantification of predation rates, indirect positive effects on plants, and foraging variation of the giant tropical ant, Paraponera clavata. J Insect Sci 2:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ester M, Kriegel H-P, Sander J, Xu X, 1996. A density-based algorithm for discovering clusters in large spatial databases with noise. In: Simoudis E, Han J, Fayyad U, editors. Proceedings of the Second International Conference on Knowledge Discovery and Data Mining. AAAI Press, pp. 226–231.
- Galen C, Newport M, 1987. Bumble bee behavior and selection on flower size in the sky pilot, Polemonium viscosum. Oecologia 74:20–23. [DOI] [PubMed] [Google Scholar]
- Giurfa M, 2012. Social learning in insects: a higher–order capacity? Front Behav Neurosci 6:57.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulson D, Peat J, Stout JC, Tucker J, Darvill B et al. , 2002. Can alloethism in workers of the bumblebee, Bombus terrestris, be explained in terms of foraging efficiency? Animal Behav 64:123–130. [Google Scholar]
- Greenleaf SS, Williams NM, Winfree R, Kremen C, 2007. Bee foraging ranges and their relationship to body size. Oecologia 153:589–596. [DOI] [PubMed] [Google Scholar]
- Heinrich B, 2004. Bumblebee Economics. Cambridge: Harvard University Press. [Google Scholar]
- Hothorn T, Bretz F, Westfall P, 2008. Simultaneous inference in general parametric models. Biom J 50:346–363. [DOI] [PubMed] [Google Scholar]
- Hunter JD, 2007. Matplotlib: a 2D graphics environment. Comput Sci Eng 9:90–95. [Google Scholar]
- Kassambara A, Kosinski M, Biecek P.. 2017. R package: survminer: Drawing Survival Curves using ‘ggplot2’ Version 0.3. https://CRAN.R–project.org/package=survminer.
- King MJ, Buchmann SL, 1996. Sonication dispensing of pollen from Solanum laciniatum flowers. Funct Ecol 10:449–456. [Google Scholar]
- King MJ, Buchmann SL, 2003. Floral sonication by bees: mesosomal vibration by Bombus and Xylocopa but not Apis (Hymenoptera: apidae), ejects pollen from poricidal anthers. J Kans Entomol Soc 76:295–305. [Google Scholar]
- Laverty TM, 1994. Bumble bee learning and flower morphology. Animal Behav 47:531–545. [Google Scholar]
- Laverty TM, Plowright RC, 1988. Flower handling by bumblebees: a comparison of specialists and generalists. Animal Behav 36:733–740. [Google Scholar]
- Loukola OJ, Perry CJ, Coscos L, Chittka L, 2017. Bumblebees show cognitive flexibility by improving on an observed complex behavior. Science 355:833–836. [DOI] [PubMed] [Google Scholar]
- Marden JH, 1987. Maximum lift production during takeoff in flying animals. J Exp Biol 130:235–258. [Google Scholar]
- Mayack C, Naug D, 2015. Starving honeybees lose self–control. Biol Lett 11:20140820.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meirovitch L, 1975. Elements of Vibration Analysis. New York (NY: ): McGraw–Hill. [Google Scholar]
- Menzel R, Müller U, 1996. Learning and memory in honeybees: from behavior to neural substrates. Annu Rev Neurosci 19:379–404. [DOI] [PubMed] [Google Scholar]
- Morgan T, Whitehorn P, Lye G, Vallejo-Marín M, 2016. Floral sonication is an innate behaviour in bumblebees that can be fine–tuned with experience in manipulating flowers. J Insect Behav 29:233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountcastle AM, Combes SA, 2013. Wing flexibility enhances load–lifting capacity in bumblebees. Proc R Soc B 280:20130531.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muth F, Francis JS, Leonard AS, 2016a. Bees use the taste of pollen to determine which flowers to visit. Biol Lett 12:20160356. doi: 10.1098/rsbl.2016.0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muth F, Papaj DR, Leonard AS, 2016b. Bees remember flowers for more than one reason: pollen mediates associative learning. Animal Behav 111:93–100. [Google Scholar]
- Nicholls EK, Hempel de Ibarra N, 2014. Bees associate colour cues with differences in pollen rewards. J Exp Biol 217:2783–2788. [DOI] [PubMed] [Google Scholar]
- Nicholls EK, Hempel de Ibarra N, Nicolson S, 2017. Assessment of pollen rewards by foraging bees. Funct Ecol 31:76–87. [Google Scholar]
- Nicolson SW, 2011. Bee food: the chemistry and nutritional value of nectar, pollen and mixtures of the two. Afr Zool 46:197–204. [Google Scholar]
- Papaj DR, Buchmann SL, Russell AL, 2017. Division of labor of anthers in heterantherous plants: flexibility of bee pollen collection behavior may serve to keep plants honest. Arthropod Plant Interact 11:307–315. [Google Scholar]
- Python Software Foundation. 2018. Python Language Reference Version 3.6.6. http://www.python.org (last accessed 26 July 2018).
- R Core Team. 2018. R: A language and environment for statistical computing Version 3.5.2. https://www.R-project.org (last accessed 25 January 2019).
- Raine N, Chittka L, 2007. Pollen foraging: learning a complex motor skill by bumblebees (Bombus terrestris). Naturwissenschaften 94:459–464. [DOI] [PubMed] [Google Scholar]
- Ramsey F, Schafer D, 2012. The Statistical Sleuth: A Course in Methods of Data Analysis. Boston (MA: ): Cengage Learning. [Google Scholar]
- Rosi-Denadai CA, Araújo PCS, de Oliveira Campos LA, Cosme L Jr, Guedes RNC, 2018. Buzz–pollination in Neotropical bees: genus–dependent frequencies and lack of optimal frequency for pollen release. Insect Sci, pp. 1–10, doi: 10.1111/1744–7917.12602. [DOI] [PubMed] [Google Scholar]
- Russell AL, Buchmann SL, Papaj DR, 2017. How a generalist bee achieves high efficiency of pollen collection on diverse floral resources. Behav Ecol 28:991–1003. [Google Scholar]
- Russell AL, Leonard AS, Gillette HD, Papaj DR, 2016. Concealed floral rewards and the role of experience in floral sonication by bees. Animal Behav 120:83–91. [Google Scholar]
- Russell AL, Papaj DR, 2016. Artificial pollen dispensing flowers and feeders for bee behaviour experiments. J Pollinat Ecol 18:13–22. [Google Scholar]
- Srinivasan MV, 2012. Bee Learning and Communication In: Seel NM, editor. Encyclopedia of the Sciences of Learning. New York (NY: ): Springer; 418–421. [Google Scholar]
- Switzer CM, Combes SA, 2017. Bumblebee sonication behavior changes with plant species and environmental conditions. Apidologie 48:223–233. [Google Scholar]
- Switzer CM, Hogendoorn K, Ravi S, Combes SA, 2016. Shakers and head bangers: differences in sonication behavior between Australian Amegilla murrayensis (blue–banded bees) and North American Bombus impatiens (bumblebees). Arthropod Plant Interact 10:1–8. [Google Scholar]
- Switzer CM, Russell AL, Papaj DR, Combes SA, Hopkins R, 2019. Sonicating bees demonstrate flexible pollen extraction without instrumental learning. Zenodo 10.5281/zenodo.2530941 (last accessed 3 February 2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therneau TM, Grambsch PM, 2000. Modeling Survival Data: Extending the Cox Model. New York (NY): Springer–Verlag. [Google Scholar]
- Vallejo-Marín M, 2018. Buzz pollination: studying bee vibrations on flowers. New Phytol doi: 10.1111/nph.15666. [DOI] [PubMed] [Google Scholar]
- Whitehorn PR, Wallace C, Vallejo MM, 2017. Neonicotinoid pesticide limits improvement in buzz pollination by bumblebees. Sci Rep 7:15562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H, 2016. ggplot2: Elegant Graphics for Data Analysis. New York (NY: ): Springer–Verlag. [Google Scholar]
- Wolf S, Chittka L, 2016. Male bumblebees, Bombus terrestris, perform equally well as workers in a serial colour–learning task. Animal Behav 111:147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood S, Scheipl F. 2017. R package: gamm4: Generalized additive mixed models using mgcv and lme4 Version 0.2–2. http://CRAN.R-project.org/package=gamm4 (last accessed 25 January 2019).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.