Abstract
Animals have evolved foraging strategies to acquire blends of nutrients that maximize fitness traits. In social insects, nutrient regulation is complicated by the fact that few individuals, the foragers, must address the divergent nutritional needs of all colony members simultaneously, including other workers, the reproductives, and the brood. Here we used 3D nutritional geometry design to examine how bumblebee workers regulate their collection of 3 major macronutrients in the presence and absence of brood. We provided small colonies artificial nectars (liquid diets) and pollens (solid diets) varying in their compositions of proteins, lipids, and carbohydrates during 2 weeks. Colonies given a choice between nutritionally complementary diets self-selected foods to reach a target ratio of 71% proteins, 6% carbohydrates, and 23% lipids, irrespective of the presence of brood. When confined to a single nutritionally imbalanced solid diet, colonies without brood regulated lipid collection and over-collected protein relative to this target ratio, whereas colonies with brood regulated both lipid and protein collection. This brood effect on the regulation of nutrient collection by workers suggests that protein levels are critical for larval development. Our results highlight the importance of considering bee nutrition as a multidimensional phenomenon to better assess the effects of environmental impoverishment and malnutrition on population declines.
Keywords: artificial diets, Bombus terrestris, bumble bees, nutritional geometry, rule of compromise
Animals have evolved behavioral strategies to acquire nutrients in amounts and balances that maximize fitness traits, such as development (Jang and Lee 2018), metabolic health (Solon-Biet et al. 2015), reproduction (Maklakov et al. 2008), and lifespan (Piper et al. 2014). These strategies are best understood using nutritional geometry (Raubenheimer and Simpson 1993; Simpson and Raubenheimer 1993, 2012), a modeling approach that describes how animals can reach and maintain an optimal nutritional state (the intake target) by adjusting their food consumption to their needs. Depending on foods available in the environment, animals can either select a nutritionally balanced food that lead them directly to their intake target, mix their intake of several individually imbalanced but complementary foods, or consume imbalanced foods according to a rule of compromise between over-ingesting some nutrients and under-ingesting others (for recent reviews see Simpson et al. 2015; Raubenheimer and Simpson 2018).
In animals whose food selection depends on social interactions, these nutritional decisions are complicated (Lihoreau et al. 2014, 2015). The challenge of group nutrition is exemplified in social insects, where foragers must balance their collection of foods to reach a colony level intake target that addresses the divergent needs of all colony members, including the non-foraging workers, the brood (larvae), and the reproductive males and females (Lihoreau et al. 2018). In these superorganisms, workers typically require carbohydrates for energy (honey bees: Paoli et al. 2014a; bumblebees: Stabler et al. 2015; ants: Wills et al. 2015) whereas queens and larvae primarily need proteins and lipids for egg laying and development (honey bees: Pirk et al. 2010; bumblebees: Stabler et al. 2015; ants: Dussutour and Simpson 2009). Nutritional geometry studies have shown that social insect foragers mix their collection of nutritionally complementary foods to reach a colony target for protein and carbohydrates (ants: Dussutour and Simpson 2009; Cook et al. 2010; Shik et al. 2016; honey bees: Hendriksma and Shafir 2016). In ants, this target ratio varies with feeding habits and colony composition, with foragers collecting more protein in the presence of brood (Dussutour and Simpson 2009) or symbiotic fungi (Shik et al. 2016). The ability of foragers to adjust nutrient collection to the collective needs is vital for the colony. At the physiological level, an excess of protein (or amino acids) reduces lifespan (honey bees: Pirk et al. 2010; Paoli et al. 2014b; ants: Dussutour and Simpson 2012; Arganda et al. 2017), whereas a high carbohydrate intake has detrimental effects on larval growth and survival (Helm et al. 2017). At the behavioral level, deficits in specific dietary lipids (e.g., omega-3) alter learning performances required for foraging (honey bees: Arien et al. 2015, 2018), a harmful effect that can potentially lead to colony collapse (Klein et al. 2017).
For bees, nutrient balancing is particularly challenging as it involves exploiting highly diverse, ephemeral and spatially distributed floral resources, in the form of liquid nectars and solid pollens. Nectar is a major source of water and carbohydrates, whereas pollen mainly contains protein, lipids, free amino acids, and other micronutrients (Wright et al. 2018). Experiments using artificial diets have shown that social bees can individually and collectively balance their acquisition of 2 nutrients simultaneously. Individual workers, or small groups of workers, balance their intake of carbohydrates and protein (or free amino acids) in nectar (honey bees: Altaye et al. 2010; Pirk et al. 2010; Paoli et al. 2014a; bumblebees: Stabler et al. 2015), as well as protein and lipids in pollen (bumblebees: Vaudo et al. 2016a, 2016b). The full-size colonies can also balance their intake of essential amino acids in pollens (honey bees: Hendriksma and Shafir 2016).
Although this is an important first step, bee colony nutrition involves collecting both nectars and pollens, and therefore regulating the acquisition of more than 2 nutrient sources simultaneously. Recent studies using 3D nutritional geometry designs show how taking into account more than 2 nutrients, and their natural associations in foods, can help bring new insights into the fundamental nutritional biology of species that would otherwise be overlooked; for instance, to explain why animals prioritize the regulation of some groups of nutrients over others (cats: Hewson-Hughes et al. 2011; mice: Solon-Biet et al. 2014) or do not appear to regulate nutrient intake at all (termites: Poissonnier et al. 2018).
Here, we used a 3D nutritional geometry design to examine how small colonies of bumblebees (Bombus terrestris) regulate their collection of protein, carbohydrates, and lipids, and how any impediment to do so affects their physiology. First, we investigated the ability of bumblebee colonies to freely regulate their nutrient collection from artificial liquid and solid diets, in the presence or absence of brood. Second, we explored the influence of brood on the nutrient collection rule of compromise used by bumblebees when confined to a single imbalanced diet. Third, we examined the effects of nutrient intake on adult emergence rate, body lipid composition and lifespan. We used artificial diets with extreme ranges in nutrient contents reported in natural foods to investigate to what extent bees can balance their nutrient collection and use rules of compromise in highly challenging conditions.
Materials and Methods
Bumblebees
Experiments were conducted between January and April 2016. Thirty-one commercial colonies of B. terrestris (Biobest, Orange, France) were used. Each colony contained about 200 workers, brood, and 1 queen. Bumblebees were maintained and tested in the laboratory at 25°C and 30–40% relative humidity, under a 12:12 h light:dark photocycle.
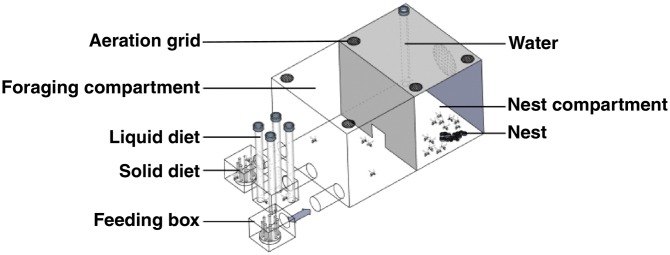
From these mother colonies, 136 microcolonies of 20 workers were set up (i.e., 4–5 microcolonies per mother colony). All workers of a given microcolony originated from the same mother colony. Each microcolony was placed in a plastic box divided in 2 compartments of the same size (Figure 1 and Supplementary Figure S1). The nest compartment was dark and contained 30 nest cells (either 30 empty cells or 20 empty cells plus 10 cells containing larvae, see details in Table 1). For each microcolony, brood and empty cells were taken from the same mother colony as the workers. Brood was in pupal stage (between 3- and 5-weeks old). Ad libitum water was provided in a gravity feeder (vertical 15 mL tube with 2 holes at the bottom from which the bumblebees could insert their proboscis and drink) in the nest compartment to avoid interfering with nutrient collection in the foraging compartment (i.e., limited access to nectar and pollen diets due to overcrowded conditions). The foraging compartment was clear and connected to 3 small feeding boxes (Figure 1), each containing either artificial nectar or pollen (see details below). Nectar was provided by 4 gravity feeders (15 mL) per feeding box. Pollen (1.5 g) was provided on 10 vertical chenille stems (2.5 cm) per feeding box (see details in Supplementary Figure S1). We used chenille stems to mimic flower stamen and facilitate collection by bees, as recommended by Russell and Papaj (2016).
Figure 1.
Schematic view of an experimental box where microcolonies of 20 bumblebee workers were kept with nest materials (either 30 empty cells or 20 empty cells with 10 cells containing larvae). Bumblebees could collect ad libitum water from a gravity feeder in the nest compartment, synthetic liquid diet (from gravity feeders) and solid diet (from chenille stems) in removable feeding boxes, during 13 days. Dimensions of the experimental box: 17.2 cm (length), 11.6 cm (width), and 9.4 cm (height). Dimensions of a feeding box: 5.7 cm (length), 5.7 cm (width), and 2.6 cm (height). See pictures in Supplementary Figure S1.
Table 1.
Summary of the choice and the no-choice experimental designs
| Experiment | Duration (days) | Solid diet | Liquid diet (%) | Mean number of empty cells at the beginning (95% CI) | Mean number of larvae at the beginning (95% CI) | Sample size behavioral assays (microcolonies) | Sample size lipid body content (bees) |
|---|---|---|---|---|---|---|---|
| Choice | 13 | Pl and pL | N10 | 27.9 (24.9–30.9) | 0 | 10 | 13 |
| N60 | 28.1 (25.9–30.3) | 0 | 10 | 12 | |||
| N10 | 19.1 (16.9–21.3) | 10.3 (9.1–11.5) | 10 | 14 | |||
| N60 | 17.4 (15.0–19.9) | 10.5 (9.3–11.7) | 10 | 15 | |||
| No-choice | 13 | pL | N10 | 28.3 (26.4–30.1) | 0 | 8 | 10 |
| N60 | 27 (25.0–29.0) | 0 | 8 | 15 | |||
| N10 | 17.9 (14.9–20.9) | 10.0 (8.6–11.4) | 8 | 13 | |||
| N60 | 17.6 (15.3–20.0) | 10.0 (9.0–11.0) | 8 | 6 | |||
| Pl | N10 | 27 (25.7–28.3) | 0 | 8 | 5 | ||
| N60 | 26.8 (25.1–28.5) | 0 | 8 | 12 | |||
| N10 | 18.6 (15.6–21.5) | 9.7 (8.8–10.6) | 8 | 6 | |||
| N60 | 17.3 (15.1–19.5) | 10.1 (8.8–11.5) | 8 | 11 | |||
| PL | N10 | 28.9 (26.6–31.2) | 0 | 8 | 12 | ||
| N60 | 27.5 (25.3–29.7) | 0 | 8 | 6 | |||
| N10 | 19.0 (15.8–22.2) | 10.8 (9.1–12.4) | 8 | 8 | |||
| N60 | 19.6 (17.2–22.1) | 10.6 (8.8–12.4) | 8 | 18 |
Artificial diets
The microcolonies were fed artificial nectars and pollens (hereafter referred as “liquid diet” and “solid diet”). Liquid diets consisted in sucrose solutions of 2 concentrations (w/w): 10% (N10%) and 60% (N60%). Solid diets were dry powders composed of lipids, protein, cellulose, and micronutrients. Three types of solid diets varying in their ratio of protein to lipids were used: low-protein, high-lipid diet (pL); high-protein, low-lipid diet (Pl); and high-protein, high-lipid diet (PL) (see details in Table 2). Nutrient selection was made based on commonly used insect diets (Cohen 2015). The protein content of all the solid diets consisted of a mixture of casein (80%) and whey protein (20%) (Nutrimuscles, Longwy, France, see composition in Supplementary Tables S1 and S2). The fat content consisted of a mixture of linseed oil (80%) (Sigma-Aldrich, Darmstadt, Germany) and cholesterol (20%) (Sigma-Aldrich, Darmstadt, Germany). Linseed oil was chosen for its high concentration in omega-3 (56%, see composition in Supplementary Table S3) which is important for brain functions (Arien et al. 2015). Cholesterol was chosen as the main source of sterols because it is essential to bees and many other sterols found in natural pollens are used for its synthesis (Herbert et al. 1980; Behmer and Nes 2003). Micronutrient contents consisted of a mixture of vitamins (25%) (Vanderzant vitamins mix, Sigma-Aldrich, Darmstadt, Germany), choline chloride (25%) (Sigma-Aldrich, Darmstadt, Germany), inositol (25%) (BVBA Acros Organics, Geel, Belgium), and ascorbic acid (25%) (Fisher Scientific, Illkirch-Graffenstaden, France). Cellulose, a non-digestible carbohydrate for bees (Roulston and Cane 2000), was used as binding agent (Sigma-Aldrich, Darmstadt, Germany). To produce the diets, we first added cholesterol into linseed oil, then protein and micronutrients, and finally the cellulose while mixing. Cellulose addition into lipids produces a homogenous powder substance. We designed artificial diets with maximum variation in nutrient ratios, within ranges reported in natural nectars and pollens (nectars: Nicolson and Thornburg 2007; pollens: Rothnie et al. 1987; Roulston and Cane 2000; Roulston et al. 2000; Somerville and Nicol 2006; Nicolson 2011; Vaudo et al. 2016a), to investigate the ability of bees to balance their nutrient collection and adjust rules of compromise in highly challenging nutritional conditions. We did not use a low-protein low-lipid diet (pl) because such diet would not yield enough nutrients for bees to reach any nutrient collection target or express rules of compromise. Preliminary observations showed that bumblebees could collect all our diets and store them in empty brood cells (Supplementary Figure S2).
Table 2.
Proportion (%) of proteins, lipids, digestible carbohydrates, cellulose and micronutrients in the 3 artificial solid diets (pL, Pl, and PL).
| Ingredients (/100 g) | pL | Pl | PL |
|---|---|---|---|
| Casein | 0.8 | 40 | 40 |
| Whey protein | 0.2 | 10 | 10 |
| Linseed oil | 16 | 0.8 | 16 |
| Cholesterol | 4 | 0.2 | 4 |
| Vitamins mix | 0.5 | 0.5 | 0.5 |
| Choline chloride | 0.5 | 0.5 | 0.5 |
| Inositol | 0.5 | 0.5 | 0.5 |
| Ascorbic acid | 0.5 | 0.5 | 0.5 |
| Cellulose | 77 | 47 | 28 |
| Total Proteins | 0.835 | 41.75 | 41.75 |
| Total Carbohydrates | 0.0326 | 1.63 | 1.63 |
| Total Lipids | 19.997 | 1.74 | 20.69 |
| Total Micronutrients | 2.041 | 3.876 | 3.88 |
Behavioral assays
Two experiments were run. In the “choice” experiment, microcolonies were given a choice between 1 type of liquid diets (N10% or N60%) and 2 types of solid diets (Pl and pL). We used Pl and pL to provide bees the opportunity to balance their diet in the broadest possible nutrient space. In the “no-choice” experiment, microcolonies were fed 1 type of liquid diet (N10% or N60%) and 1 type of solid diet (pL, Pl, or PL). Nutrient collection by microcolonies with brood and without brood was compared. Eight to 10 microcolonies were tested per condition (see summary in Table 1).
Each experiment was conducted during 13 consecutive days. Solid and liquid diets were renewed every 2–3 days by replacing the feeding boxes by new boxes containing fresh diets (Figure 1 and Supplementary Figure S1). This manipulation enabled the collection of liquid and solid diets by the bumblebees (milligrams/bumblebee) to be recorded. Collection of liquid diet was quantified by measuring the volume of diet remaining in the gravity feeders (centimeters). Collection of solid diet was quantified by measuring the weight of diet remaining on the chenille stems (milligrams) (entire feeding boxes were weighted, including the petri dish and chenille stems, see Supplementary Figure S1). The solid diet was dried (65°C for 48 h) before being weighed with a precision scale (±0.001 g; Mettler Toledo, Greifensee, Suisse). This protocol only permitted to measure food collection by bumblebees, not consumption per se. In parallel to the experiments, liquid and solid diets were placed in empty setups (feeding boxes connected to empty nest boxes) to measure volume and weight changes due to evaporation and correct the experimental measures. Sixteen of these controls were used for each type of liquid diet (N10% and N60%) and solid diet (pL, Pl, and PL). Every day, dead bumblebees were counted, removed from the boxes, and frozen at −20°C for lipid analyses (see below). For each microcolony, the number of newborn adults (i.e., emerged from the brood cells), Nb, was calculated as follows:
where Nl is the number of live individuals, Nd is the number of dead individuals, and Nlp is the number of live individuals during the previous recording.
Lipid analyses
Body lipid compositions were quantified using chloroform extraction (Cook et al. 2010; Dussutour et al. 2016; Arganda et al. 2017). Dead bumblebees were dried (65°C for 48 h) and weighed (dry mass). To extract the whole-body fat content, bumblebees were soaked in chloroform for 3 days (chloroform was changed every 24 h), dried and weighed again to obtain the body mass without lipids (lean mass). Only the bumblebees that died between Days 5 and 13 after the beginning of the experiments were used to make sure that the main variations in body composition measured were caused by variations in the nutrient contents of the artificial diets consumed. A total of 176 bumblebees (5–18 randomly chosen individuals per test condition) were analyzed (Table 1).
Statistical analyses
All statistical analyses were performed in R 3.5.1 (R Core Team 2018). From the raw liquid and solid diet collection data (weight of diet collected), average carbohydrate (C), protein (P), and lipid (L) collection by bumblebees per day were calculated:
where N is the total amount of the focal nutrient collected (C, P, or L); Di is the amount of diet i collected (weight in milligrams); Pi is the proportion of N in Di; T is the time since the beginning of the experiment (in days), Nl is the number of live bumblebees in the colony at T.
For the choice experiment, we tested how the average amount of P, C, and L collected by bumblebees per day were affected together by the liquid diet type (N10%, N60%) and brood (presence, absence), using a multivariate analysis of variance (mixed-effects MANOVA, function manova in package “stats” (R Core Team 2018)). The effects of food type (liquid diet, solid diet pL, and solid diet Pl), liquid diet type, and brood on the average amount of food collected by bumblebees per day were tested with an analysis of variance (mixed-effects ANOVA, function anova in package “stats”).
For the no-choice experiment, the effects of solid diet type (pL, Pl, PL), liquid diet type, and brood on the average amount of food collected, the average amount of lipid collected, and the average amount of protein collected by bumblebees per day were tested with a mixed-effects ANOVA (function anova in package “stats”).
For both choice and no-choice experiments, the relationship between the amount of nutrients collected per day and the number of empty brood cells in the nest were tested using an analysis of covariance (mixed-effects ANCOVA, function anova in package “stats”).
The effects of solid diet type, liquid diet type, and brood on adult emergence rate and survival were assessed using a Cox proportional hazards regression model (function coxph in package “survival” (Therneau 2015)).
The effects of solid diet type, liquid diet type, and brood on body lipid composition across conditions were tested with a mixed-effects ANOVA (function anova in package “stats”).
All statistical models accounted for any possible mother-colony effect by adding mother-colony identity as random effect. Multiple comparisons were performed with post hoc Tukey Honestly Significant Difference (HSD) tests (function glht in package “multcomp” (Hothorn et al. 2008)).
Results
Choice experiment
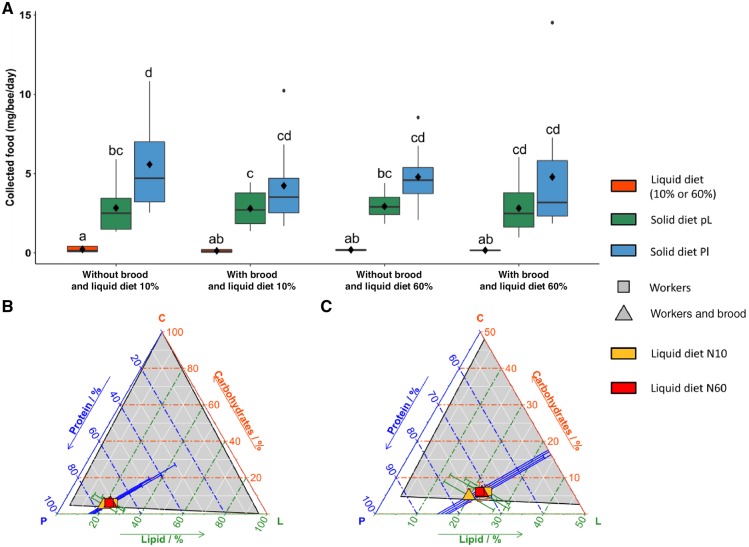
To quantify the nutrient collection target of bumblebees (i.e., amounts and balances of nutrients collected by workers and maximizing colony fitness) in the presence or absence of brood, microcolonies were given a choice between the solid Pl and the solid (pL), with either low-carbohydrate liquid diet (N10%) or high-carbohydrate liquid diet (N60%) (Table 1).
On average, bumblebees collected more Pl (3.92–5.76 95% confidence interval [CI], N = 40 colonies), than pL (2.22–3.46 95% CI, N = 40 colonies) and liquid diet (0.13–0.21 95% CI, N = 40 colonies) (Figure 2A; ANOVA, food type: F2,85 = 97.72, P < 0.001; post hoc Tukey HSD, see Supplementary Table S4). This pattern was similar in all test conditions, irrespective of the type of liquid diet used and of the presence of brood in the nest (ANOVA, liquid diet: F1,85 = 0.20, P = 0.66; brood: F1,85 = 0.01, P = 0.99; all interactions: P > 0.05). There was no significant relationship between the daily amount of food collected by bumblebees and the number of empty brood cells in the microcolonies (ANCOVA: empty cells: F1,225 = 1.17, P = 0.28; empty cells × nectar: F1,225 = 2.3, P = 0.13). This means that differences in liquid and solid diet collection between choice conditions cannot be explained by a difference in space available to store food in the nest.
Figure 2.
Choice experiment. Microcolonies of 20 bumblebees with or without brood were observed foraging on 2 types of solid diets (Pl, pL) and 1 type of liquid diet (10% or 60%) during 13 days. (A) Boxplot of the amount of liquid and solid diets collected each day per bee (milligrams). The central line is the median, the edges of the box are the 25th and 75th percentiles, the whiskers extend to the most extreme data points not considered outliers, outliers are represented by points, and diamonds represent means. Different letters above bars represent significant differences (P < 0.05, Tukey post hoc test after a mixed-effect ANOVA). (B) Nutritional geometry representation of the mean ratio of protein (P), carbohydrate (C), and lipid (L) collection by bumblebees. (C) Zoom of panel B. 2D plots are shown in Supplementary Figure S4. We calculated the average amount of each nutrient daily collected by bumblebees as the proportion of the focal nutrient divided by the proportion of that nutrient in liquid food and the 2 solid foods. Bars represent 95% CI. The gray area delimits the nutritional space in which bumblebees could navigate by eating different diets. Eight to 10 microcolonies were used per test condition (see details about sample sizes in Table 1).
When considering the nutritional composition of foods, microcolonies collected similar amounts of protein, carbohydrates and lipids irrespective of the type of liquid diet provided and of the presence of brood in the nest (MANOVA, liquid diet: F3,11 = 1.55, P = 0.26; brood: F3,11 = 0.14, P = 0.93; liquid diet × brood: F3,11 = 2.95, P = 0.08). In all choice conditions, bumblebees approached a nutrient collection ratio of 70.8% of protein (68.7–72.9 95% CI, N = 40 colonies), 6.2% of carbohydrate (5.4–7.0 95% CI, N = 40 colonies), and 23% of lipids (21.0–25.0 95% CI, N = 40 colonies) (Figure 2B), as calculated by the percentage of each macronutrient out of the total amount of the 3 macronutrients (P, C, L). Therefore, in these choice conditions, bumblebees adjusted their collection of liquid and solid diets to a nutrient ratio that is independent of colony composition.
No-choice experiment
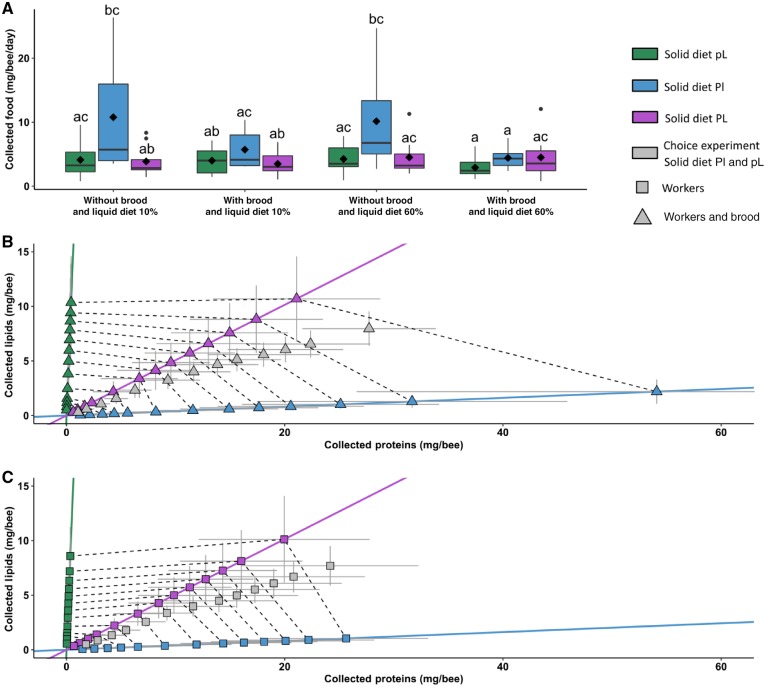
To explore the rule of compromise used by bumblebees to trade-off their collection of proteins, lipids, and carbohydrates from imbalanced foods, microcolonies were confined to 1 type of solid diet (Pl, pL, or PL) and 1 type of liquid diet (low carbohydrate [N10%] or a high carbohydrate [N60%]) (Table 1).
The total amount of food collected by bumblebees differed according to the type of solid diet and the presence of brood in the microcolony (Figure 3A; ANOVA, solid diet: F2, 50 = 7.95, P < 0.01; brood: F1, 50 = 9.88, P < 0.001; solid diet × brood: F2, 50 = 5.12, P < 0.01). Liquid diet type had no effect (ANOVA, liquid diet: F1, 50 = 0.69, P = 0.41; liquid diet × brood: F1, 50 = 0.74, P = 0.39; solid diet × nectar: F2, 50 = 0.72, P = 0.49; solid diet × liquid diet × brood: F2, 50 = 0.17, P = 0.84) on food collection. Microcolonies without brood fed Pl collected more food than microcolonies in all other conditions (Figure 3A; post hoc Tukey HSD, see Supplementary Table S5). There was no significant relationship between the daily amount of nutrients collected and the number of empty brood cells between test conditions (ANCOVA: empty cells: F1, 566 = 0.34, P = 0.56; empty cells × solid diet: F2, 566 = 0.4, P = 0.67; empty cells × liquid diet: F1, 566 = 0.01, P = 0.94; empty cells × solid diet × liquid diet: F2, 566 = 0.35, P = 0.7), meaning that these differences could not be explained by a difference in space available to store the food in the nest.
Figure 3.
No-choice experiment. Microcolonies of 20 bumblebees with or without brood were fed 1 type of solid diet (Pl, pL, PL) and 1 type of liquid diet (10% or 60%) during 13 days. (A) Boxplot of the total amount of food (liquid diet + solid diet) collected each day per bee (milligrams). The central line is the median, the edges of the box are the 25th and 75th percentiles, the whiskers extend to the most extreme data points not considered outliers, outliers are represented by points, and diamonds represent means. Different letters above bars represent significant differences (P < 0.05, Tukey post hoc test after a mixed-effect ANOVA). Mean (milligrams ± 95% CI) collection of proteins (P) and lipids (L) per bumblebee day by day in microcolonies (B) without brood or (C) with brood. Color symbols represent mean collection per day for each condition with or without brood in no-choice experiment. Gray symbols represent mean collection per day for each condition with or without brood in choice experiment Bars represent 95% CI. Lines represent the protein to lipid ratios of the diet colonies were fed. Eight to 10 microcolonies were used per test condition (see details about sample sizes in Table 1).
When considering the nutritional composition of foods, microcolonies showed different strategies in the absence and presence of brood. In the absence of brood, microcolonies fed high-lipid solid diet (pL or PL) collected similar amounts of lipids (Figure 3B, ANOVA, solid diet: F2, 18 = 15.37, P < 0.001; post hoc Tukey HSD, PL–pL: P = 0.87; Pl–pL: P < 0.001; PL–Pl: P < 0.001). However, this was not the case for microcolonies fed high-protein solid diets (Pl or PL), as microcolonies fed Pl collected more protein than those fed PL (Figure 3B, ANOVA, pollen: F2, 18 = 14.62, P < 0.001; post hoc Tukey HSD, PL–pL: P < 0.05; Pl–pL: P < 0.001; PL–Pl: P < 0.01). These results suggest that bumblebee workers prioritized lipid regulation over protein regulation, by over-collecting proteins to attain their lipid collection target.
In the presence of brood, microcolonies fed high-lipid solid diets (pL or PL) collected a similar amount of lipids (Figure 3C, ANOVA, pollen: F2, 18 = 15.55, P < 0.001; post hoc Tukey HSD, PL–pL: P = 0.67; Pl–pL: P < 0.001; PL–Pl: P < 0.001). This amount was comparable with that observed in microcolonies with no brood (Figure 3C). Interestingly, microcolonies fed high-protein solid diets (Pl or PL) also collected similar amounts of proteins (Figure 3C, ANOVA, solid diet: F2, 18 = 50.03, P < 0.001; post hoc Tukey HSD, PL–pL: P < 0.001; Pl–pL: P < 0.001; PL–Pl: P = 0.28) suggesting that, in the presence of brood, bumblebees regulated both their collection of proteins and lipids to target values.
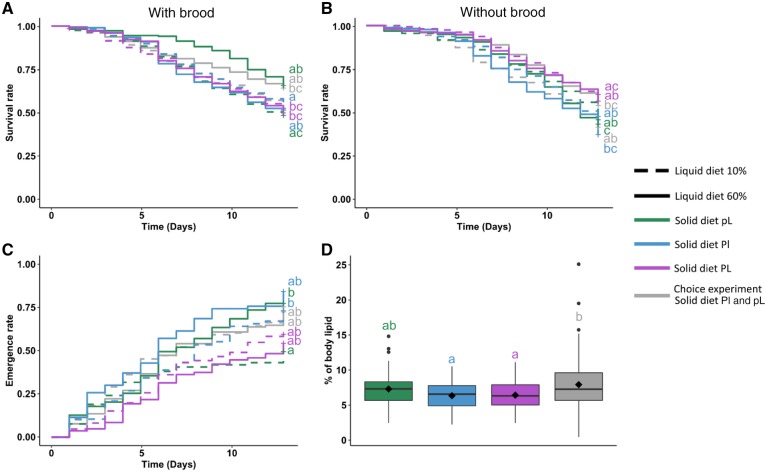
Survival
To assess the influence of diet on lifespan, we compared the survival of bumblebees in the different test conditions (Figure 4). In both the choice and the no-choice experiments, bumblebees survived longer when fed 60% liquid diet (N60%) than when fed 10% liquid diet (N10%) (Cox model, N60–N10: hazard ratio [HR]=0.72; 95% CI 0.54–0.97; P < 0.05). The presence of brood had no effect on adult survival (Cox model, Brood–No brood: HR=1.09; 95% CI 0.78–1.50; P = 0.62). In the no-choice experiment, bumblebees fed Pl had a lower survival than bumblebees fed pL and PL (Cox model, Pl–pL: HR=1.62; 95% CI 1.14–2.29; P < 0.05; Pl–PL: HR=2.12; 95% CI 1.46–3.06; P < 0.001). Bumblebees fed pL and PL had similar survival (Cox model, pL–PL: HR=1.30; 95% CI 0.90–1.87; P = 0.49). Bumblebees fed Pl in the absence of brood survived the least long (Figure 4B), whereas bumblebees fed pL and N60% in the presence of brood survived the longest (Figure 4A, post hoc Tukey HSD from Cox model, see Supplementary Table S6). Thus, overall, bumblebees fed Pl died faster than bumblebees fed high-lipid diets (pL and PL).
Figure 4.
Survival. Curves represent survival rate over 13 days for (A) microcolonies of bumblebees with brood and (B) microcolonies without brood. (C) Adult emergence rate. Curves represent emergence rate over 13 days for microcolonies with brood. Different letters above bars represent significant differences (P < 0.05, Tukey post hoc test after a Cox model). Eight to 10 microcolonies were used per test condition (see details about sample sizes in Table 1). (D) Lipid body content. Boxplot of the proportion of body content in bumblebees died at least 5 days after the start of the experiment. The central line is the median, the edges of the box are the 25th and 75th percentiles, the whiskers extend to the most extreme data points, outliers are represented by points, and diamonds represent means. Different letters above bars represent significant differences (P < 0.05, Tukey post hoc test after a mixed-effect ANOVA). About 5–18 bumblebees were analyzed per test condition (see sample size details in Table 1).
Adult emergence rate
To explore the influence of diet on larval development, we compared the emergence rate of new adults in the different test conditions. In the no-choice experiment, the rate of emergence was higher in microcolonies fed Pl than in microcolonies fed pL (Figure 4C and Supplementary Figure S3, Cox model, Pl–pL: HR=3.07; 95% CI 1.69–5.58; P < 0.01). There was no difference between all the other conditions (Cox model, pL–Choice: HR=0.52; 95% CI 0.31–0.89; P = 0.07; Pl–Choice: HR=1.60; 95% CI 0.97–2.64; P = 0.24; PL–Choice: HR=0.96; 95% CI 0.57–1.62; P = 0.99; PL–pL: HR=1.85; 95% CI 1.02–3.34; P = 0.17; PL–Pl: HR=0.60; 95% CI 0.34–1.06; P = 0.29). Therefore, Pl seemed to favor larval development. The type of liquid diet had no effect on adult emergence rate (Cox model, N60–N10: HR=0.99; 95% CI 0.68–1.45; P = 0.97; post hoc Tukey HSD from Cox model, see Supplementary Table S7). We did not observe male larvae produced by workers over the course of the observations.
Lipid body content
To assess the effect of diet on physiology, we measured the lipid body content of bumblebees in the different test conditions. The type of solid diet influenced lipid body content (Figure 4D; ANOVA, solid diet: F3, 136 = 4.91; P < 0.05), whereas neither liquid diet type, nor the presence of brood in the nest, or their interactions had a significant effect (ANOVA, liquid diet: F1, 136 = 0.68; brood: F1, 136 = 1.09, P = 0.30; all interactions: P > 0.05). Post hoc comparisons did not reveal significant difference between test conditions (post hoc Tukey HSD, Pl–pL: P = 0.96; PL–pL: P = 0.60; Choice–pL: P = 0.85; PL–Pl: P = 0.47; Choice–Pl: P = 1.00; Choice–PL: P = 0.16). However, bumblebees in the choice experiment were significantly fatter than bumblebees fed high-protein (Pl and PL) in the no-choice experiment (Figure 4D, post hoc Tukey HSD, Choice–Pl: P < 0.01; Choice–PL: P < 0.05), indicating that diet influenced lipid body content.
Discussion
We used a 3D nutritional geometry design to study how bumblebees regulate their collection of protein, carbohydrates, and lipids from artificial liquid and solid diets. Microcolonies given a choice between complementary diets self-selected foods to reach a nutrient collection target ratio of 71% proteins, 6% carbohydrates, and 23% lipids, irrespective of the presence of brood in the colony. However, when confined to an imbalanced diet, bumblebees either only regulated lipid collection or both lipid and protein collection simultaneously, depending on the presence of brood in the colony. This indicates that protein regulation is influenced by brood.
In nature, bees must extract key nutrients from plant nectars and pollens. Several recent studies show how individual workers or small groups of workers self-compose their diets to balance their acquisition of carbohydrates and proteins (honey bees: Paoli et al. 2014a, bumblebees: Stabler et al. 2015), or proteins and lipids (bumblebees: Vaudo et al. 2016a) from liquid or solid artificial diets. Here, using both diet types, we demonstrate that variations of protein, lipids, and carbohydrates, simultaneously or independently, influence food collection by microcolonies. Our data validate this novel approach by demonstrating that bumblebees did not collect artificial diets randomly, were physiologically affected by the diets, and even stored diets in empty brood cells (Supplementary Figure S1) as they would do with natural nectars and pollens (Goulson 2010).
In this study, we designed diets with extreme variation in nutrient ratio that cannot be considered as strictly equivalent to natural nectars and pollens. This was done to test the ability of bees to regulate their nutrient collection in the broadest possible nutrient space. Although this approach is useful to reveal qualitative effects of nutrition on the behavior and physiology of bees, the amplitude of the effects observed may be different when bees feed on more natural diets. Previous studies showed how bee nutrient intake depend on the type of diet proposed (Stabler et al. 2015), suggesting that the nutrient collection target ratio measured in our study could strongly differ from that of bumblebees in the field. The strong mortality observed in our experiments might be due to the extreme nature of the artificial diets used. Future studies should therefore attempt to refine diet compositions to simulate natural nutritional environments to bees. Improvements could be done, for instance, by using amino acid composition of proteins closer to that found in natural pollens (Vanderplanck et al. 2014; Moerman et al. 2015), adding micronutrients beneficial to insects (Cohen 2015), and using sterols that are more likely to be digestible for bumblebees and abundant in natural pollen such as β-sitosterol or δ5-avenasterol (Vanderplanck et al. 2014).
Exposing microcolonies to different diets showed that bumblebee colonies actively regulate their intake of proteins, carbohydrates, and lipids, thereby confirming previous observations with simpler, 2D nutritional designs (Vaudo et al. 2016a, 2016b). Differences in artificial diets and methodological approaches likely explain differences between the intake ratios we measured and those reported in previous studies (e.g., Vaudo et al. 2016b: 1.4% proteins, 98.5% carbohydrates, and 0.1% lipids). First, we varied the ratios of 3 nutrients in foods simultaneously. Second, we used microcolonies with nest materials, whereas previous studies used individual workers or small groups of workers without brood (honey bees: Altaye et al. 2010; Pirk et al. 2010; Paoli et al. 2014a; bumblebees: Stabler et al. 2015; Vaudo et al. 2016a). Third, we provided liquid sources of carbohydrates and solid sources of proteins and lipids to bees that they could store in empty brood cells, an approach that only permitted to measure food collection, not real consumption by bees. Note, however, that the fact that the body lipid composition of bumblebees varied with pollen type demonstrates that colonies consumed part of (if not all) the pollen collected. Future studies using automated tracking to quantify food collection and ingestion (including water) by individuals (e.g., Greenwald et al. 2015), and food storage in cells (e.g., Colin et al. 2018) will help understand how bumblebees balance their diet from nectar and pollen resources, and whether this varies between individuals, for instance, among members of different castes.
The presence of brood in the colony did not influence the ratio of nutrients collected by bumblebees offered complementary diets in contrast to what was observed in ants (Dussutour and Simpson 2009). This result is consistent with previous observations on another bumblebee species (B. impatiens) where foragers reached a similar protein to lipid intake ratio in a full-size colony or in a cage isolated from brood, suggesting that the nutritional needs of adult bumblebees closely match those of the larvae (Vaudo et al. 2016a). Nonetheless, we found that the presence of brood influenced food collection when colonies were confined on imbalanced diets. In these extreme conditions, bumblebees behaved as if they regulate lipids collection to reach a fixer target value of 9.09 mg/bee/day (7.97–10.21 95% CI, N = 101 colonies). By contrast, protein regulation was only evident in the presence of brood. Further experiments are needed to confirm this result in natural colonies containing all brood stages, workers, and a queen. For instance, the over-collection of protein observed in microcolonies without brood could be caused by the absence of queen pheromones that prevents the reproduction of workers and the development of ovaries that requires protein (Tasei and Aupinel 2008; Goulson 2010). It would be interesting to use colonies in which the age of workers and the development stage of larvae are controlled, since the nutritional needs of bumblebees are likely to be age or caste dependent, as this is the case in honey bees (Schmickl and Crailsheim 2002; Paoli et al. 2014a).
In our experiments, nectar collection was very low in comparison to pollen collection, suggesting that bumblebees did not give an equal importance to carbohydrates, proteins, and lipids. Since carbohydrates are a primary source of energy for flight (Brodschneider and Crailsheim 2010), energy costs should vary depending on the distance of the food sources to the nest. Previous studies show that nectar intake by bees increases with the distance to food location (honey bees: Núñez and Giurfa, 1996) and that foragers integrate nectar intake rate in their spatial decisions (bumblebees: Lihoreau et al. 2011). In our experiment, energy needs were modest as the nest boxes were relatively small and bees did not need to fly to collect food. Manipulating the distance of pollen and nectar sources from the colony would allow us to explore the potential trade-off made by foragers between collecting carbohydrates for their own energy demands or collecting proteins and lipids for the colony needs.
So, why regulate lipid and protein, and not carbohydrates as previously seen in ants (Dussutour and Simpson 2008, 2009; Cook et al. 2010; Bazazi et al. 2016)? Lipids are key for bee development, both at the larval and adult stages. Lipid metabolism participates in molting hormone production (e.g., ecdysone; Canavoso et al. 2001), larval growth and development (Vanderplanck et al. 2014), production of cuticular hydrocarbons and wax, behavioral maturation in adults (through the reduction in lipid stores), diapause, development of glands that produce brood food (Canavoso et al. 2001; Toth et al. 2005; Fliszkiewicz and Wilkaniec 2007), and learning (Arien et al. 2015, 2018). In addition, an excess of polyunsaturated fatty acids may lead to lipid peroxidation and cell damage, which could explain the difference in queen and worker lifespan, where queens are protected from peroxidation and live longer than workers (Haddad et al. 2007).
Protein is also vital for larval development (Wright et al. 2018), as confirmed by the highest emergence rates in microcolonies given high-protein diets. However, high-protein diets (or diets rich in amino acids) also reduce lifespan in many insects (e.g., Drosophila: Lee et al. 2008; field crickets: Maklakov et al. 2008; ants: Dussutour and Simpson 2009; termites: Poissonnier et al. 2018; honey bees: Altaye et al. 2010; Pirk et al. 2010; Archer et al. 2014; Paoli et al. 2014a), including bumblebees (Stabler et al. 2015). An excess of protein is believed to increase toxic nitrogen waste products and over-stimulate nutrient-sensing pathways that regulate lifespan (such as the Target of Rapamycin (TOR) pathway) (reviewed in Le Couteur et al. 2016). The fact that protein regulation was most evident in the presence of brood, as shown in ants (Dussutour and Simpson 2009), suggests that protein balance is more critical for brood development and adult maturation. Malnourished larvae develop malformations that impact lifespan, dry weight, protein content, and wing size (Brodschneider and Crailsheim 2010). Bee colonies tend to terminate brood rearing rather than produce malnourished adults (Schmickl and Crailsheim 2002), a result that may explain the difference we found in the adult emergence rates of colonies in extreme nutritional conditions of no-choice experiments. In bumblebees, information on pollen quality and its availability in the colony may be accessible to workers via empty brood cells (Dornhaus and Chittka 2005) allowing the colony to make informed foraging decisions. Our results thus suggest that protein regulation is based on cues yielding information about the nutritional state of larvae, although this will need to be confirmed with further investigations.
Nutritional geometry studies have recently changed the views about the importance of malnutrition for bee health and population declines (Wright et al. 2018). Our approach using diets varying in their ratio of 3 macronutrients shows that bee nutritional decisions are a multidimensional process that depends on colony composition. Beyond informing about nutritionally adequate mixes of plants that may help maintain sustainable bee populations (Vaudo et al. 2015), the development of solid and liquid synthetic diets for bees offers the possibility for future explorations of the spatial dimension of nutritional decisions (Lihoreau et al. 2017), by studying how the foraging patterns of bees are affected by the heterogeneity of pollen and nectars available in flowers, and how this influences plant–pollinator interactions and the critical pollination service they provide.
Supplementary Material
Acknowledgments
The authors are grateful to David Baracchi for inviting them to contribute to this special issue of Current Zoology. They also thank Mathilde Vidal, Alexandra Faure, and Avril Duchet for their technical assistance during their Master 1 internships.
Funding
This work was funded by the CNRS and a Passerelle Grant of the Research Center on Animal Cognition to A.D. and M.L. While writing S.K. was funded by a CIFRE PhD thesis (CNRS-Koppert). C.P., T.G-M., and M.L. were funded by a grant of the Agence Nationale de la Recherche to M.L. (ANR-16-CE02-0002-01).
References
- Altaye SZ, Pirk CWW, Crewe RM, Nicolson SW, 2010. Convergence of carbohydrate-biased intake targets in caged worker honeybees fed different protein sources. J Exp Biol 213:3311–3318. [DOI] [PubMed] [Google Scholar]
- Archer CR, Köhler A, Pirk CWW, Oosthuizen V, Apostolides Z et al. , 2014. Antioxidant supplementation can reduce the survival costs of excess amino acid intake in honeybees. J Insect Physiol 71:78–86. [DOI] [PubMed] [Google Scholar]
- Arganda S, Bouchebti S, Bazazi S, Le Hesran S, Puga C et al. , 2017. Parsing the life-shortening effects of dietary protein: effects of individual amino acids. Proc Biol Sci 284:20162052.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arien Y, Dag A, Shafir S, 2018. Omega-6: 3 ratio more than absolute lipid level in diet affects associative learning in honey bees. Front Psychol 9:1001.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arien Y, Dag A, Zarchin S, Masci T, Shafir S, 2015. Omega-3 deficiency impairs honey bee learning. Proc Natl Acad Sci U S A 112:15761–15766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazazi S, Arganda S, Moreau M, Jeanson R, Dussutour A, 2016. Responses to nutritional challenges in ant colonies. Anim Behav 111:235–249. [Google Scholar]
- Behmer ST, Nes WD, 2003. Insect sterol nutrition and physiology: a global overview. Adv Insect Physiol 31:1–72. [Google Scholar]
- Brodschneider R, Crailsheim K, 2010. Nutrition and health in honey bees. Apidologie 41:278–294. [Google Scholar]
- Canavoso LE, Jouni ZE, Karnas KJ, Pennington JE, Wells MA, 2001. Fat metabolism in insects. Annu Rev Nutr 21:23–46. [DOI] [PubMed] [Google Scholar]
- Cohen AC, 2015. Insect Diets: Science and Technology. 2nd edn Boca Raton (FL: ): CRC Press. [Google Scholar]
- Colin T, Bruce J, Meikle WG, Barron AB, 2018. The development of honey bee colonies assessed using a new semi-automated brood counting method: combCount. PLoS ONE 13:e0205816.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook SC, Eubanks MD, Gold RE, Behmer ST, 2010. Colony-level macronutrient regulation in ants: mechanisms, hoarding and associated costs. Anim Behav 79:429–437. [Google Scholar]
- Dornhaus A, Chittka L, 2005. Bumble bees Bombus terrestris store both food and information in honeypots. Behav Ecol 16:661–666. [Google Scholar]
- Dussutour A, Simpson SJ, 2008. Carbohydrate regulation in relation to colony growth in ants. J Exp Biol 211:2224–2232. [DOI] [PubMed] [Google Scholar]
- Dussutour A, Simpson SJ, 2009. Communal nutrition in ants. Curr Biol 19:740–744. [DOI] [PubMed] [Google Scholar]
- Dussutour A, Simpson SJ, 2012. Ant workers die young and colonies collapse when fed a high-protein diet. Proc Biol Sci 279:2402–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dussutour A, Poissonnier L-A, Buhl J, Simpson SJ, 2016. Resistance to nutritional stress in ants: when being fat is advantageous. J Exp Biol 219:824–833. [DOI] [PubMed] [Google Scholar]
- Fliszkiewicz M, Wilkaniec Z, 2007. Fatty acids and amino acids in the fat body of bumblebee Bombus terrestris L. in diapausing and non-diapausing queens. J Apic Sci 51:55–63. [Google Scholar]
- Goulson D, 2010. Bumblebees: Behaviour, Ecology, and Conservation. Oxford (NY: ): Oxford University Press. [Google Scholar]
- Greenwald E, Segre E, Feinerman O, 2015. Ant trophallactic networks: simultaneous measurement of interaction patterns and food dissemination. Sci Rep 5:12496.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad LS, Kelbert L, Hulbert AJ, 2007. Extended longevity of queen honey bees compared to workers is associated with peroxidation-resistant membranes. Exp Gerontol 42:601–609. [DOI] [PubMed] [Google Scholar]
- Helm BR, Slater GP, Rajamohan A, Yocum GD, Greenlee KJ et al. , 2017. The geometric framework for nutrition reveals interactions between protein and carbohydrate during larval growth in honey bees. Biol Open 6:872–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriksma HP, Shafir S, 2016. Honey bee foragers balance colony nutritional deficiencies. Behav Ecol Sociobiol 70:509–517. [Google Scholar]
- Herbert EW, Svoboda JA, Thompson MJ, Shimanuki H, 1980. Sterol utilization in honey bees fed a synthetic diet: effects on brood rearing. J Insect Physiol 26:287–289. [Google Scholar]
- Hewson-Hughes AK, Hewson-Hughes VL, Miller AT, Hall SR, Simpson SJ et al. , 2011. Geometric analysis of macronutrient selection in the adult domestic cat Felis catus. J Exp Biol 214:1039–1051. [DOI] [PubMed] [Google Scholar]
- Hothorn T, Bretz F, Westfall P, 2008. Simultaneous inference in general parametric models. Biom J 50:346–363. [DOI] [PubMed] [Google Scholar]
- Jang T, Lee KP, 2018. Comparing the impacts of macronutrients on life-history traits in larval and adult Drosophila melanogaster: the use of nutritional geometry and chemically defined diets. J Exp Biol 221:jeb.181115. [DOI] [PubMed] [Google Scholar]
- Klein S, Cabirol A, Devaud J-M, Barron AB, Lihoreau M, 2017. Why bees are so vulnerable to environmental stressors. Trends Ecol Evol 32:268–278. [DOI] [PubMed] [Google Scholar]
- Le Couteur DG, Solon-Biet S, Cogger VC, Mitchell SJ, Senior A et al. , 2016. The impact of low-protein high-carbohydrate diets on aging and lifespan. Cell Mol Life Sci 73:1237–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KP, Simpson SJ, Clissold FJ, Brooks R, Ballard JWO et al. , 2008. Lifespan and reproduction in Drosophila: new insights from nutritional geometry. Proc Natl Acad Sci U S A 105:2498–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lihoreau M, Chittka L, Raine NE, 2011. Trade-off between travel distance and prioritization of high-reward sites in traplining bumblebees: distance reward trade-off in bees. Funct Ecol 25:1284–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lihoreau M, Buhl J, Charleston MA, Sword GA, Raubenheimer D et al. , 2014. Modelling nutrition across organizational levels: from individuals to superorganisms. J Insect Physiol 69:2–11. [DOI] [PubMed] [Google Scholar]
- Lihoreau M, Buhl J, Charleston MA, Sword GA, Raubenheimer D et al. , 2015. Nutritional ecology beyond the individual: a conceptual framework for integrating nutrition and social interactions. Ecol Lett 18:273–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lihoreau M, Charleston MA, Senior AM, Clissold FJ, Raubenheimer D et al. , 2017. Collective foraging in spatially complex nutritional environments. Philos Trans R Soc Lond B Biol Sci 372:20160238.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lihoreau M, Gómez-Moracho T, Pasquaretta C, Costa JT, Buhl J, 2018. Social nutrition: an emerging field in insect science. Curr Opin Insect Sci 28:73–80. [DOI] [PubMed] [Google Scholar]
- Maklakov AA, Simpson SJ, Zajitschek F, Hall MD, Dessmann J et al. , 2008. Sex-specific fitness effects of nutrient intake on reproduction and lifespan. Curr Biol 18:1062–1066. [DOI] [PubMed] [Google Scholar]
- Moerman R, Vanderplanck M, Roger N, Decleves S, Wathelet B et al. , 2015. Growth rate of bumblebee larvae is related to pollen amino acids. J Econ Entomol 109:25–30. [DOI] [PubMed] [Google Scholar]
- Nicolson SW, Thornburg RW, 2007. Nectar chemistry In: Nicolson SW, Nepi M, Pacini E, editors. Nectaries and Nectar. Dordrecht: Springer Netherlands; 215–264. [Google Scholar]
- Nicolson SW, 2011. Bee food: the chemistry and nutritional value of nectar, pollen and mixtures of the two. Afr Zool 46:197–204. [Google Scholar]
- Núñez JA, Giurfa M, 1996. Motivation and regulation of honey bee foraging. Bee World 77:182–196. [Google Scholar]
- Paoli PP, Donley D, Stabler D, Saseendranath A, Nicolson SW et al. , 2014a. Nutritional balance of essential amino acids and carbohydrates of the adult worker honeybee depends on age. Amino Acids 46:1449–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoli PP, Wakeling LA, Wright GA, Ford D, 2014b. The dietary proportion of essential amino acids and Sir2 influence lifespan in the honeybee. Age (Dordr) 36:1239–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper MDW, Blanc E, Leitão-Gonçalves R, Yang M, He X et al. , 2014. A holidic medium for Drosophila melanogaster. Nat Methods 11:971–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirk CWW, Boodhoo C, Human H, Nicolson SW, 2010. The importance of protein type and protein to carbohydrate ratio for survival and ovarian activation of caged honeybees Apis mellifera scutellata. Apidologie 41:62–72. [Google Scholar]
- Poissonnier L-A, Arganda S, Simpson SJ, Dussutour A, Buhl J, 2018. Nutrition in extreme food specialists: an illustration using termites. Funct Ecol 32:2531–2541. [Google Scholar]
- R Core Team, 2018. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; https://www.R-project.org/. [Google Scholar]
- Raubenheimer D, Simpson SJ, 1993. The geometry of compensatory feeding in the locust. Anim Behav 45:953–964. [Google Scholar]
- Raubenheimer D, Simpson SJ, 2018. Nutritional ecology and foraging theory. Curr Opin Insect Sci 27:38–45. [DOI] [PubMed] [Google Scholar]
- Rothnie NE, Palmer MV, Burke DG, Sang JP, Hilliard EP et al. , 1987. Comparative analysis of fatty acids in pollen and seed of rapeseed. Phytochemistry 26:1895–1897. [Google Scholar]
- Roulston TH, Cane JH, 2000. Pollen nutritional content and digestibility for animals. Plant Syst Evol 222:187–209. [Google Scholar]
- Roulston TH, Cane JH, Buchmann SL, 2000. What governs protein content of pollen: pollinator preferences, pollen-pistil interactions, or phylogeny? Ecol Monogr 70:617. [Google Scholar]
- Russell AL, Papaj DR, 2016. Artificial pollen dispensing flowers and feeders for bee behaviour experiments. J Pollinat Ecol 18:13–22. [Google Scholar]
- Schmickl T, Crailsheim K, 2002. How honeybees (Apis mellifera L.) change their broodcare behaviour in response to non-foraging conditions and poor pollen conditions. Behav Ecol Sociobiol 51:415–425. [Google Scholar]
- Shik JZ, Gomez EB, Kooij PW, Santos JC, Wcislo WT et al. , 2016. Nutrition mediates the expression of cultivar-farmer conflict in a fungus-growing ant. Proc Natl Acad Sci U S A 113:10121–10126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson SJ, Raubenheimer D, 1993. A multi-level analysis of feeding behaviour: the geometry of nutritional decisions. Philo Trans R Soc Lond B Biol Sci 342:381–402. [Google Scholar]
- Simpson SJ, Raubenheimer D, 2012. The Nature of Nutrition: A Unifying Framework from Animal Adaptation to Human Obesity . Princeton: Princeton University Press; 256. [Google Scholar]
- Simpson SJ, Clissold FJ, Lihoreau M, Ponton F, Wilder SM et al. , 2015. Recent advances in the integrative nutrition of arthropods. Annu Rev Entomol 60:293–311. [DOI] [PubMed] [Google Scholar]
- Solon-Biet SM, McMahon AC, Ballard JWO, Ruohonen K, Wu LE et al. , 2014. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab 19:418–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solon-Biet SM, Walters KA, Simanainen UK, McMahon AC, Ruohonen K et al. , 2015. Macronutrient balance, reproductive function, and lifespan in aging mice. Proc Natl Acad Sci U S A 112:3481–3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville DC, Nicol HI, 2006. Crude protein and amino acid composition of honey bee-collected pollen pellets from south-east Australia and a note on laboratory disparity. Aust J Exp Agric 46:141. [Google Scholar]
- Stabler D, Paoli PP, Nicolson SW, Wright GA, 2015. Nutrient balancing of the adult worker bumblebee Bombus terrestris depends on the dietary source of essential amino acids. J Exp Biol 218:793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasei J-N, Aupinel P, 2008. Nutritive value of 15 single pollens and pollen mixes tested on larvae produced by bumblebee workers (Bombus terrestris, Hymenoptera: apidae). Apidologie 39:397–409. [Google Scholar]
- Therneau T, 2015. A Package for Survival Analysis in S. version 2.38. https://CRAN.R-project.org/package=survival. [Google Scholar]
- Toth AL, Kantarovich S, Meisel AF, Robinson GE, 2005. Nutritional status influences socially regulated foraging ontogeny in honey bees. J Exp Biol 208:4641–4649. [DOI] [PubMed] [Google Scholar]
- Vanderplanck M, Moerman R, Rasmont P, Lognay G, Wathelet B et al. , 2014. How does pollen chemistry impact development and feeding behaviour of polylectic bees? PLoS ONE 9:e86209.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudo AD, Tooker JF, Grozinger CM, Patch HM, 2015. Bee nutrition and floral resource restoration. Curr Opin Insect Sci 10:133–141. [DOI] [PubMed] [Google Scholar]
- Vaudo AD, Patch HM, Mortensen DA, Tooker JF, Grozinger CM, 2016a. Macronutrient ratios in pollen shape bumble bee Bombus impatiens foraging strategies and floral preferences. Proc Natl Acad Sci U S A 113:4035–4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudo AD, Stabler D, Patch HM, Tooker JF, Grozinger CM et al. , 2016b. Bumble bees regulate their intake of essential protein and lipid pollen macronutrients. J Exp Biol 219:3962–3970. [DOI] [PubMed] [Google Scholar]
- Wills BD, Chong CD, Wilder SM, Eubanks MD, Holway DA et al. , 2015. Effect of carbohydrate supplementation on investment into offspring number, size, and condition in a social insect. PLoS ONE 10: e0132440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright GA, Nicolson SW, Shafir S, 2018. Nutritional physiology and ecology of honey Bees. Annu Rev Entomol 63:327–344. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.