Abstract
Predators have both direct, consumptive effects on their prey and non-lethal effects on physiology and behavior, including reproductive decisions, with cascading effects on prey ecology and evolution. Here, we experimentally tested such non-lethal effects of exposure to increased predation risk on clutch size, egg mass, and the concentration of yolk steroid hormones in the yellow-legged gull Larus michahellis. We simulated increased predation risk by displaying stuffed predators (adult fox Vulpes vulpes, and adult buzzard Buteo buteo) to breeding adults before egg laying. The concentration of corticosterone, which has been shown to increase under exposure to maternal predation risk in other species, and of testosterone did not differ between eggs from mothers exposed to the predators and eggs from control mothers (i.e., eggs exposed to a novel object of similar size and position to the stuffed predators). The concentration of the two hormones negatively covaried. Clutch size did not vary according to experimental treatment, whereas egg mass was markedly larger in clutches from nests exposed to predators than in clutches from control nests. By increasing egg mass, mothers may reduce the risk of cooling of the eggs when incubation is impeded by predators, boost energy reserves, reduce post-natal detectability caused by food solicitation, and/or enhance development at hatching, thus increasing the chances of offspring survival. In general, our results are inconsistent with most of the few previous studies on similar non-lethal predator effects and suggest that such effects may vary among species according to ecological conditions, social behavior, and developmental mode.
Keywords: clutch size, corticosterone, egg size, predation effects, testosterone
Predation is a major force shaping the evolution of morphological, physiological, and behavioral traits of preys (Agrawal et al. 1999; Eggers et al. 2006; Fontaine and Martin 2006; Massaro et al. 2008; Peluc et al. 2008; Storm and Lima 2010; Coslovsky and Richner 2011; Giesing et al. 2011). Predators, however, also greatly impact the populations of their preys over ecological time frames (Pianka 1970; Ricklefs 2000; Kokko and Lopez-Sepulcre 2007; Griebeler et al. 2010). The most obvious ecological effect of predators is killing of preys. Recent research, however, has emphasized that the ecological effect of predators may extend far beyond those arising from the mere killing of prey (Creel et al. 2007; Creel and Christianson 2008; Travers et al. 2010; Zanette et al. 2011). Indeed, predation risk can impact the physiology and behavior of preys. Such non-lethal predator effects can vary according to whether predation risk mostly concerns adults rather than offspring/eggs, and this is expected to be reflected into the plastic physiological and behavioral response adopted by preys (Lima 2009; Travers et al. 2010). Non-lethal effects of predation on breeding birds extend from habitat choice, to social (e.g., flocking and coloniality), parental (e.g., nest and brood attendance), and offspring (e.g., begging) behavior (reviews: Caro 2005; Lima 2009). In addition, variation in predation risk affects the expression of phenotypically plastic life-history traits like clutch and egg size (Doligez and Clobert 2003; Eggers et al. 2006; Fontaine and Martin 2006). When predators mostly impact eggs/offspring, experience of high predation risk should results in a reduction in clutch size during the current reproductive event. This is expected because of low reproductive value of offspring under intense predation risk and a trade-off between current and future reproduction (Slagsvold 1984; Magnhagen 1991; Nager et al. 2000; Hauber 2003; Griebeler et al. 2010; Zanette et al. 2011). In addition, especially for territorial species, reduction in clutch size can function to decrease the nest detectability or can result from reduced foraging efficiency of laying mothers (Thomson et al. 1998; Martin et al. 2000a, 2000b; Ghalambor and Martin 2002; Fontaine and Martin 2006; Lima 2009). The relationship between predation risk and clutch size has thus been mostly shown to be negative, as expected, although with notable exceptions (Doligez and Clobert 2003; Eggers et al. 2006; Fontaine and Martin 2006; Massaro et al. 2008; Cassey et al. 2009).
The consequences of predation risk on egg size have been tested experimentally only rarely, providing contrasting results (Safriel 1975; Slagsvold 1984; Martin 1995; Cassey et al. 2009; Lima 2009; Coslovsky and Richner 2011; Zanette et al. 2011). Albeit negative effects of predation risk on egg size should be predicted (see above), a positive relationship may also be expected (e.g., Fontaine and Martin 2006), especially in colonial species where nesting sites are easily detectable. For example, large eggs produce large offspring (e.g., Amundsen 1995; Smith and Bruun 1998; Styrsky et al. 1999; reviewed in Krist 2011) with faster development that can fledge earlier (Krist 2011), thus reducing vulnerability to pre-fledging predation. In addition, large offspring can better resist peri-natal starvation periods when parental attendance is impeded by predators (e.g., Magrath 1991, 1992; Rhymer 1988; reviewed in Krist 2011), thus also resulting in reduced detectable solicitations (i.e., begging) by the hatchlings to the parents (Redondo and De Reyna 1988; Briskie et al. 1999). Finally, large eggs may reduce the negative effects of egg cooling on embryo viability when incubation is limited by the proximity of the predator to the nest (Gillooly et al. 2002).
In oviparous organisms, mothers transfer to the eggs major constituents (e.g., proteins and lipids) but also quantitatively minor components like hormones that can profoundly impact the development of the offspring, thereby having effects on morphological, physiological, as well as behavioral traits (Mousseau and Fox 1998; Bonduriansky and Day 2009). Non-lethal effects of predation can also be subtly expressed in terms of the biochemical composition of the eggs, although this hypothesis has been tested experimentally only in few studies (Cockrem and Silverin 2002; Saino et al. 2005; Coslovsky et al. 2012; Pitk et al. 2012; Morosinotto et al. 2016). Predation risk can impact the concentration of egg maternal steroid hormones. Corticosterone is the main hormonal mediator of the acute stress response via the hypothalamo–pituitary–adrenal axis in birds (Wingfield and Romero 2001; Henriksen et al. 2011; Costantini 2014). Females exposed to increased risk of predation before laying increase the amount of corticosterone that they transfer to eggs, and a similar effect also occurs in response to other forms of stress (Saino et al. 2005). Modulation of maternal corticosterone in the eggs is generally interpreted as an adaptive tool at the disposal of the mother to better equip the offspring to their post-natal environment (see Groothuis et al. 2005; von Engelhardt and Groothuis 2011; Estramil et al. 2017). In a predator–prey interaction scenario, increased egg corticosterone concentration can be adaptive because it enhances anti-predatory behavior both in adult and immature individuals. Alternatively, egg composition may simply reflect the effects of environmental factors on maternal physiology (Mousseau and Fox 1998), although evidence is accumulating that contradicts this hypothesis (Sheriff et al. 2017). In this case, increased corticosterone level in the eggs after maternal exposure to high predation risk may simply mirror the consequences of maternal stress response in terms of increased secretion of corticosterone. The effect of predation on testosterone concentration in the eggs has been rarely examined and the only two studies performed in the wild provided contrasting results (Coslovsky et al. 2012; Morosinotto et al. 2016). Egg maternal testosterone has major effects on the developing embryo as well as post-natally (Strasser and Schwabl 2004; Groothuis et al. 2005; Bonisoli-Alquati et al. 2007; Gil 2008; see Williams and Groothuis 2015). Maternal modulation of testosterone under high predation risk can be adaptive, for example, if it boosts traits growth that advance timing of fledging (Schwabl 1996; Navara et al. 2006; Ketterson et al. 2001). Again, an alternative interpretation is simply that egg composition reflects the non-adaptive consequences of the effects of predation risk on maternal physiology.
Overall, field experimental studies of the effects of predation risk on egg composition and size are few, mostly restricted to altricial species, and have provided inconsistent results. In addition, to the best of our knowledge, no study examined the effects of perceived predation risk on egg number, size, and biochemical composition in a colonial species. This is unfortunate because the impact of predation, and therefore the possible responses of the preys are expected to differ between territorial and colonial organisms (Gotmark and Andersson 1984; Robinson 1985; Rolland et al. 1998; Varela et al. 2007). Here, we tested the effects of simulated exposure to two common predators in the study area (adult fox Vulpes vulpes and adult buzzard Buteo buteo) during the period between the rapid yolk development (RYD) phase and laying on yolk concentration of corticosterone and testosterone, on clutch size and on egg mass in the yellow-legged gull Larus michahellis. We expected that corticosterone concentration increased after the predators exposure because of increased passive transfer of the hormone from the mother to the eggs and/or because of active modulation of maternal corticosterone in the eggs, if corticosterone has adaptive effects on peri- and post-natal anti-predatory behavior. We had no directional expectation on testosterone concentration in the yolk because of the lack of theoretical background on the effect of perceived risk of predation on maternal transfer of androgens to the yolk, and because current literature provided contrasting results (Coslovsky et al. 2012; Morosinotto et al. 2016).
According to life-history theory and previous experimental evidence on different species, we expected predation risk to reduce clutch size (Slagsvold 1984; Magnhagen 1991; Nager et al. 2000; Hauber 2003; Griebeler et al. 2010). Finally, the prediction on the effect on egg mass is not straightforward. According to different adaptive scenarios, an increase or a decrease in egg mass under increased perceived risk of predation of the chicks could be expected (see above).
Materials and Methods
Study organism
The yellow-legged gull is a large charadriiform widely distributed across the Mediterranean basin (see Cramp [1998] for information on the natural history of the species). Monogamous pairs lay a single clutch of 1–3 eggs per breeding season (modal clutch size is 3 eggs). Eggs are laid at 2–4 day intervals. A replacement clutch may be laid if the first clutch is lost before egg hatching. Egg size declines with laying order, particularly from the second to the third egg. The duration of the RYD phase, when maternal substances including hormones are deposited in the yolk, is unknown in this species. However, in other larids (Family Laridae), RYD occurs approximately 5–13 days before laying (Roudybush et al. 1979; Meathrel 1991; Ruiz et al. 2000). Because the timing of the RYD phase correlates with egg size (Roudybush et al. 1979), in this study we assume that the RYD phase of the yellow-legged gull also starts approximately 1 week before laying of the individual egg. The yolk of the eggs contains corticosterone and testosterone of maternal origin in amounts that vary among mothers, as observed in the same colony where the present study was conducted (Rubolini et al. 2011). Incubation of the eggs gradually starts already upon laying of the first egg, causing asynchronous hatching (hatching spread: 1–4 days). Before laying, adults in our study colony are typically found in their nesting territories inside the breeding colonies during the middle of the day (personal observation) because foraging activity typically occurs early or late during daytime and presumably also during the night.
Adult yellow-legged gulls have no predators in our study area. However, chicks may be preyed upon by rats Rattus norvegicus, foxes V.vulpes, herons (Ardea sp.), and falconiformes, as well as by feral dogs. Foxes and feral dogs can also prey on eggs. Predators are actively mobbed by colonial gulls.
Experimental procedures
In spring 2016, we identified 5 separate sub-colonies in the northern part of the Comacchio Lagoon (44° 20′ N–12° 11′ E; norther Italy). Three sub-colonies were assigned to a control treatment whereby a piece of brown cloth was placed in the center of the sub-colony, hanged on a support at ca. 1 m from the ground. The piece of cloth did not cause any apparent reaction by the nesting gulls, as expected also because artificial objects are common on the islets where the gull colonies in the Comacchio lagoon are settled. Two sub-colonies were assigned to the predator treatment. In the center of each sub-colony, we placed either a stuffed fox clearly visible from a distance on the ground or a stuffed buzzard hanged on a support ca. 1 m from the ground. Control and predator treatments were administered simultaneously. The sub-colonies were at least 100 m apart from each other (maximum inter-sub-colony distance was 1.5 km). The predators and the control cloth were presented for a period of 15 days every second day on average for 2 h late in the morning (range 9.00 am–1.00 pm), alternating them between the two sub-colonies and always in a different position (sub-colony A: day 1 = fox; day 3 = buzzard; day 5 = fox; day 7 = buzzard; and so on until day 15; and vice versa for sub-colony B). At each exposure, the position of the stuffed predator was changed by less than 3 m over the entire period of the experiment. Presentation of the predators lasted longer than any attempted predation episode ever observed during hundreds of hours of observation on the colonies in the same area since year 2005. The use of two very different predators, a nocturnal and opportunistic terrestrial predator that feeds on both eggs and chicks (i.e., the fox) and diurnal aerial one which does not attack eggs (i.e., the buzzard), their alternation in the same site as well as the long duration of their exposure were chosen to test the overall effect of a simulated environment rich in predators compared with a safe environment, which was the main purpose of our study, rather than to test for potential differences in the effects of either predator. Based on knowledge of the number of nests in the different sub-colonies from previous years, we randomly assigned one sub-colony with a large number of nests and one with a relatively small number of nests to each treatment. In addition, we established a third control sub-colony. We did not established a third sub-colony exposed to predators because we were limited by the number of stuffed predators available.
All the adults from the entire sub-colonies where the predators were placed actively reacted to the predator stimuli by mobbing them and then typically by sitting in the water at a distance while performing episodic mobbing flights to the predator. This was the case also for the fox despite it is a typically crepuscular/nocturnal predator. This reaction lasted from the presentation of the stuffed predator until it was removed, as assessed by observing the colonies from a boat from a distance, i.e., without interfering directly with the colony. This collective behavior by the colony is in marked contrast with the normal behavior in the absence of perceived predation risk, as assessed during hundreds of hours of observation of the same colonies over many years. We note that the birds invariably reacted to the stuffed predators, from the first to the last exposure, indicating that they did not habituate to them. Finally, the predator presentation on a sub-colony never affected the behavior of the adults breeding on another sub-colony.
During the overall period of experimental treatment, we monitored all the new-laid clutches and focused on those where first egg laying occurred on days 11–15 after the first predator/control exposure. This was done to be sure to consider in our analyses only clutches laid by females that started being exposed to predators prior to the RYD phase. On days 11–15 after the start of the predator or control treatment (depending on the laying date of each brood with respect to the date when the predator/control was firstly exposed), we collected all the first eggs that had just been laid in the nests within a radius of 30 m from the site where the predator or the control stimuli were located. The first-laid, collected eggs were stored at −20°C for subsequent hormonal analyses. Upon removal, the first egg was replaced with an egg collected from other nests outside the study area. The eggs that were subsequently laid after the collection of the first egg (i.e., second and third eggs of each focal clutch) were weighed to the nearest gram on the day of laying, but were not collected. No experimental nest was abandoned. In addition, we weighed all the eggs that were laid in all the additional nests found within 30 m of the stimuli (predator or control).
The number of clutches where we measured mass of all eggs was 29 and 7 for the 2 sub-colonies that were exposed to predators and 22, 7, and 4 for the control sub-colonies. Because we were limited by funding, the number of eggs analyzed for hormones was smaller than that used for mass analyses. The number of eggs from the sub-colonies used for hormone analyses was 21 and 4 for the sub-colonies that were exposed to predators and 13, 7, and 4 for the control sub-colonies.
To minimize observer bias, blinded methods were used when all data were recorded and/or analyzed.
Yolk hormone analyses
Yolk steroids were extracted from homogenized yolk samples with a double ether extraction followed by liquid column chromatography according to methods described by Schwabl (1993). Briefly, 50 mg of yolk was weighed and vortexed with 1000 μL of deionized water. Next, 3 mL of petroleum:diethyl ether (30:70 vol/vol) was added, the mixture was vortexed for 30 s and was allowed to settle for 20 min. Samples were then snap frozen and the ether fraction was decanted and dried under nitrogen. The sample was reconstituted in 1 mL 10% ethyl acetate in isooctane and steroids were separated using celite column chromatography. Testosterone was eluted in 20% ethyl acetate in isooctane and corticosterone was eluted in 50% ethyl acetate in isooctane. Testosterone and corticosterone were quantified with commercial enzyme immunossay (EIA) kits (ENZO, NY, USA). Anti-testosterone had a cross-reactivity of 100% with testosterone, 14.6% with 19-hydroxytestosterone, 7.20% with androstendione, and <1% with all other steroids. Anti-corticosterone had a cross-reactivity of 100% with corticosterone, 21.3% with deoxycorticosterone, 21.0% with desoxycorticosterone, and <1% with all other steroids. Average recoveries were 95.0% for testosterone and 93.7% for corticosterone. Average intra-assay variation was 4.9% for testosterone and 2.2% for corticosterone. Testosterone was analyzed over 4 assays and inter-assay variation was 1.9%. Corticosterone was analyzed over 2 assays and inter-assay variation was 4.4%. A single datum for corticosterone concentrations, from the control group, was excluded as it was a positive outlier, after a significant Grubb’s test.
Statistical analyses
We mainly relied on generalized linear mixed models in which the effect of sub-colony and of nest identity (models on egg mass only) were included as random effects, experimental treatment (exposure to predator or control), and laying order (models on egg mass only) were included as fixed effects, and laying date as a continuous covariate. In the analyses of corticosterone and testosterone concentrations and of egg mass, we adopted a normal error distribution while in the analysis of clutch size we adopted a Poisson error distribution. Two-way interaction terms between treatment, laying order, laying date, and egg mass (where relevant) were always initially included in the models and then all excluded in a single step because they did not significantly contribute to the models.
The test of the random effect of sub-colony in the models of hormone concentrations was performed by likelihood ratio tests based on the normal LMM described above. The test of the effect of sub-colony (random effect) on clutch size was performed by applying a Poisson GLMM while adopting Laplace approximation of likelihood estimates. In this analysis we checked that no overdispersion occurred. In the models of egg mass, the effect of the random terms of sub-colony and nest identity was tested by likelihood ratio test on the models including or, respectively, excluding the focal random effect.
To estimate effect sizes for the effect of treatment on the different variables we had to adopt a mixed strategy. For clutch size, effect size (Cohen’s d) was estimated based on Equation (22) in Nakagawa and Cuthill (2007) based on parameters provided by a normal LMM rather than a Poisson GLMM because, to the best of our knowledge, no method to estimate effect sizes from Poisson GLMM has been devised. The effect sizes for the effect of treatment on hormone concentrations were estimated simply by Equation (22) in Nakagawa and Cuthill (2007). Finally, for egg mass, the effect size was estimated based on a normal LMM where we included only the random effect of nest identity because sub-colony identity had no significant effect and, to the best of our knowledge, no method to estimate effect sizes from normal LMM with two random effects has been devised. The effect sizes were computed as Cohen’s d (Nakagawa and Cuthill 2007).
All statistical parameters are reported together with their associated standard error. All analyses were performed using SAS 9.3 statistical package. The sample for hormone analyses consisted of first-laid eggs from 25 clutches that were exposed to predators and 24 control clutches (Supplementary Table S1 for details), while the sample for the analyses of egg mass consisted of 36 entire clutches that were exposed to predators and 33 control clutches.
Results
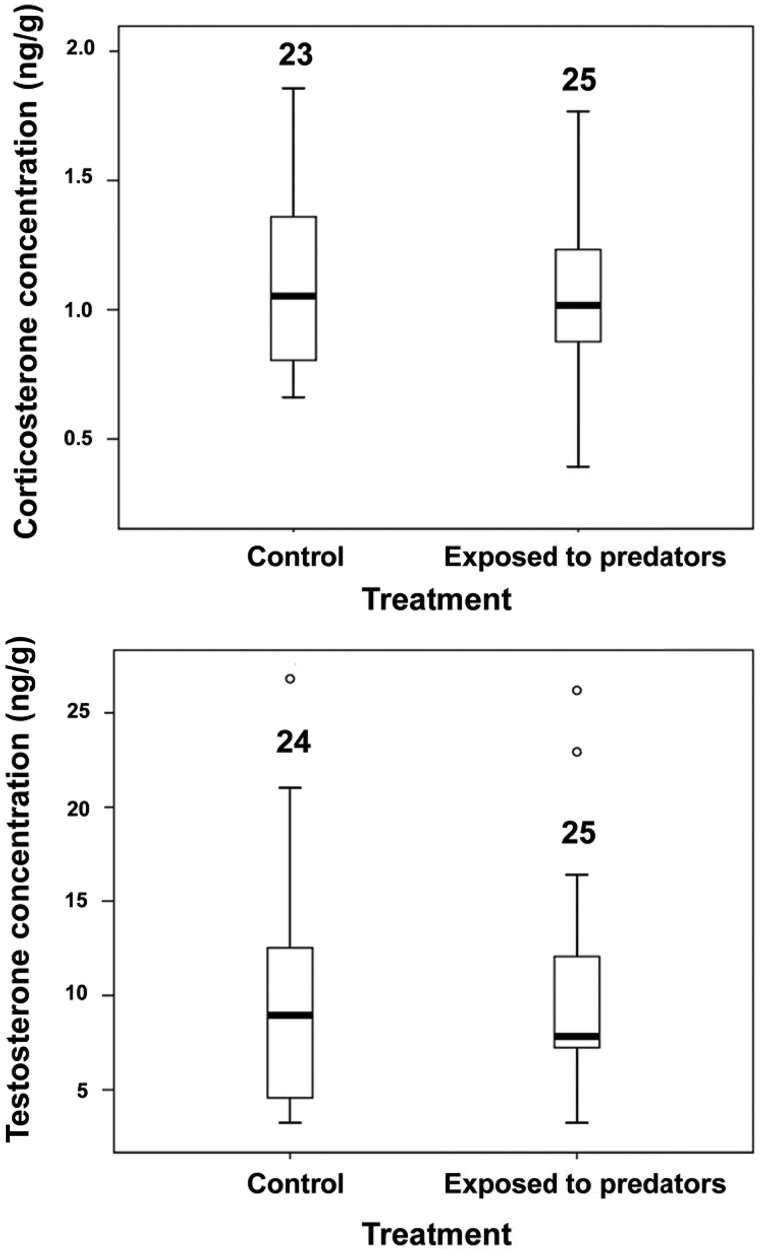
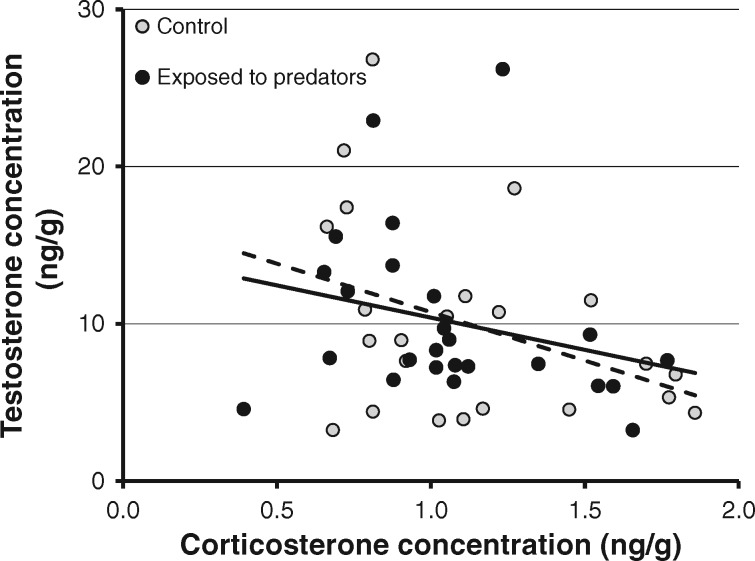
Corticosterone concentration was not significantly affected by simulated exposure to predators (Table 1 and Figure 1). The effect size (Cohen’s d) was −0.090. The significant effect of the random effect of sub-colony (Table 1) indicates that some unidentified factor caused the eggs from different sub-colonies to differ in corticosterone concentrations. Corticosterone concentration declined with laying date and did not covary with egg mass (Table 1). When included in the model, the distance between the individual nests and the place where the predator was presented did not predict corticosterone levels (details not shown). The experimental treatment did not affect testosterone concentration and there was no significant variation in testosterone concentration among colonies (Table 1 and Figure 1). The effect size was 0.030. In addition, testosterone concentration did not significantly vary with laying date and did not covary with egg mass (Table 1). In a linear mixed model with sub-colony as a random effect, the interaction term between treatment and corticosterone concentration did not predict the concentration of testosterone in the yolk (F1,44 = 0.21, P = 0.652). A simplified model excluding the interaction term showed that the concentration of testosterone significantly negatively covaried with that of corticosterone (F1,45 = 5.32, P = 0.026; Figure 2). Hence, there was a negative relationship between the concentration of these steroid hormones but the slope of the relationship did not depend on experimental treatment.
Table 1.
Linear mixed models of corticosterone and testosterone concentration, of clutch size and of egg mass in relation to treatment (exposure to predators or control)
| χ 2 | F | df | P | Coefficient (SE) | |
|---|---|---|---|---|---|
| Corticosterone concentration in first eggs | |||||
| Sub-colony | 6.11 | 0.013 | |||
| Treatment | 0.12 | 1, 41 | 0.732 | ||
| Laying date | 6.24 | 1, 41 | 0.017 | −0.093 (0.037) | |
| Egg mass | 0.16 | 1, 41 | 0.691 | 0.003 (0.008) | |
| Testosterone concentration in first eggs | |||||
| Sub-colony | 0.00 | >0.999 | |||
| Treatment | 0.01 | 1, 42 | 0.907 | ||
| Laying date | 0.57 | 1, 42 | 0.454 | 0.526 (0.695) | |
| Egg mass | 0.42 | 1, 42 | 0.523 | −0.087 (0.136) | |
| Clutch size | |||||
| Sub-colony | 0.00 | >0.999 | |||
| Treatment | 0.04 | 1, 63 | 0.837 | ||
| Laying date | 0.07 | 1, 63 | 0.787 | −0.013 (0.048) | |
| Egg mass | |||||
| Sub-colony | 0.00 | >0.999 | |||
| Nest identity | 88.26 | <0.001 | |||
| Treatment | 10.75 | 1, 122 | 0.001 | ||
| Laying order | 21.35 | 2, 122 | <0.001 | ||
| Laying date | 3.30 | 1, 122 | 0.072 | −0.588 (0.324) | |
Notes: Laying date was included as a covariate. Egg mass was also included as a covariate in models of corticosterone and testosterone concentrations. Sub-colony was included as a random effect in all models. Nest identity was included as a random effect and laying order as a fixed effect factor in the model of egg mass. χ2 refers to the likelihood ratio test comparing models including or, respectively, excluding the random effect of sub-colony. Two-way interaction terms were removed from all models as their effect was statistically non-significant.
Figure 1.
Boxplot of corticosterone and testosterone concentrations in the first laid eggs from control nests and nests that were exposed to predators. Sample sizes (number of eggs) are reported.
Figure 2.
Relationship between testosterone and corticosterone concentrations in the yolk of control and predator-exposed eggs. The regression lines for controls (dashed) and predator-exposed (continuous) eggs are presented. The slopes of the regression lines did not differ between the groups.
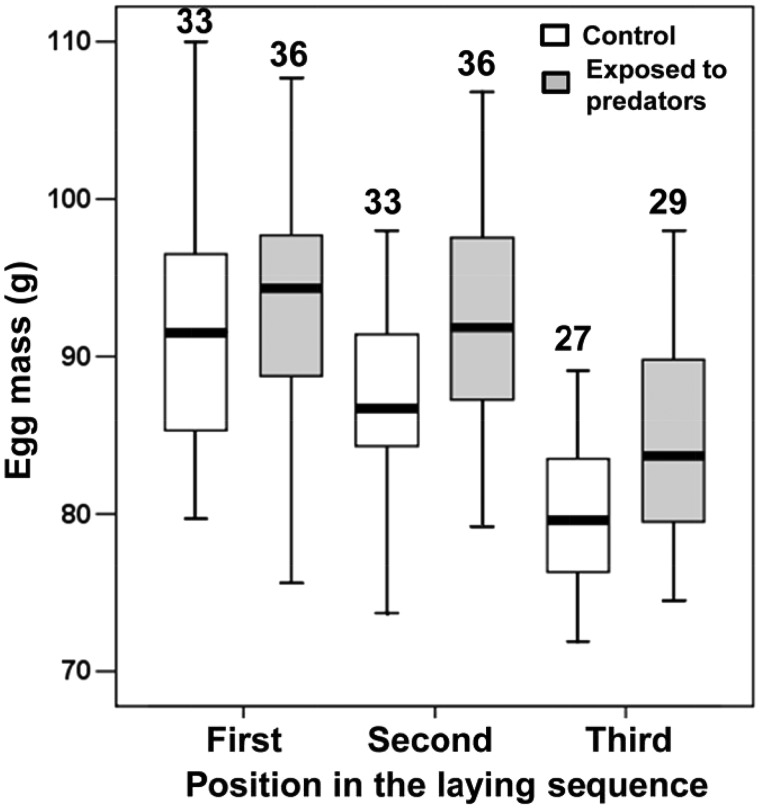
Clutch size was not predicted by experimental treatment (Table 1) with very similar mean clutch sizes in nests exposed to predators. The effect size was 0.118. Egg mass at laying was significantly larger in broods that were exposed to predators (Table 1 and Figure 3). The effect size was 0.249. The difference in average mass (4.8 g) between the 2 groups of eggs estimated as least-squares means from the model in Table 1 corresponded to 0.89 standard deviations of the mass of the eggs from the control group computed from residuals of a linear mixed model controlling for nest identity (random effect) and laying order and laying date (fixed effects). The difference in egg mass between the control and the experimental groups corresponded to ca. 0.7 standard deviations of egg mass in our population.
Figure 3.
Boxplot of mass of the eggs from control nests and nests that were exposed to predators. Sample sizes (number of eggs) are reported. The eggs from nests exposed to predators were significantly heavier than those from control nests.
In addition, egg mass significantly declined with position in the laying sequence (Table 1 and Figure 3). These effects were reciprocally independent, as shown by the non-significant effects of the two-way interaction terms that were removed from the models. Thus, independently of laying order and laying date, eggs in nests that were exposed to predators were larger than control eggs. In addition, egg mass significantly varied among nests (Table 1).
Discussion
We experimentally tested the effects of simulated exposure to predators during the egg formation period on the concentration of corticosterone and of testosterone in the eggs, and on egg mass and clutch size in the yellow-legged gull. We did not record any significant effect of experimental treatment on corticosterone concentration. This result contradicts our expectation that was based on the relatively few studies exploring the non-lethal effect of predation on transmission of corticosterone to the eggs in birds (Cockrem and Silverin 2002; Saino et al. 2005). The lack of the effect of exposure to predators was unlikely to be due to insufficient power of the statistical tests. This is the case because the size of the sample was reasonably large and the difference between the control and the predator-exposed group of eggs was minimal. The interpretation that the experimental protocol was ineffective in stimulating an anti-predator response in the gulls is also unlikely because the gulls strongly reacted to the both stuffed diurnal buzzard and nocturnal fox by actively mobbing them or by sitting in the water far from the stuffed predators. This behavior is very different from that usually adopted by undisturbed adults, that typically stay in their nesting territory within the colony during daytime. In addition, daily exposure to the predator lasted longer than any episode of alarm induced by a natural predator ever observed in the same colonies. Thus, our results may suggest that exposure to predation risk has no detectable effect on the concentration of corticosterone in the egg yolk, at least in our study system. An alternative interpretation is that the presence of the stuffed predators, while eliciting anti-predator behavior, including alarm calling and repeatedly mobbing the stuffed predators, did not trigger a stress response mediated by the hypothalamo–pituitary–adrenal axis and thus did not result in increased corticosterone secretion (e.g., Harris and Carr 2016). It is especially the case in the wild when individuals can easily escape and adjust their behavior, and hence the predator might not represent a direct threat for survival (Cockrem and Silverin 2002). This explanation is in contrast with previously observed effects of exposure to stuffed predators close to the nest on egg corticosterone concentrations (Saino et al. 2005). However, because we did not measure corticosterone levels in the adults exposed to the predators and to the control stimulus, this interpretation cannot be ruled out at present. We notice that capturing the adults at the colonies before egg laying (i.e., when the adults are not strictly linked to the nest but rather to the nesting area) is extremely difficult and, more importantly, would entail an enormous disturbance to the entire sub-colonies, thus dramatically affecting the subsequent behavior of all birds irrespectively of their experimental treatment and likely also their physiology. For these reasons, we refrained from attempting to capture the adults before egg laying. Finally, an alternative possibility is that colonial species, which are known to be less affected by predation than territorial ones (Gotmark and Andersson 1984; Robinson 1985; Rolland et al. 1998; but see Varela et al. 2007), might respond to predator exposure by releasing a lower level of stress hormones, thus resulting in a lack of detectable corticosterone transfer into the eggs. However, our results also suggest that corticosterone concentration in the eggs depends on environmental effects, as suggested by the observation that it varied among sub-colonies and also declined along the breeding season. The factors that caused such variation, however, remain unknown.
Testosterone was also apparently unaffected by experimental treatment, and its concentrations were very similar in the experimental groups, similarly to a previous study in a territorial passerine (Morosinotto et al. 2016). In the absence of clear predictions on the direct effect of exposure to predators on testosterone concentration in the eggs, and considering the previous contrasting results (Coslovsky et al. 2012; Morosinotto et al. 2016), the main reason for investigating the effect was that corticosterone and testosterone concentrations have been shown to be related in birds (Duckworth et al. 2001; Okuliarová et al. 2010; Henriksen et al. 2011; Rubolini et al. 2011). The concentrations of these hormones were negatively reciprocally correlated and the slope of relationship was independent of experimental treatment. Hence, the present study does not corroborate the idea that exposure to predators has effects on corticosterone transfer to the eggs. However, we cannot exclude the possibility that our experimental treatment might have affected the biochemical precursor of testosterone, the androstenedione, which has been shown to be reduced after the exposure to some, but not all, predators in another species (Morosinotto et al. 2016). Notably, a recent meta-analysis supported coloniality as the main factor predicting variation of egg testosterone levels across species, with more colonial species having smaller concentrations of the hormone (Bentz et al. 2016). It might be speculated that selection for reduced aggressiveness in colonial species results in smaller response to external stressful conditions in terms of allocation of steroid hormones to the eggs.
Previous studies of the effect of predation risk on clutch size showed either negative or null effects (Doligez and Clobert 2003; Eggers et al. 2006; Fontaine and Martin 2006; Massaro et al. 2008; Cassey et al. 2009). The present study thus adds to the body of evidence that predators have no effect on decisions on reproductive investment in terms of clutch size.
One general, adaptive interpretation of the lack of the effect of predation on egg hormone concentrations and clutch size is that predation risk during egg laying is a poor predictor of predation risk that the offspring will experience after hatching. This is the case because local predation risk may vary widely between laying and hatching, which occurs at least a month later. In fact, our model species differs from most of the species where the effect of predation of clutch size and egg composition has been tested, in which hatching typically occurs 14–16 days after egg laying (Cockrem and Silverin 2002; Doligez and Clobert 2003; Saino et al. 2005; Coslovsky et al. 2012; Pitk et al. 2012). Even a minimal (i.e., 1 egg) reduction in clutch size may entail a considerable seasonal fitness cost in a species where maximal clutch size is 3 eggs. This is particularly true in our study colony where large post-natal mortality typically occurs (our personal observation since 2005). In fact, third-laid chicks might function as a back-up for their larger, older siblings. Any reduction in clutch size in response to predation risk upon laying would therefore be prevented by temporal stochasticity in predation risk and large fitness cost of reduction of clutch size.
The interpretation that stochastic temporal variation in predation risk reduces the scope for adaptive flexibility in reproductive decisions is partly contradicted by the observation that experimental treatment had a highly significant, intense effect on egg mass, which was considerably larger in the predator-exposed than in the control group. The predictions on the effect of predators on egg mass were equivocal and dependent on the assumptions on the effect of large egg mass on post-natal performance under high predation risk (Cresswell 2008). We interpret the observation of the positive effect of exposure to predators on egg mass as suggestive that mothers enhanced post-natal survival prospects of their offspring by increasing their size and reserves, which could promote viability in case post-natal parental attendance is reduced by the presence of predators, by enhancing their cognitive and motor skills at hatching and perhaps also by reducing offspring need of food and thus their postural and vocal solicitation behavior to their parents. In addition, because size at hatching strongly predicts subsequent growth rates (Krist 2011), by laying large eggs parents may also reduce the duration of the period when chicks are most vulnerable to predation, at least by aerial predators. Yet an alternative interpretation is that larger eggs are less exposed to the risk of cooling when the presence of predators forces parents to leave the nest unattended and suspend incubation (e.g., Rhymer 1988). In summary, we suggest that stochasticity in local predation risk may have shrunk the scope for adaptive reduction of clutch size, which can entail considerable seasonal fitness costs, but may also have prompted laying mothers to adopt a strategy of incremental investment to boost offspring performance.
The predators used in our study have markedly different predatory strategies (i.e., the buzzard is diurnal aerial predator which do not prey on eggs while the fox is an opportunistic terrestrial predator feeding on both eggs and chicks), thus possibly driving different physiological responses in their potential preys, as observed in other species (e.g., Morosinotto et al. 2010, 2016; Cox et al. 2012). Unfortunately, our experimental approach prevented us from testing for differences in the effects of either predator species, and future studies are needed to disclose possible predator-specific physiological responses in our colonial model species.
In conclusion, we showed that exposure to increased predation risk has no detectable effect on the concentration of egg steroid hormones and on clutch size, whereas it has a marked positive effect on egg size in the yellow-legged gull, possibly to boost performance in the post-natal stages. These findings are partly inconsistent with previous evidence on the positive effect of predation risk on egg corticosterone concentration and on the negative effect on clutch size. We suggest that the effects of predation risk on reproductive decisions vary idiosyncratically among species possibly depending on predictability of predation risk, social and reproductive behavior, as well as developmental mode.
Experimental Ethics
The study was carried out under the permission of the Parco Regionale del Delta del Po (#388, 20 January 2016), and it was undertaken under the combined prescriptions of Art. 4 (1) and Art. 7 (5) of the Italian law 157/1992, which regulates studies on wild bird species. All applicable institutional and/or national guidelines for the care and use of animals were followed.
Author Contributions
M.P., A.R., C.D.P., and N.S. conceived and designed the experiment. A.R., C.D.P., D.R., M.C., and M.P. conducted the fieldwork. A.B., C.D.P., K.N., and M.P. performed the laboratory work. C.D.P., A.R., and N.S. analyzed the data. A.R., C.D.P., and N.S. wrote the manuscript. All authors gave the final approval for the publication.
Supplementary Material
References
- Agrawal AA, Laforsch C, Tollrian R, 1999. Transgenerational induction of defences in animals and plants. Nature 401:60–63. [Google Scholar]
- Amundsen T, 1995. Egg size and early nestling growth in the snow petrel. Condor 97:345–351. [Google Scholar]
- Bentz AB, Becker DJ, Navara KJ, 2016. Evolutionary implications of interspecific variation in a maternal effect: a meta-analysis of yolk testosterone response to competition. R Soc Open Sci 3:160499.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonduriansky R, Day T, 2009. Non-genetic inheritance and its evolutionary implications. Annu Rev Ecol Evol Syst 40:103–125. [Google Scholar]
- Bonisoli-Alquati A, Rubolini D, Romano M, Boncoraglio G, Fasola M et al. , 2007. Effects of egg albumen removal on yellow-legged gull chick phenotype. Funct Ecol 21:310–316. [Google Scholar]
- Briskie JV, Martin PR, Martin TE, 1999. Nest predation and the evolution of nestling begging calls. Proc R Soc Lond B 266:2153–2159. [Google Scholar]
- Caro T, 2005. Antipredator Defenses in Birds and Mammals. Chicago (IL: ): University of Chicago Press. [Google Scholar]
- Cassey P, Boulton RL, Ewen JG, Hauber ME, 2009. Reduced clutch-size is correlated with increased nest predation in exotic Turdus thrushes. Emu 109:294–299. [Google Scholar]
- Cockrem JF, Silverin B, 2002. Sight of a predator can stimulate a corticosterone response in the great tit Parus major. Gen Comp Endocrinol 125:248–255. [DOI] [PubMed] [Google Scholar]
- Coslovsky M, Richner H, 2011. Predation risk affects offspring growth via maternal effects. Funct Ecol 25:878–888. [Google Scholar]
- Coslovsky M, Groothuis T, de Vries B, Richner H, 2012. Maternal steroids in egg yolk as a pathway to translate predation risk to offspring: experiments with great tits. Gen Comp Endocrinol 176:211–214. [DOI] [PubMed] [Google Scholar]
- Costantini D, 2014. Oxidative Stress and Hormesis in Evolutionary Ecology and Physiology: A Marriage between Mechanistic and Evolutionary Approaches. Berlin: Springer. [Google Scholar]
- Cox WA, Thompson FR III, Faaborg J, 2012. Species and temporal factors affect predator-specific rates of nest predation for forest songbirds in the Midwest. Auk 129:147–155. [Google Scholar]
- Cramp S, 1998. The Complete Birds of the Western Palearctic on CD-ROM. Oxford: Oxford University Press. [Google Scholar]
- Creel S, Christianson D, 2008. Relationships between direct predation and risk effects. Trends Ecol Evol 23:194–201. [DOI] [PubMed] [Google Scholar]
- Creel S, Christianson D, Liley S, Winnie JA Jr, 2007. Predation risk affects reproductive physiology and demography of elk. Science 315:960.. [DOI] [PubMed] [Google Scholar]
- Cresswell W, 2008. Non-lethal effects of predation in birds. Ibis 150:3–17. [Google Scholar]
- Doligez B, Clobert J, 2003. Clutch size reduction as a response to increased nest predation rate in the collared flycatcher. Ecology 84:2582–2588. [Google Scholar]
- Duckworth RA, Mendonça MT, Hill GE, 2001. A condition dependent link between testosterone and disease resistance in the house finch. Proc R Soc B Biol Sci 268:2467–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers S, Griesser M, Nystrand M, Ekman J, 2006. Predation risk induces changes in nest-site selection and clutch size in the Siberian jay. Proc R Soc B Biol Sci 273:701–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estramil N, Groothuis TTG, Eens M, de Vries B, Müller W, 2017. Coadaptation of offspring begging and parental provisioning: a role for prenatal maternal effects? Horm Behav 87:129–136. [DOI] [PubMed] [Google Scholar]
- Fontaine JJ, Martin TE, 2006. Parent birds assess nest predation risk and adjust their reproductive strategies. Ecol Lett 9:428–434. [DOI] [PubMed] [Google Scholar]
- Ghalambor CK, Martin TE, 2002. Comparative manipulation of predation risk in incubating birds reveals variability in the plasticity of responses. Behav Ecol 13:101–108. [Google Scholar]
- Giesing ER, Suski CD, Warner RE, Bell AM, 2011. Female sticklebacks transfer information via eggs: effects of maternal experience with predators on offspring. Proc R Soc B Biol Sci 278:1753–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil D, 2008. Hormones in avian eggs: physiology, ecology and behavior. Adv Study Behav 38:337–398. [Google Scholar]
- Gillooly JF, Charnov EL, West GB, Savage VM, Brown JH, 2002. Effects of size and temperature on developmental time. Nature 417:70–73. [DOI] [PubMed] [Google Scholar]
- Gotmark F, Andersson M, 1984. Colonial breeding reduces predation in the common gull Larus canus. Anim Behav 32:485–492. [Google Scholar]
- Griebeler EM, Caprano T, Böhning-Gaese K, 2010. Evolution of avian clutch size along latitudinal gradients: do seasonality, nest predation or breeding season length matter? J Evol Biol 23:888–901. [DOI] [PubMed] [Google Scholar]
- Groothuis TGG, Müller W, von Engelhardt N, Carere C, Eising C, 2005. Maternal hormones as a tool to adjust offspring phenotype in avian species. Neurosci Biobehav Rev 29:329–352. [DOI] [PubMed] [Google Scholar]
- Harris BN, Carr JA, 2016. The role of the hypothalamus–pituitary–adrenal/interrenal axis in mediating predator-avoidance trade-offs. Gen Comp Endocrinol 230:110–142. [DOI] [PubMed] [Google Scholar]
- Hauber ME, 2003. Interspecific brood parasitism and the evolution of host clutch sizes. Evol Ecol Res 5:559–570. [Google Scholar]
- Henriksen R, Rettenbacher S, Groothuis TGG, 2011. Prenatal stress in birds: pathways, effects, function and perspectives. Neurosci Biobehav Rev 35:1484–1501. [DOI] [PubMed] [Google Scholar]
- Ketterson ED, Nolan V Jr, Casto JM, Buerkle CA, Clotfelter E, Grindstaff JL, Jones KJ, Lipar JL, McNabb FMA, Neudorf DL, Parker-Renga IPR, Schoech SJ, Snajdr E, 2001. Testosterone, phenotype, and fitness: a research program in evolutionary behavioral endocrinology In: Dawson A, Chaturvedi CM, editors. Avian endocrinology, pp. 19–40. New Delhi, India: Narosa Publishing House. [Google Scholar]
- Kokko H, Lopez-Sepulcre A, 2007. The ecogenetic link between demography and evolution: can we bridge the gap between theory and data? Ecol Lett 10:773–782. [DOI] [PubMed] [Google Scholar]
- Krist M, 2011. Egg size and offspring quality: a meta-analysis in birds. Biol Rev 86:692–716. [DOI] [PubMed] [Google Scholar]
- Lima SL, 2009. Predators and the breeding bird: behavioral and reproductive flexibility under the risk of predation. Biol Rev 84:485–513. [DOI] [PubMed] [Google Scholar]
- Magnhagen C, 1991. Predation risk as a cost of reproduction. Trends Ecol Evol 6:183–186. [DOI] [PubMed] [Google Scholar]
- Magrath RD, 1991. Nestling weight and juvenile survival in the blackbird Turdus merula. J Anim Ecol 60:335–351. [Google Scholar]
- Magrath RD, 1992. The effect of egg mass on the growth and survival of blackbirds: a field experiment. J Zool 227:639–654. [Google Scholar]
- Martin TE, 1995. Avian life-history evolution in relation to nest sites, nest predation, and food. Ecol Monogr 65:101–127. [Google Scholar]
- Martin TE, Martin PR, Olson CR, Heidinger BJ, Fontaine JJ, 2000a. Parental care and clutch sizes in North and South American birds. Science 287:1482–1485. [DOI] [PubMed] [Google Scholar]
- Martin TE, Scott J, Menge C, 2000b. Nest predation increases with parental activity: separating nest site and parental activity effects. Proc R Soc Lond B Biol Sci 267:2287–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massaro M, Starling-Windhof A, Briskie JV, Martin TE, 2008. Introduced mammalian predators induce behavioural changes in parental care in an endemic New Zealand bird. PLoS One 3:e2331.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meathrel CE, 1991. Variation in eggs and the period of rapid yolk deposition of the silver gull Larus novaehollandiae during a protracted laying season. J Zool 223:501–508. [Google Scholar]
- Morosinotto C, Thomson RL, Korpimäki E, 2010. Habitat selection as an antipredator behaviour in a multi-predator landscape: all enemies are not equal. J Anim Ecol 79:327–333. [DOI] [PubMed] [Google Scholar]
- Morosinotto C, Thomson RL, Ruuskanen S, Korpimäki E, Lehikoinen E et al. , 2016. Maternal transfer of androgens in eggs is affected by food supplementation but not by predation risk. J Avian Biol 47:629–641. [Google Scholar]
- Mousseau TA, Fox CW, 1998. Maternal Effects as Adaptations. Oxford: Oxford University Press. [Google Scholar]
- Nager RG, Monaghan P, Houston DC, 2000. Within-clutch trade-offs between the number and quality of eggs: experimental manipulations in gulls. Ecology 81:1339–1350. [Google Scholar]
- Nakagawa S, Cuthill IC, 2007. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev 82:591–605. [DOI] [PubMed] [Google Scholar]
- Navara KJ, Hill GE, Mary TM, 2006. Yolk testosterone stimulates growth and immunity in house finch chicks. Physiol Biochem Zool 79:550–555. [DOI] [PubMed] [Google Scholar]
- Okuliarová M, Šárniková B, Rettenbacher S, Škrobánek P, Zeman M, 2010. Yolk testosterone and corticosterone in hierarchical follicles and laid eggs of Japanese quail exposed to long-term restraint stress. Gen Comp Endocrinol 165:91–96. [DOI] [PubMed] [Google Scholar]
- Peluc SI, Sillett TS, Rotenberry JT, Ghalambor CK, 2008. Adaptive phenotypic plasticity in an island songbird exposed to a novel predation risk. Behav Ecol 19:830–835. [Google Scholar]
- Pianka ER, 1970. On r and K selection. Am Nat 104:592–597. [Google Scholar]
- Pitk M, Tilgar V, Kilgas P, Mänd R, 2012. Acute stress affects the corticosterone level in bird eggs: a case study with great tits Parus major. Horm Behav 62:475–479. [DOI] [PubMed] [Google Scholar]
- Redondo T, De Reyna LA, 1988. Locatability of begging calls in nestling altricial birds. Anim Behav 36:653–661. [Google Scholar]
- Rolland C, Danchin E, Fraipont MD, 1998. The evolution of coloniality in birds in relation to food, habitat, predation, and life-history traits: a comparative analysis. Am Nat 151:514–529. [DOI] [PubMed] [Google Scholar]
- Rhymer JM, 1988. The effect of egg size variability on thermoregulation of mallard Anas platyrhynchos offspring and its implications for survival. Oecologia 75:20–24. [DOI] [PubMed] [Google Scholar]
- Ricklefs RE, 2000. Density dependence, evolutionary optimization, and the diversification of avian life histories. Condor 102:9–22. [Google Scholar]
- Robinson SK, 1985. Coloniality in the yellow-rumped cacique as a defense against nest predators. Auk 102:506–519. [Google Scholar]
- Roudybush TE, Grau CR, Petersen MR, Ainley DG, Hirsch KV et al. , 1979. Yolk formation in some charadriiform birds. Condor 81:293–298. [Google Scholar]
- Rubolini D, Romano M, Navara KJ, Karadas F, Ambrosini R et al. , 2011. Maternal effects mediated by egg quality in the yellow-legged gull Larus michahellis in relation to laying order and embryo sex. Front Zool 8:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz X, Jover L, Pedrocchi V, Oro D, González SJ, 2000. How costly is clutch formation in the Audouin’s gull Larus audouinii?. J Avian Biol 31:567–575. [Google Scholar]
- Safriel UN, 1975. On the significance of clutch size in nidifugous birds. Ecology 56:703–708. [Google Scholar]
- Saino N, Romano M, Ferrari RP, Martinelli R, Moller AP, 2005. Stressed mothers lay eggs with high corticosterone levels which produce low-quality offspring. J Exp Zool Part A Comp Exp Biol 303A:998–1006. [DOI] [PubMed] [Google Scholar]
- Schwabl H, 1993. Yolk is a source of maternal testosterone for developing birds. Proc Nat Acad Sci U S A 90:11446–11450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabl H, 1996. Maternal testosterone in the avian egg enhances postnatal growth. Comp Biochem Physiol A Physiol 114:271–276. [DOI] [PubMed] [Google Scholar]
- Sheriff MJ, Bell A, Boonstra R, Dantzer B, Lavergne SG et al. , 2017. Integrating ecological and evolutionary context in the study of maternal stress. Integr Comp Biol 57:437–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slagsvold T, 1984. Clutch size variation of birds in relation to nest predation: on the cost of reproduction. J Anim Ecol 53:945–953. [Google Scholar]
- Smith HG, Bruun M, 1998. The effect of egg size and habitat on starling nestling growth and survival. Oecologia 115:59–63. [DOI] [PubMed] [Google Scholar]
- Storm JJ, Lima SL, 2010. Mothers forewarn offspring about predators: a transgenerational maternal effect on behaviour. Am Nat 175:382–390. [DOI] [PubMed] [Google Scholar]
- Strasser R, Schwabl H, 2004. Yolk testosterone organizes behavior and maleplumage coloration in house sparrows Passer domesticus. Behav Ecol Sociobiol 56:491–497. [Google Scholar]
- Styrsky JD, Eckerle KP, Thompson CF, 1999. Fitness-related consequences of egg mass in nestling house wrens. Proc R Soc Lond B Biol Sci 266:1253–1258. [Google Scholar]
- Thomson DL, Monaghan P, Furness RW, 1998. The demands of incubation and avian clutch size. Biol Rev Camb Philos Soc 73:293–304. [Google Scholar]
- Travers M, Clinchy M, Zanette L, Boonstra R, Williams TD, 2010. Indirect predator effects on clutch size and the cost of egg production. Ecol Lett 13:980–988. [DOI] [PubMed] [Google Scholar]
- Varela SAM, Danchin E, Wagner RH, 2007. Does predation select for or against avian coloniality? A comparative analysis. J Evol Biol 20:1490–1503. [DOI] [PubMed] [Google Scholar]
- von Engelhardt N, Groothuis TGG, 2011. Maternal hormones in avian eggs. Horm Reprod Vertebr 4:91–127. [Google Scholar]
- Williams TD, Groothuis TGG, 2015. Egg quality, embryonic development and post-hatching phenotype: an integrated perspective In: Deeming C, Reynolds SJ, editors. Nests, Eggs, and Incubation: New Ideas about Avian Reproduction. Oxford: Oxford University Press, 113–126. [Google Scholar]
- Wingfield JC, Romero LM, 2001. Adrenocortical responses to stress and their modulation in free-living vertebrates. Compr Physiol doi:10.1002/cphy.cp070411. [Google Scholar]
- Zanette LY, White AF, Allen MC, Clinchy M, 2011. Perceived predation risk reduces the number of offspring songbirds produce per year. Science 334:1398–1401. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.