Abstract
Pollinators use multiple cues whilst foraging including direct cues from flowers and indirect cues from other pollinators. The use of indirect social cues is common in social insects, such as honeybees and bumblebees, where a social environment facilitates the ability to use such cues. Bumblebees use cues to forage on flowers according to previous foraging experiences. Flowers are an essential food source for pollinators but also pose a high risk of parasite infection through the shared use of flowers leading to parasite spillover. Nevertheless, bumblebees have evolved behavioral defense mechanisms to limit parasite infection by avoiding contaminated flowers. Mechanisms underlying the avoidance of contaminated flowers by bumblebees are poorly understood. Bumblebees were recorded having the choice to forage on non-contaminated flowers and flowers contaminated by a trypan osome gut parasite, Crithidia bombi. The use of different treatments with presence or absence of conspecifics on both contaminated and non-contaminated flowers allowed to investigate the role of social visual cues on their pathogen avoidance behavior. Bumblebees are expected to use social visual cues to avoid contaminated flowers. Our study reveals that the presence of a conspecific on flowers either contaminated or not does not help bumblebee foragers avoiding contaminated flowers. Nevertheless, bumblebees whereas gaining experience tend to avoid their conspecific when placed on contaminated flower and copy it when on the non-contaminated flower. Our experiment suggests a detrimental impact of floral scent on disease avoidance behavior.
Keywords: Host-parasite interactions, social learning, pollinators, inadvertent social information, behavioral immunity, copying behavior
Bumblebees are economically important organisms involved in plant-pollinator interactions, relying on flowers as their main food resource. Thirty-five percent of human food is dependent on pollinators, with 70% of all crop species dependent upon pollination (Klein et al. 2007; Gallai et al. 2009). As with other pollinator populations, bumblebees are experiencing extreme threat of decline and parasites are an important contributing factor (Potts et al. 2010; Goulson et al. 2015). Several bumblebee species experiencing significant population decline show strong correlation with parasite spillover, where commercial bumblebee colonies transmit parasites to wild populations (Szabo et al. 2012).
Parasitic infection and associated diseases are considered major debilitating factors that dramatically reduce fitness within populations resulting in a strong evolutionary force to maintain a reduction in parasite load and leading to an arms race, known as the Red Queen Hypothesis (Kraus and Page 1998; Salathé et al. 2008a, 2008b). Parasites and their hosts develop measures to evade one another through evolution, that is, constant natural selection for an adaptation by a parasite to efficiently invade a host will be counter-adapted by the host using the same process to avoid such infection (Lively and Dybdahl 2000). Parasites are also of ecological importance as their impacts are not restricted to host species and their burden can alter several ecological interactions to a significant extent (Daszak et al. 2000; Hatcher et al. 2012).
Social insects, such as bumblebees, are more susceptible to parasitic infection because of a higher number of closely related individuals within the colony due to single mating (Schmid-Hempel and Schmid-Hempel 2000). This eases transmission and along with the stable homeostatic conditions colony life creates ideal conditions for parasite growth and maintenance (Schmid-Hempel 1998). The social organization and life-history traits make bumblebees a prime target for parasitic infection.
Due to this vulnerability, bumblebees have adapted mechanisms to ensure that parasitic burden is reduced within a colony by methods of parasite avoidance (Fouks and Lattorff 2011), minimizing the risk of parasite introduction and transmission within the colony. Colony life can, therefore, prove advantageous as it provides the unique opportunity for group-level defenses (social immunity), with each individual member of the colony collaborating to reduce the transmission of parasites within their colony (Cremer et al. 2007; Cremer and Sixt 2009).
One of the most common parasites of the bumblebee, Bombus terrestris, is the trypanosome gut parasite, Crithidia bombi. This parasite causes reduced ability to form reproductive stages and therefore formation of a new colony and an increased mortality of workers due to inefficient foraging and resource acquisition (Schmid-Hempel 2001; Gegear et al. 2006). In addition, ingestion of C. bombi by bumblebees initiates a rapid immune response, resulting in an impaired learning ability during foraging (Gegear et al. 2006; Alghamdi et al. 2008; Erler et al. 2011). C. bombi can be transmitted via the shared use of flowers by foraging bumblebees (Durrer and Schmid-Hempel 1994; Graystock et al. 2015). In response to parasite threat, bumblebees have adapted their foraging behavior to reduce parasite intake on flowers by avoiding flowers contaminated by C. bombi (Fouks and Lattorff 2011).
Fouks and Lattorff (2011) highlighted the ability of a bumblebee to choose an uncontaminated flower whilst foraging and identified learning at an individual- and colony-level as supporting mechanisms. However, there is still some uncertainty surrounding the mechanisms behind such learning. Fouks and Lattorff (2013) further investigated the role of social learning using social scent marks in foraging bumblebees in the context of disease avoidance. They revealed that the presence of social scent marks did not improve bumblebee efficiency to avoid contaminated flowers. Local enhancement is the term used to describe a type of social learning, seen in insects and especially B. terrestris, where individuals are attracted to a location because other bumblebees have been present there (Leadbeater and Chittka 2005; Kawaguchi et al. 2006). Nevertheless, bumblebees only copy the choice of other bumblebees when they previously forage in presence of another bumblebee (Avarguès-Weber and Chittka 2014). In the case of foraging, a bumblebee is therefore more likely to choose a food source where a conspecific (another bumblebee) has previously foraged (Leadbeater and Chittka 2005; Kawaguchi et al. 2006). It is stated that foraging using local enhancement is more efficient as it provides a guaranteed food source and reduces predation rate (Kawaguchi et al. 2006). Many studies have been carried out to determine the mechanisms behind choosing flowers and it has been shown that scent marking by conspecifics does not play a major role in the foraging behavior of naive bumblebees, however, local enhancement using visual cues and presence of conspecifics may be more likely to take place (Kawaguchi et al. 2006; Leadbeater and Chittka 2007, 2011). Moreover, bumblebees learn the value of social visual cues through their experience (Leadbeater and Chittka 2009), and weigh the reliability of personal and social information (Dunlap et al. 2016). Nevertheless, they demonstrate a preference for social information, as social learning can lead bumblebees to follow suboptimal foraging choices (Avarguès-Weber et al. 2018). We therefore hypothesize that colony-level learning processes may be the result of copying behavior and investigate the role of the social visual cue and associated social learning of bumblebees in the context of foraging disease avoidance. Bumblebees are then expected to use social visual cues to avoid contaminated flowers. The use of social visual cues by bumblebees should lead to higher disease avoidance efficiency when conspecifics are present and even more when present on the non-contaminated flowers.
Materials and Methods
Bumblebee maintenance and experiment preparation
Bumblebees from 3 different colonies were used for the experiment (Koppert Biological Systems, Berkel en Rodenrijs, The Netherlands). From each original colony, 1 batch of 5 and 2 batches of 20 marked bumblebees (with Opalithplättchen, ApisPro, Hohen Neuendorf, Germany) were housed in a metal cages (14.5 × 12 × 2.5 cm) containing empty honey pots on a wax frame, were provided with pollen ad libitum, and no sucrose solution was provided to motivate workers to forage. Each bee was trained to fly and feed on an artificial flower for 5 min, 3 times a day during a 1 day training period. The flower consisted of a blue foam paper (Ø 6 cm) glued onto a piece of wood placed on a plastic cylinder (Ø 2.8 cm, 4.5 cm length); an Eppendorf tube (0.2 mL) was placed in the center of the flower. The artificial flower was filled with a solution of honey water and washed after each foraging bout with ethanol (50%) (Leadbeater and Chittka 2009), and were scented with a geraniol solution (1:50 µL geraniol [99%, Roth]: HPLC grade water). Scenting artificial flowers with geraniol was done to encourage visitation (Leadbeater and Chittka 2009). No conspecific was present in this training, which could result in lack of social learning (Avarguès-Weber and Chittka 2014). The foraging training and experiment occurred in a flight arena (1 × 0.4 × 0.5 m terrarium, with the ground covered by green Kraft paper) with the flower placed toward the natural light source. After the foraging training, only the bumblebees that were observed feeding at least once were kept for the experiment. All the bumblebees were flower naive before foraging training.
Experimental set-up
Each bee was placed in a flight arena and given a choice between 2 artificial flowers (as described above), 10 cm apart and equidistant from the bumblebee entrance. Each group of bees was tested 4 times a day over a period of 4 days. In all flight arenas, the flower was washed after every foraging bout with ethanol (50%) in order to eliminate any cues that would help the bees choose between the 2 flowers, and were scented with a geraniol solution (1:50 µL geraniol [99%, Roth]: HPLC grade water) to mimic a natural floral scent, as in nature most flowers are scented (Raguso 2008). For each treatment, 1 flower was filled with a sucrose solution (50:50, v:v), and the other flower containing the same sucrose solution (50:50, v:v) including a concentration of 3000 cells * mL−1 of C. bombi (strain 076 provided by P. Schmid-Hempel, ETH Zurich).
Crithidia bombi was cultivated in cell cultures and cell number was quantified according to a standard method (Popp and Lattorff 2011; Fouks and Lattorff 2014). In order to avoid any odor or cue from the medium, C. bombi cells were washed 2 times with pure water before preparation of the sucrose solution. The position of flowers was switched regularly between the foraging bouts to avoid any position bias. The duration before the bee landed, where it landed (the contamination status of the flower, the flower position from the perspective of the bumblebee entrance into the foraging arena and the presence of a demonstrator), and the time period of feeding were recorded. When the bee spent more than 3 min without landing on a flower, it was placed back in its sub-colony.
To test how a bumblebee uses visual cues to avoid contaminated flowers, bumblebee workers were observed foraging on artificial flowers in 3 different treatments run in parallel (Supplementary Figure S1). The control treatment consisted of no visual cue on either flowers, and 2 treatments where a bumblebee conspecific was pinned onto a flower, either the contaminated or the non-contaminated one. Even if bumblebees were shown to avoid contaminated flowers, they were also prone to errors (Fouks and Lattorff 2011). That is why a bumblebee conspecific was pinned onto the contaminated flower to understand how naive forager behave, when seeing a conspecific making an error and feeding on contaminated flowers.
Control treatment
A total of 31 foraging bumblebees were recorded foraging in this treatment 3 (total number of visits: N = 34), 11 (N = 99), and 17 (N = 109) bumblebees originated respectively from Colonies I, II, and III. No conspecifics were pinned onto the flowers and therefore foraging bumblebees could not rely on a visual cue to choose a flower.
Visual cue on the non-contaminated or contaminated flower
In those treatments, a freshly snap frozen nest-mate bumblebee was pinned onto a flower serving as a conspecific. In 1 treatment, the conspecific was pinned onto the non-contaminated flower, whereas on the other treatment the conspecific was pinned onto the contaminated flower. The pinned bumblebee was changed after each foraging bout of the whole sub-colony. The first 3 days (referred to as the learning period), bumblebees were aided by a visual cue pinned with an insect-pin on the non-contaminated or contaminated flower. On the 4th day (referred to as the choice period), the conspecific was removed and no visual cue was provided to aid in flower choice and allowed ability to investigate a possible learning of the bumblebee through experience. We recorded behavior of bumblebees when a conspecific is present on a flower to understand how they are using such social cue to avoid contaminated flowers depending on which flower the social cue is situated (learning period). We then recorded their behavior when no cue is present to understand how their previous experience with the presence of a social visual cue influences later their behavior to avoid contaminated flowers (choice period). The treatment where the conspecific was pinned on the non-contaminated flower included 2 (N = 21), 10 (N = 84), and 15 (N = 93) bumblebees originated respectively from Colonies I, II, and III. The treatment where the conspecific was pinned on the contaminated flower included 4 (N = 31), 11 (N = 99), and 15 (N = 115) bumblebees originated respectively from Colonies I, II, and III.
Ethical note
All bumblebees originated from commercially-raised colonies. Bumblebee colonies were kept in the dark and only experienced day light when foraging. Bumblebees were tagged with natural resin applied on their thorax, avoiding any effect of the glue and tag on their behavior. When sacrificed, bumblebees were snap frozen (either with liquid nitrogen or moved in a freezer at −80°C) reducing distress. All bumblebees were sacrificed to further analyze their infection rate. All the bumblebees handling conformed to the legislation of experimental ethics in invertebrates and were performed to limit the impact of manipulation on bumblebee behavior and welfare.
Statistical analyses
Duration before landing on flowers
To test the effect of a visual cue on detecting contaminated or non-contaminated flowers, the time taken for a bumblebee to land on a flower during the learning period (3 first days) was compared using a generalized linear mixed model (GLMM) with a Gamma distribution as our data does not fit a normal distribution. This GLMM includes as fixed factors: flower contamination status (contaminated or not), flower position (left or right from the bumblebee), treatment (control, visual cue on contaminated or on non-contaminated flower), bumblebee experience (naive: fed on 1 flower type, i.e., contaminated or non-contaminated vs. expert: fed at least once on both flowers) and their interactions. Bumblebee experience was used as an explanatory variable to account for learning processes. Indeed, it was preferred to simple number of foraging bouts as it informs when a bumblebee can directly assess and compare reward of both flowers. This GLMM also includes as random factor: individual and colony ID of the bumblebee to account for pseudo-replication.
Frequency of visits on contaminated and non-contaminated flowers for each treatment
For each separate treatment, the frequency of flower visits was analyzed with a GLMM with a Poisson distribution to understand the overall efficiency of bumblebees to avoid contaminated flowers when no visual cue is present or when the visual cue is present on the contaminated or non-contaminated flower. This GLMM includes as fixed factors: flower contamination status, flower position and their interactions and as random factor: individual and colony ID to account for pseudo-replication.
Feeding duration
To test the effects of parasite contamination and the presence of a visual cue on the preferences of bumblebees, feeding duration was analyzed with a GLMM with a Gamma distribution as our data does not fit a normal distribution. This GLMM includes as fixed factors: flower contamination status (contaminated or not), treatment (control, visual cue on contaminated or on non-contaminated flower), period (learning [3 first days] or choice [last day]), foraging bout number (number of visits made by a bumblebee), and their interactions. Here, it was preferred to use foraging bout number as an explanatory variable as feeding duration can be influenced by temporal factors, especially after a period of starvation. This GLMM also includes as random factor: individual and colony ID of the bumblebee to account for pseudo-replication.
Effect of the visual cue on flower choice and its effect depending on the flower status
To understand the role of conspecific presence on the efficiency of bumblebee to avoid contaminated flowers, the value 0 was assigned when a bumblebee fed on a contaminated flower and 1 when it fed on a non-contaminated flower. This variable allows informed analysis on the ability of bumblebees to visit preferentially a non-contaminated flower. To understand if the presence or not of a conspecific on either contaminated or non-contaminated flower allows bumblebee foragers to perform better at avoiding contaminated flowers and then later when no cue is available (the choice period), the proportion of bumblebees feeding on the non-contaminated flower among each treatment was analyzed using a GLMM with a binomial distribution. This GLMM includes as fixed factors: treatment, flower position, period and their interactions and as random factors: individual and colony ID to account for pseudo-replication.
Moreover, to understand the impact of conspecific presence and flower contamination on the use of visual cue by foraging bumblebees, the value 0 was assigned when a bumblebee fed on a flower without conspecific and 1 when a conspecific is present. The proportion of bumblebees feeding on the flower with visual cue (including only treatments with visual cues) during the learning period was analyzed using a GLMM with binomial distribution. This GLMM includes as fixed factors: the treatment (visual cue on contaminated or non-contaminated flower), the foraging bout number, the position of the flower, and their interactions and as random factor: the individual and colony ID to account for pseudo-replication. Foraging bout number was used as an explanatory variable instead of bumblebee experience to better understand the temporal pattern of foraging in relation to the presence of a conspecific.
For all statistical analyses, the R v3.4.0 software was used (R Core Team 2017) with lme4 (Bates et al. 2015) and lmerTest (Kuznetsova et al. 2016) packages used for the GLMM calculation and model selections were automated using MuMIn package including comparisons with the null model (Bartoń 2016) using the Second-order Akaike Information Criterion (Anderson 2008). We then compared the best model with the null model using log-likelihood ratio tests (LRTs). Models were checked for over-dispersion with blmeco (Korner-Nievergelt et al. 2015), homogeneity of variances, and multi-collinearity with car (Fox and Weisberg 2011) packages, and the distribution of residuals was inspected visually. Significance of fixed factors of best models selected was obtained using Wald chi-square tests from analysis of deviance from the car package (Fox and Weisberg 2011). A post hoc Tukey test was run when the interaction of factors was significant in selected models using lsmeans package (Lenth 2016).
Results
Duration before landing on flowers
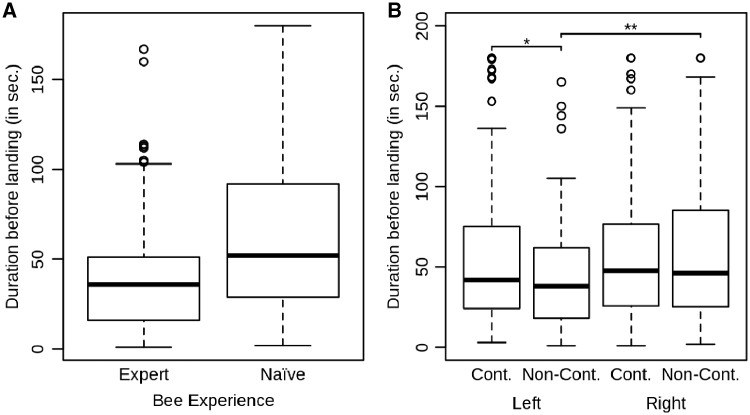
The time needed to discover a food resource is mainly dependent on bumblebee experience. Naive bumblebees needed longer to land on flowers compared with experienced bumblebees (Figure 1A). When the flower where the bumblebee landed was on their left side, they landed faster on the non-contaminated flower [mean ± standard deviation) SD = 44.5 ± 34.18 s, media N = 38 s] compared with the contaminated one (mean ± SD = 58.03 ± 49.76 s, media N = 42 s, post hoc Tukey test: z = −2.288, P < 0.05, Figure 1B); whereas when the flower was on their right side, there was no difference in duration before landing between both non-contaminated (mean ± SD = 59.73 ± 47.65 s, media N = 46 s) and contaminated flowers (mean ± SD = 56.95 ± 43.49 s, media N = 47 s, post hoc Tukey test: z = 1.429, P = 0.1529, Figure 1B). The model selection method gave the best model (Supplementary Table S1, LRT: df = 4, χ2 = 46.97, P < 0.001) as the model including bumblebee experience (df = 1, χ2 = 48.54, P < 0.001, Figure 1A), flower contamination status (df = 1, χ2 = 0.11, P = 0.745), flower position (df = 1, χ2 = 2.06, P = 0.152), and the interactions between flower contamination and position (df = 1, χ2 = 7.27, P < 0.01, Figure 1B) as fixed factors.
Figure 1.
Time spent by a bumblebee before landing on flowers among the 3 treatments. (A) Time spent by a bumblebee before landing on a flower in relation to the bumblebee’s experience (naive bumblebee fed only on 1 type of flower, expert bumblebee fed on both flowers). (B) Time spent by a bumblebee before landing on a flower in relation to flower contamination status and position. Box plots depict median, interquartile range, and non-outliers range; the dots represent the outliers. The dashed line represents the linear regression between feeding duration and foraging bout. Cont., contaminated flower; Non-Cont., non-contaminated flower. * and ** represent P-values below 0.05 and 0.01, respectively, according to post hoc Tukey tests.
Frequency of visits on contaminated and non-contaminated flowers for each treatment
Independent of the treatment, bumblebees were shown not to feed preferentially on any flowers in either position (right or left). For each treatment, the best GLMM was the null model showing no fixed factor (Supplementary Table S2).
Feeding duration
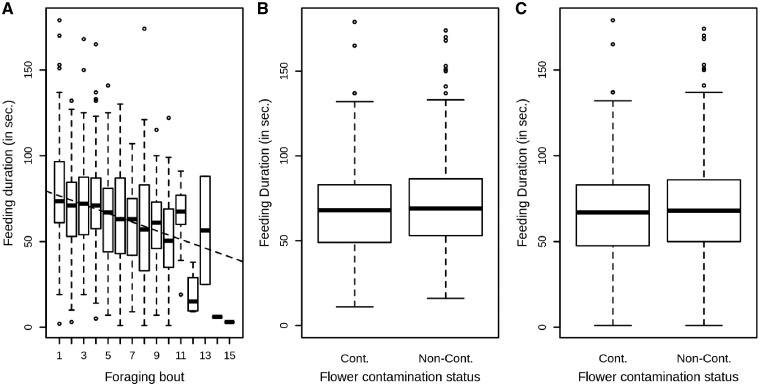
Overall, bumblebees showed a tendency to decrease significantly their feeding time periods on flowers as the number of foraging bout increased perhaps as a result of starvation experienced before the experiment and increased amount of food stored (Figure 2A). Moreover, contamination of the flower seemed to reduce feeding duration of bumblebees (Figure 2B). Indeed, the best GLMM included both foraging bout and contamination status as explanatory fixed factors (Supplementary Table S3, LRT: df = 2, χ2 = 26.43, P < 0.001). However, whilst the foraging bout effect on feeding duration was significant, flower contamination was not significant (foraging bout: df = 1, χ2 = 42.37, P < 0.001, contamination status: df = 1, χ2 = 3.07, P = 0.080, Figure 2A,C). Nevertheless, model residuals were not normally distributed and data transformations did not improve it. Residuals non-normality was due to outliers of feeding time below 11 s (21 outliers, 3.07% of the dataset), which when removed lead residuals to follow the normal distribution. While removing those outliers did not change the factors included in the best model, it made the flower contamination status to be significant (foraging bout: df = 1, χ2 = 33.85, P < 0.001, contamination status: df = 1, χ2 = 4.91, P = 0.027, Figure 2B). When removing outliers bumblebees spent significantly longer feeding on the non-contaminated flower (mean ± SD = 70.47 ± 27.49 Median=69 s) than on the contaminated one (mean ± SD = 67.93 ± 25.75 s, Median =68 s, Figure 2B).
Figure 2.
Time spent by a bumblebee feeding on flowers among the 3 treatments. (A) Feeding duration of a bumblebee on flowers in relation to the number of times (foraging bout) it fed on flower. (B) Feeding duration of a bumblebee on flowers in relation to flower contamination status without outliers below 11 s. (C) Feeding duration of a bumblebee on flowers in relation to flower contamination status with outliers below 11 s. Box plots depict median, interquartile range and non-outliers range; the dots represent the outliers. The dashed line represents the linear regression between feeding duration and foraging bout. Cont., contaminated flower; Non-Cont., non-contaminated flower.
Effect of the visual cue on flower choice and its effect depending on the flower status
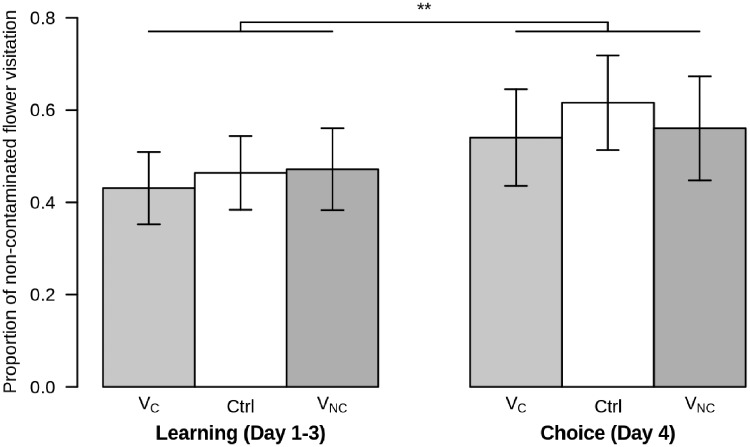
The investigation of the social visual cue role on the avoidance behavior of foraging bumblebees suggest that the presence of visual cue, regardless of the flower contamination status where the cue is present, does not increase their efficiency to avoid contaminated flowers (Figure 3 and 4A). In addition, bumblebees perform better at avoiding contaminated flowers on the last day after the learning period, when in all treatments the cue is no longer available (Figure 3). Furthermore even if treatments do not significantly impact bumblebee disease avoidance efficiency, on the last day foraging bumblebees who were never presented a social visual cue seem to have a higher success rate at avoiding contaminated flower compared with foraging bumblebees who experienced a social visual cue (Figure 3). Relying only on its personal experience may have led bumblebees to better distinguish parasite presence on flowers, as no other cue was available to choose the non-contaminated flower contrary to other treatments where bumblebees could choose flowers in relation to presence or absence of social visual cue present on it. They also perform better (in this last day) when the non-contaminated flower is placed on their left side (post hoc Tukey test: z = 3.079, P < 0.01). The best model included as explanatory variables the flower position, the period and their interactions (Supplementary Table S4, LRT: df = 3, χ2 = 18.97, P < 0.001, period: df = 1, χ2 = 8.74, P < 0.01; position: df = 1, χ2 = 2.87, P = 0.091; interactions: df = 1, χ2 = 6.65, P < 0.01).
Figure 3.
Proportion of visits by bumblebees on the non-contaminated flowers in relation to the period (learning [Day 1–3: 3 first days with social visual cue] vs. choice [Day 4: last day without cue]) and treatments. The histograms represent the means and bars represent their 95% confidence interval. VC, treatment with visual cue on the contaminated flower; Ctrl, control treatment; VNC, treatment with visual cue on the non-contaminated flower. ** represents P-values below 0.01 according to post hoc Tukey tests.
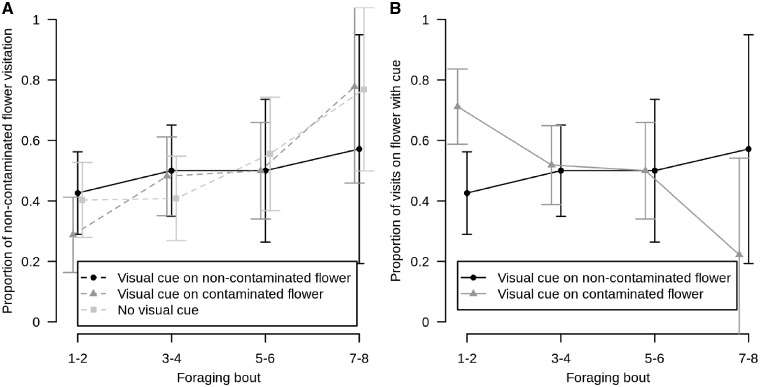
Figure 4.
Proportion of visits by bumblebees on the non-contaminated flower and on the flower with social visual cue among treatments during the learning period (the first 3 days). (A) Proportion of non-contaminated flower visitation during the learning period (Days 1–3) among the 3 treatments. (B) Proportion of visits by bumblebees on flowers with social visual cue in relation to the contamination status of the flower where the social visual cue is present. Only the learning period is represented (Days 1–3), since only during this period conspecifics were present on flowers. The points represent the means between the different bumblebees and treatment and bars represent their 95% confidence interval.
Moreover, bumblebees used the visual cue differently when it was present on the contaminated or non-contaminated flower. When the visual cue was on the contaminated flower, bumblebees on first foraging bouts utilized it, however, this reduced with increasing number of foraging bouts (Figure 4B). When the visual cue was present on the non-contaminated flower, an inverse pattern was observed (post hoc Tukey test: z = −2.125, P < 0.05, Figure 4B). Bumblebees at first seemed to avoid the flower with the visual cue, but with increasing number of foraging bouts increased their visits on flowers with the visual cue present (Figure 4B). The best model on visual cue flower visitation included the number of foraging bouts, the treatment and their interactions as explanatory variables (Supplementary Table S5, LRT: df = 3, χ2 = 10.37, P < 0.05, foraging bout number: df = 1, χ2 = 3.05, P = 0.081; treatment: df = 1, χ2 = 2.76, P = 0.097; interactions of foraging bout number and treatment: df =1, χ2 = 4.52, P < 0.05, Figure 4B).
Discussion
This study suggests that visual cues present on the flower in the form of a conspecific do not increase the ability of foraging bumblebees to initially detect parasites on contaminated flowers, and results show bumblebees rely primarily on their own experience to avoid contaminated flowers as they perform better on the last day when no cue is available. Nevertheless, these results indicate that bumblebees may use a combination of social visual cues and experience to avoid contaminated flowers across a longer time period. When conspecifics were present on the non-contaminated flower, bumblebees showed increase visitations across the foraging bout to the flower possessing the visual cue. However, in contrast, when conspecifics were present on the contaminated flower bumblebees tended to decrease visitations across foraging bouts to the flower with visual cue.
In contradiction to what was expected, the presence of a social visual cue on flowers contaminated with C. bombi or non-contaminated flowers does not increase the efficiency of bumblebees to recognize and avoid pathogen contaminated flowers. Bumblebees perform better avoiding contaminated flowers after the learning period and when the cue was removed. They also show higher efficiency when the non-contaminated flower were on their left side, in accordance with previous studies (Fouks and Lattorff 2011, 2013), certainly due to right-left asymmetries resulting in a preference for visiting a certain position (Anfora et al. 2011). However, the presence of a conspecific cannot be completely ruled out as improving pathogen avoidance efficiency. These results could be explained by 4 main factors. The foraging bumblebees were first trained to feed on flowers without the presence of a conspecific. The lack of conspecific in the first foraging bouts of a bumblebee results in lack of social learning (Avarguès-Weber and Chittka 2014). Therefore, bumblebees here may have not been able to associate flower contamination with the presence of a conspecific. In addition, the foraging bumblebees were not truly naive, as they were first trained to feed on flowers. Only naive bumblebees follow the choice of their conspecifics and therefore will feed on the same flowers as their conspecifics (Kawaguchi et al. 2007; Leadbeater and Chittka 2007; Baude et al. 2011). During this experiment, geraniol was added on all flowers to mimic natural flowers and to increase the number of bumblebees feeding on flowers. The presence of floral scent could have impaired parasite detection of foraging bumblebees and therefore the short period of experimentation was not long enough to observe significant differences. Indeed, it seems that bumblebees rely on olfactory cues to detect parasite and bacteria on flowers (Fouks and Lattorff 2013; Junker et al. 2014). Moreover, geraniol, in honey bees, leads to increased sucrose responsiveness and appetitive motivation (Barrachi et al. 2017). The presence of geraniol on flowers could then have led bumblebees to exhibit risky foraging behaviors and feed on flowers regardless of their contamination status. Contrary to previous experiments demonstrating the ability of bumblebees to avoid contaminated flowers (Fouks and Lattorff 2011, 2013), here our results demonstrate that regardless of the treatment bumblebees were not feeding preferentially on either flower. Nevertheless, bumblebees tend to reduce feeding duration on contaminated compared with non-contaminated flowers, as previously observed (Fouks and Lattorff 2013), demonstrating some disease avoidance reducing parasite uptake. The main difference between this study and Fouks and Lattorff (2013) study is the application, here, of floral scent on all flowers. Indeed, the same experimental setup has been used between both experiments (e.g., same foraging arena, flowers, control treatment, and training). The other main difference is the use of different colonies. However, despite different efficiency to avoid contaminated flower between bumblebee colonies, the less efficient colonies were still able to avoid contaminated flowers (Fouks and Lattorff 2011). The sample size being limited could also have decreased statistical power to obtain significant results. However, with similar sample size significant results have been found in Fouks and Lattorff (2013), demonstrating the ability of bumblebees to avoid contaminated flowers. Therefore, floral scents seem to be the main factor responsible for this lack of avoidance behavior, due to impairment of parasite detection and/or to increased sucrose responsiveness leading to risky foraging behaviors.
Nevertheless, the presence of a social visual cue triggers different foraging behavior in relation to the contamination status of a flower. Numerous studies have investigated choice of bumblebees when conspecifics are present on flowers (Worden and Papaj 2005; Kawaguchi et al. 2006, 2007; Leadbeater and Chittka 2007, 2009; Baude et al. 2011). While these studies differ in many aspects of their experimental design, they enable a complex understanding of foraging behavior in bumblebees. At first, naive bumblebees follow the choice of their conspecifics and therefore will feed on the same flowers as their conspecifics (Kawaguchi et al. 2007; Leadbeater and Chittka 2007; Baude et al. 2011). The strategy exhibited by naive bumblebees will be beneficial to reduce the time needed to find the first flower (Kawaguchi et al. 2007). Nevertheless, when a bumblebee is experienced or feeds on known flowers, it will tend to avoid conspecifics, seen as competitors (Kawaguchi et al. 2007; Baude et al. 2011). This foraging behavior exhibited by bumblebees is even more complex, since they need prior experience foraging with conspecifics to use social visual cues (Avarguès-Weber and Chittka 2014), they use social cues according to their previous experience (Leadbeater and Chittka 2009) and weigh the reliability of personal and social information (Dunlap et al. 2016). This leads to complex decision of bumblebees to find and feed on flowers depending on the floral cues, social cues (visual or scent), and their previous experience. Taken those findings into account, the results of this study demonstrate that bumblebees experiencing the visual cue on the non-contaminated flowers at first tend to avoid occupied flowers, whereas bumblebees experiencing the presence of the conspecific on the contaminated flower at first feed more often on flowers with the conspecific on it. The avoidance of conspecifics suggest that the foraging bumblebees perceive the conspecific as a competitor (Baude et al. 2011) or as threat as it is a dead conspecific (Abbott 2006). The latter explanation is not likely as dead conspecifics were previously and successfully utilized in other experiments (Kawaguchi et al. 2007). The copying behavior observed in foraging bumblebees from the treatment with visual cue on the contaminated flower reveals a naive behavior and local enhancement (Kawaguchi et al. 2007; Baude et al. 2011). The significant differing behavior observed between both treatments raises questions and suggests that the naive behavior observed in bees feeding on contaminated flowers with conspecific presence could be the result of increasing parasite infection rate over time. It is known that immune challenge and C. bombi infection in bumblebees impaired their ability to learn (Gegear et al. 2006; Alghamdi et al. 2008). Therefore, the copying behavior exhibited by bumblebees experiencing conspecifics on contaminated flowers could be the result of impaired learning due to a higher infection of C. bombi, explaining their tendency to rely more on conspecifics to discover flowers. This super-infection of C. bombi would be the result of food sharing inside the sub-colony, leading to a rapid immune response within a few hours (Erler et al. 2011).
Despite early contrasting behaviors observed between foraging bumblebees from the 2 treatments, with the increase of foraging bout numbers a behavioral convergence of foraging bumblebees tending to avoid contaminated flowers was observed. With bumblebees gaining experience they tend to avoid contaminated flowers irrespective on which flower the conspecific is present, suggesting the learning value of the visual cue. In the treatment where the conspecific is present on the non-contaminated flower bumblebees with experience tend to feed more often on the occupied flower, whilst bumblebees from the treatment where the conspecific is on the contaminated flower, experienced bumblebees decrease their visitation on occupied flowers (Figure 3). This suggests that bumblebees use social cues depending on their previous experience (Leadbeater and Chittka 2009). Nevertheless, further investigations with stronger disease avoidance phenotypes are needed to fully understand how bumblebees use social visual cues to avoid contaminated flowers, and could be expanded taking in account the infection status of the demonstrators and conflicting choice from multiple demonstrators.
To conclude, the presence of social visual cues does not seem to increase the efficiency of experienced bumblebees to detect and avoid contaminated flowers. Nevertheless, when bumblebees are truly naive the copying behavior they exhibit, in addition to saving time when finding flowers with good resources (Kawaguchi et al. 2007), may also reduce their chance of feeding on contaminated flowers, as experienced bumblebees in general prefer non-contaminated flowers (Fouks and Lattorff 2011). In addition, this study suggests that bumblebees can modulate the use of social information depending on their previous experience, as bumblebees through experience tend to avoid contaminated flowers irrespective of which flower the visual cue is present. Finally, the presence of floral scent on flowers seems to impair bumblebees to detect parasite present on flowers, and/or to increase their nectar responsiveness despite the presence of parasites. This raises questions about the role and impact of floral scent on disease avoidance by foraging pollinators. Many flowering plant species possess scented nectar, which may enhance pollinator attraction, deterrence of nectar robbers and florivores, antimicrobial activity, interactions with plant defense, and communication with predators and parasitoids (Raguso 2004). Then, scented nectar may also have evolved to decrease pollinator disease avoidance.
Supplementary Material
Acknowledgments
We would like to thank the Ecology class of 2012 from Martin Luther University of Halle-Wittenberg that helped us realize the experiments. We would also like to thank 3 anonymous reviewers for the helpful comments to improve the quality of our manuscript.
Funding
This work was supported by Bundesministerium für Bildung und Forschung (BMBF) (Federal Ministry of Education and Research, Germany) grant (FKZ: 0315126 to HMGL) and through support by the International Association for the Exchange of Students for Technical Experience (IAESTE) (to ER).
References
- Abbott K, 2006. Bumblebees avoid flowers containing evidence of past predation events. Can J Zool 84:1240–1247. [Google Scholar]
- Anderson DR, 2008. Model-Based Inference in the Life Sciences: A Primer on Evidence. Springer: New York. [Google Scholar]
- Anfora G, Rigosi E, Frasnelli E, Ruga V, Trona F et al. , 2011. Lateralization in the invertebrate brain: left-right asymmetry of olfaction in bumble bee Bombus terrestris. PLoS ONE 6:e18903.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alghamdi A, Dalton L, Phillis A, Rosato E, Mallon EB, 2008. Immune response impairs learning in free-flying bumble-bees. Biol Lett 4:479–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avarguès-Weber A, Chittka L, 2014. Local enhancement or stimulus enhancement? Bumblebee social learning results in a specific pattern of flower preference. Anim Behav 97:185–191. [Google Scholar]
- Avarguès-Weber A, Lachlan R, Chittka L, 2018. Bumblebee social learning can lead to suboptimal foraging choices. Anim Behav 135:209–214. [Google Scholar]
- Barracchi D, Devaud J-M, D’Ettorre P, Giurfa M, 2017. Pheromones modulate reward responsiveness and non-associative learning in honey bees. Sci Rep 7:9875.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoń K, 2018. MuMIn: Multi-Model Inference. 1.42.1. https://CRAN.R-project.org/package=MuMIn.
- Baude M, Danchin É, Mugabo M, Dajoz I, 2011. Conspecifics as informers and competitors: an experimental study in foraging bumble-bees. Proc R Soc B 278:2806–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S, 2015. Fitting linear mixed-effects models using lme4. J Stat Soft 67:35709. [Google Scholar]
- Cremer S, Armitage SAO, Schmid-Hempel P, 2007. Social immunity. Curr Biol 17:R693–R702. [DOI] [PubMed] [Google Scholar]
- Cremer S, Sixt M, 2009. Analogies in the evolution of individual and social immunity. Philos Trans Roy Soc Lond B Biol Sci 364:129–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daszak P, Cunningham AA, Hyatt AD, 2000. Emerging infectious diseases of wildlife: threats to biodiversity and human health. Science 287:443–449. [DOI] [PubMed] [Google Scholar]
- Dunlap AS, Nielsen ME, Dornhaus A, Papaj A, 2016. Foraging bumble bees weigh the reliability of personal and social information. Curr Biol 26:1195–1199. [DOI] [PubMed] [Google Scholar]
- Durrer S, Schmid-Hempel P, 1994. Shared use of flowers leads to horizontal pathogen transmission. Proc R Soc B 258:299–302. [Google Scholar]
- Erler S, Popp M, Lattorff HMG, 2011. Dynamics of immune system gene expression upon bacterial challenge and wounding in a social insect Bombus terrestris. PLoS ONE 6:e18126.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouks B, Lattorff HMG, 2011. Recognition and avoidance of contaminated flowers by foraging bumblebees Bombus terrestris. PLoS ONE 6:e26328.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouks B, Lattorff HMG, 2013. Social scent marks do not improve avoidance of parasites in foraging bumblebees. J Exp Biol 216:285–291. [DOI] [PubMed] [Google Scholar]
- Fouks B, Lattorff HMG, 2014. Comparison of two molecular diagnostic tools for the quantification of Crithidia bombi, a parasite of bumblebees. Entomol Exp App 150:191–197. [Google Scholar]
- Fox J, Weisberg S, 2011. An R Companion to Applied Regression. Thousand Oaks (CA: ): Sage. [Google Scholar]
- Gallai N, Salles JM, Settele J, Vaissière BE, 2009. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol Econ 68:810–821. [Google Scholar]
- Gegear RJ, Otterstatter MC, Thomson JD, 2006. Bumble-bee foragers infected by a gut parasite have an impaired ability to utilize floral information. Proc R Soc B 273:1073–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulson D, Nicholls E, Botías C, Rotheray EL, 2015. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347:1255957.. [DOI] [PubMed] [Google Scholar]
- Graystock P, Gouslon D, Hughes WOH, 2015. Parasites in bloom: flowers aid dispersal and transmission of pollinator parasites within and between bee species. Proc R Soc B 282:20151371.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatcher M, Dick J, Dunn A, 2012. Diverse effects of parasites in ecosystems: linking interdependent processes. Front Ecol Environ 10:186–194. [Google Scholar]
- Junker R, Romeike T, Keller A, Langen A, 2014. Density-dependent negative responses by bumblebees to bacteria isolated from flowers. Apidologie 45:467–477. [Google Scholar]
- Kawaguchi LG, Ohashi K, Toquenaga Y, 2006. Do bumble bees save time when choosing novel flowers by following conspecifics? Funct Ecol 20:239–244. [Google Scholar]
- Kawaguchi LG, Ohashi K, Toquenaga Y, 2007. Contrasting responses of bumble bees to feeding conspecifics on their familiar and unfamiliar flowers. Proc R Soc B 274:2661–2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A-M, Vaissière BE, Cane JH, Steffan-Dewenter I, Cunningham SA et al. , 2007. Importance of pollinators in changing landscapes for world crops. Proc R Soc B 274:303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korner-Nievergelt F, Roth T, Felten S, Guelat J, Almasi B et al. , 2015. Bayesian Data Analysis in Ecology Using Linear Models with R, BUGS and Stan. San Diego (CA): Academic Press. [Google Scholar]
- Kraus B, Page RE, 1998. Parasites, pathogens and polyandry in social insects. Am Nat 151:383–391. [DOI] [PubMed] [Google Scholar]
- Kuznetsova A, Brockhoff PB, Christensen RHB, 2017. lmerTest Package: Tests in Linear Mixed Effects Models. J Stat Soft 82: 1–26. [Google Scholar]
- Leadbeater E, Chittka L, 2005. A new mode of information transfer in foraging bumblebees. Curr Biol 15:R447–R448. [DOI] [PubMed] [Google Scholar]
- Leadbeater E, Chittka L, 2007. The dynamics of social learning in an insect model, the bumblebee Bombus terrestris. Behav Ecol Sociobiol 61:1789–1796. [Google Scholar]
- Leadbeater E, Chittka L, 2009. Bumble-bees learn the value of social cues through experience. Biol Lett 5:310–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leadbeater E, Chittka L, 2011. Do inexperienced bumblebee foragers use scent marks as social information? Anim Cogn 14:915–919. [DOI] [PubMed] [Google Scholar]
- Lenth RV, 2016. Least-squares means: the R Package lsmeans. J Stat Soft 69:4714. [Google Scholar]
- Lively CM, Dybdahl MF, 2000. Parasite adaptation to locally common host genotypes. Nature 405:679–681. [DOI] [PubMed] [Google Scholar]
- Popp M, Lattorff HMG, 2011. A quantitative in vitro cultivation technique to determine cell number and growth rates in strains of Crithidia bombi (Trypanosomatidae), a parasite of bumblebees. J Euk Microbiol 58:7–10. [DOI] [PubMed] [Google Scholar]
- Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O et al. , 2010. Global pollinator declines: trends, impacts and drivers. Trends Ecol Evol 25:345–353. [DOI] [PubMed] [Google Scholar]
- R Core Team, 2017. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. [Google Scholar]
- Raguso RA, 2004. Why are some floral nectars scented? Ecology 85:1486–1494. [Google Scholar]
- Raguso RA, 2008. Wake up and smell the roses: the ecology and evolution of floral scent. Annu Rev Ecol Evol Syst 39:549–569. [Google Scholar]
- Salathé M, Kouyos RD, Bonhoeffer S, 2008a. The state of affairs in the kingdom of the Red Queen. Trends Ecol Evol 23:439–445. [DOI] [PubMed] [Google Scholar]
- Salathé M, Kouyos RD, Regoes RR, Bonhoeffer S, 2008b. Rapid parasite adaptation drives selection for high recombination rates. Evolution 62:295–300. [DOI] [PubMed] [Google Scholar]
- Schmid-Hempel P, 1998. Parasites in Social Insects. Princeton (NJ: ): Princeton University Press. [Google Scholar]
- Schmid-Hempel P, 2001. On the evolutionary ecology of host-parasite interactions: addressing the question with regard to bumblebees and their parasites. Naturwissenschaften 88:147–158. [DOI] [PubMed] [Google Scholar]
- Schmid-Hempel R, Schmid-Hempel P, 2000. Female mating frequencies in Bombus spp. from Central Europe. Insectes Soc 47:36–41. [Google Scholar]
- Szabo ND, Colla SR, Wagner DL, Gall LF, Kerr JT, 2012. Do pathogen spillover, pesticide use, or habitat loss explain recent North American bumblebee declines? Conserv Lett 5:232–239. [Google Scholar]
- Worden BD, Papaj DR, 2005. Flower choice copying in bumblebees. Biol Lett 1:504–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.