Abstract
There is increasing interest in flies as potentially important pollinators. Flies are known to have a complex visual system, including 4 spectral classes of photoreceptors that contribute to the perception of color. Our current understanding of how color signals are perceived by flies is based on data for the blowfly Lucilia sp., which after being conditioned to rewarded monochromatic light stimuli, showed evidence of a categorical color visual system. The resulting opponent fly color space has 4 distinct categories, and has been used to interpret how some fly pollinators may perceive flower colors. However, formal proof that flower flies (Syrphidae) only use a simple, categorical color process remains outstanding. In free-flying experiments, we tested the hoverfly Eristalis tenax, a Batesian mimic of the honeybee, that receives its nutrition by visiting flowers. Using a range of broadband similar–dissimilar color stimuli previously used to test color perception in pollinating hymenopteran species, we evaluated if there are steep changes in behavioral choices with continuously increasing color differences as might be expected by categorical color processing. Our data revealed that color choices by the hoverfly are mediated by a continuous monotonic function. Thus, these flies did not use a categorical processing, but showed evidence of a color discrimination function similar to that observed in several bee species. We therefore empirically provide data for the minimum color distance that can be discriminated by hoverflies in fly color space, enabling an improved understanding of plant–pollinator interactions with a non-model insect species.
Keywords: chromatic signal, color model, floral color, fly pollination, plant–pollinator, vision
The hoverfly Eristalis tenax is a Batesian mimic of the honeybee and is also commonly termed the “dronefly.” Hoverflies belong to the Syrphidae, or “flower fly” family of Diptera, that actively forage on nectar and pollen-bearing flowers to derive nutrition (Holloway 1976; Gilbert 1981). The family has a ubiquitous, world-wide distribution, inhabiting all regions except Antarctica (Speight 2011). Hoverflies are becoming alternative pollinators of increasing interest for agricultural purposes (Jarlan et al. 1997; Rader et al. 2009; Jauker et al. 2012); for example, in southern Britain Eristalis spp. are an important pollinator of wild radish (Raphanus raphanistrum, Brassicaceae) and have been shown to influence flower coloration in this plant species (Kay 1976). In the Southern Hemisphere, flies including Eristalis spp. have been shown to be important introduced pollinators that can mitigate losses of native pollination vectors like bees (Stavert et al. 2018).
The increasing awareness of the contribution of flies as potentially important pollinators has been largely influenced by the global decline in wild and managed hymenopteran pollinator services (Potts et al. 2010; Inouye et al. 2015). It has been estimated that about 86% of angiosperms (Ollerton et al. 2011) and 35% of our food production crops require animal visitations to facilitate efficient pollination (Klein et al. 2007). Dipterans, in particular the families Syrphidae, Muscoidae, and Bombyliidae, have been noted as the second-most important pollinator suite after hymenopterans (Larson et al. 2001; Woodcock et al. 2014; Inouye et al. 2015).
Color vision in dipteran pollinators has been investigated using psychophysics experiments revealing color discrimination capabilities in E.tenax (Ilse 1949; Lunau and Wacht 1997), Bombylius fuliginosus (Knoll 1926), and Lucilia sp. (Fukushi 1989, 1990; Troje 1993). Electrophysiological studies have revealed that dipterans have the photoreceptors required for color vision, suggesting a potential tetrachromatic color vision system in E.tenax (Bishop 1974; Horridge et al. 1975; Tsukahara and Horridge 1977), Musca domestica, and Calliphora erythrocephala (Kirschfeld et al. 1978). Color vision has also been demonstrated in the fruit fly Drosophila melanogaster (Drosophilidae) (Menne and Spatz 1977; Schnaitmann et al. 2013), and many of the molecular mechanisms underpinning fly color processing have been carefully mapped using this model species (Morante and Desplan 2008).
The visual system of E. tenax contains multiple photoreceptors which are split up into 2 subsystems. The first subsystem comprises the photoreceptor classes R1–R6 which are principally responsible for achromatic vision and motion detection (Yamaguchi et al. 2008). The second subsystem is comprised of 4 spectral classes (R7p, R7y, R8p, and R8y) with ommatidia housing either a R7p/R8p tandem or a R7y/R8y tandem. This subsystem is thought to be primarily responsible for color vision via opponent processing of 4 input color signals (Troje 1993). Peak wavelength maxima for the 4 photoreceptors involved in color opponent processing are approximately: R7p 330 nm, R7y 340 nm, R8p 460 nm, and R8y 540 nm (Horridge et al. 1975). The achromatic and chromatic subsystems are typically thought to sample the electromagnetic spectrum independently. However, recent work on D. melanogaster mutants with restricted sets of functional photoreceptors also suggests that R1–R6 retinula cells may in some circumstances contribute to fly color perception (Schnaitmann et al. 2013).
Troje (1993) used narrow-band spectral filters in a laboratory environment to present dual-choice tests to Lucilia sp. and observed that over a wide range of reference wavelengths color stimuli were discriminated with a consistent proportion of correct choices, followed by sharp changes in choice frequency at certain wavelengths. These results led Troje (1993) to propose that blowflies have a categorical visual system where color discrimination occurs between specific hues mediated by a “simple color opponent mechanism” based on the understanding of the wiring of respective chromatic processing channels in flies. The R7p/R8p or the R7y/R8y color photoreceptor tandems integrate signals antagonistically, with the difference in the excitations of the photoreceptor tandems R7p/R8p, or the R7y/R8y, thought to be registered as either a positive or negative neural signal. For example, if the resultant difference in excitation between the R7p/R8p tandem is positive (p+), and the difference in the R7y/R8y (y+) tandem is also positive, then the stimuli eliciting this response would fall within a UV category of fly color space (Troje 1993). This opponent system thus creates 4 possible color categories: UV (p+y+), Blue (p−y+), Green (yellow appearance to humans; see methods below) (p−y−) and a theoretical Purple (p+y−) color category. Troje (1993) calculated that neutral excitation of the 2 photoreceptors within a tandem corresponding to the experimental boundaries of the 4 color categories: 407 nm for the R7p/R8p tandem separating the UV and Blue categories and 513 nm for the R7y/R8y tandem separating the Blue and Green categories. The Troje model for blowfly color vision has recently been employed to map and understand how flower visiting fly species (Syrphidae) may perceive the color signals presented by flowers (Arnold et al. 2009; Shuttleworth and Johnson 2010; Jersáková et al. 2012; Lunau 2014; Kelly and Gaskett 2014; Shrestha et al. 2016; Bergamo et al. 2018; Shrestha et al. 2019), although validation of categorical color processing for flower visiting flies remains outstanding (Shrestha et al. 2016).
The trichromatic color vision system in flower visiting hymenopteran pollinators is very well established (Briscoe and Chittka 2001) and has been extensively studied for over 100 years using psychophysics and electrophysiological methodologies (Dyer et al. 2011; Avarguès-Weber and Giurfa 2014). Frisch (1914) first demonstrated that honeybees were able to differentiate blue colored stimuli from gray shades of similar intensity using classical conditioning experiments; see Dyer et al. (2015) for review. There have since been significant advances in the methodologies used to assess color discrimination in hymenopteran pollinators (Chittka et al. 2003; Dyer and Chittka 2004a, 2004b; Dyer and Neumeyer 2005; Dyer et al. 2008, 2016). Bees trained with absolute conditioning only encounter a target color during training, which enables a coarse level of discrimination, while bees that learn a target color in the presence of distractors (termed differential conditioning) show improved learning performance (Dyer and Chittka 2004a; Giurfa 2004; Dyer and Neumeyer 2005). In addition, when color stimuli are viewed simultaneously using a star form appearing against a homogenous background presentation to enable edge color detection that excludes memory confounds, there is further improvement in color discrimination (Dyer and Neumeyer 2005). Using simultaneous viewing conditions and differential conditioning it has thus been possible to assess color discrimination of perceptually similar stimuli in Apis mellifera (Dyer and Neumeyer 2005), Bombus terrestris (Dyer et al. 2008), Tetragonula carbonaria, and Trigona cf. fuscipennis (Spaethe et al. 2014) and enable the construction of robust comparative models of bee pollinator discrimination (Garcia et al. 2017).
In the current study, we employ simultaneous color viewing conditions combined with differential conditioning to test the capacity of E.tenax to discriminate between perceptually similar color stimuli. Hence, we formally test if the categorical model of color discrimination developed by Troje (1993) for blowflies applies to flower visiting hoverflies. Specifically, we answer the questions: (i) can hoverflies discriminate the different color stimuli from the common background color, and if so, what is the discrimination threshold enabled by hoverfly color vision, and (ii) if there is evidence of discrimination within color categories, can relative color discrimination for similar colors in hoverflies be explained by a continuous monotonic function?
Materials and Methods
Location
Experiments were undertaken during February to April 2017 in the town of Daylesford located in the central highlands of Victoria (Latitude: 37.3 South, Longitude: 144 East; Altitude of 630 m a.s.l), Australia.
Eristalis tenax cohorts
Eristalis tenax individuals were obtained using standard rearing methodologies as outlined by Gladis (1994); for a more recent reference, methodologies have been revised and updated by Nicholas et al. (2018). Each cohort was housed indoors in separate 40 cm ×40 cm×40 cm (LWH) bug dorms purchased from BioFlyTech (2017). Two Ultracharge 1.2 m LED light tubes (1,700 lumens, 18 W, 240 V) were fitted into a standard Crompton tube batten. The light source was placed on a 14-h light/8-h dark cycle using a Crest 240 V 24-h timer. The mean temperature was 20.5°C (±2.9°C SD) and the mean relative humidity was 52.4% (±9.7% SD).
A total of 20 adult cohorts were created by placing 30 pupae into each bug dorm and the resultant naïve, emerging imagos formed the base cohorts for conditioning, training, and ultimately experimentation. Due to natural variability in emergence and survival, the final number of individuals per cohort colony ranged between 15 and 30 (mean 23 ± 5 SD).
Maintenance of E. tenax cohorts
The diet of E.tenax consisted of ground, desiccated Eucalyptus pollen (Saxonbee Enterprises 2018), 1 M sucrose solution, and honey. Water was supplied in a von Frisch type gravity feeder (Whitney et al. 2008).
Color stimuli
Simultaneous presentation color stimuli have previously been designed for testing honey bees (Dyer and Neumeyer 2005), bumblebees (Dyer et al. 2008), and stingless bees (T. cf. fuscipennis and T.carbonaria) (Spaethe et al. 2014). The same stimulus parameters were employed in the current study to enable some comparision between respective flower visitors. The stimuli consisted of a “neutral” gray (G0), 7 “blue,” and 7 “yellow” stimuli which were specified using the HSB color system (Table 1). For ongoing reference, color names in quotations (e.g., “blue” or “yellow”) refer to color appearance to a human observer; however, colorimetry was specified for fly perception and so respective color terms (and fly color space categories) are written in italics (e.g., Blue or Green). Color stimuli were printed using new ink cartridges in an HP Laser Jet 775F Enterprise printer on Planet Ark (80 g/m2) Bright White paper (CIE brightness 150), and laminated using UV transmitting GBC Signature 80 μm laminate. Reflectance spectra of all stimuli (Figure 1) were recorded using an Ocean Optics spectrophotometer (Ocean Optics, USA) equipped with a PX-2 pulsed xenon light source (Ocean Optics, USA) and calibrated with Ocean Optics standards following established procedures (Dyer et al. 2012; Shrestha et al. 2013). The xenon light source closely represents daylight conditions (Wyszecki and Stiles 1982; Garcia et al. 2017).
Table 1.
List of the HSB values used to create the color stimuli to assess the color discrimination capabilities of Eristalis tenax, corresponding x, y coordinates in the fly color space by Troje (1993 ) and Euclidean distances (ΔC) between G0 and each one of the colored stimuli
| Color step | HSB (H) | HSB (S) | HSB (B) | x | y | ΔC |
|---|---|---|---|---|---|---|
| B15 | 242 | 38 | 67 | −0.273 | 0.083 | 0.165 |
| B12 | 244 | 29 | 64 | −0.242 | 0.069 | 0.131 |
| B9 | 248 | 18 | 61 | −0.177 | 0.058 | 0.069 |
| B7 | 254 | 11 | 60 | −0.167 | 0.054 | 0.058 |
| B5 | 300 | 3 | 58 | −0.109 | 0.044 | 0.030 |
| B3 | 32 | 8 | 61 | −0.116 | 0.027 | 0.012 |
| B1 | 44 | 16 | 64 | −0.119 | 0.016 | 0.003 |
| G0 | 47 | 19 | 65 | −0.122 | 0.017 | 0.000 |
| Y1 | 49 | 22 | 67 | −0.134 | 0.006 | 0.016 |
| Y3 | 51 | 28 | 70 | −0.113 | −0.002 | 0.021 |
| Y5 | 52 | 34 | 73 | −0.114 | −0.008 | 0.026 |
| Y7 | 52 | 39 | 76 | −0.132 | −0.022 | 0.040 |
| Y9 | 53 | 44 | 80 | −0.132 | −0.040 | 0.058 |
| Y12 | 54 | 50 | 84 | −0.149 | −0.054 | 0.076 |
| Y15 | 54 | 56 | 89 | −0.197 | −0.066 | 0.112 |
Figure 1.
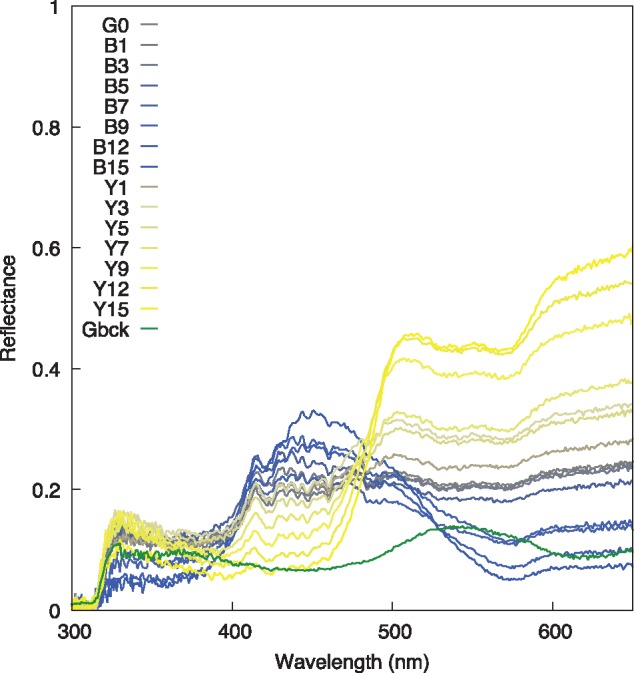
Spectral reflectance curves of the 15 color stimuli specified in Table 1. Long wavelength rich stimuli are of “yellow” appearance with Y15 having the peak reflectance above 600 nm, and progress through to the “blue” stimuli with B15 having the lowest reflectance above 600 nm. The background green color spectrum is also shown (Gbck).
The Troje (1993) opponent color theory for the blowfly Lucilia sp. is currently the only accepted basis for a color space model of fly vision that allows for quantitative analyses of flower signals (Arnold et al. 2009; Ohashi et al. 2015; Shrestha et al. 2016). We employed this model assuming a von Kries adaptation of daylight to a leaf green background and photoreceptor sensitivities for E.tenax (Lunau 2014), as described in detail (Shrestha et al. 2016). The fly color space thus has 4 quadrants, and the stimuli (Figure 1) predominantly lay in the Blue or Green regions (or categories) of the color space (Figure 2). Color distances, calculated as Euclidean distance in the color space as this is the current best practice for hymenopteran pollinators (Chittka 1992; Garcia et al. 2017, 2018), are presented in Table 1.
Figure 2.
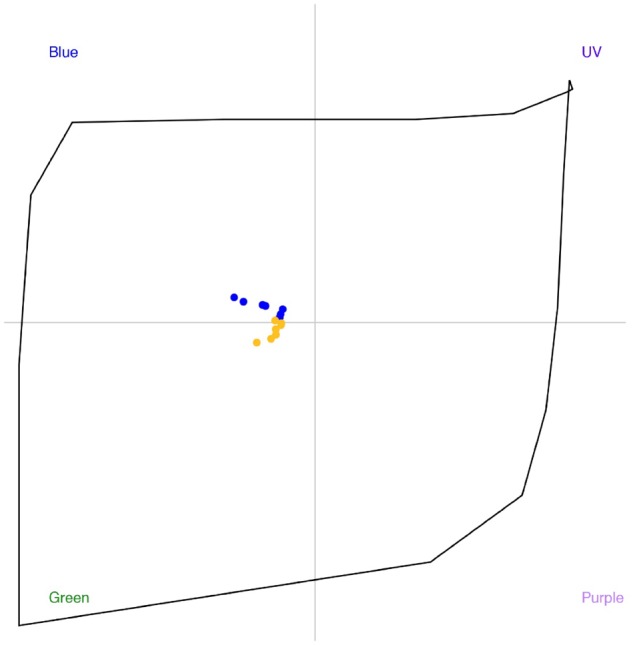
Fly color space (Troje 1993; Lunau 2014) and the loci of stimuli specified in Table 1. The quadrants of the color space indicate the 4 color categories (Blue, Green, UV, or Purple) defined based on psychophysics for the blowfly (Lucilia sp.). Blue markers indicate the color stimuli (B1, B3, B5, B7, B9, B12, and B15) and yellow markers the color stimuli (Y1, Y3, Y5, Y7, Y9, Y12, and Y15).
Preconditioning of E. tenax
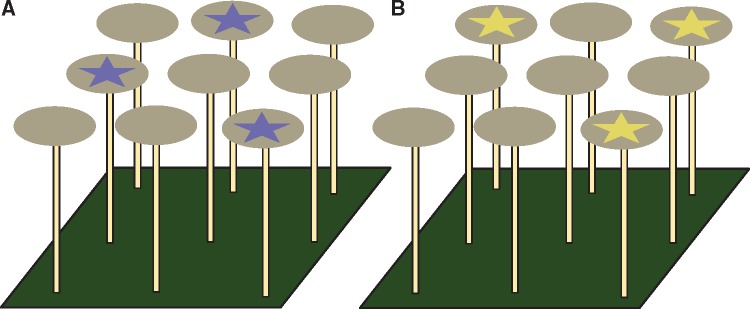
After extensive pilot trials, a preconditioning phase was implemented firstly, to begin to expose cohorts to the stimuli B15 or Y15 (50 mm diameter circular discs) and secondly, to supply young imagos with ample food and water to ensure flies were sufficiently healthy to then be conditioned to make color choices in free-flying tests. Ten cohorts were separately mass trained to B15 stimuli and another 10 cohorts were trained to Y15 stimuli. Vertically raised platforms presenting either 9 B15 or 9 Y15 stimuli were placed into each bug dorm. Platforms were made up of 9 55 mm diameter Petri dishes (within which circular stimuli were placed) and were mounted on top of 9 vertical 200 mm bamboo posts. The bamboo posts (diameter 5 mm) were fixed into a 300 mm ×300 mm “green” colored base. Ample sucrose, pollen, and honey were placed on top of each stimulus, and the fliess were able to feed and drink ad libitum for a minimum period of 3 days.
Conditioning E. tenax cohorts to stimuli
Part 1. Commencement of differential conditioning began with simultaneous presentation of star stimuli on raised platforms. Conditioning stimuli (9 B15 or Y15, star shape on a G0 background) were placed into Petri dishes as previously described and rewarded with a mixture of appetitive stimuli including ground pollen, a drop of 1 M sucrose solution, and a drop of honey. The honey was found to be initially important to encourage feeding, but the amount was reduced toward zero as the flies began regularly visiting stimuli to feed. Cohorts were placed outdoors and flies were allowed to forage on the rewarded stimuli throughout the day. Food was topped up as required. During the evening, cohorts were returned indoors where platforms were removed and water was replaced.
Part 2. During the day, cohorts were placed outdoors and were allowed to acclimatize to the environment. Following this period, the cohorts were presented with a set of 9 discs; 6 of the discs were G0 (non-rewarding) and the remaining 3 discs (rewarding) had either B15 or Y15 star-shape on a G0 background. The position of the star discs was randomly assigned among the G0 stimuli (Figure 3). A drop of 1 M sucrose was applied to the center of each star and flies were allowed to forage for 1 h. Sucrose was then topped up and 1.25 mL of ground pollen was placed next to the sucrose. Cohorts were then left to forage for another 2 h. After this 3-h period, water was replaced and platforms were removed in order to restrict food intake. Cohorts were returned indoors during the evening.
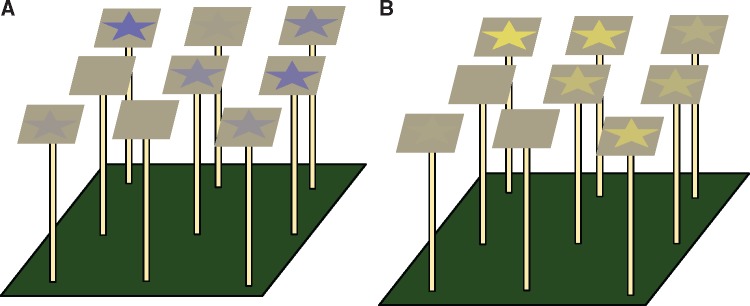
Figure 3.
Differential conditioning where Eristalis tenax flies were exposed to either “blue” star (B15) or “yellow” star (Y15) stimuli among G0 stimuli mounted on the feeding platform. (A) Diagram of B15 star circular disc stimuli on a G0 background. (B) Diagram of Y15 star circular disc stimuli on a G0 background. Stimuli positions were randomized between trials.
Color discrimination experiments
All experiments were undertaken between 11:00 and 18:30 in full sun (Wyszecki and Stiles 1982; Garcia et al. 2017), which proved successful for promoting participation in a non-rewarded test based on pilot experiments. Mean temperature was 23.4°C (±2.9°C SD) and mean relative humidity was 50.9% (±19.0 SD). The mean number of flies used in each experiment was 18 flies (±4 SD). Cohort testing order for “blue” or “yellow” stimuli was randomized, and each cohort was only tested once. Twenty-four hours prior to experimentation, cohorts were supplied with water only from the von Frisch feeder so that individual flies were hungry, but not dehydrated. This procedure was very important to ensure survival and motivation to participate in a non-rewarded experiment.
“Blue” trained cohorts were presented with 9 square 50 mm ×50 mm unrewarded stimuli mounted on top of the 9 bamboo posts: 2 G0 and 1 of each of the “blue” star stimuli (B1, B3, B5, B7, B9, B12, and B15) (Figure 4). “Yellow” trained cohorts similarly presented with 9 square unrewarded stimuli: 2 G0 square and 1 of each of the “yellow” star stimuli (Y1, Y3, Y5, Y7, Y9, Y12, and Y15) (Figure 4). Placement of stimuli was random for each experiment. The number of flies that landed on each stimulus was recorded over a 60-min period. Each cohort was only tested once to avoid pseudo replication.
Figure 4.
Color discrimination experiments. (A) A diagram example of an experimental set up for “blue” trained E. tenax. Two G0 stimuli and B1, B3, B5, B7, B9, B12, and B15 star stimuli (on a G0 background). (B) A diagram example of an experimental set up for “yellow” trained E. tenax. Two G0 stimuli and Y1, Y3, Y5, Y7, Y9, Y12, and Y15 star stimuli (on a G0 background). Stimuli position was randomized between trials.
Statistical analysis
To test if hoverflies can discriminate the differently colored stimuli from the common background color, we initially pooled the choices of the 10 different cohorts for the “blue,” or “yellow” stimuli, and used a Chi-square (χ2) statistic to test for potential differences between the frequencies of choices. We subsequently performed a test of equal proportions (Newcombe 1998) to test if the probability of choices observed for each stimulus within each set was greater than the probability of choosing the achromatic target G*. We used G* as a baseline value obtained after averaging the total frequency of landings observed for the respective neutral “gray” G01 and G02 stimuli present in experiments for either the “blue” or “yellow” stimuli tests. Chi-square and equal proportion tests were performed in R release 3.4.3 (R core team). As there was evidence that hoverfly color vision was capable of fine color discrimination within either the Blue or Green categories, we proceeded to test if relative color discrimination for similar colors in hoverflies could be explained by a continuously increasing monotonic function. To enable this we fitted 2 separate generalized linear mix models (GLMMs), 1 for each color stimulus set, using as response variable the proportion of landings observed for each target present in a stimulus set and as a fixed predictor the color dissimilarity between each colored stimulus and the achromatic target. We accounted for any potential variability between the groups of flies recruited from different cohorts by including cohort as a random term in the model determining the correlation structure between the responses observed for each group (Zuur et al. 2013). We assumed that the proportions followed a beta-distribution and used a logistic link function for our model (Zuur et al. 2013). As the hoverflies were presented with more than 2 options in each experimental observation, the proportion of choices could not be modeled assuming a binomial distribution. The use of a beta-distribution allowed us to model the proportion data directly while accounting for potential over-dispersion of the model (Zuur et al. 2013).
We fitted the model using Bayesian Markov chain Monte-Carlo techniques with Just Another Gibbs Sampler (JAGS) version 4.02 executed from the R language and environment for statistical computing release 3.4.3. The final model was constructed with 3 chains, 110,000 iterations, a burn-in of 10,000 iterations, and a thinning interval of 10. Diffuse normal priors were used for all the coefficients (Zuur et al. 2013).
Results
We first tested if E.tenax exhibited categorical color visual processing as might be expected based on the fly categorical color model proposed by Troje (1993). If this hypothesis is true, it would be expected that within a color category (e.g., Blue, Green [“yellow”], UV or Purple (Figure 2) there should be consistent choices for stimuli that can be discriminated within that category. Alternatively, if color vision is not categorical, there would be different levels of choices for stimuli above discrimination threshold within a category. Results from the Chi-square test indicate that the frequency of choices for the 9 targets making up the blue or yellow stimuli set is significantly different from each other (χ2blue =69.0, df =8, P<0.001; χ2yellow =628, df =8, P<0.001) suggesting that color discrimination in E. tenax is not consistent with the categorical color discrimination model proposed by Troje for the blowfly. Furthermore, results from the one proportion tests performed on the frequency of landing for each colored stimuli relative to G* (Table 2) indicate that the frequency of choices for the colored stimuli relative to G* increases with color dissimilarity (Figure 5).
Table 2.
Results of the proportion tests for each color stimulus
| Stimulus | Mean probability of landings (95 % CI) | χ2 | df | P-value |
|---|---|---|---|---|
| B1 | 0.107 (0.079, 0.144) | 13.5 | 1 | <0.05 |
| B3 | 0.079 (0.055, 0.112) | 2.1 | 1 | 0.074 |
| B5 | 0.079 (0.055, 0.112) | 2.1 | 1 | 0.074 |
| B7 | 0.144 (0.111, 0.184) | 43.1 | 1 | <0.001 |
| B9 | 0.113 (0.084, 0.150) | 17.0 | 1 | <0.001 |
| B12 | 0.135 (0.104, 0.175) | 34.8 | 1 | <0.001 |
| B15 | 0.223 (0.182, 0.269) | 164.0 | 1 | <0.001 |
| Y1 | 0.034 (0.022, 0.052) | 2.3 | 1 | 0.065 |
| Y3 | 0.068 (0.050, 0.092) | 45.6 | 1 | <0.001 |
| Y5 | 0.068 (0.050, 0.092) | 45.6 | 1 | <0.001 |
| Y7 | 0.107 (0.084, 0.135) | 164.0 | 1 | <0.001 |
| Y9 | 0.100 (0.078, 0.127) | 137.0 | 1 | <0.001 |
| Y12 | 0.152 (0.124, 0.184) | 388.0 | 1 | <0.001 |
| Y15 | 0.424 (0.384, 0.466) | 3830.0 | 1 | <0.001 |
For each stimulus the frequency of landings was tested versus the null hypothesis of equal landings on the plain grey, G* stimuli.
Figure 5.
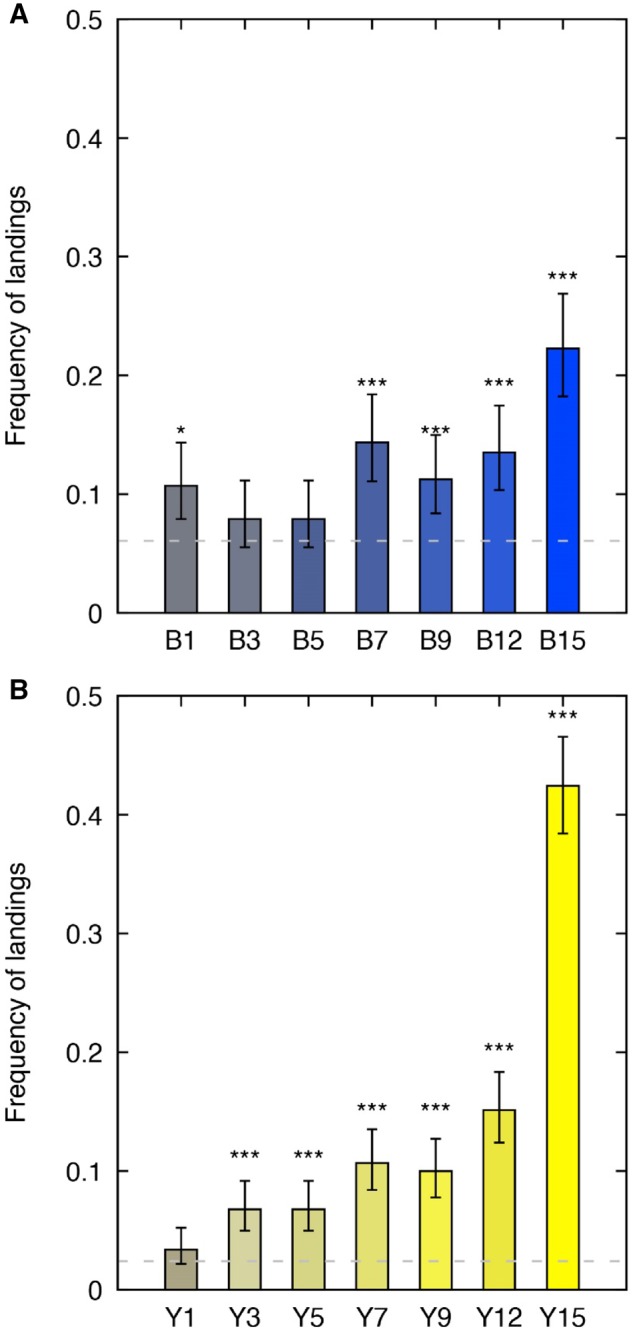
Mean frequency of landings observed for each of the (A) “blue” colour stimuli; and (B) the “yellow” color stimuli. The x-axis shows the name of each stimulus as per Table 1. Stimuli were sorted in an increasing order of distance from G0 (ΔC in Table 1). Gray shaded line indicates the respective frequency of landings for G* used as baseline for the statistical analyses. (*) indicates P-values=0.05 and (***) indicates P-values<0.001 as reported in Table 2.
Indeed, 5 of the 7 “blue” color stimuli (B1, B7, B9, B12, and B15) and 6 out of the 7 “yellow” color stimuli (Y3, Y5, Y7, Y9, Y12, and Y15) were chosen significantly more frequently than the achromatic G* stimulus (Figure 5). These results further support the idea that color discrimination in E. tenax is not categorical.
The GLMM describing the relationship between color discrimination and color distance for the “blue” and “yellow” stimuli indicates that color distance has a strong effect on the frequency of landings in E. tenax (Table 3 and Figure 6), and that there is a potential difference between the discrimination functions for the respective color stimuli.
Table 3.
Mean and 95% credibility intervals of the posterior distribution of the fixed terms defining a beta-generalized linear mixed model (beta-GLMM) describing the effect of color difference on the proportion of landings for a set of “blue” and “yellow” color stimulus by E. tenax
| “Blue” | “Yellow” | |
|---|---|---|
| βo (Intercept) | −2.42 (−2.69, −2.14 95% CI) | −3.11 (−3.39, −2.82 95% CI) |
| β1 (Slope) | 8.50 (5.80, 11.0 85% CI) | 25.3 (21.4, 29.1 95% CI) |
βi are coefficients of a linear expression η=βo+β1 * (ΔC) relating the color distance (ΔC) between a stimulus and an achromatic target by means of the function π(ΔC)=exp(η)/(1 + exp(η)) (Hardin et al. 2007). The function π(ΔC) for the “blue” and “yellow” stimuli is presented in Figure 6.
Figure 6.
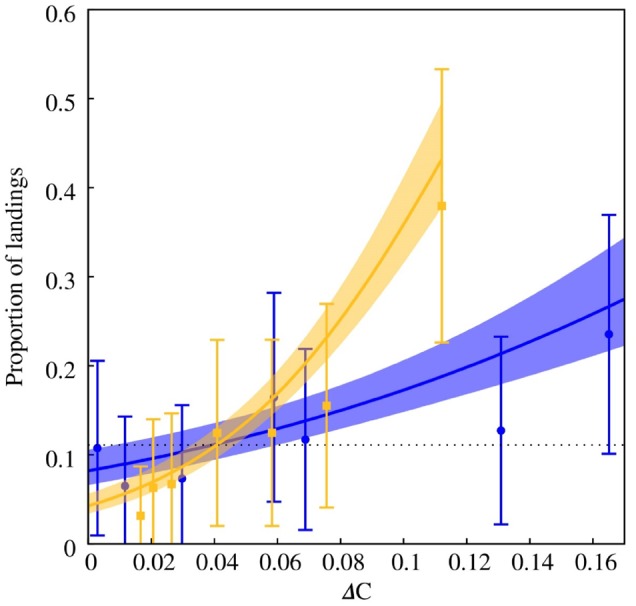
Generalized linear mixed model describing the effect of color dissimilarity on the proportion of landings for various “blue” (filled circles) and “yellow” stimuli (filled squares) by the hoverfly E. tenax. Solid lines represent the best fit model to the respective data set and shaded areas the 95% credibility intervals for each model. Markers indicate the mean proportion of landings observed for each colored stimulus during the experiment and error bars represent the standard error of the mean. Mean posterior distribution for the coefficients defining the fixed terms of the 2 functions is given in Table 3. The posterior distributions of the terms describing the random effect of the 10 different fly cohorts on the color discrimination model for the “yellow” and “blue” stimuli are provided as Supplementary Material S-1. Histograms for the random terms for each cohort suggest that there is not a major difference in variability between cohorts for either the blue or yellow stimuli.
Discussion
The hoverfly E.tenax is an important pollinator of flowering plants in both nature (Zoller et al. 2002; Fontaine et al. 2006) and agriculture (Ssymank et al. 2008; Rader et al. 2009; Stanley et al. 2013). Simultaneous color discrimination experiments with E.tenax show that these pollinators are capable of discriminating the majority of either “blue” or “yellow” color stimuli from the G0 stimulus (Figure 5 and Table 2). However, there was no evidence of a step function that would be associated with categorical processing. Indeed, we found behavioral choices were explained by a continuous monotonic function (Figures 5 and 6) rather than a categorical type response as predicted by the model of fly color vision developed for the blowfly (Troje 1993). The assumption of a categorical type color discrimination process predicts that all the individual stimuli making up a test set should be selected for, or rejected, with the same frequency. However, both the proportion test and the regression model reject this hypothesis for E.tenax. Interestingly, the “blue” color stimuli we employed in the current study do conveniently all fall within the Blue region of the Troje color space, and our “yellow” stimuli predominantly lie within the Green region of the color space, with stimulus Y1 having a locus on the boundary (Figure 2). If color choices by hoverflies were categorical, we would have expected all stimuli within the respective Blue or Green regions to be chosen at an equivalent level; or at least a step function should have existed somewhere along the continuum of colors if the categorical boundary was slightly different to what the model predictions were for our stimuli. For example, in goldfish Carassius auratus there is a sharp change in correct choices when these animals categorize colors (Poralla and Neumeyer 2006). However, our evidence that within either the Blue or Green regions of fly space hoverflies can discriminate similar colors (Figures 5 and 6) does not exclude the possibility that for the processing of dissimilar colors flies may use categorical processing, analogous to how humans perceive green, red, yellow, and blue as distinct categories (Hering 1920; Hurvich and Jameson 1957; Mollon and Jordan 1997; Kemp et al. 2015).
Given that hoverflies do not show evidence for a categorical color visual system, it is interesting to consider how their color vision should be taken into account for modeling flower colors. von Helversen (1972) hypothesized that for honeybee color choices of similar colors there should be a continuous function describing the relationship between color distances from a known target color, and the probability with which similar colors might be selected by a foraging insect. This theory was proven for honeybees by Dyer and Neumeyer (2005), and for 4 important flower visiting hymenopteran species it has recently been shown that sigmoidal functions reliably predict psychophysics results from dual-choice, flying bee experiments (Garcia et al. 2017). In the current study of hoverflies, the primary aim was to test for evidence of categorical color processing which required the simultaneous presentation of multiple stimuli, while psychophysics discriminations are more typically measured with dual choice presentations of stimuli (Dyer 2012a). Nevertheless, it is possible to obtain from the data some insights into how hoverflies do discriminate color information. The evidence that hoverflies can discriminate small color distances within a color region of the fly color space, but also the fact that with increasing color separation between the target color and the similar colors there is a significant change in choices, fits with the framework of stimulus difference proposed by von Helversen (1972), and with the biological significance of pollinator color vision empirically demonstrated for bumblebee pollinators by Dyer and Chittka (2004c). This framework suggests that even though a pollinator may be capable of discriminating fine color differences, it would be advantageous for competing flower species to increase color distance to maintain flower constant visits from biologically important flower visitors. Thus, we suggest, based on our data, that hoverflies can make small color judgments of colors with a threshold limit in the Green and Blue region of fly color space (Figure 5), but that the error rate of correct choices is not a simple step function (Figure 6) and therefore there is a range over which color information is used.
Interestingly, there was a significant difference in the rate of change for colors from the “blue” or “yellow” stimuli. Hoverfly discrimination of “yellow” stimuli was also significantly better than that for “blue” stimuli (Figure 6). This finding coincides with previous findings that hoverflies have innate preferences for “yellow” stimuli (Ilse 1949; Kugler 1950; Lunau and Wacht 1997; Lunau 2014; Lunau et al. 2018), and with recent evidence for Camponotus blandus ants that innate preferences can improve learning rates and discrimination in insects (Yilmaz et al. 2017). This effect also reveals a difference in the color distance required for the respective regions of the fly color space, which aligns with data for several bee species (Garcia et al. 2017) and humans (MacAdam 1942; Macadam 1985) that color space may not be equidistant for color measurements. We thus propose that the Troje model of fly color processing is a useful template for mapping how fly pollinators might perceive flower colors and suggest from the Euclidean distances in Table 2, that distances of 0.059 units for the blue stimulus B7 and 0.021 units for yellow stimulus Y3 are useful guides for interpreting reliable discrimination of color differences from the G0 stimulus. However, discrimination accuracy does also further increase with color salience (Figures 5 and 6), which is likely to be important for judgments of flower colors in natural conditions (Garcia et al. 2017, 2018). Now that it is understood that hoverflies can be conditioned in experiments, it would be valuable in the future to test how pairs of colors are discriminated, as has been done for several bee species.
The evidence of hoverflies’ capacity for fine color discrimination, and for blowflies’ simple color categorical visual system, suggests that environmental factors are likely to be important drivers for the evolution of color vision specific to the needs of animals in their environments. Eristalis tenax are important flower visitors and pollinator of many species (Lucas et al. 2018), and it is known that flowers have natural variability in pigmentation (Dyer et al. 2012; Garcia et al. 2018; van der Kooi et al. 2018) that requires some capacity to generalize colors to avoid accidental misclassification of a rewarding target color as an incorrect flower. However, flower visitors must also deal with the problem of avoiding similar colors that may be non-rewarding mimicss (Dafni 1984; Dyer and Murphy 2009), and so a fine color discrimination system is also important. The blowfly Lucilia sp. is necrophagous species mainly feeding on carrion and feces as a primary protein source required for development and oocyte maturation in females. Olfaction appears to be an important orientation cue (Yan et al. 2018) to efficiently locate food sources that may be random and ephemeral within a complex environments (Wall et al. 2002), and it is unlikely that fine color discrimination would serve much benefit in this task. Carbohydrates are also obtained from flowers and blowflies have been observed to also be flower visitors, however pollen load is often low in comparison to E.tenax and hymenopteran pollinators (Rader et al. 2009; Gaffney et al. 2018). Thus, our observations fit with a broad framework of how ecological conditions may shape the visual capabilities of particular species (Lythgoe 1979).
While there has been a considerable body of work on how bee flower visitors discriminate color (Chittka et al. 2003; Dyer and Chittka 2004a, 2004b; Dyer and Neumeyer 2005; Dyer et al. 2008, 2016; Garcia et al. 2017, 2018), there is a relative paucity on how flower flies use color information. One reason for this is that bees are central place feeders and it is thus possible to collect a considerable amount of data on free flying individuals (Dyer 2012b), while hoverflies are typically feeding for themselves and so become easy satiated in an experiment. To overcome this issue, we had to develop a very long procedure to rear flies that were naïve to flowers, and that would eventually enable differential conditioning to a simultaneous color discrimination task, so that we could understand color processing for broad-band color stimuli. While this is a difficult multi-day conditioning procedure, we believe the method will provide experimental access to testing more fly species to enrich our understanding of these important pollinators.
There is currently enormous interest in flower community studies and in how different pollinators may contribute to the evolution of visual or olfactory signals (Kantsa et al. 2018; Garcia et al. 2019; Howard et al. 2019). To tackle such complex phenomena, it is important to have quality empirical data to understand how animals use color information. In this study, we show that hoverflies can discriminate fine color differences, and also that they have a continuous monotonic function mediating choices depending upon color differences. This information should be of high value for future studies attempting to understand how flower flies interact with colors in complex communities.
Author Contribution
L.H., A.G.D., A.D., and M.B. established the aims and designed the experiments. L.H. and A.G.D. collected the data. L.H. and J.E.G. analyzed the data. L.H., A.G.D., A.D., and J.E.G. wrote and/or edited the manuscript.
Supplementary Material
Acknowledgments
A.G.D. and A.D. acknowledge the Australian Research Council (DP130100015 and DP16010016). We would also like to acknowledge Dr. Mani Shrestha for assistance and experimentation.
References
- Arnold SEJ, Savolainen V, Chittka L, 2009. Flower colours along an alpine altitude gradient, seen through the eyes of fly and bee pollinators. Arthropod-Plant Inte 3:27–43. [Google Scholar]
- Avarguès-Weber A, Giurfa M, 2014. Cognitive components of color vision in honey bees: how conditioning variables modulate color learning and discrimination. J Comp Physiol A 200:449–461. [DOI] [PubMed] [Google Scholar]
- Bergamo PJ, Telles FJ, Arnold SEJ, de Brito VLG, 2018. Flower colour within communities shifts from overdispersed to clustered along an alpine altitudinal gradient. Oecologia 188:223–235. [DOI] [PubMed] [Google Scholar]
- BioFlyTech, 2017. BioFlyTech ONLINE Alicante. Spain. Available from: http://bioflytech.com/en/.
- Bishop LG, 1974. An ultraviolet photoreceptor in a Dipteran compound eye. J Comp Physiol A 91:267–275. [Google Scholar]
- Briscoe AD, Chittka L, 2001. The evolution of color vision in insects. Annu Rev Entomol 46:471–510. [DOI] [PubMed] [Google Scholar]
- Chittka L, Dyer AG, Bock F, Dornhaus A, 2003. Bees trade off foraging speed for accuracy. Nature 424:388.. [DOI] [PubMed] [Google Scholar]
- Chittka L, 1992. The colour hexagon: a chromaticity diagram based on photoreceptor excitations as a generalized representation of colour opponency. J Comp Physiol A 170:533–543. [Google Scholar]
- Dafni A, 1984. Mimicry and deception. Annu Rev Ecol Syst 15:259–278. [Google Scholar]
- Dyer AG, 2012a. Psychophysics of honey bee color processing in complex environments In: Galizia C, Eisenhardt D, Giurfa M, editors. Honeybee Neurobiology and Behavior. Dordrecht: Springer; 303–314. [Google Scholar]
- Dyer AG, 2012b. The mysterious cognitive abilities of bees: why models of visual processing need to consider experience and individual differences in animal performance. J Exp Biol 215:387–395. [DOI] [PubMed] [Google Scholar]
- Dyer AG, Boyd-Gerny S, McLoughlin S, Rosa MGP, Simonov V et al. , 2012. Parallel evolution of angiosperm colour signals: common evolutionary pressures linked to hymenopteran vision. Proc R Soc B Biol Sci 279:3606–3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer AG, Boyd-Gerny S, Shrestha M, Lunau K, Garcia JE et al. , 2016. Innate colour preferences of the Australian native stingless bee Tetragonula carbonaria Sm. J Comp Physiol A 202:603–613. [DOI] [PubMed] [Google Scholar]
- Dyer AG, Chittka L, 2004a. Fine colour discrimination requires differential conditioning in bumblebees. Naturwissenschaften 91:224–227. [DOI] [PubMed] [Google Scholar]
- Dyer AG, Chittka L, 2004b. Bumblebees Bombus terrestris sacrifice foraging speed to solve difficult colour discrimination tasks. J Comp Physiol A 190:759–763. [DOI] [PubMed] [Google Scholar]
- Dyer AG, Chittka L, 2004c. Biological significance of distinguishing between similar colours in spectrally variable illumination: bumblebees Bombus terrestris as a case study. J Comp Physiol A 190:105–114. [DOI] [PubMed] [Google Scholar]
- Dyer AG, Garcia JE, Shrestha M, Lunau K, 2015. Seeing in colour: a hundred years of studies on bee vision since the work of the Nobel laureate Karl Von Frisch. Proc R Soc Vic 127:66–72. [Google Scholar]
- Dyer AG, Murphy AH, 2009. Honeybees choose “incorrect” colors that are similar to target flowers in preference to novel colors. Isr J Plant Sci 57:203–210. [Google Scholar]
- Dyer AG, Neumeyer C, 2005. Simultaneous and successive colour discrimination in the honeybee Apis mellifera. J Comp Physiol A 191:547–557. [DOI] [PubMed] [Google Scholar]
- Dyer AG, Paulk AC, Reser DH, 2011. Colour processing in complex environments: insights from the visual system of bees. Proc R Soc B Biol Sci 278:952–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer AG, Spaethe J, Prack S, 2008. Comparative psychophysics of bumblebee and honeybee colour discrimination and object detection. J Comp Physiol A 194:617–627. [DOI] [PubMed] [Google Scholar]
- Fontaine C, Dajoz I, Meriguet J, Loreau M, 2006. Functional diversity of plant–pollinator interaction webs enhances the persistence of plant communities. PLoS Biol 4:0129–0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Frisch K, 1914. Der Farbensinn und Formensinn der Biene. Zool J Physiol 37:1–238. [Google Scholar]
- Fukushi T, 1989. Learning and discrimination of coloured papers in the walking blowfly Lucilia cuprina. J Comp Physiol A 166:57–64. [DOI] [PubMed] [Google Scholar]
- Fukushi T, 1990. Colour discrimination from various shades of grey in the trained blowfly Lucilia cuprina. J Insect Physiol 36:69–75. [Google Scholar]
- Gaffney A, Bohman B, Quarrell SR, Brown PH, Allen GR, 2018. Frequent insect visitors are not always pollen carriers in hybrid carrot pollination. Insects 9:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JE, Shrestha M, Dyer AG, 2018. Flower signal variability overwhelms receptor-noise and requires plastic color learning in bees. Behav Ecol 29:1286–1297. [Google Scholar]
- Garcia JE, Shrestha M, Howard SR, Petersen P, Dyer AG, 2019. Signal or cue: the role of structural colors in flower pollination. Curr Zool 65 Available from: 10.1093/cz/zoy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JE, Spaethe J, Dyer AG, 2017. The path to colour discrimination is S-shaped: behaviour determines the interpretation of colour models. J Comp Physiol A 203:983–997. [DOI] [PubMed] [Google Scholar]
- Garcia JE, Hung Y-S, Greentree AD, Rosa MGP, Endler JA et al. , 2017. Improved color constancy in honey bees enabled by parallel visual projections from dorsal ocelli. Proc Natl Acad Sci U S A 114:7713–7718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert F, 1981. Foraging ecology of hoverflies: morphology of the mouthparts in relation to feeding on nectar and pollen in some common urban species. Ecol Entomol 6:245–262. [Google Scholar]
- Giurfa M, 2004. Conditioning procedure and color discrimination in the honeybee Apis mellifera. Naturwissenschaften 91:228–231. [DOI] [PubMed] [Google Scholar]
- Gladis T, 1994. Establishment and utilization of a mass rearing of Eristalis tenax (Diptera, Syrphidae) in the Gatersleben genebank. Insecta 1:287–294. [Google Scholar]
- Hardin JW, Hardin JW, Hilbe JM, Hilbe J, 2007. Generalized Linear Models and Extensions. Texas: Stata Press. [Google Scholar]
- von Helversen O, 1972. The relationship between difference in stimuli and choice frequency In: Wehner R, editor Information Processing in the Visual Systems of Anthropods. Berlin, Heidelberg: Springer; 323–334. [Google Scholar]
- Hering E, 1920. Outlines of a Theory of the Light Sense (Hurvich LM, Jameson D, Trans.). Cambridge, MA: Harvard University Press; 1964 (Original work published 1920). [Google Scholar]
- Holloway BA, 1976. Pollen-feeding in hover-flies (Diptera: Syrphidae). New Zeal J Zool 3:339–350. [Google Scholar]
- Horridge GA, Mimura K, Tsukahara Y, 1975. Fly photoreceptors. II. Spectral and polarized light sensitivity in the drone fly Eristalis. Proc R Soc B Biol Sci 190:225–237. [DOI] [PubMed] [Google Scholar]
- Howard SR, Shrestha M, Schramme J, Garcia JE, Avarguès-Weber A et al. , 2019. Honeybees prefer novel insect-pollinated flower shapes over bird-pollinated flower shapes. Curr Zool 65 Available from: 10.1093/cz/zoy095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurvich L, Jameson D, 1957. An opponent-process theory of color vision. Psychol Rev 64:384–404. [DOI] [PubMed] [Google Scholar]
- Ilse D, 1949. Colour discrimination in the dronefly, Eristalis tenax. Nature 163:255–256. [DOI] [PubMed] [Google Scholar]
- Inouye DW, Larson BMH, Ssymank A, Kevan PG, 2015. Flies and flowers III: ecology of foraging and pollination. J Pollinat Ecol 16:115–133. [Google Scholar]
- Jarlan A, De Oliveira D, Gingras J, 1997. Pollination by Eristalis tenax (Diptera: Syrphidae) and seed set of greenhouse sweet pepper. J Econ Entomol 90:1646–1649. [Google Scholar]
- Jauker F, Bondarenko B, Becker HC, Steffan-Dewenter I, 2012. Pollination efficiency of wild bees and hoverflies provided to oilseed rape. Agric for Entomol 14:81–87. [Google Scholar]
- Jersáková J, Jürgens A, Šmilauer P, Johnson SD, 2012. The evolution of floral mimicry: identifying traits that visually attract pollinators. Funct Ecol 26:1381–1389. [Google Scholar]
- Kantsa A, Raguso RA, Dyer AG, Olesen JM, Tscheulin T et al. , 2018. Disentangling the role of floral sensory stimuli in pollination networks. Nature Commun 9:1041.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay QON, 1976. Preferential pollination of yellow-flowered morphs of Raphinus raphanistrum by Pieris and Eristalis spp. Nature 260:643–645.772446 [Google Scholar]
- Kelly MM, Gaskett AC, 2014. UV reflectance but no evidence for colour mimicry in a putative brood-deceptive orchid Corybas cheesemanii. Curr Zool 60:104–113. [Google Scholar]
- Kemp DJ, Herberstein ME, Fleishman LJ, Endler JA, Bennett AT et al. , 2015. An integrative framework for the appraisal of coloration in nature. Am Nat 185:705–724. [DOI] [PubMed] [Google Scholar]
- Kirschfeld K, Feiler R, Franceschini N, 1978. A photostable pigment within the rhabdomere of fly photoreceptors no. 7. J Comp Physiol 125:275–284. [Google Scholar]
- Klein A-M, Vaissiere BE, Cane JH, Steffan-Dewenter I, Cunningham SA et al. , 2007. Importance of pollinators in changing landscapes for world crops. Proc R Soc B Biol Sci 274:303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll F, 1926. Insekten und Blumen. Verh Zool Bot Ges Wien 12:645. [Google Scholar]
- van der Kooi CJ, Dyer AG, Kevan PG, Lunau K, 2018. Functional significance of the optical properties of flowers for visual signalling. Ann Bot 123:263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugler H, 1950. Der blutenbesuctt der schlammfliege Eristalomyia tenax. Z Vgl Physiol 3:328–347. [PubMed] [Google Scholar]
- Larson BMH, Kevan PG, Inouye DW, 2001. Flies and flowers: taxonomic diversity of anthophiles and pollinators. Can Entomol 133:439–465. [Google Scholar]
- Lucas A, Bodger O, Brosi BJ, Ford CR, Forman DW et al. , 2018. Generalisation and specialisation in hoverfly (Syrphidae) grassland pollen transport networks revealed by DNA metabarcoding. J Anim Ecol 87:1008–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunau K, 2014. Visual ecology of flies with particular reference to colour vision and colour preferences. J Comp Physiol A 200:497–512. [DOI] [PubMed] [Google Scholar]
- Lunau K, An L, Donda M, Hohmann M, Sermon L et al. , 2018. Limitations of learning in the proboscis reflex of the flower visiting syrphid fly Eristalis tenax. PLoS ONE 13:e0194167.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunau K, Wacht S, 1997. Innate flower recognition in the hoverfly Eristalis tenax L. Mitt Dtsch Ges Allg Angew Entomol 11:481–484. [Google Scholar]
- Lythgoe JN, 1979. The ecology of vision. Oxford, UK: Clarendon Press. [Google Scholar]
- Macadam DL, 1985. Colour Measurement. Berlin, Heidelberg: Springer-Verlag. [Google Scholar]
- MacAdam DL, 1942. Visual sensitivities to color differences in daylight. J Opt Soc Am 32:247–274. [Google Scholar]
- Menne D, Spatz H-C, 1977. Colour vision in Drosophila melanogaster. J Comp Physiol 114:301–312. [Google Scholar]
- Mollon J, Jordan G, 1997. On the nature of unique hues In: Dickinson C, Murray 1, Carden D, editors. John Dalton’s Colour Vision Legacy (Reprinted). London: Taylor & Francis; 381–392. [Google Scholar]
- Morante J, Desplan C, 2008. The color-vision circuit in the medulla of Drosophila. Curr Biol 18:553–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcombe RG, 1998. Interval estimation for the difference between independent proportions: comparison of eleven methods. Statist Med 17:873–890. [DOI] [PubMed] [Google Scholar]
- Nicholas S, Thyselius M, Holden M, Nordström K, 2018. Rearing and long-term maintenance of Eristalis tenax hoverflies for research studies. J Vis Exp 135:57711.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi K, Makino TT, Arikawa K, 2015. Floral colour change in the eyes of pollinators: testing possible constraints and correlated evolution. Funct Ecol 29:1144–1155. [Google Scholar]
- Ollerton J, Winfree R, Tarrant S, 2011. How many flowering plants are pollinated by animals?. Oikos 120:321–326. [Google Scholar]
- Poralla J, Neumeyer C, 2006. Generalization and categorization of spectral colors in goldfish. II. Experiments with two and six training wavelengths. J Comp Physiol A 192:469–479. [DOI] [PubMed] [Google Scholar]
- Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O et al. , 2010. Global pollinator declines: trends, impacts and drivers. Trends Ecol Evol 25:345–353. [DOI] [PubMed] [Google Scholar]
- Rader R, Howlett BG, Cunningham SA, Westcott DA, Newstrom-Lloyd LE et al. , 2009. Alternative pollinator taxa are equally efficient but not as effective as the honeybee in a mass flowering crop. J Appl Ecol 46:1080–1087. [Google Scholar]
- Saxonbee Enterprises, 2018. Saxonbee Enterprises [online] Gidgegannup, WA, Australia. Available from: http://www.saxonbee.com.au/.
- Schnaitmann C, Garbers C, Wachtler T, Tanimoto H, 2013. Color discrimination with broadband photoreceptors. Curr Biol 23:2375–2382. [DOI] [PubMed] [Google Scholar]
- Shrestha M, Dyer AG, Boyd-Gerny S, Wong BBM, Burd M, 2013. Shades of red: bird-pollinated flowers target the specific colour discrimination abilities of avian vision. New Phytol 198:301–310. [DOI] [PubMed] [Google Scholar]
- Shrestha M, Lunau K, Dorin A, Schulze B, Bischoff M et al. , 2016. Floral colours in a world without birds and bees: the plants of Macquarie Island. Plant Biol (Stuttg.) 18:842–850. [DOI] [PubMed] [Google Scholar]
- Shrestha M, Dyer AG, Dorin A, Garcia JE, Burd M, 2019. Colour evolution within orchids depends on whether the pollinator is a bee or a fly. Plant Biol (doi: 10.1111/plb.12968). [DOI] [PubMed]
- Shuttleworth A, Johnson SD, 2010. The missing stink: sulphur compounds can mediate a shift between fly and wasp pollination systems. Proc R Soc B Biol Sci 277:2811–2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaethe J, Streinzer M, Eckert J, May S, Dyer AG, 2014. Behavioural evidence of colour vision in free flying stingless bees. J Comp Physiol A 200:485–496. [DOI] [PubMed] [Google Scholar]
- Speight MCD, 2011. Species accounts of European Syrphidae (Diptera). Syrph the Net, the Database of European Syrphidae 83:291. [Google Scholar]
- Ssymank A, Kearns CA, Pape T, Thompson FC, 2008. Pollinating flies (Diptera): a major contribution to plant diversity and agricultural production. Biodiversity 9:86–89. [Google Scholar]
- Stanley DA, Gunning D, Stout JC, 2013. Pollinators and pollination of oilseed rape crops (Brassica napus L.) in Ireland: ecological and economic incentives for pollinator conservation. J Insect Conserv 17:1181–1189. [Google Scholar]
- Stavert JR, Pattemore DE, Bartomeus I, Gaskett AC, Beggs JR, 2018. Exotic flies maintain pollination services as native pollinators decline with agricultural expansion. J App Ecol 55:1737–1746. [Google Scholar]
- Troje N, 1993. Spectral categories in the learning behaviour of blowflies. Z Naturforsch C Biosci 48:96–104. [Google Scholar]
- Tsukahara Y, Horridge GA, 1977. Visual pigment spectra from sensitivity measurements after chromatic adaptation of single dronefly retinula cells. J Comp Physiol A 114:233–251. [Google Scholar]
- Wall R, Wearmouth VJ, Smith KE, 2002. Reproductive allocation by the blow fly Lucilia sericata in response to protein limitation. Physiol Entomol 27:267–274. [Google Scholar]
- Whitney HM, Dyer A, Chittka L, Rands SA, Glover BJ, 2008. The interaction of temperature and sucrose concentration on foraging preferences in bumblebees. Naturwissenschaften 95:845–850. [DOI] [PubMed] [Google Scholar]
- Woodcock TS, Larson BMH, Kevan PG, Inouye DW, Lunau K, 2014. Flies and flowers II: floral attractants and rewards. J Pollin Ecol 12: 63–94. [Google Scholar]
- Wyszecki G, Stiles WS, 1982. Color Science: Concepts and Methods, Quantitative Data and Formulae. 2nd edn.New York: John Wiley and Sons. [Google Scholar]
- Yamaguchi S, Wolf R, Desplan C, Heisenberg M, 2008. Motion vision is independent of color in Drosophila. Proc Natl Acad Sci U S A 105:4910–4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan G, Liu S, Schlink AC, Flematti GR, Brodie BS et al. , 2018. Behavior and electrophysiological response of gravid and non-gravid Lucilia cuprina (Diptera: Calliphoridae) to carrion-associated compounds. J Econ Entomol 111:1958–1965. [DOI] [PubMed] [Google Scholar]
- Yilmaz A, Dyer AG, Rössler W, Spaethe J, 2017. Innate colour preference, individual learning and memory retention in the ant Camponotus blandus. J Exp Biol 220:3315–3326. [DOI] [PubMed] [Google Scholar]
- Zoller H, Lenzin H, Erhardt A, 2002. Pollination and breeding system of Eritrichium nanum (Boraginaceae). Plant Syst Evol 233:1–14. [Google Scholar]
- Zuur AF, Hilbe JM, Ieno EN, 2013. A Beginner’S Guide to GLM and GLMM with R: A Frequentist and Bayesian Perspective for Ecologists. Newburgh, UK: Highland Statistics Limited. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.