Abstract
In a study of gerbils with contrasting social and mating systems (group-living monogamous Mongolian gerbil Meriones unguiculatus, solitary nonterritorial promiscuous midday jird M. meridianus, and solitary territorial promiscuous pale gerbil Gerbillus perpallidus), we employed partner preference tests (PPTs) to assess among-species variation in sociability and pair-bonding patterns and tested whether the nature of contact between individuals: direct contact (DC) versus nondirect contact (NDC) affected our results. We measured male preferences as the time: 1) spent alone, 2) with familiar (partner), and 3) unfamiliar (stranger) female in the 3-chambered apparatus. Gerbil species differed strongly in sociability and male partner preferences. The time spent alone was a reliable indicator of species sociability independent of the nature of contact, whereas the pattern and level of between-species differences in male partner preferences depended on contact type: DC PPTs, unlike NDC-tests, discriminated well between monogamous and promiscuous species. In the DC-tests, stranger-directed aggression and stranger avoidance were observed both in the highly social monogamous M. unguiculatus and the solitary territorial promiscuous G. perpallidus, but not in the nonterritorial promiscuous M. meridianus. In M. unguiculatus, stranger avoidance in the DC-tests increased the time spent with the partner, thus providing evidence of a partner preference that was not found in the NDC-tests, whereas in G. perpallidus, stranger avoidance increased the time spent alone. This first comparative experimental study of partner preferences in gerbils provides new insights into the interspecific variation in gerbil sociality and mating systems and sheds light on behavioral mechanisms underlying social fidelity and pair-bonding.
Keywords: gerbils, mating systems, partner preference test, partner fidelity, social systems, stranger-directed aggression
Evolutionary models of sociality and mating systems remain intriguing and produce controversial results, often due to the lack of data and poor classifications of species behavioral traits and pair-bonding patterns, in particular (Lukas and Clutton-Brock 2013; Kappeler 2014; Kappeler and Fichtel 2016; Schradin 2017). Field studies take years of individual-based observations and enormous efforts to make conclusions on species social and mating systems (Clutton-Brock and Sheldon 2010; Taig-Johnston et al. 2017), which narrows the spectrum of model taxa for comparative analysis. To expand studies to a broader range of species, including those that are poorly studied, rare, or secretive, we need a universal operational tool, robust to exogenous inputs, which would allow classifying and comparing mating and social systems under standard conditions to control for intraspecific variation and exogenous factors. The 2-way choice partner preference test (PPT) is a good candidate tool for such a purpose and could be applied to many mammals, thereby enhancing the explanatory power of comparative studies of social and mating systems (Carp et al. 2016), but thus far, has seen limited use.
Social (not sexual) preference for a familiar opposite-sex conspecific over a stranger provides an indication of a pair bond, the inherent feature of social monogamy (Kleiman 1977). Accordingly, more time spent by the focal animal in proximity to a familiar partner than to a stranger in the PPT (outside the sexual context) is presumed to be indicative of selective partner preference, social fidelity, and pair-bonding (Williams et al. 1992; Carter et al. 1995; Young et al. 2008; Carp et al. 2016). In PPTs with nonmonogamous species (i.e., species without selective male–female bonds), males and females showed no preference for the partner, which had been interpreted as a lack of partner fidelity and social attachment (Shapiro et al. 1986; Insel et al. 1995; Lim et al. 2004).
There are 2 major types of PPTs—with and without direct contacts (DC) between the focal (selecting) animal and stimulus (selected) animals (the nondirect contact tests [NDC-tests] and direct-contact tests [DC-tests]). In the NDC-tests, a focal animal selects between caged (or anesthetized) familiar and unfamiliar opposite-sex conspecifics or their odors (Ågren and Meyerson 1977; Johnston and Rasmussen 1984; Williams et al. 1992; Roberts and Gosling 2004; Frynta et al. 2010; Carp et al. 2016); in the DC-tests, an animal selects between free-moving or tethered (to restrict movements within the experimental arena) stimulus animals (Shapiro et al. 1986; Williams et al. 1992; Parker et al. 2001; Young et al. 2008; Brandt and Macdonald 2011; Rogovin et al. 2016). DC PPTs with tethered stimulus animals in a 3-chambered apparatus (the central neutral chamber and the 2 chambers with the tethered familiar and unfamiliar opposite-sex conspecifics) have become a standard procedure to test for pair-bonding and social attachment in neurobiological studies of monogamy in voles (Hammock 2007; Young and Hammock 2007). The 3-chamber design of the PPT allows not only an assessment of preferences for the partner or stranger but also the use of the time the focal animal spends alone (in the central, socially neutral, chamber) as a measure of sociability (Shapiro et al. 1986; Parker et al. 2001; Ahern et al. 2009).
The basic difference between the 2 types of PPTs is that in the DC-tests the partner and the stranger can treat the focal animal differentially and affect its behavior, thus influencing the relative amounts of time it spends in proximity to each stimulus animal (Ågren and Meyerson 1977; Williams et al. 1992; Duarte et al. 2015). Stranger-directed intersexual aggression is one of the defining features of pair-bonding (Kleiman 1977; Young et al. 2008), and can shift the time the focal individual spends with stimulus individuals in favor of the nonaggressive partner, thereby mimicking the effect of partner fidelity and confounding conclusions regarding social attachment. Thus, it appears crucial to disentangle the mixed effects of social attachment and stranger avoidance on the time in proximity to the partner before making conclusions on partner fidelity and mating system.
So far, among rodents, PPTs have been used to study mate choice and pair-bonding (mating systems, sensu lato) mainly in arvicoline voles (Carter et al. 1995; Parker et al. 2001; Hammock 2007; Zhang et al. 2006; Ricankova et al. 2007; Young et al. 2008; Duarte et al. 2015), and also in some mice (Roberts and Gosling 2004; Brandt and Macdonald 2011; Harrison et al. 2016), the California mouse (Gubernick and Addington 1994), and mole rats (Bappert et al. 2012). Comparative studies of selective partner preferences employing PPTs so far have been limited to some vole species only and have uncovered the behavioral, physiological and neurogenetic bases of interspecific variation in sociality and mating systems (Shapiro et al. 1986; Carter et al. 1995; Insel et al. 1995; Lim et al. 2004; Young et al. 2008; Duarte et al. 2015).
Gerbils (Gerbillinae) are another useful model taxon for comparative experimental studies of sociality and pair-bonding. Gerbil species are ecologically and morphologically similar, but exhibit a diversity of social and mating systems from strictly solitary and promiscuous to family-group living and monogamous (Daly and Daly 1975; Gol'tsman et al. 1994; Gromov 1996; Tchabovsky and Bazykin 2004; Shilova and Tchabovsky 2009). However, so far, PPTs have been applied only to a single Gerbillinae species—the Mongolian gerbil Meriones unguiculatus (Ågren and Meyerson 1977; Razzoli and Valsecchi 2006).
The objective of this work was to assess the interspecific variation in sociability, stranger-directed aggression and selective partner preference in gerbils using PPTs. We performed PPTs with males of 3 species of gerbils—the Mongolian gerbil M.unguiculatus, the midday jird Meriones meridianus, and the pale gerbil Gerbillus perpallidus. We have focused on male partner preferences as is common in such studies (Winslow et al. 1993; Lim et al. 2004; Ophir et al. 2008; Solomon et al. 2009; Mabry et al. 2011; Blocker and Ophir 2016) because sexual conflict theory predicts that males are more likely to desert their partners than females (Trivers 1972). The 3 species differ in their natural history, social, and mating systems. Merionesunguiculatus is a diurnal grani-folivorous Asian gerbil, one of the most social species within the subfamily Gerbillinae (Gol'tsman et al. 1994; Gromov 1996). Typically, they live in complex family groups, composed of a breeding pair and up to 3 successive litters of offspring (Gromov 1981; Ågren et al. 1989). An adult male and a female share a nest, form a stable pair bond, take care of young, and defend the group territory from intruders by exhibiting intra- and intersexual stranger-directed aggression (Elwood and Broom 1978; Gerling and Yahr 1979; Ågren 1984; Clark and Galef 1999).
Meriones meridianus is the most closely related species to M. unguiculatus (Ito et al. 2010), but differs strongly in social and mating patterns. This nocturnal, mainly granivorous Asian gerbil lives solitarily or in loose aggregations, males occupy large overlapping home ranges that encompass smaller home ranges of several females (Shilova et al. 1983; Popov et al. 1989; Gol'tsman et al. 1994; Gromov and Vorobieva 1995). Neither males nor females exhibit strong territoriality, defending only an area around the nest burrow; female home ranges overlap, more or less, depending on resource distribution. The social structure is rather loose; the animals interact rarely and display little agonistic or amicable behavior. Males and females do not form pair bonds and meet only for mating; the young disperse soon after weaning (Verevkin 1985; Popov et al. 1989).
Much less is known about the social and mating system of G. perpallidus, a small and secretive nocturnal granivorous African gerbil. Observations under semi-natural settings in large enclosures showed that males occupied exclusive home ranges overlapping with exclusive home ranges of females. Both males and females vigorously defended their territories, and aggressive behavior predominated in both intra- and intersexual interactions (Gromov and Ilchenko 2007). Although males monopolized territories of females, they did not share the nest or form pair bonds with them, suggesting a promiscuous mating system. Soon after weaning, litters break up and young occupy exclusive home ranges (Gromov and Ilchenko 2007).
By contrasting gerbil species in PPTs, we asked: 1) Do species differ in sociability, partner fidelity, and stranger avoidance? 2) Do results of PPTs agree with the known or hypothesized species patterns of social and mating systems? 3) How do the possible confounding factors associated with the experimental paradigm (NDC-tests vs. DC-tests) and stranger aggression affect the conclusions on species sociability and selective social preferences? In particular, we predicted that males of the highly social and monogamous M. unguiculatus would spend more time with the familiar female (“partner”) than with the unfamiliar female (“stranger”), whereas males of promiscuous M. meridianus and G. perpallidus would show no preference. Moreover, since G. perpallidus appeared to be strictly solitary and aggressive, we expected its males to spend more time in the socially neutral chamber than in chambers with either female, unlike the other 2 more social species. Finally, we expected that between-species differences in the preference pattern would be more pronounced in the DC-tests where animals can interact with each other and where stimulus animals can affect the behavior of the focal animals. This first comparative experimental study of partner preferences in gerbils provides new insights into the interspecific variation in gerbil sociality and mating systems and sheds light on behavioral mechanisms underlying social fidelity and pair-bonding.
Materials and Methods
Classification of model species
Based on the available data on species social and mating systems we classified gerbils as follows: M. unguiculatus—highly social, territorial, and socially (but not sexually) monogamous (Ågren 1984; Ågren et al. 1989; Gromov 1996); M. meridianus—basically solitary, nonterritorial, and promiscuous (Gol'tsman et al. 1994; Gromov 1996); G. perpallidus—strictly solitary, territorial (Gromov and Ilchenko 2007), and presumably promiscuous.
Study animals and housing conditions
Gerbils used in this study were born to breeding pairs in established breeding colonies of the Moscow Zoo. After weaning, at 25–30 days of age, we removed litters from the parent cage and housed them under natural day–night (light:dark) conditions at room temperature as recommended for maintaining a normal breeding cycle in captive colonies of gerbils (Volodin et al. 1996) in metallic cages 50 cm × 40 cm × 40 cm with wire ceiling and glass front doors, on pine shaving bedding with a wooden box as a shelter, some hay and small branches, food (grain, carrots, and apples) available ad libitum and no water provided, because gerbils do not drink in nature and obtain water from their food. Littermates resided together until 60 days of age, when they were separated from each other and paired with opposite-sex unrelated individuals from other litters; pairmates differed in age by 1–12 days.
Males and females of the pairs were kept in 2 separate compartments of double cages (100 cm × 40 cm × 40 cm) divided by a metal partition until the first signs of female cycling (opened vagina) which appeared in G. perpallidus and M. unguiculatus at about 6 months of age and in M. meridianus at 10 months. Then, we removed a sliding metal between compartment partition to open 14.5 cm × 20 cm “window” covered with 10 mm wire mesh to prevent DCs between the male and the female, but allowing them to smell and see each other. Males and females of many species of gerbils do not tolerate each other without preliminary familiarization through olfactory, visual and auditory cues (Volodin et al. 1996).
During the next week, we checked females daily for estrous condition using vaginal smears. Vaginal secretions were collected with a lubricated sterile plastic pipette filled with saline. Slides were examined immediately to check for estrous stage as described for gerbils and other rodents (Thomas and Oommen 1999; Goldman et al. 2007). To exclude sexual context in social pair-bonding (i.e., to measure male social, not sexual, fidelity to females), we used sexually naïve males and unreceptive females in diestrus in all of our experiments. In monogamous species, social preference for the partner as compared with a stranger is a more correct measure of pair-bonding than sexual preference (Carter et al. 1995); it develops outside the sexual context and can be revealed in PPTs with unreceptive females (Williams et al. 1992; Gubernick and Addington 1994).
Experimental procedure
On the day when metestrous was observed in females from at least 2 pairs, we removed the wire-mesh dividers between “male” and “female” compartments to allow the animals of the pair DCs and free movements throughout the entire double cage for 24 h. Each partner in the pair still had his or her “own” shelter in his or her “own” compartment with “own” bedding. Twenty-four hours of cohabitation outside the sexual context was shown to be sufficient to develop a partner preference and establish a pair bond in socially monogamous rodents (Williams et al. 1992; Gubernick and Addington 1994). PPTs were conducted on the next day, if stimulus females from at least 2 pairs were in diestrus. One of the participating females was randomly assigned to be a stranger and the other female then was the partner of the focal male. On the next day, in the trial with a male from the second pair, the roles of stimulus females were reversed. Thus, each pair of stimulus females participated in tests twice on 2 consecutive days; each female first was a “stranger” and then was a “partner” and vice versa. This “role-reversal” procedure and repeated use of stimulus animals is a common practice in PPTs, which reduces animal use without confounding effects of the test order or animal experience on the test results (Insel et al. 1995; Roberts and Gosling 2004; Lim et al. 2007; Brandt and Macdonald 2011; Ahern et al. 2009; Duarte et al. 2015).
After the first trial on Day 1, the animals were returned to their home cages and resided there in pairs until the next trial on Day 2. Thus, the animals that were tested on Day 1 cohabited in pairs for 24 h, and the animals that were tested on Day 2 cohabited for 48 h. Before the trial on Day 2, females were checked for estrous cycle stage. Female gerbils exhibit prolonged diestrus (Thomas and Oommen 1999), and in this study it lasted for at least 2 days, so in all of the second-day trials, females were still in diestrus. After the 2-day series of tests, the animals were returned to their home cages, and pairs were separated by dividing the double cage with the metal opaque partition in 2 separate individual compartments.
In some DC-tests with G. perpallidus and M. unguiculatus, but not with M. meridianus, female strangers displayed agonistic behavior towards the focal male, which split the sample into tests with and without conflict. To increase and balance the sample size without increasing the number of animals used as recommended by the ASAB/ABS (2012) Guidelines for the Treatment of Animals in Behavioural Research and Teaching, the same 2-day experimental procedure, but with another strange female, was repeated after 2 months of individual housing for 2 of 10 males of G. perpallidus and for the 9 of 11 males of M. unguiculatus. Since gerbils do not discriminate between their partner and a stranger after 8 weeks of pair separation (Ågren 1980), with even shorter periods for “social memory” shown for other rodents in PPTs (voles and mole-rats, Carter et al. 1995; Bappert et al. 2012), repeated tests can be treated as independent samples. Thus, in total, 12 experiments (10 males) for G. perpallidus, 20 experiments (11 males) for M. unguiculatus and 10 experiments (10 males) for M. meridianus were conducted.
Experimental design
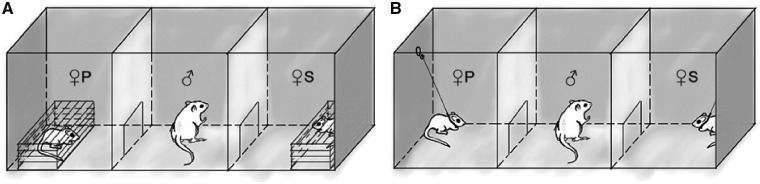
Male PPTs were conducted as 2-way choice tests in the experimental arena—a clean PLEXIGLASTM terrarium (75 cm × 25 cm × 25 cm) with transparent front wall and ceiling separated into 3 equal chambers (25 cm × 25 cm × 25 cm) by 2 transparent PLEXIGLASTM dividers, each with a 5.5 cm arc-like hole at the bottom allowed the animal easy passage between the central chamber and the side chambers. Experiments with M. meridianus and G. perpallidus, the nocturnal gerbils, were performed between 21:00 h and 02:00 h in darkness with a dim red light (∼10 lux), whereas diurnal and crepuscular M. unguiculatus were tested between 15:00 h and 20:00 h under daylight illumination (∼400–500 lux). Each trial consisted of the 2 stages—first the NDC test and then the direct-contact (DC) test (Figure 1). We opted to conduct tests in this order because the NDC condition at the initial stage of the 2-stage experiment contributes much less to the familiarization of males with stranger females than the DC-tests. Therefore, the difference in familiarity of the males to the stimulus females at the DC-stage of the experiment was maintained at the highest possible level (1 week of indirect contacts + 24 h of cohabitation before the experiment + 1 h of the NDC-test for the male and the partner versus 1 h of the NDC-test for the male and the stranger). We did not change this order from trial to trial and used the same pair of stimulus females at both stages of the experiment to standardize test conditions and exclude any possible confounding effect of variation in female personalities.
Figure 1.
Drawing of the PLEXIGLASTM testing apparatus (75 cm × 25 cm × 25 cm) used in the nondirect-contact tests (A) and direct-contact tests (B). ♀P and ♀S correspond to the female partner and female stranger, respectively; ♂ is a focal male.
NDC-tests
At the first, the NDC-stage of the trial, both stimulus females, the partner and a stranger, were confined in wire-mesh cages (25 cm × 8 cm× 8 cm) placed in the opposite side chambers of the apparatus. The side chambers were randomly assigned in the first trial to the female partner and a female stranger, and each female was placed in her assigned chamber at both stages of the experiment. In each trial thereafter, the side chambers were switched between the stimulus females to counterbalance the location (left or right) of the partner and the stranger. The male was placed in the central (socially neutral) chamber separated from the side chambers by metal sliding partitions. Animals were left for 10 min to habituate, and then the partitions were raised, and the male was free to move around the entire arena and approach and investigate olfactory, visual, and auditory cues from each female, but without DC. The test was terminated after 59 min, if, at that moment, the male was in the central chamber, that is, in the starting position; otherwise, we waited until he had entered the central chamber. Thus, the duration of tests varied from 59 to 65 min.
DC-tests
After the NDC-stage of the experiment, the male and both females were removed from experimental chambers, and the PLEXIGLAS™ surfaces were cleaned with 96% ethanol. Then, the females were fitted with collars made from a plastic zip-tie 3 mm in width and tethered with a flexible steel wire fishing leader (0.1 mm × 200 mm) attached to their collars and anchored to hooks in the upper middle area of the side walls within their respective chambers with swivel clips (Figure 1). The wire allowed the females to move throughout their chambers but prevented them from entering the central chamber or blocking the entrance opening to their chamber. The females were left to habituate to the tethering for 20 min. Preliminary observations showed that after 10–15 min of acclimation the tethered females behaved normally (see section “Ethical note”). Tethering was shown not to interfere with normal activities in stimulus animals in PPTs (Blocker and Ophir 2016). Five minutes before the end of the female habituation period, we returned the male to the central chamber for acclimation, and 5 min later removed the partitions to start the DC-stage of the experiment which lasted, likewise, for about an hour (59–63 min). During the DC-stage the male could move freely throughout the entire arena and interact with females directly. Contacts between males and females included neutral interactions (sniffing), affiliative behaviors (side-by-side sitting, allogrooming, submissive postures, climbing-on, climbing-under), and agonistic behaviors (lunges, sideway threats, upright postures with/without boxing, attacks, chasing, short locked fights without biting, fleeing, and contact avoidance) described for gerbils elsewhere (Ågren and Meyerson 1977; Hurtado-Parrado et al. 2015).
During both the NDC- and DC stages of the experiment, 2 observers, who were blind to which female was the stranger and which female was the partner, manually scored the number and duration (to the nearest second) of the male’s visits to each chamber (the central and the 2 side chambers) using specified keys on the keyboard of laptops with automatic time recording with the help of macros in a MS Excel-designed spreadsheet. The observers sat in front of the arena at a distance of 2 m, and animals did not show any reaction to their presence. Chamber entries were characterized by the animal entering the chamber with all 4 paws. The time estimates of the 2 observers differed by less than 1% and were averaged for the analysis. The experiments were filmed using a monochrome CCD camera from above the arena and recorded on a digital video-recorder at normal speed. Video was used to classify tests as “conflict” and “nonconflict” based on the presence or absence of agonistic interactions between the male and the female. Tests were classified as “conflict” if any of the agonistic behaviors listed above were recorded more than 5 times during the entire experiment; otherwise they were classified as “nonconflict”. All conflicts occurred between the male and the female strangers, and both males and females displayed agonistic behavior in the “conflict” situations.
Ethical note
Females were collared and tethered 20 min before the DC-stage of the experiment to allow them to habituate and had remained tethered until the end of experiment, for a total of 1.5 h. The collars did not have any visible adverse effects on the females; the fur around the neck was not disturbed, and after the experiment the females moved and behaved normally. No drop in body mass was recorded in subsequent daily weighing during the week after the experiments. Tethering was shown not to interfere with normal activities in stimulus animals or affect their welfare thereafter, and is commonly used in PPTs with various rodent species (Young and Hammock 2007; Blocker and Ophir 2016). Contacts between the animals were monitored by the observers to prevent the escalation of aggressive interactions. No acute aggression with repeated biting, continual fighting bouts or persistent harassment of the tethered females by the male was recorded and no signs of physical injury were found. At the end of the study, the animals were returned to the breeding colonies of the Moscow Zoo. No special permission for use of gerbils in behavioral research is required in the Russian Federation. The study protocol met all the requirements of animal welfare standards accepted by the Moscow Zoo as a member of the European Association of Zoos and Aquaria (EAZA) and World Association of Zoos and Aquaria (WAZA). The study was conducted in accordance with the ASAB/ABS (2012) Guidelines for the Treatment of Animals in Behavioural Research and Teaching and with the laws of the Russian Federation, the country where the research was conducted.
Analysis
Social preferences of a focal animal in the 2-way PPTs outside the sexual context are traditionally assessed by the time in proximity to a particular stimulus animal (amounts of time in one or another side chamber) and/or time in “quiet” peaceful contact, particularly, in side-by-side body contact (huddling) indicative of an affiliative bond (Shapiro et al. 1986; Carter et al. 1995; Hammock et al. 2005; Razzoli and Valsecchi 2006; Young and Hammock 2007; Brandt and Macdonald 2011). Though time in “quiet” contact may be a more reliable indicator of the partner preference than the time in proximity, both metrics are highly correlated (Ahern et al. 2009) and have produced similar results in PPTs in many studies (Gubernick and Nordby 1993; Gubernick and Addington 1994; Cho et al. 1999; Hammock et al. 2005; Blocker and Ophir 2016). Time in body contact can be used in the DC-tests only, and since we have tested males with both caged and tethered females to assess the effect of the testing paradigm, we have used only the time in proximity (i.e., the time in a chamber with one or another female) as a universal and commonly accepted metric of social preference (Brandt and Macdonald 2011; Carp et al. 2016). We used the time in the central (socially neutral) chamber as a measure of sociability (Ågren and Meyerson 1977; Shapiro et al. 1986; Insel et al. 1995; Parker et al. 2001; Ahern et al. 2009).
For every test, we summed the time the focal male spent in each chamber, and since the duration of experimental trials varied, we calculated the proportions of time spent in the neutral, the partner’s and the stranger’s chambers out of the total duration of the experiment. The distributions of time proportions were skewed and did not conform to the assumptions of normality (Shapiro–Wilk’s W test, P < 0.05); thus, we transformed the variables using the arcsine-square-root transformation (the untransformed data are presented in Table 2 and Figure 2).
Table 2.
Portions of time (Medians; 1st–3rd quartiles; untransformed data) the male spent in each of the 3 chambers (Partner/Central/Stranger) for the 2 testing paradigms (NDC and DC) in G. perpallidus, M. unguiculatus, and M. meridianus
| Testing paradigm |
|||||||
|---|---|---|---|---|---|---|---|
| NDC-test |
DC-test |
||||||
| Partner | Central | Stranger | Partner | Central | Stranger | ||
|
G. perpallidus
|
|||||||
| No conflict | 0.13; | 0.14; | 0.40; | 0.11; | 0.10; | 0.51; | |
| 0.08–0.46 | 0.07–0.51 | 0.10–0.78 | 0.07–0.45 | 0.04–0.49 | 0.13–0.83 | ||
| Conflict | 0.18; | 0.44; | 0.16; | 0.18; | 0.69 *; | 0.10; | |
| 0.08–0.53 | 0.08–0.80 | 0.09–0.29 | 0.10–0.31 | 0.47–0.83 | 0.07–0.18 | ||
| M. unguiculatus | |||||||
| No conflict | 0.74; | 0.05; | 0.14; | 0.71; | 0.02; | 0.27; | |
| 0.23–0.88 | 0.03–0.09 | 0.07–0.59 | 0.14–0.88 | 0.005–0.03 | 0.09–0.71 | ||
| Conflict | 0.50; | 0.06; | 0.35; | 0.93; | 0.02; | 0.05; | |
| 0.12–0.85 | 0.04–0.09 | 0.09–0.73 | 0.55–0.94 | 0.01–0.11 | 0.04–0.19 | ||
| M. meridianus | |||||||
| No conflict | 0.35; | 0.08; | 0.53; | 0.37; | 0.02; | 0.56; | |
| 0.22–0.42 | 0.05–0.17 | 0.38–0.67 | 0.18–0.64 | 0.02–0.04 | 0.21–0.75 | ||
Note: For G. perpallidus and M. unguiculatus, the data are presented separately for the tests with and without stranger aggression (Conflict/No conflict) in DC-tests. No conflicts were observed in M. meridianus. Medians for tests with significant (P < 0.05) differences among chambers (based on the analysis of the transformed data, see statistics in the text) are marked with bold font, medians for significantly preferred chamber are underlined (when there are 2 equally preferred chambers, the medians are underlined for both chambers).
The difference between the Central and the Stranger chambers is borderline (P = 0.05).
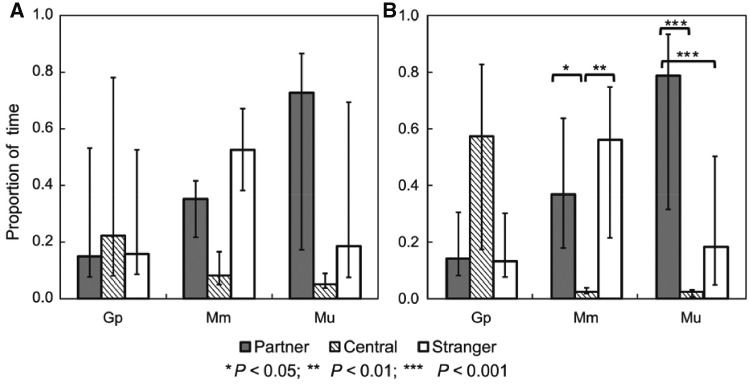
Figure 2.
Time allocation across the 3 chambers (Partner, Central, and Stranger) by males of G. perpallidus (Gp), M. unguiculatus (Mu), and M. meridianus (Mm) in NDC tests (A) and DC tests (B). Untransformed data: Medians, 25–75% percentiles. Significant differences between chambers (revealed by the Tukey HSD test for the transformed data) are indicated by asterisks. For other comparisons and statistics see the text.
The standard PPTs with the “role-reversal” design with 2 pairs of stimulus females for the 2 focal males suggested the use of a 2-level repeated measures analysis (dependent measurements for females in every test and for the same female in different tests). However, we constructed models which did not include dependent measurements from any female within any combination of the factors levels. Given the short social memory of gerbils (Ågren 1980), we assumed the independence of measurements for familiar partners in repeated trials. Since the design was unbalanced and the data were heteroscedastic, we used robust semi-parametric repeated measures models (RMM) with Wald-type statistics (RM function in R package MANOVA.RM; Friedrich et al. 2017) to analyze the effects of the species, the testing paradigm (NDC vs. DC) and the chamber type (Neutral, Partner, Stranger) on the proportions of time the male spent in each chamber. Initial models included all 2-way and 3-way factor interactions, but since the 3-way interactions were nonsignificant, we excluded them from the final models. Post-hoc comparisons were performed with the Tukey honestly significant difference (HSD) test (lsmeans in the lsmean package in R; Lenth 2016).
The analysis consisted of 3 main blocks:
Male sociability model. To assess the variation in male sociability, we tested among-species differences in the time the male spent alone versus the total time the male spent in chambers with the female partner and the stranger, with species as the main factor and the testing paradigm (NDC vs. DC) as the sub-factor in the RMM. All 3 species were included in the analysis.
Model of male social preference in M. meridianus. In 8 of 12 DC tests with G. perpallidus and in 8 of 20 tests with M. unguiculatus, the animals displayed stranger-directed aggression. No conflicts were recorded in the DC-tests with M. meridianus, and thereby the full model with both the “species factor” and the “conflict factor” was incomplete and contained missing cells. Thus, we performed a separate repeated measures analysis for M. meridianus. The model for M. meridianus included the experimental paradigm (NDC vs. DC) and the chamber type (Neutral, Partner, Stranger) as the 2 sub-factors and the time in the chamber as the response variable.
Model of male social preference in G. perpallidus and M. unguiculatus. Because the consistency of the male preferences between the 2 testing paradigms in G. perpallidus and M. unguiculatus was very low, we utilized 2 separate models for the NDC- and DC-tests with the species and the conflict (yes/no) as the between factors and the chamber type as the within-effect. Though conflicts could occur in the DC-tests only, we included the “conflict factor” also in the NDC model to control for the possible inherent differences in attractiveness between females in the “conflict” and “nonconflict” samples (i.e., for each species we divided the sample of males in the NDC-tests into 2 subsamples: males involved/not involved in the conflict with a female stranger at the next, DC-stage of experiment).
In addition to the 3 main blocks of analyses, we performed 2 more statistical procedures to understand and illustrate the structure of our data thoroughly. First, to assess consistency in male preferences (i.e., in the time allocation across the 3 chambers) between the 2 stages of the test (NDC and DC), we estimated the strength of linear correlation between the 2 stages for all 3 time variables (i.e., times in each of 3 chambers) in all 3 species. Second, to visualize and assess the effect of any conflict between the focal male and the female stranger on the consistency of the male preferences in the 2 subsequent stages of the experiment, we conducted 3 simple linear regression analyses with portions of time spent by the focal male in the neutral, the partner’s and the stranger’s chambers during the first NDC-stage as predictors for the time the male spent in the same chambers during the DC-stage. We then used the residuals from the regression models as new dependent variables to reveal the pattern and the size of male response to the variation in the testing paradigm. We expected that a stronger effect of the testing paradigm on time allocation across the 3 chambers would be reflected in the higher residuals, and, if the conflict with a female stranger affected the male choice, the size of residuals would differ between the tests with and without conflict. We performed factorial analysis of variance for each residual variable (Partner Residual, Stranger Residual, and Neutral Residual) with the species (G. perpallidus vs. M. unguiculatus) and the conflict (yes/no) as factors.
Statistical analyses were performed with software packages STATISTICA v. 13.0 (StatSoft, Tulsa, OK, USA) and R 3.4.1 (R Development Core Team 2017). All tests were 2-tailed with a significance level of 0.05.
Results
Male choice between the neutral chamber and females (male sociability)
Species differed considerably in the time the male spent in the neutral chamber (Semi-parametric repeated measures analysis: Wald-type χ2 = 22.3, P = 0.0001; Figure 2). Males of M. meridianus and M. unguiculatus preferred chambers with females over the neutral chamber (the difference between species in time spent in the neutral chamber was not statistically significant - Tukey post-hoc test: P = 0.6). On the contrary, males of G. perpallidus generally spent about half of the test duration alone, substantially greater than did males of M. meridianus (Tukey post-hoc test: P = 0.002) and M. unguiculatus (P < 0.0001). The testing paradigm (NDC vs. DC) did not affect the time in the neutral chamber (χ2 = 0.8, P = 0.4). Accordingly, in 5 of 12 NDC tests and in 7 of 12 DC-tests, males of G. perpallidus spent more than half of the test time in the neutral chamber. In contrast, in almost all tests, males of both Meriones species spent more than 50% of time in the chambers with females—in 9 of 10 (NDC-tests) and 9 of 10 (DC) for M. meridianus, in 19 of 20 (NDC) and 20 of 20 (DC) for M. unguiculatus.
Male choice between familiar and strange females (male social preference)
Consistency of male preferences between the testing paradigms
In M. meridianus, the correlation between the NDC- and DC-tests was strong and significant for the time the male spent with the female stranger and in the neutral chamber, but not significant for the time he spent with the partner (Table 1). Thus, the male preferences were more or less consistent between the testing paradigms for this species. In contrast, in both G. perpallidus and M. unguiculatus, the time the male spent in each of 3 chambers at the NDC-stage was not correlated with the time he spent in the same chamber at the DC-stage of the test (Table 1).
Table 1.
Pearson’s correlation coefficients (r) between the time the male spent in the particular chamber (Neutral, Partner, or Stranger) at the NDC- and DC-stages of the test
| Chamber | Species |
||
|---|---|---|---|
| G. perpallidus (N = 12) | M. unguiculatus (N = 20) | M. meridianus (N = 10) | |
| Neutral | r=0.44, P = 0.15 | r=0.29, P = 0.2 | r=0.84, P = 0.002 |
| Partner | r=0.58, P = 0.05 | r=0.33, P = 0.15 | r=0.55, P = 0.1 |
| Stranger | r=0.27, P = 0.4 | r=0.34, P = 0.1 | r=0.77, P = 0.01 |
Note: The significant correlations (P < 0.05) are indicated by bold font.
Male social preference in M. meridianus
In M. meridianus, the time the male spent in each chamber varied among chamber types (Neutral, Partner, Stranger; Semi-parametric repeated measures analysis: Wald-type χ 2= 8.9, P = 0.01) and between the testing paradigms (NDC vs. DC; χ2 = 24.3, P < 0.0001; Table 2 and Figure 2). However, the paired comparisons within testing paradigms indicated that males of M. meridianus did not show preference for the female partner or stranger: the amounts of time the focal males spent with female partners and strangers did not differ significantly in both the NDC- and the DC-tests (Tukey post-hoc test: P = 0.8 and P = 0.9, respectively). Moreover, in the NDC-tests we did not find differences in the amounts of time the males spent in each of the 3 chambers (Tukey post-hoc test: Neutral - Partner, P = 0.6; Neutral – Stranger, P = 0.06). The only significant differences in time allocation across chambers were observed in the DC-tests, where males preferred to stay with either of the females than to be alone (Tukey post-hoc test: Neutral – Partner, P = 0.02; Neutral – Stranger, P = 0.001).
Male preferences in G. perpallidus and M. unguiculatus and the conflict effect
In G. perpallidus and M. unguiculatus, the differences in time allocation across the 3 chambers and between-species differences were not significant in the NDC-tests (Table 2 and Figure 2). On the contrary, under the DC testing paradigm, time allocation across the 3 chambers varied significantly, differed between species, and was affected by the conflict between the male and the female stranger (Table 3). Males of G. perpallidus in the DC-test generally spent more time in the neutral chamber than males of M. unguiculatus (Tukey post-hoc test: P = 0.002) and showed no preference between the female partner and the stranger whether the conflict with the female stranger occurred (P = 1.0) or not (P = 0.9). In the tests with conflicts, G. perpallidus males spent slightly more time alone than with the female stranger (P = 0.05).
Table 3.
Effects of species (G. perpallidus, N = 12, vs. M. unguiculatus, N = 20), conflict (yes/no), and chamber type (Neutral, Partner, Stranger) on the proportions of time the male spent in each chamber in semi-parametric RMs for the NDC and DC tests
| Factor | Testing paradigm |
|
|---|---|---|
| NDC | DC | |
| Species (GP/MU) | χ2=3.0, df=1, P = 0.09 | χ2=11.2, df=1, P = 0.0008 |
| Conflict (yes/no) | χ2=0.7, df=1, P = 0.4 | χ2=5.9, df=1, P = 0.01 |
| Chamber type (N, P, S) | χ2=3.8, df=2, P = 0.15 | χ2=6.4, df=2, P = 0.04 |
| Species* Conflict | χ2<0.0001, df=1, P = 1.0 | χ2=0.16, df=1, P = 0.7 |
| Species* Chamber | χ2=7.0, df=2, P = 0.03 | χ2=20.2, df=2, P < 0.0001 |
| Conflict* Chamber | χ2=0.6, df=2, P = 0.5 | χ2=6.0, df=2, P = 0.05 |
Note: Response variables were transformed (arcsine-square-root transformation). χ2 corresponds to Wald-type statistics. Three-level interactions between predictors were not significant (P > 0.1). Significant main effects (P < 0.05) are marked with bold.
In contrast to G. perpallidus, in M. unguiculatus, the conflict with the female stranger affected male preferences (Table 2). In the nonconflict situation, males of M. unguiculatus showed no preference for either female—amounts of time did not vary significantly between the partner and the stranger chambers (P = 0.7) and were significantly greater than the time spent alone (P < 0.0001 and P = 0.04, respectively). However, in the tests with conflicts, males strongly preferred the familiar partner over a stranger (Tukey post-hoc test: P = 0.003), and the time the male spent with the latter did not differ significantly from the time spent alone (P = 1.0). In both G. perpallidus and M. unguiculatus, the presence/absence of conflict with a female stranger and its effect on male preferences at the DC-stage could not be predicted from the NDC-tests results: at the NDC-stage males did not differ in time allocation across chambers regardless of whether or not they were involved in conflicts with female strangers at the subsequent DC-stage (Table 3) which excluded the possible confounding effect of females’ personality (“attractiveness”) on male choice.
The pattern and the size of male response to the testing paradigm
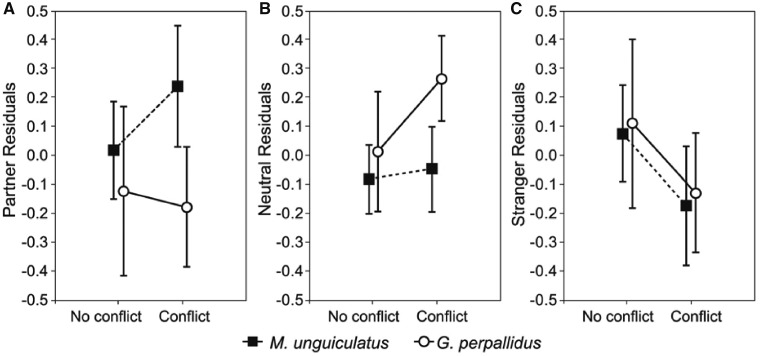
Males of G. perpallidus and M.unguiculatus differed in their response to the variation in the testing paradigm as indicated by significant between-species differences in the Partner Residuals—(higher in M. unguiculatus; factorial ANOVA: F1, 28 = 6.5, P = 0.02; Figure 3) and Neutral Residuals (higher in G. perpallidus; F1, 28 = 6.8, P = 0.01). Thus, at the DC-stage of the test, males of M. unguiculatus increased the time they spent with the female partner, whereas G. perpallidus males increased the time they spent alone. The effect of conflict was not significant for either Partner or Neutral Residual (both P > 0.05). Stranger Residual did not differ between species (F1, 28 = 0.1, P = 0.7), but was influenced by conflict with the female stranger: in both M. unguiculatus and G. perpallidus, Stranger Residuals were significantly lower in a conflict situation than if no conflict occurred (F1, 28 = 5.1, P = 0.03). In all tests, the factor interactions (Species*Conflict) were not significant (P > 0.1). Thus, at the DC-stage of the test, males of both species reduced the time with the female stranger if she displayed aggressive behavior; but whereas males of M. unguiculatus limited the time with the aggressive female in favor of more time with the female partner, males of G. perpallidus re-allocated more time to be alone.
Figure 3.
Partner (A), Neutral (B), and Stranger (C) residuals in G. perpallidus and M. unguiculatus for the tests with and without aggression (Conflict/No conflict) between the male and a female stranger. Residuals are from the regression model with the variables from the NDC-stage of the test (portions of time spent by the male in the partner’s, neutral and the stranger’s chambers) as predictors for the corresponding variables from the DC-stage. See statistics in the text; means ± 95 CI are presented.
Discussion
Male sociability and selective social preferences
In general, PPTs have revealed that gerbil species differ in sociability, male partner preferences and pair-bonding in agreement with our expectations based on the species-specific patterns of social and mating systems. As predicted, the lowest sociability combined with the lack of preference for either female partner or stranger was shown by males of G. perpallidus, supporting the results of observations in enclosures that suggested solitary living, strong territoriality and promiscuity for this poorly-studied, secretive species (Gromov and Ilchenko 2007). Males of M. meridianus were likewise unselective in choosing females, but, unlike males of G. perpallidus, displayed no stranger-directed aggression and were quite sociable, avoiding staying alone, as was expected for this solitary, but socially tolerant and nonterritorial promiscuous gerbil. Males of social and monogamous M. unguiculatus predictably displayed high sociability combined with the intersexual stranger-directed aggression and preference for the female partner over a stranger, 2 defining features of pair-bonding (Kleiman 1977; Young et al. 2008). In previous studies, M. unguiculatus also demonstrated intersexual stranger-directed aggression and selective partner preference in PPTs (Razzoli and Valsecchi 2006).
PPTs appear to be an effective tool to uncover interspecific variation and species-specific patterns of social and mating systems in rodent species beyond gerbils. Comparative studies of North American voles showed that social and monogamous species, unlike solitary nonmonogamous voles, spent less time alone and demonstrated stranger-directed aggression and preference for a familiar partner over a stranger which correlated with between-species differences in hormone regulation and neurogenetic patterns (Shapiro et al. 1986; Williams et al. 1992; Carter et al. 1995; Insel et al. 1995; Young and Wang 2004; Lim et al. 2004; Young et al. 2008). Intersexual stranger-directed aggression and selective social (not sexual) partner preference were shown in PPTs with the monogamous Peromyscus californicus (Gubernick and Nordby 1993; Gubernick and Addington 1994). Social and sexual fidelity of males toward their partner combined with aggression toward an unfamiliar female demonstrated in PPTs was assumed to maintain pair-bonds and families of Ansell’s mole-rats (Fukomys anselli, Bappert et al. 2012). Also, PPTs were effective in revealing pair-bonding and monogamous patterns in poorly studied or secretive species of Eurasian voles (Ricankova et al. 2007; Duarte et al. 2015).
The effect of testing paradigm and stranger-directed aggression on male preference pattern
On the whole, PPTs discriminated well among gerbil species and revealed species differences in sociality and pair-bonding patterns. However, the diagnostic power of the test and, thereby, conclusions about species-specific male preferences depended on the experimental set-up. The time in the neutral chamber has been the most robust to changes in the testing paradigm and can be taken as a reliable measure of sociability. This metric discriminated strictly solitary and socially intolerant G. perpallidus from the other 2, more social, species and was effective also as a clear-cut indicator of sociability in PPTs with voles (Shapiro et al. 1986; Insel et al. 1995).
On the contrary, the measure of partner preference was sensitive to the testing paradigm. The NDC-tests poorly discriminated among species and were not sensitive enough to reveal species-specific partner preference patterns. In particular, in the NDC-tests, males of monogamous M. unguiculatus showed no selective preference for either female. Earlier, PPTs with the caged animals also failed to reveal a preference for a female partner in this species (Ågren and Meyerson 1977).
Differences among species in social preferences were more pronounced in the DC PPTs and reflected species-specific patterns of gerbil social and mating systems. The results of the DC-tests depended on the behavioral context and allowed an assessment of the role of social attachment, on the one hand, and stranger-directed aggression, on the other, to selective partner preference. Both of them (separately or combined) can affect the time allocation by the test animal across the chambers. Not surprisingly, the results of the DC-tests in the nonconflict situation were similar to the results of the NDC-tests and less efficient in discriminating species than the DC-tests with conflicts. Accordingly, the preference pattern was consistent between the testing paradigms for nonaggressive M. meridianus and inconsistent for G. perpallidus and M. unguiculatus, the species that exhibited stranger-directed aggression. In both species, males similarly avoided an aggressive stranger, but in different ways—males of solitary G. perpallidus moved to the neutral chamber, that is, preferred being alone, whereas males of social M. unguiculatus reallocated time in favor of the partner. These different outcomes of similar behavioral interactions reflect species differences in sociality and mating systems and elucidate mechanisms underlying selective partner preference in M. unguiculatus. In particular, these results show that both social attachment to the partner and stranger avoidance contribute to the preference for familiar females in males of M. unguiculatus and maintain pair-bonds in this monogamous species.
In conclusion, PPTs appear to provide a comprehensive, effective, and powerful tool for comparative analysis of social and mating systems and shed light on interspecific variation in sociality and male-female relationships of gerbils and underlying mechanisms. The results of PPTs reveal that sociability, partner preferences, partner fidelity, and stranger avoidance differ strongly even between very closely-related species of gerbils as has been shown for voles of North America (Shapiro et al. 1986; Carter et al. 1995; Insel et al. 1995; Lim et al. 2004; Young et al. 2008). Broad possibilities to modify, combine and tune various designs of the PPT can help to answer many specific questions on mate choice, sociability, pair-bond formation and maintenance, territoriality and social motivation. So far, the use of the DC PPTs has been mainly confined to some arvicoline voles, and among them to the prairie vole, Microtus ochrogaster, whereas in order to answer evolutionary questions on pair-bonding, mating and social systems, a wider range of species and taxa should be studied (e.g., Carp et al. 2016; Rogovin et al. 2016). This will allow us to uncover between-species differences in social and mating preferences, as well as underlying behavioral mechanisms under standardized laboratory conditions, which will help to classify species (especially, secretive, rare or poorly-studied species like G. perpallidus; see also Duarte et al. 2015) more rigorously and will increase the power of comparative studies and evolutionary models of sociality.
Author Contributions
A.V.T., L.E.S., and N.L.O. designed the study; A.V.T., N.L.O., L.E.S., A.S., O.N.I., and S.R.S. collected the data; A.V.T. and N.A.V. performed the analysis and wrote the manuscript with support from A.S. and N.L.O. All authors contributed substantially to revisions of the draft paper.
Acknowledgments
Comments from 3 anonymous reviewers and Y. Chabovskaya were very useful and appreciated. Michael Birman helped with English wording.
Funding
The study was supported by the Russian Foundation for Basic Research (grant 16-04-01376).
References
- ASAB/ABS, 2012. Guidelines for the treatment of animals in behavioural research and teaching. Anim Behav 83:301–309. [DOI] [PubMed] [Google Scholar]
- Ågren G, 1980. Persistence of male/female social relationships in the Mongolian gerbil Meriones unguiculatus. Behav Processes 5:373–379. [Google Scholar]
- Ågren G, 1984. Pair formation in the Mongolian gerbil. Anim Behav 32:528–535. [Google Scholar]
- Ågren G, Meyerson BJ, 1977. Influence of gonadal hormones on the behaviour of pair-living Mongolian gerbils Meriones unguiculatus towards the cagemate versus a non-cagemate in a social choice test. Behav Processes 2:325–335. [DOI] [PubMed] [Google Scholar]
- Ågren G, Zhou Q, Zhong W, 1989. Ecology and social behaviour of Mongolian gerbils Meriones unguiculatus at Xilinhot, Inner Mongolia, China. Anim Behav 37:11–27. [Google Scholar]
- Ahern TH, Modi ME, Burkett JP, Young LJ, 2009. Evaluation of two automated metrics for analyzing partner preference tests. J Neurosci Methods 182:180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bappert MT, Burda H, Begall S, 2012. To mate or not to mate? Mate preference and fidelity in monogamous Ansell’s mole-rats, Fukomys anselli, Bathyergidae. Folia Zool 61:71–83. [Google Scholar]
- Blocker TD, Ophir AG, 2016. A preference to bond? Male prairie voles form pair bonds even in the presence of multiple receptive females. Anim Behav 122:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt R, Macdonald DW, 2011. To know him is to love him? Familiarity and female preference in the harvest mouse Micromys minutus. Anim Behav 82:353–358. [Google Scholar]
- Carp SB, Rothwell ES, Bourdon A, Freeman SM, Ferrer E et al. , 2016. Development of a partner preference test that differentiates between established pair bonds and other relationships in socially monogamous titi monkeys Callicebus cupreus. Am J Primatol 78:326–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, DeVries AC, Getz LL, 1995. Physiological substrates of mammalian monogamy: the prairie vole model. Neurosci Biobehav R 19:303–314. [DOI] [PubMed] [Google Scholar]
- Cho MM, DeVries AC, Williams JR, Carter CS, 1999. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles Microtus ochrogaster. Behav Neurosci 113:1071–1079. [DOI] [PubMed] [Google Scholar]
- Clark MM, Galef JBG, 1999. A testosterone-mediated trade-off between parental and sexual effort in male mongolian gerbils Meriones unguiculatus. J Comp Psychol 113:388–395. [DOI] [PubMed] [Google Scholar]
- Clutton-Brock T, Sheldon BC, 2010. Individuals and populations: the role of long-term, individual-based studies of animals in ecology and evolutionary biology. Trends Ecol Evol 25:562–573. [DOI] [PubMed] [Google Scholar]
- Daly M, Daly S, 1975. Socio-ecology of Saharan gerbils, especially Meriones libycus. Mammalia 39:289–312. [Google Scholar]
- Duarte MA, da Luz Mathias M, Bastos-Silveira C, 2015. Pair-bonding behaviour of the sister species Microtus lusitanicus and M. duodecimcostatus. J Ethol 33:213–223. [Google Scholar]
- Elwood RW, Broom DM, 1978. The influence of litter size and parental behaviour on the development of Mongolian gerbil pups. Anim Behav 26:438–454. [Google Scholar]
- Friedrich S, Konietschke F, Pauly M, 2017. GFD: an R package for the analysis of general factorial designs. J Stat Softw 79:1–18.30220889 [Google Scholar]
- Frynta D, Volfová R, Fraňková-Nováková M, Stejskal V, 2010. Oestrous females investigate the unfamiliar male more than the familiar male in both commensal and non-commensal populations of house mice. Behav Processes 83:54–60. [DOI] [PubMed] [Google Scholar]
- Gerling S, Yahr P, 1979. Effect of the male parent on pup survival in Mongolian gerbils Meriones unguiculatus. Anim Behav 27:310–311. [Google Scholar]
- Goldman JM, Murr AS, Cooper RL, 2007. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B 80:84–97. [DOI] [PubMed] [Google Scholar]
- Gol'tsman ME, Popov SV, Chabovskiĭ AV, Borisova NG, 1994. The sociality syndrome: a comparative study of the behavior of gerbils. Zh Obshch Biol 55:49–69. [In Russian] [PubMed] [Google Scholar]
- Gromov VS, 1981. Social-organization of family groups of the clawed jird Meriones unguiculatus in natural colonies. Zool Zh 60:1683–1694. [In Russian] [Google Scholar]
- Gromov VS, 1996. The spatial relationships and social structure of gerbils in the genus Meriones (Gerbillinae, Rodentia). Zh Obshch Biol 58:35–54. [In Russian] [PubMed] [Google Scholar]
- Gromov VS, Ilchenko OG, 2007. Use of space and social organization in Gerbillus perpallidus under semi-natural conditions. Zool Zh 86:1131–1140. [In Russian] [Google Scholar]
- Gromov VS, Vorobieva TV, 1995. The behavior of midday gerbils Meriones meridianus under semi-free maintenance. 1. The social organization and the use of space. Zool Zh 74:94–109. [In Russian] [Google Scholar]
- Gubernick DJ, Addington RL, 1994. The stability of female social and mating preferences in the monogamous California mouse Peromyscus californicus. Anim Behav 47:559–567. [Google Scholar]
- Gubernick DJ, Nordby JC, 1993. Mechanisms of sexual fidelity in the monogamous California mouse Peromyscus californicus. Behav Ecol Sociobiol 32:211–219. [Google Scholar]
- Hammock EA, 2007. Gene regulation as a modulator of social preference in voles. Adv Genet 59:107–127. [DOI] [PubMed] [Google Scholar]
- Hammock EAD, Lim MM, Nair HP, Young LJ, 2005. Association of vasopressin 1a receptor levels with a regulatory microsatellite and behavior. Genes Brain Behav 4:289–301. [DOI] [PubMed] [Google Scholar]
- Harrison N, Lopes PC, König B, 2016. Oxytocin and social preference in female house mice Mus musculus domesticus. Ethology 122:571–581. [Google Scholar]
- Hurtado-Parrado C, González CH, Moreno LM, González CA, Arias M et al. , 2015. Catalogue of the behaviour of Meriones unguiculatus f. dom. (Mongolian gerbil) and wild conspecies, in captivity and under natural conditions, based on a systematic literature review. J Ethol 33:65–86. [Google Scholar]
- Insel TR, Preston S, Winslow JT, 1995. Mating in the monogamous male: behavioral consequences. Physiol Behav 57:615–627. [DOI] [PubMed] [Google Scholar]
- Ito M, Jiang W, Sato JJ, Zhen Q, Jiao W et al. , 2010. Molecular phylogeny of the subfamily Gerbillinae (Muridae, Rodentia) with emphasis on species living in the Xinjiang - Uygur autonomous region of China and based on the mitochondrial cytochrome b and cytochrome c oxidase subunit II genes. Zoolog Sci 27:269–278. [DOI] [PubMed] [Google Scholar]
- Johnston RE, Rasmussen K, 1984. Individual recognition of female hamsters by males: role of chemical cues and of the olfactory and vomeronasal systems. Physiol Behav 33:95–104. [DOI] [PubMed] [Google Scholar]
- Kappeler PM, 2014. Lemur behaviour informs the evolution of social monogamy. Trends Ecol Evol 29:591–593. [DOI] [PubMed] [Google Scholar]
- Kappeler PM, Fichtel C, 2016. The evolution of Eulemur social organization. Int J Primatol 37:10–28. [Google Scholar]
- Kleiman DG, 1977. Monogamy in mammals. Q Rev Biol 52:39–69. [DOI] [PubMed] [Google Scholar]
- Lenth RV, 2016. Least-squares means: the R package lsmeans. J Stat Softw 69:1–33. [Google Scholar]
- Lim MM, Wang Z, Olazábal DE, Ren X, 2004. Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature 429:754. [DOI] [PubMed] [Google Scholar]
- Lim MM, Liu Y, Ryabinin AE, Bai Y, Wang Z et al. , 2007. CRF receptors in the nucleus accumbens modulate partner preference in prairie voles. Horm Behav 51:508–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas D, Clutton-Brock TH, 2013. The evolution of social monogamy in mammals. Science 341:526–530. [DOI] [PubMed] [Google Scholar]
- Mabry KE, Streatfeild CA, Keane B, Solomon NG, 2011. avpr1a length polymorphism is not associated with either social or genetic monogamy in free-living prairie voles. Anim Behav 81:11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ophir AG, Campbell P, Hanna K, Phelps SM, 2008. Field tests of cis-regulatory variation at the prairie vole avpr1a locus: association with V1aR abundance but not sexual or social fidelity. Horm Behav 54:694–702. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Phillips KM, Lee TM, 2001. Development of selective partner preferences in captive male and female meadow voles Microtus pennsylvanicus. Anim Behav 61:1217–1226. [Google Scholar]
- Popov SV, Tchabovsky AV, Shilova SA, Shchipanov NA, 1989. Mechanisms of formation of the spatial-and-ethological population structure in the Midday gerbil. Fauna Ecol Rodents 17:5–57. [In Russian] [Google Scholar]
- R Core Team. 2016. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, http://www.R-project.org/. [Google Scholar]
- Razzoli M, Valsecchi P, 2006. Different social bonds produce differential effects on behaviour and physiology in Mongolian gerbils. Ethol Ecol Evol 18:289–306. [Google Scholar]
- Ricankova V, Sumbera R, Sedlacek F, 2007. Familiarity and partner preferences in female common voles Microtus arvalis. J Ethol 25:95–98. [Google Scholar]
- Roberts SC, Gosling LM, 2004. Manipulation of olfactory signaling and mate choice for conservation breeding: a case study of harvest mice. Conserv Biol 18:548–556. [Google Scholar]
- Rogovin KA, Khrushchova AM, Shekarova ON, Vasilieva NA, Vasilieva NY, 2016. Females choose gentle, but not healthy or macho males in Campbell dwarf hamsters (Phodopus campbelli Thomas 1905). Curr Zool 63:545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schradin C, 2017. Comparative studies need to rely both on sound natural history data and on excellent statistical analysis. R Soc Open Sci 4:170346.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro LE, Austin D, Ward SE, Dewsbury DA, 1986. Familiarity and female mate choice in two species of voles (Microtus ochrogaster and Microtus montanus). Anim Behav 34:90–97. [Google Scholar]
- Shilova S, Derviz N, Shilov A, Stchipanov N, Marova I et al. , 1983. Some features of territorial distribution and behavior in Meriones meridianus (Rodentia, Cricetidae) under the conditions changed by anthropogenic effects. Zool Zh 62:916–921. [In Russian] [Google Scholar]
- Shilova SA, Tchabovsky AV, 2009. Population response of rodents to control with rodenticides. Curr Zool 55:81–91. [Google Scholar]
- Solomon NG, Richmond AR, Harding PA, Fries A, Jacquemin S et al. , 2009. Polymorphism at the avpr1a locus in male prairie voles correlated with genetic but not social monogamy in field populations. Mol Ecol 18:4680–4695. [DOI] [PubMed] [Google Scholar]
- Taig-Johnston M, Strom MK, Calhoun K, Nowak K, Ebensperger LA et al. , 2017. The ecological value of long-term studies of birds and mammals in Central America, South America and Antarctica. Rev Chil Hist Nat 90:7. [Google Scholar]
- Tchabovsky A, Bazykin G, 2004. Females delay dispersal and breeding in a solitary gerbil Meriones tamariscinus. J Mammal 85:105–112. [Google Scholar]
- Thomas BB, Oommen MM, 1999. Reproductive biology of the South Indian gerbil Tatera indica cuvieri under laboratory conditions. Mammalia 63:341–348. [PubMed] [Google Scholar]
- Trivers R, 1972. Parental Investment and Sexual Selection. Cambridge: Biological Laboratories, Harvard University. [Google Scholar]
- Verevkin MV, 1985. Reproductive biology of the midday jird Meriones meridianus. Zool Zh 64:276–281. [Google Scholar]
- Volodin IA, Ilchenko OG, Popov SV, 1996. Peschanki: Soderzhanie i Demographiya Populyatsii Raznykh Vidov v Nevole (Gerbils: Management and Demography of Different Species in Captivity). Moscow: Moscow Zoo. [In Russian] [Google Scholar]
- Williams JR, Catania KC, Carter CS, 1992. Development of partner preferences in female prairie voles Microtus ochrogaster: the role of social and sexual experience. Horm Behav 26:339–349. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR, 1993. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature 365:545.. [DOI] [PubMed] [Google Scholar]
- Young KA, Liu Y, Wang Z, 2008. The neurobiology of social attachment: a comparative approach to behavioral, neuroanatomical, and neurochemical studies. Comp Biochem Phys C 148:401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Hammock EA, 2007. On switches and knobs, microsatellites and monogamy. Trends Genet 23:209–212. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z, 2004. The neurobiology of pair bonding. Nat Neurosci 7:1048.. [DOI] [PubMed] [Google Scholar]
- Zhang J, Shi D, Sun L, 2006. The effect of male competition on female choice in Brandt’s vole Lasiopodomys brandti. Folia Zool 55:123. [Google Scholar]