Abstract
Angle dependent colors, such as iridescence, are produced by structures present on flower petals changing their visual appearance. These colors have been proposed to act as signals for plant–insect communication. However, there is a paucity of behavioral data to allow for interpretations of how to classify these colors either as a signal or a cue when considering the natural conditions under which pollination occurs. We sampled flowers from 6 plant species across various viewpoints looking for changes in the visual appearance of the petals. Spectral characteristics were measured with different instruments to simulate both the spectral and spatial characteristics of honeybee’s vision. We show the presence of color patches produced by angle dependent effects on the petals and the calyx of various species; however, the appearance of the angle dependent color patches significantly varies with viewpoint and would only be resolved by the insect eye at close distances. Behavior experiments with honeybees revealed that pollinators did not use angle dependent colors to drive behavior when presented with novel flower presentations. Results show that angle dependent colors do not comply with the requirements of a signal for plant–pollinator communication since the information transmitted by these colors would be unreliable for potential, free-flying pollination vectors. We thus classify angle dependent colors produced by micro- and ultra-structures as being a cue (a feature which has not evolved for communication), and observe no evidence supporting claims of these angle dependent colors having evolved as visual signal.
Keywords: approach angle, color, flower, iridescence, photography, pollination, vision
Flowering plants around the world have evolved a wide range of flower types displaying a striking gamut of colors using a variety of different pigments (Faegri and Pijil 1966; Scogin 1983; Rausher 2008; Tanaka et al. 2008; Dyer et al. 2012; Ng et al. 2018). Petal colors attract flower visitors, like bees or birds (Varassin et al. 2001; Shrestha et al. 2013), which facilitate the efficient transfer of pollen between conspecific plants (Chittka and Menzel 1992; Chittka et al. 1999). In recent times, there has been an increasing number of reports of different optical phenomena producing angle dependent coloration through the interaction of optical radiation with microstructures on flowers belonging to distantly related clades to produce visual effects including iridescence (Whitney et al. 2009b; Vignolini et al. 2015), mirror-like reflectance (gloss) (Vignolini et al. 2012; van der Kooi et al. 2017), and “halos” (Moyroud et al. 2017). The optical principles leading to the production of angle dependent colorations such as iridescence and mirror-like reflection (gloss) are produced by interference of incident light caused by the presence of nano, and ultra-structures of different refractive order regularly or quasi-regularly ordered on the petal surface (van der Kooi et al. 2018, 2017); for this reason, such colors are commonly referred to as structural colors to differentiate them from colors produced by the selective absorption of light as those produced by pigments (Srinivasarao 1999; Nassau 2001). In the present manuscript, we will thus refer interchangeably to both angle dependent and structural colors, as our primary question is how such colors may be used by bee pollinators in a way that would fit the formal definition for signal.
Accordingly to various authors, angle dependent colors have evolved to produce visual signals to potential pollinators (Whitney et al. 2009b; Moyroud et al. 2017). However, it currently remains unresolved as to whether such optical effects are indeed biologically significant when considering the sensory capabilities of important pollinators like bees (Morehouse and Rutowski 2009; van der Kooi et al. 2015). To understand if angle dependent color in flower can be classified as a signal when considering plant–pollinator interactions, it is essential to recognize in what circumstances visual information can be effectively transferred to a potential pollinator. Thus, to understand flower evolution, it is necessary to understand bee–pollinator perception.
The use of the term signal when referring to angle dependent colors in plants implies that these colors allow for an effective visual communication between plant (sender) and insect (receiver). More precisely, these type of colors should comply with 3 conditions to be considered as a signal: (a) effectively transmit information from the signaler to the receiver, (b) have evolved for this particular purpose, and (c) both parties should benefit from producing and monitoring these colors (Smith and Harper 2003; Bradbury and Vehrencamp 2011). Visual traits producing stimuli that do not meet the fore mentioned 3 criteria may be defined as a cue (Bradbury and Vehrencamp 2011). Unlike signals, cues have not specifically evolved for communication purposes and may be produced as a secondary effect or by-product of inherent anatomical characteristics to the emitter (Bradbury and Vehrencamp 2011).
In contrast to structural colors produced by flower petals, angle dependent colors produced by animals, as for example bird feathers (Finger et al. 1992), are known to be effectively used as signals for visual communication. For example, female peacocks use the color produced by the iridescent plumage of males to detect and visit mates (Loyau et al. 2007), the quality of the structural color in house sparrows is correlated with the nutritional condition of the bird (McGraw et al. 2002), plumage structural coloration of eastern bluebirds acts as honest signal of male quality and females matting with the most colorful males receive benefits from their mates (Siefferman and Hill 2003); and female starlings use structural coloration to rank male attractiveness (Bennett et al. 1997).
Flower colors produced by pigments can be classified as a visual signal (van der Kooi et al. 2018) as this type of color complies with the 3 requirements for effective communication between plant and insect. Flowers relying on hymenopterans to reproduce typically offer small nutritional rewards to their visitors (Goulson 1999) and have often evolved colors that maximizes their discriminability considering the visual system of important pollinators (Chittka and Menzel 1992; Dyer et al. 2012; Shrestha et al. 2013; Bukovac et al. 2016). Furthermore, insect pollinators like bees constantly visit conspecific rewarding flowers that are easily recognized to maximize their nutritional intake, and this flower constancy promotes the evolution of flower color signals that best correspond to the visual capabilities of important pollinators (Chittka et al. 1999; Burns and Dyer 2008; Shrestha et al. 2013). However evidence supporting the role of angle dependent colors in flowers as visual signals remain tenuous when considering natural environments (Morehouse and Rutowski 2009; van der Kooi et al. 2015, 2018).
A fundamental requirement for petal color to serve as a signal for visual communication is that this trait should unambiguously transmit information from the flower to the insect (Smith and Harper 2003). Most pigment-based colors present in flower petals retain their chromatic appearance independently from viewing angle as they produce diffuse reflection (Lee 2005). This means that a pollinator approaching a flower from any direction will perceive the color independent of the angle of illumination. However, this may not be the case with angle dependent colors since by definition there can be significant changes in appearance depending on the direction of illumination and approach of a prospective pollinator (van der Kooi et al. 2015, 2018).
Let us consider the case of a hypothetical flower displaying a color pattern consisting of angle dependent color patches produced by 2 different phenomena, plus a diffuse, angle independent color produced by pigment (Figure 1). In our example, as in naturally occurring flowers, a pollinator may approach from any inclination angle (ϕ) along the vertical axis (red arrow in Figure 1), and from any orientation angle (θ) along the horizontal plane (green arrow in Figure 1). Furthermore, one of the angle dependent colors may result from Fraunhofer Diffraction produced by a grating as reported for Hibiscus trionum (Whitney et al. 2009b), where the intensity of the reflected radiation varies with viewing angle (Hecht 2002). The second angle dependent color may be the result of mirror-like reflectance as the type of angle dependent coloration observed in several species of the genus Ranunculus (family Ranunculaceae) (Galsterer et al. 1999; van der Kooi et al. 2017). In both cases, as in many examples of angle dependent colors, a pollinator would only see the angle dependent colors when approaching the flower at those specific angles where the petal microstructure allows for constructive interference of the radiation reflected by the petals (Hecht 2002; van der Kooi et al. 2016). For this reason it is of value to assess potential changes in the visual appearance of a flower by collecting information from different angles as those used by a free flying pollinator (van der Kooi et al. 2015), using calibrated digital images that allow to recover measurements of total reflectance from digital values (Garcia et al. 2014).
Figure 1.
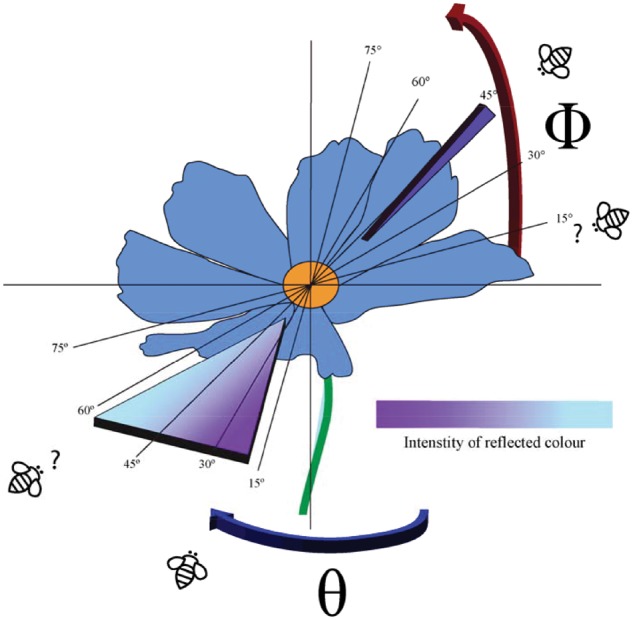
Diagram depicting a hypothetical flower whose color is the result of angle dependent and independent colors. The perceived appearance of the angle dependent colors depends on the optical phenomena producing them and view angle which is a combination of the inclination angle along the y-axis (ϕ, red arrow) and the orientation angle along the x-axis (θ, blue arrow). In this particular example, one of the angle dependent colors is a mirror-like reflectance only visible at a particular inclination angle (top right quadrant). The second angle dependent color is produced by a simple diffraction effect where the intensity of a given reflected color changes with angle (bottom left quadrant) here represented by the graded color. The third color is produced by radiation reflected by the pigment which produces a solid, diffuse color angle independent from view point (solid blue color). Depending on the particular approach angle, an insect pollinator will perceive different aspects of the angle dependent component of the color pattern (question marks). However, the appearance of the diffuse pigment color will remain the same independent from view point.
To communicate information that can drive an insect pollinator behavior as expected from a signal (Smith and Harper 2003; Bradbury and Vehrencamp 2011), the petal appearance resulting from the joint effect of the structural and pigment coloration should remain constant across all directions used by an insect to approach a flower. Alternatively, the insect has to be able to detect and identify a flower independently from changes in its appearance resulting from approaching the target from different directions (Figure 1). The latter condition implies that the pollinator has to use the overall change in appearance induced by the angle dependent colors as information for identifying the flower sending the signal (de Premorel et al. 2017). Laboratory measurements of the optical properties of various petals showing angle dependent colorations suggest that the former hypothesis does not hold true for several species. Some color effects produced by nano and ultra-structures such as iridescence (Whitney et al. 2009b) and specular reflection (Vignolini et al. 2012) are only visible at specific angles. However, studies considering changes in petal appearance due to angle dependent coloration under natural-like illuminations (van der Kooi et al. 2015; Vignolini et al. 2015) have not formally tested for the potential correlation between angle and changes in the visual appearance of the petals as perceived by potential pollinators.
Whether hymenopteran insect pollinators use visual information produced by the structural coloration to drive decisions remains a topic for debate (van der Kooi et al. 2018). Some authors have addressed this question through the use of a discrimination paradigm where bumblebees (Bombus terrestris) were trained to discriminate between angle dependent and diffuse colorations on artificial targets mimicking petal structural colors (Whitney et al. 2009b; de Premorel et al. 2017). In these experiments, bumblebees learned to discriminate between the angle dependent and angle independent colorations following an appetitive aversive differential conditioning which significantly improve learning in bees (Avarguès-Weber et al. 2010). Using this conditioning procedure each bee received a sucrose reward when choosing the iridescent targets, and was punished with a quinine solution when choosing the non-iridescent distractor. Although results from these experiments show that bumblebees can readily learn to discriminate angle dependent colorations from their angle independent counterparts after extensive conditioning, these experiments do not prove that under natural circumstances structural color are used as signals by insect pollinators.
In recent years it has become clear that understanding how a bee pollinator uses their color vision in a natural setting requires careful consideration of what motivates and modulates the attention of individuals (Dyer 2012). Testing on color vison in both honeybees (Giurfa 2004; Reser et al. 2012; Garcia et al. 2018) and bumblebees (Dyer and Chittka 2004; Garcia et al. 2018) shows that bees trained with absolute conditioning (i.e. target stimuli in isolation) only enables a relatively coarse level of discrimination. In contrast, bees trained with differential conditioning (i.e. rewarded target stimuli vs. non-rewarded and perceptually similar distractor stimuli) acquire fine color discrimination. Differential conditioning results in the formation of a long-term memory (Dyer and Chittka 2004; Dyer and Garcia 2014), which has also been recently reported in other hymenopterans such as ants (Yilmaz et al. 2017). The use of appetitive-aversive conditioning, where choices for the correct distractor are punished with a bitter tasting quinine solution further improve color discrimination (Chittka et al. 2003), probably via modulation of attention (Avarguès-Weber et al. 2010). The question then becomes which type of condition is most appropriated for evaluating hypotheses about flower signal evolution. By comparing either absolute or differential conditioning functions for either honey or bumblebees to pigment-based flower color signals (Dyer et al. 2012; Garcia et al. 2018) or the flower constancy behavior of bees (Dyer 2006), it has been shown that for natural conditions absolute conditioning is the correct behavioral paradigm to use for understanding how bee pollinators use visual information in a way that might drive flower evolution. For example, color discrimination under absolute conditioning explains how insect pollinators may cope with the color variability observed in natural flowers to maintain flower constancy, and allow for “imperfect” camouflage in spiders preying on visiting honeybees (Garcia et al. 2018).
Here we address the important question of the reliability of structural color under simulated natural lighting conditions when considering both viewpoint and the spectral and spatial characteristics of the visual system of the honey bee (Apis mellifera). We used linearized digital images, which express total reflectance at each pixel location (Stevens et al. 2007; Garcia et al. 2013a), and a mechano-optical device which produces images with an spatial resolution close to that measured for honeybees (Knowles and Dartnall 1977; Williams and Dyer 2007). To fully understand the extent to which angle dependent colors are biologically relevant, we test free-flying honeybees, trained under absolute condition, on their capacity to use visual information from the different patterns produced by angle dependent patterns to drive decisions.
We specifically test the role of angle dependent coloration on a biologically relevant discrimination task as it is already known that honeybees (Giurfa et al. 1996; Dyer et al. 2008) and bumblebees (Spaethe et al. 2001; Dyer et al. 2008; predominantly use achromatic vision mediated by the long wavelength sensitive photoreceptor for flower detection. Therefore, color information is not used for flower detection in bees.
If a flower’s structural color does constitute a visual signal which provides information to the pollinator such that it may modify its behavior, one can hypothesize that: (i) color patches produced by angle dependent colors are perceivable when considering the visual acuity of a bee. (ii) it is robust enough as to enable flower identification independently from viewing angle and (iii) it is readily discriminable from pigment color. For the first hypothesis to be true, small color patches responsible for angle dependent colors in flowers should be easily discerned when observed through an optical device with the same resolution as that of the compound eye of a pollinator such as a honeybee. For the second hypothesis to hold true, the visual appearance of the color pattern of a flower should be independent from view point. Finally, for the third hypothesis, pollinators should be able to learn and recognize the pattern produced by a given angle dependent coloration when asked to choose between this option and a solid color whose appearance is independent from viewing angle. Altogether the null hypothesis framework is that angle dependent colors are only incidental effects.
Materials and Methods
Plant material
Flowers from 6 insect pollinated plant species: (a) Alyogyne huegelii, (b) Solanum laciniatum, (c) Lycianthesrantonnetii (previously Solanum rantonnetii), (d) Tropaeolum majus, (e) Hibiscus heterophyllus, and (f) Pelargonium rodneyanum (Figure 2) were collected from a botanical garden at Monash University, Clayton campus, Victoria, Australia during late Austral spring 2014 (September–November). Four native species to Australia: (a) A. huegelii, (b) S. laciniatum, (e) H. heterophyllus, and (f) P. rodneyanum were grown in the native plant section of the garden as an indigenous food plant; while the 2 naturalized species (L. rantonnetti and T. majus) were cultivated in a separate section of the garden. Flowers were placed inside a cooler at about 15°C and immediately brought to the lab for spectrophotometric measurement and photographic recording to ensure that petal microstructures potentially producing angle dependent coloration were preserved (Vignolini et al. 2015).
Figure 2.
RGB representation of flowers from the 6 plant species used for our experiments: (A) Alyogyne huegelii, (B) Solanum laciniatum, (C) Lycianthes rantonnetii, (D) Tropaeolum majus, (E) Hibiscus heterophyllum, and (F) Pelargonium rodneyanum.
Our samples include 4 species from the closely related orders: Brassicales (T. majus), Geraniales (P. rodneyanum), and Malvales (A. huegelii and H. heterophyllus) (Wikström et al. 2001), whereas S. laeciniatum and L. rantonnetii belong to the order Solanales. The 2 species of order Malvales were chosen to compare with Hibiscus trionum, the plant species for which angle dependent, iridescent colorations were first reported (Whitney et al. 2009b). The orders Brassicales and Geraniales are the closest to Malvales that serve as comparison between 2 groups whereas the remaining species of our sample, while S. laciniatum and L. rantonnetii, serve as a potential outgroup for comparison. These species were selected to compare within and outside the order to which H. trionium belongs to test if iridescence (Whitney et al. 2009) is a property observed in other plant groups. Moreover, these plants were also selected to understand plant–pollinator interactions in a broader phylogenetic scale.
Spectrophotometry
Spectra were measured from 300 to 700 nm using an Ocean Optics USB2000+ Spectrometer (Ocean Optics Inc., USA) equipped with quartz optics and connected to a PX-2 pulsed xenon light source (Ocean Optics Inc., USA, 2011). The spectrophotometer was controlled using the software package Spectra Suite (Ocean Optics, USA), and calibrated before each measurement to avoid drift from electrical noise. Reflectance profiles were measured relative to a Lambertian, PTF WS-1 reflectance standard (Ocean Optics, USA). Mean reflectance spectrum for each species corresponds to multiple spectral measurements of 3 different flowers as described in Dyer et al. (2012) and Shrestha et al. (2013).
Scanning electron microscopy (SEM) imaging
We prepared replicas of the petal surfaces following methods described by van der Kooi et al. (2014). Briefly, sepals and petal were pressed into a dental impression material that solidifies within minutes. Positive surface replicates were subsequently generated by filling the mould with transparent nail polish, creating a cast. Casts were sputtered gold coated and images were acquired using a Scanning Electron Microscope (Philips XL30) at the RMIT Microscopy and Microanalysis Facility (RMMF), at RMIT University, Melbourne, Australia. We used 30 KV current with spot size 5 and magnification ranges 6,000×–1,800× with a 10 mm working distance from the sample to the current beam.
Photographic recording and image processing
Flower samples were located on a platform 55 cm high and inserted in a black cardboard shield to minimize potential reflection from background. To account for variations in the size and location of structural color patches arising from changes in viewing point, we recorded a total of 37 images for each flower within a hemisphere (dome) sampling grid centered at the flower sample. Sampling viewpoints were defined in terms of spherical coordinates using 3 parameters: (i) the angle on the x–y plane (azimuth, ϴ) created from the x-axis to the camera’s position, (ii) the angle between the x–y plane and the camera position (inclination, ϕ), and (iii) the distance (radius, r) between the center of the flower and the camera at each ϴ, ϕ combination. These viewpoints represented typical approach angles observed for several bee species (Apis sp., Bombus sp., Trigona sp.) foraging in natural environments (Garcia et al. 2018; Dyer AG, Shrestha M, personal observation); refer to van der Kooi et al. (2015) for discussion.
Sampled azimuth angles ranged from 0° to 315° at 45° intervals. Five different inclination angles (ϕ = 15°, 30°, 45°, 60°, and 75°) plus the zenith (ϕ = 90°) position were sampled for each orientation position excepting for ϴ = 45°. At this azimuth angle, only the inclination ϕ = 45° was sampled to prevent shadowing the illumination produced by the main light source. The light source consisted of a bare bulb (uncoated) Broncolor Pulso F2 flash lamp (Bron Elektronik, Switzerland) connected to a Broncolor Graffit A2 power pack (Bron Elektronik, Switzerland) raised 2.00 m from the floor and aimed perpendicular to a white ceiling of 4.10 m height. This arrangement simulated a lighting condition typical of open environments where the light reaching the target is made up by the mixture of the direct light emitted by a point source (the Sun) and the indirect light reflected or refracted by the sky, and resulted in a realistic environmental illumination difference ratio of about 8:1 (3 photographic stops) (Salvaggio 2009). For each flower, an additional image was recoded at the zenith of the sample.
Images were recorded with a calibrated Canon EOS 40D digital camera (Canon Inc., Japan) equipped with a 100-mm electro focused macro lens (Canon Inc., Japan). Images were stored as native RAW files and encoded into 8-bit, Adobe 1998 color space (Adobe Systems Incorporated 2005) TIFF files using the Adobe Camera Raw v.7.3 plug-in available as part of the Adobe Design and Web Premium Suite CS6 (Adobe Corp., USA). TIFF files were linearized to recover values equivalent to the total number of photons captured by each of the color channels, analogous to P-values sensu (Wyszecki and Stiles 1982; Chittka 1992) making up the RGB image at each pixel location. Linearization was carried out using look up tables (LUTs) specifically constructed for our imaging device (Garcia et al. 2013a, 2014). Recovered P-values were subsequently transformed into physiological receptor excitations, or E-values (Chittka 1992), by applying the Naka and Rushton transformation to accurately model pollinator color perception (Naka and Rushton 1966; Chittka 1992; Spaethe et al. 2001; Dyer et al. 2007; Whitney et al. 2009b).
Spectral threshold
Since ultraviolet (UV) reflecting patches are present in several flower species (Chittka et al. 1994; Kevan et al. 2001), we assessed our sample of flower species to evaluate if any flower reflected sufficient UV radiation to be perceivable when considering the spectral sensitivity of typical hymenopteran pollinators (Peitsch et al. 1992). In the current study, we considered flowers with apparent structural colors, but no modulation of the UV-sensitive photoreceptors of a bee (Figure 3 and Supporting Information S-1).
Figure 3.
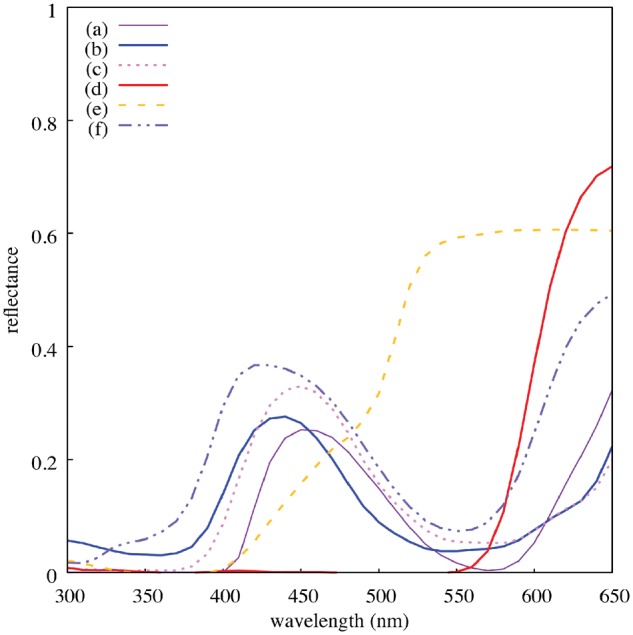
Reflectance spectra from the 6 plant species sampled for the study: (a) A. heugelii, (b) S. laciniatum, (c) L. rantonnetii, (d) T. majus, (e) H. heterophyllum, and (f) P. rodneyanum. Excepting from L. rantonnetti, selected species did not modulate the UV photoreceptor of the honeybee. As our imaging system had no sensitivity to this spectral interval, this species was excluded from subsequent analyses.
We employed the hexagon color space (Chittka 1992; Whitney et al. 2009b; Dyer et al. 2012; Garcia et al. 2017) to (a) model the chromatic appearance of the pigment coloration of each species and (b) to identify the color difference required by an angle dependent color patch to be distinguishable from its pigment background. We set a color distance of 0.04 hexagon units (hu) as the color discrimination threshold required by a honeybee to discriminate between angle dependent (structural) and angle independent (diffuse) colors. “Blue” color stimuli differing by 0.04 hu can be discriminated by a honeybee trained under an appetitive aversive conditioning about 96% of the time as predicted by the color discrimination function for this species modeled from behavioral data (Dyer and Neumeyer 2005; Garcia et al. 2017). Therefore, for each of the flower species used for our experiment, we represented the color discrimination threshold as a circle with a radius of 0.04 hexagon units centered at the x–y coordinates corresponding to the color produced by the pigment reflectance spectrum for each species (Figure 4).
Figure 4.
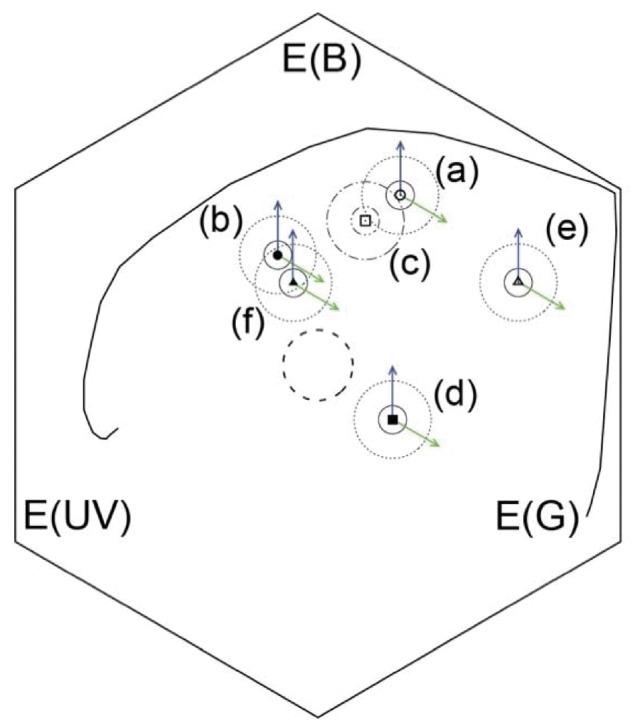
Representation of the petal colors corresponding to the reflectance spectra in Figure 3 in the hexagon color space (Chittka 1992): (a) A. huegelii (open circle), (b) S. laciniatum (solid circle), (c) L. rantonnetii (open square), (d) T. majus (closed square), (e) H. heterophyllum (open triangle), and (f) P. rodneyanum (closed triangle). Circles surrounding the markers indicating each flower species represent the discrimination threshold for a typical hymenopteran pollinator trained with differential conditioning when discrimination color differences of 0.04 hu (solid circle) and 0.11 hu (dashed circle). Arrows represent the shift in color space expected from increasing the photoreceptor excitation values (E-vectors) by either the medium (E(B), solid blue arrow) or long (E(G), solid green arrow) photoreceptors here modeled by the transformed linear response of the green and blue color channels of a characterized digital camera (Garcia et al. 2013a, 2014). Photoreceptor excitation values corresponding to the point of intersection between the vector and the discrimination threshold are considered as being perceptually different from the pigment-produced color, and thus used as threshold values for differentiating structural from pigment colouration (refer to text for details).
We then established spectral discrimination threshold values for the 405–505 nm and 450–600 nm spectral intervals corresponding to the regions sensed by the respective “blue” and “green” channels of our camera system (Garcia et al. 2014) for each one of the flower sample species. Spectral threshold values were obtained from a pair of E-vectors (i.e. modulation of color space excitation values), which systematically increased in the number of photon catches for the blue and green photoreceptors from those corresponding to the measured spectral reflectance for each flower and represented as the origin of the discrimination circle (blue and green arrows in Figure 4). The intersection point between each of the respective vectors and their corresponding color discrimination circle was then established as a threshold value for identifying petal regions where the structural color was perceptually different from the pigment-based hue in the linearized images. The result of the threshold operation consisted on a set of binary masks incorporating white for selected pixels, but black otherwise, representing petal regions with angle dependent color patches perceivable as being different from the pigment background for either the “blue” or the “green” channels of the linearized images.
Spatial threshold
The size of the lens and diameter of the rhabdoms making up most insect’s compound eyes limit their spatial resolution (resolving power) to less than about 1 cycle per degree of visual angle (cpd) (Land 1997). Large, simple lenses such as those present in vertebrate eyes and photographic optics typically have a minimum resolving power well above this limit (Kirschfeld 1976; Land 1997; Williams and Dyer 2007). This means that structural coloration patches, although potentially perceivable as being of different color on a photographic image, may not necessarily be resolved by an insect eye (van der Kooi et al. 2015). To account for this potential limitation, we recorded images corresponding to the threshold, binary masks using a mechano-optical device constructed on the optical principle of ray selectors (Knowles and Dartnall 1977; Williams and Dyer 2007). The device consisted on an array of about 4,500 black tubes, 31 cm long with a diameter of 3 mm stacked in a 36 ×38 cm wooden frame which projected a single image on a piece of architecture tracing paper of the same dimensions. This arrangement produced images with a spatial resolution of about 0.24 cpd (Williams and Dyer 2007), very close to the 0.23 cpd corner resolution limit behaviorally determined for free flying honeybees (Srinivasan and Lehrer 1988). This visual acuity principle also approximately fits with how other bees like bumblebees use visual information to find flowers in complex-type environments (Spaethe et al. 2001; Dyer et al. 2008, 2016).
Binary mask images and their corresponding non-linear RGB representations were displayed on an LED 27″ Thunderbolt Display (Apple Corp., USA) with a resolution of 2,560 ×1,440 pixels. Images were resized such that when projected through the mechano-optical device at a distance of 0.3 cm produced an image of sufficient size to cover a piece of architectural drafting paper attached to the device’s wooden frame. This set-up replicated how a bee’s compound eye may resolve the flower at close range (Williams and Dyer 2007). Images projected on the tracing paper corresponding to the different azimuth and inclination angles for each species were photographed using the same Canon 40D camera used for recording the flower samples.
TIFF images containing the mechano-optical representation of the spectral threshold masks and their corresponding non-linear RGB images were then segmented following protocols for measuring and analyzing color patterns (Garcia et al. 2013b) to identify and measure: (a) the petal’s area corresponding to the angle dependent coloration and (b) the total visible area. These 2 variables were subsequently used for calculating the ratio of petal area occupied by angle dependent color patches (RAD) on each image by applying Equation (1).
| (1) |
Statistical analysis of images
To test the reliability of the color signal produced by the structural color component of the color pattern, we measured the correlation between RAD and azimuth and inclination angles using a measure of linear–circular association (Pewsey et al. 2013). We calculated the Mardia’s Rank correlation coefficient for linear–circular association between the linear variable RAD, and the circular variables azimuth and inclination independently. In both cases, we tested for the null hypothesis of independence (Mardia 1976). Statistical analyses were performed using code by Pewsey et al. (2013) written for the statistical package R v.3.2.1 (Core Team R 2015).
Behavioral testing and statistical analysis
To test if honeybees could use angle dependent colorations as a signal we conducted behavioral experiments using the images of S. laciniatum as stimuli (Figure 5, third column, panels I–L) as this flower presents the highest proportion of angle dependent color patches relative to the entire petal surface (RAD) when considering free-flying bee vision (see the “Results” section).
Figure 5.
Composite images indicating regions of perceivable structural color in T. majus (first column, panels A–D) A. huegelii (second column, panels E–H), and S. laciniatum (third column, panels I–L). Areas of structural coloration potentially perceivable to a honeybee are indicated with cyan color if not present on the petal area or red color otherwise. Panels E, G, I, and K depict RGB representations of A. huegelii (panels E and G) and S. laciniatum (panels I and K) at 1:5 and 1:3 magnification ratios. Panels F, H, J, and I correspond to the same RGB images after being projected by a mechano-optical device: A. huegelii (panels F and H) and S. laciniatum (panels J and L). In panels F, H, I, and J, the red color indicates potentially perceivable structural color regions when considering both spectral and spatial threshold values set by the properties of the honeybee’s visual system. Scale bars on panels A–E, G, I, and K represent the flower’s size; on panels F, H, J, and L, scale represent the size of the projected image. T. majus images represent viewing angles: ϴ = 0°, ϕ = 75° (panel A); ϴ = 90°, ϕ = 30° (panel B); ϴ = 135°, ϕ = 60° (panel C); and ϴ = 315°, ϕ = 60° (panel D). Images corresponding to: A. huegelii (second column) and S. laciniatum (third column) represent viewing points at which the percentage of structural to visible color area (RAD) were maximal for each species: (E, F) ϴ = 90°, ϕ = 30°; (G, H) ϴ = 225°, ϕ = 15°; (I, G) ϴ = 180°, ϕ = 15°; and (K, L) ϴ = 270°, ϕ = 30°.
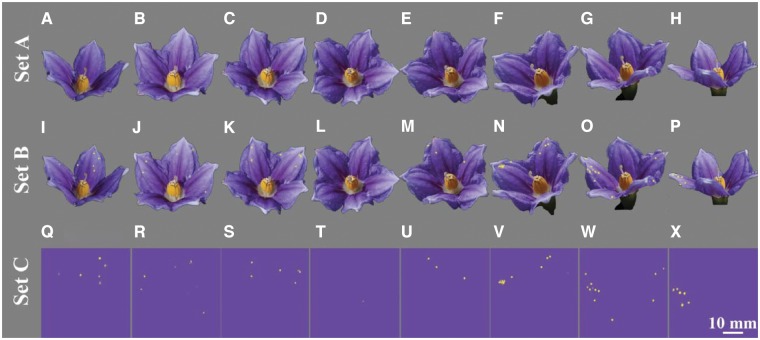
Eight images representing the different azimuth angles (ϴ) measured at ϕ = 45° were used for the behavioral experiment. Stimuli were created from the RGB images recorded for measuring the RAD of S. laciniatum and divided into different sets (Figure 6). Set A consisted of 8 images of this species viewed at different ϴ angles but without any indication of the presence of angle dependent coloration (Figure 6 panels A–H). Set B contained the same images, but additionally included the pattern created by the patches produced by the angle dependent color at each viewpoint (Figure 6 panels I–P). Patches in this image set corresponded to colors which are potentially perceivable by a honeybee as predicted by our color modeling (see spectral and spatial threshold subsections above). The pattern produced by patches of angle dependent colors at the different inclination and orientation angles considered were indicated with a strong “yellow” color as this promotes the most rapid learning of spatial stimuli by honeybees (Morawetz et al. 2013). Set C (Figure 6 panels Q–X) displayed such patterns in insolation on a sample of the petal pigment color to control for potential innate color preference effects (Morawetz et al. 2013).
Figure 6.
Images of the 8 stimuli triplets used for the behavioral experiments. Each column represents an image of S. laciniatum at an inclination angle (ϕ)=15° and various orientation (ϴ) angles: (A, I, Q) 0°; (B, J, R) 45°; (C, K, S) 90°; (D, L, T) 135°; (E, M, U) 180°; (F, N, V) 225°; (G, O, W) 270°; and (H, P, X) 315°. Images on Set A (first row) represent flowers of Solanum at different ϴ angles but without indication of angle dependent color effects. Set B represents the same ϴ angles as in Set A, but the pattern produced by the perceived angle dependent color patches at each viewpoint is indicated with a “yellow” color which is easily discriminated from the pigment color by a bee. Set C represents the same angle dependent color patterns as in Set B, but excludes visual information about flower morphology. The violet color making the background of images in Set C correspond to a printer ink interpretation of the petal color of images in Sets A and B (see details in Supporting Information S-2).
The use of the multiple stimuli sets allowed behavioral testing to determine if the patterns produced by angle dependent coloration could influence bee choices in a way that would be consistent with the definition of a signal. For the signal hypothesis to be true, a bee would need to reliably identify a stimulus by the pattern produced by the angle dependent patches, independent from the azimuth position. The alternative hypothesis would suggest that angle dependent colors are a cue that bees may only use in limited circumstances.
We individually trained marked honeybees (n = 13) using absolute conditioning to 4 of the 8 stimuli presenting patches produced by the angle dependent coloration (Supporting Information S-3 panel a). The 4 stimuli were randomly selected for each bee from the 8 different azimuth positions. For any testing run, all 4 training stimuli were simultaneously presented on a rotating screen which enabled realistic testing of honeybees using ecologically relevant stimuli (Stejskal et al. 2015). The absolute conditioning phase length was 30 choices (landing and drinking of sucrose) which is twice as long as bees typically take to learn color signals considering absolute conditioning (Giurfa 2004; Dyer 2012). This training regime mimics a potential signal that a bee would likely encounter to identify a flower in natural settings. Bees were rewarded with 15 µL drops of 50% sucrose and allowed to return to the hive if satiated.
A learning test of 20 unconditioned choices was conducted after the absolute conditioning phase. Each bee was given a non-rewarded learning test where 2 of the 4 angle dependent color patches used as training stimuli were presented against 2 gray stimuli (Supporting Information S-3 panel b). Following the learning test, a transfer test and conflict test were conducted in pseudo-random order using the same protocol as the learning test with 4–8 refresher choices conducted between each test to maintain bee motivation. The transfer test presented bees with 2 of the 4 stimuli not used during training (Set A vs. matched Set B in Figure 6, Supporting Information S-3 panel c) to determine whether bees would prefer flowers presenting the angle-dependant coloration information. The conflict test presented bees with the remaining stimuli not used during the transfer test (Set A vs. Set C in Figure 6, Supporting Information S-3 panel d). The conflict test would determine whether bees prefer to visit flowers with no angle-dependant coloration information or a colored stimulus with angle-dependant color information presented.
To determine whether bees had learned to associate flowers of S. laciniatum presenting angle dependent colorations with a reward of sucrose, the “proportion of correct choices” data from all 3 tests were estimated by means of 3 independent generalized linear mixed models (GLMMs) assuming a binomial distribution for the binary response, and bee ID number as random effect to account for the repeated measurements (Zuur et al. 2009). The models only included the intercept term as fixed factor allowing for testing if the observed proportion of choices was different from chance expectation (Ho: proportion of choices for choices for target =0.5). Models were fitted using the routine glmer available as part of the package lme4 (Bates et al. 2015) for the R statistical language and programming environment. Overdispersion and residual plots were constructed for each model to validate the GLMM assumptions.
Results
Scanning electron microscope imaging
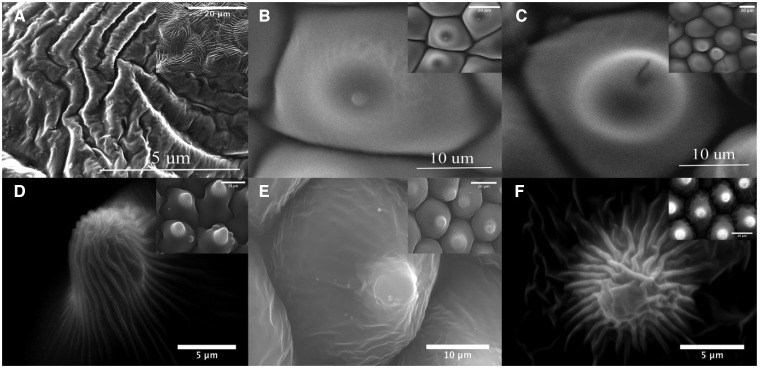
Scanning electron microscopy images reveal 3 different cell types on the petals of our flower sample (Figure 7): (i) tabular rugose-striated cells in A. huegelii, (ii) conically shaped cells in H. heterophillum and T. majus, (iii) flat, hexagonal cells in P. rodneyanum, and (iv) papillate cells in L. rantonnetii and S. laciniatum. Alyogynehuegelii presents quasi-parallel striations with separations smaller than 1 μm (Figure 7 panel A), while epidermal cells of T. majus and P. rodneyanum show distinctive radially striated crests with separations smaller than 1 μm (Figure 7 panels D and F).
Figure 7.
Scanning electronic microscope images showing details of petal features present on the adaxial surfaces of the 6 species used for our study at various magnifications to accommodate for differences in feature size: (A) A. huegelii (6,000×), (B) S. laciniatum (3,383×), (C) L. rantonnetii (3,294×), (D) T. majus (3,406×), (E) Hibiscus heterophyllum (3,159×), and (F) P. rodneyanum (3,228×). Insets on each panel depict a less augmented version of each image. In all insets the scale bar represents 20 μm. All SEM images were acquired using a Philips XL30 SEM microscope.
Imaging experiments
We evaluated the reliability of a signal produced by angle dependent colors within the 405–505 nm (“blue”) and 450–600 nm (“green”) spectral intervals for 5 plant species: A. huegelii, S. laciniatum, T. majus, H. heterophyllus, and P. rodneyanum; when considering viewing angle, the spectral characteristics of the visual system of the honeybee and a color discrimination threshold value of 0.04 hexagon units (Table 1). Two of the species, H. heterophyllus and P. rodneyanum, did not present angle dependent color patches which could be discriminated from the pigment background about 95% of the time as being different from the pigment background in either the “green” or “blue” spectral intervals (Table 1). Most of the angle dependent color patches in T. majus corresponded to the “blue” spectral region and were found the calyx region which is not involved in plant sexual reproduction (first column Figure 5A–D,); for this reason, data corresponding to this species were excluded from subsequent analyses. Alyogynehuegelii (second column Figure 4e–h) and S. laciniatum (third column Figure 4i–l) only presented angle dependent coloration perceivable as being different from the pigment background in the “green” spectral region.
Table 1.
Threshold E-values for the “blue” and “green” spectral regions for the 5 plant species showing no modulation in the UV region when considering green adaptation background and a color discrimination threshold value of 0.04 hexagon units (second column)
| Species\spectral parameters | Threshold E-value |
Mean maximum E-value |
Is iridescence perceivable? |
|||
|---|---|---|---|---|---|---|
| “Green” | “Blue” | “Green” | “Blue” | “Green” | “Blue” | |
| Alyogyne huegelii | 0.417 | 0.764 | 0.412±0.09 | 0.472±0.05 | Yes | No |
| Solanum laciniatum | 0.406 | 0.786 | 0.477±0.04 | 0.497±0.02 | Yes | No |
| Lycianthes rantonnetii a | NI | NI | NI | NI | NI | NI |
| Tropaeolum majus | 0.328 | 0.050 | 0.018±0.002 | 0.196±0.05 | No | Yesb |
| Hibiscus heterophyllum | 0.804 | 0.709 | 0.493±0.01 | 0.378±0.08 | No | No |
| Pelargonium rodneyanum | 0.572 | 0.847 | 0.350±0.11 | 0.474±0.05 | No | No |
Mean E-values and standard deviations corresponding to the maximum E-value obtained on each of the n = 37 linearized images representing various viewing points recorded for each species
Solanum rantonnetii potentially modulates the UV-sensitive photoreceptor in the honeybee; however, as this spectral region is beyond our current system capability, this species was not included in the reported results. NI, not included.
Perceivable iridescence mainly corresponds to flower regions not involved in sexual reproduction (Figure 5).
Threshold binary masks corresponding to A. huegelii and S. laciniatum were subsequently imaged with the mechano-optical device to obtain spatial measurements of optically resolvable angle dependent color patches by a honeybee. Examples of some of the resulting images are presented in Figure 5F, H, J, L.
We found a significant negative correlation between the area of the petal occupied by angle dependent color patches and the total area petal for the 2 species [Kendall’s tau (τ)A. huegelii =−0.312, P = 0.011; τS.laciniatum =−0.335, P = 0.004]. For this reason, RAD values were used for the remaining analyses.
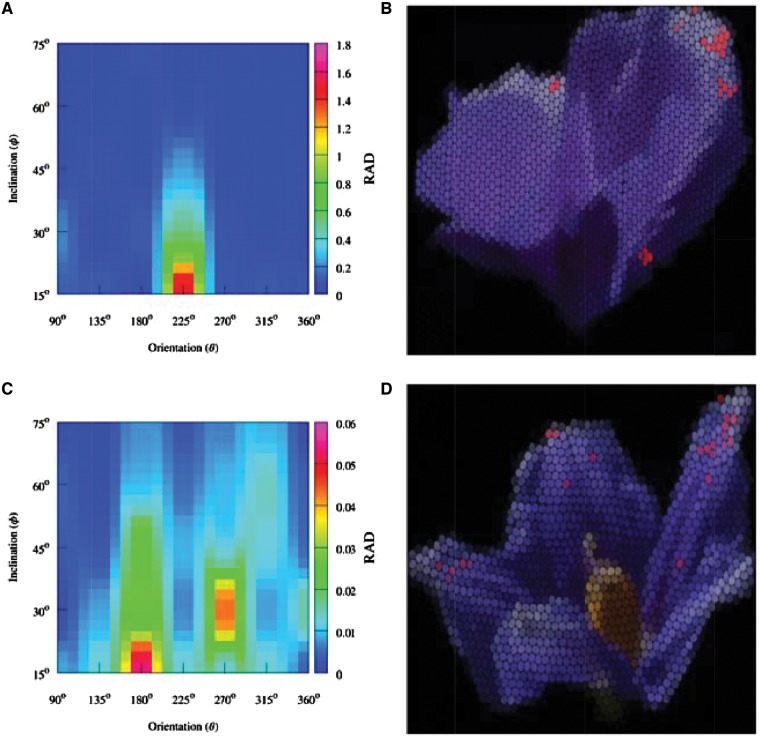
The RAD values significantly varied across the measured orientation and inclination angles, and in different ways for either the A. huegelii and S. laciniatum flowers (Figure 8, Supplementary Information videos V1 and V2, respectively). Alyogynehuegelii presented a larger RAD area than S. laciniatum, but the latter was characterized by having a greater number of RAD areas than its counterpart (Figure 8). Linear–circular correlation analysis and tests for independence evidenced different relationships between the size of the petal area displaying a perceptually different structural color, and the viewing angle in either A. huegelii or S. laciniatum. While structural coloration in A. huegelii was independent from azimuth angle [Mardia’s rank correlation coefficient (U)ϴA.huegelii =777.0, P = 0.825] it was dependent on inclination (UϕA.huegelii =1.96 ×104, P = 0.008). Structural coloration in S. laciniatum was dependent on azimuth (UϴS.laciniatum =1.43× 104, P = 0.026) but independent from inclination (UϕS.laciniatum =9.81 ×103, P = 0.098).
Figure 8.
Color map representing the ratio of angle dependent color areas [indicated as red dots on panels (B) and (D)] to total visible area (RAD) as a function of orientation (x-axis) and inclination (y-axis) for A. huegelli [panel (A)] and S. laciniatum [panel (C)]. Panels (B) and (D) show and RGB representation of A. huegelli and S. laciniatum, respectively, as produced by the mechano-optical device used to simulate the image produced by the honeybee compound eye (Knowles and Dartnall 1977; Williams and Dyer 2007), at the orientation and inclination position showing the largest area of angle dependent coloration for each species. On panels (B) and (D) image regions where angle dependent coloration is discriminable from the pigment background 95 % of the time are indicated by a red color to aid visual interpretation by human observers.
Behavioral experiments
Figure 9 summarized the results of the 3 behavioral tests carried out. In the learning test, honeybees chose the target displaying the angle dependent coloration significantly more times than the gray stimulus (mean proportion of correct choices for angle dependent coloration [μadc =0.737 (0.679, 0.792 95% CI), z = 7.04, P < 0.001], thus demonstrating they had associated the images of S. laciniatum with a reward of sucrose.
Figure 9.
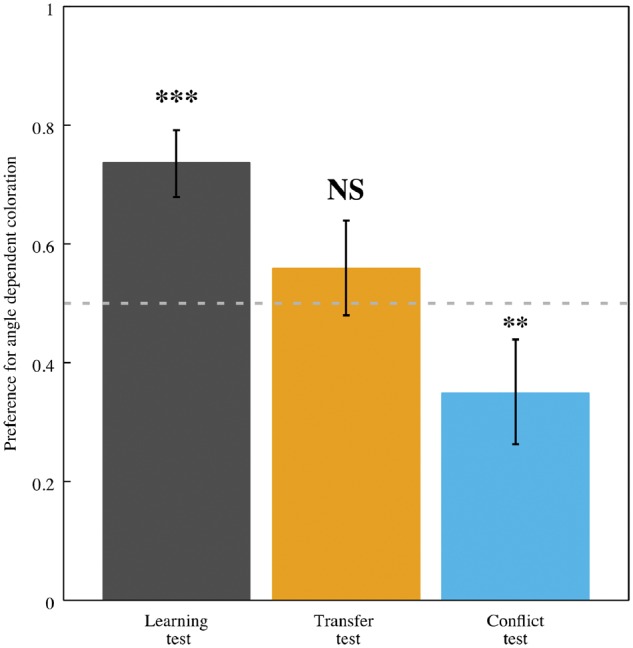
Mean proportion of honeybee choices for angle dependent stimuli when presented against different alternative stimuli: an achromatic, gray target without angle dependent or independent color (learning test), flowers with patterns produced by angle dependent colors at different orientation and inclination positions against the same flowers without the angle dependent patterns (transfer test); and, flowers at different orientation and inclination angles without the corresponding angle dependent color patterns against stimuli showing the respective angle dependent patterns on an uniform background with the same color displayed by the petals. ***P < 0.001, **P < 0.01, NS non-significant at α=0.05.
Two further tests were conducted following the learning test: a transfer test (Set A vs. matched Set B in Figure 6, Supporting Information S-3 panel c) and a conflict test (Set A vs. matched Set C in Figure 6, Supporting Information S-3 panel d). For the transfer test, bees were presented with 2 of the 4 stimuli not used during initial training against the matched versions of these stimuli that lacked angle dependent colors. If the signal hypothesis holds true, then bees must be able to perform this task above chance expectation (50%). Between each of the 3 tests, 4–8 refresher landings were presented to ensure motivation.
In the transfer test, where images representing novel azimuth angles plus angle dependent color marks were presented against the images recorded at the same angles but without presenting the pattern produced by the angle dependent coloration, bees did not show a preference for images displaying flowers with angle dependent coloration [μadc =0.559 (0.480, 0.639 95% CI), z = 1.58, P = 0.114], thus suggesting that honeybees did not use these patches to inform their choices.
Finally, we performed a conflict test—bees were presented with the remaining stimuli not used for the transfer test against the pattern produced by the angle dependent coloration for the selected viewpoint on a square displaying the same color as S. laciniatum. Three outcomes were possible from the conflict test: (i) bees prefer the original flower even without angle dependent colors, OR (ii) there is a conflict caused by the angle dependent patches and flower information being presented; if we see chance performance in this test. Alternatively (iii), if bees have learnt to use the angle dependent color pattern as a signal, they would significantly prefer to choose the patterns produced by angle dependent colors on the purple squares. If bees could do the learning test, but not the transfer test, and in the conflict test they preferred the flower, then there is no evidence supporting the hypothesis of bees using angle dependent color signals in the presence of a strong pigment color signal. However, we can only consider angle dependent colors as being a signal if bees perform significantly above chance expectation in the learning and transfer tests, and do not prefer the flower in the conflict test. Bees did not show a significant preference for the colored squares containing the respective angle dependent color patches [μadc =0.349 (0.263, 0.439 95% CI)], but instead preferred the flower images which did not present angle dependent colors (z =−3.40, P < 0.001) thus suggesting that the signal hypothesis does not hold true in the context of our experiments.
Discussion
Insect pollination is essential for a large number of plant species, and for many flowering plants there is evidence that specific floral traits enhance successful repeat visits from flower constant pollinators (Waser 1986; Fenster et al. 2004; Sargent and Ackerly 2008; Schiestl and Johnson 2013; Ohashi et al. 2015). Recent reports that bumblebees can be trained in lab conditions with appetitive-aversive differential conditioning to learn iridescent colors (Whitney et al. 2009b, 2016; Moyroud et al. 2017; de Premorel et al. 2017) have raised the interesting possibility that structural coloration may have evolved in evolutionary distantly related flower species to serve as a signal to enhance plant–pollinator visual communication. However, several studies have questioned this interpretation because flowers are typically viewed by potential insect pollinators in complex environments where structural color is unlikely to be a robust source of information for a free-flying insect (Morehouse and Rutowski 2009; van der Kooi et al. 2014, 2015). Furthermore, the possibility that such stimuli may often be beyond the resolution of an insect compound eye has also been raised (van der Kooi et al. 2015). In the current study, we were able to employ recent advances in our understanding of how to model bee pollinator color (Garcia et al. 2017, 2018) and spatial vision (Dyer and Williams 2005; Howard et al. 2018) to formally test the potential role of angle dependent colors as potential signals for bees. Furthermore, we tested the hypothesis derived from image analysis regarding the potential use of angle dependent colors as visual signals by pollinating insects using free-flying honeybees.
The precise role of structural colors for plant–pollinator visual communication has largely remained unresolved, probably due to the synonymous use of the words cue and signal in the literature. However, these 2 words have different meanings in the context of biological communication (Smith and Harper 2003; Bradbury and Vehrencamp 2011) and would imply different evolutionary and behavioral relationships between flowers and their pollinators. For angle dependent colors to be considered salient visual signals for communicating with pollinators, as previously proposed for target flower discrimination (Whitney et al. 2009; Moyroud et al. 2017), it is necessary that these colors transfer meaningful information to a bee such that it can reliably identify a flower irrespective of viewing angle, and that it has evolved specifically for this purpose. Our results, however, indicate that this is not the case. Interestingly, bird predators in natural conditions cannot use angle dependent, iridescent colors reflected by the ventral wing of Battus philenor butterflies for prey identification (Pegram et al. 2015), also suggesting structural colors may be of limited value for visual signaling when viewing angle is variable. In one of the species (T. majus), angle dependent colors potentially discriminable from the pigment background color were found on the calyx, a flower part different from the petals (Figure 5a–c) and thus would likely serve no value in communicating with a potential pollinator. Similar optical effects have also been reported for other plant parts not involved with pollination such as fruits (Lee 1991), and on the leaves of non-flowering plants such as the red algae Chondrus crispus (Chandler et al. 2015) and the fern Selaginella (Hébant and Lee 1984).
Finally, angle dependent color patches in A. huegelii. and S. laciniatum fail to unambiguously transfer information to a bee pollinator due to: (a) the significant correlation between size of the petal area displaying such colors with viewing angle (Spectral limitations) and (b) the difficulty of resolving these patches by the insect compound eye (Spatial limitations).
Our behavioral experiments formally tested the hypotheses arising from the imaging results (Figure 9). When required to learn angle dependent color information from a variety of biologically plausible azimuth positions, bees subsequently showed no preference for flowers images containing angle dependent color patterns when presented against flower images without such information in a transfer test. This was despite the fact that bees had learnt the flower image as rewarding in the learning tests. To be classified as signal particular visual information must allow for the unique identification of individual flowers, but this was not the case for angle dependent colors perceived by bee pollinator for our biologically plausible scenario. Specifically, in the transfer test bees were unable to use angle dependent patterns to identify a target flower and in the conflict test bees actually chose to prefer solid, flower colors rather than angle dependent color patterns (Figure 9). This means that angle dependent colors as those produced by ultra-structures are very unlikely to be a robust signal in complex natural conditions.
Spectral limitations
The correlation of viewing angle with the size of the area presenting perceivable structural coloration means that a bee could only uniquely identify a flower when approaching at a specific set of angles. If the angle dependent coloration serves as a signal for communication, the information transmitted by these colors would be unreliable for a free flying bee in a natural environment unless individual bees always approached different flowers from exactly the same viewpoint (Figure 1); and unlikely scenario in complex and competitive environments (Garcia et al. 2018). In contrast, pigment coloration transmits color information independently from angle due to its diffuse nature (Lee 2005), thus effectively reducing the ambiguity introduced by chromatic variation produced by changes in view point typical of structural colors (Doucet and Meadows 2009; de Premorel et al. 2017).
The correlation between view point and the size of the petal area displaying angle dependent colorations perceptually discriminable from the pigment background also limits the usefulness of structural colors as a mechanism to boost pigment color in flowers as it has also been proposed (Glover and Whitney 2010). Although optical and physiological properties of plants such as ultrastructure and heliotropism have been shown to significantly increase the temperature of internal flower parts, potentially increasing pollen growth and accelerate ovule fertilization in some species (van der Kooi et al. 2017; Wilts et al. 2018), its effect on pollinator attraction remains inconclusive (Totland 1996). Even though it is possible that under specific illumination conditions heliotropism and or ultrastructures may increase the effect of angle dependent coloration in certain species (Figures 5, 7), the production of such a coloration seems to be incidental rather than evolved as expected from a signal. For example, Totland (1996) showed that insect visitation was not affected by the alignment of Ranunculus acris, an heliotropic genus known to present angle dependent coloration (van der Kooi et al. 2017), relative to the sun.
Spatial limitations
Another important aspect to consider is the small size of the patches produced by structural colors (van der Kooi et al. 2015) observed in the 2 sampled species presenting potentially perceivable structural colors. Most of the angle dependent patches in A. huegelii and S. laciniatum occupy an area of less than 1% of the petal visible area (Figure 8), which can only be resolved by an hymenopteran at close range due to the optical properties of the compound eye (Kirschfeld 1976; Srinivasan and Lehrer 1988; Land 1997). Although it remains to be specifically tested if small patches of angle dependent colorations might improve the efficiency of, or act as, “nectar guides,” such a possibility would not necessarily imply that angle dependent colors act as a signal for visual communication.
Firstly, petal marks are very likely to be resolved well after an insect pollinator has made the decision to land onto a petal; therefore, nectar guides are unlikely to serve for unambiguously identifying a flower from afar by an approaching insect as expected from a salient signal evolved for visual communication between plant (emitter) and insect pollinator (receiver). Both optical modeling an experimental behavioral data suggest that bee-sized insect pollinators cannot perceive such markings over long distances, in particular if they reflect short wavelength radiation as the L (long wavelength) photoreceptor is responsible for detecting small objects in honeybees and bumblebees through achromatic vision (Giurfa 1996; Hempel de Ibarra et al. 2009, 2015), while the hypothesis of a signaling role of structural colors is formulated in the context of color discrimination (Whitney et al. 2009, 2016). Secondly, the presence of petal marks does not seem to increase the number of pollinator visits as evidenced both by bumblebees (Manning 1956) or specialized pollinating flies (Hansen et al. 2012). Therefore, angle dependent colors present in nectar guides, if any, are more likely to act as an orientation cue rather than as a salient signal for visual communication between plant and insect, or as a signal for plant identification as previously hypothesized (Whitney et al. 2009; Moyroud et al. 2017). However, this does not exclude the possibility of structural colors present in nectar guides, if any, could serve as short distance visual signals for improving flower handling after landing.
It is possible that large bees like bumblebees that have chromatic processing channels with equivalent resolution to achromatic spatial channels (Dyer et al. 2008) may in some cases be able to resolve angle dependent color patches from our flower samples as suggested by experiments using artificial targets (Whitney et al. 2009b, 2016; Moyroud et al. 2017; de Premorel et al. 2017). However, behavioral testing of bumblebees detecting either wild-type or mixta-mutant flowers suggests that changes in petal structure have no significant effect on the efficiency of bees detecting flowers (Dyer et al. 2007). For honeybees, chromatic processing is coarser than the achromatic channel (Giurfa et al. 1996; Dyer et al. 2008) and so it is unlikely that honeybees, or smaller bees, could ever see patches of angle dependent coloration as a chromatic source of information unless the bee has practically already landed on the flower. The relatively small size of the patches observed in flowers reported as presenting angle dependent colorations (Whitney et al. 2009b; Vignolini et al. 2015; Moyroud et al. 2017, Figure 5 this study) may explain how bumblebees could slowly learn angle dependent colorations such as iridescence using ideal iridescent targets in controlled lab conditions. Indeed, when trained with appetitive-aversive differential conditioning, bumblebees took about 80 choices to achieve an accuracy of about 75% when discriminating artificial, iridescent stimuli (Whitney et al. 2009b), compared with a discrimination task between 2 disimilar pigment colors where bumblebees took 20 choices to achieve a sucess rate of more than 90% (Dyer and Chittka 2004). Honeybees are known to be able to use salient small local cues to make decisions if specifically trained to do so (Avargues-Weber et al. 2015), but the results of our behavioral experiments using free-flying individuals show that in the presence of an angle independent color as that produced by the pigment background, bees did not use angle dependent color to make reliable repeat decisions in natural environments. The results obtained from our behavioral experiments are very likely to apply to a wide range of angle dependent colorations independently from the specific optical phenomena.
Structural colors act as cues in flowers
How can we then classify the role of patches produced by structural coloration in pollination? We agree that angle dependent colors could be treated as a cue in the context that the structural color can be correlated with a particular physical trait of the plant (Bradbury and Vehrencamp 2011), for example the particular texture of a flower’s epidermal cells (Whitney et al. 2009b; Vignolini et al. 2012; van der Kooi et al. 2017; Moyroud et al. 2017). However from the classic definition of signals for communication, this does not imply that structural coloration has indeed evolved to transmit useful information to the observer as expected from a signal (Smith and Harper 2003), a proposed explanation for the presence of micro and ultrastructures on the petals of plants distantly related (Moyroud et al. 2017; Wilts et al. 2018). The fact that a pollinator can positively identify angle dependent colors displayed by artificial targets from that produced by a pigment in laboratory conditions under carefully specified lighting conditions is thus insufficient evidence for regarding iridescence and other structural colors as a being visual signals as evidenced by the results of our behavioral experiments. Furthermore, the increasing number of plants species reported to display structural coloration by organs not related with pollination such as leaves and fruits (Hébant and Lee 1984; Lee 1991; Chandler et al. 2015) and the calyx (Figure 5a–c) strongly suggests that the structures producing angle dependent colors may likely serve the plant for functions other than visual communication such as an aid to increase photosynthetic activity (Hébant and Lee 1984) or increasing the temperature of specific areas of a flower (Wilts et al. 2018).
Before we can classify structural coloration in plants as an example of a visual signaling comparable to that observed in some animal species, attention must be given to answer the 3 important questions that differentiate a signal from a cue in this specific context: (i) what is the possible information potentially transmitted from the plant to the pollinator by angle dependent coloration? (ii) Is there a mutual gain by the production and monitoring of these colors? and (iii) How feasible is the perception of angle dependent color patches when considering the physiological characteristics of the pollinators and ecological setting where pollination takes place?
One possible avenue for further exploration is whether iridescence or other forms of angle dependent colors may provide useful information in low light or forest environments which have very different lighting conditions (Endler 1993) to what was tested in the current study; or if the micro and nano structures responsible for angle dependent colorations have evolved for a different purpose such as water repellence (Koch et al. 2009; Whitney et al. 2011), facilitate pollinator manipulation (Whitney et al. 2009a), temperature modulation (Koch et al. 2009; Wilts et al. 2018), or to facilitate detection in specific illumination and viewing conditions. However, we encourage future work to engage the formal framework of signaling for possible plant–pollinator iterations as here presented, and ideally mapping the complexity of the UV + B + G photoreceptor modulation.
Author Contributions
J.E.G., M.S., S.R.H., and A.G.D. designed the experiments, collected and analyzed data, and wrote the manuscript. P.P. designed the experiment, collected data, and wrote the manuscript.
Supplementary Material
Acknowledgments
A.G.D. acknowledges the Australian Research Council [DP130100015]. M.S. and A.G.D. express their gratitude to Monash University for permitting collecting plant samples from their gardens and providing the facilities required for recoding the spectral data. The authors also acknowledge the facilities, and the scientific and technical assistance of the RMIT University Microscopy and Microanalysis Facility (RMMF), a linked laboratory of the Australian Microscopy and Microanalysis Research Facility. We also thank Chaitali Dekiwadia, for her assistance and expertise during sample preparation.
References
- Avarguès-Weber A, de Brito Sanchez MG, Giurfa M, Dyer AG, 2010. Aversive reinforcement improves visual discrimination learning in free-flying honeybees. PLoS ONE 5:e15370.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avargues-Weber A, Dyer AG, Ferrah N, Giurfa M, 2015. The forest or the trees: preference for global over local image processing is reversed by prior experience in honeybees. Proc R Soc B Biol Sci 282:20142384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, Walker S, 2015. Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. [Google Scholar]
- Bennett ATD, Cuthill IC, Partridge JC, Lunau K, 1997. Ultraviolet plumage colors predict mate preferences in starlings. Proc Natl Acad Sci U S A 94:8618–8621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury JW, Vehrencamp SL, 2011. Principles of Animal Communication. Sunderland (MA: ): Sinauer Associates. [Google Scholar]
- Bukovac Z, Dorin A, Finke V, Shrestha M, Garcia J et al. , 2016Assessing the ecological significance of bee visual detection and colour discrimination on the evolution of flower colours. Evol Ecol 31:153–172. [Google Scholar]
- Bukovac Z, Shrestha M, Garcia JE, Burd M, Dorin A et al. , 2017. Why background colour matters to bees and flowers. J Comp Physiol A 203:369–380. [DOI] [PubMed] [Google Scholar]
- Burns JG, Dyer AG, 2008. Diversity of speed–accuracy strategies benefits social insects. Curr Biol 18:R953–R954. [DOI] [PubMed] [Google Scholar]
- Chandler CJ, Wilts BD, Vignolini S, Brodie J, Steiner U et al. , 2015. Structural colour in Chondrus crispus. Sci Rep 5:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittka L, 1992. The colour hexagon: a chromaticity diagram based on photoreceptor excitations as a generalized representation of colour opponency. J Comp Physiol A 170:533–543. [Google Scholar]
- Chittka L, Dyer AG, Bock F, Dornhaus A, 2003. Bees trade off foraging speed for accuracy. Nature 424:388.. [DOI] [PubMed] [Google Scholar]
- Chittka L, Menzel R, 1992. The evolutionary adaptation of flower colours and the insect pollinators’ colour vision. J Comp Physiol A 171:171–181. [Google Scholar]
- Chittka L, Shmida A, Troje N, Menzel R, 1994. Ultraviolet as a component of flower reflections, and the colour perception of hymenoptera. Vision Res 34:1489–1508. [DOI] [PubMed] [Google Scholar]
- Chittka L, Thomson JD, Waser NM, 1999. Flower constancy, insect psychology, and plant evolution. Naturwissenschaften 86:361–377. [Google Scholar]
- Doucet SM, Meadows MG, 2009. Iridescence: a functional perspective. J R Soc Interface 6:S115–S132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer AG, 2006. Discrimination of flower colours in natural settings by the bumblebee species Bombus terrestris (Hymenoptera: Apidae). Entomol Gen 28:257–268. [Google Scholar]
- Dyer AG, 2012. Psychophysics of honey bee color processing in complex environments In: Galizia CG, Eisenhardt D, Giurfa M, editors. Honeybee Neurobiology and Behavior: A Tribute to Randolf Menzel. Dordrecht: Springer Netherlands, 303–314. [Google Scholar]
- Dyer AG, Boyd-Gerny S, McLoughlin S, Rosa MGP, Simonov V et al. , 2012. Parallel evolution of angiosperm colour signals: common evolutionary pressures linked to hymenopteran vision. Proc R Soc B Biol Sci 279:3606–3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer AG, Chittka L, 2004. Fine colour discrimination requires differential conditioning in bumblebees. Naturwissenschaften 91:224–227. [DOI] [PubMed] [Google Scholar]
- Dyer AG, Chittka L, 2004. Bumblebees (Bombus terrestris) sacrifice foraging speed to solve difficult colour discrimination tasks. J Comp Physiol A 190:759–763. [DOI] [PubMed] [Google Scholar]
- Dyer AG, Garcia JE, 2014. Color difference and memory recall in free-flying honeybees: forget the hard problem. Insects 5:629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer AG, Neumeyer C, 2005. Simultaneous and successive colour discrimination in the honeybee (Apis mellifera). J Comp Physiol A 191:547–557. [DOI] [PubMed] [Google Scholar]
- Dyer AG, Spaethe J, Prack S, 2008. Comparative psychophysics of bumblebee and honeybee colour discrimination and object detection. J Comp Physiol 194:617–627. [DOI] [PubMed] [Google Scholar]
- Dyer AG, Streinzer M, Garcia J, 2016. Erratum to: flower detection and acuity of the Australian native stingless bee Tetragonula carbonaria Sm. J Comp Physiol A 202:629–639. [DOI] [PubMed] [Google Scholar]
- Dyer AG, Whitney HM, Arnold SEJ, Glover BJ, Chittka L, 2007. Mutations perturbing petal cell shape and anthocyanin synthesis influence bumblebee perception of Antirrhinum majus flower colour. Arthropod Plant Interact 1:45–55. [Google Scholar]
- Dyer AG, Williams SK, 2005. Mechano-optical lens array to simulate insect vision photographically. Imaging Sci J 53:209–213. [Google Scholar]
- Endler JA, 1993. The color of light in forests and its implications. Ecol Monogr 63:2–27. [Google Scholar]
- Endler JA, Thery M, 1996. Interacting effects of lek placement, display behavior, ambient light, and color patterns in three neotropical forest-dwelling birds. Am Nat 148:421–452. [Google Scholar]
- van der Faegri KP, 1966. Principles of Pollination Ecology. New York: Pergamon Press. [Google Scholar]
- Fenster CB, Armbruster WS, Wilson P, Dudash MR, Thomson JD, 2004. Pollination syndromes and floral specialization. Annu Rev Ecol Evol Syst 35:375–403. [Google Scholar]
- Finger E, Burkhardt D, Dyck J, 1992. Avian plumage colors. 79:187–188. [Google Scholar]
- Galsterer S, Musso M, Asenbaum A, Fürnkranz D, 1999. Reflectance measurements of glossy petals of Ranunculus lingua (Ranunculaceae) and of non-glossy petals of Heliopsis helianthoides (Asteraceae). Plant Biol 1:670–678. [Google Scholar]
- Garcia JE, Dyer AG, Greentree AD, Spring G, Wilksch PAPA, 2013a. Linearisation of RGB camera responses for quantitative image analysis of visible and UV photography: a comparison of two techniques. PLoS ONE 8:e79534.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JE, Greentree AD, Shrestha M, Dorin A, Dyer AG, 2014. Flowers through the lens: quantitative measurement with visible and ultraviolet digital photography. PLoS ONE 9:e96646.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JE, Rohr D, Dyer AG, 2013b. Trade-off between camouflage and sexual dimorphism revealed by UV digital imaging: the case of Australian Mallee Dragons (Ctenophorus fordi). J Exp Biol 216:4290–4298. [DOI] [PubMed] [Google Scholar]
- Garcia JE, Shrestha M, Dyer AG, 2018. Flower signal variability overwhelms receptor-noise and requires plastic color learning. Behav Ecol 29:1286–1297. [Google Scholar]
- Garcia JE, Spaethe J, Dyer AG, 2017. The path to colour discrimination is S-shaped: behaviour determines the interpretation of colour models. J Comp Physiol A 203:983–997. [DOI] [PubMed] [Google Scholar]
- Giurfa M, 2004. Conditioning procedure and color discrimination in the honeybee Apis mellifera. Naturwissenschaften 91:228–231. [DOI] [PubMed] [Google Scholar]
- Giurfa M, Vorobyev M, Kevan P, Menzel R, 1996. Detection of coloured stimuli by honeybees: minimum visual angles and receptor specific contrasts. J Comp Physiol A 178:699–709. [Google Scholar]
- Glover BJ, Whitney HM, 2010. Structural colour and iridescence in plants: the poorly studied relations of pigment colour. Ann Bot 105:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulson D, 1999. Foraging strategies of insects for gathering nectar and pollen, and implications for plant ecology and evolution. Perspect Plant Ecol Evol Syst 2:185–209. [Google Scholar]
- Hansen D, van der Niet T, Johnson S, 2012. Floral signposts: testing for the significance of visual ‘nectar guides’ for pollinator behaviour and plant fitness. Proc R Soc B 279:634–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hébant C, Lee DW, 1984. Ultrastructural basis and developmental control of blue iridescence in Selaginella leaves. Am J Bot 71:216–219. [Google Scholar]
- Hecht E, 2002. Optics. Reading: Addison-Wesley. [Google Scholar]
- Heindl M, Winkler H, 2003. Interacting effects of ambient light and plumage color patterns in displaying Wire-tailed Manakins (Aves, Pipridae). Behav Ecol Sociobiol 53:153–162. [Google Scholar]
- Hempel de Ibarra N, Langridge K, Vorobyev M, 2015. More than colour attraction: behaviorual functions of flower patterns. Curr Opin Insect Sci 12:64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempel de Ibarra N, Vorobyev M, 2009. Flower patterns are adapted for detection by bees. J Comp Physiol A 195:319–323. [DOI] [PubMed] [Google Scholar]
- Howard SR, Avarguès-Weber A, Garcia JE, Greentree AD, Dyer AG, 2018. Numerical ordering of zero in honey bees. Science 360:1124–1126. [DOI] [PubMed] [Google Scholar]
- Kevan PG, Chittka L, Dyer AG, 2001. Limits to the salience of ultraviolet: lessons from colour vision in bees and birds. J Exp Biol 204:2571–2580. [DOI] [PubMed] [Google Scholar]
- Kirschfeld K, 1976. The resolution of lens and compound eyes In: Zettler F, Weiler R, editors. Neural Principles in Vision. Berlin: Springer Heidelberg, 354–370. [Google Scholar]
- Knowles A, Dartnall HJ, 1977. The Photobiology of Vision. London: Academic Press. [Google Scholar]
- Koch B, Bhushan B, Barthlott W, 2009. Multifunctional surface structures of plants: an inspiration for biomimetics. Prog Mater Sci 54:137–178. [Google Scholar]
- van der Kooi CJ, Dyer AG, Kevan PG, Lunau K, 2018. Functional significance of the optical properties of flowers for visual signalling. Ann Bot (doi: 10.1093/aob/mcy119). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kooi CJ, Dyer AG, Stavenga DG, 2015. Is floral iridescence a biologically relevant cue in plant–pollinator signalling?. New Phytol 205:18–20. [DOI] [PubMed] [Google Scholar]
- van der Kooi CJ, Elzenga JTM, Dijksterhuis J, Stavenga DG, 2017. Functional optics of glossy buttercup flowers. J R Soc Interface 14:20160933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kooi CJ, Elzenga JTM, Staal M, Stavenga DG, 2016. How to colour a flower: on the optical principles of flower coloration. Proc R Soc B Biol Sci 283:20160429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kooi CJ, Wilts BD, Leertouwer HL, Staal M, Elzenga JTM et al. , 2014. Iridescent flowers? Contribution of surface structures to optical signaling. New Phytol 203:667–673. [DOI] [PubMed] [Google Scholar]
- Land MF, 1997. Visual acuity in insects. Annu Rev Entomol 42:147–177. [DOI] [PubMed] [Google Scholar]
- Lee DW, 1991. Ultrastructural basis and function of iridescent blue colour of fruits in Elaeocarpus. Nature 349:260–262. [Google Scholar]
- Lee H-C, 2005. Introduction to Color Imaging Science. Cambridge: Cambridge University Press. [Google Scholar]
- Loyau A, Gomez D, Moureau B, Théry M, Hart NS et al. , 2007. Iridescent structurally based coloration of eyespots correlates with mating success in the peacock. Behav Ecol 18:1123–1131. [Google Scholar]
- Manning A, 1956. The effect of honey-guides. Behaviour 9:114–139. [Google Scholar]
- Mardia K, 1976. Linear–circular correlation coefficients and rhythmometry. Biometrika 63:403–405. [Google Scholar]
- McGraw KJ, Mackillop EA, Dale J, Hauber ME, 2002. Different colors reveal different information: how nutritional stress affects the expression of melanin- and structurally based ornamental plumage. J Exp Biol 205:3747–3755. [DOI] [PubMed] [Google Scholar]
- Morawetz L, Svoboda A, Spaethe J, Dyer AG, 2013. Blue colour preference in honeybees distracts visual attention for learning closed shapes. J Comp Physiol A 199:817–827. [DOI] [PubMed] [Google Scholar]
- Morehouse NI, Rutowski RL, 2009. Comment on “Floral iridescence, produced by diffractive optics, act as a cue for animal pollinators.” Science 325:1072.. [DOI] [PubMed] [Google Scholar]
- Moyroud E, Wenzel T, Middleton R, Rudall PJ, Banks H et al. , 2017. Disorder in convergent floral nanostructures enhances signalling to bees. Nature 550:469–474. [DOI] [PubMed] [Google Scholar]
- Naka KI, Rushton WAH, 1966. S-potentials from colour units in the retina of fish (Cyprinidae). J Physiol 185:536–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassau K, 2001. The Physics and Chemistry of Color. New York: John Wiley & Sons, Inc. [Google Scholar]
- Ng J, Freitas LB, Smith SD, 2018. Stepwise evolution of floral pigmentation predicted by biochemical pathway structure. Evolution (doi:10.1111/evo.13589). [DOI] [PubMed] [Google Scholar]
- Ng L, Garcia JE, Dyer AG, 2018. Why colour is complex: evidence that bees perceive neither brightness nor green contrast in colour signal processing. Facets 3:800–817. [Google Scholar]
- Ohashi K, Makino TT, Arikawa K, 2015. Floral colour change in the eyes of pollinators: testing possible constraints and correlated evolution. Funct Ecol 29:1144–1155. [Google Scholar]
- Pegram K, Han H, Rutowski R, 2015. Warning signal efficacy: assessing the effects of color iridescence, and time of day in the field. Ethology 121:861–873. [Google Scholar]
- Peitsch D, Fietz A, Hertel H, de Souza J, Ventura DF et al. , 1992. The spectral input systems of hymenopteran insects and their receptor-based colour vision. J Comp Physiol A 170:23–40. [DOI] [PubMed] [Google Scholar]
- Pewsey A, Ruxton GD, NeuhäUser M, 2013. Circular Statistics in R. Oxford, UK: Oxford University Press. [Google Scholar]
- de Premorel G, Giurfa M, Andraud C, Gomez D, 2017. Higher iridescent-to-pigment optical effect in flowers facilitates learning, memory and generalization in foraging bumblebees. Proc R Soc B Biol Sci 284:20171097.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausher MD, 2008. Evolutionary transitions in floral color. Int J Plant Sci 169:7–21. [Google Scholar]
- Reser DH, Wijesekara Witharanage R, Rosa MGP, Dyer AG, 2012. Honeybees (Apis mellifera) learn color discrimination via differential conditioning independent of long wavelength (green) photoreceptor modulation. PLoS ONE 7:e48577.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvaggio N, 2009. Basic Photographic Materials and Processes. Amsterdam: Focal Press/Elsevier. [Google Scholar]
- Sargent RD, Ackerly DD, 2008. Plant–pollinator interactions and the assembly of plant communities. Trends Ecol Evol 23:123–130. [DOI] [PubMed] [Google Scholar]
- Schiestl FP, Johnson SD, 2013. Pollinator-mediated evolution of floral signals. Trends Ecol Evol 28:307–315. [DOI] [PubMed] [Google Scholar]
- Scogin R, 1983. Visible floral pigments and pollinators In: Jones CE, Little RJ eds. Handbook of Experimental Pollination Biology, New York: Scientific and Academic Editions, 160–172. [Google Scholar]
- Shrestha M, Dyer AG, Boyd-Gerny S, Wong BBM, Burd M, 2013. Shades of red: bird-pollinated flowers target the specific colour discrimination abilities of avian vision. New Phytol 198:301–310. [DOI] [PubMed] [Google Scholar]
- Siefferman L, Hill GE, 2003. Structural and melanin coloration indicate parental effort and reproductive success in male eastern bluebirds. Behav Ecol 14:855–861. [Google Scholar]
- Smith JM, Harper D, 2003. Animal Signals (Harvey P, May RM, eds). Oxford: Oxford University Press. [Google Scholar]
- Spaethe J, Tautz J, Chittka L, 2001. Visual constraints in foraging bumblebees: flower size and color affect search time and flight behavior. Proc Natl Acad Sci U S A 98:3898–3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan MV, Lehrer M, 1988. Spatial acuity of honeybee vision and its spectral properties. J Comp Physiol A 162:159–172. [Google Scholar]
- Srinivasarao M, 1999. Nano-optics in the biological world: beatles, butterflies, birds and moths. Chem Rev 99:1935–1962. [DOI] [PubMed] [Google Scholar]
- Stejskal K, Streinzer M, Dyer A, Paulus HF, Spaethe J, 2015. Functional significance of labellum pattern variation in a sexually deceptive orchid (Ophrys heldreichii): Evidence of individual signature learning effects. PLoS ONE 10:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M, Párraga CA, Cuthill IC, Partridge JC, Troscianko TS, 2007. Using digital photography to study animal coloration. Biol J Linn Soc 90:211–237. [Google Scholar]
- Tanaka Y, Sasaki N, Ohmiya A, 2008. Biosynthesis of plant pigments: anthocyanins, betalains and carotenoids. Plant J 54:733–749. [DOI] [PubMed] [Google Scholar]
- Totland O, 1996. Flower heliotropism in an alpine population of Ranunculus acris (Ranunculaceae): effects on flower temperature, insect visitation, and seed production. Am J Bot 83:452–458. [Google Scholar]
- Varassin IG, Trigo JR, Sazima M, 2001. The role of nectar production, flower pigments and odour in the pollination of four species of Passiflora (Passifloraceae) in south-eastern Brazil. Bot J Linn Soc 136:139–152. [Google Scholar]
- Vignolini S, Davey MP, Bateman RM, Rudall PJ, Moyroud E et al. , 2012. The mirror crack’d: both pigment and structure contribute to the glossy blue appearance of the mirror orchid, Ophrys speculum. New Phytol 196:1038–1047. [DOI] [PubMed] [Google Scholar]
- Vignolini S, Moyroud E, Hingant T, Banks H, Rudall PJ et al. , 2015. The flower of Hibiscus trionum is both visibly and measurably iridescent. New Phytol 205:97–101. [DOI] [PubMed] [Google Scholar]
- Waser NM, 1986. Flower constancy: definition, cause, and measurement. Am Nat 127:593–603. [Google Scholar]
- Whitney HM, Chittka L, Bruce TJA, Glover BJ, 2009a. Conical epidermal cells allow bees to grip flowers and increase foraging efficiency. Curr Biol 19:948–953. [DOI] [PubMed] [Google Scholar]
- Whitney HM, Kolle M, Andrew P, Chittka L, Steiner U et al. , 2009b. Floral iridescence, produced by diffractive optics, acts as a cue for animal pollinators. Science 323:130–133. [DOI] [PubMed] [Google Scholar]
- Whitney HM, Poetes R, Steiner U, Chittka L, Glover BJ, 2011. Determining the contribution of epidermal cell shape to petal qettability using isogenic Antirrhinum lines. PLoS ONE 6:e17576.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney HM, Reed A, Rands SA, Chittka L, Glover BJ, 2016. Flower iridescence increases object detection in the insect visual system without compromising object identity. Curr Biol 26:802–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikström N, Savolainen V, Chase MW, 2001. Evolution of the angiosperms: calibrating the family tree. Proc R Soc B Biol Sci 268:2211–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S, Dyer A, 2007. A photographic simulation of insect vision. J Ophthalmic Photogr 29:10–14. [Google Scholar]
- Wilts BD, Rudall PJ, Moyroud E, Gregory T, Ogawa Y et al. , 2018. Ultrastructure and optics of the prism-like petal epidermal cells of Eschscholzia californica (California poppy). New Phytol 219:1124–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyszecki G, Stiles WS, 1982. Color Science Concepts and Methods, Quantitative Data and Formulae. New York: John Wiley & Sons, Inc. [Google Scholar]
- Yilmaz A, Dyer AG, Rössler W, Spaethe J, 2017. Innate colour preference, individual learning and memory retention in the ant Camponotus blandus. J Exp Biol 220:3315–3326. [DOI] [PubMed] [Google Scholar]
- Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM et al. , 2009. Mixed Effects Models and Extensions in Ecology with R Extensions in Ecology with R. New York: Springer. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.