Abstract
Plant–pollinator interactions have a fundamental influence on flower evolution. Flower color signals are frequently tuned to the visual capabilities of important pollinators such as either bees or birds, but far less is known about whether flower shape influences the choices of pollinators. We tested European honeybee Apis mellifera preferences using novel achromatic (gray-scale) images of 12 insect-pollinated and 12 bird-pollinated native Australian flowers in Germany; thus, avoiding influences of color, odor, or prior experience. Independent bees were tested with a number of parameterized images specifically designed to assess preferences for size, shape, brightness, or the number of flower-like shapes present in an image. We show that honeybees have a preference for visiting images of insect-pollinated flowers and such a preference is most-likely mediated by holistic information rather than by individual image parameters. Our results indicate angiosperms have evolved flower shapes which influence the choice behavior of important pollinators, and thus suggest spatial achromatic flower properties are an important part of visual signaling for plant–pollinator interactions.
Keywords: angiosperm, Apis mellifera (European honeybee), bird-pollinated, flower, insect-pollinated, pollinator
Studies on the co-evolution of pollinators and angiosperms have found that floral phenotypes may have evolved due to their selection by different functional groups of pollinators (Fenster et al. 2004, 2006). Flowers utilize a variety of signals, cues, and traits in order to attract or deter specific pollinators (Lunau et al. 2011; van der Kooi et al. 2018) as animals exhibit different sensory capabilities. Plant communication has developed specific plant–pollinator relationships which maximize signal quality and reception (Chittka and Menzel 1992). The difference in evolutionary pathways of flower color for plants that have evolved for insect or for bird pollination has been observed in different sites around the world (Chittka and Menzel 1992; Rausher 2008; Des Marais and Rausher 2010; Dyer et al. 2012; Shrestha et al. 2013). Bird-pollinated flowers generally reflect long wavelength radiation (Raven 1972), which has been shown as evidence of spectral signals tuning to important pollinators, independent of phylogenetic constraints (Shrestha et al. 2013). Analogous type changes also occur at the short wavelength (UV) region of the spectrum for insect pollinators (Lunau et al. 2011).
Pollinators have preferences for shapes, sizes, and patterns of real and artificial flowers (Lehrer et al. 1995; Johnson and Dafni 1998; Dafni and Kevan 1997). For example, beetles prefer “bowl-shaped” flowers, while small bees prefer flowers which consist of broken outlines (Dafni and Kevan 1997). Bee-flies prefer larger dissected flower models (Johnson and Dafni 1998), and honeybees prefer larger flowers to smaller ones (Martin, 2004). Studies demonstrate that the prefernce of pollinators for spatial charateristics of flowers may be a driver of flower evolution (Giurfa et al. 1999; Lázaro and Totland 2014; Gómez et al. 2016). Furthermore, the morphology of flowers constrains access to morphologically complex flower species (Krishna and Keasar 2018). Bees recognize a number of different flower characteristics which they use to make decisions on which flowers to forage from. These signals, cues or traits include scent (Raguso 2008), color (Giurfa et al. 1995), shape (Lehrer et al. 1995), size (Martin 2004), or symmetry (Giurfa et al. 1996). Given that honeybee foragers have shown preferences for flower-like shapes (Lehrer et al. 1995), symmetry (Lehrer et al. 1995; Giurfa et al. 1996), larger sizes (Martin 2004), and/or different spatial frequencies (lower spatial frequencies when viewing images from a distance and higher spatial frequencies when viewing images at close range; Lehrer et al. 1995), which represent the resolution of bee vision, we tested whether such preferences may indeed exist for real-flowers.
As a number of floral spectral signals have evolved to attract birds or bees for pollination, we hypothesize that differences in flower morphology between insect- and bird-pollinated flowers could be an additional signal which may be used to attract pollinators. While some insect- and bird-pollinated flowers may share similar morphologies, there are some flowers for respective pollinator groups that appear different in morphologies (Cronk and Ojeda 2008) and thus in the current study we randomly selected flowers from our Australian flower data base to test the potential preference question. By using achromatic images of Australian native flowers (Shrestha et al. 2013), which exclude confounding factors of flower color and scent, it is possible to get insights into whether honeybees have a preference for certain natural flower shapes. Research has demonstrated that bumblebees view flowers and images of flowers as similar (Thompson and Plowright 2014), which validate the use of 2D-printed pictures in our study. European honeybees Apis mellifera were tested in Germany as within this region there are no bird-pollinated flowers and no occurrence of the Australian native flowers used in this study, thus enabling insights into how innate preferences may influence the pollinator decisions for choosing flowers.
Materials and Methods
Study site and species
Experiments were conducted in the bee training facilities at the Johannes Gutenberg University in Germany with free-flying honeybee foragers A.mellifera. Individual bees were marked on the abdomen or thorax with a colored mark for identification. One bee was tested at a time and overall a total of 422 individual honeybees were tested. A gravity feeder which provided 5–10% sucrose solution was used to maintain a regular number of bees available for testing. Foragers from different hives were recruited to the feeder to use as a food source and individuals in our experiments were collected from this feeder. We collected 1 individual at a time for participation in the experiments. To collect a honeybee from the feeder, the bee was picked-up using a plexi glass spoon containing a higher concentration of sucrose than the feeder (50% sucrose solution). The bee was taken to the rotating screen apparatus and placed on one of the platforms which contained 50% sucrose solution (Figure 1). Once bees were consistently coming back to the apparatus instead of the feeder for a higher reward, the experiments began.
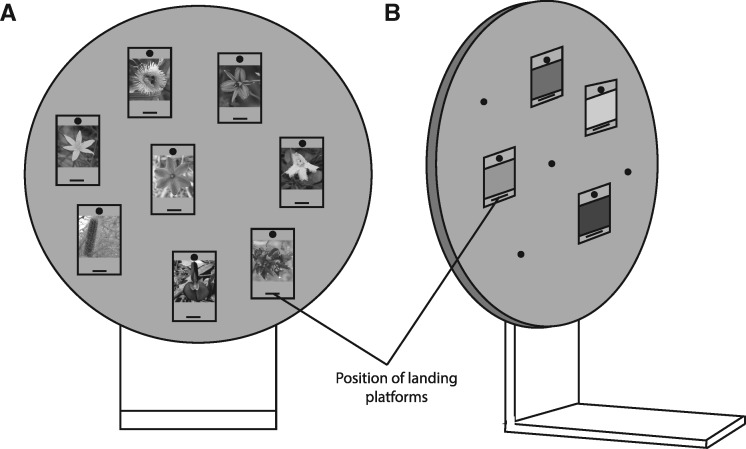
Figure 1.
Schematic of the rotating screen apparatus where the (A) achromatic flower images were presented to bees (front view). Shown are examples of insect- and bird-pollinated flower images presented on hangers with landing platforms located below images on the hangers. (B) The rotating screen with control stimuli presented to bees (side view). Shown is the test for brightness preference.
Apparatus
Honeybees were trained to visit a vertical rotating screen, 50 cm in diameter (Dyer et al. 2008; see Figure 1). By using this screen, the spatial arrangement of stimulus choices could be randomly arranged, thus excluding position cues. The apparatus was able to be rotated between choices and bouts to randomize the position of the stimuli, but was not constantly rotating. Stimuli were presented vertically on 6 ×8 cm hangers with a landing platform attached below the presentation area (Figure 1). A standard gray plastic was used for the screen, hangers, and landing platforms (Dyer et al. 2008). Hangers and surrounding screen areas were cleaned with 20% ethanol solution and then dried between landings and before each test were conducted to exclude the use of olfactory cues.
Experiment 1: Preference for bird- versus Insect-pollinated flowers
Stimuli
Stimuli used for the study consisted of 24 achromatic photographs of Australian native flowers with known pollinators chosen from our databases (Shrestha et al. 2013; Burd et al. 2014). Flowers were chosen for the experiment based on the quality of the collected images from previous field work to exclude photographer bias for the current study (Shrestha et al. 2013; Burd et al. 2014). Twelve of the flowers were identified as exclusively insect-pollinated (Figure 2A) and 12 were exclusively bird-pollinated (Figure 3A). As these flowers were novel to European honeybee pollinators in Germany, we could determine that results were not caused by familiarity with flowers from previous foraging experience. Images of flowers were cropped to 6 ×6 cm squares. The color images were transformed into achromatic grayscale images using the program ImageJ (version 1.50) by discarding the red and blue layers of the original RGB images and keeping only the layer produced by the green channel (Figures 2A and 3A and Supplementary Figure S1). We selected the green channel as the wavelengths sensed by this channel map closely between camera and bee green photoreceptor sensitivities (Garcia et al. 2014) which are known to be important for how free-flying bees perform spatial tasks (Giger and Srinivasan 1996; Hempel de Ibarra and Giurfa 2003; Stach et al. 2004; Morawetz et al. 2013; Avarguès-Weber et al. 2014). The images were printed on EPSON Archival Matte Paper, Super A3, 192 g/m2 and laminated with Avery Dennison® DOL 1480 3D Matte (Matte Clear Super Conformable Cast Overlaminate). A radiometer (Instrument Systems SPECTRO 320 Optical Scanning Spectrometer) was used to ensure stimuli were monochromatic images in the green-receptor channel. Chromatic contrast (0.05 units for the white paper) was also calculated in a Hexagon color space (Chittka 1992) and was well below the threshold of 0.11 Hexagon units that bees perceive as different from an achromatic background (Dyer et al. 2012). For information on flower size, see Supplementary Table S1.
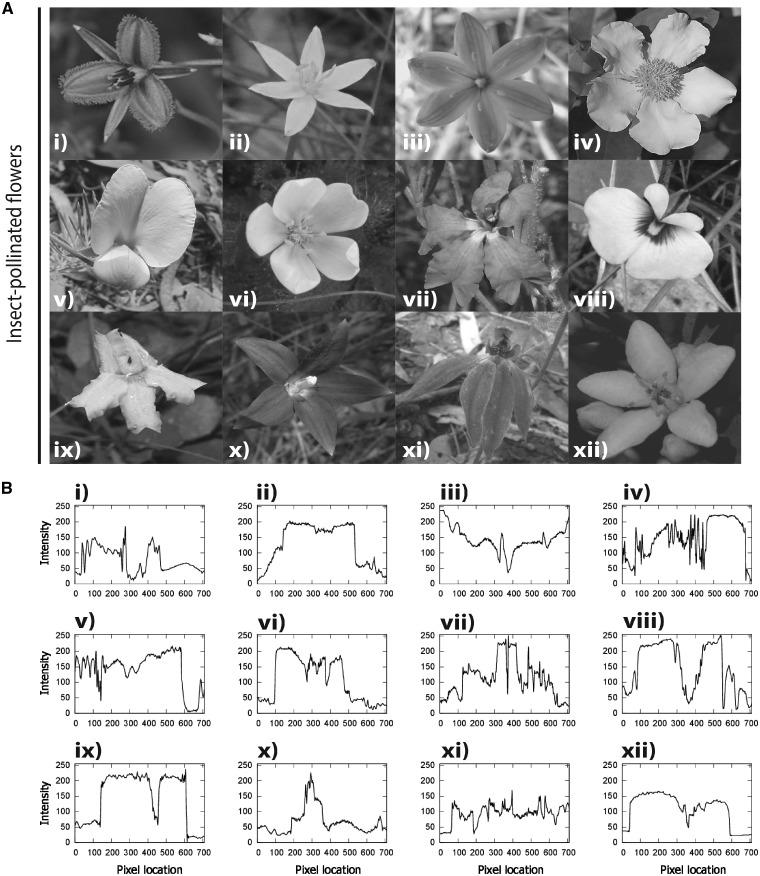
Figure 2.
(A) The 12 insect-pollinated flowers used in the experiments which are native to Australia. The color images of the flowers (i–xii) were converted into achromatic grayscale images by selecting the layer corresponding to the green channel of the original RGB images. (B) The corresponding brightness profiles for the insect-pollinated flower images taken along a linear transect sampled across the middle of the image on the horizontal axis in (A). Species names: (i) Thysanotus juncifolius, (ii) Tricoryne elatior, (iii) Chamaescilla corymbosa, (iv) Hibbertia scandens, (v) Gompholobium huegelii, (vi) Drosera whittakeri, (vii) Dampiera stricta, (viii) Eutaxia microphylla, (ix) Goodenia lanata, (x) Wahlenbergia gloriosa, (xi) Caladenia carnea, and (xii) Philotheca myoporoides. See Supplementary Figure S1A for full color images.
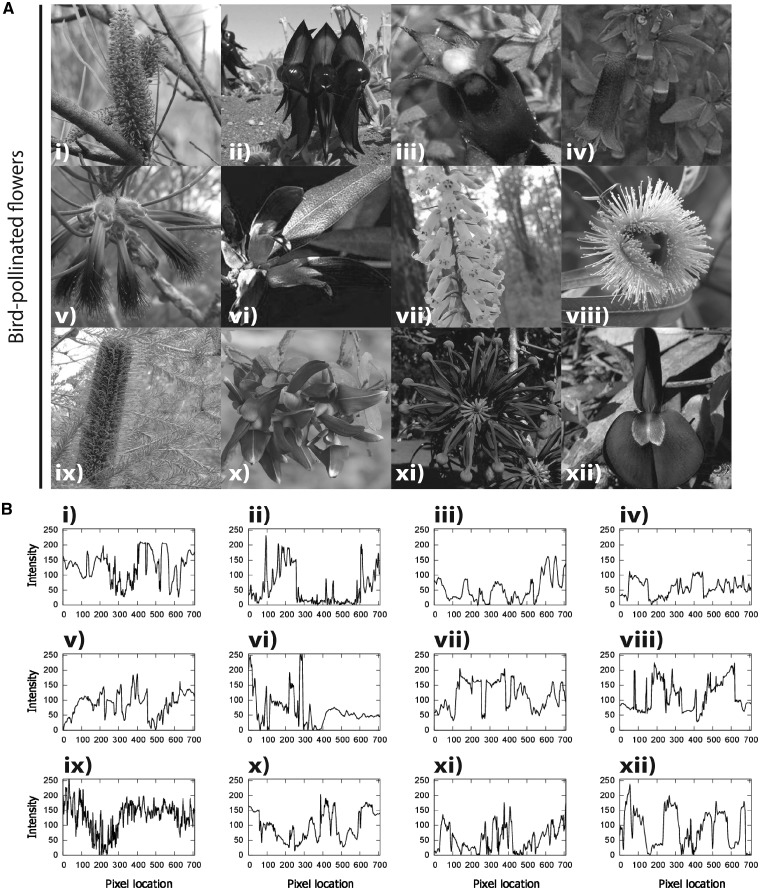
Figure 3.
(A) The 12 bird-pollinated flowers used in the experiments which are native to Australia. The color images of the flowers (i–xii) were converted into achromatic grayscale images by selecting the layer corresponding to the green channel of the original RGB images. (B) The corresponding brightness profiles for the bird-pollinated flower images taken along a linear transect sampled across the middle of the image on the horizontal axis in (A). Species names: (i) Hakea francissiana, (ii) Swainsona formosa, (iii) Astroloma ciliatum, (iv) Corea pulchella, (v) Calothamnus rupestris, (vi) Gastrolobium celsianum, (vii) Epacris impressa, (viii) Eucalyptus sp., (ix) Banksia ericifolia, (x) Templetonia retusa, (xi) Stenocarpus sinuatus, and (xii) Kennedia prostrata. See Supplementary Figure S1B for full color images.
Priming phase
We primed 138 individual honeybees over 24 rewarded choices to land on platforms and become familiar with the apparatus using a 10 µL drop of 50% sucrose solution placed on each of the 8 hanger platforms. This type of priming was found necessary during pilot studies to enable a very high level of motivation from the bees for the subsequent non-rewarded testing. We began counting these priming choices when bees could land on the hanger platforms without assistance. During the priming phase, 6 × 6 cm squares of sand-blasted aluminium were presented on hangers as a spectrally neutral stimulus. The sand blasted aluminium reflects radiation equally from 300 to 650 nm (Dyer et al. 2016) and are thus achromatic for bee perception. After individual bees landed and imbibed the sucrose, they were gently removed from the apparatus using a transparent spoon with sucrose on it and placed behind an opaque screen about 1 m from the rotating screen while the apparatus and hangers were cleaned (Dyer et al. 2008). After this procedure, bees could either choose to land on the apparatus hangers for a reward again or return to the hive to deposit the sucrose.
Testing phase
After the priming phase, we conducted 1 test with 8 pseudo-randomly chosen flower image stimuli from our image database of 24 flowers by using dice rolls (Figure 2A: 4 different insect-pollinated flowers; Figure 3A: 4 different bird-pollinated flowers, Figure 1A). The flower stimuli were placed on the hangers and 10 µL drop of water was used instead of sucrose in the associated platforms as the test was unrewarded. We recorded the number of choices (touches of platforms or images) for a total of 24 choices in this test thus each image had an equal chance of being chosen. A touch was defined as any contact to the platform or flower image during the test.
Statistical analysis
Bee preference analysis
To determine whether bees had any preference to insect- or bird-pollinated flower images, we estimated the mean of the insect-pollinated choices from the intercept of a generalized linear mixed model only including the intercept as predictor. Choices were recorded as binary responses giving a value of 1 for choices made to insect-pollinated flowers and zero otherwise. Subject (individual bees) was included as a random variable to account for the repeated measurements. The model was estimated using the routine “glmer” available as part of the “lme4” package written for the R statistical language (R Core Development Team 2016).
Image analysis
We also analyzed the flower images to determine if contrast or line length of the flower images used were significantly different in terms of insect-pollinated (Figure 2B) or bird-pollinated images (Figure 3B). For all images, the brightness profiles were constructed from pixel values of a linear transect sampling going from the leftmost pixel location to the rightmost location along the central axis of the image. Contrast for each image was calculated as the root mean square of the pixel intensity values (Bex and Makous 2002) for the entire image. Contrast values for the 2 image groups (bird-pollinated or insect-pollinated) were compared by means of an independent t-test. Contrast analyses were performed in MATLAB release 2016b. The flowers line length was analyzed using ImageJ by tracing the perimeter of the flowers and measuring the line length. The line lengths of the bird-pollinated and insect-pollinated flowers were then compared by means of a 2-tailed t-test. The t-tests were carried out in SPSS version 24.
Experiment 2: Honeybee preferences to different aspects of the flower images
Stimuli
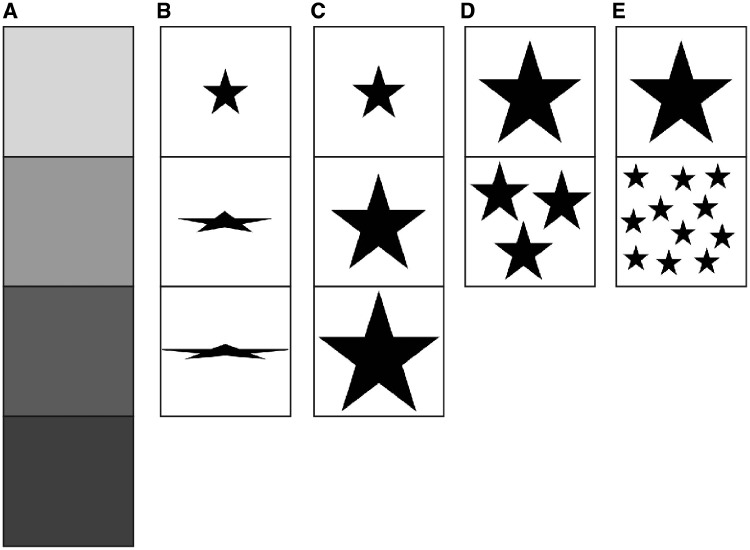
There were 5 control tests that were conducted to check preferences for (i) 4 different levels of brightness, (ii) 3 different elongations of a typical flower-shape, (iii) 3 different flower sizes, (iv) a preference for 1 versus 3 flower shapes, and (v) a preference for 1 versus 11 flower shapes in an image (Figures 1 and 4). The stimuli for this control experiment were developed using the previous tested images of flowers in Experiment 1. We tested for a preference to brightness using 4 stimuli of different levels of brightness 10%, 20%, 35%, and 50% (Figure 4A) which mirror the biologically relevant range of reflectance values for the most common flowers (Chittka et al. 1994; van der Kooi et al. 2016). We tested for a preference to shape using a familiar flower-like star shape (Lehrer et al. 1995), which was elongated, using 3 different stimuli: 1× elongation (none), 2× elongation, and 3× elongation (Figure 4B). We tested for a flower size preference in the image using 3 differently sized flower-like stimuli: small, medium, and large (Figure 4C). We also assessed in 2 tests the preference for images containing 1 flower-like stimulus versus 3 (few; Figure 4D) or 1 versus 11 (many; Figures 1 and 4E) flower-like stimuli. We tested the bees’ preferences for number of flower-like elements in an image as insect-pollinated flowers in our stimuli set (Figure 2) typically consist of 1 large flower-shaped element in an area of the plant, while bird-pollinated flowers in our stimuli set (Figure 3) often have inflorescence (multiple flowers in a single area).
Figure 4.
Samples of the control stimuli used in experiments. (A) Representation of brightness stimuli (10%, 20%, 35%, and 50%). (B) Shape stimuli with elongation of a star-shaped flower-like image at 1× elongation, 2× elongation, and 3× elongation. (C) Size stimuli showing small, medium, and large surface areas of flower-like images with the areas derived from the flower sizes used in part 1. (D) Stimuli used for the flower number experiment of 1 versus 3. (E) Stimuli used for the flower number experiment of 1 versus 11.
Priming phase
The priming phase was identical to Experiment 1.
Testing phase
After the priming phase, a total of 280 bees participated in one of the control tests in which stimuli were either manipulated for (i) brightness (n = 78), (ii) shape elongation (n = 61), (iii) size (n = 65), or (iv–v) number of elements (1 versus 3: n = 34; 1 versus 11: n = 42) and were placed on the hangers. Testing order was random. Ten choices were recorded per bee. A choice was defined as any contact to the platform or stimulus during the test.
Statistical analysis
In Experiment 2, we used a set of generalized linear mixed models (glmm) initially including choice number (sequence) and stimuli parameter as fixed terms to test for potential bee preferences for different visual aspects of the flower images and a potential effect of choice number (sequence of choices). We followed a classical model reduction analytical framework to test for significant effects of the 2 fixed factors. Bees participating on tests for brightness, amount of elongation, and size could select from more than 2 options; therefore, we assumed that the response variable, that is, the stimulus chosen on each trial, followed a multinomial distribution (Faraway 2005). Models for the flower number experiments assumed a binomial distribution for the response variable. Subject (individual bees) was included as a random effect on all models to account for the repeated measurements (Zuur et al. 2009).
The stimulus options with (i) a brightness level of 20%, (ii) 1× elongation, and (iii) medium size were selected as baseline for the multinomial models. The baselines were chosen as (i) 20% as this was similar to the priming brightness level, (ii) 1× elongation as this means there was no elongation in this stimulus, and (iii) medium size as this was the average size of flowers in the images in Experiment 1. Images depicting 1 flower were designed as the “correct” answer for the (iv–v) binomial models. All choice comparisons were done relative to the baseline following standard protocols (Faraway 2005).
Multinomial models were fitted using Bayesian interference with Monte Carlo Markov Chain methods with the routine MCMCglmm (Hadfield 2010), available for the R statistical language. Multivariate normal distributions with mean vector zero and large variance were used as diffuse priors for the fixed and random terms (Hadfield 2010). Models were run with 210,000 iterations, a thinning interval of 1,000 and discarding the first 10,000 iterations as burnin phase. By the end of the simulation phase, chains in all models had an autocorrelation value <0.1.
Binomial models were also fitted using Bayesian techniques. Diffuse normal priors were assumed for the fixed terms while half-Cauchy priors were assumed for the random terms (Zuur et al. 2015). Fitting of the binomial models was done in JAGS (Hornik et al. 2003) for R using the same number of iterations, thinning, and burnin parameters used for the multinomial model.
Posterior distributions of the regression model coefficients were subsequently used to evaluate if the magnitudes of the model’s coefficients were different from zero. For the multinomial models, coefficient values including zero demonstrate that there is no difference between the number of choices observed for the respective trait and the chosen baseline (Supplementary Table S2).
Results
Experiment 1
Bee preference analysis
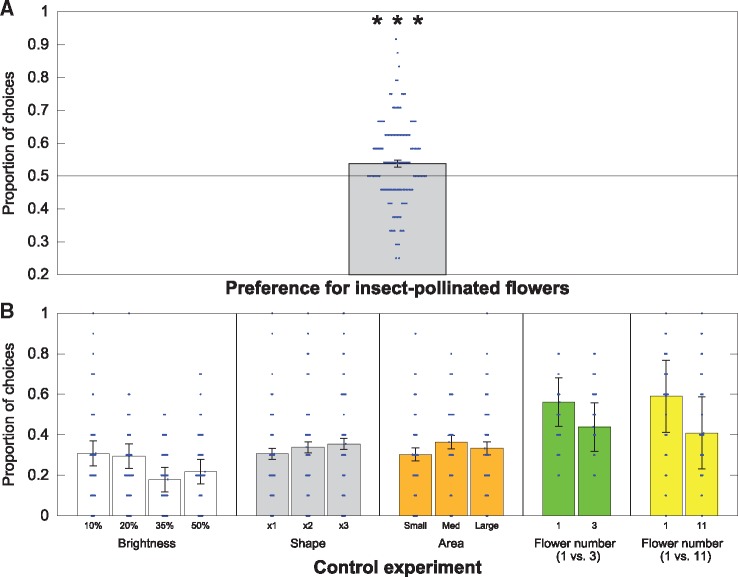
Honeybees (n = 138) significantly preferred insect-pollinated flower images compared with bird-pollinated flower images at a level of 53.8 ± 1.1% (mean ± standard error of the mean) which was significantly different from chance level (H0 =50%, z = 3.556, P < 0.0001). Thus, honeybees had a significant preference for novel insect-pollinated flower images (Figure 5A).
Figure 5.
The results of the preferences tests for Experiments 1 and 2. (A) The mean proportion of choices made for insect-pollinated flower images (gray) during the preference test. This column shows the mean±95% confidence intervals (CIs). The solid black line shows chance expectation at 50%. Significance from chance level performance is indicated by ***≥0.001. Blue dots indicate the raw data, depicted as a bee-swarm plot, of each individual bee’s preference for insect-pollinated flowers (n = 138). (B) The mean proportion of choices made for each of the 5 preference control experiments: brightness (white; n = 78); shape (gray; n = 61), area (orange; n = 65), flower number test 1 versus 3 (green; n = 34), and 1 versus 11 (yellow; n = 42). The columns show the mean±95% CIs. Blue dots indicate the raw data, depicted as a bee-swarm plot, of each individual bee’s preference for each option in the tests.
Image analysis
The contrast values of the images (n = 12) were normally distributed for both insect-pollinated images (W = 0.960, df = 12, P = 0.780; Figure 2B and Supplementary Figure S2) and also for the 12 images of bird-pollinated flowers (W = 0.958, df = 12, P = 0.753; Figure 3B and Supplementary Figure S3). We conducted an independent-sample t-test between the contrast values for the 2 groups and found no significant differences (t = 1.692, df = 17.255, P = 0.109).
The line length of the bird-pollinated and insect-pollinated flowers was not significantly different (independent samples t-test: t =−0.728, df =22, P = 0.475). The area of flowers was also not significantly different (independent samples t-test: t = 0.928, df = 22, P = 0.364); thus, the contrast nor the line length nor the area could be considered a driver of bee preference.
Experiment 2
Zero was included in all the 95% credible intervals for the trial coefficient in all models. This suggests that bees were generally showing similar choices at the beginning and end of the 10 choices in the tests. Therefore, reduced models only including the intercept were subsequently fitted to the data to test for differences in the total number of choices for each trait modification relative to the baseline chosen for each trait. Analyses revealed that bees did not choose any of the modified traits for shape (n = 61), brightness (n = 78), or number of petals (1 versus 3: n = 34; 1 versus 11: n = 42) (Figure 5B). However, bees chose the small flowers less frequently relative to the normal sized images (n = 65; Figure 5B andTable 1).
Table 1.
Percentage of bee choices for each option in each of the 5 tests
| Brightness | |||||
| 10% | 20% | 35% | 50% | ||
| 30.77% | 29.49% | 17.95% | 21.79% | ||
| Shape | |||||
| x1 | x2 | x3 | |||
| 30.65% | 33.87% | 35.48% | |||
| Size | |||||
| Small | Medium | Large | |||
| 30.30% | 36.36% | 33.33% | |||
| 1 versus 3 shapes | |||||
| 1 shape | 3 shapes | ||||
| 56.10% | 43.90% | ||||
| 1 versus 11 shapes | |||||
| 1 shape | 11 shapes | ||||
| 59.09% | 40.91% | ||||
Discussion
Considering flowers presented to honeybees were novel (flowers were native to Australia whereas our honeybee population was located and tested in Germany), we propose that the preference for insect-pollinated flowers was not a direct result of familiarity with flowers through foraging. Based on our results, we thus suggest that the choice for insect-pollinated flowers based on shape is an effect due to an evolved preference rather than through familiarity with specific flowers. This position would be consistent with theories of innate shape preference present in bees proposed by Lehrer et al. (1995). In addition, our control tests suggest that honeybees prefer to choose flowers based on an overall, global view of the flower images rather than on a single parameter. This interpretation fits with how honeybees are known to prefer to process visual input using global holistic information rather than local elemental features (Zhang et al. 1992; Avarguès-Weber et al. 2015, 2018; Howard et al. 2017b). However, we acknowledge that it is also possible that the observed preference for insect-pollinated flowers could alternatively be a result of familiarity of foraging on “similar” insect-pollinated flowers throughout an individual bee’s lifetime. For example, Verguts and Chen (2017) suggested that an individual animal undergoes “evolution” at an individual level throughout its lifetime as it learns and experiences its own environment, thus bees in our experiment may demonstrate a preference for insect-pollinated flowers due to their previous individual experience. Future work with fully naïve bees could help inform the mechanisms underpinning the observed effect of a preference for certain flower morphologies.
Consistent with the current study, honeybees have previously demonstrated a preference for larger flowers of the species, Mimulus guttatus (Martin 2004). In both studies, the selection by honeybees against smaller sizes is possibly due to the lower visibility of the smaller flower-like shape. Other previous works have demonstrated that flower size plays a significant role in plant–pollinator interactions. For example, larger flower sizes may be caused by selection pressures to advertise a higher reward quality or quantity (Ashman and Stanton 1991; Campbell et al. 1991; Cohen and Shmida 1993; Benitez-Vieyra et al. 2010, 2014), thus resulting in a preference against smaller flowers. This is evident in flowers of Turnera ulmifolia L., where nectar production and petal length (an indication of flower size) were positively correlated in an environment where signal accuracy was selected for by pollinators (Benitez-Vieyra et al. 2010). Bees can reliably learn and process size (Howard et al. 2017a) but the size factor alone could not explain the observed preference for insect-pollinated flowers as there were no significant size differences between the images of the flower types. Our investigation of potential elemental factors that might influence bee preferences did not find any significant effect of flower elongation, nor brightness on bee choices. This result is consistent with recent findings that image brightness is not processed by honeybees when using color vision to detect flowers, and indeed brightness appears an unreliable visual cue in complex environments (Ng et al. 2018; van der Kooi et al. 2018).
The results in our current study suggest 2 potential evolutionary mechanisms. The first involves the evolution of flowers to suit pollination by insects such as honeybees due to the bees preference for certain morphologies. This possibility is supported by previous research demonstrating that evolution of flower color occurred through flowers tuning to the relative sensitivity of the plant’s most important pollinators (Chittka and Menzel 1992; Rausher 2008; Des Marais and Rausher 2010; Dyer et al. 2012; Shrestha et al. 2013). The second possible mechanism would be the evolution of bees to prefer morphologies of insect-pollinated flowers as those are the flowers from which it would be easiest to receive nutrition compared with bird-pollinated flowers. As a result, over time bees may have developed evolutionary relevant recognition of insect-pollinated flowers and be able to generalize that familiarity to novel flower comparisons, as discussed above. The preference for insect-pollinated flower shapes could also be a result of a combination of these 2 mechanisms, where insect-pollinated plants and insects, specifically bees, co-evolved.
Our results suggest that the recognition and preference for insect-pollinated flowers by honeybees is innate as bees in Germany had not previously encountered the species of flowers which we presented. In addition, if flowering plants have evolved to suit morphological preferences of bees, Europe and Australia have been separated for many millions of years (with honeybees arriving in Australia within the last 200 years; Paton 1993, 1996), meaning the coevolution of this plant–pollinator system is a deep rooted evolutionary occurrence. Such a phylogenetically conserved effect of the visual system of bee pollinators is plausible as flower colors in Australia have evolved to suit color discrimination of native bee pollinators; and the distribution of colors is the same as regions of the world where honeybees were the dominant influence on flower coloration evolution (Chittka and Menzel 1992; Dyer et al. 2012). Thus, our new evidence suggests that native Australian pollinators may also have a similar preference for flower-shape.
Author Contributions
S.R.H., M.S., J.S., and A.G.D. were involved in experimental design and creation of the stimuli. S.R.H., J.S., J.E.G., and A.G.D. were involved in data collection. S.R.H. and J.E.G. were involved in data analysis. S.R.H. wrote the manuscript. All authors were involved in the interpretation of data and editing of the manuscript.
Supplementary Material
Acknowledgments
We wish to thank Lars Hoppe, Mona Schmitt, and the other students involved in data collection at Johannes Gutenberg Universität, Mainz, Germany. S.R.H. acknowledges the Australian Government Research Training Program Scholarship. A.A.-W. acknowledges the Fyssen Foundation and University Toulouse 3 for financial support. These results were first presented at the XI International Symposium on Pollination (2018) in Berlin, Germany.
References
- Ashman TL, Stanton M, 1991. Seasonal variation in pollination dynamics of sexually dimorphic Sidalcea oregana ssp. spicata (Malvaceae). Ecology 72:993–1003. [Google Scholar]
- Avarguès-Weber A, d’Amaro D, Metzler M, Dyer AG, 2014. Conceptualization of relative size by honeybees. Front Behav Neurosci 8:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avarguès-Weber A, d’Amaro D, Metzler M, Finke V, Baracchi D et al. , 2018. Does holistic processing require a large brain? Insights from honeybees and wasps in fine visual recognition tasks. Front Psychol 9:1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avarguès-Weber A, Dyer AG, Ferrah N, Giurfa M, 2015. The forest or the trees: preference for global over local image processing is reversed by prior experience in honeybees. Proc R Soc B 282:20142384.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitez-Vieyra S, Fornoni J, Pérez-Alquicira J, Boege K, Domínguez CA, 2014. The evolution of signal–reward correlations in bee- and hummingbird-pollinated species of Salvia. Proc R Soc B 281:20132934.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitez-Vieyra S, Ordano M, Fornoni J, Boege K, Domínguez C, 2010. Selection on signal–reward correlation: limits and opportunities to the evolution of deceit in Turnera ulmifolia L. J Exp Biol 23:2760–2767. [DOI] [PubMed] [Google Scholar]
- Bex PJ, Makous W, 2002. Spatial frequency, phase, and the contrast of natural images. JOSA A 19:1096–1106. [DOI] [PubMed] [Google Scholar]
- Burd M, Stayton CT, Shrestha M, Dyer AG, 2014. Distinctive convergence in Australian floral colours seen through the eyes of Australian birds. Proc R Soc B 281:20132862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DR, Waser NM, Price MV, Lynch EA, Mitchell RJ, 1991. Components of phenotypic selection: pollen export and flower corrolla width in Ipomopsis aggregata. Evolution 45:1458–1467. [DOI] [PubMed] [Google Scholar]
- Chittka L, 1992. The colour hexagon: a chromaticity diagram based on photoreceptor excitations as a generalized representation of colour opponency. J Comp Physiol A 170:533–543. [Google Scholar]
- Chittka L, Menzel R, 1992. The evolutionary adaptation of flower colours and the insect pollinators’ colour vision. J Comp Physiol A 171:171–181. [Google Scholar]
- Chittka L, Shmida A, Troje N, Menzel R, 1994. Ultraviolet as a component of flower reflections, and the colour perception of Hymenoptera. Vision Res 34:1489–1508. [DOI] [PubMed] [Google Scholar]
- Cohen D, Shmida A, 1993. The evolution of flower display and reward. Evol Biol 27:197–243. [Google Scholar]
- Cronk Q, Ojeda I, 2008. Bird-pollinated flowers in an evolutionary and molecular context. J Exp Bot 59:715–727. [DOI] [PubMed] [Google Scholar]
- Dafni A, Kevan PG, 1997. Flower size and shape: implications in pollination. Isr J Plant Sci 45:201–211. [Google Scholar]
- Des Marais DL, Rausher MD, 2010. Parallel evolution at multiple levels in the origin of hummingbird pollinated flowers in Ipomoea. Evolution 64:2044–2054. [DOI] [PubMed] [Google Scholar]
- Dyer AG, Boyd-Gerny S, McLoughlin S, Rosa MG, Simonov V et al. , 2012. Parallel evolution of angiosperm colour signals: common evolutionary pressures linked to hymenopteran vision. Proc R Soc B 279:3606–3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer AG, Rosa MG, Reser DH, 2008. Honeybees can recognise images of complex natural scenes for use as potential landmarks. J Exp Biol 211:1180–1186. [DOI] [PubMed] [Google Scholar]
- Dyer AG, Boyd-Gerny S, Shrestha M, Lunau K, Garcia JE et al. , 2016. Innate colour preferences of the Australian native stingless bee Tetragonula carbonaria Sm. J Comp Physiol A 202:603–613. [DOI] [PubMed] [Google Scholar]
- Faraway JJ, 2005. Extending the Linear Model with R: Generalized Linear, Mixed Effects and Nonparametric Regression Models. Florida, USA: CRC Press. [Google Scholar]
- Fenster CB, Armbruster WS, Wilson P, Dudash MR, Thomson JD, 2004. Pollination syndromes and floral specialization. Annu Rev Ecol Evol Syst 35:375–403. [Google Scholar]
- Fenster CB, Cheely G, Dudash MR, Reynolds RJ, 2006. Nectar reward and advertisement in hummingbird-pollinated Silene virginica (Caryophyllaceae). Am J Bot 93:1800–1807. [DOI] [PubMed] [Google Scholar]
- Garcia JE, Greentree AD, Shrestha M, Dorin A, Dyer AG, 2014. Flower colours through the lens: quantitative measurement with visible and ultraviolet digital photography. PLoS One 9:e96646.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giger A, Srinivasan M, 1996. Pattern recognition in honeybees: chromatic properties of orientation analysis. J Comp Physiol A 178:763–769. [Google Scholar]
- Giurfa M, Dafni A, Neal PR, 1999. Floral symmetry and its role in plant-pollinator systems. Int J Plant Sci 160:S41–S50. [DOI] [PubMed] [Google Scholar]
- Giurfa M, Eichmann B, Menzel R, 1996. Symmetry perception in an insect. Nature 382:458–461. [DOI] [PubMed] [Google Scholar]
- Giurfa M, Nunez J, Chittka L, Menzel R, 1995. Colour preferences of flower-naive honeybees. J Comp Physiol A 177:247–259. [Google Scholar]
- Gómez JM, Torices R, Lorite J, Klingenberg CP, Perfectti F, 2016. The role of pollinators in the evolution of corolla shape variation, disparity and integration in a highly diversified plant family with a conserved floral bauplan. Ann Bot 117:889–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadfield JD, 2010. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J Stat Softw 33:1–22.20808728 [Google Scholar]
- Hempel de Ibarra NH, Giurfa M, 2003. Discrimination of closed coloured shapes by honeybees requires only contrast to the long wavelength receptor type. Anim Behav 66:903–910. [Google Scholar]
- Hornik K, Leisch F, Zeileis A, 2003. JAGS: a program for analysis of Bayesian graphical models using Gibbs sampling. Proceedings of the 3rd International Workshop on Distributed Statistical Computing. Vienna, Austria, 20–22 March 2003.
- Howard SR, Avarguès-Weber A, Garcia J, Dyer AG, 2017a. Free-flying honeybees extrapolate relational size rules to sort successively visited artificial flowers in a realistic foraging situation. Anim Cogn 20:627–638. [DOI] [PubMed] [Google Scholar]
- Howard SR, Avarguès-Weber A, Garcia JE, Stuart-Fox D, Dyer AG, 2017b. Perception of contextual size illusions by honeybees in restricted and unrestricted viewing conditions. Proc R Soc B 284:20172278.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S, Dafni A, 1998. Response of bee‐flies to the shape and pattern of model flowers: implications for floral evolution in a mediterranean herb. Funct Ecol 12:289–297. [Google Scholar]
- Krishna S, Keasar T, 2018. Morphological complexity as a floral signal: from perception by insect pollinators to co-evolutionary implications. Int J Mol Sci 19:1681.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lázaro A, Totland Ø, 2014. The influence of floral symmetry, dependence on pollinators and pollination generalization on flower size variation. Ann bot 114:157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer M, Horridge G, Zhang S, Gadagkar R, 1995. Shape vision in bees: innate preference for flower-like patterns. Philos Trans R Soc B 347:123–137. [Google Scholar]
- Lunau K, Papiorek S, Eltz T, Sazima M, 2011. Avoidance of achromatic colours by bees provides a private niche for hummingbirds. J Exp Biol 214:1607–1612. [DOI] [PubMed] [Google Scholar]
- Martin NH, 2004. Flower size preferences of the honeybee (Apis mellifera) foraging on Mimulus guttatus (Scrophulariaceae). Evol Ecol 6:777–782. [Google Scholar]
- Morawetz L, Svoboda A, Spaethe J, Dyer AG, 2013. Blue colour preference in honeybees distracts visual attention for learning closed shapes. J Comp Physiol A 199:817–827. [DOI] [PubMed] [Google Scholar]
- Ng L, Garcia JE, Dyer AG, 2018. Why colour is complex: evidence that bees perceive neither brightness nor green contrast in colour signal processing. Facets 3:800–817. [Google Scholar]
- Paton DC, 1993. Honeybees in the Australian environment. Bioscience 43:95–103. [Google Scholar]
- Paton D, 1996. Overview of feral and managed honeybees in Australia: distribution, abundance, extent of interactions with native biota, evidence of impacts and future research. Australian Nature Conservation Agency.
- R Core Development Team, 2016. R: A Language and Environment for Statistical Computing. Vienna, Austria: Available from: http://www. R-project. org. [Google Scholar]
- Raguso RA, 2008. Wake up and smell the roses: the ecology and evolution of floral scent. Annu Rev Ecol Evol Syst 39:549–569. [Google Scholar]
- Rausher MD, 2008. Evolutionary transitions in floral color. Int J Plant Sci 169:7–21. [Google Scholar]
- Raven PH, 1972. Why are bird‐visited flowers predominantly red? Evolution 26:674.. [DOI] [PubMed] [Google Scholar]
- Shrestha M, Dyer AG, Boyd-Gerny S, Wong B, Burd M, 2013. Shades of red: bird‐pollinated flowers target the specific colour discrimination abilities of avian vision. New Phytol 198:301–310. [DOI] [PubMed] [Google Scholar]
- Stach S, Benard J, Giurfa M, 2004. Local-feature assembling in visual pattern recognition and generalization in honeybees. Nature 429:758–761. [DOI] [PubMed] [Google Scholar]
- Thompson EL, Plowright CM, 2014. How images may or may not represent flowers: picture–object correspondence in bumblebees (Bombus impatiens)? Anim Cogn 17:1031–1043. [DOI] [PubMed] [Google Scholar]
- van der Kooi CJ, Dyer AG, Kevan PG, Lunau K, 2018. Functional significance of the optical properties of flowers for visual signalling. Ann Bot. doi: 10.1093/aob/mcy119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kooi CJ, Elzenga JTM, Staal M, Stavenga DG, 2016. How to colour a flower: on the optical principles of flower coloration. Proc R Soc B 283:20160429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verguts T, Chen Q, 2017. Numerical cognition: learning binds biology to culture. Trends Cogn Sci 21:409–424. [DOI] [PubMed] [Google Scholar]
- Zhang S, Srinivasan M, Horridge G, 1992. Pattern recognition in honeybees: local and global analysis. Proc R Soc B 248:55–61. [Google Scholar]
- Zuur AF, Hilbe JM, Ieno EN, 2015. A Beginner’s Guide to GLM and GLMM with R. Newburgh: Highland Statistics. [Google Scholar]
- Zuur AF, Ieno EN, Saveliev AA, 2009. Mixed Effects Models and Extensions in Ecology with R: Springer. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.