Abstract
Topical beta-blocker formulations are commonly used to treat infantile hemangiomas (IHs); however, the skin concentrations and drug permeation through the skin have not been quantified. Microneedles (MNs) may increase local skin concentrations, which could further enhance lesion clearance and improve dosing regimens. The objective of this study was to quantify skin concentrations and drug permeation of two beta-blockers, propranolol and timolol, in vitro after application to intact skin and skin pretreated with solid MNs of two lengths. Propranolol skin concentrations and drug permeation were significantly higher than timolol skin concentrations for all study conditions, which is likely due to the lipophilic nature of propranolol compared to the hydrophilicity of timolol. Propranolol skin concentrations were significantly influenced by dosing regimen, as skin concentrations increased with increasing drug application. Pretreatment of the skin with solid 250 μm and 500 μm length MNs increased local skin concentrations of timolol; propranolol skin concentrations did not significantly increase after MN pretreatment. Propranolol and timolol permeation through the skin increased after MN pretreatment with both MN lengths for both compounds. Taken together, solid MN pretreatment prior to application of topical timolol may be beneficial for deep or mixed IHs upon further optimization of the MN treatment paradigm.
Keywords: microneedle, beta-blocker, propranolol, timolol, infantile hemangioma
1. Introduction
Oral beta-adrenergic receptor antagonists (or “beta-blocker”) formulations have become the standard of care for infantile hemangiomas (IHs), due to superior lesion clearance compared to previous treatment options (Malik et al. 2013). To minimize the systemic side effects commonly observed with systemic beta-blocker use, topical beta-blocker therapy options have also been explored (Chakkittakandiyil et al. 2012; Kunzi-Rapp 2012; Painter et al. 2016). While propranolol was the first beta-blocker used to treat IHs (Leaute-Labreze et al. 2008) and since has become widely studied for this indication, timolol maleate has also been widely studied for topical application for IH treatment (Painter et al. 2016) due to the commercial availability of timolol ophthalmic drops. For both propranolol and timolol, topical treatment is typically applied multiple times a day (Painter et al. 2016), which can reduce adherence to therapy (Gupta et al. 2009).
There are many advantages of topical drug delivery over traditional routes of administration for skin disorders, including localized delivery drug directly to the site of action, minimization of systemic side effects, and ease of use (Guy 2010). Despite these advantages, topical drug delivery can be challenging due to the barrier function of the skin provided by the lipid matrix of the stratum corneum (SC). Passive diffusion of therapeutic compounds to the lower layers of the skin is restricted to small, moderately lipophilic compounds. For drugs that fit these strict physicochemical properties, potent drugs are often ideal, as drug delivery through the skin is limited to milligrams per day or less (Guy 2010). As such, few drugs fit these rigid criteria. To improve the dermal delivery of compounds that do not have the physicochemical properties necessary for passive delivery into the skin, a variety of enhancement techniques such as microneedles (MNs) have been developed.
MNs are micron-scale projections that temporarily bypass the SC to create aqueous micropores (or microchannels) through which drugs that are typically considered skin impermeable can reach the underlying tissues (Kim et al. 2012; Prausnitz et al. 2008). MNs are considered to be painless (Kaushik et al. 2001) and safe (Donnelly et al. 2009), particularly compared to hypodermic needles. Additionally, MN delivery parameters can be altered for the desired indication based on the MN geometry (MN length and/or number). Solid MNs are applied in a two-step “poke and press” method, in which micropores are created by insertion of the MN array, followed by the application of a drug formulation on top of the treatment site (Kim et al. 2012). A wide range of compounds have been delivered using the poke and press method, including small hydrophilic molecules (Brogden et al. 2013; Donnelly et al. 2008; Li et al. 2010; Wermeling et al. 2008), large biotherapeutics (Martanto et al. 2004), and nanoparticles (Coulman et al. 2009; McAllister et al. 2003; Zhang et al. 2010), for both topical and transdermal indications.
To date, the primary focus when evaluating topical beta-blocker formulations for IHs has been efficacy and safety. However, the clinically effective concentration of drug within the skin after application of these topical formulations has not been quantified. These skin concentrations will be necessary for comparison when developing future topical formulations to reduce the number of applications required per day. If optimized properly, MNs may be able to increase local skin concentrations of drug, thus contributing to improved dosing regimens. The objective of this study was to quantify the skin concentrations and drug permeation profiles of two beta-blockers used for IH treatment, propranolol and timolol, after application using various dosing regimens that mimic what is used in a clinical setting. Additionally, the MN-mediated delivery of propranolol and timolol using solid MN arrays was evaluated. The skin concentrations and drug permeation through skin pretreated with two lengths of solid MNs was evaluated.
2. Methods
2.1. Materials
(±) Propranolol hydrochloride, acetonitrile, ethanol, Hank’s balanced salts, methanol, metoprolol (+) tartate (+), and gentamicin sulfate were obtained from Sigma Aldrich (St. Louis, MO). Formic acid was obtained from J.T. Baker (Avanto Performance Materials, Inc., Center Valley, PA). HEPES ((4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid)) and sodium bicarbonate were obtained from Research Products International (Mount Prospect, IL). Phosphate buffered saline was obtained from Amresco LLC (Solon, OH). Timolol maleate ophthalmic solution USP (0.5% w/v; Sandoz, Holzkirchen, Germany) was purchased through the University of Iowa Hospital and Clinics Central Pharmacy. Stainless steel Dermarollers® of 250 μm and 500 μm length were purchased on Amazon. Full thickness Yucatan porcine skin was obtained from Sinclair Bio-Resources (Columbia, MO).
2.2. Diffusion study conditions
The in vitro permeation and skin retention of drug were evaluated using an in-line diffusion set-up (PermeGear, Inc., Hellertown, PA). Full thickness Yucatan porcine skin (Sinclair Bio-Resources, Columbia, MO) was used for the diffusion studies. The hair was clipped, and the skin was cleaned to remove excess subcutaneous fat and stored at −20°C until the day of the study. The skin was thawed, cut into the appropriate size, and the thickness of each individual piece was measured. The mean ± SD skin thickness was 1.7 ± 0.2 mm. Skin was placed in the diffusion cells and allowed to equilibrate with the receiver fluid. The receiver fluid was HEPES buffer with 10% ethanol, pH 7.4, modified with Hank’s balanced salts and sodium bicarbonate, delivered at a flow rate of 25 μL/min. The receiver fluid and diffusion cells were maintained at 37°C throughout the study.
Skin concentrations and drug permeation were evaluated for two formulations: a 0.5% w/v propranolol solution made in 1X PBS, and a commercially available 0.5% w/v timolol ophthalmic eyedrop preparation. Each dose was 200 μL, delivering 1 mg of drug per application. For intact skin studies, three dosing regimens were evaluated. Single dose studies were run for 24, 36, 48, and 72 hours, with one-time drug application at baseline for all studies. Once a day studies (drug application every 24 hours) were run for 24, 48, and 72 hours, resulting in one, two, and three drug applications, respectively. Twice a day studies (drug application every 12 hours) were run for 24, 36, and 48 hours, resulting in two, three, and four drug applications, respectively. For solid MN studies, stainless steel Dermarollers® of 250 μm and 500 μm length were rolled across the diffusion area in four perpendicular lines, 5 times in each direction; this is similar to previously published methods (Pawar etal. 2013)To mimic the support of the tissue underlying the skin seen in vivo, the skin was placed on a polydimethylsiloxane polymer block during MN insertion. To confirm that MN treatment was successful in breaching the skin barrier, changes in transepidermal water loss (TEWL) were evaluated post-MN treatment (n=3 for each MN condition). The TEWL of intact porcine skin was made using a single probe open-chamber evaporimeter (CyberDERM Inc., Broomall, PA) by placing the probe on the skin surface for approximately 30 seconds or until the measurements stabilized. TEWL measurements were repeated again immediately after MN insertion. All studies were run in triplicate.
2.3. Quantification of skin drug concentration
Drug was extracted from the skin according to published methods (Kelchen et al. 2018). At the end of each study, the excess formulation on the surface was gently blotted off with a KimWipe®. The skin was rinsed three times with distilled water and gently blotted with a paper towel between rinses. The surface of the skin was then tape stripped twice, excess skin around the diffusion area was removed, and the diffusion area was cut into nine small pieces. The weight of the skin was recorded. The skin was sonicated (1510 Ultrasonic cleaner, Branson, Danbury, CT) in methanol for 10 minutes, followed by two cycles of homogenization (5 minutes at 5 m/s) (Bead Mill 4, Fisher Scientific, Hampton, NH). Homogenized skin samples were centrifuged at 3155 xg for 30 minutes (Centrifuge 5804R, Eppendorf AG, Hamburg, Germany), the supernatant was removed and centrifuged at 17,000 xg for 30 minutes (accuSpin Micro 17R, FisherScientific, Hampton, NH) to pellet any remaining pieces of skin. The supernatant was diluted with mobile phase and analyzed via LC-MS. The receiver samples were also diluted appropriately and analyzed via LC-MS. The amount of drug retained in the skin was normalized to the weight of the skin sample, and skin drug concentrations are reported as μmol drug/g skin.
2.4. Liquid chromatography-mass spectrometry (LC-MS) methods
Samples were separated on a Phenomenex Synergi Polar RP 80A column (C18, 4 μm, 250 mm × 2 mm, Torrance, CA) with a guard column, and the column temperature was maintained at 40°C. The mobile phase (0.075% v/v formic acid in water and acetonitrile in a 65:35 ratio) was delivered at a flow rate of 0.380 mL/min. The LC-MS system (Shimadzu, Columbia, MD, USA) operated using atmospheric pressure chemical ionization (APCI) in positive-ion detection mode. The injection volume was 20 μL. Single ion monitoring mode was used to detect propranolol (m/z 260.30), timolol (m/z 317.30), and the internal standard metoprolol (m/z 268.30) at retention times of 4.7 minutes, 2.7 minutes, and 2.9 minutes, respectively. Propranolol and timolol were linear over a range of 8–2000 ng/mL.
2.5. Data analysis
Statistical analysis was completed using GraphPad Prism 7 (GraphPad Software, Inc., La Jolla, CA). Skin concentrations and cumulative drug permeation from different study lengths within the same dosing regimens were compared using a one-way ANOVA with Tukey’s multiple comparison. Comparisons in skin concentrations and cumulative drug permeation across dosing regimens at the same study length were compared using a Student’s t-test. The skin concentrations and cumulative drug permeation for the same study length of the same dosing regimen were compared using a Student’s t-test. TEWL measurements, skin concentrations, and cumulative drug permeation were compared using a Student’s t-test between intact and MN-treated skin within the same MN condition. A p-value of <0.05 was considered statistically significant.
3. Results
3.1. Intact skin retention from various dosing regimens
3.1.1. Propranolol
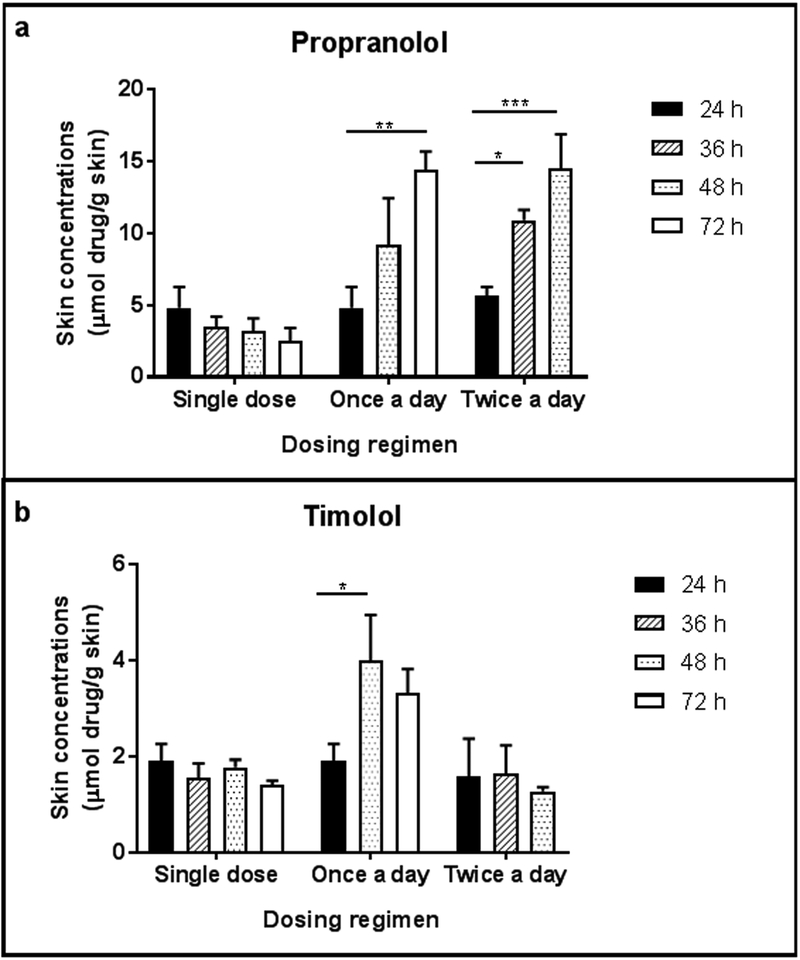
After application of a single dose, the amount of propranolol retained in the skin at 24 hours was 0.883 μmol propranolol, which is equivalent to 229.08 μg, and the recovered dose of propranolol in the skin was 22.91% after 24 hours. After a single application of propranolol, skin concentrations decreased as the study length increased (Figure 1a; Table 1). However, there was no significant difference in skin concentrations between the four time points (p=0.121). Following application of propranolol every 24 hours, skin concentrations increased with increasing study length. A 0.90-fold increase in skin concentrations was observed between 24 and 48 hour studies, and a 0.57-fold increase was observed in skin concentrations between 48 and 72 hour studies. Skin concentrations were significantly different at 24 and 72 hours (p<0.01). After application of propranolol every 12 hours, skin concentrations also increased with increasing study length. Skin concentrations increased 0.93-fold between the 24 and 36 hour studies and increased 0.33-fold between the 36 and 48 hour studies. The skin concentrations were significantly higher at 36 hours (p<0.05) and 48 hours (p<0.001) compared to 24 hours.
Figure 1.
Skin concentrations (μmol drug/g skin) after application of (a) 0.5% w/v propranolol in PBS solution or (b) 0.5% w/v timolol ophthalmic solution to full thickness excised porcine skin under various dosing regimens (single dose, once a day dosing, or twice a day dosing) for various study lengths; n=3 for each condition. Bars represent mean ± SD. *: p<0.05; **: p<0.01; ***: p<0.001
Table 1.
Intact skin concentrations (μmol drug/g skin) of propranolol and timolol under various dosing regimens (n=3)
| Study | Drug application | Study length | Skin concentrations (μmol drug/g skin) | |
|---|---|---|---|---|
| Propranolol | Timolol | |||
| Single dose study | At the beginning of the study | 24 hours | 4.82 ± 1.45 | 1.91 ± 0.35 |
| 36 hours | 3.45 ± 0.77 | 1.56 ± 0.29 | ||
| 48 hours | 3.22 ± 0.85 | 1.77 ± 0.16 | ||
| 72 hours | 2.53 ± 0.87 | 1.41 ± 0.08 | ||
| Once a day study | Every 24 hours | 24 hours | 4.82 ± 1.45 | 1.91 ± 0.35 |
| 48 hours | 9.17 ± 3.27 | 4.00 ± 0.95 | ||
| 72 hours | 14.41 ± 1.28 | 3.33 ± 0.49 | ||
| Twice a day study | Every 12 hours | 24 hours | 5.66 ± 0.61 | 1.57 ± 0.80 |
| 36 hours | 10.92 ± 0.71 | 1.63 ± 0.60 | ||
| 48 hours | 14.54 ± 2.36 | 1.27 ± 0.09 | ||
3.1.2. Timolol
After application of a single dose, the amount of timolol retained in the skin at 24 hours was 0.447 μmol timolol, which is equivalent to 141.33 μg, and the recovered dose of timolol in the skin was 14.13% after 24 hours. After a single application of timolol eyedrops, there were no significant differences in skin concentrations between the four study lengths (Figure 1b; Table 1, p=0.1368). After application every 24 hours, skin concentrations were significantly greater at 48 hours compared to 24 hours, but not significantly different than 72 hours (p=0.4632). After application of timolol every 12 hours, skin concentrations were not significantly different between the three study lengths (p=0.7298).
3.1.3. Comparison between beta-blockers
For all dosing conditions, timolol skin concentrations were lower than propranolol skin concentrations from the same dosing regimen (Table 1). For the single dose regimen, propranolol skin concentrations were significantly greater than timolol for all time points (p<0.05) except at 72 hours (p=0.0921). For the once a day dosing regimen, propranolol skin concentrations were significantly greater for the 24 hour and 72 hour study lengths (p<0.05), but not at 48 hours (p=0.0582). For the twice a day dosing regimen, propranolol skin concentrations were significantly higher for all study lengths (p<0.050).
3.2. Drug permeation through intact skin from various dosing regimens
3.2.1. Propranolol
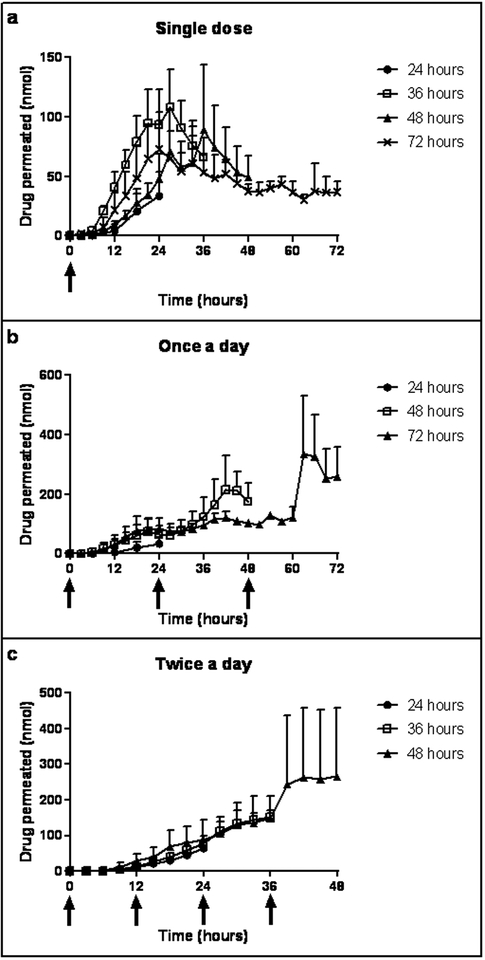
Drug permeation profiles after a single dose of propranolol are shown in Figure 2a. For the 24 hour study, the amount of drug in the receiver increased throughout the length of the study. For 36–72 hour studies, the amount of drug in the receiver peaked at approximately 24–27 hours and then decreased over the study. Drug permeation profiles after once a day dosing of propranolol are shown in Figure 2b. The profiles for the three studies were similar for the first 24 hours. Drug permeation increased after drug application at 24 hours for both the 48 and 72 hour study, and drug permeation increased further for the 72 hour study after drug application at 48 hours. While not exactly overlapping, the cumulative drug permeation within the first 24 hours was similar between all study lengths of the single dose study and the once a day study (p=0.2216). Drug permeation profiles after twice a day dosing of propranolol are shown in Figure 2c. The profiles for the three studies follow a similar pattern for the first 24 hours; the 36 hour and 48 hour studies also remain similar until 36 hours. The amount of drug permeated increased at approximately 39 hours with very little change afterwards.
Figure 2.
Permeation profiles of propranolol into a receiver fluid after application of a 0.5% w/v propranolol in PBS solution to full thickness excised porcine skin (a) once (b) once a day and (c) twice a day for various study lengths; n=3 for each condition. Data points represent mean ± SD.
3.2.2. Timolol
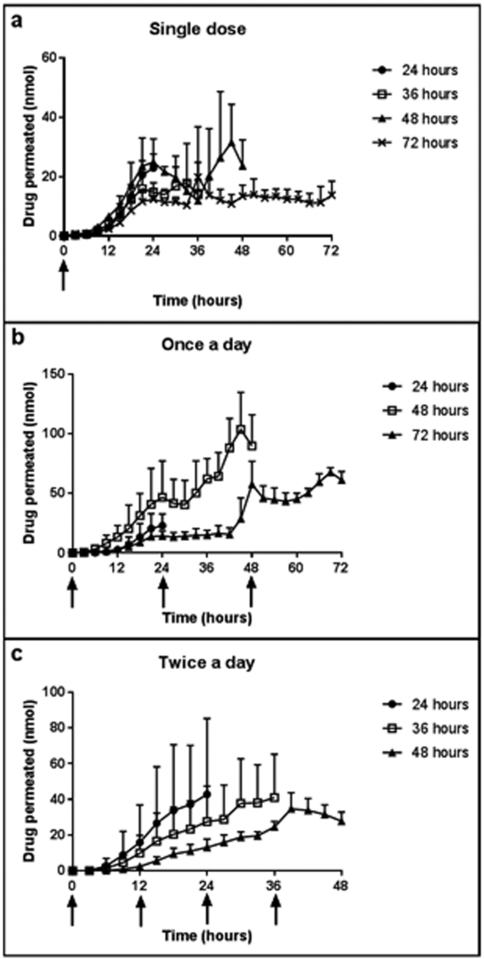
Drug permeation profiles after application of a single dose of timolol eyedrops are shown in Figure 3a. Similar to propranolol, the amount of drug in the receiver increased throughout the length of the 24 hour study. For the 36–72 hour studies, the amount of the drug in the receiver peaked around 21–24 hours. For the 48 hour study, a second peak was observed at approximately 45 hours. Drug permeation profiles after once a day dosing are shown in Figure 3b. At 24 hours (after application of the second dose), the amount of drug in the receiver for the 48 hour study increased rapidly, with the amount of drug peaking at 45 hours. The amount of drug in the receiver remained constant after application of the second dose at 24 hours for the 72 hour length study; the amount of drug increased after application of the third dose at 48 hours. While not exactly overlapping, the cumulative drug permeation within the first 24 hours was similar between all study lengths of the single dose study and the once a day study (p=0.1469). Drug permeation profiles after twice a day dosing are shown in Figure 3c. The amount of drug permeated increased quickly after the application of the second dose at 12 hours. Similar increases were seen after application at 24 hours and 36 hours.
Figure 3.
Permeation profiles of timolol into a receiver fluid after application of a 0.5% w/v timolol ophthalmic solution to full thickness excised porcine skin (a) once (b) once a day and (c) twice a day for various study lengths; n=3 for each condition. Data points represent mean ± SD
3.2.3. Comparison between beta-blockers
Cumulative propranolol permeation through the skin was greater compared to timolol for all study lengths and all dosing regimens. For the single dose studies, this difference was not significantly different at 24 hours (p=0.2443) but was significant for the longer study lengths (p<0.05). For the once a day dosing studies, significant differences were not observed at 48 hours (p=0.1370), but these differences were significant at 72 hours (p=0.0086). For twice a day dosing, the differences between cumulative propranolol permeation and cumulative timolol permeation were not significant for any time point (p>0.05).
3.3. Barrier disruption after solid MN insertion
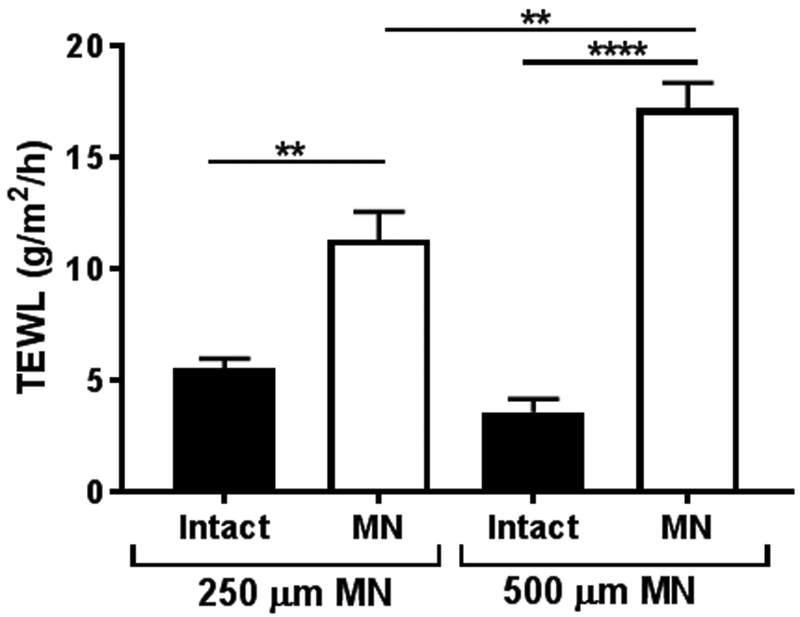
The extent of barrier disruption after solid MN application was evaluated using TEWL (Figure 4). After application with the Dermarollers®, TEWL values were significantly increased for the 250 μm length MNs (intact and MN-treated skin: 5.56 ± 0.43 g/m2/h vs. 11.33 ± 1.24 g/m2/h, respectively; p=0.0016) and 500 μm length MNs (intact and MN-treated skin: 3.60 ± 0.59 g/m2/h vs. 17.21 ± 1.14 g/m2/h, respectively; p<0.0001). There were no significant differences in baseline TEWL for intact skin (p=0.1144), though post-MN TEWL values were significantly different between the two MN lengths (p=0.0038).
Figure 4.
TEWL measurements (n=3) for intact skin and skin treated with solid MNs of two lengths (250 μm and 500 μm length). TEWL measurements were significantly increased after MN treatment for both MN lengths. Bars represent mean ± SD. **: p<0.01; ****: p<0.0001
3.4. Skin retention and drug permeation through MN pretreated skin
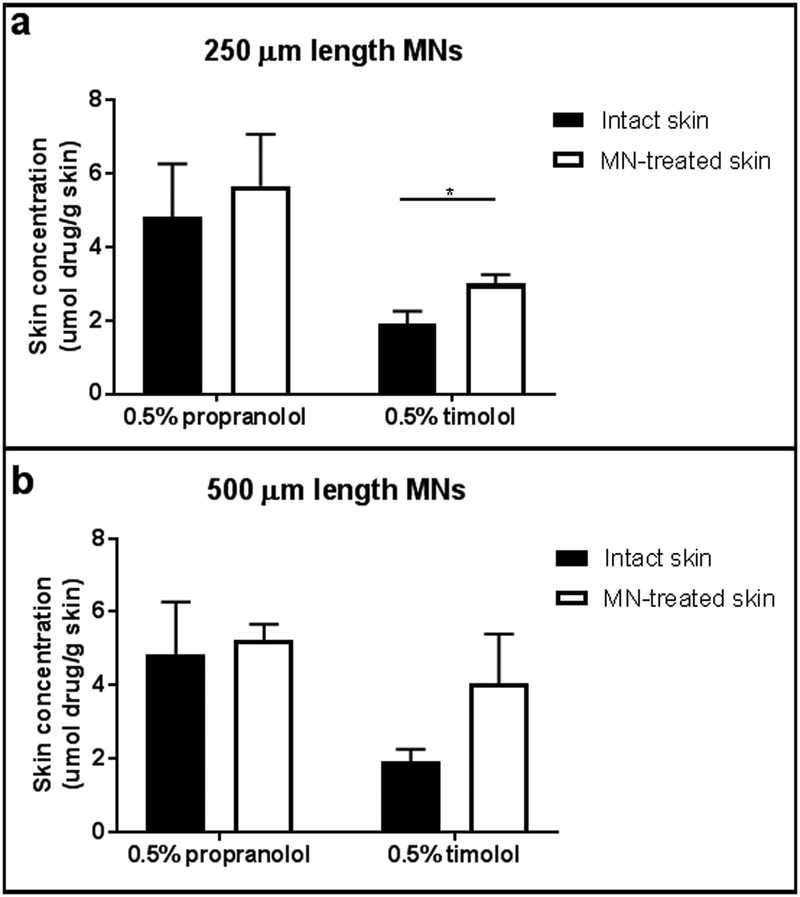
The skin concentrations of propranolol and timolol for intact skin and skin treated with 250 μm MNs are shown in Figure 5a. There was no significant difference in propranolol skin concentrations between intact (4.82 ±1.45 μmol drug/g skin) and MN pretreated skin (5.66 ± 1.41 μmol drug/g skin; p=0.5117). MN pretreated skin had significantly greater timolol skin concentrations compared to intact skin (intact: 1.91 ± 0.35 μmol drug/g skin; MN: 3.00 ± 0.25 μmol drug/g skin; p=0.0118).
Figure 5.
Skin concentrations (n=3) of propranolol and timolol for intact skin (black bars) and skin pretreated with (a) 250 μm solid MNs or (b) 500 μm solid MNs (white bars). Timolol skin concentrations were significantly increased after insertion with the 250 μm length MNs; no significant differences in skin concentrations were observed for timolol after insertion with the 500 μm length MNs or for propranolol after insertion with either MN length. Bars represent mean ± SD. *: p<0.05
The skin concentrations of propranolol and timolol for intact and skin treated with 500 μm MNs are shown in Figure 5b. Propranolol skin concentrations were not significantly different between the intact and MN treated skin (4.82 ±1.45 μmol drug/g skin vs. 5.22 ± 0.44 μmol drug/g skin, respectively; p=0.6712). Timolol skin concentrations were increased after solid MN pretreatment (intact: 1.91 ± 0.35 μmol drug/g skin; MN: 4.04 ± 1.36 μmol drug/g skin); this nearly reached statistical significance (p=0.0584).
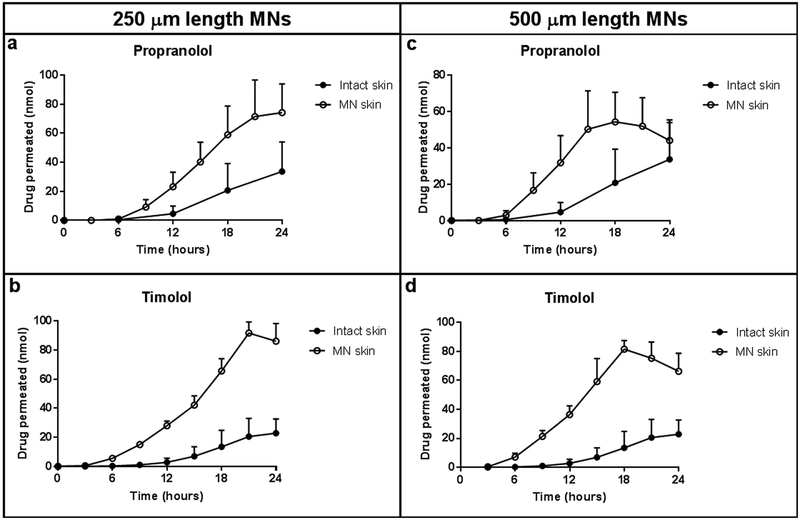
Drug permeation through the skin was similar between intact and 250 μm length MN pretreated skin for the first 6 hours for both propranolol (Figure 6a) and timolol (Figure 6b). After the first 6 hours, drug permeation through MN pretreated skin was greater than intact skin for both drugs. The cumulative drug permeation through the MN pretreated skin after 24 hours was significantly greater compared to intact skin for both propranolol (intact: 56.63 ± 43.93 nmol; MN: 278.68 ± 73.82 nmol; p=0.0110) and timolol (intact: 68.01 ± 44.42 nmol; MN: 334.98 ± 35.18 nmol; p=0.0012).
Figure 6.
Drug permeation profiles of (a, c) propranolol and (b, d) timolol for intact skin (closed circles) and skin pretreated with 250 μm length MNs (a,b) or 500 μm length MNs (c,d), (open circles); n=3. For both MN lengths, drug permeation of propranolol and timolol was similar between intact and MN pretreated skin conditions until approximately 6 hours, after which drug permeation was greater for MN pretreated skin. Drug permeation through the skin decreased after 18 hours, due to a depletion of drug solution from the donor chamber. Data points represent mean ± SD.
Similar to the 250 μm length condition, MN pretreatment with 500 μm length MNs increased drug permeation through the skin for both propranolol (Figure 6c) and timolol (Figure 6d). For both drugs, drug permeation was similar between intact and MN pretreated skin until approximately 6 hours, after which the drug permeation through MN pretreated skin increased. The cumulative drug permeation through MN pretreated skin over 24 hours through the MN pretreated skin was significantly increased compared to intact skin for both propranolol (intact: 56.63 ± 43.93 nmol; MN: 251.77 ± 86.53 nmol; p=0.0266) and timolol (intact: 68.01 ± 44.42 nmol; MN: 347.28 ± 18.19 nmol; p=0.0005).
4. Discussion
IHs are benign vascular tumors that can present as bright red lesions on the skin in children less than a year of age. IHs are one example of a dermatological disease that would benefit from topical drug delivery, as the current standards of care (systemic beta-blockers) are associated with serious adverse events in pediatric populations, such as hypoglycemia, bronchospasms, bradycardia, and hypotension (Leaute-Labreze et al. 2017). Topical beta-blockers have already been explored in a clinical setting, though the specific drug, dose, dosing regimen, and vehicle used in previous studies varies widely, as well as the outcome measures to determine efficacy. Despite inconsistency in these variables, topical beta-blockers are effective at improving lesion appearance and achieving lesion clearance. Adverse events related to topical beta-blocker treatment are relatively infrequent and mild, including pruritus and mild sleep disturbance. This indicates the improved safety profile of these formulations compared to oral propranolol (Painter et al. 2016).
Despite the benefits and lack of adverse events with topical therapy, there are no approved formulations that have been specifically optimized for dermal delivery in this indication. Much of the literature regarding topical beta-blocker delivery for IHs has been focused on the efficacy and safety of this route of administration. However, the retention of the drug within the skin has not been evaluated; therefore, it is unclear what skin concentrations of these beta-blockers are necessary in order to achieve the desired lesion clearance. This is likely due to the invasive nature of the methods required to obtain this information in vivo, including skin biopsies, microdialysis, collection of blister fluid, or tape stripping (Herkenne et al. 2008). Previous work has evaluated the effect of occlusion and dosing volume on permeation of beta-blockers in vitro through a human epidermal membrane, but that study did not provide information about local tissue concentrations in conjunction with permeation (Zhang et al 2015). In the current study, in vitro skin retention and drug permeation of two beta-blockers, propranolol and timolol, were evaluated under various dosing regimens. These drug concentrations will be valuable for ongoing formulation development purposes, as they provide a target concentration for future topical formulations. Additionally, solids MNs were evaluated with the goal of increasing local skin drug concentrations compared to intact skin using a control formulation.
4.1. Effect of beta-blocker physicochemical properties
While there are a number of beta-blockers currently on the market, two beta-blockers in particular have gained attention for their role in treating IHs. Propranolol was the first beta-blocker to be discovered as an effective treatment for IHs and has since become the standard of care for these lesions (Leaute-Labreze et al. 2017), and 96% of surveyed pediatric dermatologists prescribed oral propranolol for IHs in 2013 (Kumar et al. 2015). Studies have evaluated the efficacy of topical propranolol (Price et al. 2018), but these studies are limited in comparison to timolol. Timolol is readily available as an ophthalmic formulation, and is therefore commonly used topically, rather than orally. In Kumar et al.’s 2013 survey of pediatric dermatologists, 91% of responding physicians reported they prescribed topical timolol for IH treatment, particularly for superficial IHs (97%) (Kumar et al. 2015).
While propranolol and timolol are both non-selective beta-blockers, they differ in lipophilicity (Table 2), with propranolol being more lipophilic than timolol (Zhang et al. 2015). Previous work has shown that the log Ko/w for propranolol (3.48) is approximately twice as large as that for timolol (1.79) (Modamio et al. 2000; Zhang et al. 2015). As it is widely agreed that drug permeation through the lipid matrix of the SC is the primary pathway for most compounds (Guy et al. 1988), lipophilic compounds partition into the SC more readily than hydrophilic compounds. Pawar et al. showed that both the skin retention and flux of beta-blockers from passive diffusion increased with increasing lipophilicity (Pawar et al. 2013). Because of this it was expected that propranolol would partition into the SC more readily than timolol, which was observed. As timolol is hydrophilic in nature, it likely was unable to partition into the lipid matrix of the SC, which would result in both low skin concentrations and drug permeation into the receiver solution.
Table 2.
Physicochemical properties of propranolol and timolol
| Propranolol | Timolol | |
|---|---|---|
| Structure | 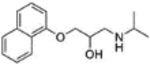 |
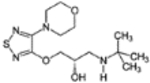 |
| Molecular weight (g/mol) | 259.3 | 316.4 |
| Log P | 3.0 | 1.8 |
| pKa | 9.5 | 9.2 |
In the current study, propranolol had significantly higher skin concentrations than timolol for the majority of the study lengths under the different dosing regimens (Table 1). This is ideal for skin disorders such as IHs, as the drug is readily available at the site of action. Additionally, propranolol had higher permeation into the receiver fluid than timolol. This trend was also observed by Zhang et al., who found that both the percentage of applied dose delivered across the skin and the cumulative drug permeation was higher for the lipophilic propranolol compared to the more hydrophilic timolol (Zhang et al. 2015). While this might seem concerning since systemic propranolol can cause adverse events, previous evaluation of serum levels after topical application of propranolol (Schneider et al. 2014) and timolol (Weibel et al. 2016) in IH patients were found to be below 0.2 ng/mL for both compounds (this is well below the 60 ng/ml detected in the serum after oral dosing). In vivo, there were no reported side effects associated with systemic exposure for either propranolol or timolol (Schneider et al. 2014; Weibel et al. 2016).
4.2. Effect of dosing regimen
Currently, there is no standard for the frequency of application for topical beta-blocker formulations. Rather, the dosing regimen for the topical products is often clinician dependent. These dosing intervals can range from twice daily up to five times per day (Painter et al. 2016). However, comparison of efficacy between studies using different dosing regimens proves difficult due to the variation in dose and outcome measures. Our current study evaluated skin concentrations and drug permeation after a single application, as well as two dosing regimens (once a day and twice a day). These dosing schedules were selected to mimic the regimens previously reported in clinical studies (Painter et al. 2016), and could ideally provide an objective target for comparison between formulations and regimens.
Single dose studies were completed to evaluate the elimination of drug from the skin over time. Skin concentrations of propranolol at 24 hours after a single dose were slightly lower than a previous study (Pawar et al. 2013) using a similar vehicle (1.67 mg drug/g skin vs 1.25 mg drug/g skin in the current study); this difference may be attributed to a larger applied dose (5 mg vs our 1 mg) and occluded dosing conditions (compared to our non-occluded conditions). The cumulative drug permeation in our study was also in line with cumulative drug permeation under similar study conditions after taking dose into consideration (Zhang et al. 2015). As the length of study increased, propranolol retention in the skin decreased without additional drug application. However, at 72 hours, approximately half of the drug that was present at 24 hours remained in the skin. This is not surprising, given that propranolol is a lipophilic drug and therefore may form a reservoir in the lipophilic SC. Drug permeation into the receiver from 48–72 hours indicated a steady release from the skin into the receiver solution. For timolol, there were no significant differences in skin concentrations between the four study lengths after a single dose, although a slight downwards trend was observed when comparing the 24 hour and 72 hour studies. The drug permeation into the receiver increased until approximately 24 hours, after which it decreased for all study lengths. Notably, a second peak appears for the 48 hour study, in which the amount of drug begins to increase at approximately 36 hours and reaches a second peak at 45 hours. It is not entirely clear why this peak occurred. This second peak had much larger error bars in comparison to the remaining data points, which makes the permeation profile for this study length more difficult to interpret at the later time points. It is possible that the increased variability later in the study relates to the different amount of subcutaneous tissue present in the full thickness skin samples. Drug reservoirs in the skin may have formed and saturated at different time points for each skin sample, which could produce large variations in the amount of drug permeating through the skin at the later points. These trends would need to be explored in in vivo pharmacokinetic studies to determine if variability changes over time in vivo as well.
As topical formulations carry the risk of surface loss for each applied dose, parents are typically instructed to apply topical beta-blocker formulations to IHs multiple times a day (Painter et al. 2016). Frequency of dose application can influence the skin concentrations of a topically applied product, with additional dosing contributing to the amount of drug retained in the skin. In this study, propranolol skin concentrations increased with the application of additional doses every 24 hours and every 12 hours, with significant differences in skin concentrations observed between various time points. Additionally, twice a day application for 48 hours resulted in skin concentrations similar to those observed with once a day dosing for 72 hours. For timolol, the increase in dosing frequency did not appear to influence the skin concentrations nearly as much as propranolol. As timolol is a hydrophilic beta-blocker, it is possible the drug remained on the surface of this skin, rather than partitioning into the SC. For both propranolol and timolol, increases in the amount of drug permeated were observed after application of an additional dose. As many previous studies have focused on a single application of beta-blockers for 24 hours, understanding the extent of drug retention in the skin after multiple doses will be valuable going forward, as these situations are more clinically relevant.
4.3. Effect of solid MN pretreatment
Drug delivery using solid MNs is partly dependent on the degree to which the barrier of the skin has been breached. TEWL measures the movement of water across the skin to the external environment and often serves as a surrogate marker for barrier function. As intact skin prevents the excess movement of water out of the body, TEWL values are relatively low when the barrier is intact (4–10 g/m2/h) and increase after barrier disruption (Boer et al. 2016). In this study, solid MNs significantly increased TEWL in vitro after MN pretreatment for two different needle lengths (250 μm and 500 μm), confirming sufficient disturbance to the SC after MN insertion. The longer MNs created a greater change in TEWL compared to the shorter needles, which is consistent with previous reports that MN length influences the extent of barrier disruption (Gupta et al. 2011; Kelchen et al. 2016).
Solid MNs have most commonly been used to increase the systemic delivery of skin impermeable compounds across the SC to permit systemic absorption. However, some studies have shown that MNs can be used to increase local skin concentrations as well (Choi et al. 2017; Donnelly et al. 2008; Zhang et al. 2012). In the current study, MNs of both 250 and 500 μm length increased skin concentrations of timolol, but propranolol skin concentrations after MN treatment remained similar to intact skin. These differences in the change of skin concentrations is likely due to the simple differences in lipophilicity between the two beta-blockers. As micropores created by MN insertion are aqueous in nature, it is likely that the hydrophilic timolol utilized these micropores more readily to facilitate drug diffusion to the underlying epidermis and dermis. Alternatively, propranolol, which partitions into intact skin more readily than timolol due to the lipophilic path of drug delivery in the SC, may utilize the intact skin as the primary pathway for drug diffusion, rather than the aqueous micropores. This trend has previously been shown by Pawar et al, who found the enhancement ratio for skin retention between MN treated skin and intact skin decreased with increasing lipophilicity of various beta-blockers (Pawar et al. 2013). Although timolol was not evaluated in that study, acebutolol, which has a similar logP to timolol, was found to have an enhancement ratio of 1.87 after MN treatment. This is similar to the enhancement ratio for skin retention in this study for timolol, which was 1.57 for the 250 μm length and 2.12 for the 500 μm length. Skin retention enhancement ratios for propranolol in the present study were found to be 1.17 and 1.08 for 250 μm and 500 μm length MNs, respectively, which is also similar to the enhancement ratios (1.04) previously observed (Pawar et al. 2013).
While the increase in skin concentrations for timolol using MNs was encouraging, drug permeation through the skin was also increased after MN pretreatment. This initially appears concerning for IH treatment, as systemic exposure can cause serious adverse events. However, increased drug permeation into the deeper layers of the skin may be ideal for mixed or deep IHs, as these lesions are often affect the lower layers of the dermis and subcutaneous tissue (Leaute-Labreze et al. 2017).
The use of MNs in a pediatric population is overall limited. To our knowledge factors such as micropore closure rates, which could affect the duration of drug delivery through the skin, have not been studied in an infant population. There has been conflicting evidence regarding the competence of the barrier function of the skin in children (Fluhr et al. 2010; Harpin et al. 1983; Lund et al. 1997; Nikolovski et al. 2008). Therefore, it is unclear if the micropore closure rates would be comparable to those seen in adults (several hours to several days depending on conditions such as MN geometry and occlusion status) (Gupta et al. 2011), or if prolonged micropore lifetimes are observed in infants and young children. Additionally, the effect of MN treatment on IH lesions is not known. Intralesional injections of corticosteroids have been used to treat infantile hemangiomas, including those in the periorbital region of the face (Xu et al. 2014). MNs are considered to be less invasive compared to traditional hypodermic needles; therefore, we expect that MNs would not pose any additional risks when applied to lesional skin compared to intralesional treatments.
4.4. Limitations
Some limitations exist in this work. First, full thickness skin was used for evaluation of the skin retention and permeation of propranolol and timolol. For lipophilic compounds such as propranolol, drug concentrations within the skin may be artificially inflated with full thickness skin due to the formation of a depot within the dermis. Additionally, full thickness skin may artificially decrease the drug permeation through the skin into the receiver fluid. Therefore, caution must be exercised when comparing these results to other studies, such as studies that use split thickness skin. Second, a single dose (0.5% propranolol or timolol) was evaluated. This dose chosen due to the commercial availability of a 0.5% timolol ophthalmic solution, which has been used in a wide range of studies evaluating efficacy (Painter et al. 2016). In order to compare the skin concentrations between propranolol and timolol, the same dose was used for propranolol. However, a wide range of doses have been used for both propranolol and timolol, including 1–3% propranolol and 0.25–0.5% timolol (Painter et al. 2016). The effect of dose on skin concentrations and drug permeation through both intact and MN treated skin will be necessary in future studies. Finally, MN insertion using manual methods may result in high inter- and intra-individual variability due to differences in application force (van der Maaden et al. 2014). Because of this, the insertion of the MNs may have varied during MN treatment if consistent pressure was not applied.
5. Conclusions
The lipophilicity of propranolol allowed for greater skin concentrations compared to timolol, as propranolol was able to partition into the lipophilic SC. Increasing the frequency of application increased skin concentrations and drug permeation of propranolol, while timolol appeared less influenced by dosing regimen. Solid MNs increased the skin concentrations of hydrophilic timolol, while the more lipophilic propranolol did not demonstrate appreciable changes in skin concentrations after MN treatment. Drug permeation through the skin was also increased after MN pretreatment, which may be ideal for mixed or deep IHs. These results provide benchmarks for comparison during future optimization of formulation and MN parameters that could improve the dosing schedule for treating IH lesions.
Acknowledgements
Research reported in this publication was supported by the National Institutes of General Medical Sciences of the National Institutes of Health under Award Number R35GM124551.
6. References
- Boer M, Duchnik E, Maleszka R, Marchlewicz M, Postepy dermatologii i alergologii 33, 1 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogden NK, Banks SL, Crofford LJ, Stinchcomb AL, Pharm. Res 30, 8 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakkittakandiyil A, Phillips R, Frieden IJ, Siegfried E, Lara-Corrales I, Lam J, Bergmann J, Bekhor P, Poorsattar S, Pope E, Pediatr. Dermatol 29, 1 (2012) [DOI] [PubMed] [Google Scholar]
- Choi SY, Kwon HJ, Ahn GR, Ko EJ, Yoo KH, Kim BJ, Lee C, Kim D, Dermatol. Ther 30, 6 (2017) [DOI] [PubMed] [Google Scholar]
- Coulman SA, Anstey A, Gateley C, Morrissey A, McLoughlin P, Allender C, Birchall JC, Int. J. Pharm 366, 1–2 (2009) [DOI] [PubMed] [Google Scholar]
- Donnelly RF, Morrow DI, McCarron PA, Woolfson AD, Morrissey A, Juzenas P, Juzeniene A, Iani V, McCarthy HO, Moan J, Control J. Release 129, 3 (2008) [DOI] [PubMed] [Google Scholar]
- Donnelly RF, Singh TR, Tunney MM, Morrow DI, McCarron PA, O’Mahony C, Woolfson AD, Pharm. Res 26, 11 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluhr JW, Darlenski R, Taieb A, Hachem JP, Baudouin C, Msika P, De Belilovsky C, Berardesca E, Exp. Dermatol 19, 6 (2010) [DOI] [PubMed] [Google Scholar]
- Gupta G, Mallefet P, Kress DW, Sergeant A, Br. J. Dermatol 161, 2 (2009) [DOI] [PubMed] [Google Scholar]
- Gupta J, Gill HS, Andrews SN, Prausnitz MR, Control J. Release 154, 2 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy RH, Handb. Exp. Pharmacol 197 (2010) [DOI] [PubMed] [Google Scholar]
- Guy RH, Hadgraft J, Pharm. Res 5, 12 (1988) [DOI] [PubMed] [Google Scholar]
- Harpin VA, Rutter N, J. Pediatr 102, 3 (1983) [DOI] [PubMed] [Google Scholar]
- Herkenne C, Alberti I, Naik A, Kalia YN, Mathy FX, Preat V, Guy RH, Pharm. Res 25, 1 (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik S, Hord AH, Denson DD, McAllister DV, Smitra S, Allen MG, Prausnitz MR, Anesth. Analg 92, 2 (2001) [DOI] [PubMed] [Google Scholar]
- Kelchen MN, Brogden NK, Pharm. Res 35, 12 (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelchen MN, Siefers KJ, Converse CC, Farley MJ, Holdren GO, Brogden NK, Control J. Release 225, (2016) [DOI] [PubMed] [Google Scholar]
- Kim YC, Park JH, Prausnitz MR, Advanced drug delivery reviews 64, 14 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar MG, Coughlin C, Bayliss SJ, Pediatr. Dermatol 32, 2 (2015) [DOI] [PubMed] [Google Scholar]
- Kunzi-Rapp K, Pediatr. Dermatol 29, 2 (2012) [DOI] [PubMed] [Google Scholar]
- Leaute-Labreze C, Dumas E la Roque de, Hubiche T, Boralevi F, Thambo JB, Taieb A, N. Engl. J. Med 358, 24 (2008) [DOI] [PubMed] [Google Scholar]
- Leaute-Labreze C, Harper JI, Hoeger PH, Lancet 390, 10089 (2017) [DOI] [PubMed] [Google Scholar]
- Li WZ, Huo MR, Zhou JP, Zhou YQ, Hao BH, Liu T, Zhang Y, Int. J. Pharm 389, 1–2 (2010) [DOI] [PubMed] [Google Scholar]
- Lund CH, Nonato LB, Kuller JM, Franck LS, Cullander C, Durand DJ, J. Pediatr 131, 3 (1997) [DOI] [PubMed] [Google Scholar]
- Malik MA, Menon P, Rao KL, Samujh R, J. Pediatr. Surg 48, 12 (2013) [DOI] [PubMed] [Google Scholar]
- Martanto W, Davis SP, Holiday NR, Wang J, Gill HS, Prausnitz MR, Pharm. Res 21, 6 (2004) [DOI] [PubMed] [Google Scholar]
- McAllister DV, Wang PM, Davis SP, Park JH, Canatella PJ, Allen MG, Prausnitz MR, Proc. Natl. Acad. Sci. U. S. A 100, 24 (2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modamio P, Lastra CF, Mariño EL, Int. J. Pharm 194, 2 (2000) [DOI] [PubMed] [Google Scholar]
- Nikolovski J, Stamatas GN, Kollias N, Wiegand BC, J. Invest. Dermatol 128, 7 (2008) [DOI] [PubMed] [Google Scholar]
- Painter SL, Hildebrand GD, Surv. Ophthalmol 61, 1 (2016) [DOI] [PubMed] [Google Scholar]
- Pawar KR, Smith F, Kolli CS, Babu RJ, J. Pharm. Sci 102, 10 (2013) [DOI] [PubMed] [Google Scholar]
- Prausnitz MR, Langer R, Nat. Biotechnol 26, 11 (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price A, Rai S, McLeod RWJ, Birchall JC, Elhassan HA, J. Eur. Acad. Dermatol. Venereol (2018) [DOI] [PubMed] [Google Scholar]
- Schneider M, Reimer A, Cremer H, Ruef P, World J Pediatr. 10, 4 (2014) [DOI] [PubMed] [Google Scholar]
- van der Maaden K, Sekerdag E, Jiskoot W, Bouwstra J, The AAPS journal 16, 4 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel L, Barysch MJ, Scheer HS, Konigs I, Neuhaus K, Schiestl C, Rentsch K, Muller DM, Theiler M, Pediatr. Dermatol 33, 2 (2016) [DOI] [PubMed] [Google Scholar]
- Wermeling DP, Banks SL, Hudson DA, Gill HS, Gupta J, Prausnitz MR, Stinchcomb AL, Proc. Natl. Acad. Sci. U. S. A 105, 6 (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Jia R, Ge S, Lin M, Fan X, Paediatr J. Child Health 50, 4 (2014) [DOI] [PubMed] [Google Scholar]
- Zhang Q, Chantasart D, Li SK, J. Pharm. Sci 104, 5 (2015) [DOI] [PubMed] [Google Scholar]
- Zhang W, Gao J, Zhu Q, Zhang M, Ding X, Wang X, Hou X, Fan W, Ding B, Wu X, Wang X, Gao S, Int. J. Pharm 402, 1–2 (2010) [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brown K, Siebenaler K, Determan A, Dohmeier D, Hansen K, Pharm. Res 29, 1 (2012) [DOI] [PubMed] [Google Scholar]