Abstract
Blood-Injection-Injury (BII) phobia is an anxiety disorder that may be accompanied by vasovagal fainting during confrontation with the feared stimuli. The underlying pattern of autonomic regulation has been characterized as a diphasic response, with initial increases in heart rate and blood pressure that are typical of a fight-flight response, and subsequent drops in blood pressure and/or heart rate that may precipitate vasovagal fainting. Tensing skeletal muscles of the arms, legs, and trunk (applied tension) has been proposed as a technique to cope with this dysregulation. This review critically examines the empirical basis for the diphasic response and its treatment by applied tension in BII phobia. An alternative perspective on the psychophysiology of BII phobia and vasovagal fainting is offered by focusing on hypocapnia that leads to cerebral blood flow reductions, a perspective supported by research on neurocardiogenic and orthostatically-induced syncope. The evidence may indicate a role for respiration-focused coping techniques in BII phobia.
Keywords: Blood-injection-injury phobia, diphasic response, vasovagal syncope, respiration, hyperventilation, applied tension
Blood-injection-injury phobia is a Specific Phobia that affects about 4% of the population in the United States (Stinson et al., 2007). Patients experience marked and persistent fear or apprehension when confronted with stimuli such as blood, injuries, wounds, mutilations, needles, or injections. According to the DSM-IV-TR (American Psychiatric Association, 2000), the fear that is cued by the presence or anticipation of the feared stimulus (e.g., receiving an injection or seeing blood) is deemed unreasonable and excessive. While adult sufferers recognize their exaggerated response, this feature can be absent in children. If the situation is not avoided altogether, the afflicted person endures the phobic object (e.g., blood) or situation (e.g., going to the doctor) with intense anxiety or distress. Consequently, significant interference with the person’s normal routine, including occupational and social functioning, is observed and a necessary diagnostic criteria. Furthermore, the disorder can lead to serious consequences for patients’ health, such as avoidance of necessary medical attention (including medical check-ups, life saving surgical procedures, care for chronic conditions), complications in parenthood (e.g., problems with perinatal care, inadequate response to children’s injuries, avoidance of pregnancy altogether), and limitations in their choice of potential professions (e.g., exclusion from most health-related professions). Surprisingly, the literature on this condition has been rather sparse, which likely stems from a general misperception of the clinical psychology and psychiatry community that the condition is well explored in terms of its psychophysiological dynamics and treatment options. However, as we will discuss below, a closer look at the published research reveals a substantial lack of evidence in key questions regarding its basic psychophysiological mechanisms and the effects of treatment modalities.
1. The anatomy of the perfect storm: Dramatic conceptions of the vasovagal syncope in BII phobia
The most distinct feature of the psychophysiology of BII phobia is its culmination in a vasovagal syncope. The typical response pattern has been described in the literature as an event comprising two phases, the diphasic (alternatively, using the Latin prefix: biphasic) response (e.g., Engel, 1978; Kozak and Montgomery, 1981; Thyer and Curtis, 1985). Accordingly, the initial phase is characterized by an increase in heart rate (HR; tachycardia) and blood pressure (BP), as is typically expected from fear responses of the fight-flight type. The subsequent second phase is characterized by a sharp fall in HR (bradycardia) as well as BP (hypotension) leading to reduced cerebral blood flow and ultimately fainting (e.g., Graham et al., 1961).
This second phase makes BII phobia a unique phenomenon among the anxiety disorders and has provoked a plethora of functional interpretations and speculations about its adaptivity (e.g., Alboni et al., 2008; Bracha et al. 2007; Marks, 1988). In contrast, psychophysiological research on BII phobia has remained sparse and authoritative volumes on anxiety dedicate only minimal space to this unique phenomenon (Barlow, 2002). Consequently, common knowledge in this area draws heavily on earlier research on emotional fainting (Engel, 1978; Graham et al., 1961) and textbook entries for BII phobia tend to focus strongly on fainting and the diphasic response pattern as fundamental characteristics of this disorder (Barlow & Durand, 2005).
An earlier interpretation by George Engel (Engel & Romano, 1947) viewed the BII-phobic response as a conflict between stimulating and inhibitory activities leading to a profound disruption of the autonomic homeostasis. The first phase, in which the person has the primitive impulse to escape injury, was identified as Cannon’s fight-flight response that includes preparation for action. Sympathetic activation was thought to be the dominating autonomic influence in this phase, which would shunt larger volumes of blood to the skeletal muscles. At the same time, situational circumstances or inner conflicts prohibited the person from acting out these impulses. In the absence of muscle activation, disproportionate pooling of blood in the periphery would occur and culminate in fainting. HR decelerations were viewed as a marker of these inhibitory forces. The authors speculate that the response could be functional as a secondary line of defense in that awareness of the threatening stimulus would be disrupted through loss of consciousness. Engel (1978) later interpreted the second phase as a co-activation or alternating activation of a fight-flight response and a parasympathetically mediated “conservation-withdrawal” response. Graham et al. (1961), on the other hand, proposed that the fainting in the second phase of the diphasic response was a “physiological expression of the sudden cessation of anxiety” (p.505) that had dominated the first phase. Powerful antagonistic reflexes that had developed during this first phase to counteract the massive sympathetic cardiovascular activation due to anxiety were suddenly unopposed in the second phase. He interpreted this stage, which may result in asystole, a fall in BP, or apnea, as a relief-reaction with resignation or giving-up (“all is lost”) and compared it to playing dead or actual death. Thus, earlier interpretations of the BII phobic response conjure up powerful images of a struggle of life’s polarities between death and survival through fight or flight. Although dramatic metaphors and functional interpretations such as these can stimulate and guide research, they may also distract and divert attention from important aspects of the phenomenon.
In this review, we will discuss some of the problems that arise in examining the character of psychophysiological responses in BII phobia. We will explore problems and inconsistencies in the literature regarding the definition of fainting, the conceptualization of the diphasic response, and the current psychophysiological treatment rationale for blood phobia. We will then explore new approaches to understanding and treating vasovagal syncope in BII phobia, which will mainly focus on disturbances in the respiratory system and their interaction with experiential processes.
2. The problem of defining fainting: Who is susceptible and who is not, and exactly to what?
The uniqueness of the anxiety response to BII stimuli comes from the frequent observation of fainting or near-fainting episodes in these patients (Marks, 1988). However, a closer reading of the literature shows that this core phenomenon is often not well defined.
2.1. The phenomenon of syncope and its differential diagnosis
The definition of fainting in the medical literature typically involves an episode of loss of consciousness (e.g., Merriam Webster Medical Dictionary, 2009). Commonly referred to as syncope [Footnote 1], it is viewed as a global cerebral hypoperfusion leading to loss of consciousness that is transient, has a rapid onset, a short duration (usually in the range of 20 s), and a spontaneous recovery (Moya et al., 2009). It is thus distinguished from longer loss of consciousness (e.g., coma). The cause for loss of consciousness should also be non-traumatic (thus excluding e.g., concussions) [Footnote 2] and a further distinction is made from other non-traumatic loss of consciousness by epileptic seizures, metabolic disorders, or intoxication. Other distinctions are made from rare conditions such as cataplexy, which is a loss of muscle tone triggered by strong emotions (often laughter) that can lead to postural collapse but is accompanied by full consciousness (Thijs et al., 2004). This condition occurs often in the context of narcolepsy that can itself be confused with syncopal episodes, as it is accompanied by spontaneous irresistible sleep episodes or excessive daytime sleepiness.
Syncope is also distinguished from psychogenic pseudosyncope, which is a condition that simulates syncope by overt signs of loss of postural tone or motionlessness and closed eyes, but with no measurable drops in BP and HR or EEG delta activity that are typical for syncope with loss of consciousness (Moya et al., 2009; Benbadis & Chichkova, 2006; Thijs et al., 2004). Attacks can be frequent, up to several times per day, without clearly recognizable triggers. Possible causes and conditions leading to such episodes are conversion disorders, factitious disorder (the more current term for Münchhausen Syndrome, an intentionally feigning of illness), or malingering.
2.2. Classifications of syncope
Syncope itself can be classified according to its causes or autonomic regulatory abnormalities. Categories of syncope by causes include syncope due to orthostatic hypotension, cardiac syncope (cardiovascular), or reflex (neurally-mediated) syncope (see Table 1, modified from Moya et al., 2009). Of these, only the latter would be the relevant pathophysiological category including BII phobia, with the focus on the emotional distress component eliciting the syncopal event. Alternatively, categorizing syncope by abnormalities in cardiovascular regulation, Thijs et al. (2004) distinguished syncope by (i) insufficient cardiac output, with major underlying conditions being arrhythmias and structural heart defects, (ii) reduced vascular tone leading to low BP, with orthostatic hypotension and autonomic failure as underlying conditions, (iii) insufficient filling of the circulation due to hypovolemic states, and (iv) neural cardiovascular dysregulation with patterns of vagally mediated bradycardia, vasodilation due to sympathetic withdrawal, or mixed patterns of both. This latter category is again the one where BII phobia may fit best, as the focus is on the neural dysregulation of cardiac and vascular components (thus vasovagal syncope).
Table 1.
Classification of syncope (modified from Moya et al., 2009)
| • Reflex (neurally-mediated) syncope |
| – Vasovagal: e.g., by emotional stress, pain, blood phobia, orthostatic stress |
| – Situational: e.g., by coughing, gastrointestinal stimulation, post-micturition |
| – Carotid sinus syncope |
| – Atypical forms (no apparent triggers and/or atypical presentation) |
| • Syncope due to orthostatic hypertension |
| – Primary autonomic failure: e.g., pure autonomic failure, Parkinsons’ disease |
| – Secondary autonomic failure: e.g., diabetes, spinal cord injuries |
| – Drug-induced orthostatic hypertension; e.g., by alcohol, diuretics, vasodilators |
| – Volume depletion: haemorrhage, diarrhea, vomiting |
| • Cardiac syncope (cardiovascular) |
| – Arrhythmia as a primary cause: bradycardia due to sinus node dysfunction, supraventricular tachycardia, drug-induced bradycardia or tachycardia |
| – Structural disease: cardiac (e.g. acute myocardial infarction) or others (e.g. pulmonary hypertension) |
Regardless of the classification, determining that syncope or fainting is due to BII phobia-related fears requires detailed differential diagnosis. This task is complicated by the fact that other non-syncopal conditions (as described in the paragraph above) can be accompanied by similar loss of consciousness or can mimic it. It could be expected that exploration of fainting history in the context of specific BII-related stimuli should guarantee sufficient specificity and sensitivity of the diagnosis. [Footnote 3] However, in relying on retrospective self-report, it is not always guaranteed that patients will remember circumstances of fainting well, especially when episodes date further back (e.g., childhood) or when retrograde amnesia had occurred (Parry and Kenny, 2002).
2.3. Syncope in BII phobia and orthostatic stress; more similarities than differences?
The distinction between various types of syncope may be less straightforward than it appears. This is particularly evident for BII-related and orthostatic vasovagal syncopes. Van Lieshout et al. (1991; 1997) distinguished two main responses: (a) a “central” vasovagal response characterized by descending pathways from cortical, limbic, and hypothalamic centers and triggered by emotional stress and pain, and (b) a “peripheral” response type in which the stimulus comes from various peripheral cardiovascular sites, in particular the heart, and is triggered by orthostatic stress. Because the latter is much easier to elicit experimentally, typically by tilt-table testing or lower body negative pressure, much more detailed knowledge has been accumulated on the peripheral response type. Orthostatic stress can also be applied in doses that would almost certainly lead to fainting, even in individuals that are ordinarily not susceptible to syncope (Zhang et al., 1998). The literature on BII phobia has typically drawn heavily on regulatory events observed in response to such tests (Sarlo et al., 2008). In a rare comparison of these subtypes, Accurso et al. (2001) showed a strong susceptibility of BII-phobia patients to faint under tilt-table testing. It is therefore possible that in BII phobia, an underlying predisposition for autonomic dysregulation interacts with an acquired fear of BII-related situations. This would allow drawing at least tentative conclusions for BII-phobia related fainting from this body of research. Interestingly, syncope related to BII stimuli, but not to orthostatic stress, is generally viewed as a form of “emotional fainting.” However, emotional concomitants of the latter are not studied well. At least during phases of presyncope in orthostatic stress tests, probands appear to develop feelings described as distress (Lagi et al., 2001) or “feeling uncomfortable in an ill-defined way” (Van Lieshout et al., 2003, p.835). It is conceivable that information about such tests and their potential consequences can trigger some level of discomfort or apprehension, at least in individuals with concerns over physical symptoms.
2.4. Soft Gray-Outs or Hard Black-Outs?
Additional problems arise in the psychophysiological literature from the definition of fainting itself. In some studies, fainters were characterized by their reported history of fainting in response to the sight of blood, injuries, or injections (e.g. Gerlach et al., 2006; Vögele et al., 2003), but it is not always explicit whether researchers’ and patients’ definitions of fainting actually adhered to the criterion of rapid-onset, transient loss of conscience. Some authors have even included in their definition of “fainter” patients who came close to fainting during BII-relevant stimulus exposure (Öst, Lindahl, et al., 1984) or reported a history of feeling close to fainting when exposed to BII stimuli (Vögele et al., 2003). The origins of such “soft criteria” of fainting might also lie in Graham et al.’s (1961) earlier definition of fainting as a sudden drop in BP and pulse rate accompanied by reports of disturbance of consciousness described as feeling “dizzy,” lightheaded,” or “woozy.” The authors further contended that “it is unrealistic to insist on complete loss of consciousness as a criterion of fainting, since it is clear […] that the vasovagal fainting is not an all-or-nothing reaction but occurs in various degrees” (p.494). This is in line with the idea of syncope as a dimensional phenomenon, where partial syncopes are only weaker expressions of the full-blown “hard-criterion” syncope. The implicit assumption is also that studying the pathophysiology of pre-syncopal patients will generate useful information about the syncopal patient. However, it is sometimes debated whether pre-syncope and syncope share the same underlying mechanisms (Moya et al., 2009).
Indeed, vasovagal syncope may be accompanied by prodromal symptoms of nausea, lightheadedness, or sweating (so-called “pre-syncopal” episodes), but this is not always the case (Moya et al., 2009). The majority of psychophysiological studies that often include a component of exposure to feared stimuli (e.g., films of surgery, blood- and injury pictures) have documented pre-syncopal episodes (or “gray-outs,” Engel & Romano, 1947), while documentations of self-reported loss of consciousness (“black-outs”) are rare (see e.g., Steptoe & Wardle, 1988). [Footnote 4] Reasons for incomplete fainting can be multiple, such as variations in stimulus material (content, duration, personal significance), initiation of coping strategies, or adherence issues during exposure (e.g., subtle or overt avoidance strategies such as averting the gaze or premature termination of exposure). Because it is often difficult to prevent or exclude such influences, it will remain an open question as to whether the patient would have fainted under slightly altered conditions or whether the patient is only prone to pre-syncope. Self-report of prior loss of consciousness could be a discriminating factor. However, complicating matters, the extent of loss of consciousness may be difficult to determine for the patient, particularly retrospectively. Recent guidelines mention the potential underestimation of retrograde amnesia (Moya et al., 2009; see also Parry and Kenny, 2002), which could make accurate retrospective reports impossible. On the other hand, it is also not guaranteed that patients are always able to prospectively determine the likelihood of impending syncope. For example, it is common for individuals with panic disorder to report feelings of faintness (Meuret et al., 2006), even though actual fainting rarely happens. In one study, participants were selected based on reports of feeling faint at the sight of blood; however, when further questioned, only a subset (53%) reported actual past fainting (Steptoe and Wardle, 1988). Furthermore, in a study by Lumley and Melamed (1992), BII patients who reported feelings of faintness did not necessarily show a drop in BP during exposure to feared stimuli. Finally, determining syncopal loss of consciousness from behavioral observation is also not always straightforward. In a study by Thijs et al. (2008), psychology students viewing a syncopal episode on video were able to correctly remember less that half of its symptomatic features.
Considerations for studying pre-syncope rather than full syncope could be both of a practical and ethical nature. In terms of practicality, the prevalence of pre-syncope can be expected to be higher because a number of patients never fulfill “hard criteria” for syncope (Graham et al., 1961). On the ethical side, the perceived risks of states with full-blown loss of consciousness, massive cardiac slowing, and postural collapse may deter researchers, especially outside medical settings. Increasingly stricter institutional review boards may prohibit the study of such phenomena, especially without physician oversight. In addition, for ethical reasons, patients are always allowed to self-terminate exposure in the experimental situation (Öst, Sterner, et al., 1984), thus some of them will avoid transitioning from pre-syncope to complete loss of consciousness.
2.5. Conclusion: The trouble with determining fainting history
Thus, for a number of reasons, subgroups of “fainters” among blood-sensitive participants or BII-phobia patients may have been heterogeneous in previous studies. Not surprisingly, only few studies have reported informative findings comparing subgroups of fainters and non-fainters, such as qualitatively different patterns of cardiovascular reactivity to BII-related material in blood-sensitive individuals with and without history of fainting (Vögele et al., 2003). Actual fainting in response to BII-related material in laboratory or treatment exposure has typically been observed in considerably fewer participants than expected from reports of fainting history (Steptoe & Wardle, 1988) and pre-syncopal experiences have not necessarily been restricted to those reporting prior fainting experience or phobic responses to BII material (Ritz, Wilhelm, et al., 2005). It is conceivable that problems with the definition and exploration of the patients’ history of fainting, which also extend to published observations of fainting in the laboratory, have contributed to this state of the literature.
3. The diphasic response pattern I: Is it there at all?
3.1. Diphasic response: problems with the standard canon
The diphasic response has become the standard canon of psychophysiological BII phobia research. Yet a closer examination of the published research reveals surprisingly little consistency in the demonstration of this response pattern that is thought to be so typical. Part of the problem is the lack of a shared definition with strict criteria of what should constitute this unique response pattern. The lack of rigor in distinguishing it from other patterns of activation and deactivation, which are relatively common in confrontation with experimental stimuli, has been a particular shortcoming.
Early accounts of diphasic cardiovascular patterns date back to George Engel and David Graham (Engel & Romano, 1947; Romano & Engel, 1945; Graham et al., 1961). Whereas Engel did not view the diphasic pattern as an essential manifestation in BII phobia, Graham proposed that the biphasic response would always be present. In the work of both authors, the clearest demonstrations of a cardiovascular adjustment pattern with two phases, including the initial rise in HR and BP followed by subsequent falls, were based on case studies. In systematic observations, a pre-exposure baseline was usually taken as a reference for subsequent activation or deactivation during exposure. In Graham et al. (1961), data from two groups of blood donors who fainted during blood draws showed increases in SBP and HR before venipuncture and falls in these parameters below baseline level following the procedure. DBP values showed increases with subsequent decreases, but they did not exhibit falls below baseline.
There are a number of problems with using such observations as evidence for diphasic responses. First, to qualify as syncopal, one would eventually expect exceptionally low levels of HR and/or BP for the particular person. Second and related, comparison with control situations would be needed to demonstrate the uniqueness. Third, these observations bring up the question of specificity of the physiological response pattern across channels.
3.2. What is the reference for diphasic responding?
Regarding the first problem, there is probably little clinical relevance for BII phobia in a diphasic response pattern that does not culminate in exceptionally low levels of HR and BP for the particular individual or group of individuals. On average, one would expect values to drop below a quiet baseline steady-state reference level. Studies have not always been strict in applying such criteria. Graham et al. (1961) interpreted diastolic response patterns as diphasic even when they did not show decreases below initial levels. Similarly, Page (2003) observed patterns of BP adaptation during a 2-min blood or needle slide presentation that did not clearly culminate in a significant deactivation below baseline levels. Qualitative inspection of the data of Öst et al. 1984 also showed considerable falls in HR and BP values during late stages of surgery film viewing. However, subsequent quantification focused on change scores of initial minus peak values and recovery minus peak values, rather than initial minus recovery values, which could have supported their interpretation of a relevant deactivation statistically. Gerlach et al. (2006) observed no falls below initial levels of HR and BP in a group of BII phobia patients undergoing venipuncture, but two participants that fainted showed such falls.
Using initial levels as a reference could both facilitate and impede the demonstration of “diphasic” responding. Initial levels could be elevated by anticipatory anxiety in patients that expect confrontation, a common problem for laboratory studies of anxiety. This would make it easier to demonstrate subsequent falls below initial levels in the second phase, but could make it more difficult to demonstrate substantial increases in the first phase. Obtaining a less tainted baseline (possibly control days without exposure) would be essential for such studies.
3.3. Is there anything different in responding diphasically?
When taking an initial baseline as reference for the diphasic response, the observed pattern of change may be indistinguishable from benign psychophysiological adaptations that can show similar response patterns. Challenging laboratory stressors, particularly those requiring some type of active coping, clearly can be expected to increase HR and BP substantially (Obrist, 1976). Such task-related activations and the subsequent deactivation in recovery from the task could take on the shape of a diphasic response. Even more relevant, the behavioral treatment of anxiety by exposure is based on the idea that during exposure, the patient first exhibits a steady increase in physiological activation, anxiety, and arousal, which then should be resolved during later stages of exposure through processing of fear-incompatible experience (Foa & Kozak, 1986). There are also observations that a pronounced situational stress activation can be followed by rebound, a phenomenon in which the system deactivates below baseline levels (Gellhorn, 1970). Similarly, novel stimuli elicit orienting responses that entail HR decelerations below initial levels (e.g., Turpin, 1986; Öhman et al., 2000). If the intention is to distinguish the BII-related diphasic response from such phenomena, control conditions are needed that account for the dynamic range achieved by such conditions alone.
Studies have rarely provided adequate control conditions for comparison. Three studies of surgery film exposure that used neutral film clips as a comparison were not able to demonstrate HR falls below neutral levels (Sarlo et al., 2002; Steptoe and Wardle, 1988; Vögele et al., 2003). The latter two studies reported lower values of DBP during surgery compared to controls, but the observations were not substantiated by adequate significance tests. In the research of the first author, a dual criterion was adopted for which a diphasic response during surgery films was only identified if in subsequent 10-s averages across the film period, (1) the initial activation exceeded the level of a film stimulus of negative valence, and (2) the subsequent deactivation exceeded that during viewing of a neutral film (Ritz, Wilhelm, et al., 2005). According to these criteria, a minority of the 12 examined patients showed a sufficient biphasic pattern: one in HR and SBP, and the other in HR, SBP, and DBP. In addition, three of the 14 control participants also showed biphasic patterns according to this criterion: one in SBP and DBP and two others in HR (Ritz, Wilhelm, Meuret, et al., 2005). Sarlo et al. (2008), who compared a surgery film with a landscape film and cockroach invasion or spider films, were also not able to demonstrate a biphasic pattern in HR and BP on average, when taking into account the levels reached during these neutral and negative comparison films.
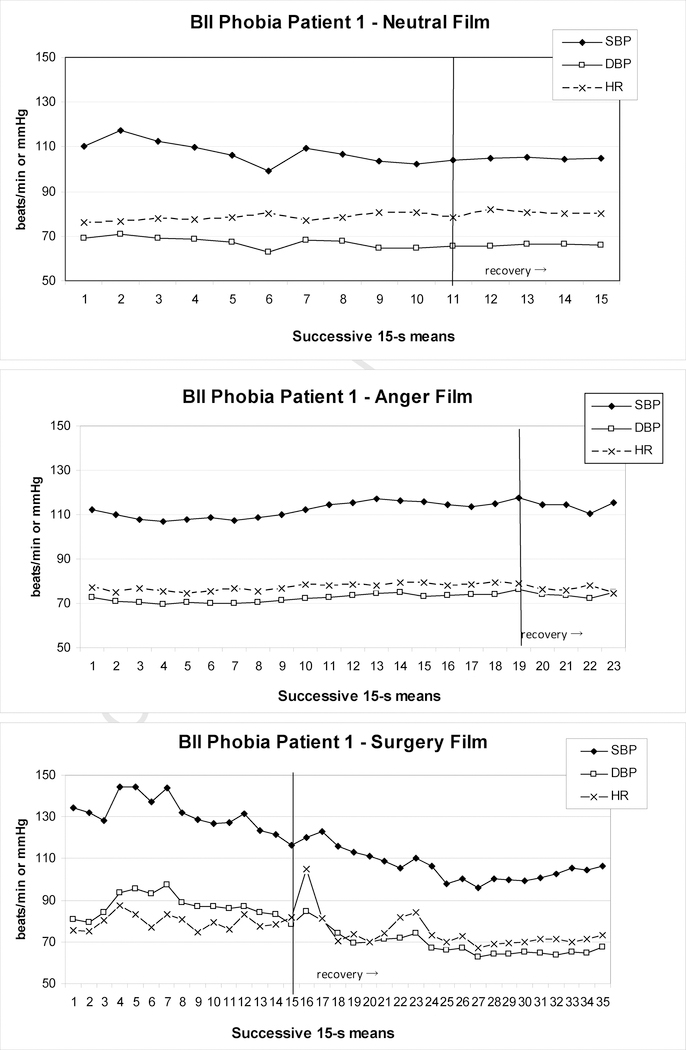
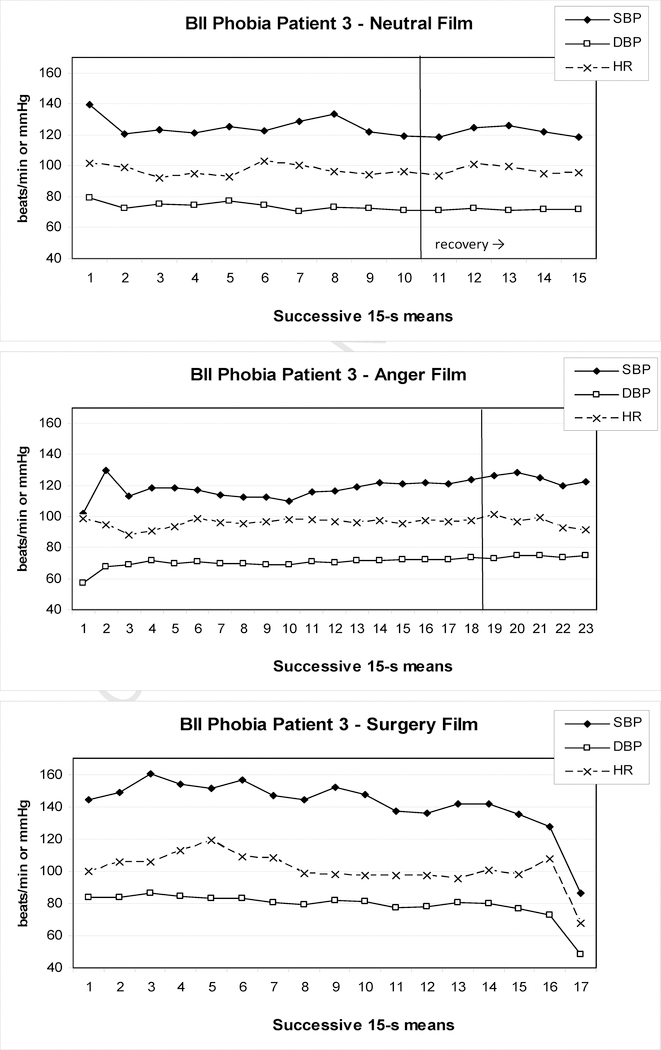
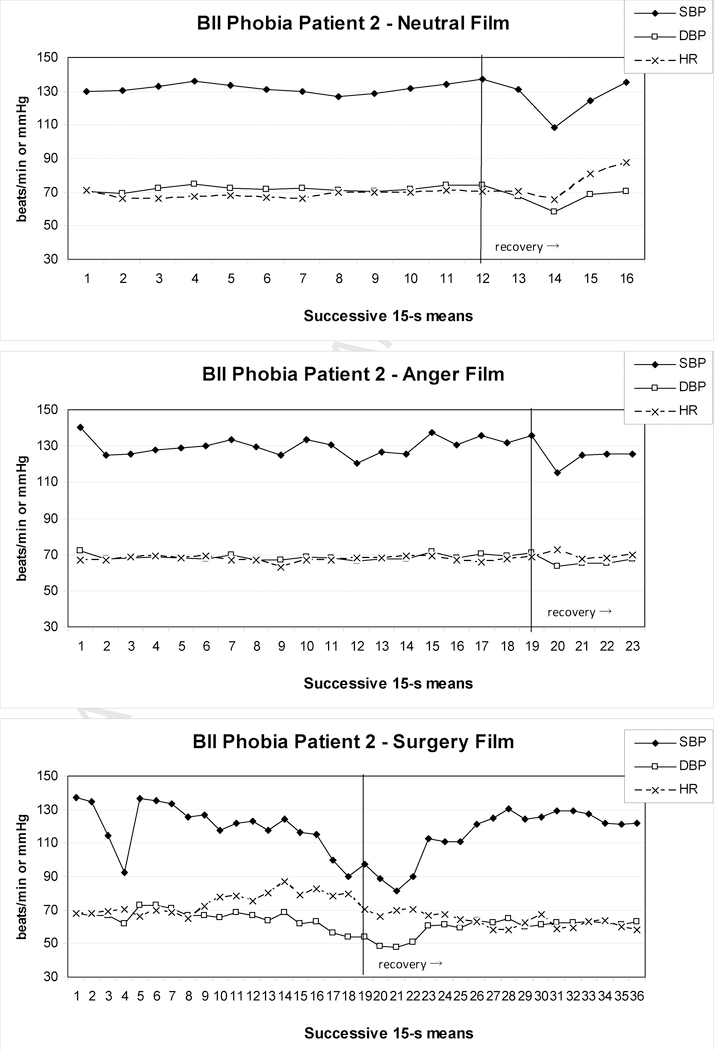
Examples of BII-phobia patients from our recent study (Ayala et al., 2010) are shown in Figures 1 to 3.These patients either reported pre-syncopal sensations (e.g., indicating feelings of impending faint) or showed typical overt signs of pre-syncope, such as pallor, nausea, or profuse sweating. In addition, pronounced drops in either HR or BP consistent with syncope were observed and two patients terminated the surgery film prematurely. In Patient 1 (Figure 1), SBP initially rose to 145 mmHg during the surgery film and after some fluctuation then began to drop steadily, which continued into the 5-min recovery period. Peak SBP and HR were higher than during the anger film, but their minima never fell below neutral film levels during the surgery film; only during the recovery period such minima were reached for SBP, but not before 2–3 min into the recovery. DBP maxima during the surgery film again exceeded the anger film, but at no point during the film or recovery did the levels clearly fall below neutral film levels. In Patient 2 (Figure 2), who showed signs of presyncope but managed to watch the film to the end, DBP barely and SBP never exceeded values during control films, although levels dropped below the neutral control towards the end of presentation. In contrast, HR was elevated at this point, but did not drop below neutral before 1 min into the recovery. Patient 3 (Figure 3) showed a more substantial drop in HR and BP beginning approximately 240 s into the film before self-termination of the presentation. Although these drops are drastic in the final 15s of the film, the overall pattern during surgery film presentation cannot easily be interpreted as a diphasic one. Only comparison with HR and BP levels during the anger and neutral films reveals considerably higher peak values, and lower minima, respectively, for the surgery film.
Figure 1.
Heart rate and blood pressure of one BII-phobia patient during exposure to a neutral (upper panel), anger (center panel), and surgery (lower panel) film; the patient showed presyncopal symptoms and terminated the surgery film prematurely after 219 s, but remained in the sitting position during the subsequent 5-min recovery period.
Figure 3.
Heart rate and blood pressure of one BII-phobia patient during exposure to a neutral (upper panel), anger (center panel), and surgery (lower panel) film; the patient showed presyncopal symptoms and terminated the surgery film prematurely after 258 s; the ensuing posture change to the lying position rendered the Finapres blood pressure recordings uninterpretable during recovery from the surgery film.
Figure 2.
Heart rate and blood pressure of one BII-phobia patient during exposure to a neutral (upper panel), anger (center panel), and surgery (lower panel) film; the patient showed presyncopal symptoms, but viewed the complete film and remained in the sitting position during the subsequent 5-min recovery period.
Overall, the pattern of cardiovascular change in these presyncopal stages rarely adhered to an idealized diphasic pattern, particularly when using more stringent criteria. Without including recovery recordings (as done in most BII phobia laboratory studies), most instances of diphasic change would have been missed completely. Given this heterogeneity and issues in timing, it is difficult to argue for a predictive value of a diphasic response pattern. It should be noted that establishing stricter criteria for a diphasic response is not simply an academic exercise in scientific parsimony or conservatism. Syncopal or pre-syncopal responses are distinct and clinically problematic events for BII phobia patients. A better understanding of their underlying psychological and physiological processes would require an identification of aspects that are unique to such events and distinguish them from other more commonly known psychophysiological response patterns. Paradigms such as the diphasic response pattern will be of little heuristic value if they do not reflect this uniqueness sufficiently. While these pattern analyses remained on a purely descriptive level, we will speculate below about mechanisms of presyncope in invoking hyperventilation as a possible contributor (see Sections 4 and 5.2.3).
3.4. Only somewhat or completely diphasic-which physiological parameters are important?
The mixed findings across physiological indices lead to the third problem: In which physiological parameters should the diphasic response be manifested to qualify as a clinically relevant response pattern?
3.4.1. Heart rate, blood pressure, or both?
The literature has focused strongly on falls in HR and BP; however, are both necessary components of the vasovagal syncope that is unique to the anxiety response in BII phobia? One could also question whether HR and BP both are equally important as major indicators of the vasovagal response. Thomas Lewis (1932), who had introduced the term “vasovagal syncope,” described two phenomena: the bradycardia with massive HR slowing and a vasodilation with an excessive fall in BP. In this, the vasomotor phenomenon was thought to be the more important element. Similarly, earlier work of George Engel on the “vasodepressor syncope” (Engel & Romano, 1947) focused to a greater extent on the latter mechanism, although the varying importance of bradycardia vs. vasodilation across individuals was already reflected in their times (see Romano & Engel, 1945, discussion chaired by Franz Alexander). Earlier research also suggested that the initial phase before onset of syncope is characterized by increases in both HR and BP (Graham et al., 1961; Engel & Romano, 1947). Research using orthostatic stress paradigms (e.g., tilt-table testing) with patients suffering from neurally mediated syncope has substantiated these observations by finding bradycardic and vasodepressor as well as mixed types of syncope (Van Lieshout et al., 1991; 1997). Among these subtypes, the vasodepressor type is more common and more likely to culminate in full fainting. As already observed by Lewis (1932), administration of atropine can abolish the bradycardia but not the BP drop. Thus, it appears that observations of BP may be more relevant in predicting subsequent fainting than marked bradycardia.
3.4.2. Specific markers of autonomic branches
It could be argued that markers more specific to each branch of the autonomic nervous system would be better indicators for studying vasovagal responses. Indices such as HR or BP are ambiguous with respect to the underlying autonomic regulation (Berntson et al., 1991). However, to characterize underlying autonomic regulatory adjustments, research will increasingly need to focus on parameters that are thought to tap selectively into one aspect of the autonomic regulation at a particular end-organ, such as T-wave amplitude or pre-ejection period as a marker of beta-adrenergic activity and respiratory sinus arrhythmia (RSA) as an indicator of cardiac parasympathetic control.
Studies of blood-sensitive individuals or BII-phobia patients have rarely used these more specific markers of autonomic control. Sarlo et al. (2002; 2008) measured T-wave amplitude or pre-ejection period, respectively, during film presentation and observed initial decreases followed by subsequent increases in these indices, suggesting elevated and reduced sympathetic activity, respectively. In both studies, only the initial increase in sympathetic activity, not the subsequent decrease, exceeded the levels reached during neutral and negative comparison films. In the same two studies, analysis of RSA remained uninformative, but no control for influences of respiration rate and volume had been attempted. It has been known for some time that increases in respiration rate can reduce RSA while increases in tidal volume can increase it, independent of changes in vagal outflow (Hirsch & Bischop, 1981; Grossman, Karemaker & Wieling, 1991; Ritz & Dahme, 2006). Given that marked increases in tidal volume paired with no substantial changes in respiration rate have been observed in BII-phobia patients during exposure to surgery films (Ritz et al., 2009; Ayala et al., 2010), a lack of control for tidal volume effects could lead to inflated RSA values suggesting a parasympathetic discharge. Two more studies that had implemented at least one form of respiratory control with RSA produced similarly negative findings (Gerlach et al., 2006; Steptoe & Wardle, 1988).
3.4.3. Baroreflex control
A number of studies have also explored baroreflex sensitivity, with the idea that an insufficient response of the baroreflex to falls in BP could predispose to syncope. Indeed, reductions in baroreflex sensitivity have been demonstrated in presyncopal states by some studies, although the literature is not consistent, probably also due to methodological differences (e.g., Iacoviello et al., 2008; Samniah et al., 2004). Most research has focused on orthostatically-induced syncope, and none of studies have explored baroreflex function relative to a diphasic response pattern. One study that also included individuals with a history of emotional fainting showed reduced baroreflex sensitivity during pain stimulation but not mental arithmetic (Ader et al., 1991). We are not aware of any reports on BII phobia-related syncope.
3.4.4. Humoral factors
have also been explored as a potential key to syncope. Again, most evidence is available from orthostatic stress testing, with some studies finding decreases in noradrenaline and/or increases in adrenaline leading up to or following syncope (Van Lieshout et al., 1991; 1997). The latter could be of particular interest because adrenaline serves as a powerful vasodilator and could thus explain the massive fall in BP. In one case study of a participant with fainting history in the medical context, Vingerhoets (1984) observed spontaneous fainting during a protocol of repeated blood draws. Adrenaline and noradrenaline increased before syncope and decreased after it, with increases in cortisol from pre- to post-syncope also evident. The author’s speculations were in line with Graham et al.’s (1961) diphasic response paradigm, which describes adrenaline as an indicator of the initial fight-flight phase followed by cortisol secretion as an indicator of a subsequent conservation-withdrawal phase. The search for the “unknown fainting factor” (Van Lieshout et al., 1991) has led to the exploration of a further number of humoral indices, among these, vasopressin, pancreatic polypeptide, and endogenous opiates. Among the most interesting ones, high baseline levels of adenosine and further increases during syncope have been observed patients with neurocardiogenic syncope (Shen et al., 1996; Saadjian et al., 2002). Adenosine has both bradycardic and vasodilatory effects. In the central nervous system, adenosine is known to inhibit excitatory neurotransmission and to induce sleep (Gallopin et al., 2005). However, for the indices studied so far, diphasic patterns have not been demonstrated, nor have these indices been used successfully to explain potential diphasic patterns in HR or BP.
Taken together, the few studies directly relevant to BII phobia that have used more selective markers of autonomic or humoral regulation have not been very instructive with regard to prevalence or mechanisms of diphasic response patterns. Although not ultimately informative about the underlying autonomic regulation, BP and HR are useful for characterizing the pattern of cardiovascular adjustments, which is probably of greater clinical relevance because it informs more directly about relevant adverse events (underperformance of the heart, blood pooling in the periphery) that are most obviously linked to fainting in the respective patient. However, other hemodynamic indices such as cardiac output, total peripheral resistance, or myocardial contractility, which could improve the understanding of cardiovascular dynamics, have rarely been explored in BII phobia-related fainting (Sarlo et al., 2008). Such measures could also address hypotheses about the role of cardiac filling in BII-phobia syncope (Henry, 1984), which have not receive unequivocal support in other research (Mitro & Hijová, 2006; van Lieshout et a., 1997).
3.5. The elusive pattern: is it diphasic, monophasic, or polyphasic?
What are possible reasons for failures to show diphasic responses in more recent BII-phobia research? Given the lack of convincing demonstrations of diphasic responses, are there any other patterns observed? Are such alternative patterns clinically relevant, or can only diphasic patterns inform us about eventual outcomes in terms of fainting? In the following, we will address these issues and argue for a wider perspective on regulatory adjustments preceding syncope.
3.5.1. Methodological issues preventing the observation of a diphasic response
Reasons for no apparent diphasic response patterns can be numerous. Under the assumption that this response is prototypical for BII-related syncope, it could simply be that the examined patient is not prone to fainting and consequently would not show a diphasic response pattern. Indeed, it can be expected that a diphasic response pattern, if deemed a useful concept, would only be seen in a subgroup of BII-phobia patients (Cisler et al., 2009). Reported findings suggest that this response pattern is a manifestation much rarer than originally thought. However, the overlap between diphasic responses and fainting history are also not perfect. For example, the two participants who became pre-syncopal in our initial study (Ritz, Wilhelm, Gerlach, et al., 2005) did not display diphasic patterns according to our strict criteria of BP or HR exceeding a negative reference and falling below a neutral reference level (see 3.2).
Additional methodological reasons for not demonstrating a diphasic response are listed in Table 2. For example, lack of the second phase of the diphasic response may be due to brief exposures: Lumley and Melamed (1992) used a film presentation as short as 60-s, which may have been too short as a number of patients develop pre-syncopal symptoms only after a few minutes (Engel, 1978). In other studies, premature termination of exposure by the patients may have prohibited the observation of diphasic patterns, as in our initial study with 3 out of 12 patients terminating one or both of the two 5-min surgery films prematurely (Ritz, Wilhelm, Gerlach, et al., 2005) or in Öst, Sterner et al. (1984) with 14 out of 18 patients terminating a 30-min surgery film early. Another issue is the choice of the correct time window for capturing a diphasic response. In addition, a considerable number of patients may actually show strongest symptoms after termination of exposure (Graham et al., 1961; Kleinknecht et al., 1990). This intriguing phenomenon would be missed if measurements were not extended beyond exposure into a sufficiently long recovery period. Figures 1 and 2 provide examples of this problem: SBP and HR reach minima only during recovery and only at this point justify the identification of a diphasic pattern (according to criteria introduced above under 3.3).
Table 2.
Reasons for unsuccessful demonstrations of diphasic response patterns in BII phobia patients during experimental exposure
| • Patient does not have a proneness to syncope |
| • Ineffectiveness of experimental stimulus material |
| – Lack of thematic relevance |
| – Duration of stimulus too short |
| • Not enough time for development of diphasic pattern |
| • Truncation of second phase of diphasic pattern |
| – Premature termination of presentation by patient |
| – Successful coping efforts of patient |
| – Lack of compliance with instructions (e.g., looking away) |
| • Anxious anticipation during prior reference period: No pronounced first phase of diphasic pattern |
| • Time window of analysis not suitable: Response after termination of presentation |
| • Too large averaging intervals: Peaks and troughs “smoothened out” |
| • Too low sampling of relevant parameters (e.g., too few blood pressure measurements) |
| • Patient in supine posture |
Data reduction problems can also interfere with observing diphasic patterns. Given the nature of the diphasic response as a phasic event, studying its temporal dynamics across subsequent 10–20 s averages of BP and HR values would be appropriate, whereas larger averaging intervals could result in the relevant peaks and troughs being “smoothed out.” Similarly, sampling at too low resolution could result in these peaks and valleys being missed. For BP monitoring, only continuous beat-by-beat recordings allow for sufficiently frequent assessments. On the other hand, artifactual diphasic patterns could also be produced by aspects of the exposure protocol: Most experiments use the sitting posture, but pre-syncopal or syncopal participants are often brought into the supine posture to let them recover by redirecting blood to vital areas. This posture change alone can cause BP and HR to drop and be therefore uninformative or misleading in that it simulates the second leg of the diphasic pattern.
3.5.2. Alternative patterns
With this in mind, it is not surprising that demonstration of biphasic patterns in BII phobia are rare and that there is no evidence that such a response pattern is representative for this population of patients during an exposure situation. It must be considered that a diphasic pattern may not be a necessary autonomic manifestation for actual fainting. Studies have frequently observed monophasic patterns. Lumley and Melamed (1992) observed only increases in HR during a surgery film. On the other hand, BII patients in Sarlo et al. (2008) showed continuous falls in SBP, which started from the outset of their surgery film presentation lasting slightly longer than 5 min. None of the patients in these studies were reported to have fainted. Patterns observed in our studies for patients reaching presyncopal levels were also mixed. While some showed response patterns that were suggestive of overall diphasic changes, such changes were exclusive to some cardiovascular parameters (HR, SBP, DBP) and not to others (Ritz, Wilhelm, Meuret, et al., 2005) (see also Figure 1).
It is possible that the narrow focus on identifying diphasic patterns distracts from an open exploration of the actual nature of BII phobia-related fainting behavior. Important aspects of this phenomenon may be overlooked by trying to fit it into the mold of the diphasic response. Research on orthostatically elicited vasovagal syncopes has shown fainting without sharp drops in BP and HR (e.g., Dan et al., 2002). Alternatively, autonomic dynamics leading up to syncope can show characteristics that allow the distinction of more than two phases. Julu et al. (2003) administered tilt-table tests and lower body negative pressure and terminated testing when SBP fell to 80 mmHg and pre-syncopal symptoms occurred. They described four phases: (i) following vertical tilt, full compensation of the initial BP drop occurs within 30 s, which is followed by (ii) a tachycardia phase, subsequently followed by (iii) an instability phase with massive BP fluctuations, and finally by (iv) pre-syncope, in which BP and HR fall below initial levels (falls in BP preceded falls in HR in a majority of cases). Phases (ii) to (iv) seem to be most relevant for BII phobia and could be interpreted as a triphasic response pattern. Instability phases have not been explored well in BII phobia research. They may be of particular significance as manifestations of the mechanisms that are central to the eventual breakdown of a normal autonomic or central nervous system regulation, such as failure of the baroreflex.
3.5.3. Conclusion: the heuristic value and clinical relevance of a “pattern”
Ultimately, the value of any model of the BII phobia-related autonomic dysregulation will be determined by its ability to inform about the irrevocable decline into syncope and treatment options that can counter it. Postulating a diphasic pattern with an initial sympathetic activation may only contribute to the understanding of the second phase, if the latter is indeed a regulatory consequence of the former (e.g., in the sense of a pronounced counter-regulation, rebound phenomenon). Such a dependency of the second on the first phase has not been ascertained. Thus, any other experiential or physiological event or chain of events that is able to predict syncope by regularly preceding it could replace the diphasic response paradigm.
4. The biphasic response pattern II: Is that all there is?
Is there significant research beyond the focus on peripheral cardiovascular adjustments in BII phobia? What kind of physiological mechanisms and pathways may also contribute to the eventual fainting responses in BII-phobia patients? In the following, we will widen the perspective on vasovagal fainting and explore potential factors linked to respiration and their interaction with experiential states in BII phobia.
4.1. Hyperventilation in BII phobia
4.1.1. Empirical findings
In referring back to Cannon’s work on the fight-flight response, Engel and Romano (1947) pointed out that hyperventilation was part of the response pattern. However, subsequent work has focused on the cardiovascular regulation and not considered respiratory adjustments in exposure to BII phobia-relevant stimuli in greater depth. Mostly qualitative observations and case studies have been published that reported hyperventilation or deep breathing in BII-phobia patients (e.g., Elmore et al., 1980; Foulds, 1993; Steptoe & Wardle, 1988). Thyer and Curtis (1985) estimated on the basis of behavioral observation that approximately half of their patients hyperventilated during exposure sessions. Indeed, in two of our own studies, we demonstrated that BII-phobia patients have a tendency to hyperventilate when exposed to surgery film scenes (Ayala et al., 2010; Ritz, Wilhelm, et al., 2005). The basis of this hyperventilation seems to be a marked increase in tidal volume, rather than an increase in respiration rate (Ritz et al., 2009). Overall, patients showed strong elevations in minute ventilation together with increases in indices of tidal volume irregularity, sighing, and thoraco-abdominal asynchrony. PCO2 showed phasic decreases below 30mmHg, which is sometimes taken as the level that is indicative of full-blown hyperventilation (Bass & Gardner, 1985). In our recent study, more tonic reductions in mean end-tidal PCO2 levels below 30mmHg were observed in 10 patients (16.7 %) during the surgery film and 16 patients (26.7 %) during recovery (0% and 5%, respectively, for healthy controls) (Ayala et al., 2010). These participants showed average PCO2 drops of 8–9mmHg, while those with values above 30mmHg averaged 2–3 mmHg. Interestingly, hyperventilation was maximal in the first minute of recovery from the exposure and then gradually returned to pre-film levels throughout the following 4 min. In both studies, tidal volume and minute ventilation, but not respiration rate, as well as breathing irregularity correlated with the experience of a variety of symptoms, most prominently shortness of breath, lightheadedness, dizziness, and faintness (Ayala et al., 2010; Ritz et al., 2009).
Table 3 shows cardiovascular and respiratory data of 3 patients and 2 controls that displayed pre-syncopal episodes in our recent study while exposed to a 5-min surgery film (Ayala et al., 2010) (see Figures 1 to 3 for their cardiovascular patterns). All five participants showed an onset of deep and irregular breathing well before drops in HR or BP falls began. The findings coincide with those of Gerlach et al. (2006) that showed increases in minute ventilation in BII-phobia patients during venipucture and massive increases in this parameter during the recovery period in two patients that fainted.
Table 3.
Mean respiratory parameters for healthy controls and BII-phobia patients that became pre-syncopal during the 5-min surgery film or recovery from it
| Mean PCO2 (mm Hg) |
Respiration Rate (breaths/min) |
Tidal volume (ml) |
Minute ventilation (l/min) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| QS | SUR | SUR-R | QS | SUR | SUR-R | QS | SUR | SUR-R | QS | SUR | SUR-R | |
| Controls (n=2) | 38.5 (1.1) | 35.5 (1.8) | 30.4 (1.2) | 15.9 (0.0) | 15.8 (2.3) | 15.6 (0.4) | 349.9 (78.3) | 372.0 (25.6) | 515.0 (123.4) | 5.59 (1.22) | 5.92 (1.17) | 8.54 (0.65) |
| BII Patients (n=3) | 38.3 (1.4) | 36.5 (4.3) | 30.1 (5.5) | 15.9 (0.4) | 12.6 (2.7) | 14.9 (4.6) | 373.1 (14.3) | 672.7 (168.8) | 990.0 (165.0) | 5.95 (0.40) | 8.22 (2.29) | 14.69 (4.44) |
Note: QS=quiet sitting baseline; SUR=surgery film; SUR-R=surgery film 1-min recovery
Although measurements of respiration had been implemented in some BII studies, they often failed to fully consider all aspects of breathing that might play a role in hyperventilation. In terms of the respiratory pattern, hyperventilation (breathing in excess of metabolic demand) can be brought about by increasing the rate of breathing and/or the depth of breathing. However, studies have generally focused on respiration rate, such as through the measurement with a single strain gauge (e.g., Kozak & Miller, 1985; Steptoe & Wardle, 1988; Vögele et al., 2003) or visual observation of breathing (e.g., Cohn et al., 1976), rather than implementing a full assessment of the breathing pattern including volume or direct measures of expired CO2. Thus, true hyperventilatory states may have been overlooked in most of these studies. Respiratory inductance plethysmography and capnography currently provide more precise techniques for capturing these aspects of ventilation noninvasively (Ritz et al., 2002; Meuret et al., 2004).
4.1.2. Functional significance of deep breaths in BII phobia
The observed increases in tidal volume in relation to BII-relevant stimulus material could be interpreted in at least three ways. First, they could be part of the fight-flight pattern: The organism increases minute ventilation in preparation for action, but no actual muscular action ensues, thus leading to metabolically unjustified ventilation, which is the definition for hyperventilation. Second, they could be part of a coping strategy. The lay recommendation to “take a deep breath” is a popular antidote for many anxieties and tensions in life. Steptoe and Wardle (1988) observed that blood-sensitive participants that fainted during exposure to a surgery film also reported taking deep breaths to cope with the aversive material. While such sigh breathing may produce short-term relief from symptoms, it is also dysfunctional in that it lowers PCO2 levels and may thereby contribute to symptoms and fear levels in susceptible individuals (Papp et al., 1993; Abelson et al., 2001; Wilhelm, Trabert, et al., 2001). And third, yet another function of deep sigh breaths could be an involuntary attempt to open up the airway passages, which show evidence of constriction when patients hyperventilate. Hyperventilation is known to lead to airway narrowing (van den Elshout et al., 1991). In the BII-phobia patients of the first author’s initial study (Ritz, Wilhelm, et al., 2005), an analysis of respiratory resistance, obtained continuously throughout exposure by forced oscillation, demonstrated increases in resistance that were equal or greater than those of asthma patients exposed to the same surgery films (Ritz et al., in press). Intermittent deep breaths have been shown to be functional in reducing airway resistance, probably by re-lengthening the contracted smooth muscles of the airways (Lavoie et al., 2009). The dyspnea perceived by patients when exposed to BII stimuli may trigger such deep breaths.
Hyperventilation is not a trivial response for BII-phobia patients. While basic research and treatment literatures are divided over the specificity of hyperventilation for panic disorder (e.g., Meuret et al., 2005; Roth, Wilhelm & Pettit, 2005; Wilhelm, Gerlach, et al., 2001; Van den Hout et al., 1992), such concerns are secondary in the context of BII phobia-specific responding. Hyperventilation certainly cannot serve as a diagnostic marker for BII phobia, given that it is also common to other anxiety disorders or states (Roth, 2005). However, even if hyperventilation were a nonspecific response to fear exposure or a mere concomitant correlate of anxiety states, it could have problematic consequences that are specific for BII-phobia patients. Cerebral blood vessels are highly sensitive to changes in CO2 levels. Because hypocapnia leads to both cerebral vasoconstriction (Claassen et al., 2007) and peripheral vasodilation (Norcliff-Kaufmann et al., 2008), it is likely to contribute to pooling of blood in the periphery and reductions in cerebral blood flow (CBF), and could thus become a critical factor in inducing fainting.
4.2. Hyperventilation in neurocardiogenic and orthostatically induced syncope
4.2.1. Empirical findings
The significance of hyperventilation in syncope in general has been recognized for some time. In earlier reports, respiratory abnormalities such as frequent yawning, sighing, or hypocapnia have been observed during or preceding the development of syncope induced by orthostatic stress (e.g., Weissler et al., 1957; Epstein et al., 1968; see also Lipsitz et al. 1998; van Lieshout et al., 1991). More recently, these observations were further substantiated by head-up tilt table testing. Carey et al. (2001) found on average small reductions (−3.8 mmHg) in end-tidal PCO2 in 7 patients with neurally mediated syncope and healthy controls before syncope, whereas Norcliff-Kaufmann et al. (2008) observed marked falls in end-tidal PCO2 to levels around 21–22 mmHg at presyncope in a sample of 43 patients and controls that fainted. Novak et al. (1998) demonstrated hypocapnia (falls of almost 10 mmHg) and reductions in cerebral perfusion in 30 patients with orthostatic intolerance. CO2 rebreathing was able to reverse these effects. Gradual and drastic falls in PCO2 were also seen in a study by Lagi et al. (2001), starting 160 s before syncope, whereas BP decreases were observed to begin only 60 s after that point.
The hypocapnia observed before syncope in these studies seemed to be largely driven by increases in tidal volume rather than respiration rate. Likewise, Lipsitz et al. (1998) observed marked increases in tidal volume 90–180s before onset of syncope. All but one of 25 of their syncopal individuals at least doubled or tripled their amplitude of slow breaths. Novak et al. (1998) observed no significant change in the overall average respiration rate during tilt-table testing, but reported qualitative observations of episodes of rhythmic breathing interrupted by deep breaths, faster respiration rate, irregular respiration, or apneas. Lagi et al. (2001) found PCO2 falls to begin in an initial phase of irregular and deep breathing accompanied by reductions in CBF. They proposed four phases leading up to syncope; following the first phase of “deep breathing,” a second phase ensued during which patients complained of “discomfort,” followed by a third phase marked by the onset of falls in mean BP, and a fourth and final phase characterized by syncope. Carey at al. (2001) concluded that “it would appear that hypocapnia due to an increase in respiratory amplitude forms an intrinsic part of the pathophysiology of vasovagal syncope” (p. 356). An additional function of the tidal volume-driven increase in ventilation, beyond those considered earlier (see 4.1.2), could be a “respiratory pump” effect, which may be an attempt to increase venous return by producing a larger negative intrathoracic pressure (Lagi et al., 2001; Lipsitz et al., 1998). Overall, these findings closely match our recent observations with BII-phobia patients during exposure to surgery films (Ayala et al., 2010; Ritz et al., 2005; 2009). More research is needed to determine factors that lead to hyperventilation, which is essentially an override of respiration that is controlled by feedback from blood gas tension levels, in particular arterial PCO2. On a cautionary note, as most of the cited studies have employed end-tidal measures of PCO2 levels, the extent to which these dissociate from arterial PCO2 in syncope by orthostatic stress testing requires further exploration (Serrador et al., 2006).
4.2.2. Hyperventilation, fainting, and the “propensity factor X”
How does hyperventilation translate into fainting in syncopal patients if it does not lead to fainting in other anxiety disorders (e.g., Alpers et al., 2005; Wilhelm, Gerlach, et al., 2001; Meuret et al., 2008)? In healthy individuals, voluntary hyperventilation from a PCO2 of 40 down to 20 mmHg leads to reductions in CBF of approximately 50% (Claassen et al., 2007), which is markedly more than can be achieved through orthostatic stress by lower body negative pressure alone (Levine et al., 1994). At 50–60% reduction of CBF, symptoms of mental confusion and problems with cerebral oxygenation set in (van Lieshout et al., 2003). In patients with recurrent vasovagal syncope, hyperventilation appears to change the pressure threshold at which the blood flow in cerebral vessels ceases and vessel walls start collapsing (critical closing pressure), in that higher pressures than are present under normocapnia are sufficient to induce this state (Carey et al., 2001). However, reports of syncope induced by hypocapnia alone a rare (e.g., David et al., 2003) or limited to special maneuvers that combine voluntary hyperventilation with straining and postural stress (the “fainting lark,” van Lieshout et al., 2003). Still, although adding voluntary hyperventilation to orthostatic stress leads to additional reductions in CBF, it does not lead to fainting in healthy individuals (Levine et al., 1994). Thus, in most cases, hypocapnia may be “an important factor accentuating cardiovascular embarrassment” in syncope (Weissler et al., 1957, p.881), but most probably, additional predisposing factors must be invoked to explain actual fainting.
Individuals with a tendency to faint also seem to have a greater sensitivity of the vasculature to respond to CO2 changes. Norcliff-Kaufmann et al. (2008) observed stronger reductions in an index of forearm vascular resistance to end-tidal pCO2 changes in patients with neurally mediated syncope compared to healthy controls. Thus, hyperventilation in combination with abnormalities in the vasomotor regulation could provide the critical synergy for syncopal events. Critchley and co-workers reported evidence for structural variations in midbrain and medulla regions involved in BP and baroreflex regulation in patients with neurocardiogenic syncope (Beacher et al., 2009), which could serve as dispositional factors contributing to vasomotor abnormalities and thus fainting risk. Other risk factors could be genetic. In a small study, Hohoff et al. (2009) recently found significant enhancement of respiratory and cardiac responses to venipuncture in BII-phobia patients explained by polymorphism of the adenosine A2A receptor gene. In unrelated research, polymorphism of the adenosine A2A receptor gene has also been linked to a greater likelihood of fainting in tilt-table testing (Sadjian et al., 2009). Exploration of similar genetic variabilities and their association with cerebrovascular responses and fainting would be highly interesting. Whatever the as of yet unknown propensity factor, hyperventilation in interaction with this factor may become a very specific predictor or marker of fainting in BII-phobic individuals.
4.3. Respiratory fluctuations in cardiovascular indices
Respiration could also complicate the picture of physiological adjustments to BII or orthostatic stress exposure in other ways. It is well-known that breathing excursions systematically modulate HR and BP (Hirsch & Bischop, 1981; Grossman et al., 1991; Eckberg, 2003). Such fluctuation can be exaggerated when breathing becomes deeper and/or slower. Above (see 3.4), we have already addressed this as a problem in estimating cardiac vagal activity by RSA. In addition, increases in respiratory amplitudes as observed in BII phobia and patients with various forms of syncope could simulate phases of “instability” in cardiovascular indices. For example, the instability phase observed by Julu et al. (2003) showed HR and BP oscillation periods ranging from 9–12 s, which could translate into slow breathing at 5–7 breaths/min. In this region, low-frequency cardiovascular fluctuations related to baroreflex regulation are located, which can be superimposed with fluctuations in RSA when respiration rate slows down to this frequency (resonance frequency; Lehrer et al., 2003). The result will be marked increases in overall oscillations, quite similar to those observed by Julu et al. (2003). It remains to be demonstrated whether respiration rate indeed becomes so slow in these presumed instability phases, as most studies have shown little change in respiration rate on average in presyncope or syncope. However, when analyzing cardiovascular dynamics in individual patients, even single long breaths (such as in sighing) can simulate patterns of marked fluctuations that would need to be accounted for. The shorter the time windows for averaging become in an analysis of the temporal dynamics of cardiovascular signals, the more pronounced will be the influence of respiratory fluctuations.
Almost all studies of BII phobia and syncope in general have ignored this source of influence on cardiovascular parameters. To estimate cardiac vagal activity in a more conservative way, a number of adjustment procedures have been proposed that should become a standard part of the experimenter’s instrumentation (Grossman & Taylor, 2007; Ritz et al., 2001; 2006; Wilhelm et al., 2004).
4.4. Is there a role for apnea?
Another aspect of the respiratory response that has not received sufficient attention is apneas. Romano & Engel (1945) already mentioned the possibility of hyperventilation-induced apnea as a cause for a reflex-like cardiac standstill. Graham et al. (1961) noted that in their observations of fainting, asystole, as well as falls in BP and apnea, presented “a picture indistinguishable from death” (p. 505). Similarly, apneas were noted by other investigators in periods leading up to syncope (e.g., Weissler et al., 1957). Depression of ventilation is normally observed after a period of hyperventilation, which can also result in complete cessation of breathing excursions (Meah & Gardner, 1994). Typically, apnea is accompanied by a marked increase in sympathetic vasoconstrictor tone in the skeletal muscles (Jänig, 2006; Khayat et al., 2004) and cerebral vasodilation through its accompanying hypercapnia (Przybylowski et al., 2003).
It could be speculated whether such mechanisms are defective in BII-phobia patients and vasovagal syncope in general. Indeed, during tilt-table testing-induced vasovagal syncope, muscle sympathetic nerve activity is reduced in late phases (Jardine et al., 2002; see also Sarlo et al., 2008). In recovering from hyperventilation, apneas or partial depression of ventilation may not be accompanied by sufficient vasoconstrictor input, thus contributing to peripheral blood pooling. Furthermore, the cerebro dilatory response to apnea-induced hypercapnia could be compromised. It could be further speculated whether oxygen desaturation during depressed ventilation or apneas (Ohi et al., 1994) is responsible for drastic falls in HR or even cardiac arrest, as is seen in neonates with immaturity of respiratory control (Abu-Shaweesh & Martin, 2008), pallid breath-holding spells in childhood (Stephenson, 1978), or in cases of apnoeic asphyxia in adults (Daly et al., 1979). Thus, it could be instructive for future studies to focus on autonomic dynamics associated with ventilatory depression following cessation of hyperventilation, or full apneas, which may be characteristics of more severe faints (Graham et al., 1961). Patients who faint during recovery from BII-stimulus exposure may do so when hyperventilation ceases because of removal of the phobic stimulus. A key factor for actual fainting outcomes could be the exact time course as well as the duration of either hyperventilation and/or depressed ventilation or apnea.
4.5. Respiration and the experiential quality of BII stimuli
With a strong focus of psychophysiological BII-phobia research on cardiovascular dysregulation and the diphasic response pattern, aspects related to the emotional quality and cognitive processing of the BII-phobia experience have not received sufficient attention. An exception is the role of disgust as a key response to BII-stimulus exposure, which has received a more in-depth treatment recently (for a review see Cisler et al., 2009). Here, we will briefly address perspectives on the role of disgust and cognition as they emanate from the study of respiratory processes in BII phobia.
4.5.1. Disgust
is a substantial component of the BII-phobic experience. Regularly, self-report of disgust is found to be as strong as self-report of fear or anxiety for BII patients in exposure to relevant stimuli (Cisler et al., 2009). There are also indications that BII-phobia patients have a heightened disgust sensitivity in specific domains, such as body envelope violations, contamination, or death (e.g., de Jong & Merckelbach, 1998; Sawchuk et al., 2000; Tolin et al., 1997), although studies are not consistent (see also Sawchuk et al., 2002). Page (1994; 2003) proposed that strong feelings of disgust could be the critical factor in bringing the patient to syncope: Following an initial sympathetic activation by fear, homeostatic counter-regulation by the parasympathetic system would set in, which would then be joined by the additional parasympathetic activation associated with the disgust experience. However, findings regarding the contribution of this experiential component to the vasovagal syncope are not convincing. Page (2003) observed more pronounced increases and subsequent decreases in BP to presentations of blood and needle slides in blood- and injection-anxious students who were high versus low in disgust sensitivity, but without substantial decreases below initial levels, responses fell short of the most common criterion for a diphasic response. Gerlach et al. (2006) observed little change in RSA throughout a protocol of venipuncture and no association with reported disgust, which was significantly lower than anxiety in this situation. Similarly, van Overveld et al. (2009) found no association between RSA or salivary flow rate and blood-injury sensitivity in students when exposed to injury-related guided imagery. However, the latter study was limited because of a lack of respiratory control of RSA and both studies may have been limited in that the venipuncture situation did not entail the supposedly phobia-relevant factor of contamination-proneness (Page, 2003) and the guided imagery did not contain images of blood.
In our own studies, we also observed almost equally strong anxiety and disgust responses to surgery film presentations in BII-phobia patients. Interestingly, the respiratory system was sensitive to the disgust experience. Patients with greater disgust responses also showed greater drops in PCO2 (Ritz et al., 2005) or stronger increases in ventilation (Ayala et al., 2010; Ritz et al., 2009) during and/or following film presentations. Patients also showed significant drops in PCO2 during a pure contamination-relevant disgust film, a prolonged scene of transferring ground up insects to a bowl using the mouth. Different from the surgery film, PCO2 levels quickly returned to normal levels during recovery from the disgust film. Nevertheless, higher nausea ratings were associated with stronger ventilation and lower PCO2 during disgust recovery in patients. Thus, the role of the disgust experience in BII-related respiratory dysregulation deserves further attention. Adding to the list of potential functions of deep breaths, it could be speculated that they are an attempt to cope with symptoms of nausea that result from the disgust experience.
4.5.2. Perceived control
Another potentially important experiential variable linked to the BII-phobia experience is perceived control. Although fear of loss of control is among the key symptoms of anxiety disorders, particularly panic disorder (Barlow, 2002), the actual loss of control is most evident, physical, and absolute in BII phobia-related fainting. Surprisingly little is known about the involvement of control cognitions in pre-syncopal and syncopal dynamics. Prodromal experiences in orthostatic syncope have been described in detail by Van Lieshout et al. (2003). This involves diffuse symptoms of epigastric discomfort, nausea, sweating, and “the desire to sit down or leave the room” (p. 835) in early stages, and subsequent sensations of lightheadedness, fatigue, palpitations, and blurred vision through reduced retinal perfusion. No closer empirical examinations of cognitive processes leading up to syncope have been reported. However, earlier insights into antecedents of vasovagal syncope were gained by retrospective reports (Sledge, 1978). Confirming Engel’s interpretation of the conflictual character of the emotional fainters’ psychological situation, the author observed in a number of cases, vague feelings of being bodily or socially harmed (injury, social embarrassment, humiliation) that occurred in combination with submission to a threatening situation and the perceived need to deny the threat (e.g., a medical examination situation that was experienced as inescapable and refusal to submit to it would have appeared as cowardly). These accounts seem to suggest that fear of losing control may indeed be central to the syncopal experience. Consistent with this, BII-phobia patients typically rate their experience during surgery film viewing as particularly low on the affective dimension of submissiveness – dominance (i.e., as having little control over the situation; Ritz, Wilhelm, Gerlach, et al., 2005). Findings of Hermann et al. (2007) that suggest a reduction in inhibitory control by the medial prefrontal cortex in BII-phobia patients compared to controls exposed to blood and wound pictures may be additionally instructive in this context. Interestingly, hyperventilation has been linked to experiential states of overwhelming distress, pain, passive coping, or failure to mount an adequate coping response (Boiten et al., 1994). Future studies need to explore the temporal dynamics in perceived control and hyperventilation during BII-phobic exposure. Together, both could be powerful prodromal symptoms or early warning signs of an impending vasovagal syncope.
4.6. Conclusion: Outside the diphasic straightjacket
It appears that the role of respiration in BII-related vasovagal syncope should receive more attention. Observations of hyperventilation have been excluded by a narrow focus on the cardiovascular regulation and the search for diphasic response patterns in much of the psychophysiological BII-phobia research. If respiration had been considered at all, it was restricted to measurements of respiration rate, which seems to play a negligible role in vasovagal syncope. It may be time to strip off the straightjacket of the diphasic response if progress should be made in understanding the psychophysiological mechanisms behind BII phobia. Alternative definitions of phases (such as those proposed by Lagi et al., 2001) can help direct attention to critical phenomena that have been largely overlooked. Beyond the purely academic interest in mechanisms, such perspectives may help improve the prediction of fainting responses in individual patients and ultimately inform behavioral therapies of BII phobia in new ways.
5. Treatment of BII phobia – no more questions?
5.1. Applied tension technique: the perfect antidote to the perfect storm?
5.1.1. Development and rationale of applied tension
The behavioral treatment of BII phobia has been dominated for some time by the applied tension technique (Öst & Sterner, 1987). In this technique, the patients learn to systematically tense and release skeletal muscle sites for brief periods of time as soon as feelings of faintness appear. The psychophysiological rationale is that such basic activities increase venous return, BP, and cerebral perfusion. They also lead to a vagal withdrawal, thus potentially truncating the bradycardia component of the vasovagal response. The technique echoes the earlier work of Engel and Romano (1947), who instructed their patients to begin running on the spot in order to avoid fainting, with the idea that this would help resolve the stalemate between activating fight-flight impulses and inhibitory forces countering them. In the reported case studies, this strategy was successful in keeping patients from fainting. Kozak and Montgomery (1981) first devised the technique of using static tense and release sequences of major muscle sites (thorax, arms, legs) for BII phobia patients, with the explicit goal of augmenting venous return. Öst and colleagues expanded on these ideas with a number of controlled studies that generally showed some success, even with short one to five-session treatments employing this technique. However, as outlined in our recent review (Ayala, Meuret, et al., 2009), with the five original studies from this team only and no independent replications, there is rather limited empirical evidence for the efficacy of the technique. In addition, effect sizes for a number of outcome measures and observed physiological changes did not demonstrate a clear advantage over other treatment approaches such as exposure alone (see also 5.1.4).
5.1.2. Fine tuning of the applied tension technique
To date, little attention has been directed towards the psychophysiology of implementing applied tension, thus questions on how to optimize the technique in terms of targeted muscle sites, duration and frequency of muscle contractions, and ideal timing of their initiation remain largely unanswered. An exception is the study of Bodycoat et al. (2000) that compared the effect of two patterns of muscle tension, constant tension versus rhythmic tension of arm, chest and leg muscles, on BP in a nonphobic sample of 45 adults. Constant tension, which was similar to that described by Öst and Sterner (1987), involved tensing for 15 s and then releasing the tension for 15 s. Rhythmic tension, which was based on Foulds et al. (1990), required tension for 5 seconds and release for 5 seconds, with both conditions continuing the pattern for 2 minutes. Differences in DBP emerged, with rhythmic tension showing a significantly greater increase than constant tension. In both conditions, DBP declined immediately after termination of tension, with no significant differences in DBP between conditions during the release period. Thus, the extended break in constant tension may be more likely to allow for presyncopal symptoms to emerge, thus supporting the use of rhythmic tension. The authors also varied the manipulation of respiration rate on three levels, with very fast pacing (60 breaths/min), normal pace, and a slow pace with breath holding during tension, but this factor did not influence their findings (respiration was not measured though, thus this finding is difficult to interpret). The results have yet to be replicated in clinical samples under conditions of imminent syncope.
5.1.3. Physical maneuvers to prevent fainting in other populations
Muscle tension techniques similar to applied tension have been developed and successfully tested in other populations. For example, Ditto et al. (2003) observed a reduction in blood donation-related symptoms (including faintness, weakness, nausea) and a reduced need for chair reclining in women when instructing them in repeated 5-s tension of arm and leg muscles during and after a blood draw. The usefulness of physical maneuvers such as squatting, leg muscle pumping (tiptoeing), and/or tensing (leg crossing) has been demonstrated in aborting or delaying fainting responses due to orthostatic challenges in patients with autonomic failure or unspecified vasovagal syncope (Krediet et al., 2002; Kim et al., 2005; ten Harkel et al., 1994; van Dijk et al., 2005; van Lieshout et al., 1992; Wieling et al., 1993). In at least one study, the sample included patients with a history of vasovagal syncope due to pain or unusually strong emotions (Krediet et al., 2005).
5.1.4. Applied tension for BII phobia – the final answer?
Thus, muscle tension techniques seem to be well suited as the primary intervention tool for behavioral BII-phobia treatment. In fact, textbooks seem to suggest that it is the major if not exclusive behavioral intervention for BII phobia (e.g., Barlow & Durand, 2005). Therefore, the applied tension technique has become the twin brother of the diphasic response as the dominating canon of BII-phobia treatment. However, this development is problematic in at least three ways. First, treatment cannot be reduced to this single “somatic” intervention element only. Effective treatment will always include elements of other common cognitive behavior treatments, such as cognitive restructuring and exposure. The former is at least rudimentarily provided by the psychophysiological rationale for fainting, as well as applied tension as the technique countering the response. The latter is an integral part of the training, because learning to choose the right timing for initiating the tension maneuvers relative to the onset of presyncopal symptoms is viewed as critical for its effectiveness (e.g. Öst et al., 1991; Hellström et al., 1996) and requires experimentation with the feared situation. Indeed, a more differentiated approach is provided by clinical handbooks of specific phobia treatment that outline elements such as cognitive restructuring and exposure and reserve applied tension for those patients with a fainting history (e.g., Craske et al., 2006). Second it is not at all clear if applied tension is the best treatment available. As mentioned above (5.1.1), there is limited evidence on efficacy (Ayala, Meuret, et al., 2009), and physiological changes and effects sizes of other outcome measures do not suggest a clear superiority of applied tension over other techniques: in particular, exposure alone and/or applied relaxation demonstrated equal if not stronger effects in reductions on standard measures of the phobia, such as the Fear Survey Schedule (Wolpe and Lang, 1964) or the Mutilation Questionnaire (Klorman et al., 1974); e.g., the range of effect sizes (g) was 1.82–2.13 versus 2.73–3.73 for five sessions of applied tension versus exposure across post-treatment and follow-up for improvement in these questionnaire measures.
Third, there are inconsistencies between the rationale and application of the technique, which suggests that the limits and full extent of its effects are not fully understood yet. Most importantly, there are no differences in effectiveness of the technique between patients who faint (defined by an initial exposure test; Hellström et al., 1996) and those who do not, although the rationale holds that its key effects should unfold when presyncopal symptoms or BP drops set in. In at least three earlier studies of Öst et al. (1989;1991; Öst, Lindahl, et al., 1984), in which applied tension was compared to applied relaxation or in vivo exposure, non-fainters consistently profited equally from treatment. (Unfortunately, these studies did not clearly break down syncopal or presyncopal signs between treatment groups.). The fact that applied tension is not more or less effective in patients that show only increases in cardiovascular activation (the first leg of the presumed diphasic response) during stimulus confrontation makes the psychophysiological rationale of the technique as counteracting hypotension less convincing. In addition, at follow-up, patients often reported that they no longer found that they needed to utilize the technique, which argues for additional factors that may contribute nonspecifically to the effects of the technique.
Overall, the notion of applied tension being the perfect BII-phobia treatment is probably as premature as the concept of the diphasic response, which provided its underlying psychophysiological rationale. Because the technique seems to be sufficiently effective (Ayala, Meuret, et al., 2009), its seemingly convincing rationale may have distracted from a critical evaluation of the actual complexity of the treatment, the lack of superiority over other treatments, and the lack of specificity in addressing fainting responses. In consequence, little attention has been directed to developing alternative treatments that might address important aspects of the psychophysiological dysregulation such as hyperventilation.
5.2. Targeting respiration in BII phobia – an alternative treatment modality?
Considering the lack of apparent benefits of hyperventilation during BII exposure and the potential risk associated with it (see 4.1), targeting respiratory regulation appears to be a promising avenue for behavioral interventions. Despite the prevailing focus on cardiovascular dynamics in BII phobia-related syncope, there are at least two instances in the past literature where respiratory processes had been addressed.
5.2.1. Respiratory relief
Orwin (1971) outlined a treatment, respiratory relief, as a behavioral intervention that combined graded exposure to phobic stimuli with a respiratory maneuver. In this method, patients were instructed to hold their breath at maximum expiration by closing the mouth and pinching their nostrils. The re-initiation of breathing was then paired precisely with the moment of the phobic stimulus presentation. The relief from anxiety developed during the breath-hold was thought to counteract the phobic fear and ultimately lead to its extinction. Typically, the breath-hold was 15 s or longer when tolerated, and trials were repeated 8–10 times in 1–2-min intervals. No adverse effects of this maneuver were reported [Footnote 5]. Orwin and Duffy (1972) utilized the technique to successfully treat an individual with a history of fear and fainting in response to blood. Subsequently, Orwin (1973) experimented with adding CO2 in concentrations of 30 to 70% at re-initiation of breathing after breath-holding. Of two patients with BII phobia, one treated with this “augmented respiratory relief” technique and the other one with regular respiratory relief, both were reported to be symptom free after treatment; however, the former had relapsed at 3–6 months follow-up (Orwin et al., 1975). Longo and vom Saal (1984) suggested that respiratory relief increases partial pressure of CO2, thus working against hyperventilation and the anxiety associated with the phobic response. It could also be speculated that autonomic adjustments during extended breath-holding, such as increases in muscle sympathetic nerve activity (Jänig, 2006), helped to counteract hemodynamic derangements, such as the pooling of the blood in the periphery, that is seen in syncope.
The technique is an interesting contrast to biological theorizing and experimental findings on panic disorder. From this perspective, the respiratory relief maneuver could be expected to lead to symptomatic worsening in panic patients, given the likelihood of CO2 build-up that has been suspected of triggering a suffocation alarm system in panic patients (Klein, 1993; Roth et al., 2005). Breath-holding has been shown to lead to anxiety and/or panic symptoms, although its specificity to panic is debated (see e.g., Asmundsen & Stein, 1994; Nardi et al., 2008; Roth et al. et al., 1998). There is also a solid literature on panic induction by CO2 challenge tests (e.g., Fyer et al., 1987; Papp et al., 1993; Perna et al., 1994), which may be part of the reason the augmented respiratory relief intervention has received little further scrutiny. But physiological mechanisms could be quite different between these anxiety disorders, thus there would be no a priori reason for avoiding the further exploration of such intervention techniques in BII-phobia patients. Indeed, CO2-rebreathing has been used to counteract hypocapnia-induced reductions in CBF in vasovagal syncope (Novak et al., 1998) and has been recommended for further scrutiny as an intervention technique (Carey et al., 2001). However, before recommending respiratory relief maneuvers, research would need to explore the autonomic regulation linked to breath-holding and apneas in vasovagal syncopes (see 4.4).
5.2.2. Respiratory control maneuvers
Foulds (1993) suspected that muscle tension techniques in themselves would not be effective enough to raise CBF levels sufficiently to avoid fainting. He considered the possibility that the cerebral effects produced by muscle tensing were “caused by increased metabolic production of CO2, i.e. partially reversing hyperventilation, rather than by reversing a decrease in HR and blood pressure” (p. 144). Thus, a more efficient treatment would be to combine muscle tension and respiratory control exercises that aimed at slowing the breath to avoid hyperventilation (Clark et al., 1985). Measurements of CBF velocity and HR were obtained throughout the therapy. Prior to treatment, the response of the patient to voluntary hyperventilation was assessed, showing a marked increase in HR (from 72 to 138 bpm) accompanied by a 48% drop in CBF. When the patient was confronted with a phobic stimulus, a response pattern similar to voluntary hyperventilation was seen - CBF decreased while both HR and reports of dizziness increased, which was eventually followed by a drop in BP and HR. Following training in both muscle tension and respiratory control techniques, the patient gradually improved across five exposure sessions. However, at a seven-month follow-up test exposure to a venipuncture video, the patient showed again an HR increase and dizziness followed by pronounced bradycardia. Initiation of muscle tension did not seem to abolish this response. Interestingly, at the same time the patient felt fully in control of the situation and only mildly anxious.
5.2.3. Hypoventilation techniques
Based on prior work targeting hyperventilation in panic disorder patients (Meuret et al., 2008; 2004; 2001), we recently pilot-tested a hypoventilation technique that attempted to target the respiratory dysregulation observed in BII patients (Ayala, Ritz, et al. 2009). For that purpose, we designed an economic intervention element with minimal requirements for therapist involvement and a high level of standardization. It consisted of a 14-min training video showing a patient who receives advice from a therapist in modifying her breathing pattern when confronted with BII-related stimuli. Sixty BII-phobia patients were randomly assigned to receive instructions in this counter-hyperventilation technique, applied tension, or pure relaxation. The techniques to counter hyperventilation included demonstration and practice of slow, shallow, and abdominal breathing during confrontation with feared BII stimuli. In contrast to classical breathing retraining or the respiratory control technique, which emphasize “deep” abdominal breathing (Schmidt et al., 2000; Craske et al., 1997), the shallowness aspect aimed to produce a certain degree of hypoventilation, which counters the patients’ tendency to hyperventilate by taking deep breaths (Ritz et al., 2009). The outcome of these three minimal interventions was measured during exposure to a surgery film, compared to a similar exposure on a day prior to the intervention.
Overall, the techniques resulted in reductions in anxiety, disgust experience, and physical symptoms. Patients undergoing breathing instructions reached higher levels of PCO2 during exposure than the other groups. Both the instructions in breathing and muscle tension lead to similar reductions in anxiety, whereas changes in relaxation instruction were minimal. The three groups experienced equal increases in perceived control. Although participants in the applied tension group reported the highest success in applying the technique after the short instructions, breathing instructions were viewed as equally helpful in reducing the phobic response. Self-reported history of fainting to BII stimuli (partial or complete, with loss of consciousness) did not influence the outcome. Feelings of faintness occurred again in a small minority of patients and only two patients needed to be reclined. None of these patients were in the hypoventilation group. Findings suggest that such breathing instructions may be a useful treatment component for BII phobia. In a subsequent study, we will explore the impact of this training on CBF during exposure.
5.2.4. Countering gray-outs and black-outs: effects on cerebral blood flow?
In devising the respiratory control technique, Foulds (1993) suggested that applied tension may not be able to impact BP enough to raise CBF above the threshold necessary to prevent syncope. By using muscle tension, CBF only increased by 8–15% in BII-phobia patients (see also Foulds et al., 1990). The extent to which muscle tension can contribute to stabilizing CBF in patients prone to BII-related vasovagal syncope is an open question. Studies attempting to decrease orthostatic hypotension due to autonomic failure (e.g., ten Harkel et al., 1994; van Lieshout et al., 1992) noted that a small increase in BP produced considerable improvements in orthostatic tolerance. Wieling et al. (1993) suggested that small changes in BP through increased venous return of blood flow from the legs may be sufficient to prevent syncope by increasing CBF minimally above the threshold necessary.
Similarly, the contribution of breathing techniques to CBF increases under exposure requires further exploration. As outlined above (see 4.2.2), hyperventilation in itself has rarely been shown to initiate fainting, unless additional predisposing factors are considered. Thus, countering hyperventilation alone may not impact CBF sufficiently, unless hypoventilation leads to increases in PCO2 above normal levels. Following voluntary hyperventilation, rebreathing to increase PCO2 from 20 to 60mmHg leads to an almost three-fold increase in CBF, and more than 100% from a baseline of 40mmHg (Claassen et al., 2007). On the other hand, one report indicated that orthostatic stress was tolerated for a longer time by the addition of inspired CO2, but eventual presyncope was not prevented (Blaber et al., 2001). Thus, the potential of therapeutic hypoventilation techniques for improving CBF is considerable, but may often not be sufficient to address fainting completely. In his single-case report, Foulds (1993) used the pragmatic strategy of combining both techniques, respiratory control and muscle tension, which seemed to be sufficient to counteract fainting during the course of therapy. It also must be considered that some of the beneficial effects of muscle tension on CBF may unfold through the increase in CO2 production by the accompanying increase in metabolic activity (Foulds, 1993). A combination of both techniques may therefore be effective through multiple and partly overlapping mechanisms.
Eventually, the exact time sequence of changes in respiratory and circulatory parameters may best inform on the clinical importance of targeting one versus the other dysregulated process, hyperventilation or peripheral BP falls, behaviorally. Some studies suggest that drops in HR or BP may lag considerably behind the onset of hyperventilation (see 4.1.1 and 4.2.1) or CBF reductions (Dan et al., 2002). Lipsitz et al. (1998) generally found deep breaths coinciding with BP drops in healthy volunteers developing syncope during tilt-table testing, yet substantial drops in HR occurred at a later stage. Both BII patients from our recent study (Ayala et al., 2010) presented in Figures 1 and 2 showed their first deep sigh breaths well before more substantial drops in HR and BP were observed. Studies also suggest that patients may show reduced CBF before syncope despite a lack of notable reductions in HR and BP (Sung et al., 2000). In addition, in healthy individuals under higher levels of orthostatic stress, PCO2 changes explain substantially more of the variance in CBF than mean arterial BP changes (Mitsis et al., 2006). Thus, in preventing syncope, targeting CBF changes with a hypoventilation technique at an early stage of presyncope may be at least as critical as targeting BP and HR at a later stage with a muscle tension technique. Countering hyperventilation at its very onset could reverse CBF reductions at least partially, whereas physical maneuvers or muscle exercises could be initiated at a later stage in case drops in BP and/or HR should still set in. Consideration for differential primary mechanisms, either respiratory or cardiovascular, in patients that lead to syncope is also warranted. Subtype analysis of regulatory patterns may prove to be a successful strategy to tailor treatments to the individual and avoid the one-size-fits all approach that has dominated the BII-phobia treatment literature.
5.3. Cognitions – the neglected factor in BII phobia treatment research
Considering the experiential uniqueness of the distressing fainting response, it would appear consequent to address cognitive factors more explicitly in research and treatment of BII phobia. The observations that (i) fainting status was not related to success of treatment of BII-phobia patients with applied tension (Hellström et al., 1996) and/or other treatment techniques (e.g. Öst et al., 1991) and (ii) that patients at follow-up reported not using the technique but were nevertheless further improved, implies that some general, fainting-nonspecific factors are at work that have not been explored well. This seems to make the dominating psychophysiological rationale of the applied tension treatment, the truncation of the second phase of the diphasic response, appear as obsolete. It could be speculated that conveying a sense of control in situations that are actual or imagined threats to bodily integrity or health might be a powerful intervention in itself. Changes in perceived control could explain the fact that some patients treated with the applied tension technique reported no longer needing the technique at follow-up (Öst et al., 1989, 1991). Breathing instructions may be similarly powerful agents conveying bodily control to the patient, in addition to their psychophysiological effects of reducing hyperventilation. Lay instructions to “take a deep breath” to overcome anxiety may speak to that, as well as the demonstrated positive impact of paced breathing instructions (Clark & Hirschman, 1990; Harris et al., 1976) or instructions in “focused breathing” (Arch & Craske, 2006) on negative affect.
Instruction and training in applied tension or breathing techniques, along with a psychophysiological rationale that is appealing to the patient, may provide a powerful “rational placebo” or safety aid (Gerlach et al., 2006; Ayala, Meuret, et al., 2009) that primarily boosts patients’ feelings of control. Such objections have originally been raised against breathing retraining interventions in panic disorder treatment (e.g. Craske et al., 1997; Garssen et al., 1992; Schmidt et al., 2000; for a review see Meuret et al., 2003). It could be suspected that the dissociation between vasovagal responses and perceived control observed by Foulds (1993) in his case-study at follow-up, despite initiation of muscle exercises, may be indicative of such a mechanism. However, the interpretation of a technique as placebo or a mechanistically effective treatment modality ultimately hinges on the availability of a parsimonious and valid psychophysiological rationale. A promising avenue is the exploration of mediators and moderators of treatment (Meuret et al., 2009), which have demonstrated that in panic disorder treatment, capnometry-assisted reductions in hyperventilation are prospectively associated with an increasing sense of control and decline in panic symptom severity, but not vice versa.
In therapy application, cognitive and physical intervention elements are probably never implemented in an idealized, pure form. Beyond their successful combination in exposure and cognitive therapy interventions for specific phobias (Choy et al., 2007), it would be impossible to administer in vivo exposure without at least minimal cognitive elements, such as provision of a treatment rationale and discussion of the patient’s experience and thus their cognitive integration. For BII phobia, exposure training would also require physical contingencies against possible fainting, which in the simplest form includes posture changes to sitting or lying down and basic physical maneuvers, such as raising the legs above head level. Teaching these physical maneuvers as coping techniques will always include appealing to cognitive processes, whether in explanation of their rationale, directing attention to prodromal symptoms, estimating likelihood of phobic responses, etc. Beyond that, genuine cognitive techniques that address irrational expectations and beliefs need to be considered (Thompson, 1999). Patients with differences in fainting status will require varying balances of both approaches. For fainters, the emphasis may initially be on teaching physical maneuvers to counteract actual fainting responses. During the further course of treatment, with the development of a greater sense of control over presyncopal episodes, the emphasis may then shift gradually towards additional cognitive factors, such as irrational beliefs about bodily harm or catastrophic cognitions and automatic negative thoughts related to BII stimuli, fainting, and associated fear (e.g., White & Sellwood, 1995; Thompson, 1999). Non-fainters on the other hand may profit at an initial stage from physical maneuvers as nonspecific agents providing a sense of control, thus lowering the threshold for in-sensu or in-vivo confrontation with the feared BII stimuli.
5.4. Treatment of BII - summary and conclusion
Much remains to be learned about the optimal approach to treating BII phobia. Although applied tension has shown promise, independent replications are needed for demonstrating its efficacy, particularly in long-term follow-up. To date, the psychophysiological mechanisms through which the technique unfolds its effects are not well understood. The treatment also seems to work, despite its rationale, for patients that do not have a tendency to faint. This underscores the necessity of devising mechanism studies, which will also need to explore the role of cognitive factors, such as perceived control through learning of coping skills. As an alternative or additional approach to treatment, hypoventilation training seems to hold promise. It may more directly target problematic reductions in CBF and the ensuing fainting responses. Future studies also need to determine possible differential indications of these treatments for subgroups of BII-phobia patients.
6. Overall conclusion and outlook
The psychophysiological approach to BII phobia has been plagued by inconsistencies in the basic definition of fainting and uncertainties about the core regulatory abnormalities underlying syncopal episodes as well as the rationale for its treatment. The paradigm of the diphasic response has not been reflected well in its conceptualization and validity for the BII-phobic responding and the usefulness of the applied tension technique may have been overstated. The narrow focus on these two concepts as “cornerstones” of BII-phobia research and treatment has probably impeded progress in the understanding of the disorder and the development of intervention techniques that target the dysregulation more efficiently in its various facets. At this point, the psychophysiological literature on BII phobia and its treatment appears to produce more questions than answers. This is surprising for a disorder that, in conventional receptions of the literature, has been thought to be well understood. We have proposed a number of new angles of inquiry into the psychophysiological regulation in BII phobia, most importantly focusing on respiratory disturbances and their interaction with cerebral perfusion and experiential aspects of BII phobia. It is our hope that this will provide new perspectives for future research and help motivate greater research efforts to further elucidate this debilitating disorder.
Footnotes
Throughout the text we will use the terms “fainting” and “syncope” interchangeably.
As one reviewer noted, falls as a consequence of syncope can lead to concussions, which may prolong the period of loss of consciousness.
It should be noted that for the observer, it is often challenging to determine that the cause of fainting is a BII-related stimulus. An anecdotal event from a college in the US may serve as an example. During a regular psychology undergraduate class, one student suddenly felt faintness and subsequently lost consciousness. While the instructor tried to help the student, a second student also developed symptoms of faintness. At this point the instructor called for emergency help and the building was evacuated, the rumor being that carbon monoxide poisoning might have occurred. After it was determined that no emergency was present, it was discovered that the class had viewed a video clip that included an invasive medical procedure. The instructor had not been aware of a potential association between this type of trigger and the fainting response. While the first student had not been able to comment on the event, the second student informed the instructor that the reason for his faintness was concern over what happened to the first student.
In gray-outs, disturbances of retinal perfusion begin to affect color vision; in black-outs, perfusion is more severely compromised so that the retina ceases to function (Van Lieshout et al., 2003).
It could be speculated that the maneuver may lead to similar outcomes as the “fainting lark” (van Lieshout et al., 2003). However, the critical difference is probably that the breath hold was expiratory rather than inspiratory, thus preventing a build-up in intrathoracic pressure similar to that in the Valsalva maneuver, and that no postural change was associated with it.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abelson JL, Weg JG, Nesse RM, Curtis GC, 2001. Persistent respiratory irregularity in patients with panic disorder. Biological Psychiatry 49, 588–595. [DOI] [PubMed] [Google Scholar]
- Abu-Shaweesh JM, Martin RJ, 2008. Neonatal apnea: what’s new? Pediatric Pulmonology 43, 937–44. [DOI] [PubMed] [Google Scholar]
- Accurso V, Winnicki M, Shamsuzzaman AS, Wenzel A, Johnson AK, Somers VK, 2001. Predisposition to vasovagal syncope in subjects with blood/injury phobia. Circulation 104, 903–907. [DOI] [PubMed] [Google Scholar]
- Ader PSJ, France C, Ditto B, 1991. Baroreflex sensitivity at rest and during stress in individuals with a history of vasovagal syncope. Journal of Psychosomatic Research 35, 591–597. [DOI] [PubMed] [Google Scholar]
- Alboni P, Alboni M, Bertorelle G, 2008. The origin of vasovagal syncope: to protect the heart or to escape predation? Clinical Autonomic Research 18, 170–178. [DOI] [PubMed] [Google Scholar]
- Alpers GW, Wilhelm FH, Roth WT, 2005. Psychophysiological assessment during exposure in driving phobic patients. Journal of Abnormal Psychology 114, 126–39. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association., 2000. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition Text Revision (DSM-IV TR). American Psychiatric Press, Washington, DC. [Google Scholar]
- Arch JJ, Craske MG, 2006. Mechanisms of mindfulness: emotion regulation following a focused breathing induction. Behaviour Research and Therapy 44, 1849–1858. [DOI] [PubMed] [Google Scholar]
- Asmundson GJ, Stein MB, 1994. Triggering the false suffocation alarm in panic disorder patients by using a voluntary breath-holding procedure. The American Journal of Psychiatry 151, 264–266. [DOI] [PubMed] [Google Scholar]
- Ayala ES, Meuret AE, Ritz T, 2009. Treatments for blood-injury-injection phobia: a critical review of current evidence. Journal of Psychiatric Research 43, 1235–1242. [DOI] [PubMed] [Google Scholar]
- Ayala ES, Meuret AE, Ritz T, 2010. Confrontation with blood and disgust stimuli precipitates respiratory dysregulation in blood-injury-injection phobia. Biological Psychology 84, 88–97. [DOI] [PubMed] [Google Scholar]
- Ayala ES, Ritz T, Meuret AE, 2009. A novel biobehavioral approach to treating bloodinjury-injection phobia. Paper presented at the 2009 Annual Conference of the ADAA, Santa Ana Pueblo, NM, March 12–15. [Google Scholar]
- Barlow DH, 2002. Anxiety and its disorders: The nature and treatment of anxiety and panic (2nd ed.). Guildford Press, New York, NY. [Google Scholar]
- Barlow DH, Durand VM 2005. Abnormal psychology: An integrative approach (4th ed.). Thomson Wadsworth, Belmont, CA. [Google Scholar]
- Bass C, Gardner WN, 1985. Respiratory and psychiatric abnormalities in chronic symptomatic hyperventilation. British Medical Journal (Clinical Research Ed.) 290, 1387–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beacher FD, Gray MA, Mathias CJ, Critchley HD, 2009. Vulnerability to simple faints is predicted by regional differences in brain anatomy. Neuroimage 47, 937–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbadis SR, Chichkova R, 2006. Psychogenic pseudosyncope: an underestimated and provable diagnosis. Epilepsy & Behavior 9, 106–110. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS, 1991. Autonomic determinism: The modes of autonomic control, the doctrine of autonomic space, and the laws of autonomic constraint. Psychological Review 98, 459–487. [DOI] [PubMed] [Google Scholar]
- Blaber AP, Bondar RL, Moradshahi P, Serrador JM, Hughson RL, 2001. Inspiratory CO2 increases orthostatic tolerance during repeated tilt. Aviation, Space, and Environmental Medicine 72, 985–991. [PubMed] [Google Scholar]
- Bodycoat N, Grauaug L, Olson A, Page AC, 2000. Constant versus rhythmic muscle tension in applied tension. Behaviour Change 17, 97–102. [Google Scholar]
- Boiten F, Frijda NH, Wientjes CJE, 1994. Emotions and respiratory patterns: Review and critical analysis. International Journal of Psychophysiology, 17, 103–128. [DOI] [PubMed] [Google Scholar]
- Bracha HS, Bienvenu OJ, Eaton WW, 2007. Testing the Paleolithic-human-warfare hypothesis of blood-injection phobia in the Baltimore ECA Follow-up Study--towards a more etiologically-based conceptualization for DSM-V. Journal of Affective Disorders 97, 1–4. [DOI] [PubMed] [Google Scholar]
- Carey BJ, Eames PJ, Panerai RB, Potter JF, 2001. Carbon dioxide, critical closing pressure and cerebral haemodynamics prior to vasovagal syncope in humans. Clinical Science (London, England: 1979) 101, 351–358. [PubMed] [Google Scholar]
- Choy Y, Fyer AJ, Lipsitz JD, 2007. Treatment of specific phobia in adults. Clinical Psychology Review 27, 266–286. [DOI] [PubMed] [Google Scholar]
- Cisler JM, Olatunji BO, Lohr JM, 2009. Disgust, fear, and the anxiety disorders: a critical review. Clinical Psychology Review 29, 34–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claassen JA, Zhang R, Fu Q, Witkowski S, Levine BD, 2007. Transcranial Doppler estimation of cerebral blood flow and cerebrovascular conductance during modified rebreathing. Journal of Applied Physiology 102, 870–877. [DOI] [PubMed] [Google Scholar]
- Clark DM, Salkovskis PM, Chalkley AJ, 1985. Respiratory control as a treatment for panic attacks. Journal of Behavior Therapy and Experimental Psychiatry 16, 23–30. [DOI] [PubMed] [Google Scholar]
- Clark ME, Hirschman R, 1990. Effects of paced respiration on anxiety reduction in a clinical population. Biofeedback and Self-Regulation 15, 273–284. [DOI] [PubMed] [Google Scholar]
- Cohn CK, Kron RE, Brady JFA, 1976. Acase of blood/injury/illness phobia treated behaviorally. Journal of Nervous and Mental Disease 162, 65–68. [DOI] [PubMed] [Google Scholar]
- Craske MG, Antony MM, Barlow DH, 2006. Mastering your fears and phobias Therapist guide, 2nd ed. New York, NY, Oxford University Press. [Google Scholar]
- Craske MG, Rowe M, Lewin M, Noriega-Dimitri R, 1997. Interoceptive exposure versus breathing retraining within cognitive-behavioural therapy for panic disorder with agoraphobia. The British Journal of Clinical Psychology 36, 85–99. [DOI] [PubMed] [Google Scholar]
- Daly MD, Angell-James JE, Elsner R, 1979. Role of carotid-body chemoreceptors and their reflex interactions in bradycardia and cardiac arrest. Lancet 1, 764–767. [DOI] [PubMed] [Google Scholar]
- Dan D, Hoag JB, Ellenbogen KA, Wood MA, Eckberg DL, Gilligan DM, 2002. Cerebral blood flow velocity declines before arterial pressure in patients with orthostatic vasovagal presyncope. Journal of the American College of Cardiology 20, 1039–1045. [DOI] [PubMed] [Google Scholar]
- David JE, Yale SH, Vidaillet HJ, 2003. Hyperventilation-induced syncope: no need to panic. Clinical Medicine & Research 1, 137–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong PJ, Merckelbach H, 1998. Blood-injection-injury phobia and fear of spiders: Domain specific individual differences in disgust sensitivity. Personality and Individual Differences 24, 153–158. [Google Scholar]
- Ditto B, France CR, Lavoie P, Roussos M, Adler PS, 2003. Reducing reactions to blood donation with applied muscle tension: A randomized controlled trial. Transfusion 43, 1269–1275. [DOI] [PubMed] [Google Scholar]
- Eckberg DL, 2003. The human respiratory gate. Journal of Physiology 548, 339–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore RT, Wildman RW, Westfield JS 1980. The use of systematic desensitization in the treatment of blood phobia. Journal of Behaviour Therapy and Experimental Psychiatry 11, 277–279. [Google Scholar]
- Engel GL, Romano J, 1947. Studies of syncope: IV. Biologic interpretation of vasodepressor syncope. Psychosomatic Medicine 9, 288–294. [DOI] [PubMed] [Google Scholar]
- Engel GL, 1978. Psychological stress, vasodepressor (vasovagal) syncope, and sudden death. Annals of Internal Medicine 89, 403–412. [DOI] [PubMed] [Google Scholar]
- Epstein SE, Stampfer M, Beiser GD, 1968. Role of the capacitance and resistance vessels in vasovagal syncope. Circulation 37, 524–533. [DOI] [PubMed] [Google Scholar]
- Foa EB, Kozak MJ, 1986. Emotional processing of fear: exposure to corrective information. Psychological Bulletin 99, 20–35. [PubMed] [Google Scholar]
- Foulds J, Wiedmann K, Patterson J, Brooks N, 1990. The effects of muscle tension on cerebral circulation in blood-phobic and non-phobic subjects. Behaviour Research and Therapy 28, 481–486. [DOI] [PubMed] [Google Scholar]
- Foulds J, (1993). Cerebral circulation during treatment of blood-injury phobia: a case study. Behavioural Psychotherapy 21, 137–146. [Google Scholar]
- Fyer MR, Uy J, Martinez J, Goetz R, Klein DF, Fyer A, Liebowitz MR, Gorman J, 1987. CO2 challenge of patients with panic disorder. The American Journal of Psychiatry 144, 1080–1082. [DOI] [PubMed] [Google Scholar]
- Gallopin T, Luppi PH, Cauli B, Urade Y, Rossier J, Hayaishi O, Lambolez B, Fort P, 2005. The endogenous somnogen adenosine excites a subset of sleep-promoting neurons via A2A receptors in the ventrolateral preoptic nucleus. Neuroscience 134,1377–1390. [DOI] [PubMed] [Google Scholar]
- Garssen B, de Ruiter C, Van Dyck R, 1992. Breathing retraining: A rational placebo? Clinical Psychology Review 12, 141–153. [Google Scholar]
- Gellhorn E, 1970. The emotions and the ergotrophic and trophotropic systems. Psychologische Forschung 34, 48–94. [DOI] [PubMed] [Google Scholar]
- Gerlach AL, Spellmeyer G, Vögele C, Huster R, Stevens S, Hetzel G, Deckert J, 2006. Blood-injury phobia with and without a history of fainting: disgust sensitivity does not explain the fainting response. Psychosomatic Medicine 68, 331–339. [DOI] [PubMed] [Google Scholar]
- Graham DT, Kabler JD, Lunsford LJ, 1961. Vasovagal fainting: A diphasic response. Psychosomatic Medicine 23, 493–507. [DOI] [PubMed] [Google Scholar]
- Grossman P, Karemaker J, Wieling W, 1991. Prediction of tonic parasympathetic cardiac control using respiratory sinus arrhythmia: The need for respiratory control. Psychophysiology 28, 201–216. [DOI] [PubMed] [Google Scholar]
- Grossman P, Taylor EW, 2007. Toward understanding respiratory sinus arrhythmia: relations to cardiac vagal tone, evolution and biobehavioral functions. Biological Psychology 74, 263–285. [DOI] [PubMed] [Google Scholar]
- Harris VA, Katkin ES, Lick JR, Habberfield T, 1976. Paced respiration as a technique for the modification of autonomic response to stress. Psychophysiology 13, 386–391. [DOI] [PubMed] [Google Scholar]
- Hellström K, Fellenius J, Öst LG, 1996. One versus five sessions of applied tension in the treatment of blood phobia. Behaviour Research and Therapy 34,101–112. [DOI] [PubMed] [Google Scholar]
- Henry JP, 1984. On the triggering mechanism of vasovagal syncope. Psychosomatic Medicine 46, 91–93. [DOI] [PubMed] [Google Scholar]
- Hermann A, Schäfer A, Walter B, Stark R, Vaitl D, Schienle A, 2007. Diminished medial prefrontal cortex activity in blood-injection-injury phobia. Biological Psychology 75, 124–130. [DOI] [PubMed] [Google Scholar]
- Hirsch JA, Bishop B, 1981. Respiratory sinus arrhythmia in humans: How breathing pattern modulates heart rate. American Journal of Physiology 241, H620–H629. [DOI] [PubMed] [Google Scholar]
- Hohoff C, Domschke K, Schwarte K, Spellmeyer G, Vögele C, Hetzel G, Deckert J, Gerlach AL, 2009. Sympathetic activity relates to adenosine A(2A) receptor gene variation in blood-injury phobia. Journal of Neural Transmission 116, 659–662. [DOI] [PubMed] [Google Scholar]
- Iacoviello M, Guida P, Forleo C, Sorrentino S, D’Alonzo L, Favale S, 2008. Impaired arterial baroreflex function before nitrate-induced vasovagal syncope during head-up tilt test. Europace 10, 1170–1175. [DOI] [PubMed] [Google Scholar]
- Jänig W, 2006. Integrative Action of the Autonomic Nervous System: Neurobiology of Homeostasis. Cambridge University Press, Cambridge, MA. [Google Scholar]
- Jardine DL, Melton IC, Crozier IG, English S, Bennett SI, Frampton CM, Ikram H, 2002. Decrease in cardiac output and muscle sympathetic activity during vasovagal syncope. American Journal of Physiology. Heart and Circulatory Physiology 282, H1804–H1809. [DOI] [PubMed] [Google Scholar]
- Julu PO, Cooper VL, Hansen S, Hainsworth R, 2003. Cardiovascular regulation in the period preceding vasovagal syncope in conscious humans. The Journal of Physiology 549, 299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khayat RN, Przybylowski T, Meyer KC, Skatrud JB, Morgan BJ, 2004. Role of sensory input from the lungs in control of muscle sympathetic nerve activity during and after apnea in humans. Journal of Applied Physiology 97, 635–640. [DOI] [PubMed] [Google Scholar]
- Kim KH, Cho JG, Lee KO, Seo TJ, Shon CY, Lim SY, Yun KH, Sohn IS, Hong YJ, Park HW, Kim JH, Kim W, Ahn YK, Jeong MH, Park JC, Kang JC, 2005. Usefulness of physical maneuvers for prevention of vasovagal syncope. Circulation 69, 1084–1088. [DOI] [PubMed] [Google Scholar]
- Klein DF, 1993. False suffocation alarms, spontaneous panics, and related conditions. An integrative hypothesis. Archives of General Psychiatry 50, 306–317. [DOI] [PubMed] [Google Scholar]
- Kleinknecht RA, Lenz J, Ford G, DeBerard S, 1990. Types and correlates of blood/injury-related vasovagal syncope. Behaviour Research and Therapy 28, 289–295. [DOI] [PubMed] [Google Scholar]
- Klorman R, Weerts TC, Hastings JE, Melamed BG, Lang PJ, 1974. Psychometric description of some specific-fear questionnaires. Behavior Therapy 5, 401–409. [Google Scholar]
- Kozak MJ, Montgomery GK, 1981. Multimodal behavioral treatment of recurrent injury-scene, elicited fainting (vasodepressor syncope). Behavioural Psychotherapy 9, 316–321. [Google Scholar]
- Kozak MJ, Miller GA, 1985. The psychophysiological process of therapy in a case of injury-scene-elicited fainting. Journal of Behavioral Therapy and Experimental Psychiatry 16, 139–145. [DOI] [PubMed] [Google Scholar]
- Krediet CT, de Bruin IG, Ganzeboom KS, Linzer M, van Lieshout JJ, Wieling W, 2005. Leg crossing, muscle tensing, squatting, and the crash position are effective against vasovagal reactions solely through increases in cardiac output. Journal of Applied Physiology 99, 1697–1703. [DOI] [PubMed] [Google Scholar]
- Krediet CT, van Dijk N, Linzer M, van Lieshout JJ, Wieling W, 2002. Management of vasovagal syncope: Controlling or aborting faints by leg crossing and muscle tensing. Circulation 106, 1684–1689. [DOI] [PubMed] [Google Scholar]
- Lagi A, Cencetti S, Corsoni V, Georgiadis D, Bacalli S, 2001. Cerebral vasoconstriction in vasovagal syncope: any link with symptoms? A transcranial Doppler study. Circulation 104, 2694–2698. [DOI] [PubMed] [Google Scholar]
- Lavoie TL, Dowell ML, Lakser OJ, Gerthoffer WT, Fredberg JJ, Seow CY, Mitchell RW, Solway J, 2009. Disrupting actin-myosin-actin connectivity in airway smooth muscle as a treatment for asthma? Proceedings of the American Thoracic Society 6, 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer PM, Vaschillo E, Vaschillo B, Lu SE, Eckberg DL, Edelberg R, Shih WJ, Lin Y, Kuusela TA, Tahvanainen KU, Hamer RM, 2003. Heart rate variability biofeedback increases baroreflex gain and peak expiratory flow. Psychosomatic Medicine 65, 796–805. [DOI] [PubMed] [Google Scholar]
- Levine BD, Giller CA, Lane LD, Buckey JC, Blomquist CG, 1994. Cerebral versus systemic hemodynamics during graded orthostatic stress in humans. Circulation 90, 298–306. [DOI] [PubMed] [Google Scholar]
- Lewis T, 1932. A lecture on vasovagal syncope and the carotid sinus mechanism. BMJ 1, 873–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsitz LA, Hayano J, Sakata S, Okada A, Morin RJ, 1998. Complex demodulation of cardiorespiratory dynamics preceding vasovagal syncope. Circulation 98, 977–983. [DOI] [PubMed] [Google Scholar]
- Longo DJ, vom Saal W, 1984. Respiratory relief therapy. A new treatment procedure for the reduction of anxiety. Behavior Modification 8, 361–378 [DOI] [PubMed] [Google Scholar]
- Lumley MA, Melamed BG, 1992. Blood phobics and nonphobics: Psychological differences and affect during exposure. Behaviour Research and Therapy 30, 425–434. [DOI] [PubMed] [Google Scholar]
- Marks I, 1988. Blood-injury phobia: A review. The American Journal of Psychiatry 145, 1207–1213. [DOI] [PubMed] [Google Scholar]
- Meah MS, Gardner WN, 1994. Post-hyperventilation apnoea in conscious humans. Journal of Physiology 477, 527–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuret AE, Wilhelm FH, Ritz T, Roth WT, 2003. Breathing training in panic disorder treatment. Useful intervention or impediment to therapy? Behavior Modification 27, 731–754. [DOI] [PubMed] [Google Scholar]
- Meuret AE, Ritz T, Dahme B, Roth WT, 2004. Therapeutic use of ambulatory capnography In Gravenstein J, Jaffe M, Paulus D (Eds.), Capnography, Clinical Aspects. Cambridge University Press, Cambridge, MA. [Google Scholar]
- Meuret AE, Ritz T, Wilhelm FH, Roth WT, 2005. Voluntary hyperventilation in the treatment of panic disorder. Clinical Psychology Review, 25, 285–306. [DOI] [PubMed] [Google Scholar]
- Meuret AE, Rosenfield D, Hofmann SG, Roth WT, 2009. Changes in respiration mediate changes in fear of bodily sensations in panic disorder. Journal of Psychiatric Research 43, 634–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuret AE, White KS, Ritz T, Roth WT, Hofmann SG, Brown TA, 2006. Panic attack symptom dimensions and their relationship to illness characteristics in panic disorder. Journal of Psychiatric Research 40, 520–527. [DOI] [PubMed] [Google Scholar]
- Meuret AE, Wilhelm FH, Ritz T Roth WT, 2008. Feedback of end-tidal pCO2 as a therapeutic approach for panic disorder. Journal of Psychiatric Research 42, 560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuret AE, Wilhelm FH, Roth WT, 2001. Respiratory biofeedback-assisted therapy in panic disorder. Behavior Modification 25, 584–605. [DOI] [PubMed] [Google Scholar]
- Mitro P, Hijová E, 2006. Myocardial contractility and cardiac filling measured by impedance cardiography in patients with nitroglycerine-induced vasovagal syncope Pacing and Clinical Electrophysiology 29, 1–8. [DOI] [PubMed] [Google Scholar]
- Mitsis GD, Zhang R, Levine BD, Marmarelis VZ, 2006. Cerebral hemodynamics during orthostatic stress assessed by nonlinear modeling. Journal of Applied Physiology 101, 354–366. [DOI] [PubMed] [Google Scholar]
- Moya A, Sutton R, Ammirati F, Blanc JJ, Brignole M, Dahm JB, Deharo JC, Gajek J, Gjesdal K, Krahn A, Massin M, Pepi M, Pezawas T, Granell RR, Sarasin F, Ungar A, van Dijk JG, Walma EP, Wieling W, 2009. Guidelines for the diagnosis and management of syncope (version 2009): the Task Force for the Diagnosis and Management of Syncope of the European Society of Cardiology (ESC). European Heart Journal 30, 2631–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardi AE, Nascimento I, Valença AM, Lopes FL, Zin WA, Mezzasalma MA, Versiani M, 2008. A breath-holding challenge in panic disorder patients, their healthy first-degree relatives, and normal controls. Respiratory Physiology & Neurobiology 133, 43–47. [DOI] [PubMed] [Google Scholar]
- Norcliffe-Kaufmann LJ, Kaufmann H, Hainsworth R, 2008. Enhanced vascular responses to hypocapnia in neurally mediated syncope. Annals of Neurology 63, 288–294. [DOI] [PubMed] [Google Scholar]
- Novak V, Spies JM, Novak P, McPhee BR, Rummans TA, Low PA, 1998. Hypocapnia and cerebral hypoperfusion in orthostatic intolerance. Stroke 29,1876–1881. [DOI] [PubMed] [Google Scholar]
- Obrist PA, 1976. The cardiovascular-behavioral interaction - as it appears today. Psychophysiology 13, 95–107. [DOI] [PubMed] [Google Scholar]
- Ohi M, Chin K, Hirai M, Kuriyama T, Fukui M, Sagawa Y, Kuno K, 1994. Oxygen desaturation following voluntary hyperventilation in normal subjects. American Journal of Respiratory and Critical Care Medicine 149, 731–738. [DOI] [PubMed] [Google Scholar]
- Öhman A, Hamm AO, Hugdahl K, 2000. Cognition and the autonomic nervous system. Orienting, Anticipation, and conditioning In: Cacioppo JT, Tassinary LG, Berntson GG, (Eds.), Handbook of psychophysiology, 2nd Ed. Cambridge University Press, Cambridge, MA: pp.533–567. [Google Scholar]
- Orwin A, Duffy T, 1972. Breathe less, fear less: a new method used to treat blood phobia. Nursing Mirror and Midwives Journal 134, 25–26. [PubMed] [Google Scholar]
- Orwin A, le Boeuf A, Dovey J, James S, 1975. A comparative trial of exposure and respiratory relief therapies. Behaviour Research and Therapy 13, 205–214. [DOI] [PubMed] [Google Scholar]
- Orwin A 1973. Augmented respiratory relief. A new use of CO 2 therapy in the treatment of phobic conditions: a preliminary report on two cases. The British Journal of Psychiatry: the journal of mental science 122, 171–173. [DOI] [PubMed] [Google Scholar]
- Orwin A, 1991. Respiratory relief: a new and rapid method for the treatment of phobic states.The British Journal of Psychiatry: the journal of mental science 119, 635–637. [DOI] [PubMed] [Google Scholar]
- Öst LG, Fellenius J, Sterner U, 1991. Applied tension, exposure in vivo, and tension only in the treatment of blood phobia. Behaviour Research and Therapy 29, 561–574. [DOI] [PubMed] [Google Scholar]
- Öst LG, Hellström K, Kaver A, 1992. One versus five sessions of exposure in the treatment of injection phobia. Behaviour Research and Therapy 23, 263–282. [Google Scholar]
- Öst LG, Lindahl IL, Sterner U, Jerremalm A, 1984. Exposure in vivo versus applied relaxation in the treatment of blood phobia. Behaviour Research and Therapy 22, 205–216. [DOI] [PubMed] [Google Scholar]
- Öst LG, Sterner U, 1987. Applied tension. A specific behavioral method for treatment of blood phobia. Behaviour Research and Therapy 25, 25–29. [DOI] [PubMed] [Google Scholar]
- Öst LG, Sterner U, Fellenius J, 1989. Applied tension, applied relaxation, and the combination in the treatment of blood phobia. Behaviour Research and Therapy 27, 109–121. [DOI] [PubMed] [Google Scholar]
- Öst LG, Sterner U, Lindahl IL, 1984. Physiological responses in blood phobics. Behaviour Research and Therapy 22, 109–117. [DOI] [PubMed] [Google Scholar]
- Page AC, 1994. Blood-injury phobia. Clinical Psychology Review 14, 443–461. [Google Scholar]
- Page AC, 2003. The role of disgust in faintness elicited by blood and injection stimuli. Journal of Anxiety 17, 45–58. [DOI] [PubMed] [Google Scholar]
- Papp LA, Klein DF, Gorman JM, 1993. Carbon dioxide hypersensitivity, hyperventilation, and panic disorder. The American Journal of Psychiatry 150, 1149–1157. [DOI] [PubMed] [Google Scholar]
- Parry SW, Kenny RA, 2002. Vasovagal syncope masquerading as unexplained falls in an elderly patient. Canadian Journal of Cardiology 18, 757–758. [PubMed] [Google Scholar]
- Perna G, Battaglia M, Garberi A, Arancio C, Bertani A, Bellodi L, 1994. Carbon dioxide/oxygen challenge test in panic disorder. Psychiatry Research 52, 159–171. [DOI] [PubMed] [Google Scholar]
- Przybyłowski T, Bangash MF, Reichmuth K, Morgan BJ, Skatrud JB, Dempsey JA, 2003. Mechanisms of the cerebrovascular response to apnoea in humans. Journal of Physiology 548:323–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz T, Dahme B, DuBois AB, Folgering H Fritz GK, Harver AR, Kotses H, Lehrer PM, Ring C, Steptoe A, Van de Woestijne KP, 2002. Guidelines for mechanical lung function measurements in psychophysiology. Psychophysiology 39, 546–567. [DOI] [PubMed] [Google Scholar]
- Ritz T, Dahme B, 2006. Implementation and interpretation of heart rate variability measures in psychosomatic medicine: Practice against better evidence? Psychosomatic Medicine 68, 617–627. [DOI] [PubMed] [Google Scholar]
- Ritz T, Thöns M, Dahme B, 2001. Modulation of respiratory sinus arrhythmia by respiration rate and volume: Stability across postures and volume variations. Psychophysiology 38, 858–862. [PubMed] [Google Scholar]
- Ritz T, Wilhelm FH, Gerlach AL, Kullowatz A, Roth WT, 2005. End-tidal pCO2 in blood phobics during viewing of emotion- and disease-related films. Psychosomatic Medicine 67, 661–668. [DOI] [PubMed] [Google Scholar]
- Ritz T, Wilhelm FH, Meuret AE, Gerlach AL, Roth WT, 2005. Vasovagal syncope in blood phobia: Evidence for a biphasic response? Depression and Anxiety 22, 229 (Abstract) [Google Scholar]
- Ritz T, Wilhelm FH, Meuret AE, Gerlach AL, Roth WT, in press. Airway response to emotion- and disease-specific films in asthma, blood phobia, and health. Psychophysiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz T, Wilhelm FH, Meuret AE, Gerlach AL, Roth WT, 2009. Do blood phobia patients hyperventilate during exposure by breathing faster, deeper, or both? Depression and Anxiety 26, E60–E67. [DOI] [PubMed] [Google Scholar]
- Romano J, Engel GL, 1945. Studies of syncope: III. Differentiation between Vasodepressor and Hysterical Fainting. Psychosomatic Medicine 7, 3–15. [Google Scholar]
- Roth WT, 2005. Physiological markers for anxiety: panic disorder and phobias. International Journal of Psychophysiology 258, 190–198. [DOI] [PubMed] [Google Scholar]
- Roth WT, Wilhelm FH, Pettit D, 2005. Are current theories of panic falsifiable? Psychological Bulletin 131, 171–192. [DOI] [PubMed] [Google Scholar]
- Roth WT, Wilhelm FH, Trabert W, 1998. Voluntary breath holding in panic and generalized anxiety disorders. Psychosomatic Medicine 60, 671–679. [DOI] [PubMed] [Google Scholar]
- Saadjian AY, Gerolami V, Giorgi R, Mercier L, Berge-Lefranc JL, Paganelli F, Ibrahim Z, By Y, Guéant JL, Lévy S, Guieu RP, 2009. Head-up tilt induced syncope and adenosine A2A receptor gene polymorphism. European Heart Journal 30,1510–1515. [DOI] [PubMed] [Google Scholar]
- Saadjian AY, Lévy S, Franceschi F, Zouher I, Paganelli F, Guieu RP, 2002. Role of endogenous adenosine as a modulator of syncope induced during tilt testing. Circulation 30, 106, 569–574. [DOI] [PubMed] [Google Scholar]
- Samniah N, Sakaguchi S, Ermis C, Lurie KG, Benditt DG, 2004. Transient modification of baroreceptor response during tilt-induced vasovagal syncope. Europace 6, 48–54. [DOI] [PubMed] [Google Scholar]
- Sarlo M, Palomba D, Angrilli A, Stegagno L, 2002. Blood phobia and spider phobia: Two specific phobias with different autonomic cardiac modulations. Biological Psychology 60, 91–108. [DOI] [PubMed] [Google Scholar]
- Sarlo M, Buodo G, Munafò M, Stegagno L, Palomba D, 2008. Cardovascular dynamics in blood phobia: Evidence for a key role of sympathetic activity in vulnerability to syncope. Psychophysiology 45, 1038–1045. [DOI] [PubMed] [Google Scholar]
- Sawchuk CN, Lohr JM, Westendorf DH, Meunier SA, Tolin DF, 2002. Emotional responding to fearful and disgusting stimuli in specific phobics. Behaviour Research and Therapy 40, 1031–1046. [DOI] [PubMed] [Google Scholar]
- Sawchuk CN, Lohr JM, Tolin DF, Lee TC, & Kleinknecht RA, 2000. Disgust sensitivity and contamination fears in spider and blood-injection-injury phobias. Behaviour Research and Therapy 38, 753–762. [DOI] [PubMed] [Google Scholar]
- Schmidt NB, Woolaway-Bickel K, Trakowski J, Santiago H, Storey J, Koselka M, Cook J, 2000. Dismantling cognitive-behavioral treatment for panic disorder: questioning the utility of breathing retraining. Journal of Consulting and Clinical Psychology 68, 417–424. [DOI] [PubMed] [Google Scholar]
- Serrador JM, Hughson RL, Kowalchuk JM, Bondar RL, Gelb AW, 2006. Cerebral blood flow during orthostasis: role of arterial CO2. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology 290, R1087–1093. [DOI] [PubMed] [Google Scholar]
- Shen WK, Hammill SC, Munger TM, Stanton MS, Packer DL, Osborn MJ, Wood DL, Bailey KR, Low PA, Gersh BJ, 1996. Adenosine: potential modulator for vasovagal syncope. Journal of the American College of Cardiology 28, 146–154. [DOI] [PubMed] [Google Scholar]
- Sledge WH, 1978. Antecedent psychological factors in the onset of vasovagal syncope. Psychosomatic Medicine 40, 568–579. [DOI] [PubMed] [Google Scholar]
- Stephenson JBP, 1978. Reflex anoxic seizures (“white breath-holding”): non-epileptic vagal attacks. Archives of Disease in Children 53, 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A Wardle J, 1988. Emotional fainting and the psychophysiologic response to blood and injury: Autonomic mechanisms and coping strategies. Psychosomatic Medicine 50, 402–417. [DOI] [PubMed] [Google Scholar]
- Stinson FS, Dawson DA, Patricia Chou S, Smith S, Goldstein RB, June Ruan W, Grant BF, 2007. The epidemiology of DSM-IV specific phobia in the USA: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychological Medicine 37, 1047–1059. [DOI] [PubMed] [Google Scholar]
- Sung RY, Du ZD, Yu CW, Yam MC, Fok TF, 2000. Cerebral blood flow during vasovagal syncope induced by active standing or head up tilt. Archive of Disease in Children 82, 154–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ten Harkel AD, van Lieshout JJ, Wieling W, 1994. Effects of leg muscle pumping and tension on orthostatic arterial pressure: A study in normal subjects and patients with autonomic failure. Clinical Science 87, 553–558. [DOI] [PubMed] [Google Scholar]
- Thijs RD, Wagenaar WA, Middelkoop HA, Wieling W, van Dijk JG, 2008. Transient loss of consciousness through the eyes of a witness. Neurology 71, 1713–1718. [DOI] [PubMed] [Google Scholar]
- Thijs RD, Wieling W, Kaufmann H, van Dijk G, 2004. Defining and classifying syncope. Clinical Autonomic Research: Official journal of the Clinical Autonomic Research Society 14 (Suppl. 1), 4–8. [DOI] [PubMed] [Google Scholar]
- Thompson A, 1999. Cognitive-behavioural treatment of blood-injury-injection phobia: A case study. Behaviour Change 16, 182–190. [Google Scholar]
- Thyer BA, Curtis GC, 1985. On the diphasic nature of vasovagal fainting associated with blood-injury-illness phobia. The Pavlonian Journal of Biological Science 20, 84–87. [DOI] [PubMed] [Google Scholar]
- Tolin DF, Lohr JM, Sawchuk CN, Lee TC, 1997. Disgust and disgust sensitivity in blood-injection-injury and spider phobia. Behaviour Research and Therapy 35, 949–953. [DOI] [PubMed] [Google Scholar]
- Turpin G, 1986. Effects of stimulus intensity on autonomic responding: the problem of differentiating orienting and defense reflexes. Psychophysiology 23, 1–14. [DOI] [PubMed] [Google Scholar]
- van den Elshout FJJ, van Herwaarden CLA, Folgering HTM, 1991. Effects of hypercapnia and hypocapnia on respiratory resistance in normal and asthmatic subjects. Thorax 46, 28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hout MA, Hoekstra R, Arntz A, Christiaanse M, Ranschaert W, Schouten E, 1992. Hyperventilation is not diagnostically specific to panic patients. Psychosomatic Medicine 53, 182–191. [DOI] [PubMed] [Google Scholar]
- van Dijk N, de Bruin IG, Gisolf J, de Bruin-Bon HA, Linzer M, van Lieshout JJ, Wieling W, 2005. Hemodynamic effects of leg crossing and skeletal muscle tensing during free standing in patients with vasovagal syncope. Journal of Applied Physiology 98, 584–590. [DOI] [PubMed] [Google Scholar]
- van Lieshout JJ, ten Harkel AD, Wieling W, 1992. Physical manoeuvres for combating orthostatic dizziness in autonomic failure. Lancet 339, 897–898. [DOI] [PubMed] [Google Scholar]
- van Lieshout JJ, Wieling W, Karemaker JM, 1997. Neural circulatory control in vasovagal syncope. Pacing and Clinical Electrophysiology 20, 753–763. [DOI] [PubMed] [Google Scholar]
- van Lieshout JJ, Wieling W, Karemaker JM, Eckberg DL, 1991. The vasovagal response. Clinical Science 81, 575–586 [DOI] [PubMed] [Google Scholar]
- van Lieshout JJ, Wieling W, Karemaker JM, Secher NH, 2003. Syncope, cerebral perfusion, and oxygenation. Journal of Applied Physiology 94, 833–848. [DOI] [PubMed] [Google Scholar]
- van Overveld WJ, de Jong PJ, Peters ML, 2009. Digestive and cardiovascular responses to core and animal-reminder disgust. Biological Psychology 80,149–157. [DOI] [PubMed] [Google Scholar]
- Vingerhoets AJ, 1984. Biochemical changes in two subjects succumbing to syncope. Psychosomatic Medicine 46:95–103. [DOI] [PubMed] [Google Scholar]
- Vögele C, Coles J, Wardle J, Steptoe A, 2003. Psychophysiologic effects of applied tension on the emotional fainting response to blood and injury. Behaviour Research and Therapy 41, 139–155. [DOI] [PubMed] [Google Scholar]
- Weissler AM, Warren JV, Estes EH, Mcintosh HD, Leonard JJ, 1957. Vasodepressor syncope; factors influencing cardiac output. Circulation 15, 875–882. [DOI] [PubMed] [Google Scholar]
- White C, Sellwood W, 1995. Cognitive factors in the maintenance of injection phobia. Behavioural and Cognitive Psychotherapy 23, 57–61. [Google Scholar]
- Wieling W, van Lieshout JJ, van Leeuwen AM, 1993. Physical manoeuvres that reduce postural hypotension in autonomic failure. Clinical Autonomic Research: The Official Journal of Clinical Autonomic Research Society 3, 57–65. [DOI] [PubMed] [Google Scholar]
- Wilhelm FH, Gerlach AL, Roth WT, 2001. Slow recovery from voluntary hyperventilation in panic disorder. Psychosomatic Medicine 63, 638–649. [DOI] [PubMed] [Google Scholar]
- Wilhelm FH, Trabert W, Roth WT, 2001. Physiologic instability in panic disorder and generalized anxiety disorder. Biological Psychiatry 49, 596–605. [DOI] [PubMed] [Google Scholar]
- Wilhelm FH, Grossman P, Coyle MA, 2004. Improving estimation of cardiac vagal tone during spontaneous breathing using a paced breathing calibration. Biomedical Science and Instruments 40, 317–324. [PubMed] [Google Scholar]
- Wolpe J, & Lang PJ (1964). A fear schedule for use in behavior therapy. Behaviour Research and Therapy, 2, 27–30. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zuckerman JH, Levine BD, 1998. Deterioration of cerebral autoregulation during orthostatic stress: insights from the frequency domain. Journal of Applied Physiology 85, 1113–1122. [DOI] [PubMed] [Google Scholar]