Abstract
Prader–Willi syndrome (PWS) is a complex genetic imprinting disorder characterized by childhood obesity, short stature, hypogonadism/hypogenitalism, hypotonia, cognitive impairment, and behavioral problems. Usually PWS occurs sporadically due to the loss of paternally expressed genes on chromosome 15 with the majority of individuals having the 15q11-q13 region deleted. Examples of familial PWS have been reported but rarely. To date 13 families have been reported with more than one child with PWS and without a 15q11-q13 deletion secondary to a chromosome 15 translocation, inversion, or uniparental maternal disomy 15. Ten of those 13 families were shown to carry microdeletions in the PWS imprinting center. The microdeletions were found to be of paternal origin in nine of the ten cases in which family studies were carried out. Using a variety of techniques, the microdeletions were identified in regions within the complex SNRPN gene locus encompassing the PWS imprinting center. Here, we report the clinical and genetic findings in three adult siblings with PWS caused by a microdeletion in the chromosome 15 imprinting center inherited from an unaffected father that controls the activity of genes in the 15q11-q13 region and summarize the 13 reported cases in the literature.
Keywords: chromosome 15, familial imprinting center, microdeletion, Prader-Willi syndrome (PWS)
1 |. INTRODUCTION
Prader–Willi syndrome (PWS) is a complex genomic imprinting disorder caused by a number of different genetic mechanisms leading to the loss of gene expression from the paternal chromosome 15. The most common defect is a deletion of the chromosome 15q11-q13 region historically reported to occur in about 70% of cases, followed by maternal uniparental disomy (both chromosome 15s inherited from the mother) in 30%. The remaining patients have an imprinting center defect either due to a microdeletion or epimutation. Two typical PWS deletion subtypes (Type I or Type II) are commonly reported, while atypical deletions which may be larger or smaller in size than the typical deletions are less common. More clinical differences are seen in those with the typical larger 15q11-q13 Type I deletion compared with the smaller Type II deletion or maternal disomy 15 (Bittel, Kibiryeva, & Butler, 2006; Butler, Bittel, Kibiryeva, Talebizadeh, & Thompson, 2004; Zarcone et al., 2007). PWS was the first reported human disorder found to be caused by errors in the differential expression of genes depending on the parent of origin or genomic imprinting.
PWS is characterized by failure to thrive and feeding difficulties in infancy and later, an uncontrollable appetite leading to obesity. Short stature, hypogonadism, hypotonia, and cognitive/behavior problems are common features. The incidence is one in 10,000–30,000 with most cases due to sporadic events; however, 13 familial cases of PWS have been reported (Bingeliene, Shapiro, & Chung, 2015; Burke, Kousseff, Gleeson, O’Connell, & Delvin, 1987; Buiting et al., 2000; Clarren & Smith, 1977; Ishikawa, Kanayama, & Wada, 1987; Ishikawa, Kibe, & Wada, 1996; Jancar, 1971; Lubinsky et al., 1987; McEntagart, Webb, Hardy, & King, 2000; Ming et al., 2000; Ohta et al., 1999; Orstavik et al., 1992; Reis et al., 1994; Teshima et al., 1996). Historically, the first familial cases were reported in 1971 in two affected brothers with PWS, one having a dizygotic twin sister without PWS (Jancar, 1971). They had normal chromosome studies at that time prior to the discovery of the chromosome 15q11-q13 deletion reported in 1981 (Ledbetter et al., 1981). The mother was reported to have a below average intelligence but their father and two other siblings were reported as physically and cognitively normal.
In the 13 families reported, the subjects met the major and minor criteria for a clinical diagnosis of PWSas proposed by Holm et al. (1993). The major criteria are neonatal hypotonia, feeding problems, rapid weight gain, characteristic facial features (narrow face, down-turned mouth), hypogonadism, developmentaldelay, hyperphagia, and cytogenetic abnormalitiesin the 15q11-q13 region. The minor criteria are decreased fetal movement, characteristic behavioral problems (tantrums, obsessive-compulsive behavior), sleep disturbances, short stature, hypopigmentation, small hands and/ or feet, eye abnormalities, thick saliva, speech problems, and skin picking. Ten of the 13 familial cases with PWS in the literature were found to have microdeletions affecting the imprinting center (Bingeliene, Shapiro, & Chung, 2015; Buiting et al., 1995; Buiting et al., 2000; Ishikawa et al., 1987, 1996; McEntagart, Webb, Hardy, & King, 2000; Ming et al., 2000; Ohta et al., 1999; Orstavik et al., 1992; Reis et al., 1994; Teshima et al., 1996). The microdeletions were passed to the subjects from the paternal grandmother through their father. Prader-Willi syndrome arises in the grandchild because the microdeletion is passed directly to the offspring by the father. The other three PWS families showed no chromosome 15q deletion (Burke et al., 1987; Clarren & Smith, 1977; Jancar, 1971). However, microdeletions in those subjects cannot be ruled out as analysis of the PWS imprinting region was not carried out. Here, we report three adult siblings with PWS caused by a microdeletion in the PWS imprinting center within the 15q11-q13 region inherited from the father and summarize relevant literature.
2 |. CLINICAL REPORTS
2.1 |. Subjects
2.1.1 |. Sibling ABCF31
Sibling ABCF31 is a 40-year-old female with a history of PWS confirmed at the age of 23 years via fluorescence in situ hybridization (FISH) using a 15q11-q13 SNRPN probe. She was born 4 weeks premature to non-consanguineous parents. There were no complications during the pregnancy but decreased intrauterine movements were noted. She weighed 2.36 kg (2nd percentile) with APGAR scores of 5 and 6. She was hypotonic, essentially never cried, and had a poor suck but did not require tube feedings. She remained hospitalized for 10 days after delivery secondary to elevated bilirubin levels and feeding issues. She walked at 12 months and her verbal skills were considered normal. She had mild intellectual development delay with full-scale IQ testing of 59 at the age of 17 years. Her appetite began to increase at 4 years of age and was noted to be overweight by 7 years. Her maximum weight was 138.8 kg (99th percentile) at the age of 26 years. She is currently 154.94 cm (10th percentile) in height and weighs 72.57 kg (82nd percentile) while in residential care. She has a history of significant food-seeking behaviors and has stolen food at home and from stores in the community in the past.
She was diagnosed in the past with bipolar affective disorder type I, impulse-control disorder, depressive disorder, not otherwise specified, mood disorder, not otherwise specified, personality change secondary to PWS, and nonverbal learning disorder. Her history of mood symptoms noted at 19 years of age included argumentativeness with manipulative behaviors. She had baseline demeanor changes with agitation, restlessness, irritable mood, and increased rate of speech and was admitted to the Prader–Willi Homes of Oconomowoc (PWHO) in Wisconsin at 26 years of age. She has a history of anxiety, skin picking, asking repetitive questions, verbal and physical aggression, agitation, and rapid mood shifts. She made multiple suicidal threats in the past, as well as threats to elope during adulthood. She also drank a bottle of body lotion during this one year period but did not require hospitalization following the ingestion. She was appropriately monitored and treated at PWHO by clinical staff under the care of a psychiatrist. Her family noted social withdrawal, sadness, and increased sleep but she was not hospitalized for psychiatric problems. Her current DSM V psychiatric diagnoses include personality change secondary to PWS, bipolar affective disorder type I, skin picking disorder, intellectual developmental disorder-mild, and learning disorder.
Additionally, she was diagnosed with morbid obesity, hypertension, and diabetes mellitus type II; all of which resolved with weight loss and exercise. She has also suffered from chronic sinusitis, chronic constipation, acid reflux, enuresis, osteopenia, borderline thrombocytopenia, and irregular menstrual spotting while on oral contraceptives. Her medical history includes a nondisplaced oblique fracture of the base of a toe, levoscoliosis of the lumbar spine-mild to moderate leftward curve, moderate S-shaped thoracolumbar spine, leftward curvature of mid-upper lumbar spine with subtle rotary component, a lumbar L1 compression fracture, and degenerative disk disease.
2.2 |. Sibling ABCM26
Sibling ABCM26 is the 35-year-old brother of Sibling ABCF31 and ABCM51. He was diagnosed with PWS at 18 years of age. His mother received fertility drugs prior to conception. He was born 7 weeks premature with a double footling breech presentation but delivered vaginally. No complications were reported during the pregnancy. He weighed 2.18 kg (1st percentile) at birth, was hypotonic and cried little, with a poor suck and feeding problems. He required orogastric tube feeds for the first 8 weeks of life and remained in the neonatal intensive care unit for 6 weeks after birth. A systolic heart murmur was identified shortly after birth which resolved by 2 years of age. He had seizure-like activity on one occasion shortly after birth with a negative neurologic workup and without reoccurrence.
He rolled over independently at 5 months and walked by 12 months. He was able to produce four word sentences by 16 months; toilet trained by 3 years and began reading at a normal age. He had mild intellectual developmental delay with full-scale IQ testing of 65 at the age of 18 years. Significant hypotonia continued and increased appetite began at 3 years of age. His weight gain increased by 5 years of age and was overweight by 7 years. His maximum weight of 120.2 kg (>99th percentile or 2.7 Z-score) occurred at 18 years of age. His current weight is 82.37 kg (80th percentile) with a current height of 157 cm (< 1st percentile or −2.7 Z-score).
He was diagnosed with major depressive disorder, depressive disorder, not otherwise specified, anxiety disorder, not otherwise specified, posttraumatic stress disorder, obsessive-compulsive disorder, oppositional defiant disorder, intermittent explosive disorder, personality change secondary to a PWS, nonverbal learning disorder and a provisional diagnosis of mixed receptive, and expressive learning disorder. His parents became concerned about depressive symptoms including social withdrawal and sadness between the ages of 11 and 12 years. He has an extensive history of self-harm which began at 13 years of age including inserting objects under his skin, gouging, burning, and biting himself at times to the point of tissue loss. A suicide attempt occurred at 17 years of age when he overdosed on acetaminophen and Motrin. He later attempted suicide by hanging.
He has had multiple previous psychiatric hospitalizations with four admissions to The Children’s Institute (TCI) in Pittsburgh, PA. His first psychiatric hospitalization was at 16 years of age followed by three additional psychiatric hospitalizations at 17 years. At the age of 18, he was admitted to TCI secondary to excessive food-seeking behavior, morbid obesity, and ongoing depressive symptoms with suicidal ideation. A second admission occurred at 19 years of age for 45 days secondary to significant depressive symptoms including a self-inflicted laceration. At discharge, he was referred to Prader–Willi Homes of Oconomowoc (PWHO) for residential care. He had two brief hospitalizations at Winnebago Mental Health Institute in Wisconsin (WMHI) at the age of 19 years for depressive symptoms and suicidal ideation but then his mood appeared to stabilize without major incidents of stealing or self-harm until the age of 20 years. He was initially admitted to Rogers Memorial Hospital after jabbing a pen into his arm that required medical attention as well as depressive symptoms and suicidal ideation. While at Rogers Memorial, he stabbed himself with a fork and was subsequently transferred back to WMHI. He remained there for 2 months. He had another admission to WMHI at 21 years of age but was transferred to TCI where he was hospitalized for 2 months for the third time secondary to significant depression, suicidal ideation, and self-mutilation. He was admitted again at 21 years of age to TCI for significant depressive symptoms, suicidal ideation, and self-mutilation. He also has a history of pulling head and eyebrow hair in secret or when angry.
He has rare verbal outbursts, but displays intermittent skin picking and rectal digging. He has a history of significant food-seeking behaviors, consuming inedible objects (cigarette butts) and has stolen edible items from stores, peers, and also his family. He broke into a freezer at his parents’ home with his brother and stole food on at least one occasion. He also has a history of chronic kidney disease stage III with an atrophic left kidney, degenerative disc disease, chronic constipation with a history of rectal digging, enuresis, scoliosis, status post abdominal, and inguinal hernia repairs, osteoporosis, anemia, gastroesophageal reflux disease, positive PPD (purified protein derivative) but further workup was negative for tuberculosis and he had two subarachnoid hemorrhages at 25 years of age, secondary to a fall and striking head on concrete. He required 6 weeks of inpatient rehabilitation and displayed a possible change in articulation since the injury, per parents. His current DSM V psychiatric diagnoses include personality change secondary to PWS, other specified depressive disorder, other specified obsessive-compulsive, and related disorder (repeatedly placing objects under skin and rectal picking), borderline intellectual function, learning, and language disorder and other medication-induced movement disorder (neuroleptic-induced Parkinsonism and potential lithium contribution to bilateral hand tremor).
2.3 |. Sibling ABCM51
Sibling ABCM51 is the33-year-old brother of Sibling ABCF31 and Sibling ABCM26. His parents were 33 years old at conception. His mother was placed on bed-rest for the last trimester, secondary to history of previous premature birth. He was born 3 weeks premature and weighed 2.41 kg (3rd percentile) with APGAR scores of 7 and 9. At birth he was hypotonic, but had a suck reflex and did not require tube feedings or other feeding assistance. He remained hospitalized for 6 days after birth secondary to elevated bilirubin levels. He was first diagnosed with PWS at the age of 16 years along with his two older siblings. He had a maximum weight of 161.4 kg (>99th percentile or 3.5 Z-score) at 18 years and currently weighs 88.9 kg (89th percentile, and stands at 167.64 cm (11th percentile). He has a history of significant food-seeking behaviors and has stolen edible items from his family at home.
He rolled over independently by 3 months, crawled at 8 months, and walked at 11 months. He has the most delayed verbal/language skills of the three siblings with difficulties in word retrieval. He was toilet trained by 2.75 years of age. He had early intervention services: PT, OT, and speech therapy, beginning at approximately 4 years old until kindergarten. He had mild intellectual developmental delay with full-scale IQ testing of 70 at the age of 16 years. His appetite began to increase at 5 years of age and at 9 years was noted to be overweight. His parents stated that he was more physically active and stronger than his two older siblings and was more active in utero and continuing through childhood. He was more adventurous about exploring his surroundings as a toddler, more likely to try new things and to take more physical risks than his siblings. He is more physically active than the typical individual with PWS as he participated in downhill skiing and wrestling in middle school.
His parents became concerned at approximately 12 years of age, due to anger, oppositional defiant disorder symptoms, and anxiety. His psychiatric symptoms were not as severe as his two older siblings; however, he was admitted to The Children’s Institute (TCI) in Pittsburgh, PA at 18 years of age. His maximum weight occurred prior to this hospitalization. At discharge from TCI he was then admitted to PWHO, which was his first group home admission.
He has an extensive history of verbal aggression, threatening group home staff (particularly female staff) and family members. He has significant tantrums, rare impulsive suicidal ideation, minimal skin picking, hoarding, and elopement. He has not been hospitalized solely for psychiatric reasons nor has he had a history of suicide attempts or psychotic symptoms. He was previously diagnosed with attention deficit hyperactivity disorder, oppositional defiant—disorder, anxiety disorder secondary to a general medical condition, personality change secondary to PWS, nonverbal learning disorder − mixed, expressive, and receptive language disorder, borderline intellectual functioning and mild intellectual developmental delay. His current DSM V psychiatric diagnoses include personality change secondary to PWS, generalized anxiety disorder, oppositional defiant disorder, attention deficit hyperactivity disorder, borderline intellectual function, and learning and language disorder.
At 3 years of age, he underwent surgery for bilateral undescended testes and an inguinal hernia. His medical history also includes an overactive bladder and urinary incontinence, idiopathic thrombocytopenic purpura, lower back pain, patellofemoral chondrosis with resultant chronic left knee pain, chronic constipation, gastroesophageal reflux disease (GERD), and sleep apnea. He was prescribed CPAP for sleep apnea but refused treatment. He also has diabetes mellitus type II and chronic nasal congestion.
3 |. GENETIC TESTING METHODS
3.1 |. DNA collection and extraction
All individuals agreed to participate and signed informed consent forms approved by the institutional review board at the University of Kansas Medical Center before entry into the study. Saliva was collected using a Saliva DNA Collection and Preservation Device (Norgen Biotek Corporation, Thorold, Ontario, CA) and genomic DNA isolated and purified using a Saliva DNA Isolation Kit (Norgen Biotek Corporation) according to manufacturer’s protocol.
3.2 |. Methylation specific-multiplex ligation probe amplification (MS-MLPA®)
MS-MLPA reagents and kits were obtained from MRC-Holland (Amsterdam, the Netherlands) and MS-MLPA carried out on all three siblings and their parents using the ME028-B2 kit containing sequence-specific probes along the length of the 15q11.2-q13 region. The B2 kit contains 48 MLPA probes for copy number detection and methylation status using methylation-sensitive restriction enzymes. Approximately, 50 ng of genomic DNA was used for each of the MS-MLPA reactions and carried out according to manufacturer’s instructions. The MS-MLPA PCR products were sent to GENEWIZ (Frederick, MD) for fragment analysis, a commercially available sequencing laboratory for a fee for service.
3.3 |. High-resolution microarrays
High-resolution microarray analysis was carried out on subjects ABCM51 and ABCF31 using an Affymetrix™ Genome-Wide Human SNP Array 6.0 (Affymetrix, Inc., Santa Clara, CA) performed on 3 μg of intact genomic DNA undertaken at the Genomics Core facility at KUMC. Genomic DNA from ABCM51 was also analyzed using an Infinium Omni5Exome-4 BeadChip array (Illumina, Inc., San Diego, CA) by Victorian Clinical Genetics Services at the Murdoch Children’s Research Institute in Victoria, Australia.
3.4 |. Digital droplet PCR (ddPCR)
Digital droplet PCR (ddPCR) studies were performed on the subjects and their parents in triplicate to confirm the location of the imprinting center deletion. Three different primers were designed and FAM-tagged probe sets were located in the PWS imprinting center on chromosome 15 (IC1, IC2, and SRNPN exon 1). The primers and FAM tagged probe sets were purchased from Integrated DNA Technologies (Coralville, IA). We also purchased validated GABRβ3 HEX ddPCR copy number assay probe from Bio-Rad (UniqueAssay IDdHsaCP2506453-Hercules, CA)touse as a reference, as it is outside the PWS imprinting center but in the 15q11-q13region. Prepared reactions were undertaken inthe Genomics Core at the University of Kansas–Lawrence. Data were analyzed using the QuantaSoft Analysis Pro software available from Bio-Rad.
4 |. RESULTS AND DISCUSSION
Thirteen families were previously reported in the literature with more than one child with PWS and without a recognized 15q11-q13 deletion secondary to a chromosome 15 translocation, inversion, or uniparental maternal disomy 15 (Table 1). Three of the families were diagnosed with PWS before the use of high-resolution chromosome analysis. However, when data were available in the literature reports, the subjects met the major and minor consensus diagnostic criteria for PWS (Table 2). Jancar (1971) reported on two brothers with PWS; both were hypotonic at birth, had developmental delays, hypogonadism, and acromicria, were of short stature and had obesity at a young age. The older brother was reported to have feeding difficulties, high myopia, and dry oral mucosa and to be restless at times. His diagnostic score was 7.5 and younger brother’s score was 5.
TABLE 1.
Diagnostic tests and results from studies of 13 cases of familial Prader-Willi syndrome
| Reference | Subjects | Testing/Results |
|---|---|---|
| Jancar (1971) | Two PWS brothers clinically diagnosed. One had an unaffected female dizygotic twin. | Chromosomal investigation displayed normal male karyotypes. Done prior to high-resolution chromosome analysis. |
| 1. Hall and
Smith (1972) 2. Clarren and Smith (1977) |
Two PWS male maternal first cousins clinically diagnosed. | Diagnosed clinically only. Done prior to high-resolution chromosome analysis. |
| Burke et al. (1987) | Three PWS sisters clinically diagnosed and four unaffected siblings. | All three affected sisters, an unaffected sister and their mother, had normal karyotypes by high-resolution chromosome analysis. |
| 1. Lubinsky et
al. (1987) 2. Buiting et al. (1995) |
1. Four PWS siblings clinically diagnosed (two females, one died at 10 months from pneumonia, and two males). 2. Two affected brothers (family “U“ in Buiting et al., 1995). | 1. High-resolution chromosome analysis showed normal karyotypes for all siblings and parents. 2. Quantitative DNA dosage analysis for 5’ SNRPN exons showed exons −1 and 0 deleted but exon 1 was intact in both male siblings and father. |
| 1. Ishikawa et
al. (1987) 2. Ishikawa et al. (1996) |
1. Two PWS sisters and one unaffected brother. 2. Two siblings, no sex given (family “PWS-J“ in Ohta et al., 1999). | 1. One sister with a normal karyotype and one with a terminal deletion of distal Xq. 2. SNRPN cosmid showed exons 4–10 deleted. Fathers’ DNA not available. |
| 1. Orstavik et
al. (1992) 2. Sutcliffe et al. (1994) |
Family “O“. PWS brother and sister with an older brother who died at 7 days of age from respiratory distress. | FISH analysis with SNRPN cosmid showed SNRPN deletion in PWS siblings, father and paternal grandmother. |
| Reis et al. (1994) | Family “S“. PWS brother and sister. | Densitometry studies showed proximal SNRPN deletion in both siblings and their paternal grandmother. |
| Teshima et al. (1996) | Two PWS brothers (“PWS-T“ in Ohta et al., 1999). | FISH analysis with SNRPN cosmid showed SNRPN deleted in siblings and father. |
| Ohta et al. (1999) | Family “PWS-P“. PWS male and female siblings. | Both had normal karyotypes. FISH analysis with SNRPN probe showed SNRPN deleted in siblings and father. |
| Ming et al. (2000) | PWS male and female paternal first cousins. | FISH analysis with SNRPN cosmid showed SNRPN deleted in the cousins, their fathers and two paternal aunts. |
| McEntagart et al. (2000) | PWS brother and sister. | Microsatellite analysis showed SNRPN deletion in the siblings, their father and paternal grandmother. |
| Buiting et al. (2000) | Two PWS sisters. | Southern blot analysis identified deletion in the imprinting center in the siblings, their father, an aunt, an uncle and their paternal grandmother. |
| Bingeliene et al. (2015) | Two PWS brothers and their PWS half-sister who died of obstructive sleep apnea. | All three carried a 15q11-q13 paternal chromosome deletion. Reported paternal grandfather had a history of PWS (unconfirmed). |
The deletions were paternal in origin in which genetic studies were completed in the above cases. PWS = Prader-Willi syndrome. FISH = fluorescence in situ hybridization.
TABLE 2.
Consensus diagnostic criteria for Prader–Willi syndrome in individuals with imprinting defects
| Three Siblings with Prader-Willi Syndrome | Bingeliene et al. [2015] | Buiting et al. [2000] | McEntagart et al. [2000] | Ming et al. [2000] | Ohta et al.[1999] | Teshima et al. [1996] | Reis et al. [1994] | Orstavik et al. [1992]; Sutcliffe et al. [1994] | Ishikawa et al. [1987] and [1996] | Lubinsky et al. [1987]; Buiting et al. [1995] | Burke et al. [1987] | Hall & Smith [1972]; Clarren and Smith [1977] | Jancar [1971] | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ABC F31 | ABC M26 | ABC M51 | M 20yr | M 23yr | F* 24yr | F 5yr | F 2yr | M 10yr | F 6yr | M 0.5yr | F 16yr | F 4yr | M 3yr | M 7yr | M 12yr | M 29yr | F 26yr | M* 0.1 yr | M 12yr | F 7yr | F 22yr | F 19yr | F* 0.8yr | M 26yr | F 27yr | M 21 yr | F 26yr | F 22yr | F 26yr | M 17yr | M 22yr | M 22yr | M 20yr | |
| Major Criteria | ||||||||||||||||||||||||||||||||||
| Neonatal hypotonia | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||
| Feeding problems | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||||||||
| Excessive or rapid weight gain | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||||||||||||||||||
| Characteristic face (narrow face, almond shaped eyes, down turned mouth) | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||||||
| Hypogonadism | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||||||
| Developmental delay | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |||||
| Hyperphagia | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |||||||||||||||||
| Chromosome 15q 11-q13 abnormality | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||||||
| Minor Criteria | ||||||||||||||||||||||||||||||||||
| Decreased fetal movement | + | + | + | + | + | + | + | |||||||||||||||||||||||||||
| Characteristic behavior (tantrums, violent outbursts, stealing) | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |||||||||||||||||||
| Sleep disturbances | + | + | + | + | + | + | ||||||||||||||||||||||||||||
| Short stature | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||||||||||
| Hypopigmentation compared to family | ||||||||||||||||||||||||||||||||||
| Small hands/feet | + | + | + | + | + | + | + | + | + | + | + | + | + | |||||||||||||||||||||
| Eye findings (esotropia, myopia) | + | + | + | + | + | + | ||||||||||||||||||||||||||||
| Thick saliva | + | + | + | + | + | + | + | |||||||||||||||||||||||||||
| Speech problems | + | + | + | |||||||||||||||||||||||||||||||
| Skin picking | + | + | + | + | + | + | + | + | + | |||||||||||||||||||||||||
| Total** | 10 | 10 | 9.5 | 10 | 10 | 2 | 7.5 | 6.5 | 9 | 8.5 | 7 | 7.5 | 6 | 7 | 4 | 7 | 5.5 | 5.5 | 1.5 | 10 | 9 | 7.5 | 7 | 3 | 9.5 | 9 | 9 | 8 | 7.5 | 7.5 | 7 | 6 | 7.5 | 5 |
Died prior to data analysis or incomplete data. M= male; F = female
Diagnostic scoring criteria from Holm et al. (1993). Major criteria are worth one point each. Minor criteria are worth half point. A total score of five points are required for diagnosis of children with PWS at 3 years of age or younger. Four of which should come from the major group. A total score of eight is necessary for diagnosis of PWS at 3 years of age to adulthood. Five or more points should come from the major group. Other features of PWS noted but not discussed.
Two male maternal first cousins were reported by Hall and Smith in 1972 and again by Clarren and Smith in 1977 with reduced fetal movement, hypotonia at birth, developmental delays, hyperphagia, hypogonadism, small feet, and characteristic facial features. The diagnostic score was 6 in the older cousin and 7 in the younger cousin. The three sisters reported by Burke et al. in 1987 were hypotonic at birth, gained weight rapidly, had hypogonadism, developmental delay, short stature, and dysmorphic facial features characteristic of PWS. Their diagnostic scores were 7.5, 7.5, and 8.
Ten of those 13 families carried microdeletions in the PWS imprinting center (Table 1). Family studies were carried out in 9 of the 10 cases and the microdeletions were found to be of paternal origin. These microdeletions included regions within the complex SNRPN gene locus encompassing the PWS imprinting center and identified by using a variety of techniques. The microdeletions resulted in altered maternal and paternal imprints during gametogenesis as the paternally expressed genes could not be activated due to this imprinting center microdeletion (Buiting et al., 1995). In seven of the families, the father carried the same SNRPN deletion on his maternal chromosome 15 and in four families; the paternal grandmother carried the SNRPN deletion on her maternally contributed chromosome 15. These data further support that lesions in the PWS imprinting center can change the epigenotype on chromosome 15 from a paternal epigenotype to a maternal epigenotype in regards to methylation and gene expression. Failure to erase the imprint makes it impossible to set a new correct imprint in the germline. The maternal imprint could not be erased in the paternal germline in those with PWS imprinting center deletions.
PWS caused by an imprinting center defect falls within two classes: microdeletion or epimutation. Patients with PWS and epimutations on the paternal chromosome will not switch the imprint throughout the imprinted area in the 15q11-q13 region. This failure to switch the imprint is a prefertilization imprint-switch error since the imprinting center has no known function in somatic cells; however, a postzygotic event cannot be excluded (Buiting et al., 1998, 2003; Bürger et al., 1997; El-Maarri et al., 2001; Nicholls & Knepper, 2001; Xin, Allis, & Wagstaff, 2001). An analysis of 51 PWS subjects with an imprinting defect found that 86% of the subjects carried epimutations and not microdeletions occurring spontaneously without other DNA changes. The absence of other DNA mutations may indicate that the PWS imprinting center contains several redundant elements or can withstand sequence changes. Epimutations show biparental inheritance of chromosome 15 DNA markers, PWS DNA-methylation, and gene-expression patterns throughout the imprinted domain within the 15q11-q13 region (Buiting et al., 1995; Glenn et al., 1993; Reis et al., 1994; Saitoh et al., 1996; Sutcliffe et al., 1994). Although epimutations have been reported to account for the largest percentage of imprinting defects, the recurrence risk appears to be negligible (Buiting et al., 1998, 2003). However, when PWS results from a microdeletion in the imprinting center carried by the father, the recurrence risk is 50% (Buiting et al., 2000, 1995). If the deletion is only passed through the maternal line, then no phenotypic effect will occur but her son(s) would be at a 50% risk of having children with PWS. Her daughters’ sons would also be at risk of having children with PWS. Here, we provide an example of PWS in three siblings who inherited a microdeletion in the PWS imprinting center on chromosome 15 from their father. Previous DNA testing of the paternal grandmother showed a defect or deletion on chromosome 15 that was found in the father in the imprinting center and passed to the siblings with PWS. The father has two siblings; one sister without children and one brother with two daughters. The father also had three maternal aunts, one paternal aunt and two paternal uncles. There were no other individuals affected with PWS.
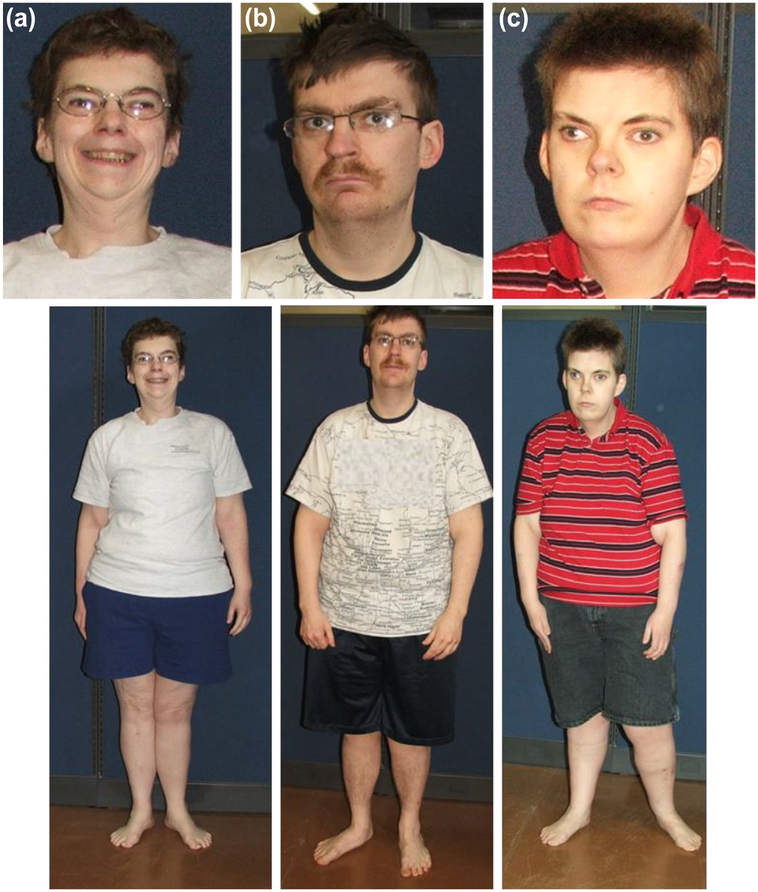
Our PWS siblings demonstrate the dysmorphism and clinical features with behavioral problems seen in PWS (Table 2). At birth all three siblings were hypotonic, one of the major criteria for a PWS diagnosis. Siblings ABCF31 and ABCM26 had a poor suck but did not require tube feedings. The youngest sibling, ABCM51, had a suck at birth but no other feeding problems reported. The siblings were developmentally delayed and had onset of childhood obesity, short stature, hypogonadism, cognitive dysfunction and characteristic behavioral problems including skin picking, depression, food-seeking, and stealing with obsessive-compulsive behaviors. All three siblings exhibited characteristic dysmorphic facial features-narrow head, a small down-turned mouth, almond-shaped eyes and strabismus, and central obesity (Figure 1).
FIGURE 1.
Clinical photographs of the three siblings with PWS. a) ABCF31, 40 year old female. b) ABCM26, 35 year old male. c) ABCM51, 33 year old male. The siblings display the typical characteristic facial features of PWS including a narrow face, almond-shaped eyes and a small down-turned mouth and central abdominal obesity.
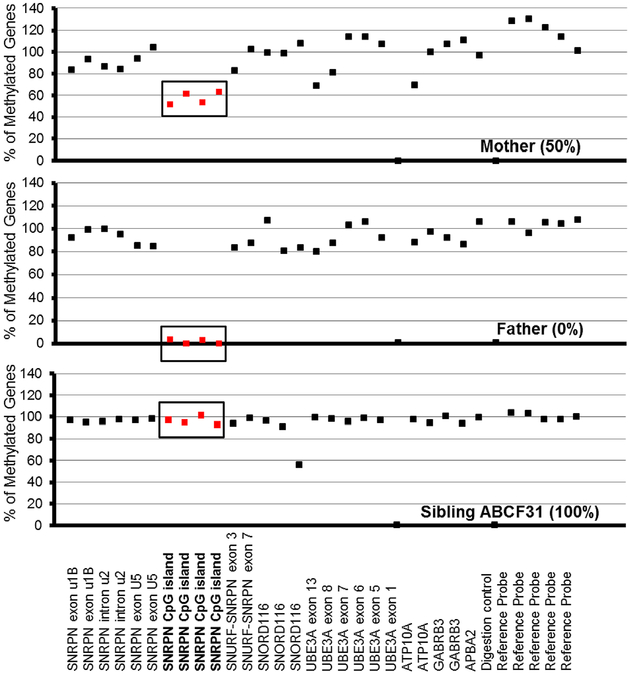
The PWS imprinted genes are only methylated on the maternally contributed allele and not on the paternal allele; therefore, the fragments from the genes in this region are digested by about 50% in unaffected individuals during MS-MLPA analysis. The MS-MLPA B2 kit contains five probes that are about 50% digested in control samples, including four from the SNRPN region. The DNA fragments from imprinted genes on chromosome 15 are 100% methylated in PWS subjects regardless of the genetic subtype (Henkhaus et al., 2012). MS-MLPA of the siblings demonstrated the typical 100% methylation pattern for PWS as shown in Figure 2. The father displayed 0% methylation for the four probes in the SNRPNregion(Figure 2). Since the normal methylation signal from the active paternal allele is 0% and 100% from the maternal allele, the unaffected father should display a normal 50% methylation pattern. However, he displayed 0% methylation for the probes in the SNRPN region, indicating a SNRPN deletion including the imprinting center on his maternal allele and 0% methylation pattern for his active paternal allele (Hassan & Butler, 2016). The MS-MLPA copy number analysis of all three siblings and their father detected a heterozygous deletion of the SNRPN region in the PWS imprinting center (Figure 3). Twelve probes from the MS-MLPA B2 kit detected a partial deletion of the SNRPN locus containing the imprinting center and ranged from SNRPN intron u2 through the SNRPN locus and SNORD 116 snoRNA cluster located at 15q11.2. This analysis excluded a large deletion or uniparental disomy 15 and classified the PWS siblings as having an imprinting defect due to a microdeletion. A normal methylation pattern and copy number were observed in their mother (Figure 3).
FIGURE 2.
Methylation patterns generated using Methylation Specific-Multiplex Ligation Probe Amplification B2 kit (MS-MLPA-B2). The mother displayed a normal methylation pattern for the four methylation sensitive fragments digested in the SNRPN region (in bold). The father displayed an abnormal methylation pattern (0%) in the SNRPN region due to loss on his maternal chromosome 15. All three siblings (only ABCF31 shown) displayed the typical PWS methylation pattern (~80–100%) of the four SNRPN probes. UBE3A exon 1 and one other digestion control probe were used during methylation analysis.
FIGURE 3.
Copy number generated using MS-MLPA-B2 kit. The mother has a normal copy number of 2 for all gene fragments analyzed of chromosome 15. The father and all three siblings (only ABCF31 shown) were deleted (showing a copy number of 1) for 12 probes in and around the SNRPN region (triangles).
High-resolution microarray analysis was also performed on genomic DNA in two of the siblings using the Affymetrix 6.0 SNP array. We identified microdeletions in the imprinting center for ABCM51 and ABCF31. ABCF31 had a microdeletion located at the 15q11.2 region (145 kbp) that includes SNURF-SNRPN and SNORD116 while ABCM51 carried a somewhat smaller deletion (129kbp). The Illumina 5 M microarray technology was also utilized and a 375 kbp microdeletion was found at 15q11.2 for ABCM51. The variation in size may be due to the difference in the amount of probe coverage for each microarray platform. Neither microarray analysis was undertaken with sibling ABCM26 or the parents due to the cost of microarray testing.
Deletion status was also assessed by using digital droplet PCR (ddPCR) for validation of aforementioned results performed at the University of Kansas Genomics Core facility with probes designed to detect microdeletions in the PWS imprinting center spanning chr15: 25,170,003–25,200,184 (hg19). All three PWS siblings plus their father showed a copy number reduction using three probes with ddPCR, indicating that they carried a deletion in the imprinting region. The probes that covered an area within the imprinting center were: IC2 (hg19 chr15:25,170,055), IC1 (hg19 chr15:25,181,550), and SNRPN exon1 (hg19 chr15:25,200,126). The PWS siblings’ mother displayed a normal copy number for the same probes, indicating she did not carry a microdeletion in the PWS imprinting center (data not shown). Comparison of MS-MLPA and microarray analysis of DNA from the PWS siblings verified the authenticity of our ddPCR results.
We encourage the reporting of other families with more than one affected individual with PWS not due to chromosome 15 trans-locations or inversions and detailed genetic analysis to identify the genetic defect within the imprinting center and impact on phenotype. These studies will allow a better understanding of the cause and diagnosis of this rare obesity-related genetic syndrome.
ACKNOWLEDGMENTS
We thank the siblings and their parents for participation in this study. We would also like to thank Mandilea Bandt for collecting the saliva samples from the PWS siblings and Dr. Ann Manzardo for her comments on the manuscript. We acknowledge National Institute of Child Health and Human Development (NICHD-HD02528) and Prayer-Will Support PWS Organization (Family & Friends of Kyleigh Ellington).
Funding information
National Institute of Child Health and Human
Development, Grant number: HD02528;
Prayer–Will Support PWS Organization
(Family & Friends of Kyleigh Ellington)
Footnotes
CONFLICTS OF INTEREST
There are no conflicts of interest to report for any of the authors.
REFERENCES
- Bingeliene A, Shapiro CM, & Chung SA (2015). Three siblings with Prader-Willi syndrome: Brief review of sleep and Prader-Willi syndrome. Case Reports in Neurological Medicine, 2015, Article ID 278287, 5 pp. 10.1155/2015/278287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittel DC, Kibiryeva N, & Butler MG (2006). Expression of 4 genes between chromosome 15 breakpoints 1 and 2 and behavioral outcomes in Prader-Willi syndrome. Pediatrics, 118 (e1276–), e1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buiting K, Saitoh S, Gross S, Dittrich B, Schwartz S, Nicholls RD, & Horsthemke B (1995). Inherited microdeletions in the Angelman and Prader-Willi syndromes define an imprinting centre on human chromosome 15. Nature Genetics, 9, 395–400. [DOI] [PubMed] [Google Scholar]
- Buiting K, Dittrich B, Gross S, Lich C, Färber C, Buchholz T, & Horsthemke B (1998). Sporadic imprinting defects in Prader-Willi syndrome and Angelman syndrome: Implications for imprint-switch models, genetic counseling, and prenatal diagnosis. American Journal of Human Genetics, 63(1), 170–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buiting K, Färber C, Kroisel P, Wagner K, Brueton L, Robertson ME, … Horsthemke B (2000). Imprinting centre deletions in two PWS families: Implications for diagnostic testing and genetic counselling. Clinical Genetics, 58, 284–290. [DOI] [PubMed] [Google Scholar]
- Buiting K, Groß S, Lich C, Gillessen-Kaesbach G, El-Maarri O, & Horsthemke B (2003). Epimutations in Prader-Willi and Angelman syndromes: A molecular study of 136 patients with an imprinting defect. American Journal of Human Genetics, 72, 571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bürger J, Buiting K, Dittrich B, Gross S, Lich C, Sperling K, Reis A (1997). Different mechanisms and recurrence risks of imprinting defects in Angelman syndrome. American Journal of Human Genetics, 61, 88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke CM, Kousseff BG, Gleeson M, O’Connell BM, & Delvin JG (1987). Familial Prader-Willi syndrome. Archives of Internal Medicine, 147, 673–675. [PubMed] [Google Scholar]
- Butler MG, Bittel DC, Kibiryeva N, Talebizadeh Z, & Thompson T (2004). Behavioral differences among subjects with Prader-Willi syndrome and type I or type II deletion and maternal disomy. Pediatrics, 113, 565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarren SK, & Smith DW (1977). Prader-Willi syndrome: Variable severity and recurrence risk. American Journal of Diseases of Children, 131, 798–800. [DOI] [PubMed] [Google Scholar]
- El-Maarri O, Buiting K, Peery EG, Kroisel PM, Balaban B, Wagner K, Horsthemke B (2001). Maternal methylation imprints on human chromosome 15 are established during or after fertilization. Nature Genetics, 27, 341–344. [DOI] [PubMed] [Google Scholar]
- Glenn CC, Nicholls RD, Robinson WP, Saitoh S, Niikawa N, Schinzel A, Driscoll DJ (1993). Modification of 15q11-q13 DNA methylation imprints in unique Angelman and Prader-Willi patients. Human Molecular Genetics, 2, 1377–1382. [DOI] [PubMed] [Google Scholar]
- Hall BD, & Smith DW (1972). Prader-Willi syndrome: A resumé of 32 cases including an instance of affected first cousins, one of whom is of normal stature and intelligence. The Journal of Pediatrics, 81, 286–293. [DOI] [PubMed] [Google Scholar]
- Hassan M, & Butler MG (2016). Prader-Willi syndrome and atypical submicroscopic 15q11-q13 deletions with or without imprinting defects. European Journal of Medical Genetics, 59, 584–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkhaus RS, Kim SJ, Kimonis VE, Gold JA, Dykens EM, Driscoll DJ, & Butler MG(2012).Methylation-specificmultiplexligation-dependentprobe amplification and identification of deletion genetic subtypes in Prader-Willi syndrome. Genetic Testing and Molecular Biomarkers, 16, 178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm VA, Cassidy SB, Butler MG, Hanchett JM, Greenswag LR, Whitman BY, & Greenburg F (1993). Prader-Willi syndrome: Consensus diagnostic criteria. Pediatrics, 91, 398–402. [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T, Kanayama M, & Wada Y (1987). Prader-Willi syndrome in two siblings: One with normal karyotype, one with a terminal deletion of distal Xq. Clinical Genetics, 32, 295–299. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Kibe T, & Wada Y (1996). Deletion of Small Nuclear Ribonucleoprotein Polypeptide N (SNRPN) in Prader-Willi syndrome detected by fluorescence in situ hybridization: Two sibs with the typical phenotype without cytogenetic deletion in chromosome 15q. American Journal of Medical Genetics, 62, 350–352. [DOI] [PubMed] [Google Scholar]
- Jancar J (1971). Prader-Willi syndrome. Journal of Intellectual Disability Research, 15, 20–29. [Google Scholar]
- Ledbetter DH, Riccardi VM, Airhart SD, Strobel RJ, Keenan BS, & Crawford JD (1981). Deletions of chromosome 15 as a cause of the Prader-Willi syndrome. New England Journal of Medicine, 304, 325–329. [DOI] [PubMed] [Google Scholar]
- Lubinsky M, Zellweger H, Greenswag L, Larson G, Hansmann I, & Ledbetter D (1987). Familial Prader-Willi syndrome with apparently normal chromosomes. American Journal of Medical Genetics, 28, 37–43. [DOI] [PubMed] [Google Scholar]
- McEntagart ME, Webb T, Hardy C, & King MD (2000). Familial Prader-Willi syndrome: Case report and a literature review. Clinical Genetics, 58, 216–223. [DOI] [PubMed] [Google Scholar]
- Ming JE, Blagowidow N, Knoll JH, Rollings L, Fortina P, McDonald-McGinn DM, Zackai EH (2000). Submicroscopic deletion in cousins with Prader-Willi syndrome causes a grandmatrilineal inheritance pattern: Effects of imprinting. American Journal of Medical Genetics, 92, 19–24. [DOI] [PubMed] [Google Scholar]
- Nicholls RD, & Knepper JL (2001). Genome organization, function, and imprinting in Prader-Willi and Angelman syndromes. Annual Review of Genomics and Human Genetics, 2, 153–175. [DOI] [PubMed] [Google Scholar]
- Ohta T, Gray TA, Rogan PK, Buiting K, Gabriel JM, Saitoh S,. Nicholls RD. (1999). Imprinting-mutation mechanisms in Prader-Willi syndrome. American Journal of Human Genetics, 64, 397–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orstavik KH, Tangsrud SE, Kiil R, Hansteen IL, Steen-Johnson J, Cassidy SB, Brondum-Nielsom K (1992). Prader-Willi syndrome in a brother and sister without cytogenetic or detectable molecular genetic abnormality at chromosome 15q11q13. American Journal of Medical Genetics, 44, 534–538. [DOI] [PubMed] [Google Scholar]
- Reis A, Dittrich B, Greger V, Buiting K, Lalande M, Gillessen-Kaesbach G, Horsthemke B (1994). Imprinting mutations suggested by abnormal DNA methylation patterns in familial Angelman and Prader-Willi syndromes. American Journal of Human Genetics, 54, 741–747. [PMC free article] [PubMed] [Google Scholar]
- Saitoh S, Buiting K, Rogan PK, Buxton JL, Driscoll DJ, Arnemann J, & Nicholls RD (1996). Minimal definition of the imprinting center and fixation of chromosome 15q11-q13 epigenotype by imprinting mutations. Proceedings of the National Academy of Sciences of the United States of America, 93(15), 7811–7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe JS, Nakao M, Christian S, Orstavik KH, Tommerup N, Ledbetter DH, & Beaudet AL (1994). Deletions of a differentially methylated CpG island at the SNRPN gene define a putative imprinting control region. Nature Genetics, 8, 52–58. [DOI] [PubMed] [Google Scholar]
- Teshima I, Chadwick D, Chitayat D, Kobayashi J, Ray P, Shuman C, Weksberg R (1996). FISH detection of chromosome 15 deletions in Prader-Willi and Angelman syndromes. American Journal of Medical Genetics, 62, 216–223. [DOI] [PubMed] [Google Scholar]
- Xin Z, Allis CD, & Wagstaff J (2001). Parent-specific complementary patterns of histone H3 lysine 9 and H3 lysine 4 methylation at the Prader-Willi syndrome imprinting center. American Journal of Human Genetics, 69, 1389–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarcone J, Napolitano D, Peterson C, Breidbord J, Ferraioli S, Caruso-Anderson M, Thompson T (2007). The relationship between compulsive behaviour and academic achievement across the three genetic subtypes of Prader-Willi syndrome. Journal of Intellectual Disability Research, 51, 478–487. [DOI] [PMC free article] [PubMed] [Google Scholar]