Summary
The Ca2+/calmodulin(CaM)-dependent protein kinase II (CaMKII) was touted as a memory molecule, even before its involvement in long-term potentiation (LTP) was shown. The enzyme has not disappointed, with subsequent demonstrations of remarkable structural and regulatory properties. Its neuronal functions now extend to long-term depression (LTD), and last year saw the first direct evidence for memory storage by CaMKII. Although CaMKII may have taken the spotlight, it is a member of a large family of diverse and interesting CaM kinases. Our aim is to place CaMKII in context of the other CaM kinases and then review certain aspects of this kinase that are of current interest.
Keywords: CaMKII, synapse, LTP, LTD, memory, calmodulin, DAPK
Introduction
The Ca2+/calmodulin(CaM)-dependent protein kinase II (CaMKII) is a ubiquitous mediator of cellular Ca2+ signals but is best known for its functions in the brain, where its expression level exceeds a staggering 1% of total protein (especially in hippocampus and neocortex). Forty years of extensive research have firmly established CaMKII as a central regulator of neuronal plasticity and of cognitive functions such as learning. However, only last year has seen the first direct experimental support for a long-claimed role of CaMKII in the storage of declarative memory. The central role of CaMKII in both induction and maintenance of LTP has long been textbook dogma. However, recent studies indicate that (i) the mechanisms underlying LTP maintenance likely differ from the original proposal, and that (ii) CaMKII also mediates the opposing form of synaptic plasticity, LTD. These findings will be discussed here, in context of the complex CaMKII regulation (and its structural basis) that ultimately underlies any of the diverse functions of CaMKII. But first, we will clarify some common misconceptions about CaMKII and provide a brief overview of the large CaMK family.
CaMKII and the CaMK family: early history and a brief overview
The introduction of CaMKs was one of the many contributions of the late Paul Greengard: Despite its numerical designation, CaMKII was among the first Ca2+/CaM-regulated kinases described (Schulman and Greengard, 1978a, b), while CaMKI was introduced a year later (Huttner and Greengard, 1979). In the same year, CaM regulation was described to mediate Ca2+-sensitivity also for myosin light chain kinase (MLCK; Yagi et al., 1978) and for phosphorylase kinase (PhK; Cohen et al., 1978). While PhK was the first protein kinase to be purified and characterized (in the Nobel-winning work of Krebs and Fisher; Krebs and Fischer, 1956; Krebs et al., 1958), it wasn’t until 20 years later that it’s intrinsic regulatory δ-subunit was recognized to be CaM. In 1978, the name CaMKII was not yet established (and calmodulin still went by several earlier names). The current nomenclature is based on the order of elution from a fractionation column loaded with brain extract, and included CaMKI to IV (Yamauchi and Fujisawa, 1980). This nomenclature is somewhat misleading in several ways: In addition to suggesting a different order of discovery, it suggests that there are only four CaMKs. By contrast, there are now over 80 CaMKs described (Manning et al., 2002), and even CaMKII is a family of four closely related kinases (Tombes et al., 2003; see also next sub-section and Figure 1A). The nomenclature also suggests that CaMKI to IV are closely related. This is only partially true: Whereas CaMKI and IV are indeed very closely related, CaMKII is evolutionarily closer to PhK. More importantly, while CaMKI/IV and CaMKII are at least members of related sister-groups, CaMKIII is now termed eukaryotic elongation factor 2 (eEF2) kinase and is now classified as an atypical kinase, not as a CaMK. Thus, while CaM-regulation is the name-giving feature of the CaMK family, not all CaM-regulated kinases are CaMKs. This exception also includes CaMK kinases (CaMKKs), which are CaM-regulated upstream-activating kinases for CaMKI and IV (as well as for AMPK and Akt). Conversely, not all kinases that are phylogenetically classified as CaMKs are regulated by CaM. For instance, while death associated protein kinase (DAPK) 1 and 2 are indeed activated by CaM, DAPK3 is not and does not even contain a CaM binding site. In fact, several whole CaMK subfamilies lack CaM regulation, including AMPKs and RSKs. And, as also indicated in the next sub-sections, some CaMKs may not function as kinases at all.
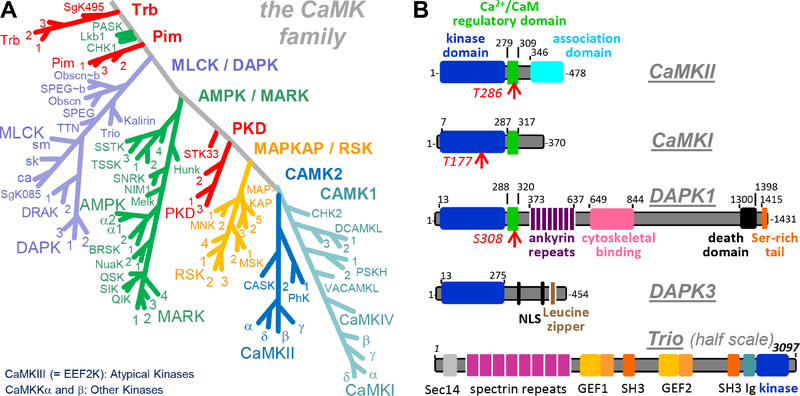
Figure 1. The CaMK family.
(A) The CaMKII family tree within the human kinome (based on (Manning et al., 2002)). Also indicated are the different CaMK subfamilies as well as the classification of some CaM-regulated kinases that are not part of the CaMK family.
(B) Examples of CaMK domain organization: CaMKII, CaMKI, DAPK1, DAPK3, and Trio in schematic representation. DAPK3 and Trio are examples of CaMKs without CaM binding regulatory domain; DAPK1 and Trio are examples of CaMKs with complex domain structure. CaMKII contains a unique association domain that mediates 12meric holoenzyme assemblies (see Figure 3).
The CAMK1 family contains 12 members, including CaMKIV and the four CaMKI isoforms. Like CaMKII, both CaMKI and IV are multifunctional kinases that have also been implicated in neuronal plasticity and gene regulation (Schulman, 1993; Takemoto-Kimura et al., 2017; Wayman et al., 2008). CaMKI and II have also been implicated in different phases of cell cycle control (Skelding et al., 2011), a function prominently shared with the CAMK1 family member ChK2 (Zannini et al., 2014).
The extended MLCK or DAPK family contains 16 members, including four MLCK isoforms, three DAPK isoforms, and two DRAK isoforms. All of these aforementioned kinases accept myosin light chain as a substrate, but many have additional substrates. Notably, while the MlcK isoforms and DAPK1 and 2 (also called DRP-1) are activated by Ca2+/CaM, DRAK1 and 2 and DAPK3 (also named ZIPK) lack a CaM-binding regulatory domain (reviewed in Shiloh et al., 2014). Other prominent family members include trio and kalirin (also named trad), which are large multi-domain proteins (as are DAPK1 and 2) that are better known for their RhoGEF function (Figure 1B). Indeed, CaMKIIα is thought to potentiate synaptic strength at least in part through regulating this GEF activity of trio and kalirin (Herring and Nicoll, 2016a). While trio has been classified to contain a MLCK-related kinase domain immediately after its discovery (Debant et al., 1996), nothing is known about the kinase activity of trio or kalirin; however, both appear to lack a CaM-binding regulatory domain.
The extended MAPKAP or RSK family contains 11 members. As their name (MAPK-activated protein kinases) indicates, these CaMKs are typically not activated by Ca2+/CaM, but by upstream phosphorylation events (both within and outside of the activation loop). Notably, the four RSK and two MSK isoforms have two kinases domains, and only their C-terminal kinase domains (CTKDs) belongs to the CaMK (and MAPKAP) family, while their N-terminal kinase domains (NTKDs) are AGC kinases (the kinase family that contains PKA, PKC, and PKG, and is closely related to CaMKs ). The only known function of the CTKD is to activate the NTKD, as a built-in kinase kinase. While RSKs (or p90 RSKs) were identified and named as ribosomal S6 kinases, the predominant S6 kinases are the p70 S6K1 and 2 that contain only the NTKD (reviewed in Romeo et al., 2012). The three MAPKAP kinase isoforms contain a single kinase domain, and are best known for their transcriptional regulation related to inflammation (reviewed in Gaestel, 2006).
The extended AMPK or MARK family contains 24 members and has also been termed CaMK-like (CAMKL); neither AMPKs nor MARKs are regulated by Ca2+/CaM, but most appear to be regulated by phosphorylation in their activation loop and additional sites. MARKs are best known for phosphorylating and regulating microtubule-associated proteins (including tau), but also have several other functions, including cell cycle regulation (reviewed in Marx et al., 2010; Matenia and Mandelkow, 2009). AMPKs are prominently known for their integrative role in regulating energy balance and metabolism (reviewed in Herzig and Shaw, 2018; Lin and Hardie, 2018).
Several small families including PKD, Pim, and Trb contain 1–4 members each that are generally not regulated by CaM. PKDs share similar domains with PKCs that mediate regulation by diacylglycerol and phorbol esters, but their kinase domains differ phylogenetically and regarding substrate selection (reviewed in Fu and Rubin, 2011; Rozengurt, 2011). Pim kinases are oncogenes that are actively studied for their role in multiple cancers (reviewed in Jinesh et al., 2016; Nawijn et al., 2011). The Tribbles kinases Trb1–3 and SgK495 are all predicted to be catalytically inactive pseudokinases (reviewed in Eyers et al., 2017).
CaM kinases in humans and other species can differ; for instance, DRAK1 is present in humans (and in dog and rabbit) but is absent in mouse. On the other hand, mouse contains 25 kinases not present in human; most of the genes coding for these additional murine kinases lack introns, indicating unusual evolutionary emergence (reviewed in Caenepeel et al., 2004). While all vertebrates appear to have four CaMKII genes, unicellular eukaryotes as well as C. elegans and Drosophila have only a single CaMKII gene (reviewed in Tombes et al., 2003).
Several CaM kinases are pseudokinase, including CASK and the Trb family. Pseudokinases differ at conserved sites of the ATP binding pocket, which results in dramatically reduced ATP affinity; thus, they are generally considered kinase dead. However, at high cellular ATP concentrations (~4 mM), many pseudokinases (including CASK and Trb) may bind ATP. However, cellular Mg2+ is typically essential for ATP binding to kinases, whereas Mg2+ actually inhibits ATP binding to CASK (Mukherjee et al., 2010). Even if pseudokinases cannot function as kinases, they do have cellular functions (reviewed in Boudeau et al., 2006; Reiterer et al., 2014).
The CAMK2 family contains 7 members: four CaMKII isoforms that are encoded by separate genes, two PhK isoforms, and CASK. The CaMKIIα, β, γ, and δ isoforms are highly homologous and show differential but overlapping expression patterns among tissues, brain regions, and during development, with a being most brain-specific, and γ and δ being expressed early and ubiquitously (Bayer et al., 1999; Cook et al., 2018; Tobimatsu and Fujisawa, 1989). The largest biochemical difference to date remains the specific high affinity binding of the β isoform to F-actin (Borgesius et al., 2011; Fink et al., 2003; Kim et al., 2015; O’Leary et al., 2006; Shen et al., 1998). More striking difference can be introduced by alternative splicing, which can abolish F-actin binding in the βe variant (O’Leary et al., 2006) or can insert a nuclear localization signal in most mammalian CaMKII isoforms (i.e. in α, γ and δ but not β; Brocke et al., 1995; Cook et al., 2018; Ma et al., 2014; Srinivasan et al., 1994). All CAMK2 members bind CaM, however, CASK is a pseudokinase with limited kinase activity (Mukherjee et al., 2010) that is thought to fulfill primarily structural functions (mainly at presynaptic nerve terminals). Thus, the CAMK2 family contains members that are multifunctional kinases with broad substrate ranges (CaMKIIs), members that appear to be dedicated to specific substrates (PhKs), and a member that is a pseudokinase (CASK). The CaMKIIα gene also encodes the non-kinase scaffolding protein aKAP that completely lacks the kinase domain (Bayer et al., 1998; Bayer et al., 1996). Notably, while CaMKIIs are highly catalytically competent, their multimeric holoenzyme structure and high concentration in brain is thought to additionally enable structural functions (Hojjati et al., 2007; Kim et al., 2015).
Structural basis for CaMK regulation
CaMKII has an N-terminal kinase domain that is typical for CaMKs, followed by a CaM-binding regulatory domain (Figure 1B). However, its C-terminal association domain is unique, and enables formation of 12meric holoenzymes (compare Figure 3). All three domains act in concert in the complex regulation and tuning of CaMKII activity.
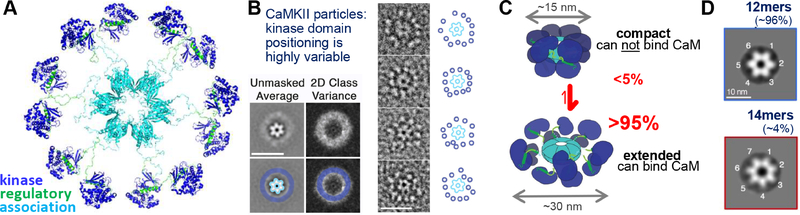
Figure 3.
The CaMKII holoenzyme structure based on EM tomography (Myers et al., 2017).
(A) A pseudoatomic model of the CaMKII holoenzyme in the preferred average conformation.
(B) Image analysis of individual CaMKII holoenzymes shows that the CaMKII association domain assemblies are very static, but that the kinase domain positioning is highly flexible; as a result, essentially no individual holoenzyme is in the average conformation shown in panel A.
(C) While a crystal structure has suggested an additional compact conformation (in which the CaM binding region is not accessible), most of the holoenzymes are in an activation-competent extended conformation, both in vitro and within cells.
(D) While most CaMKII holoenzymes are 12meric, a minority can be found in a 14meric conformation, which may represent a transition state that enables subunit exchange.
The kinase domain
Like all typical protein kinases, the kinase domains of CaMKs are comprised of a small and a large lobe, with the ATP binding site in the cleft between them (Figure 2A). The ADP/ATP exchange that occurs during each catalytic cycle of kinase activity is largely driven by the difference in cellular concentration of these nucleotides, not by significantly different affinities. With a kD for ATP of ~8 μM, CaMKII is quite typical. Notably, at least for kinases that have similar affinities for ADP and ATP (such as PkA and CaMKII), the ADP/ATP exchange is thought to be the rate limiting step of the catalytic cycle (one per ~50 ms), while the actual phospho-transfer can be rather fast (one per ~2 ms)(Grant and Adams, 1996; Lew et al., 1997; Zhou and Adams, 1997). Consequently, kinases that are present in similar amounts as their substrates -as is the case for CaMKII at the synapse- may mediate phosphorylation on a remarkably fast time-scale that could approach the range of electrophysiological responses. In such a scenario each kinase molecule may only be required for one phospho-transfer during any given stimulus.
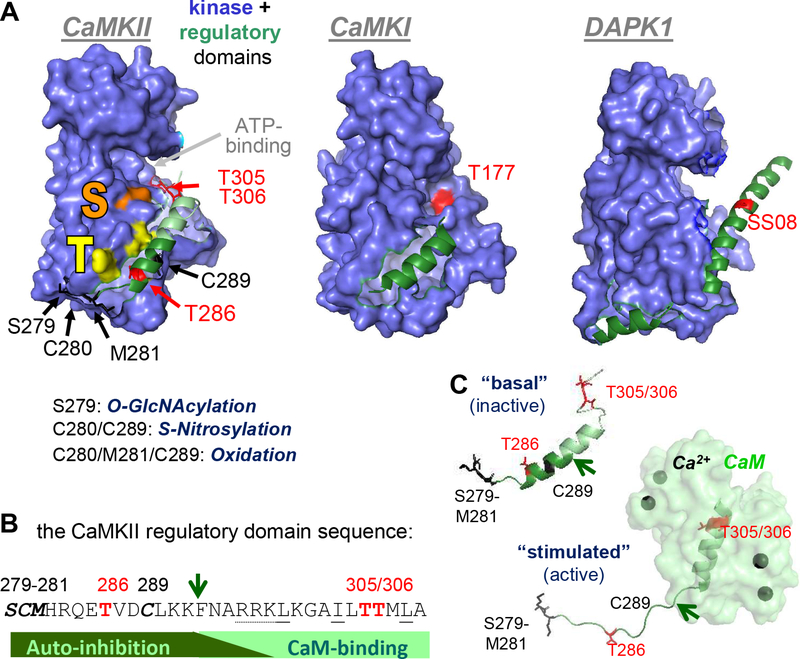
Figure 2.
The CaMKII kinase and regulatory domains, with regulatory phosphorylation sites indicated in red; sites for other posttranslational modifications in black.
(A) Structure of the kinase and regulatory domains of CaMKII, compared to CaMKI and DAPK1. In CaMKII, the substrate binding “S-site” is indicated in orange; the nearby “T-site” that interacts with T286 of the regulatory domain in the basal state (and with GluN2B upon activation) is indicated in yellow.
(B) Amino acid sequence of the CaMKII regulatory domain, with sites of posttranslational modification by phosphorylation, oxidation/nitrosylation and GlcNacylation indicated.
(C) Structural transition of the CaMKII regulatory structure in response to Ca2+/CaM binding (Rellos et al., 2010). The arrow is for orientation and for comparison to the sequence in panel C.
A common regulatory feature is the “activation loop” that is located within the large lobe of a kinase domain, near the ATP-binding pocket. The name of this loop is based on the fact that many CaMKs (including CaMKI and IV) and other kinases (including PKA and PKC) require phosphorylation within the activation loop for proper folding of the ATP-binding pocket, and thus for optimal kinase activity. By contrast, other kinases (including CaMKII and DAPK1) do not even contain a phosphorylatable residue in this loop. While CaMKII is prominently regulated by T286 autophosphorylation, this phosphorylation is not required for activity and the site is not located in the kinase domain but in the regulatory domain (Figure 2A).
The regulatory domain
The regulatory domain contains a Ca2+/CaM binding site that overlaps with an autoinhibitory region, and binding of Ca2+/CaM relieves the autoinhibitory block. In CaMKII, this domain mainly regulates substrate access, although it also mildly affects nucleotide binding. Many CaMKs contain homologous regulatory domains (for example CaMKII, CaMKI and DAPK1; Figure 2A) and their CaM binding is the name giving feature of the kinase family; however, as already mentioned, about half of all CaMKs lack this CaM binding domain.
CaM binding can be directly suppressed by phosphorylation of the CaM binding region. For instance, DAPK1 autophosphorylates at S308 in its basal state, making phosphatase action an additional requirement for DAPK1 activation. While CaMKII can autophosphorylate at T305/306 to disrupt CaM binding, this reaction involves a more complex cycle of regulation and prior generation of “autonomous” kinase activity by T286 autophosphorylation. As discussed in the next section, autonomous CaMKII activity can also be generated by several other posttranslational modification of the regulatory domain (Figure 2B). Interestingly, the entire the CaMKII regulatory domain can exist in a helical conformation (and this is how the domain first crystallized; Rosenberg et al., 2005). However, in the basal state, only the N-terminal portion is helical, while activation by Ca2+/CaM binding induces helical conformation of the C-terminus and causes a disordered conformation of the N-terminus (Chao et al., 2011; Rellos et al., 2010)(Figure 2C).
The CaMKII holoenzyme structure
Unique among CaMKs, CaMKII forms holoenzymes composed of 12 identical or similar subunits (Figure 3); the C-terminal association domains of the α, β, γ, and δ isoforms can form homo- or hetero-meric assemblies with no known isoform preference. The kinase domain of each subunit is activated separately by Ca2+/CaM binding to the regulatory domain of the same subunit, but the holoenzyme structure allows regulatory inter-subunit autophosphorylation (as described in the next section). Electron microscopy (EM) tomography enabled reconstruction of the CaMKII holoenzyme in an activation competent conformation (Myers et al., 2017) (Figure 3A), with pseudo-atomic resolution enabled by fitting of previous individual crystal structures of the kinase domain or association domains (Hoelz et al., 2003; Rellos et al., 2010). While the model represents the preferred average kinase domain positioning, hardly any individual holoenzyme was found in this conformation: While the association domain assembly was static, kinase domain positioning was very flexible (Myers et al., 2017)(Figure 3B). Notably, a crystal structure of the holoenzyme (that used a variant lacking the linker between regulatory and association domain) showed a compact conformation that is incompetent for Ca2+/CaM binding (Chao et al., 2011), which raised the possibility for additional regulatory complexity. However, the large majority of CaMKII appears to exist in the activation competent extended conformation (Figure 3C), both in vitro and within cells (Myers et al., 2017).
In addition to 12mers, a small percentage of holoenzymes were found as 14mers (Figure 3D)(Myers et al., 2017). While the 12meric conformation is preferred by full-length CaMKII, the association domains by themselves instead preferably assemble into 14mers. Remarkably, 14meric association domain assemblies can be formed after proteolytic cleavage of the kinase domains from 12meric holoenzymes in vitro (Rosenberg et al., 2006). Thus, even though the holoenzymes are extremely stable, subunit exchange can occur, and the 14mers may represent intermediate states of such exchange (Bhattacharyya et al., 2016; Rosenberg et al., 2006; Stratton et al., 2014). The function of this subunit exchange is currently unknown, but it may include mundane repair of damaged holoenzymes and/or exciting new plasticity mechanisms, for instance by changing the holoenzyme subunit composition at stimulated synapses. In support of the latter speculation are the facts that (i) CaMKII is required for the “synaptic tagging” that is involved in input-specificity of LTP (Moncada et al., 2011; Sajikumar et al., 2007), (ii) subunit exchange can be triggered by CaMKII activation (Bhattacharyya et al., 2016; Stratton et al., 2014), (iii) only the CaMKIIα isoform mRNA is localized and translated in dendrites (Burgin et al., 1990; Mayford et al., 1996), while (iv) the CaMKIIβ isoform has been implicated specifically as an “inverse synaptic tag” for the neighboring non-potentiated synapses (Okuno et al., 2012). In this scenario, local CaMKIIα translation at activated synapses would aid local replacement of the β isoform. Indeed, there is recent indication that the association domain interactions may be weaker for the β compared to the α isoform (Cook et al., 2018), which may promote such isoform-linked subunit exchange.
While each kinase subunit within a holoenzyme is activated separately by direct binding of Ca2+/CaM to its regulatory domain, the holoenzyme structure allows for additional, more sophisticated regulation (see next section). Additionally, the multivalent nature of the holoenzyme may aid protein-protein interactions in several ways: by increasing the apparent affinity through avidity effects and by enabling “crosslinking” via simultaneous binding to multiple different partners (reviewed in Colbran, 2004; Coultrap and Bayer, 2012; Hell, 2014). Many of the CaMKII protein interactions are mediated by its kinase domain, and the highly flexible nature of the kinase domain positioning should significantly aid the ability of a holoenzyme to engage in multiple of such interactions simultaneously.
CaMKII “autonomy”: Multiple mechanisms for molecular memory
CaMKII activity is stimulated by Ca2+/CaM binding, but several mechanisms can subsequently generate Ca2+-independent “autonomous” CaMKII activity. These mechanisms have been regarded as forms of molecular memory (as they enable CaMKII to “remember” past Ca2+-stimuli by sustained activity) and include the following: Autophosphorylation at t286, binding to the NMDA-receptor subunit GluN2B, nitrosylation at C280/C289, oxidation at M281/C289, and GlcNAcylation at S279. (Note: all amino acid positions are for the a isoform; the homologous positions in the other isoforms are one number higher). Each of these mechanisms has unique features that are discussed below. However, they share several important themes: All require an initial Ca2+/CaM-stimulus, likely to displace the regulatory domain and optimize exposure the relevant residues for modification or for protein-protein interaction (and all of the modifications are within the regulatory domain; see Figure 2B–D). Indeed, the initial Ca2+ requirement enables the function as a memory mechanism of these initial Ca2+ stimuli. Then, the modification or binding is thought to prevent the regulatory domain from re-forming its full inhibitory interactions with the kinase domain, even after Ca2+/CaM has dissociated. Importantly, these autonomy mechanisms generate only partial activity that can be further stimulated by Ca2+/CaM. This mechanism still allows molecular memory of past Ca2+ stimuli, but also prevents complete uncoupling from subsequent Ca2+ stimuli. The different autonomy mechanisms are listed here in descending order of the approximate level of autonomous activity they induce.
Thr286 phosphorylation enables frequency decoding and computation
Autophosphorylation was the first CaMKII autonomy mechanism to be described (Lai et al., 1986; Lou et al., 1986; Miller and Kennedy, 1986; Saitoh and Schwartz, 1985; Schworer et al., 1986) and was later shown to occur at T286 (Colbran et al., 1989; Lou and Schulman, 1989; Miller et al., 1988). Of all known autonomy mechanisms, T286 phosphorylation generates the highest level of autonomy. However, even this autonomy is significantly less than fully stimulated activity (approximately 20%, but with the value depending on reaction conditions). Significantly higher levels of autonomy have been reported, but this requires special mechanisms that are specific to a few special substrates (Coultrap et al., 2010; Coultrap et al., 2014a; Woolfrey et al., 2018). As discussed later, these special substrates appear to be linked to the more recently discovered functions of CaMKII autonomy also in LTD (Coultrap et al., 2014a; Woolfrey et al., 2018).
CaMKII T286 autophosphorylation occurs as inter-subunit reaction within a holoenzyme, and was proposed to provide long-term information storage: It was thought that one autonomous autophosphorylated subunit would rapidly phosphorylate its neighbors, thereby also counteracting ongoing phosphatase activity (until the entire holoenzyme has been dephosphorylated). However, subsequent studies instead indicated that T286 is much more suited for short-term information storage that enables computation: (i) T286 autophosphorylation requires Ca2+/CaM binding to two neighboring subunits, i.e. not only to the subunit acting as kinase, but also to the substrate subunit that is being phosphorylated (because T286 is not accessible for phosphorylation until Ca2+/CaM displaces the regulatory domain; Hanson et al., 1994; Rich and Schulman, 1998)(Figure 4A). Thus, the mechanism cannot store information beyond phosphatase action, as any re-phosphorylation requires a new Ca2+-signal in order to make t286 again accessible for phosphorylation. However, (ii) this dual role of CaM in the autophosphorylation reaction enables CaMKII to act as a frequency-sensitive detector (Bayer et al., 2002; De Koninck and Schulman, 1998; Fujii et al., 2013), which can compute input stimulation and produce graded CaMKII activation (Figure 4B,C).
Figure 4. CaMKII autonomy generated by T286 autophosphorylation: mechanism, frequency detection, and spike counting.
(A) CaMKII autophosphorylation at T286 occurs primarily within holoenzymes and exclusively as a reaction between two subunits. Ca2+/CaM stimulates the process and has to be bound to the subunit that is being phosphorylated, in order to make T286 accessible (compare also Figure 2B). Therefore, the reaction can only occur in presence of Ca2+/CaM, and making CaMKII autonomous can only substitute for the function of Ca2+/CaM in activating one kinase subunit, but not for the substrate-directed function.
(B) The dual role of CaM in T286 autophosphorylation enables frequency detection. During submaximal Ca2+-spikes, some CaM molecules bind to some subunits of a CaMKII holoenzyme, and then dissociate during the spike interval, and so on. However, at higher frequencies (with spike intervals in the range of the CaM dissociation time) additional CaM molecules accumulate before all of the initial CaM molecules have dissociated. This increases the chance of CaM binding to neighboring subunits and thereby the autophosphorylation at T286. Panels A and B are derived from a previous review (Coultrap and Bayer, 2012).
(C) T286 phosphorylation can be induced at lower Ca2+-spike frequencies, but then takes longer time (and more spikes) to reach the same level. (Adapted from (De Koninck and Schulman, 1998).
(D) Spikes of glutamate uncaging are integrated by CaMKII within hippocampal neurons in a T286-dependent manner, as measured using a FRET sensor of CaMKlI activity. (Adapted from (Chang et al., 2017).
The underlying frequency mechanism is illustrated in Figure 4B: During sub-maximal Ca2+-spikes, Ca2+/CaM will bind and activate some subunits of a CaMKII holoenzyme; during the spike intervals, Ca2+/CaM will dissociate and the kinase will deactivate. It is likely that a given Ca2+ transient activates only a subset of CaMKII subunits because of the high CaMKII amount relative to the amount of free CaM available for activation. At low frequencies, these activation/deactivation cycles will repeat; however, at higher frequencies, with the spike intervals in the range of the CaM dissociation time, Ca2+/CaM will build up on the holoenzyme (as new Ca2+/CaM associates before all previously bound Ca2+/CaM has dissociated). This build-up increases the chance of Ca2+/CaM binding to neighboring subunits, which in turn increases the chance of autophosphorylation at T286. Thus, the same number of Ca2+-spikes will elicit more T286 autophosphorylation when delivered at higher frequencies. Figure 4B illustrates this frequency-detection in an all-or-none fashion. However, even at low frequencies, the chance for autophosphorylation is not zero, as some Ca2+/CaM will bind to neighboring subunits during any spike; then, during subsequent spikes, binding of a single Ca2+/CaM to the neighbor of the phosphorylated subunit is sufficient to induce another autophosphorylation reaction (compare Figure 4A). Thus, significant CaMKII T286 phosphorylation can be generated not only by brief high-frequency stimulation (such as during LTP induction), but also by low-frequency stimulation when it is extended for a long enough time (such as during typical LTD induction)(Coultrap et al., 2014a; Marsden et al., 2010). In effect, T286 autophosphorylation reflects both spike count and frequency in an integrated fashion (Figure 4C,D), a computational paradigm that was indeed directly indicated by the original biochemical experiments (De Koninck and Schulman, 1998) and recently shown to occur within neurons (Chang et al., 2017).
CaMKII binding to the NMDA-receptor subunit GluN2B: computation by competition
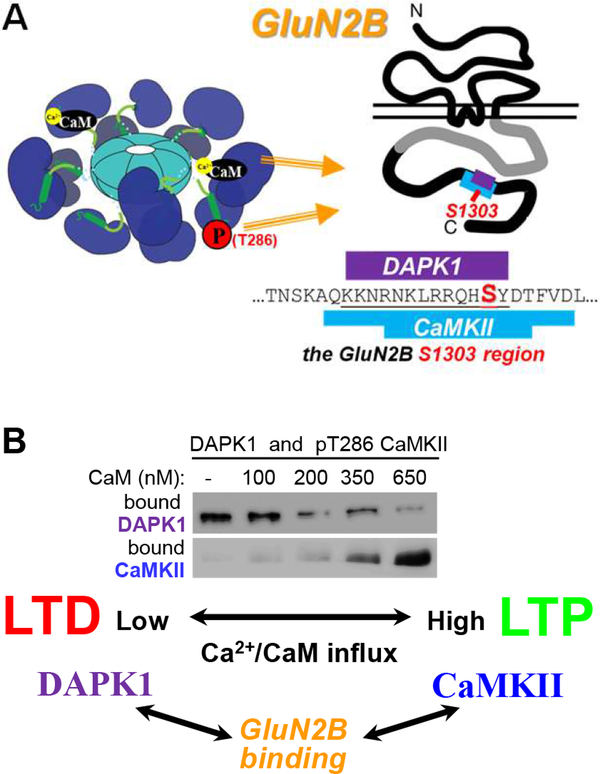
CaMKII binding to GluN2B is sufficiently induced by either Ca2+/CaM or T286 autophosphorylation (Figure 5A). Once the binding is fully established, the inducing stimulus is no longer required, thus making this another molecular memory mechanism (Bayer et al., 2001; Bayer et al., 2006). In contrast to T286 phosphorylation, this memory mechanism is resistant to erasure by phosphatases, and may thus be more suitable for prolonged information storage. The CaMKII/GluN2B binding directly induces autonomous kinase activity (Bayer et al., 2001), but this form of autonomy cannot functionally substitute for T286 phosphorylation in LTP induction. Thus, a more important consequence of GluN2B binding may be in directing CaMKII localization: The binding to GluN2B mediates further accumulation of CaMKII at excitatory synapses in response to LTP stimuli (Bayer et al., 2001; Bayer et al., 2006; Halt et al., 2012). Notably, while LTD stimuli also induce the T286 phosphorylation that should be sufficient to induce CaMKII binding to GluN2B, they do not induce synaptic accumulation of CaMKII. The reason is active suppression of CaMKII/GluN2B binding during LTD stimuli by another CaMK family member, DaPk1 (Goodell et al., 2017). CaMKII and DAPK1 compete for GluN2B binding in vitro (Figure 5B); CaMKII wins after LTP-types stimuli while DAPK1 wins after LTD-type stimuli (Goodell et al., 2017).This is likely due to the essentially opposite regulation of their binding: CaMKII binding to GluN2B is induced by Ca2+/CaM and inhibited by GluN2B phosphorylation at S1303 (Bayer et al., 2001; O’Leary et al., 2011; Strack et al., 2000), while DAPK1 binding is disrupted by Ca2+/CaM and enhanced by GluN2B S1303 phosphorylation (Goodell et al., 2017). Opposing regulation of these two related CaMKs is enabled by distinct non-homologous binding mechanisms: CaMKII binding is mediated by its kinase domain (Bayer et al., 2001; Bayer et al., 2006; Robison et al., 2005), whereas DAPK1 binding is not (Goodell et al., 2017). Likewise, the Ca2+/CaM regulation is mediated via the regulatory domain for CaMKII (Bayer et al., 2001), but via a different site for DAPK1 (Goodell et al., 2017). Taken together, this CaMKII/DAPK1 competition adds another unexpected layer of biochemical computation that, in contrast to T286 phosphorylation, can distinguish between LTP- and LTD-stimuli and outlast phosphatases.
Figure 5. CaMKII binding to GluN2B: mechanisms and competition with DAPK1.
(A) CaMKII binding to GluN2B can be induced by either Ca2+/CaM or T286 phosphorylation alone. However, after the binding is established, no stimulus is required anymore. The binding site on GluN2B is around S1303, overlapping with the proposed DAPK1 binding site.
(B) CaMKII and DAPK1 compete for binding to GluN2B in vitro, with CaMKII winning during LTP-type stimuli (high Ca2+-concentrations) and DAPK1 winning during LTD-type stimuli (low Ca2+-concentrations). (Adapted from (Goodell et al., 2017).
CaMKII nitrosylation, oxidation, and GIcNAcylation: functions beyond pathology?
Various posttranslational modifications can occur in the CaMKII regulatory domain (see Figure 2C,D). Regulatory modulation of CaMKII by oxidation of M281 or by GlcNAcylation of S279 were first described for the CaMKIIδ isoform in the context of cardiac pathology (Erickson et al., 2008; Erickson et al., 2013)(reviewed in Anderson et al., 2011; Daniels et al., 2015). For GlcNAcylation, generation of autonomous activity has not been shown directly, but the process generates a conformational change detected by a FRET-based readout that is consistent with activation. For oxidation, the requirement of M281 was later extended to C289 (Coultrap et al., 2014b; Erickson et al., 2015), and both principle mechanisms were found also for the CaMKIIα isoform. Autonomy induced by S-nitrosylation was first described for CaMKIIα (Coultrap and Bayer, 2014), and subsequently for β and δ (Coultrap et al., 2014b; Erickson et al., 2015). While CaMKIIα requires concurrent nitrosylation of C280 and C289, the other isoforms require only nitrosylation of their C289 homologue (and they, in fact, lack a Cys homologous to C280). CaMKII autonomy generated by nitrosylation or oxidation can cause neuronal cell death (Coultrap and Bayer, 2014), but potential physiological functions in neuronal plasticity are not yet known. However, CaMKII gets hypo-nitrosylated with aging (due to increased expression of the NO-limiting enzyme GSNOR), which is correlated with aging-related deficiencies in LTP (Zhang et al., 2017). While this finding is intriguing, a direct causal relationship of CaMKII hypo-nitrosylation and lTp mal-functions remains to be tested.
“Inhibitory” phosphorylation caps further stimulation of autonomous CaMKII
Dissociation of Ca2+/CaM from T286-phosphorylated CaMKII triggers inhibitory autophosphorylation at T305/306 (Colbran and Soderling, 1990; Hanson and Schulman, 1992; Lou and Schulman, 1989). T305/306 residues are located in the CaM binding region of the regulatory domain, and Ca2+/CaM dissociation is required in order to make them accessible for phosphorylation by the autonomous kinase. T306 (but not T305) can also be autophosphorylated by basal CaMKII activity without stimulation, but at an extremely slow rate (õne reaction per 20 min) that may not be sufficient to counteract basal phosphatase activity within cells (Colbran, 1993). Interestingly, unlike T286 phosphorylation, autonomy generated by nitrosylation does not efficiently phosphorylate T305/306 (Coultrap and Bayer, 2014) and CaMKII binding to GluN2B even blocks this phosphorylation (Bayer et al., 2001). Conversely, T305/T306 phosphorylation inhibits binding to GluN2B and reduces targeting to the PSD (Barcomb et al., 2014; Elgersma et al., 2002).
The “inhibitory” T305/306 phosphorylation prevents re-binding and stimulation by Ca2+/CaM. Thus, the main effect is not inhibition per se, but prevention of stimulation to maximal activity. Specifically, kinase activity of the T286/305/306 triple-phosphorylated CaMKII is capped at the level of autonomous activity, i.e. ~20% of maximal activity. Notably, this form of activity (autonomy without further Ca2+/CaM-stimulation) is thought to favor phosphorylation of LTD-related CaMKII substrates (Coultrap et al., 2014a), and overexpression of a corresponding triple-phosphomimetic T286/305/306D mutant indeed promotes synaptic depression (Barcomb et al., 2014; Pi et al., 2010), while genetic block of this phosphorylation instead facilitates LTP (Elgersma et al., 2002). Thus, T305/306 phosphorylation could provide another potential computational switch to decide if T286-phosphorylated CaMKII promotes LTP or LTD. However, the requirement -or even the occurrence- of T305/306 phosphorylation during LTD still remains undetermined. Clearly, neither the holoenzyme mechanisms governing T305/306 phosphorylation nor its consequences have been fully explored.
CaMKII in induction versus maintenance of LTP and memory
CaMKII plays a number of important roles in synaptic plasticity, but is it also the memory molecule that maintains LTP and memory? Or is it “only” a key trigger for other maintenance mechanisms? It certainly acts on key substrates for the induction of LTP. During prototypical LTP at the hippocampal CA3 to CA1 synapse, Ca2+ influx through NMDA-type glutamate receptors (NMDARs) activates CaMKII, which in turn potentiates AMPA-type glutamate receptor (AMPAR) currents through a variety of mechanisms. Direct phosphorylation of the GluA1 subunit at S831 causes an increase in single channel conductance (Benke et al., 1998; Derkach et al., 1999; Kristensen et al., 2011), and a variety of indirect mechanisms cause an increase in synaptic channel number (reviewed in Diering and Huganir, 2018; Herring and Nicoll, 2016b; Shepherd and Huganir, 2007). These indirect mechanisms include CaMKII-mediated phosphorylation of regulatory proteins of small GTPases (such as SynGAP and trio/kalirin; Araki et al., 2015; Herring and Nicoll, 2016a; Walkup et al., 2015; Zhu et al., 2002) and of AMPA-receptor associated proteins (such as TARPs; Opazo et al., 2010; Park et al., 2016; Straub and Tomita, 2012). In addition to stimulation by Ca2+/CaM, two other CaMKII mechanisms are required for the full upregulation of glutamate receptor function during LTP: CaMKII T286 phosphorylation and GluN2B binding (Barria and Malinow, 2005; Giese et al., 1998; Halt et al., 2012).
CaMKII autonomy in LTP: T286 is for processing, GluN2B-binding is for storage?
The requirement for CaMKII and its autophosphorylation at T286 for LTP induction has been well established for almost 30 years (Giese et al., 1998; Malinow et al., 1989; Silva et al., 1992). A role of autonomous CaMKII also in the maintenance phase of LTP has been postulated, but was never demonstrated. The hypothesis can be tested by inhibitors that block autonomous activity, e.g. by ATP- or substrate-competitive inhibitors that are now available but not by the traditional allosteric inhibitors that are competitive with Ca2+/CaM (Neef et al., 2018; Pellicena and Schulman, 2014). Substrate-competitive peptide inhibitors (such as tatCN21) that block both stimulated and autonomous CaMKII activity with the same potency (Deng et al., 2017; Vest et al., 2007) efficiently block LTP induction but do not block LTP maintenance if added 15 min after induction (Figure 6A,B)(Buard et al., 2010). Similarly, rapid optogenetic release of another substrate-competitive peptide inhibitor in neurons indicates that CaMKII activity is no longer required even 1 min after LTP induction (Murakoshi et al., 2017). Furthermore, optical probes of CaMKII activation indicate that T286 phosphorylation acutely contributes to the integration of Ca2+ signals during LTP induction, but is then largely reversed in dendritic spines at least within 2 min after LTP stimuli (Chang et al., 2017; Lee et al., 2009). This is consistent with prior studies that used more traditional methods: While an increase in T286-phosphorylated CaMKII has been detected long after LTP, this also correlated with an increase in total CaMKII and the increase was seen only in the neuronal soma, but not in dendrites or spines (Fukunaga et al., 1993; Ouyang et al., 1997). Together, these findings strongly indicate that T286 phosphorylation is not required for the maintenance phase of LTP. Interestingly, a more persistent increase in T286 phosphorylation has been detected instead following LTD stimuli (Figure 6C)(Marsden et al., 2010; Mockett et al., 2011), but a possible function in maintenance of LTD has not yet been examined.
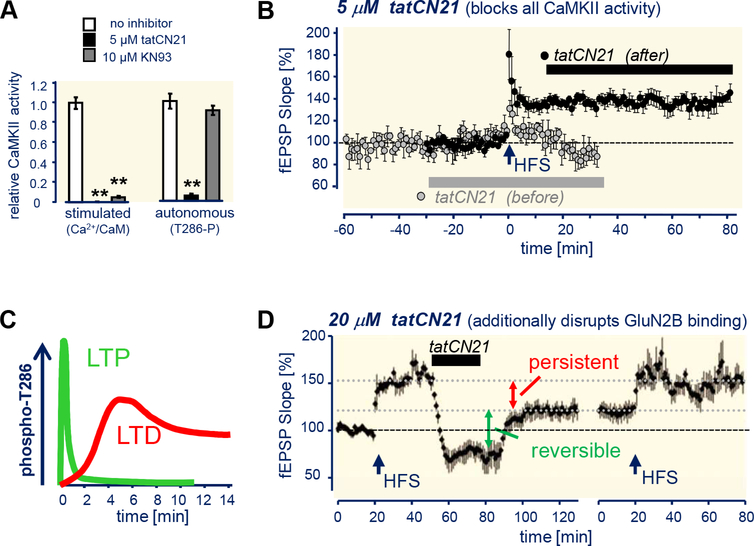
Figure 6. CaMKII T286 phosphorylation and GluN2B binding in LTP induction versus maintenance.
(A) tatCN21 but not KN93 inhibits autonomous CaMKII. (Adapted from (Vest et al., 2010).
(B) tatCN21 (5 μM) blocks LTP induction by high-frequency stimulation (HFS) but does not interfere with its maintenance. (Adapted from (Buard et al., 2010).
(C) CaMKII T286 autophosphorylation is more rapidly reversed after LTP than LTD. (As previously reviewed in Coultrap and Bayer, 2012).
(D) Higher tatCN21 concentrations (20 pM) that additionally disrupt CaMKII/GluN2B binding persistently reverse LTP maintenance. LTP can be re-established by additional HFS stimulation, indicating complete washout of the drug and slice health (Adapted from (Sanhueza et al., 2011).
It has been argued that T286 phosphorylation could nonetheless be required for LTP maintenance, but that it has evaded detection because it is required only for a small pool of the synaptic CaMKII (Lisman et al., 2012). Indeed, there are two pools of synaptically targeted CaMKII that are also expected to be able to evade inhibition by the peptide inhibitors in the functional assays described above: CaMKII bound to either GluN2B or densin-180, as the binding of these proteins and the inhibitors should be mutually exclusive (because they occur at the same site on CaMKII, the “T-site” that also interacts with the regulatory domain region around T286; see Figure 2)(Bayer et al., 2001; Jiao et al., 2011; Vest et al., 2007). GluN2B-bound CaMKII is the more likely relevant candidate, as GluN2B mutations that abolish CaMKII binding indeed impair LTP (Barria and Malinow, 2005; Halt et al., 2012) while densin-180 knockout instead impairs LTD (Carlisle et al., 2011). However, in this scenario, GluN2B binding would be more important for lTp maintenance than T286 phosphorylation. Indeed, increasing the peptide inhibitor concentrations to levels that additionally disrupt the CaMKII/GluN2B binding did reduce synaptic strength and reversed LTP (Figure 6D)(Barcomb et al., 2016; Sanhueza et al., 2011). Furthermore, while neither constitutive CaMKII knockout nor acute CaMKII inhibition appear to affect basal synaptic transmission, a “medium-term” CRISPR-based deletion/replacement recently indicated a requirement of CaMKII also in basal transmission (Incontro et al., 2018). Thus, CaMKII indeed appears to be a “memory molecule” that is important for maintenance of synaptic strength, as originally proposed. However, T286 phosphorylation is most likely important only for the signal processing during the induction of LTP, while GluN2B binding may additionally enable the information storage provided by maintenance of LTP.
Does a “dead” CaMKII disrupt memories, and if so, how?
After decades of speculation, last year saw the first direct evidence for an involvement of CaMKII signaling not only in the generation but also in the storage of hippocampus-dependent memory in rats (Rossetti et al., 2017). In this study, CaMKII signaling was disrupted in vivo by transient virusmediated overexpression of the kinase-dead CaMKII K42M mutant in the hippocampus (Figure 7A,B). The same approach had previously shown that transient expression of this CaMKII mutant in the nucleus accumbens shell reverses maladaptive addiction-related memory; specifically, it reversed the increase in amphetamine self-administration that is triggered by pre-exposing rats to amphetamine (Loweth et al., 2013). The CaMKII K42M mutation prevents kinase activity by disabling binding of nucleotides, including ATP. Its overexpression should not affect the total CaMKII activity within a cell per se, but it could interfere with CaMKII autonomy generated by either T286 phosphorylation or GluN2B binding: As T286 autophosphorylation occurs between subunits of a holoenzyme, insertion of kinase-dead subunits should interfere with this process. GluN2B binding does not require kinase activity (Barcomb et al., 2013), but is dramatically enhanced by nucleotide binding (O’Leary et al., 2011); thus, the K42M mutant should reduce this binding. Indeed, the K42M mutant does not show any significant binding to GluN2B in heterologous cells or neurons (O’Leary et al., 2011). As discussed above, phosphatase-resistant GluN2B binding is the more likely of the two mechanisms to mediate long-term information storage, and there is additional indication in favor of the GluN2B mechanism: Memory is stable in T286A mice when induced by massed learning (Irvine et al., 2011; Radwanska et al., 2011; Villers et al., 2014), and injection of a peptide inhibitor in vivo (at concentrations expected to interfere with activity but not GluN2B binding) prevented learning but not memory or recall (Buard et al., 2010). Further, the LTP-induced phosphorylation of GluA1 at S831 requires CaMKII binding to GluN2B (Halt et al., 2012), and transient K42m expression persistently reduced phosphorylation of this site (Loweth et al., 2013). Nonetheless, it remains to be determined which specific CaMKII mechanisms are involved in the storage of memory. In fact, an additional possible mechanism has just been described last month: CaMKII binding to the GTPase-regulatory protein Tiam1 promotes the persistence of structural LTP of dendritic spines and this binding is reduced by the a similar CaMKII mutation, K42R (Saneyoshi et al., 2019).
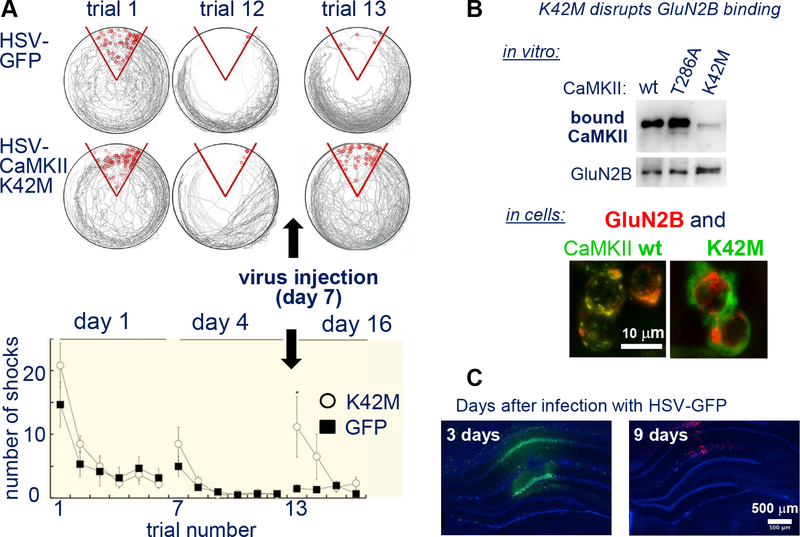
Figure 7. CaMKII function in memory: erasure by a kinase dead K42M mutant.
(A) Transient viral (HSV) expression of the CaMKII K42M mutant (but not of GFP control) caused erasure of conditioned place avoidance. Rats were placed on a rotating platform that will eventually bring the rat into a shock zone (red), that the rats can identify by spatial cues. Over a course of twelve 10 min trials, rats learn to avoid the shock, as measured by the reduction of shocks received. The memory was retained in a subsequent trial twelve days later, but not after CaMKII K42M expression. (Adapted from (Rossetti et al., 2017).
(B) The CaMKII K42M mutation impairs CaMKII binding to GluN2B in vitro in heterologous cells (adapted from (O’Leary et al., 2011); this effect is indirect: K42M prevents the nucleotide binding to CaMKII that positively regulates GluN2B binding.
(C) GFP expression in hippocampus after infection with HSV-GFP is transient. However, while this has been shown also for a different GFP-CaMKII mutant, it remains to be formally demonstrated for GFP-CaMKII K42M mutant.
Both of the studies that used viral overexpression of the K42M mutant are very convincing, yet both leave distinct small loop holes that allow for alternative interpretation: In each study, the conclusion depends on the transient nature of the expression, i.e. on the K42M mutant no longer being expressed at the time of memory testing (otherwise, the effect could be on acute memory recall rather than on memory storage). Indeed, the HSV-based viral vectors used in both studies typically cause suitable transient expression, and the addiction-related study showed that CaMKII expression levels were increased transiently and then back to baseline by the time of testing (Loweth et al., 2013). However, as the overexpressed mutant was not tagged, it is formally possible that the K42M mutant was still expressed, and that overall expression levels appeared normal due to downregulation of endogenous wild type kinase. In contrast, the study on hippocampal memory used GFP-tagged mutants, yet tested the expression only for GFP and for a less informative mutant but not for the more important K42M mutant (Rossetti et al., 2017). While expression of GFP or the other CaMKII mutant was no longer detected at the time of testing (Figure 7C), it is formally possible that the K42M mutant is significantly more stable. Thus, despite very compelling recent evidence, the matter of CaMKII functions in memory storage may still not be settled completely.
Beyond LTP: CaMKII functions in LTD and at inhibitory synapses
While postsynaptic functions of CaMKII in excitatory LTP have been featured most prominently, functions on the presynaptic side and at other synapse types have been known for a long time. This includes, for instance, the CaMKII function in regulating dopamine biosynthesis (Nose et al., 1985; Sumi et al., 1991), and some of the first described CaMKII substrates are pre-synaptic (Kennedy et al., 1983). More recently, an essential function of CaMKII also in LTD has become evident, and the first mechanisms that allow CaMKII to mediate two opposing directions of synaptic plasticity are emerging. Notably, in LTD, CaMKII functions may additionally include hetero-synaptic communication to inhibitory synapses. These two emerging postsynaptic topics will be discussed here.
CaMKII functions in LTD
Like LTP, NMDAR-dependent LTD is blocked by CaMKII inhibition, knock-out, or T286A mutation (Figure 8A)(Coultrap et al., 2014a). But how can CaMKII mediate both LTP and LTD? It appears that at least two distinct and independent mechanisms are required: (i) differential CaMKII trafficking and (ii) differential substrate selection. While normal LTP requires the synaptic accumulation of CaMKII that is mediated by GluN2B binding, LTD does not (Halt et al., 2012). As described above, DAPK1 activation during LTD suppresses synaptic CaMKII accumulation, and this suppression mechanism is indeed required for LTD (Goodell et al., 2017). Thus, differential synaptic targeting appears to enable CaMKII mediating two opposing forms of synaptic plasticity (Figure 8B). Notably, the LTD-induced DAPK1 activation requires dephosphorylation of its regulatory domain at S308 by calcineurin (Goodell et al., 2017), a Ca2+-dependent phosphatase that is well established to be required for LTD (Mulkey et al., 1994; Zeng et al., 2001). Thus, LTD requires a concerted action of Ca2+-dependent kinases and phosphatases (in contrast to traditional models in which kinases mediate lTp and phosphatases mediate LTD).
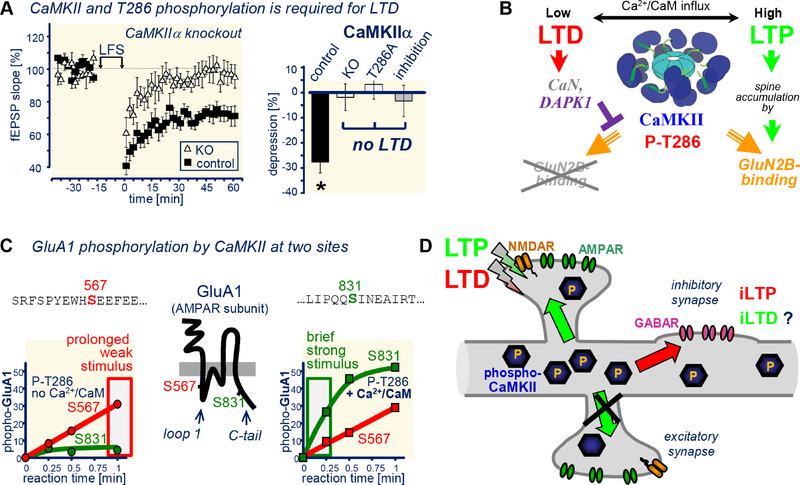
Figure 8. CaMKII in LTD: mechanisms that enable roles in opposing forms of plasticity.
(A) CaMKII requirement in LTD: Low frequency (LFS)-induced LTP is blocked by CaMKII knockout (KO), T286A mutation, and by acute inhibition (with 5 μM tatCN21; adapted from (Coultrap et al., 2014a).
(B) LTP-specific CaMKII targeting to excitatory synapses is mediated by LTD-specific suppression of such targeting by calcineurin-dependent activation of dApKI (adapted from (Goodell et al., 2017).
(C) Differential phosphorylation of GluA S567 versus S831, with S567 being an unusual substrate that is equally well phosphorylated by stimulated and autonomous activity, and S831 being a typical substrate that is more readily phosphorylated when additionally stimulated with Ca2+/CaM (adapted from (Coultrap et al., 2014a). Notably, CaMKII prefers an Arg in the −3 position, but this is lacking in both of these substrate sites on GluA1.
(D) Both LTP and LTD induce CaMKII T286 phosphorylation, but LTP-stimuli trigger input-specific CaMKII movement to the stimulated excitatory synapses, while LTD-stimuli instead trigger CaMKII movement to inhibitory synapses. At excitatory synapses CaMKII is required for LTP and LTD; at inhibitory synapses, it is at least required for the iLTP that is induced by excitatory LTD.
A second difference is that LTP-type stimuli (strong but brief) versus LTD-type stimuli (weak but prolonged) lead to differential substrate site preference of CaMKII on GluA1, e.g. at S831 versus S567 (Coultrap et al., 2014a). Indeed, this differential phosphorylation should promote LTP versus LTD, as S831 phosphorylation increases AMPAR conductance (Derkach et al., 1999; Kristensen et al., 2011) while S567 phosphorylation reduces synaptic AMpAr localization (Lu et al., 2010). Notably, while LTP-induced S831 phosphorylation requires CaMKII binding to GluN2B (Halt et al., 2012), the differential substrate site selection on GluA1 also occurs in solution in vitro, and is thus supported by additional mechanisms that are completely independent of targeting (Coultrap et al., 2014a): While S831 is a regular CaMKII substrate for which phosphorylation by autonomous CaMKII can be further promoted by additional Ca2+/CaM stimulation, S567 is a “high-autonomy” substrate that is phosphorylated equally well by autonomous CaMKII in the presence or absence of Ca2+/CaM. Indeed, this mechanism favors S831 phosphorylation during strong and brief LTP-like stimuli, while prolonged (LTD-like) stimuli instead favor s567 phosphorylation (Figure 8C)(Coultrap et al., 2014a). An even more extreme high-autonomy substrate is AKAP79/150, a synaptic anchoring protein for PKA and calcineurin: Autonomous CaMKII phosphorylates this substrate even better in absence of Ca2+/CaM, resulting in apparent autonomy of over 300%. The underlying mechanism is Ca2+-dependent CaM binding to the substrate site, which protects it from phosphorylation during Ca2+-stimuli (Woolfrey et al., 2018); the mechanism underlying the high autonomy towards GluA1 S567 is still unknown, but differs from AKAP79/150 (Coultrap et al., 2014a; Woolfrey et al., 2018). CaMKII activity is required for the LTD-induced removal of AKAP79/150 from dendritic spines, which is in turn required for the spine shrinkage thought to be the structural correlate of LTD (Woolfrey et al., 2018). However, while direct CaMKII mediated phosphorylation contributes to the AKAP79/150 movement (by disrupting interactions with F-actin), a more important function may be CaMKII signaling that leads to de-palmitoylation of AKAP79/150. While it is currently unknown how this de-palmitoylation event is regulated by CaMKII, we speculate that most LtD-promoting CaMKII substrates will be similar to GluA1 S567 or AKAP79/150, i.e. “high-autonomy substrates” that are equally or better phosphorylated by autonomous CaMKII in absence of Ca2+/CaM.
Based on the substrate-selection model described above, the prediction is that further stimulation of autonomous CaMKII (to reach maximal activity) would promote LTP, while autonomous CaMKII without further stimulation would promote LTD. Indeed, CaMKII mutants that are constitutively autonomous (by T286D mutation) promote synaptic potentiation when they can be further stimulated, but promote synaptic depression when further stimulation is prevented (by T305/306 phosphorylation, T305/306D mutation, or other mutations that prevent CaM binding)(Barcomb et al., 2014; Pi et al., 2010). This is also consistent with earlier findings that expression of truncated CaMKII 1–290 (Hayashi et al., 2000): this truncation completely lacks auto-inhibition and thus generates maximal activity, rather than the lower level of autonomous activity.
Forms of lTd that are dependent on metabotropic glutamate receptors (mGluRs) can also be regulated by CaMKII. However, this will require further investigation, as it is currently solely based on inhibitor studies that reported conflicting directions of regulation (Bernard et al., 2014; Mockett et al., 2011; Schnabel et al., 1999).
CaMKII at inhibitory synapses
Less attention has been focused on the action of CaMKII at inhibitory synapses, even though it has been known for over 20 years that CaMKII can potentiate the inhibitory GABAA-R currents: Infusing hippocampal neurons with autonomous CaMKII persistently enhanced inhibitory postsynaptic potentials (Wang et al., 1995). More recently, it has been shown that CaMKII T286 phosphorylation indeed mediates long-term potentiation of inhibitory synapses (iLTP) in response to excitatory LTD-type stimulation of NMDARs, in both hippocampal cultures (Marsden et al., 2010) and acute slices (Chiu et al., 2018). This CaMKII-dependent iLTP may involve several phosphorylation sites, most likely including S383 on the GaBAA-R β3 subunit (Houston et al., 2007; Petrini et al., 2014; Saliba et al., 2012). Similar to excitatory lTp, iLTP also appears to require CaMKII translocation, in this case to inhibitory synapses (Cook et al., 2019; Marsden et al., 2010). However, while synaptic CaMKII translocation during excitatory LTP is input specific (Zhang et al., 2008), the accumulation of CaMKII during the iLTP that is induced by excitatory NMDAR stimulation is instead an example for trans-synaptic communication (Figure 8D). In this case, the communication of excitatory lTd stimuli to additionally induce iLTP at inhibitory synapses further tips the excitatory/inhibitory balance towards inhibitory synapses, thereby further coordinating the neuronal response in one direction. In another example of trans-synaptic communication, LTD-type NMDAR stimuli can also cause CaMKII-dependent strengthening of nearby electrical gap-junction synapses (Turecek et al., 2014). Whether or not CaMKII bi-directionally regulates not only excitatory but also inhibitory (and maybe electrical) synapses remains to be determined.
Closing remarks
CaMKII has been studied extensively over the past 40 years, and much has been learned about its complex regulation and many neuronal functions; in fact, much more than could be summarized in this review. Nonetheless, much remains to be discovered, with some of the “known unknowns” pointed out here. Additionally, we expect that new discoveries on the “unknown unknowns” of this intriguing kinase will continue to surprise and inform us for years to come.
Acknowledgements
We thank all members of the Bayer lab, for helpful discussions and for critical reading of the manuscript. We are grateful to Sarah Cook (University of Colorado) and Dr. Steve Reichow (Portland State University) for additional help with several Figures. The work was funded in part by National Institutes of Health grants R01NS081248, R01NS080851, and R01NS110383 (to K.U.B.).
Footnotes
Declaration of interests: The University of Colorado holds the patent rights for tatCN21, its derivatives, and its uses (PCT/US08/077934 “Compositions and methods for improved CaMKII inhibitors and uses thereof”). K.U.B. is co-founder of Neurexus Therapeutics, LLC; H.S. is cofounder of Allosteros Therapeutics, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson ME, Brown JH, and Bers DM (2011). CaMKII in myocardial hypertrophy and heart failure. J Mol Cell Cardiol 51, 468–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki Y, Zeng M, Zhang M, and Huganir RL (2015). Rapid dispersion of SynGAP from synaptic spines triggers AMPA receptor insertion and spine enlargement during LTP. Neuron 85, 173–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcomb K, Buard I, Coultrap SJ, Kulbe JR, O’Leary H, Benke TA, and Bayer KU (2014). Autonomous CaMKII requires further stimulation by Ca2+/calmodulin for enhancing synaptic strength. FASEB J 28, 3810–3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcomb K, Coultrap SJ, and Bayer KU (2013). Enzymatic activity of CaMKII is not required for its interaction with the glutamate receptor subunit GluN2B. Mol Pharmacol 84, 834–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcomb K, Hell JW, Benke TA, and Bayer KU (2016). The CaMKII/GluN2B Protein Interaction Maintains Synaptic Strength. J Biol Chem 291, 16082–16089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barria A, and Malinow R (2005). NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron 48, 289–301. [DOI] [PubMed] [Google Scholar]
- Bayer KU, De Koninck P, Leonard AS, Hell JW, and Schulman H (2001). Interaction with the NMDA receptor locks CaMKII in an active conformation. Nature 411, 801–805. [DOI] [PubMed] [Google Scholar]
- Bayer KU, De Koninck P, and Schulman H (2002). Alternative splicing modulates the frequency-dependent response of CaMKII to Ca(2+) oscillations. EMBO J 21, 3590–3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer KU, Harbers K, and Schulman H (1998). alphaKAP is an anchoring protein for a novel CaM kinase II isoform in skeletal muscle. EMBO J 17, 5598–5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer KU, LeBel E, McDonald GL, O’Leary H, Schulman H, and De Koninck P (2006). Transition from reversible to persistent binding of CaMKII to postsynaptic sites and NR2B. J Neurosci 26, 1164–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer KU, Lohler J, and Harbers K (1996). An alternative, nonkinase product of the brain-specifically expressed Ca2+/calmodulin-dependent kinase II alpha isoform gene in skeletal muscle. Mol Cell Biol 16, 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer KU, Lohler J, Schulman H, and Harbers K (1999). Developmental expression of the CaM kinase II isoforms: ubiquitous gamma- and delta-CaM kinase II are the early isoforms and most abundant in the developing nervous system. Brain research Molecular brain research 70, 147–154. [DOI] [PubMed] [Google Scholar]
- Benke TA, Luthi A, Isaac JT, and Collingridge GL (1998). Modulation of AMPA receptor unitary conductance by synaptic activity. Nature 393, 793–797. [DOI] [PubMed] [Google Scholar]
- Bernard PB, Castano AM, Bayer KU, and Benke TA (2014). Necessary, but not sufficient: insights into the mechanisms of mGluR mediated long-term depression from a rat model of early life seizures. Neuropharmacology 84, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya M, Stratton MM, Going CC, McSpadden ED, Huang Y, Susa AC, Elleman A, Cao YM, Pappireddi N, Burkhardt P, et al. (2016). Molecular mechanism of activation-triggered subunit exchange in Ca(2+)/calmodulin-dependent protein kinase II. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgesius NZ, van Woerden GM, Buitendijk GH, Keijzer N, Jaarsma D, Hoogenraad CC, and Elgersma Y (2011). betaCaMKII plays a nonenzymatic role in hippocampal synaptic plasticity and learning by targeting alphaCaMKII to synapses. J Neurosci 31, 10141–10148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudeau J, Miranda-Saavedra D, Barton GJ, and Alessi DR (2006). Emerging roles of pseudokinases. Trends Cell Biol 16, 443–452. [DOI] [PubMed] [Google Scholar]
- Brocke L, Srinivasan M, and Schulman H (1995). Developmental and regional expression of multifunctional Ca2+/calmodulin-dependent protein kinase isoforms in rat brain. J Neurosci 15, 6797–6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buard I, Coultrap SJ, Freund RK, Lee YS, Dell’Acqua ML, Silva AJ, and Bayer KU (2010). CaMKII “autonomy” is required for initiating but not for maintaining neuronal long-term information storage. J Neurosci 30, 8214–8220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgin KE, Waxham MN, Rickling S, Westgate SA, Mobley WC, and Kelly PT (1990). In situ hybridization histochemistry of Ca2+/calmodulin-dependent protein kinase in developing rat brain. J Neurosci 10, 1788–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caenepeel S, Charydczak G, Sudarsanam S, Hunter T, and Manning G (2004). The mouse kinome: discovery and comparative genomics of all mouse protein kinases. Proc Natl Acad Sci U S A 101, 11707–11712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlisle HJ, Luong TN, Medina-Marino A, Schenker L, Khorosheva E, Indersmitten T, Gunapala KM, Steele AD, O’Dell TJ, Patterson PH, et al. (2011). Deletion of densin-180 results in abnormal behaviors associated with mental illness and reduces mGluR5 and DISC1 in the postsynaptic density fraction. J Neurosci 31, 16194–16207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JY, Parra-Bueno P, Laviv T, Szatmari EM, Lee SR, and Yasuda R (2017). CaMKII Autophosphorylation Is Necessary for Optimal Integration of Ca(2+) Signals during LTP Induction, but Not Maintenance. Neuron 94, 800–808 e804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao LH, Stratton MM, Lee IH, Rosenberg OS, Levitz J, Mandell DJ, Kortemme T, Groves JT, Schulman H, and Kuriyan J (2011). A mechanism for tunable autoinhibition in the structure of a human Ca2+/calmodulin- dependent kinase II holoenzyme. Cell 146, 732–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CQ, Martenson JS, Yamazaki M, Natsume R, Sakimura K, Tomita S, Tavalin SJ, and Higley MJ (2018). Input-Specific NMDAR-Dependent Potentiation of Dendritic GABAergic Inhibition. Neuron 97, 368–377 e363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P, Burchell A, Foulkes JG, Cohen PT, Vanaman TC, and Nairn C (1978). Identification of the Ca2+-dependent modulator protein as the fourth subunit of rabbit skeletal muscle phosphorylase kinase. FEBS Lett 92, 287–293. [DOI] [PubMed] [Google Scholar]
- Colbran RJ (1993). Inactivation of Ca2+/calmodulin-dependent protein kinase II by basal autophosphorylation. J Biol Chem 268, 7163–7170. [PubMed] [Google Scholar]
- Colbran RJ (2004). Targeting of calcium/calmodulin-dependent protein kinase II. Biochem J 378, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbran RJ, Smith MK, Schworer CM, Fong YL, and Soderling TR (1989). Regulatory domain of calcium/calmodulin-dependent protein kinase II. Mechanism of inhibition and regulation by phosphorylation. J Biol Chem 264, 4800–4804. [PubMed] [Google Scholar]
- Colbran RJ, and Soderling TR (1990). Calcium/calmodulin-independent autophosphorylation sites of calcium/calmodulin-dependent protein kinase II. Studies on the effect of phosphorylation of threonine 305/306 and serine 314 on calmodulin binding using synthetic peptides. J Biol Chem 265, 11213–11219. [PubMed] [Google Scholar]
- Cook SG, Bourke AM, O’Leary H, Zaegel V, Lasda E, Mize-Berge J, Quillinan N, Tucker CL, Coultrap SJ, Herson PS, et al. (2018). Analysis of the CaMKIIalpha and beta splice-variant distribution among brain regions reveals isoform-specific differences in holoenzyme formation. Sci Rep 8, 5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook SG, Goodell DJ, Restrepo S, Arnold DB, and Bayer KU (2019). Simultaneous live-imaging of multiple endogenous proteins reveals a mechanism for Alzheimer’s- related plasticity impairment. Cell Rep 27, 658–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coultrap SJ, and Bayer KU (2012). CaMKII regulation in information processing and storage. Trends in neurosciences 35, 607–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coultrap SJ, and Bayer KU (2014). Nitric oxide induces Ca2+-independent activity of the Ca2+/calmodulin-dependent protein kinase II (CaMKII). J Biol Chem 289, 19458–19465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coultrap SJ, Buard I, Kulbe JR, Dell’Acqua ML, and Bayer KU (2010). CaMKII autonomy is substrate-dependent and further stimulated by Ca2+/calmodulin. J Biol Chem 285, 17930–17937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coultrap SJ, Freund RK, O’Leary H, Sanderson JL, Roche KW, Dell’Acqua ML, and Bayer KU (2014a). Autonomous CaMKII mediates both LTP and LTD using a mechanism for differential substrate site selection. Cell Reports 6, 431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coultrap SJ, Zaegel V, and Bayer KU (2014b). CaMKII isoforms differ in their specific requirements for regulation by nitric oxide. FEBS Lett 588, 4672–4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels L, Bell JR, Delbridge LM, McDonald FJ, Lamberts RR, and Erickson JR (2015). The role of CaMKII in diabetic heart dysfunction. Heart Fail Rev 20, 589–600. [DOI] [PubMed] [Google Scholar]
- De Koninck P, and Schulman H (1998). Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations. Science 279, 227–230. [DOI] [PubMed] [Google Scholar]
- Debant A, Serra-Pages C, Seipel K, O’Brien S, Tang M, Park SH, and Streuli M (1996). The multidomain protein Trio binds the LAR transmembrane tyrosine phosphatase, contains a protein kinase domain, and has separate rac-specific and rho- specific guanine nucleotide exchange factor domains. Proc Natl Acad Sci U S A 93, 5466–5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng G, Orfila JE, Dietz RM, Moreno-Garcia M, Rodgers KM, Coultrap SJ, Quillinan N, Traystman RJ, Bayer KU, and Herson PS (2017). Autonomous CaMKII Activity as a Drug Target for Histological and Functional Neuroprotection after Resuscitation from Cardiac Arrest. Cell Rep 18, 1109–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkach V, Barria A, and Soderling TR (1999). Ca2+/calmodulin-kinase II enhances channel conductance of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate type glutamate receptors. Proc Natl Acad Sci U S A 96, 3269–3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diering GH, and Huganir RL (2018). The AMPA Receptor Code of Synaptic Plasticity. Neuron 100, 314–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgersma Y, Fedorov NB, Ikonen S, Choi ES, Elgersma M, Carvalho OM, Giese KP, and Silva AJ (2002). Inhibitory autophosphorylation of CaMKII controls PSD association, plasticity, and learning. Neuron 36, 493–505. [DOI] [PubMed] [Google Scholar]
- Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O’Donnell SE, Aykin-Burns N, et al. (2008). A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell 133, 462–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JR, Nichols CB, Uchinoumi H, Stein ML, Bossuyt J, and Bers DM (2015). S-Nitrosylation Induces Both Autonomous Activation and Inhibition of Calcium/Calmodulin-dependent Protein Kinase II delta. J Biol Chem 290, 25646–25656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JR, Pereira L, Wang L, Han G, Ferguson A, Dao K, Copeland RJ, Despa F, Hart GW, Ripplinger CM, et al. (2013). Diabetic hyperglycaemia activates CaMKII and arrhythmias by O-linked glycosylation. Nature 502, 372–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyers PA, Keeshan K, and Kannan N (2017). Tribbles in the 21st Century: The Evolving Roles of Tribbles Pseudokinases in Biology and Disease. Trends Cell Biol 27, 284–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink CC, Bayer KU, Myers JW, Ferrell JE Jr., Schulman H, and Meyer T (2003). Selective regulation of neurite extension and synapse formation by the beta but not the alpha isoform of CaMKII. Neuron 39, 283–297. [DOI] [PubMed] [Google Scholar]
- Fu Y, and Rubin CS (2011). Protein kinase D: coupling extracellular stimuli to the regulation of cell physiology. EMBO Rep 12, 785–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H, Inoue M, Okuno H, Sano Y, Takemoto-Kimura S, Kitamura K, Kano M, and Bito H (2013). Nonlinear decoding and asymmetric representation of neuronal input information by CaMKIIalpha and calcineurin. Cell Rep 3, 978–987. [DOI] [PubMed] [Google Scholar]
- Fukunaga K, Stoppini L, Miyamoto E, and Muller D (1993). Long-term potentiation is associated with an increased activity of Ca2+/calmodulin-dependent protein kinase II. J Biol Chem 268, 7863–7867. [PubMed] [Google Scholar]
- Gaestel M (2006). MAPKAP kinases - MKs - two’s company, three’s a crowd. Nat Rev Mol Cell Biol 7, 120–130. [DOI] [PubMed] [Google Scholar]
- Giese KP, Fedorov NB, Filipkowski RK, and Silva AJ (1998). Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science 279, 870–873. [DOI] [PubMed] [Google Scholar]
- Goodell DJ, Zaegel V, Coultrap SJ, Hell JW, and Bayer KU (2017). DAPK1 Mediates LTD by Making CaMKII/GluN2B Binding LTP Specific. Cell Rep 19, 2231–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BD, and Adams JA (1996). Pre-steady-state kinetic analysis of cAMP-dependent protein kinase using rapid quench flow techniques. Biochemistry 35, 2022–2029. [DOI] [PubMed] [Google Scholar]
- Halt AR, Dallpiazza RF, Zhou Y, Stein IS, Qian H, Juntti S, Wojcik S, Brose N, Silva AJ, and Hell JW (2012). CaMKII binding to GluN2B is critical during memory consolidation. EMBO J 31, 1203–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson PI, Meyer T, Stryer L, and Schulman H (1994). Dual role of calmodulin in autophosphorylation of multifunctional CaM kinase may underlie decoding of calcium signals. Neuron 12, 943–956. [DOI] [PubMed] [Google Scholar]
- Hanson PI, and Schulman H (1992). Inhibitory autophosphorylation of multifunctional Ca2+/calmodulin-dependent protein kinase analyzed by site-directed mutagenesis. J Biol Chem 267, 17216–17224. [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, and Malinow R (2000). Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science 287, 2262–2267. [DOI] [PubMed] [Google Scholar]
- Hell JW (2014). CaMKII: claiming center stage in postsynaptic function and organization. Neuron 81, 249–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring BE, and Nicoll RA (2016a). Kalirin and Trio proteins serve critical roles in excitatory synaptic transmission and LtP . Proc Natl Acad Sci U S A 113, 2264–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring BE, and Nicoll RA (2016b). Long-Term Potentiation: From CaMKII to AMPA Receptor Trafficking. Annu Rev Physiol 78, 351–365. [DOI] [PubMed] [Google Scholar]
- Herzig S, and Shaw RJ (2018). AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol 19, 121–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoelz A, Nairn AC, and Kuriyan J (2003). Crystal structure of a tetradecameric assembly of the association domain of Ca2+/calmodulin-dependent kinase II. Mol Cell 11, 1241–1251. [DOI] [PubMed] [Google Scholar]
- Hojjati MR, van Woerden GM, Tyler WJ, Giese KP, Silva AJ, Pozzo-Miller L, and Elgersma Y (2007). Kinase activity is not required for alphaCaMKII-dependent presynaptic plasticity at CA3-CA1 synapses. Nat Neurosci 10, 1125–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston CM, Lee HH, Hosie AM, Moss SJ, and Smart TG (2007). Identification of the sites for CaMK-II-dependent phosphorylation of GABA(A) receptors. J Biol Chem 282, 17855–17865. [DOI] [PubMed] [Google Scholar]
- Huttner WB, and Greengard P (1979). Multiple phosphorylation sites in protein I and their differential regulation by cyclic AMP and calcium. Proc Natl Acad Sci U S A 76, 5402–5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incontro S, Diaz-Alonso J, Iafrati J, Vieira M, Asensio CS, Sohal VS, Roche KW, Bender KJ, and Nicoll RA (2018). The CaMKII/NMDA receptor complex controls hippocampal synaptic transmission by kinase-dependent and independent mechanisms. Nat Commun 9, 2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine EE, Danhiez A, Radwanska K, Nassim C, Lucchesi W, Godaux E, Ris L, and Giese KP (2011). Properties of contextual memory formed in the absence of alphaCaMKII autophosphorylation. Mol Brain 4, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Jalan-Sakrikar N, Robison AJ, Baucum AJ, 2nd, Bass, M.A., and Colbran, R.J. (2011). Characterization of a central Ca2+/calmodulin-dependent protein kinase Ilalpha/beta binding domain in densin that selectively modulates glutamate receptor subunit phosphorylation. J Biol Chem 286, 24806–24818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinesh GG, Mokkapati S, Zhu K, and Morales EE (2016). Pim kinase isoforms: devils defending cancer cells from therapeutic and immune attacks. Apoptosis 21, 1203–1213. [DOI] [PubMed] [Google Scholar]
- Kennedy MB, McGuinness T, and Greengard P (1983). A calcium/calmodulin-dependent protein kinase from mammalian brain that phosphorylates Synapsin I: partial purification and characterization. J Neurosci 3, 818–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Lakhanpal G, Lu HE, Khan M, Suzuki A, Hayashi MK, Narayanan R, Luyben TT, Matsuda T, Nagai T, et al. (2015). A Temporary Gating of Actin Remodeling during Synaptic Plasticity Consists of the Interplay between the Kinase and Structural Functions of CaMKII. Neuron 87, 813–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs EG, and Fischer EH (1956). The phosphorylase b to a converting enzyme of rabbit skeletal muscle. Biochim Biophys Acta 20, 150–157. [DOI] [PubMed] [Google Scholar]
- Krebs EG, Kent AB, and Fischer EH (1958). The muscle phosphorylase b kinase reaction. J Biol Chem 231, 73–83. [PubMed] [Google Scholar]
- Kristensen AS, Jenkins MA, Banke TG, Schousboe A, Makino Y, Johnson RC, Huganir R, and Traynelis SF (2011). Mechanism of Ca2+/calmodulin-dependent kinase II regulation of AMPA receptor gating. Nat Neurosci 14, 727–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y, Nairn AC, and Greengard P (1986). Autophosphorylation reversibly regulates the Ca2+/calmodulin-dependence of Ca2+/calmodulin-dependent protein kinase II. Proc Natl Acad Sci U S A 83, 4253–4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Escobedo-Lozoya Y, Szatmari EM, and Yasuda R (2009). Activation of CaMKII in single dendritic spines during long-term potentiation. Nature 458, 299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew J, Taylor SS, and Adams JA (1997). Identification of a partially rate-determining step in the catalytic mechanism of cAMP-dependent protein kinase: a transient kinetic study using stopped-flow fluorescence spectroscopy. Biochemistry 36, 6717–6724. [DOI] [PubMed] [Google Scholar]
- Lin SC, and Hardie DG (2018). AMPK: Sensing Glucose as well as Cellular Energy Status. Cell Metab 27, 299–313. [DOI] [PubMed] [Google Scholar]
- Lisman J, Yasuda R, and Raghavachari S (2012). Mechanisms of CaMKII action in longterm potentiation. Nature reviews Neuroscience 13, 169–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou LL, Lloyd SJ, and Schulman H (1986). Activation of the multifunctional Ca2+/calmodulin-dependent protein kinase by autophosphorylation: ATP modulates production of an autonomous enzyme. Proc Natl Acad Sci U S A 83, 9497–9501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou LL, and Schulman H (1989). Distinct autophosphorylation sites sequentially produce autonomy and inhibition of the multifunctional Ca2+/calmodulin-dependent protein kinase. J Neurosci 9, 2020–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loweth JA, Li D, Cortright JJ, Wilke G, Jeyifous O, Neve RL, Bayer KU, and Vezina P (2013). Persistent reversal of enhanced amphetamine intake by transient CaMKII inhibition. J Neurosci 33, 1411–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Isozaki K, Roche KW, and Nicoll RA (2010). Synaptic targeting of AMPA receptors is regulated by a CaMKII site in the first intracellular loop of GluA1. Proc Natl Acad Sci U S A 107, 22266–22271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Groth RD, Cohen SM, Emery JF, Li B, Hoedt E, Zhang G, Neubert TA, and Tsien RW (2014). gammaCaMKII shuttles Ca(2)(+)/CaM to the nucleus to trigger CREB phosphorylation and gene expression. Cell 159, 281–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinow R, Schulman H, and Tsien RW (1989). Inhibition of postsynaptic PKC or CaMKII blocks induction but not expression of LTP. Science 245, 862–866. [DOI] [PubMed] [Google Scholar]
- Manning G, Whyte DB, Martinez R, Hunter T, and Sudarsanam S (2002). The protein kinase complement of the human genome. Science 298, 1912–1934. [DOI] [PubMed] [Google Scholar]
- Marsden KC, Shemesh A, Bayer KU, and Carroll RC (2010). Selective translocation of Ca2+/calmodulin protein kinase IIalpha (CaMKIIalpha) to inhibitory synapses. Proc Natl Acad Sci U S A 107, 20559–20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx A, Nugoor C, Panneerselvam S, and Mandelkow E (2010). Structure and function of polarity-inducing kinase family MARK/Par-1 within the branch of AMPK/Snf1-related kinases. FASEB J 24, 1637–1648. [DOI] [PubMed] [Google Scholar]
- Matenia D, and Mandelkow EM (2009). The tau of MARK: a polarized view of the cytoskeleton. Trends Biochem Sci 34, 332–342. [DOI] [PubMed] [Google Scholar]
- Mayford M, Baranes D, Podsypanina K, and Kandel ER (1996). The 3’-untranslated region of CaMKII alpha is a cis-acting signal for the localization and translation of mRNA in dendrites. Proc Natl Acad Sci U S A 93, 13250–13255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SG, and Kennedy MB (1986). Regulation of brain type II Ca2+/calmodulin-dependent protein kinase by autophosphorylation: a Ca2+-triggered molecular switch. Cell 44, 861870. [DOI] [PubMed] [Google Scholar]
- Miller SG, Patton BL, and Kennedy MB (1988). Sequences of autophosphorylation sites in neuronal type II CaM kinase that control Ca2(+)-independent activity. Neuron 1, 593604. [DOI] [PubMed] [Google Scholar]
- Mockett BG, Guevremont D, Wutte M, Hulme SR, Williams JM, and Abraham WC (2011). Calcium/calmodulin-dependent protein kinase II mediates group I metabotropic glutamate receptor-dependent protein synthesis and long-term depression in rat hippocampus. J Neurosci 31, 7380–7391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada D, Ballarini F, Martinez MC, Frey JU, and Viola H (2011). Identification of transmitter systems and learning tag molecules involved in behavioral tagging during memory formation. Proc Natl Acad Sci U S A 108, 12931–12936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee K, Sharma M, Jahn R, Wahl MC, and Sudhof TC (2010). Evolution of CASK into a Mg2+-sensitive kinase. Sci Signal 3, ra33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey RM, Endo S, Shenolikar S, and Malenka RC (1994). Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature 369, 486–488. [DOI] [PubMed] [Google Scholar]
- Murakoshi H, Shin ME, Parra-Bueno P, Szatmari EM, Shibata ACE, and Yasuda R (2017). Kinetics of Endogenous CaMKII Required for Synaptic Plasticity Revealed by Optogenetic Kinase Inhibitor. Neuron 94, 37–47 e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers JB, Zaegel V, Coultrap SJ, Miller AP, Bayer KU, and Reichow SL (2017). The CaMKII holoenzyme structure in activation-competent conformations. Nat Commun 8, 15742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawijn MC, Alendar A, and Berns A (2011). For better or for worse: the role of Pim oncogenes in tumorigenesis. Nat Rev Cancer 11, 23–34. [DOI] [PubMed] [Google Scholar]
- Neef S, Steffens A, Pellicena P, Mustroph J, Lebek S, Ort KR, Schulman H, and Maier LS (2018). Improvement of cardiomyocyte function by a novel pyrimidine-based CaMKII-inhibitor. J Mol Cell Cardiol 115, 73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nose PS, Griffith LC, and Schulman H (1985). Ca2+-dependent phosphorylation of tyrosine hydroxylase in PC12 cells. J Cell Biol 101, 1182–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary H, Lasda E, and Bayer KU (2006). CaMKIIbeta association with the actin cytoskeleton is regulated by alternative splicing. Mol Biol Cell 17, 4656–4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary H, Liu WH, Rorabaugh JM, Coultrap SJ, and Bayer KU (2011). Nucleotides and phosphorylation bi-directionally modulate Ca2+/calmodulin-dependent protein kinase II (CaMKII) binding to the N-methyl-D-aspartate (NMDA) receptor subunit GluN2B. J Biol Chem 286, 31272–31281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuno H, Akashi K, Ishii Y, Yagishita-Kyo N, Suzuki K, Nonaka M, Kawashima T, Fujii H, Takemoto-Kimura S, Abe M, et al. (2012). Inverse synaptic tagging of inactive synapses via dynamic interaction of Arc/Arg3.1 with CaMKIIbeta. Cell 149, 886–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opazo P, Labrecque S, Tigaret CM, Frouin A, Wiseman PW, De Koninck P, and Choquet D (2010). CaMKII triggers the diffusional trapping of surface AMPARs through phosphorylation of stargazin. Neuron 67, 239–252. [DOI] [PubMed] [Google Scholar]
- Ouyang Y, Kantor D, Harris KM, Schuman EM, and Kennedy MB (1997). Visualization of the distribution of autophosphorylated calcium/calmodulin-dependent protein kinase II after tetanic stimulation in the CA1 area of the hippocampus. J Neurosci 17, 5416–5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Chavez AE, Mineur YS, Morimoto-Tomita M, Lutzu S, Kim KS, Picciotto MR, Castillo PE, and Tomita S (2016). CaMKII Phosphorylation of TARPgamma-8 Is a Mediator of LTP and Learning and Memory. Neuron 92, 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicena P, and Schulman H (2014). CaMKII inhibitors: from research tools to therapeutic agents. Front Pharmacol 5, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrini EM, Ravasenga T, Hausrat TJ, Iurilli G, Olcese U, Racine V, Sibarita JB, Jacob TC, Moss SJ, Benfenati F, et al. (2014). Synaptic recruitment of gephyrin regulates surface GABAA receptor dynamics for the expression of inhibitory LTP. Nat Commun 5, 3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi HJ, Otmakhov N, Lemelin D, De Koninck P, and Lisman J (2010). Autonomous CaMKII can promote either long-term potentiation or long-term depression, depending on the state of T305/T306 phosphorylation. J Neurosci 30, 8704–8709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radwanska K, Medvedev NI, Pereira GS, Engmann O, Thiede N, Moraes MF, Villers A, Irvine EE, Maunganidze NS, Pyza EM, et al. (2011). Mechanism for long-term memory formation when synaptic strengthening is impaired. Proc Natl Acad Sci U S A 108, 18471–18475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiterer V, Eyers PA, and Farhan H (2014). Day of the dead: pseudokinases and pseudophosphatases in physiology and disease. Trends Cell Biol 24, 489–505. [DOI] [PubMed] [Google Scholar]
- Rellos P, Pike AC, Niesen FH, Salah E, Lee WH, von Delft F, and Knapp S (2010). Structure of the CaMKIIdelta/calmodulin complex reveals the molecular mechanism of CaMKII kinase activation. PLoS Biol 8, e1000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich RC, and Schulman H (1998). Substrate-directed function of calmodulin in autophosphorylation of Ca2+/calmodulin-dependent protein kinase II. J Biol Chem 273, 28424–28429. [DOI] [PubMed] [Google Scholar]
- Robison AJ, Bass MA, Jiao Y, MacMillan LB, Carmody LC, Bartlett RK, and Colbran RJ (2005). Multivalent interactions of calcium/calmodulin-dependent protein kinase II with the postsynaptic density proteins NR2B, densin-180, and alpha-actinin-2. J Biol Chem 280, 35329–35336. [DOI] [PubMed] [Google Scholar]
- Romeo Y, Zhang X, and Roux PP (2012). Regulation and function of the RSK family of protein kinases. Biochem J 441, 553–569. [DOI] [PubMed] [Google Scholar]
- Rosenberg OS, Deindl S, Comolli LR, Hoelz A, Downing KH, Nairn AC, and Kuriyan J (2006). Oligomerization states of the association domain and the holoenyzme of Ca2+/CaM kinase II. FEBS J 273, 682–694. [DOI] [PubMed] [Google Scholar]
- Rosenberg OS, Deindl S, Sung RJ, Nairn AC, and Kuriyan J (2005). Structure of the autoinhibited kinase domain of CaMKII and SAXS analysis of the holoenzyme. Cell 123, 849–860. [DOI] [PubMed] [Google Scholar]
- Rossetti T, Banerjee S, Kim C, Leubner M, Lamar C, Gupta P, Lee B, Neve R, and Lisman J (2017). Memory Erasure Experiments Indicate a Critical Role of CaMKII in Memory Storage. Neuron 96, 207–216 e202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E (2011). Protein kinase D signaling: multiple biological functions in health and disease. Physiology (Bethesda) 26, 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh T, and Schwartz JH (1985). Phosphorylation-dependent subcellular translocation of a Ca2+/calmodulin-dependent protein kinase produces an autonomous enzyme in Aplysia neurons. J Cell Biol 100, 835–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajikumar S, Navakkode S, and Frey JU (2007). Identification of compartment- and process-specific molecules required for “synaptic tagging” during long-term potentiation and long-term depression in hippocampal CA1. J Neurosci 27, 5068–5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliba RS, Kretschmannova K, and Moss SJ (2012). Activity-dependent phosphorylation of GABAA receptors regulates receptor insertion and tonic current. EMBO J 31, 29372951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saneyoshi T, Matsuno H, Suzuki A, Murakoshi H, Hedrick NG, Agnello E, O’Connell R, Stratton MM, Yasuda R, and Hayashi Y (2019). Reciprocal Activation within a Kinase-Effector Complex Underlying Persistence of Structural LTP. Neuron 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanhueza M, Fernandez-Villalobos G, Stein IS, Kasumova G, Zhang P, Bayer KU, Otmakhov N, Hell JW, and Lisman J (2011). Role of the CaMKII/NMDA receptor complex in the maintenance of synaptic strength. J Neurosci 31, 9170–9178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnabel R, Palmer MJ, Kilpatrick IC, and Collingridge GL (1999). A CaMKII inhibitor, KN-62, facilitates DHPG-induced LTD in the CA1 region of the hippocampus. Neuropharmacology 38, 605–608. [DOI] [PubMed] [Google Scholar]
- Schulman H (1993). The multifunctional Ca2+/calmodulin-dependent protein kinases. Curr Opin Cell Biol 5, 247–253. [DOI] [PubMed] [Google Scholar]
- Schulman H, and Greengard P (1978a). Ca2+-dependent protein phosphorylation system in membranes from various tissues, and its activation by “calcium-dependent regulator”. Proc Natl Acad Sci U S A 75, 5432–5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman H, and Greengard P (1978b). Stimulation of brain membrane protein phosphorylation by calcium and an endogenous heat-stable protein. Nature 271, 478479. [DOI] [PubMed] [Google Scholar]
- Schworer CM, Colbran RJ, and Soderling TR (1986). Reversible generation of a Ca2+- independent form of Ca2+(calmodulin)-dependent protein kinase II by an autophosphorylation mechanism. J Biol Chem 261, 8581–8584. [PubMed] [Google Scholar]
- Shen K, Teruel MN, Subramanian K, and Meyer T (1998). CaMKIIbeta functions as an F- actin targeting module that localizes CaMKIIalpha/beta heterooligomers to dendritic spines. Neuron 21, 593–606. [DOI] [PubMed] [Google Scholar]
- Shepherd JD, and Huganir RL (2007). The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol 23, 613–643. [DOI] [PubMed] [Google Scholar]
- Shiloh R, Bialik S, and Kimchi A (2014). The DAPK family: a structure-function analysis. Apoptosis 19, 286–297. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Stevens CF, Tonegawa S, and Wang Y (1992). Deficient hippocampal long-term potentiation in a-calcium-calmodulin kinase II mutant mice. Science 257, 201–206. [DOI] [PubMed] [Google Scholar]
- Skelding KA, Rostas JA, and Verrills NM (2011). Controlling the cell cycle: the role of calcium/calmodulin-stimulated protein kinases I and II. Cell Cycle 10, 631–639. [DOI] [PubMed] [Google Scholar]
- Srinivasan M, Edman CF, and Schulman H (1994). Alternative splicing introduces a nuclear localization signal that targets multifunctional CaM kinase to the nucleus. J Cell Biol 126, 839–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strack S, McNeill RB, and Colbran RJ (2000). Mechanism and regulation of calcium/calmodulin-dependent protein kinase II targeting to the NR2B subunit of the N- methyl-D-aspartate receptor. J Biol Chem 275, 23798–23806. [DOI] [PubMed] [Google Scholar]
- Stratton M, Lee IH, Bhattacharyya M, Christensen SM, Chao LH, Schulman H, Groves JT, and Kuriyan J (2014). Activation-triggered subunit exchange between CaMKII holoenzymes facilitates the spread of kinase activity. Elife 3, e01610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub C, and Tomita S (2012). The regulation of glutamate receptor trafficking and function by TARPs and other transmembrane auxiliary subunits. Curr Opin Neurobiol 22, 488495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumi M, Kiuchi K, Ishikawa T, Ishii A, Hagiwara M, Nagatsu T, and Hidaka H (1991). The newly synthesized selective Ca2+/calmodulin dependent protein kinase II inhibitor KN-93 reduces dopamine contents in PC12h cells. Biochem Biophys Res Commun 181, 968–975. [DOI] [PubMed] [Google Scholar]
- Takemoto-Kimura S, Suzuki K, Horigane SI, Kamijo S, Inoue M, Sakamoto M, Fujii H, and Bito H (2017). Calmodulin kinases: essential regulators in health and disease. J Neurochem 141, 808–818. [DOI] [PubMed] [Google Scholar]
- Tobimatsu T, and Fujisawa H (1989). Tissue-specific expression of four types of rat calmodulin-dependent protein kinase II mRNAs. J Biol Chem 264, 17907–17912. [PubMed] [Google Scholar]
- Tombes RM, Faison MO, and Turbeville JM (2003). Organization and evolution of multifunctional Ca(2+)/CaM-dependent protein kinase genes. Gene 322, 17–31. [DOI] [PubMed] [Google Scholar]
- Turecek J, Yuen GS, Han VZ, Zeng XH, Bayer KU, and Welsh JP (2014). NMDA receptor activation strengthens weak electrical coupling in mammalian brain. Neuron 81, 1375–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vest RS, Davies KD, O’Leary H, Port JD, and Bayer KU (2007). Dual Mechanism of a Natural CaMKII Inhibitor. Mol Biol Cell 18, 5024–5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vest RS, O’Leary H, Coultrap SJ, Kindy MS, and Bayer KU (2010). Effective post-insult neuroprotection by a novel Ca(2+)/ calmodulin-dependent protein kinase II (CaMKII) inhibitor. J Biol Chem 285, 20675–20682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villers A, Giese KP, and Ris L (2014). Long-term potentiation can be induced in the CA1 region of hippocampus in the absence of alphaCaMKII T286-autophosphorylation. Learn Mem 21, 616–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkup W.G.t., Washburn L, Sweredoski MJ, Carlisle HJ, Graham RL, Hess S, and Kennedy MB (2015). Phosphorylation of synaptic GTPase-activating protein (synGAP) by Ca2+/calmodulin-dependent protein kinase II (CaMKII) and cyclin-dependent kinase 5 (CDK5) alters the ratio of its GAP activity toward Ras and Rap GTPases. J Biol Chem 290, 4908–4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RA, Cheng G, Kolaj M, and Randic M (1995). Alpha-subunit of calcium/calmodulin- dependent protein kinase II enhances gamma-aminobutyric acid and inhibitory synaptic responses of rat neurons in vitro. J Neurophysiol 73, 2099–2106. [DOI] [PubMed] [Google Scholar]
- Wayman GA, Lee YS, Tokumitsu H, Silva AJ, and Soderling TR (2008). Calmodulin- kinases: modulators of neuronal development and plasticity. Neuron 59, 914–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolfrey KM, O’Leary H, Goodell DJ, Robertson HR, Horne EA, Coultrap SJ, Dell’Acqua ML, and Bayer KU (2018). CaMKII regulates the depalmitoylation and synaptic removal of the scaffold protein AKAP79/150 to mediate structural long-term depression. J Biol Chem 293, 1551–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi K, Yazawa M, Kakiuchi S, Ohshima M, and Uenishi K (1978). Identification of an activator protein for myosin light chain kinase as the Ca2+-dependent modulator protein. J Biol Chem 253, 1338–1340. [PubMed] [Google Scholar]
- Yamauchi T, and Fujisawa H (1980). Evidence for three distinct forms of calmodulin- dependent protein kinases from rat brain. FEBS Lett 116, 141–144. [DOI] [PubMed] [Google Scholar]
- Zannini L, Delia D, and Buscemi G (2014). CHK2 kinase in the DNA damage response and beyond. J Mol Cell Biol 6, 442–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H, Chattarji S, Barbarosie M, Rondi-Reig L, Philpot BD, Miyakawa T, Bear MF, and Tonegawa S (2001). Forebrain-specific calcineurin knockout selectively impairs bidirectional synaptic plasticity and working/episodic-like memory. Cell 107, 617–629. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wu K, Su W, Zhang DF, Wang P, Qiao X, Yao Q, Yuan Z, Yao YG, Liu G, et al. (2017). Increased GSNOR Expression during Aging Impairs Cognitive Function and Decreases S-Nitrosation of CaMKIIalpha. J Neurosci 37, 9741–9758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YP, Holbro N, and Oertner TG (2008). Optical induction of plasticity at single synapses reveals input-specific accumulation of alphaCaMKII. Proc Natl Acad Sci U S A 105, 12039–12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, and Adams JA (1997). Participation of ADP dissociation in the rate-determining step in cAMP-dependent protein kinase. Biochemistry 36, 15733–15738. [DOI] [PubMed] [Google Scholar]
- Zhu JJ, Qin Y, Zhao M, Van Aelst L, and Malinow R (2002). Ras and Rap control AMPA receptor trafficking during synaptic plasticity. Cell 110, 443–455. [DOI] [PubMed] [Google Scholar]