Abstract
Corticosterone is the primary metabolic steroid in birds, and is vital for maintaining homeostasis. However, the relationship between baseline corticosterone and reproduction is unclear, and we lack an understanding of how differences in baseline corticosterone at one stage of the breeding cycle influence reproductive effort at later stages. In a wild population of house wrens, we quantified the concentration of corticosterone in yolks of freshly laid eggs as an integrated measure of maternal physiology, and related this to a behavioral measure of stress reactivity made during the nestling period, namely, the latency with which females resumed parental activities following a standardized disturbance at their nest (setting up a camera to record provisioning). Females that recently produced eggs containing higher corticosterone concentrations, which were significantly repeatable within females, took longer to resume activity related to parental care (i.e., feeding and brooding young) following the disturbance. Moreover, a female’s latency to resume parental activities negatively predicted her provisioning of nestlings with food and the condition of these young at fledging, but did not predict the number fledged. We cross-fostered offspring prior to hatching, so these effects on maternal behavior are independent of any prenatal maternal effects on nestlings via the egg. These results are consistent with earlier findings suggesting that females with higher baseline corticosterone during egg laying or early incubation tend to prioritize self-maintenance over reproduction compared with females with lower baseline corticosterone, and suggest that a female’s latency to return to her nest and resume parental care following a disturbance might represent a simple, functional measure of maternal stress reactivity.
Keywords: birds, corticosterone, life history, maternal effect, parental care, stress reactivity
Introduction
Corticosterone is the primary metabolic steroid in birds, and mediates the physiological and behavioral responses of individuals to changes in their environment to maintain homeostasis (Hau et al. 2016). At baseline levels, corticosterone varies according to the energetic demands of foraging, migration, reproduction, and immunity (Romero 2002; Landys et al. 2006; Romero et al. 2009; Monaghan and Spencer 2014). In response to an acute stressor, however, such as an increased risk of predation, decreased food availability, or competitive social interactions, corticosterone can rapidly increase well above baseline, initiating an emergency life-history stage comprising a suite of physiological and behavioral responses that prioritize immediate survival (Wingfield et al. 1998; Sapolsky et al. 2000; Ricklefs and Wikelski 2002; Romero et al. 2009; Legagneux et al. 2016).
Stress-induced corticosterone appears to be negatively associated with reproduction, as might be expected if an individual is prioritizing survival over reproduction (e.g., Wingfield et al. 1998; Sapolsky et al. 2000). However, the relationship between baseline corticosterone and reproduction is less clear, and both positive and negative correlations between baseline corticosterone and fitness have been documented (Bonier et al. 2009a; Ouyang et al. 2011, 2013a,b; Henderson et al. 2017; Deviche et al. 2017). These contrasting results have led to the emergence of two competing hypotheses, the CORT-adaptation hypothesis, which posits that higher baseline corticosterone reflects an increased capacity to meet the increased energetic demands of reproduction (Bonier et al. 2009a,b), and the CORT-fitness hypothesis, which suggests that higher baseline corticosterone is indicative of individuals of lower body condition and, thus, less capable of successful reproduction (Bonier et al. 2009a,b).
At least some of the ambiguity concerning the association between baseline corticosterone and fitness can be attributed to the fact that the sign of the association can vary according to the stage of the breeding cycle (Bonier et al. 2009b; Ouyang et al. 2011, 2013a; Schoenle et al. 2017; Deviche et al. 2017). In red-winged blackbirds (Agelaius phoeniceus), for example, increased baseline corticosterone has been shown to be negatively associated with incubation effort and decreased clutch mass, suggesting that females shift reproductive investment away from reproduction and towards self-maintenance (Schoenle et al. 2017). Similarly, in house wrens (Troglodytes aedon), females with a higher baseline corticosterone at the beginning of incubation were more likely to abandon their nesting attempt after capture than females with lower baseline levels (Lothery et al. 2014). In tree swallows (Tachycineta bicolor), baseline corticosterone during incubation is negatively correlated with the number of offspring fledged, but during the nestling provisioning period, higher maternal corticosterone is associated with increased growth of nestlings, suggesting that increased corticosterone during this demanding stage of the breeding cycle promotes increased parental provisioning (Bonier 2009b).
However, we still lack a clear understanding of how differences in baseline corticosterone at one stage of the breeding cycle influence reproductive effort at later stages, and the extent to which such differences are associated with sensitivity to stressors and differences in behavior in the natural habitat. In the present study, we capitalized on a measure of baseline corticosterone in female house wrens quantified in an earlier study (Bowers et al. 2015a), the concentration of corticosterone in the yolks of their eggs, and related this to a behavioral measure of stress reactivity made during the nestling period, namely, the latency with which females resumed parental activities following a standardized disturbance at their nest (viz., setting up a camera to record provisioning behavior). As a lipophilic steroid, corticosterone accumulates in the yolk during egg formation (McCormick 1998; Hayward and Wingfield 2004; Okuliarová et al. 2010; Almasi et al. 2012; Pitk et al. 2012; Bowers et al. 2016). Thus, egg-yolk corticosterone can serve as a non-invasive, integrative measure of maternal physiology around the time of egg production that is not influenced by researcher- or restraint-induced stress (Bowers et al. 2015a). Corticosterone production has been characterized by a high degree of within-individual repeatability, at least in plasma (Angelier et al. 2010; Schoenemann and Bonier 2018), so we might also expect some repeatability in yolk-corticosterone concentrations within females over time.
Increases in baseline maternal corticosterone during egg production or incubation might reflect the prioritizing of self-maintenance over reproduction compared with females with lower baseline corticosterone (Bonier 2009b; Lothery et al. 2014; Schoenle et al. 2017), but elevated levels may also reflect preparative changes in maternal physiology for the energetic demands associated with post-natal parental care (e.g., Breuner 2011; Sheriff and Love 2013; Bowers et al. 2015a, 2016), in which case corticosterone and parental care may be generally positively correlated. We tested these alternative hypotheses by relating maternal corticosteroid levels prior to hatching to the latency with which females provisioned their young after hatching. If the former hypothesis is correct, we predicted that corticosterone concentrations within the yolks of eggs would be positively correlated with the latency with which females resumed parental activity following a disturbance (i.e., greater corticosterone would be associated with greater latency), whereas the latter hypothesis predicts the reverse. Moreover, if the latency with which females resume parental care following a disturbance reflects their ability to cope with a stressor, then such a latency might also impose costs on their ability to rear offspring of high reproductive value. We predicted, therefore, that the latency with which females resumed parental activity following the disturbance would negatively predict the rate at which they provisioned offspring with food after hatching. Finally, we predicted that, if the latency with which females resumed parental care is indicative of post-natal maternal care in general, then this latency should be negatively correlated with the number and body condition of the offspring they reared to fledging.
Methods
Study area and species
House wrens are secondary-cavity-nesting songbirds with a widespread distribution across North and South America (biology summarized in Johnson 2014). We studied a migratory population breeding in secondary deciduous forest in McLean County, Illinois, USA (40.665°N, 88.89°W) during the 2012 and 2013 breeding seasons (May through August). Females select a mate that is defending a nesting site and produce one egg per day until their clutch is completed. Only females incubate eggs, but both parents provision offspring with food after hatching, and the length of the nestling period is typically ca. 15 days (Bowers et al. 2013, 2014a). House wrens readily accept nestboxes for nesting, and the nestboxes in this study were spaced 30 m apart along north-south transects separated by 60 m and mounted atop 48.3-cm diameter aluminum predator baffles on 1.5-m poles (Lambrechts et al. 2010 provide further details on nestboxes). During incubation or early in the nestling-rearing stage, we captured adults inside nestboxes or by using mist nets near the box. We measured their body mass (± 0.1 g) and tarsus length (± 0.1 mm), and banded them with a unique U. S. Geological Survey leg band; males received three additional colored bands in a unique combination so they could be identified without needing to be recaptured (males are more difficult to capture than females). All research activities complied with current laws of the United States of America and were in accordance with the Illinois State University Institutional Animal Care and Use Committee (Protocol Nos. 05–2010, 04–2013), United States Geological Survey banding permit 09211, and U.S. Fish and Wildlife Service collecting permit MB692148–0. All applicable institutional and national guidelines for the care and use of animals were followed.
Procedures
Females and their eggs in this study represent a subset from an earlier study investigating consequences of immune activation on maternal care (Bowers et al. 2015a). That study involved three treatments: experimental, control, and natural (unmanipulated) females. Experimental females (omitted from this study) had recently produced a clutch of eggs, were injected with a saline solution containing lipopolysaccharide to induce an immune response, and had their current clutch of eggs collected to force the females to produce a replacement clutch while mounting the immune response. Control females were treated the same way, but were injected with saline only. Natural females were not manipulated directly, but were matched with control and experimental females for cross-fostering of eggs. The current study focuses exclusively on females in the control and natural groups (Bowers et al. 2015a). Thus, control females raised an unmanipulated clutch, whereas the unmanipulated females raised a replacement clutch, but, given the patterns we are analyzing in this study, the only effect this difference could have on our results is the potential production of noise in our data; it could not create any sort of bias.
We cross-fostered clutches among females such that the control females received eggs produced by natural, unmanipulated females, whereas natural females received eggs produced by control and experimental females (Fig. 1 in Bowers et al. 2015a). In other words, control females reared unmanipulated eggs and unmanipulated parents reared eggs from a replacement clutch. All females in this study received five foster eggs (mean ± SD of natural, non-foster clutches = 5.4 ± 0.9 eggs), so that the condition of offspring produced to fledging could be used as an unbiased, functional proxy of post-hatching parental care, and we also recorded maternal provisioning at the nest using digital video cameras. Maternal effects, including those induced by maternally derived corticosterone within the egg, often affect nestling begging and growth (Love and Williams 2008; Smiseth et al. 2011; Bowers et al. 2016; Weber et al. 2018); thus, that the females here reared unrelated foster offspring means their maternal behavior was independent of any pre-natal maternal effects on offspring begging solicitations.
Figure 1.

Within-female consistency in yolk-corticosterone concentrations. Individual eggs were analyzed with nest within female as a random effect, and plotted are least-squares means ± SE for each set of paired clutches.
Provisioning of nestlings by females was video-recorded on brood-day 4 or 5, an age at which nestling growth is most rapid, and the amount of food delivered by parents at this age is positively correlated with nestling growth, fledging success, and the recruitment of offspring to the breeding population (Bowers et al. 2014b; Bowers et al. 2015a). Videos of maternal provisioning were obtained in the same manner for all females (N = 65). This involved placing a small, pocket-sized digital video camera in a cell-phone holster at the end of a pole ca. 1.5 m from the nestbox and recording parental activity at the nest for ca. 1.5 h, which provides an accurate representation of what are consistent among-individual differences in parental behavior over longer observation periods and multiple days, at least in other species in which this has been quantified (e.g., eastern kingbirds Tyrannus tyrannus; Murphy et al. 2015). Poles and dummy cameras had been in place for a day prior to filming. Thus, our visit to the nest caused us to have a brief (< 5 min) presence at the nest, which had a noticeable effect on resident birds (see also Murphy et al. 2015). For example, females were usually inside the nestbox brooding their ectothermic nestlings as we approached, and our approach always caused them to fly away from the nest. Resident birds also typically produced aggressive, “scolding” vocalizations in response to our presence, even in those cases where a bird was not in the nestbox initially. Thus, even though a bird may not have been in the nestbox upon our initial approach, our presence at the nest did not go unnoticed. All nests were included in analyses, regardless of whether the female was in the nestbox initially, to avoid creating a self-selected sample. In most cases, the resident male or female returned to the nest within ca. 10 min of our departure. If the male returned first, he sang at the nest, which was generally followed shortly thereafter by the female’s return and entrance into the nestbox and resumption of normal parental behavior. Once a recording began, there was always a latency period before any birds returned to the nest, and, in all instances, our hour-long observation did not begin until parents resumed normal parental behavior.
Although females generally returned to the nest within 10 min, they varied widely in their latency to return to the nest following this disturbance, and we hypothesized that this latency might be related to general “fearfulness” or sensitivity to the putatively stressful event. Thus, we predicted that a female’s latency to return to their nest following this disturbance when rearing nestlings might be related to the concentration of corticosterone in the eggs they had recently produced. For a set of these females, we collected the eggs they produced on the day each was laid to quantify the extent of corticosterone accumulation within egg yolks as a measure of baseline corticosterone concentration, which we expected to be positively correlated with maternal stress reactivity. We collected eggs on the morning each was laid, replacing the fresh eggs with artificial eggs that females readily accepted and incubated. We measured the concentration of yolk corticosterone through competitive-binding radioimmunoassay (RIA) using a standardized protocol (Paitz et al. 2011); additional details on this procedure can be found in Bowers et al. (2015a). In the current study, we analyzed yolk corticosterone of eggs laid by control females to assess within-female repeatability in this trait over time (N = 22 paired clutches from 11 control females). These females initiated their replacement clutch 5.73 ± 0.64 d (mean ± SD) following the collection of their initial clutch, and there was no effect of the saline injection and forcing of females to produce a replacement clutch on egg-yolk corticosterone (Bowers et al. 2015a). For a subset of these females (N = 7), we were able to collect their eggs for steroid quantification and then provide them with foster eggs produced by unmanipulated females, the offspring from which they subsequently reared. We obtained measures of corticosterone from both the first clutch and replacement clutches. Among these control females, there was no difference in egg-yolk corticosterone levels between first and replacement clutches (Bowers et al. 2015a); thus, the measure we used in our analysis was the replacement clutch as this was produced closer to the timing of hatching for their foster clutch. We also recorded the provisioning behavior of these females, and used the concentration of corticosterone in the eggs they had recently produced as a reflection of maternal corticosterone production.
After recording parental behavior, we subsequently monitored the progress of each nest. Nestlings were banded, weighed, and had their tarsus length measured on brood-day 11 (where brood-day 0 is the day hatching begins within a nest), at which point body mass has reached an asymptote and positively predicts recruitment and future reproductive success in the study population (Bowers et al. 2014b, 2015b); residual body mass likewise predicts adult female reproductive success (Sakaluk et al. 2018). Nestlings were placed back in their nests, which we subsequently monitored daily to check for fledging beginning on brood-day 13.
Data and analyses
We used SAS (version 9.4) for all analyses, all tests are two-tailed (α = 0.05), and we converted data to z-scores prior to analysis to obtain standardized parameter estimates, which provide a measure of effect size (Schielzeth 2010). We used Satterthwaite’s degrees-of-freedom approximation, which can result in non-integer denominator degrees of freedom. We first used individual eggs, nested within the paired clutches as a random effect, to estimate within-female repeatability in the concentration of corticosterone within their eggs (Lessells and Boag 1987; Nakagawa and Schielzeth 2010). We then used a linear model to test whether the concentration of corticosterone in egg yolks predicted a female’s latency to return to her nest following our disturbance when setting up video recordings, and we included egg-yolk mass as a covariate because we suspected that other components of maternal allocation might explain some of the variation in this trait. For this analysis, we used the average corticosterone concentration per yolk within a clutch, because, even though we had measures of yolk corticosterone from multiple eggs within a clutch, we only had one latency value for each nest. There was one female in this analysis with a very low latency to return to the nest and who also had produced the clutch with the lowest average corticosterone concentration (see Results). We have no objective a priori reason to omit that datum, but excluding this nest from our analysis reveals a comparably large effect size (standardized parameter estimate ± SE = 0.780 ± 0.386) as that reported in our manuscript (see Results). We then used linear models on the larger set of females for which we had provisioning data to test whether a female’s latency to return to the nest predicted (i) the rate at which she provisioned food to offspring after our departure, (ii) the body condition of offspring prior to fledging, and (iii) the number of young fledged from the nest. We employed this model using linear terms, as we did not have a good reason, a priori or theoretical, to include a non-linear/quadratic term in our original analysis. However, including a quadratic term reveals patterns that are qualitatively similar to what we report in the Results. For our analysis of nestling body condition, we analyzed individual body mass as the dependent variable, and included tarsus length to control for the skeletal size of nestlings. For graphical purposes, we plotted the body condition of individual nestlings as the residual from a mass × tarsus linear regression, to be more consistent with our analyses and interpretation of size-adjusted mass as a measure of condition. We also included brood size and hatching date as covariates in this analysis, and nest identity as a random effect to account for the non-independence of nestlings within the same brood.
Results
Among females for which we had multiple clutches with which to estimate yolk corticosterone (each egg from 22 paired clutches), repeatability of yolk-corticosterone concentration between clutches was 0.622 (F9, 12.1 = 4.29, P = 0.011), indicating within-female consistency in this trait over time (Fig. 1). For a subset of these females (N = 7), we were able to provide them with foster offspring after we had collected their eggs for hormone assays. Among these females, their latency to return to the nest following our disturbance to begin recording was strongly correlated with the concentration of corticosterone in the yolks of the eggs they had recently produced (standardized parameter estimate ± SE = 0.830 ± 0.211, F1, 4 = 15.48, P = 0.017; Fig. 2), while controlling for variation in the amount of yolk allocated to eggs. There was a marginally non-significant tendency for increases in yolk mass to predict a reduced latency to resume parental care (standardized parameter estimate ± SE = –0.539 ± 0.211, F1, 4 = 6.53, P = 0.063).
Figure 2.
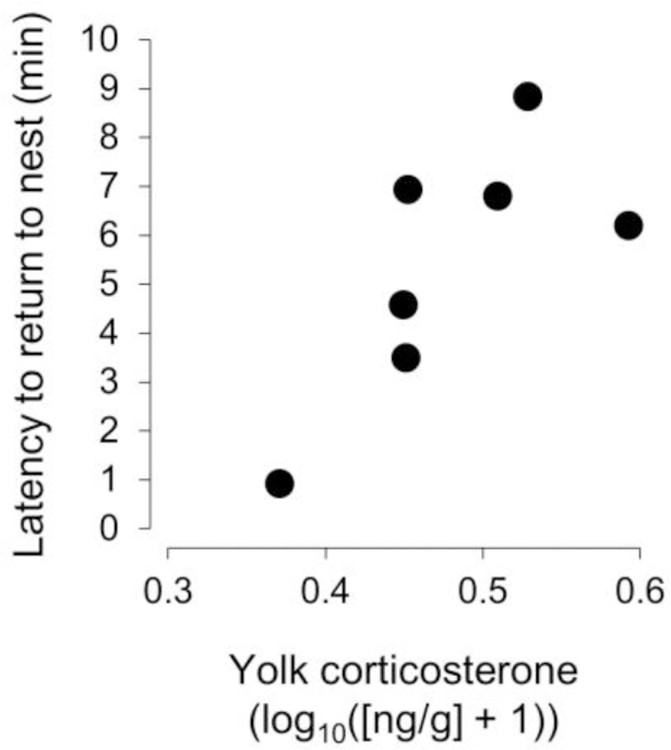
Latency of a female to return to her nest following a disturbance (i.e., an experimenter setting up a video camera at the nest) in relation to the concentration of corticosterone in the yolks of the eggs (clutch means) she produced.
For the larger set of females for which we had provisioning data, but not yolk-corticosterone data, there was a negative correlation between their latency to return to the nest and the amount of food they delivered to nestlings per unit time (parameter estimate ± SE = –0.315 ± 0.120, F1, 63 = 6.92, P = 0.011; Fig. 3A). This latency was also negatively correlated with the pre-fledging body condition of the unrelated foster offspring they reared (Table 1; Fig. 3B). There was no correlation between a female’s latency to return to the nest and the number of foster offspring surviving to fledging (parameter estimate ± SE = 0.123 ± 0.126, F1, 61 = 0.95, P = 0.335; effect of hatching date on nestling survival: parameter estimate ± SE = 0.100 ± 0.126, F1, 61 = 0.63, P = 0.429).
Figure 3.

(A) Rate with which females provisioned food to unrelated foster offspring after hatching, and (B) The pre-fledging body mass of these offspring in relation to the latency with which females returned to the nest following disturbance. Plotted in (B) are residuals from a log-mass × log-tarsus linear regression to reflect size-adjusted body mass, or condition (nestlings with positive values have increased mass relative to what would be predicted from their skeletal size), of individual nestlings for graphical purposes.
Table 1:
Factors affecting pre-fledging mass of foster offspring (N = 228 nestlings from 57 different broods). Data were analyzed using a linear mixed model with brood as a random effect.
| Estimate ± SE | F | df | P | |
|---|---|---|---|---|
| Female latency to resume parental care | −0.219 ± 0.090 | 5.86 | 1, 50.0 | 0.019 |
| Brood size | 0.047 ± 0.099 | 0.22 | 1, 56.3 | 0.642 |
| Hatching date | 0.100 ± 0.093 | 1.15 | 1, 51.3 | 0.289 |
| Nestling tarsus length | 0.534 ± 0.050 | 114.43 | 1, 206.0 | < 0.001 |
| Intercept | 0.013 ± 0.096 | |||
Discussion
Females producing eggs containing higher concentrations of corticosterone took significantly longer to return to the nest to resume parental care following a brief, investigator-instigated disturbance. Moreover, a female’s latency to resume care negatively predicted the rate at which she provisioned foster nestlings with food and the condition of these young prior to fledging, a trait associated with increases in recruitment and longevity of nestlings in the breeding population in subsequent years (Bowers et al. 2014b; see also Tinbergen and Boerlijst 1990; Young 1996; Both et al. 1999; Naef-Daenzer et al. 2001 for similar results in other species). These results are consistent with earlier findings suggesting that females with higher baseline corticosterone during egg laying or early incubation tend to prioritize self-maintenance over reproduction compared with females with lower baseline corticosterone (Bonier 2009a; Lothery et al. 2014; Schoenle et al. 2017), although it is important to note that there is really no consensus on the directionality of the effects of corticosteroids on the relationship between self-maintenance and reproduction. Our data suggest that a female’s latency to return to her nest and resume normal parental activity following a disturbance may represent a simple, functional measure of maternal stress reactivity that shapes the trade-off between reproductive effort and self-maintenance, and is underlain by among-female differences in physiological state.
We found that concentrations of corticosterone within eggs were significantly repeatable within females, as expected if lipophilic steroids that accumulate within eggs during egg formation are generally reflective of maternal physiology. Indeed, the circulating concentration of corticosterone in plasma has also been shown to be highly repeatable (Angelier et al. 2010; Schoenemann and Bonier 2018). Given that repeatability represents the maximum level of additive genetic variation that can be observed in a trait, these results suggest that corticosterone production could be heritable and, thus, potentially responsive to selection (see also Hayward et al. 2005; Jenkins et al. 2014), although the heritability may vary from year to year (Ouyang et al. 2011) or be hormone specific (Tschirren et al. 2009). Thus, given the negative correlation between maternal glucocorticoid production and levels of maternal care observed in the current study, one might expect selection to favor reduced sensitivity to stressors, such that parents are less-strongly affected by disturbances or visits by potential predators at their nests, but not if reduced stress-reactivity ultimately reduces maternal fitness (e.g., by reducing survival).
We caution, however, against drawing any inferences regarding the relative fitness, condition, or quality of females of varying baseline corticosterone during breeding until such data are available. The direction of net selection on either stress reactivity or glucocorticoid production is unclear, as variation in these traits in natural populations is likely subject to a number of opposing selective pressures. Moreover, subtle increases in corticosterone production may be adaptive in some cases while maladaptive in others (Love and Williams 2008; Sheriff et al. 2010; Bowers et al. 2015a; Weber et al. 2018), suggesting a high degree of context-dependence (see also Sheriff et al. 2017). For example, chronic elevations in baseline glucocorticoid levels are generally thought to induce putatively negative fitness consequences (Bonier et al. 2009a,b; Schoech et al. 2011). However, we previously found that transient elevations in maternal corticosterone during egg production can lead to increases in (i) maternal allocation to eggs, (ii) nestling begging post-hatching, (iii) the size of the offspring they subsequently rear to fledging, and (iv) recruitment of these offspring as breeders in the local population in future years (Bowers et al. 2016; Weber et al. 2018). We also observed an effect of maternal corticosterone in reducing the stress reactivity of their offspring, an effect manifested via both pre- and post-natal maternal effects (Weber et al. 2018; see also Schoech et al. 2012).
In these previous studies, maternal corticosterone was manipulated only during egg laying, and, in Weber et al. (2018), the eggs the females subsequently produced were cross-fostered among nests prior to or shortly after hatching, and yet we detected lasting effects of the increased maternal corticosterone on post-natal allocation, much later into the nesting cycle than might be expected given the normally rapid clearance of corticosteroids (e.g., half-life of ca. 66 min reported by Weitzman et al. 1971). Comparative studies of adult birds reveal that stress reactivity is generally muted during reproduction, whereas in stressful times outside the breeding period, HPA axis activity is upregulated. Likewise, stress reactivity is lower during periods of food abundance, but higher when there may be insufficient food to support reproduction (Krause et al. 2016; Wingfield et al. 2018). Thus, stress reactivity may be an important component of the classic life-history trade-off between growth and survival among neonates, and between reproduction and survival among adults. However, baseline glucocorticoids often increase within breeding vs. non-breeding individuals, as expected if rearing young requires mobilizing energetic reserves in general (Hennin et al. 2015, 2016; Lamarre et al. 2017).
Given the link between glucocorticoids and metabolic rate (Jimeno et al. 2018) and in promoting foraging (Angelier et al. 2007; Crossin et al. 2012), circulating glucocorticoids may provide an important source of variation in maternal investment, particularly if it enhances the ability of females to acquire and assimilate food in the wild (Merkling et al. 2017). For example, in a multi-year study, Hennin et al. (2015) recently obtained a large sample of female common eiders (Somateria mollissima) shortly before clutch initiation, and found a pronounced increase in baseline corticosterone leading up to egg laying, which was strongly positively associated with increases in body mass (see also Hennin et al. 2016), very-low-density lipoprotein, and vitellogenin, each of which is essential to mediating maternal investment in eggs (Hennin et al. 2015). It is worth noting, however, that the species studied by Hennin et al. (2016) is a capital breeder, whose reproductive allocation is dependent upon stored nutritional reserves, whereas our study species is an income breeder in which allocation varies with current resource availability. Thus, glucocorticoids could have different effects in these distantly related species with very different life histories. Nonetheless, we recently found that experimental increases in maternal glucocorticoids in our study species led them to produce clutches with more eggs, and allocated more yolk to these eggs (Bowers et al. 2016; Weber et al. 2018), consistent with that previous study. Moreover, we found in the current study that, while maternal corticosterone within eggs was positively correlated with their latency to resume normal parental behavior, there was a negative correlation, albeit marginally non-significant, between this latency and the amount of yolk that females had recently allocated to their eggs.
In conclusion, we found concentrations of corticosterone within eggs to be repeatable within females and predictive of their post-natal parental behavior expressed 2–3 weeks later. Specifically, the latency with which females resumed parental activities following our disturbance during nestling rearing was positively related to the amount of corticosterone that had accumulated within their eggs. This latency was also negatively correlated with maternal provisioning effort in general, and a reduction in nestling pre-fledging mass. These results suggest that females with greater baseline corticosterone levels may favor self-maintenance at a cost to reproduction. At the same time, the latency with which females resumed parental care was negatively correlated with the amount of yolk allocated to eggs, further suggesting that post-hatching maternal effort is generally consistent with pre-natal allocation to eggs. Thus, our results suggest that the latency to resume parental activities following a standardized disturbance may provide a realistic, behavioral proxy for stress reactivity and underlying differences in physiological state.
What is already known:
The relationship between baseline corticosterone and reproduction in birds is unclear, with both positive and negative correlations between baseline corticosterone and fitness having been documented. Although relationships between baseline corticosterone and reproductive effort have been reported, exactly how differences in baseline corticosterone at one stage of the breeding cycle influence reproductive effort at later stages is unclear.
What this study adds:
We quantified the concentration of corticosterone in yolks of freshly laid eggs and related this to the latency with which female house wrens resumed parental care following a standardized disturbance later at their nest when they were provisioning nestlings. Females producing eggs containing higher corticosterone concentrations took longer to return to the nest to resume parental activities following the disturbance, and provisioned their nestlings at a lower rate, resulting in young of lower body condition at fledging. Thus, our results suggest that latency to resume parental care can be a useful behavioral proxy for stress reactivity that is underlain by individual differences in physiological state.
Acknowledgments
We thank the 2012–2013 Wren Crews for field assistance, the ParkLands Foundation (Merwin Preserve), the Illinois Great Rivers Conference of the United Methodist Church and the Sears and Butler families for the use of their properties, and two anonymous reviewers who provided helpful comments that improved the manuscript. Financial support was provided by NSF grants IBN-0316580, IOS-0718140, and IOS-1118160; NIH grant R15HD076308; the School of Biological Sciences, Illinois State University; and student-research grants from the Animal Behavior Society, the American Museum of Natural History’s Frank M. Chapman Memorial Fund, and the Beta Lambda Chapter of the Phi Sigma Biological Honor Society at Illinois State University.
Literature Cited
- Almasi B, Rettenbacher S, Müller C, Brill S, Wagner H, and Jenni L 2012. Maternal corticosterone is transferred into the egg yolk. Gen Comp Endocrinol 178:139–144. [DOI] [PubMed] [Google Scholar]
- Angelier F, Clément-Chastel C, Gabrielsen GW, and Chastel O 2007. Corticosterone and time–activity budget: an experiment with black-legged kittiwakes. Horm Behav 52:482–491. [DOI] [PubMed] [Google Scholar]
- Angelier F, Wingfield JC, Weimerskirch H, and Chastel O 2010. Hormonal correlates of individual quality in a long-lived bird: a test of the ‘corticosterone-fitness hypothesis.’ Biol Lett 6:846–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonier F, Martin PR, Moore IT, and Wingfield JC 2009a. Do baseline glucocorticoids predict fitness? Trends Ecol Evol 24:634–642. [DOI] [PubMed] [Google Scholar]
- Bonier F, Moore IT, Martin PR, Robertson RJ 2009b. The relationship between fitness and baseline glucocorticoids in a passerine bird. Gen Comp Endocrinol 163:208–213. [DOI] [PubMed] [Google Scholar]
- Both C, Visser ME, and Verboven N 1999. Density-dependent recruitment rates in great tits: the importance of being heavier. Proc R Soc Lond B 266:465–469. [Google Scholar]
- Bowers EK, Sakaluk SK, and Thompson CF 2013. Sibling cooperation influences the age of nest leaving in an altricial bird. Am Nat 181:775–786. [DOI] [PubMed] [Google Scholar]
- Bowers EK, Nietz D, Thompson CF, and Sakaluk SK 2014a. Parental provisioning in house wrens: effects of varying brood size and consequences for offspring. Behav Ecol 25:1485–1493. [Google Scholar]
- Bowers EK, Hodges CJ, Forsman AM, Vogel LA, Masters BS, Johnson BGP, Johnson LS, Thompson CF, and Sakaluk SK 2014b. Neonatal body condition, immune responsiveness, and hematocrit predict longevity in a wild bird population. Ecology 95:3027–3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers EK, Bowden RM, Sakaluk SK, and Thompson CF 2015a. Immune activation generates corticosterone-mediated terminal reproductive investment in a wild bird. Am Nat 185:769–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers EK, Thompson CF, and Sakaluk SK 2015b. Persistent sex-by-environment effects on offspring fitness and sex-ratio adjustment in a wild bird population. J Anim Ecol 84:473–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers EK, Bowden RM, Thompson CF, and Sakaluk SK 2016. Elevated corticosterone during egg production elicits increased maternal investment and promotes nestling growth in a wild songbird. Horm Behav 83:6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuner CW 2011. Stress and reproduction in birds. Pp. 129–151 in Norris DO, and Lopez KH, eds. Hormones and Reproduction of Vertebrates, Volume 4 Elsevier, London. [Google Scholar]
- Crossin GT, Trathan PN, Phillips RA, Gorman KB, Dawson A, Sakamoto KQ, and Williams TD 2012. Corticosterone predicts foraging behavior and parental care in macaroni penguins. Am Nat 180:E31–E41 [DOI] [PubMed] [Google Scholar]
- Deviche P, Bittner S, Gao S, and Valle S 2017. Roles and mechanistic bases of glucocorticoid regulation of avian reproduction. Integr Comp Biol 57:1184–1193. [DOI] [PubMed] [Google Scholar]
- Hau M, Casagrande S, Ouyang JQ, and Baugh AT 2016. Glucocorticoid-mediated phenotypes in vertebrates: multilevel variation and evolution. Adv Study Behav 48:41–115. [Google Scholar]
- Hayward LS and Wingfield JC 2004. Maternal corticosterone is transferred to avian yolk and may alter offspring growth and adult phenotype. Gen Comp Endocrinol 135:365–371. [DOI] [PubMed] [Google Scholar]
- Hayward LS, Satterlee DG, and Wingfield JC 2005. Japanese quail selected for high plasma corticosterone response deposit high levels of corticosterone in their eggs. Physiol Biochem Zool 78:1026–1031. [DOI] [PubMed] [Google Scholar]
- Henderson LJ, Evans NP, Heidinger BJ, Herborn KA, and Arnold KE 2017. Do glucocorticoids predict fitness? Linking environmental conditions, corticosterone and reproductive success in the blue tit, Cyanistes caeruleus. R. Soc. Open Sci 4:170875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennin HL, Legagneux P, Bêty J, Williams TD, Gilchrist HG, Baker TM, and Love OP 2015. Pre-breeding energetic management in a mixed-strategy breeder. Oecologia 177:235–243. [DOI] [PubMed] [Google Scholar]
- Hennin HL, Wells-Berlin AM, and Love OP 2016. Baseline glucocorticoids are drivers of body mass gain in a diving seabird. Ecol Evol 6:1702–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins BR, Vitousek MN, Hubbard JK, and Safran RJ 2014. An experimental analysis of the heritability of variation in glucocorticoid concentrations in a wild avian population. Proc R Soc B 281:20141302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimeno B, Hau M, and Verhulst D 2018. Corticosterone levels reflect variation in metabolic rate, independent of ‘stress’. Sci Rep 8:13020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LS 2014. House wren (Troglodytes aedon), version 2.0. In Poole AF, ed. The Birds of North America Ithaca, NY: Cornell Lab of Ornithology; https://doi.org/10.2173/bna.380 . Retrieved from the Birds of North America: https://doi.org/10.2173/bna.380https://birdsna.org/Species-Account/bna/species/houwre. Retrieved from the Birds of North America: https://birdsna.org/Species-Account/bna/species/houwre . [Google Scholar]
- Krause JS, Pérez JH, Chmura HE, Meddle SL, Hunt KE, Gough L, Boelman N, and Wingfield JC 2016. The stress response is attenuated during inclement weather in parental, but not in pre-parental, Lapland longspurs (Calcarius lapponicus) breeding in the Low Arctic. Horm Behav 83:68–74. [DOI] [PubMed] [Google Scholar]
- Lamarre V, Franke A, Love OP, Legagneux P, and Bêty J 2017. Linking pre-laying energy allocation and timing of breeding in a migratory arctic raptor. Oecologia 183:653–666. [DOI] [PubMed] [Google Scholar]
- Lambrechts MM, Adriaensen F, Ardia DR, Artemyev AV, Atiénzar F, Bańbura J, Barba E, et al. 2010. The design of artificial nestboxes for the study of secondary hole-nesting birds: a review of methodological inconsistencies and potential biases. Acta Ornithol 45:1–26. [Google Scholar]
- Landys MM, Ramenofsky M, and Wingfield JC 2006. Actions of glucocorticoids at a seasonal baseline as compared to stress-related levels in the regulation of periodic life processes. Gen Comp Endocrinol 148:132–149. [DOI] [PubMed] [Google Scholar]
- Legagneux P, Hennin H, Gilchrist HG, Williams TD, Love OP, and Bêty J 2016. Unpredictable perturbation reduces breeding propensity regardless of pre-laying reproductive readiness in a partial capital breeder. J Avian Biol 47:880–886. [Google Scholar]
- Lessells CM and Boag PT 1987. Unrepeatable repeatabilities: a common mistake. Auk 104:116–121. [Google Scholar]
- Lothery CJ, Thompson CF, Lawler ML, and Sakaluk SK 2014. Food supplementation fails to reveal a trade-off between incubation and self-maintenance in female house wrens. PLoS ONE 9:e106260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love OP and Williams TD 2008. The adaptive value of stress‐induced phenotypes: effects of maternally derived corticosterone on sex‐biased investment, cost of reproduction, and maternal fitness. Am Nat 172:E135–E149. [DOI] [PubMed] [Google Scholar]
- McCormick MI 1998. Behaviorally induced maternal stress in fish influences progeny quality by a hormonal mechanism. Ecology 79:1873–1883. [Google Scholar]
- Merkling T, Blanchard P, Chastel O, Glauser G, Vallat-Michel A, Hatch SA, Danchin E, and Helfenstein F 2017. Reproductive effort and oxidative stress: effects of offspring sex and number on the physiological state of a long-lived bird. Funct Ecol 31:1201–1209. [Google Scholar]
- Monaghan P, and Spencer KA 2014. Stress and life history. Curr Biol 24:R408–R412. [DOI] [PubMed] [Google Scholar]
- Murphy MT, Chutter CM, and Redmond LJ 2015. Quantification of avian parental behavior: what are the minimum necessary sample times? J Field Ornithol 86:41–50. [Google Scholar]
- Naef-Daenzer B, Widmer F, and Nuber M 2001. Differential post-fledging survival of great and coal tits in relation to their condition and fledging date. J Anim Ecol 70:730–738. [Google Scholar]
- Nakagawa S, and Schielzeth H 2010. Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol Rev 85:935–956. [DOI] [PubMed] [Google Scholar]
- Okuliarová M, Šárniková B, Rettenbacher S, Škrobánek P, and Zeman M 2010. Yolk testosterone and corticosterone in hierarchical follicles and laid eggs of Japanese quail exposed to long-term restraint stress. Gen Comp Endocrinol 165:91–96. [DOI] [PubMed] [Google Scholar]
- Ouyang JQ, Hau M, and Bonier F 2011. Within seasons and among years: when are corticosterone levels repeatable? Horm Behav 60:559–564. [DOI] [PubMed] [Google Scholar]
- Ouyang JQ, Muturi M, Quetting M, and Hau M 2013a. Small increases in corticosterone before the breeding season increase parental investment but not fitness in a wild passerine bird. Horm Behav 63:776–81. [DOI] [PubMed] [Google Scholar]
- Ouyang JQ, Sharp P, Quetting M, and Hau M 2013b. Endocrine phenotype, reproductive success and survival in the great tit, Parus major. J Evol Biol 26:1988–98. [DOI] [PubMed] [Google Scholar]
- Paitz RT, Bowden RM, and Casto JM 2011. Embryonic modulation of maternal steroids in European starlings. Proc Roy Soc B 278:99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitk M, Tilgar V, Kilgas P, and Mänd R 2012. Acute stress affects the corticosterone level in bird eggs: a case study with great tits (Parus major). Horm Behav 62:475–479. [DOI] [PubMed] [Google Scholar]
- Ricklefs RE and Wikelski M 2002. The physiology/life-history nexus. Trends Ecol Evol 17:462–468. [Google Scholar]
- Romero LM 2002. Seasonal changes in plasma glucocorticoid concentrations in free-living vertebrates. Gen Comp Endocrinol 128:1–24. [DOI] [PubMed] [Google Scholar]
- Romero LM, Dickens MJ, and Cyr NE 2009. The reactive scope model – a new model integrating homeostasis, allostasis, and stress. Horm Behav 55:375–389. [DOI] [PubMed] [Google Scholar]
- Sakaluk SK, Thompson CF, and Bowers EK 2018. Experimental manipulation of incubation period reveals no apparent costs of incubation in house wrens. Anim Behav 137:169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, and Munck AU 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrinol Rev 21:55–89. [DOI] [PubMed] [Google Scholar]
- Schielzeth H 2010. Simple means to improve the interpretability of regression coefficients. Methods Ecol Evol 1:103–113. [Google Scholar]
- Schoech SJ, Rensel MA, and Heiss RS 2011. Short- and long-term effects of developmental corticosterone exposure on avian physiology, behavioral phenotype, cognition, and fitness: a review. Curr Zool 57:514–530. [Google Scholar]
- Schoech SJ, Rensel MA, and Wilcoxen TE 2012. Here today, not gone tomorrow: long-term effects of corticosterone. J Ornithol 153:217–226. [Google Scholar]
- Schoenemann KL and Bonier F 2018. Repeatability of glucocorticoid hormones in vertebrates: a meta-analysis. PeerJ 6:e4398; DOI 10.7717/peerj.4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenle LA, Dudek AM, Moore IT, and Bonier F 2017. Red-winged blackbirds (Agelaius phoeniceus) with higher baseline glucocorticoids also invest less in incubation and clutch mass. Horm Behav 90:1–7. [DOI] [PubMed] [Google Scholar]
- Sheriff MJ, Krebs CJ, and Boonstra R 2010. The ghosts of predators past: population cycles and the role of maternal programming under fluctuating predation risk. Ecology 91:2983–2994. [DOI] [PubMed] [Google Scholar]
- Sheriff MJ, and Love OP 2013. Determining the adaptive potential of maternal stress. Ecol Lett 16:271–280. [DOI] [PubMed] [Google Scholar]
- Sheriff MJ, Bell A, Boonstra R, Dantzer B, Lavergne SG, McGhee KE, MacLeod KJ, Winandy L, Zimmer C, and Love OP 2017. Integrating ecological and evolutionary context in the study of maternal stress. Integr Comp Biol 57:437–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiseth PT, Scott MP, and Andrews C 2011. Hormonal regulation of offspring begging and mediation of parent-offspring conflict. Anim Behav 81:507–517. [Google Scholar]
- Tinbergen JM and Boerlijst MC 1990. Nestling weight and survival in individual great tits (Parus major). J Anim Ecol 59:1113–1127. [Google Scholar]
- Tschirren B, Sendecka J, Groothuis TGG, Gustafsson L, and Doligez B 2009. Heritable variation in maternal yolk hormone transfer in a wild bird population. Am Nat 174:557–564. [DOI] [PubMed] [Google Scholar]
- Weber BM, Bowers EK, Terrell KA, Falcone FJ, Thompson CF, and Sakaluk SK 2018. Pre- and post-natal effects of experimentally manipulated maternal corticosterone on growth, stress reactivity, and survival of nestling house wrens. Funct Ecol 32:1995–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzman ED, Fukushima D, Nogeire C, Roffwarg H, Gallagher TF, and Hellman L 1971. Twenty-four hour pattern of the episodic secretion of cortisol in normal subjects. J Clinic Endocrinol Metab 33:14–22. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Maney DL, Breuner CW, Jacobs JD, Lynn S, Ramenofsky M, and Richardson RD 1998. Ecological bases of hormone-behavior interactions: the “emergency life history stage.” Am Zool 38:191–206. [Google Scholar]
- Wingfield JC, Hau M, Boersma PD, Romero LM, Hillgarth N, Ramenofsky M, Wrege P, et al. 2018. Effects of El Niño and La Niña Southern Oscillation events on the adrenocortical responses to stress in birds of the Galapagos Islands. Gen Comp Endocrinol 259:20–33. [DOI] [PubMed] [Google Scholar]
- Young BE 1996. An experimental analysis of small clutch size in tropical house wrens. Ecology 77:472–488. [Google Scholar]


